Abstract
The Lewis acidic framework Ti sites in Ti-Beta and Si-Beta catalysts were compared by FT-IR and NMR characterization methods before they were applied to the conversion of four butenes. The results showed that Si-Beta has fewer Lewis acid sites and abundant weak Brønsted acidic silanol nests, which play an important role in conversions between n-butene, cis-2-butene, and trans-2-butene. The conversions for these butenes over Si-Beta were always higher than those over a series of Ti-Beta catalysts with gradient-varied Lewis acidic framework Ti sites and silanols. This is because isobutene can only oligomerize, which requires stronger acidity, so its conversion over Si-Beta was lower than those over Ti-Beta zeolites. For a series of Ti-Beta catalysts with different abundances of Lewis acidic Ti sites, the more Lewis acid sites it had, the higher the conversions for the four butenes.
1. Introduction
Zeolites are indispensable catalysts in chemistry and chemical engineering due to their unique pore structures and adjustable acid properties [1,2]. This adjustability allows the formation of zeolites with Brønsted and Lewis acid sites [3] of different strengths [4] and contents [5], both of which determine their properties and application scope in refining, petrochemical processing, and fine chemicals. Olefin reactions catalyzed by solid acids are crucial processes in chemistry [6,7]. A deep understanding of double-bond and skeletal isomerization reaction mechanisms of olefins catalyzed by solid acid catalysts is important in catalyst development [8,9]. Butene isomers, i.e., n-butene, cis-2-butene, trans-2-butene, and isobutene, show simple structures and are, therefore, effective probe molecules in mechanistic research [10] and in industrial applications [11]. For example, butene isomers are important chemical materials and feedstocks in isomerization [12], alkylation [13], and oligomerization reactions [14].
The Brønsted and Lewis acid sites of zeolites supply protons and accept electrons, respectively, allowing them to promote reactions by different mechanisms [15]. The Brønsted acid sites of zeolites comprise bridged hydroxy groups, so they promote hydrocarbon-conversion reactions, such as isomerization, oligomerization, and aromatization, by the classical carbenium mechanism [16]. In addition, silanol nests in zeolites are another significant source of weak acidity that has a catalytic effect [17,18,19,20,21,22]. Silanols in zeolites also show weak Brønsted acidity, which promotes addition to double bonds [17], but cannot protonate double bonds following carbenium ions mechanism. Lewis acid sites [23] originating from reactive oxygen sites and silanol nests [24] in Si-Beta have been shown to catalyze butene groups to alkoxyl groups.
In contrast, Lewis acid sites in zeolites are complex, mainly containing extra-framework Al species such as Al(OH)3, Al(OH)2+, AlOH2+, and Al3+ [25,26,27]. Tetrahedral framework metal sites, such as Sn [21], Ti [28], Zr [29], and Lewis acidic metal ions, also exhibit Lewis acidity, which catalyzes olefin reactions via π-/σ-allyl [30,31] and σ-alkyl species [1,31,32] as intermediates. Tri-coordinated Al [33] and Si+ [34] in the framework of the zeolite also show Lewis acidities. Furthermore, the cleavage of strained three-membered rings in amorphous SiO2 also produces Si+ and reactive oxygen species (ROS). Mechanisms of double-bond reactions of butenes on Lewis acid sites are rare.
However, a mechanistic comparison of silanol as a weak Brønsted acid and Lewis acidic metal sites in M-Beta zeolites for the conversion of butenes has yet to be reported. Therefore, in the present study, Ti-Beta zeolites with different contents of framework Ti sites as well as Si-Beta with silanol nests alone were prepared by gas-phase impregnation and dealumination methods. Then, four butene isomer probe molecules were used to compare the effects of Lewis acidic Ti sites and silanol nests in Ti-Beta zeolites on butene conversion.
2. Results and Discussion
2.1. Crystal Structure and Textural Properties
The Si/Al ratio of Si-Beta was over 1200, suggesting that all aluminum of Beta had been removed, which was verified by ICP-AES. Si-Beta and Ti-Beta with typical BEA topological structures were analyzed by XRD (Figure 1a), which confirmed that both dealumination and gas-phase impregnation do not destroy the zeolite framework. The position of the (302) diffraction peak moved from 22.54° for zeolite Beta to 22.76° for Si-Beta, which confirmed the gradual contraction of the BEA matrix. The peak shift from 22.76° for Si-Beta to 22.58° for Ti-Beta is evidence of the lattice expansion of the structure, which suggests the incorporation of Ti into skeletal silanol nests of Si-Beta [35].
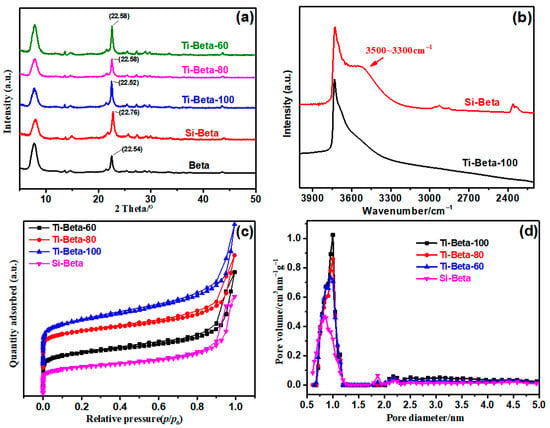
Figure 1.
(a) XRD patterns, (b) FT-IR spectra, (c) Ar adsorption–desorption isotherms, and (d) micropore size distributions of samples.
All samples show type-I adsorption and desorption isotherms (Figure 1c), which further indicates that dealumination does not destroy the micropores in accordance with the XRD results. The silanol nests in Si-Beta occupy its channel space and, therefore, decrease the micropore volume (Table 1), which is confirmed by the micropore size-distribution results (Figure 1d). The presence of hydrogen-bonding silanol nests is verified by a broad band at 3500–3300 cm−1 in the FT-IR spectra (Figure 1b), which confirms the presence of vacant T-sites [36,37,38]. For Ti-Beta-100, the intensity of this broad band decreases dramatically when Ti is loaded into the silanol nests.

Table 1.
Textural and acidic properties of Si-Beta and Ti-Beta from Py-IR.
The dealumination of Beta decreases the micropores and increases the mesopores. Compared to textural properties of Beta, both the Smic and micropore volume of Beta decreased (Table 1). The incorporation of Ti into the framework of Si-Beta forms Si-O-Ti while also removing silanol nests, which releases more micropore volume (Table 1). Therefore, with the incorporation of Ti from Ti-Beta-100 to Ti-Beta-60, the surface areas of micropores (Smic) increase and the external surface areas (Sext) decrease. The incorporation of Ti into the Si-Beta framework was confirmed by UV–Vis DRS (Figure 2a) and XPS analyses (Figure 2b). The band at 226 nm is due to charge transfer from O2− to Ti4+, which verifies the successful incorporation of Ti atoms into the zeolite frameworks [39]. The absence of absorption signals above 330 nm indicates that bulky TiO2 or Ti-enriched species were not formed in Ti-Beta [34]. Generally, the Ti 2P binding energy values of 460.0 and 465.7 eV are assigned to the 2p 3/2 and 2p 1/2 photoelectrons of tetrahedrally coordinated framework Ti(IV) species [40], which verifies the incorporation of Ti into silanol nests. The silica/alumina ratio of pure silica zeolite Si-Beta (over 1200) and the silica/alumina ratio of Ti-Beta were determined by ICP-AES (Table 1). SEM micrographs (Figure 2c) and EDS elemental mapping (Figure 2d) show the highly dispersed Ti in Ti-Beta.
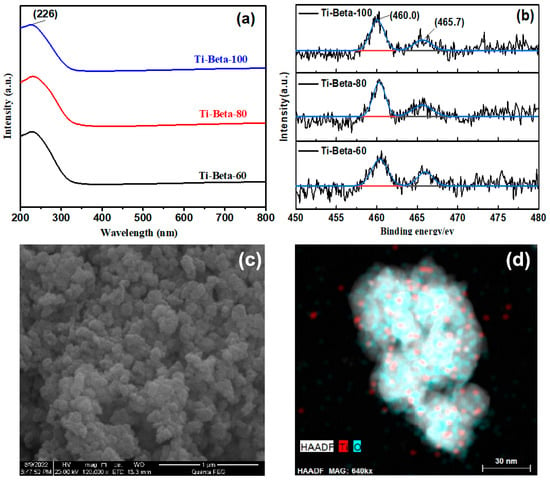
Figure 2.
(a) UV–Vis and (b) Ti 2p XPS spectra, (c) SEM images, and (d) EDS elemental mapping of Ti in Ti-Beta-100 by HAADF-STEM.
2.2. Acidic Properties
The acidic properties of Si-Beta and Ti-Beta were quantified by NH3-TPD and Py-IR. The acid sites in Si-Beta comprise a small amount of Lewis acid sites and a large amount of silanol nests showing aprotonic acidity with peaks that are not well defined [41] and are distinguished by bands at 1453 cm−1 and 1446–1439 cm−1 (dashed boxes in Figure 3c). The silanol nests and Lewis acid sites can be distinguished by the 19a and 8b band modes of pyridine adsorbed on samples at 1483 cm−1 and 1495 cm−1, respectively [42]. Si-Beta presents Lewis acids from Si+ and reactive oxygen sites, which are formed from the cleavage of strained three-membered rings when the probe molecules such as pyridine are adsorbed on Si-Beta, as verified by the Raman signal at 607 cm−1 (Figure 2b) [43]. The abundance of silanol nests is much greater than that of Lewis acid sites, which were both weak acid sites, in Si-Beta. Lewis acid sites in Ti-Beta originate from framework metallic Ti [21]. The amounts of Lewis acidic Ti sites and silanols were gradient-varied to generate Ti-Beta-100, Ti-Beta-80, and Ti-Beta-60 (Table 1). The three-membered rings are absent in Ti-Beta, owing to the incorporation of Ti into the framework of Si-Beta, as verified by Raman spectroscopy (Figure 3b). The large amount of silanol nests is indicated by the NH3 desorption signal at about 100 °C (Figure 3a). With the incorporation of Ti into the silanol nests, the NH3 desorption peak areas mainly representing the amount of silanols decrease from Si-Beta to Ti-Beta-60.
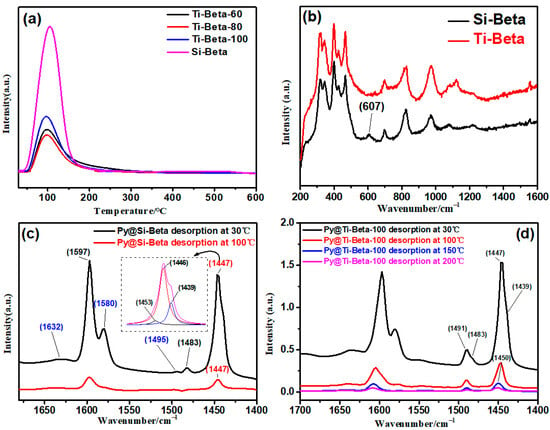
Figure 3.
(a) NH3-TPD, (b) Raman and Py-IR spectra of pyridine adsorbed on (c) Si-Beta and (d)Ti-Beta-100.
Lewis acidic Ti sites and silanol in Ti-Beta were distinguished after TMPO probe adsorption by 31P SSNMR spectra with and without cross-polarization (Figure 4a,b). Chemical shift at 56.0 ppm is ascribed to the 31P signals of TMPO adsorbed on Lewis acidic Ti sites [34], while the shift at 38.8 ppm is ascribed to the 31P signal of TMPO adsorbed on silanol. Silanol nests are only present in Si-Beta (Figure 4a). There are Lewis acid sites and silanol in Ti-Beta, which are distinguished by 31P MAS NMR spectra with cross-polarization (Figure 4b). The signals at 56.1 ppm and 38.3 ppm are ascribed to the 31P signals of TMPO adsorbed on Lewis acidic Ti sites and silanols, respectively.
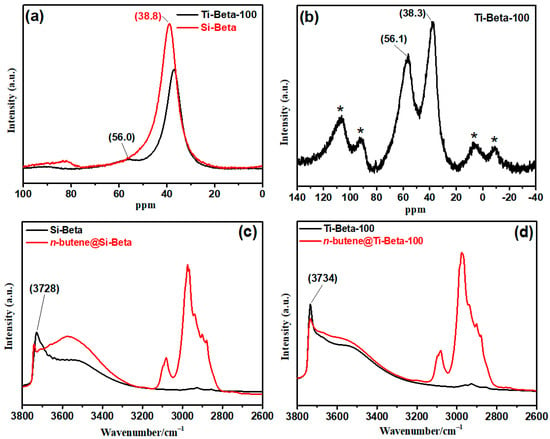
Figure 4.
31P MAS NMR spectra of TMPO adsorbed on samples obtained (a) without and (b) with cross-polarization and the FT-IR spectra of n-butene adsorbed on (c) Si-Beta and (d) Ti-Beta-100. * represents spinning side band.
2.3. Catalytic Performance
The effects of the different acid sites in the Si-Beta and Ti-Beta samples on the transformation of four different butenes were investigated using a microreactor (Figure 5 and Table 2, Table 3, Table 4 and Table 5). Both Lewis acids and silanols participate in double-bond isomerization reactions [24]. Double-bond migration reactions of n-butene follow the abstraction–addition (AB–AD) mechanism, which is realized on Lewis acid sites first and then silanols. During the above process, a hydrogen in the CH2 group of n-butene is abstracted (AB) by Ti in Ti-Beta to produce σ-bonded allylic species as an intermediate. After a structural transformation of the allylic species, the abstracted hydrogen can be added to the first carbon atom to produce cis-2-butene and trans-2-butene products (Scheme 1). Conversely, cis–trans isomerization between cis-2-butene and trans-2-butene follows an addition–abstraction (AD–AB) mechanism, which is realized on silanols first and then Lewis acid sites. During this process, a silanol hydrogen is added to the double bond of cis-2-butene to generate an alkyl group. The dehydrogenation of the methylene group is facilitated, and trans-2-butene is produced. This pathway is defined as an AD–AB mechanism as shown in Scheme 1.
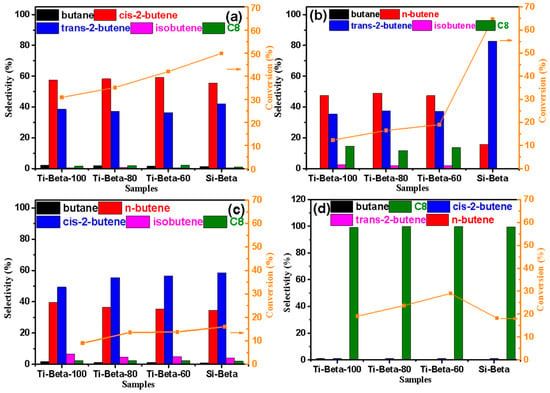
Figure 5.
Catalytic transformation performance for (a) n-butene, (b) cis-2-butene, (c) trans-2-butene, and (d) isobutene on Si-Beta and Ti-Beta zeolites.

Table 2.
Product distribution of n-butene isomerization on samples.

Table 3.
Product distribution of cis-2-butene isomerization on samples.

Table 4.
Product distribution of trans-2-butene isomerization on samples.

Table 5.
Product distribution of isobutene oligomerization on samples.
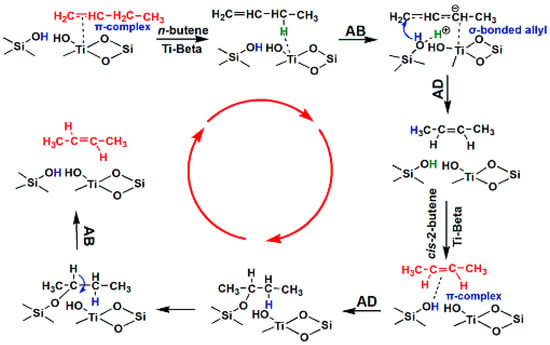
Scheme 1.
Mechanisms of n-butene isomerization reactions on Ti-Beta.
Thus, the Lewis acid sites from framework Ti in Ti-Beta are beneficial for the double-bond isomerization of n-butene, cis–trans isomerization between cis-2-butene and trans-2-butene, and oligomerization reactions of isobutene. Accordingly, as the abundances of Lewis acid sites and silanols in the Ti-Beta increase and decrease, respectively, the higher the conversions of the butenes. Accordingly, extrapolated to the hydrogenation and dehydrogenation of long-chain alkenes and alkanes on metal sites as Lewis acid sites, the more metallic sites are beneficial to the activation of long-chain hydrocarbons within a certain range of metal loading. The more metallic sites in catalysts, the higher the conversion of hydrocarbons in reactions.
Compared with the oligomerization reaction of isobutene, which needs stronger acids, the double-bond isomerization reactions, including double-bond migration and cis–trans isomerization, occur more readily for n-butene and 2-butene. Although the acid strength of silanols is lower than that of Lewis acidic Ti sites, the larger amount of silanols in Si-Beta has a large influence on the double-bond isomerization of n-butene and 2-butene. Therefore, the conversions of n-butene, cis-2-butene, and trans-2-butene on Si-Beta are higher than those on Ti-Beta, as shown in Figure 5. Accordingly, in contrast with light olefins, the isomerizations of long-chain olefins need stronger acids such as metallic Lewis acid sites, which cannot occur on Si-Beta with abundant silanols. The participation of silanols in the transformation of n-butene was explored using FT-IR spectra to characterize n-butene adsorption on Si-Beta and Ti-Beta (Figure 4c,d). A large amount of silanol in Si-Beta participates in the conversion of n-butene, as its consumption is indicated by the decrement of its signal at 3728 cm−1. Conversely, very little silanol in Ti-Beta participates in the reaction. Thus, silanol nests as weak Brønsted acid sites are the main active sites in Si-Beta, while Ti Lewis acid sites are the main active sites in Ti-Beta. However, the oligomerization of isobutene requires strong acidity. The reactive oxygen sites and silanols in Si-Beta are weak Brønsted acid sites. Accordingly, the oligomerization rate of isobutene on Si-Beta is lower than those on the Ti-Beta zeolites.
3. Materials and Methods
3.1. Preparation of Samples
Si-Beta zeolite was obtained by the dealumination of Beta, which was purchased from Shanghai Xinnian Petrochemical Additives Co., Ltd. (Shanghai, China). The Si/Al ratio of zeolite Beta was 10.6. The dealumination of Beta was realized by 13.5 mol/L nitric acid aqueous solution at 100 °C for 12 h in which the ratio of nitric acid aqueous solution (mL) to Beta zeolite (g) was twenty to one. All aluminum species of Beta were removed by concentrated nitric acid aqueous solution completely. The obtained dealuminated zeolite named Si-Beta was subsequently washed by deionized water, dried, and calcinated at 550 °C for 6 h.
Ti-Beta-X (X are 100, 80, and 60 representing the ratio of Si to Ti) zeolites were obtained by the gas-phase impregnation method [21]. Si-Beta without calcination was pretreated at 200 °C for 12 h under vacuum in order to remove the impurity. Then, certain amounts of Si-Beta and titanocene dichloride (Cp2TiCl2) precursor powders were uniformly mixed under nitrogen atmosphere. Finally, the mixture was spattering uniformly on a crucible and transferred into a tube furnace rapidly. The tube furnace was sealed, vacuumized, and heated at 550 °C for 6 h. During this process, Cp2TiCl2 precursor was gasified into vapor and impregnated on Si-Beta. The obtained Ti-Beta zeolites were finally calcinated in a muffle furnace at 550 °C for 6 h.
3.2. Characterizations
X-ray diffraction (XRD) technology was used to analyze phases of Ti-Beta-X zeolites with Cu Kα radiation (40 kV, 40 mA). X-ray diffractometer model was Bruker D8 Avance (Bruker, Ettlingen, Germany).
Textural properties of Ti-Beta zeolites were measured by an AutosorbiQ analyzer at 77 K. Total surface area and microporous information were calculated by the Brunauer–Emmett–Teller (BET) equation and t-plot method, respectively. Total surface area is calculated by following Formula (1) including parameters such as equilibrium pressure (P); the amount of adsorption under P pressure (V); the constant related in adsorption heat (C); the amount of adsorption on the first layer when argon is completely covered (Vm); the saturated vapor pressure at experimental temperature (P0). Microporous information is obtained and calculated by following Formula (2) with several parameters including relative pressure represented by P/P0, monolayer saturated adsorption capacity (mL, STP) represented by Vm, and the average monolayer thickness of argon (nm) represented by tm. The pore size distribution is obtained by nonlocal density functional theory (NLDFT) assuming argon adsorption in cylindrical zeolite pores in the micropore ranges [44,45].
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was used to detect the silica/alumina ratio of Si-Beta and the silica/titanium ratio of Ti-Beta, which were carried out on an Optima 2100DV optical emission spectrometer (PerkinElmer, San Diego, CA, USA).
Framework Ti metal in Ti-Beta was verified by a spherical integrating detector (UH4150) in an ultraviolet and visible diffuse reflectance spectrometer (UV-Vis DRS) (Hitachi, Tokyo, Japan) with BaSO4 as the reference. The scanning speed was 10 nm/s with 1 nm step size and 5 nm slit width.
The morphology of zeolites was observed by a scanning electron microscope (SEM) (FEI QUANTA 400). The electron acceleration voltage was 10 kV. The element mapping of Ti-Beta was performed by high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) (FEI Talos F200A) and an energy-dispersive spectrometer (EDS) (FEI, Lausanne Switzerland).
Acidic properties of zeolites were characterized by pyridine-adsorbed infrared (Py-IR) spectroscopy and ammonia temperature-programmed desorption (NH3-TPD). Py-IR spectroscopy was performed on a Bruker VERTEX 70 spectrometer (Bruker, Ettlingen, Germany). The self-supporting wafer samples were pretreated at 350 °C for 1 h under vacuum. Then, samples were saturated and adsorbed with pyridine vapor until equilibrium at 30 °C. Finally, pyridine was desorbed at 100, 150, and 200 °C, respectively, and spectra were measured in the range of 400–4000 cm−1 at a resolution of 2 cm−1. Py-IR spectra were deconvoluted by the Gaussian function. NH3-TPD was conducted on a chemisorption analyzer (Micromeritics autochem II, Micromeritics, Norcross, GA, USA). The samples were first pretreated at 350 °C under helium for 1 h followed by saturated adsorption of ammonia at 30 °C. Then, the samples were purged by helium for 30 min to desorb physically absorbed NH3 and heated to 600 °C. Signals of desorbed ammonia were measured with a mass spectrometer quantitatively.
An ESCALAB 250Xi spectrometer with an Al Kα radiation multichannel detector (Thermo Scientific, Waltham, MA, USA) was used to test the Ti 2p X-ray photoelectron spectroscopy (XPS) of Ti-Beta zeolites. Ti 2p binding energies were corrected with regard to the C 1 s peak at 284.8 eV.
Prior to the adsorption of the probe molecule TMPO, samples were pretreated at 420 °C for at least 12 h under vacuum (below 10−3 Pa). Then, a desirable and known amount of TMPO in CH2Cl2 was syringed into the sample vessel containing the dehydrated samples followed by the removal of the CH2Cl2 solvent by evacuation at ca. 50 °C. Subsequently, the guest/host sample system was further subjected to a baking treatment at 150 °C for at least 1 h. The above thermal treatment is crucial in ensuring a uniform adsorption of TMPO on sites of the catalyst. Finally, the sample was transferred into a zirconia (ZrO2) MAS rotor and sealed with a gastight Kel-F cap under inert environment. The well-pretreated sample packed in the rotor was inserted into a MAS probe head for subsequent 31P MAS SSNMR experiments. All 31P NMR experiments were performed on a Bruker Avance III 600 WB spectrometer with a resonance frequency of 243 MHz and a spinning rate of 12 kHz (Bruker, Ettlingen, Germany). 31P MAS NMR spectra were recorded with a recycle delay of 10 s.
3.3. Catalyst Test
Isomerization reactions of butenes were performed on a stainless steel microreactor with a reaction tube (6 mm i.d.; length, 300 mm). Before the reaction, catalysts were pretreated at 623 K for 1 h to remove physically adsorbed water. The isomerization reactions of butenes were performed at atmospheric pressure, a weight hourly space velocity (WHSV) of 6 h−1, and 423 K. The reactant composition was 10% butene and 90% helium (molar ratio). Reaction products were analyzed by online gas chromatography (Agilent, Santa Clara, CA, USA) equipped with two FID detectors in which PONA and Al2O3 columns were applied to analyze heavy and light hydrocarbon, respectively.
4. Conclusions
A series of Ti-Beta zeolites were prepared based on Si-Beta by gas-impregnation methods. The physicochemical properties of the samples were compared by several characterization methods, especially FT-IR and NMR spectroscopies. The acidic properties of Si-Beta and Ti-Beta are markedly different. Weakly proton-acidic silanol nests are the main active sites in Si-Beta, while Lewis acidic Ti sites and silanol nests are the main active sites in Ti-Beta. The number of Lewis acidic sites increases with the increasing abundance of framework Ti sites from Ti-Beta-100 to Ti-Beta-60. Mutual conversions between n-butene, cis-2-butene, and trans-2-butene can be achieved readily on both the weak protonic acid sites in Si-Beta and the Lewis acidic Ti sites. Although the acid strength of silanols is lower than that of Lewis acidic Ti sites, the large amount of silanols has an important influence in the conversions of n-butene, cis-2-butene, and trans-2-butene. However, isobutene can only oligomerize on stronger acid sites. Therefore, Lewis acidic Ti sites in Ti-Beta are more beneficial to the oligomerization of isobutene.
Author Contributions
Conceptualization, J.-P.C. and Y.Y.; formal analysis, F.Y. and S.G.; investigation, M.X.; writing—original draft preparation, F.Y.; writing—review and editing, J.-P.C. and S.G.; funding acquisition, F.Y. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by the National Natural Science Foundation of China (22208370) (22408386), the Natural Science Foundation of Shanxi Province (Grant No. 202203021212005), and the Open Sharing Fund for the Large-Scale Instruments and Equipment of China University of Mining and Technology (CUMT) (DYGX-2023-070).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Mengjiao Xing was employed by the company State Power Investment Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Busca, G. Acid catalysts in industrial hydrocarbon chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.M.; Jentys, A.; Lercher, J.A. Steaming of Zeolite BEA and Its Effect on Acidity: A Comparative NMR and IR Spectroscopic Study. J. Phys. Chem. C 2011, 115, 8005–8013. [Google Scholar] [CrossRef]
- Chu, Y.; Yi, X.; Li, C.; Sun, X.; Zheng, A. Brønsted/Lewis acid sites synergistically promote the initial C–C bond formation in the MTO reaction. Chem. Sci. 2018, 9, 6470–6479. [Google Scholar] [CrossRef] [PubMed]
- Katada, N.; Suzuki, K.; Noda, T.; Sastre, G.; Niwa, M. Correlation between Brønsted Acid Strength and Local Structure in Zeolites. J. Phys. Chem. C 2009, 113, 19208. [Google Scholar] [CrossRef]
- Müller, S.; Liu, Y.; Kirchberger, F.M.; Tonigold, M.; Sanchezsanchez, M.; Lercher, J.A. Hydrogen Transfer Pathways during Zeolite Catalyzed Methanol Conversion to Hydrocarbons. J. Am. Chem. Soc. 2016, 138, 15994–16003. [Google Scholar] [CrossRef]
- Hatice, E.; Basbug, A.; Jones, G.R.; Eva, H. Branching Regulation in Olefin Polymerization via Lewis Acid Triggered Isomerization of Monomers. Angew. Chem. Int. Ed. 2020, 59, 4743–4749. [Google Scholar]
- Kenton, E.H.; Andrew, T.Y.W.; Omar, K.F.; Justin, M.N. The Dependence of Olefin Hydrogenation and Isomerization Rates on Zirconium Metal-Organic Framework Structure. ACS Catal. 2022, 12, 13671–13680. [Google Scholar]
- Sheng, D.; Zhang, Y.; Song, Q.; Xu, G.; Peng, D.; Hou, H.; Xie, R.; Shan, D.; Liu, P. Isomerization of 1-Butene to 2-Butene Catalyzed by Metal-Organic Frameworks. Organometallics 2020, 39, 51–57. [Google Scholar] [CrossRef]
- Rodrigo, J.C.; Claudio, J.A.M. Theoretical study of protonation of butene isomers on acidic zeolite: The relative stability among primary, secondary and tertiary alkoxy intermediates. Phys. Chem. Chem. Phys. 2002, 4, 375–380. [Google Scholar]
- Jennifer, P.; Kieran, P.S.; Shashank, S.N.; Weronika, W.; Henry, J.C. Theoretical Study of the Reaction of Hydrogen Atoms with Three Pentene Isomers: 2-Methyl-1-butene, 2-Methyl-2-butene, and 3-Methyl-1-butene. J. Phys. Chem. A 2020, 124, 10649–10666. [Google Scholar]
- Mikhail, V.P.; Dmitry, P.I.; Alexander, S.K.; Konstantin, A.D. Gas-Phase Selective Oxidation of Butenes in the C4 Fraction by Nitrous Oxide. Ind. Eng. Chem. Res. 2022, 61, 8607–8615. [Google Scholar]
- Alexander, G.; Joaquín, S.A.; Li, W.; Notker, R.; Günther, R. The origin of the particle-size-dependent selectivity in 1-butene isomerization and hydrogenation on Pd/Al2O3 catalysts. Nat. Commun. 2021, 12, 6098. [Google Scholar]
- Zhang, X.; Li, H.; Du, Y.; Chen, X.; Wang, P.; Wang, L.; Feng, X.; Yang, C.; Li, S. Elucidating effect of acid strength on isomerization mechanisms of butene over solid acid catalysts in C4 alkylation. Fuel 2023, 339, 127397. [Google Scholar] [CrossRef]
- Fabian, B.; Joachim, S. Dimerization of Linear Butenes and Pentenes in an Acidic Zeolite (H-MFI). Angew. Chem. Int. Ed. 2021, 60, 3529–3533. [Google Scholar]
- Kissin, Y.V. Chemical Mechanisms of Catalytic Cracking over Solid Acidic Catalysts: Alkanes and Alkenes. Chem. Rev. 2000, 43, 85–146. [Google Scholar] [CrossRef]
- Hansford, R.C. Mechanism of Catalytic Cracking. Ind. Eng. Chem. 2002, 39, 849–852. [Google Scholar] [CrossRef]
- Kondo, J.N.; Yoda, E.; Ishikawa, H.; Wakabayashi, F.; Domen, K. Acid Property of Silanol Groups on Zeolites Assessed by Reaction Probe IR Study. J. Catal. 2000, 191, 275–281. [Google Scholar] [CrossRef]
- SATO, H. Acidity Control and Catalysis of Pentasil Zeolites. Chem. Rev. 1997, 39, 395–424. [Google Scholar] [CrossRef]
- Ishikawa, H.; Yoda, E.; Konko, J.N.; Wakabayashi, F.; Domen, K. Stable Dimerized Alkoxy Species of 2-Methylpropene on Mordenite Zeolite Studied by FT-IR. J. Phys. Chem. B 1999, 103, 5681–5686. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; García, H.; Semmer-Herlédan, V.; Valencia, S. Active sites for the liquid-phase beckmann rearrangement of cyclohexanone, acetophenone and cyclododecanone oximes, catalyzed by beta zeolite. J. Catal. 1998, 177, 267–272. [Google Scholar] [CrossRef]
- Tang, B.; Dai, W.; Wu, G.; Guan, N.; Li, L.; Hunger, M. Improved Postsynthesis Strategy to Sn-Beta Zeolites as Lewis Acid Catalysts for the Ring-Opening Hydration of Epoxides. ACS Catal. 2014, 4, 2801–2810. [Google Scholar] [CrossRef]
- Finocchio, E.; Busca, G.; Rossini, S.; Cornaro, U.; Piccoli, V.; Miglio, R. FT-IR characterization of silicated aluminas, active olefin skeletal isomerization catalysts. Catal. Today. 1997, 33, 335–352. [Google Scholar] [CrossRef]
- Yi, F.; He, P.; Chen, H.; He, Y.; Tao, Z.; Li, T.; Zhao, G.; Yun, Y.; Wen, X.; Yang, Y.; et al. Mechanisms of Double-Bond Isomerization Reactions of n-Butene on Different Lewis Acids. ACS Catal. 2021, 11, 11293–11304. [Google Scholar] [CrossRef]
- Yi, F.; Chen, Y.; Tao, Z.; Hu, C.; Yi, X.; Zheng, A.; Wen, X.; Yun, Y.; Yang, Y.; Li, Y. Origin of weak Lewis acids on silanol nests in dealuminated zeolite Beta. J. Catal. 2019, 380, 204–214. [Google Scholar] [CrossRef]
- Hölderich, W.F.; Röseler, J.; Heitmann, G.; Liebens, A.T. The use of zeolites in the synthesis of fine and intermediate chemical. Catal. Today. 1997, 37, 353–366. [Google Scholar] [CrossRef]
- Brodu, N.; Manero, M.H.; Andriantsiferana, C.; Pic, J.S.; Valdés, H. Role of Lewis acid sites of ZSM-5 zeolite on gaseous ozone abatement. Chem. Eng. J. 2013, 231, 281–286. [Google Scholar] [CrossRef]
- Marques, J.P.; Gener, I.; Ayrault, P.; Bordado, J.C.; Lopes, J.M.; Ribeiro, F.R.; Guisnet, M. Infrared spectroscopic study of the acid properties of dealuminated BEA zeolites. Microporous Mesoporous Mater. 2003, 60, 251–262. [Google Scholar] [CrossRef]
- Peng, W.; Takayuki, K.A.; Yashima, T. Characterization of Titanium Species Incorporated into Dealuminated Mordenites by Means of IR Spectroscopy and 18O-Exchange Technique. J. Phys. Chem. 1996, 100, 10316–10322. [Google Scholar]
- Song, S.; Di, L.; Wu, G.; Dai, W.; Guan, N.; Li, L. Meso-Zr-Al-beta zeolite as a robust catalyst for cascade reactions in biomass valorization. Appl. Catal. B 2017, 205, 393–403. [Google Scholar] [CrossRef]
- Busca, G. The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys. Chem. Chem. Phys. 1999, 1, 723–736. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Toktarev, A.V.; Stepanov, A.G. Solid-State NMR Characterization of the Structure of Intermediates Formed from Olefins on Metal Oxides (Al2O3 and Ga2O3). J. Phys. Chem. C 2012, 116, 21430–21438. [Google Scholar] [CrossRef]
- Yamamoto, Y. From σ-to π-Electrophilic Lewis Acids. Application to Selective Organic Transformations. J. Org. Chem. 2007, 72, 7817–7831. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Liu, K.; Chen, W.; Li, J.; Xu, S.; Li, C.; Xiao, Y.; Liu, H.; Guo, X.; Liu, S.; et al. Origin and Structural Characteristics of Tri-coordinated Extra-framework Aluminum Species in Dealuminated Zeolites. J. Am. Chem. Soc. 2018, 140, 10764–10774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Liu, S.B.; Deng, F. 31P NMR Chemical Shifts of Phosphorus Probes as Reliable and Practical Acidity Scales for Solid and Liquid Catalysts. Chem. Rev. 2017, 117, 12475–12531. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Hua, Z.; Ge, T.; Zhou, X.; Chen, L.; Yan, Z.; Shi, J. Post-synthesis of hierarchically structured Ti-β zeolites and their epoxidation catalytic performance. Chin. J. Catal. 2015, 36, 906–912. [Google Scholar] [CrossRef]
- Janas, J.; Gurgul, J.; Socha, R.P.; Kowalska, J.; Nowinska, K.; Shishido, T.; Che, M.; Dzwigaj, S. Influence of the Content and Environment of Chromium in CrSiBEA Zeolites on the Oxidative Dehydrogenation of Propane. J. Phys. Chem. C 2009, 113, 13273–13281. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Janas, J.; Gurgul, J.; Socha, R.P.; Shishido, T.; Che, M. Do Cu(II) ions need Al atoms in their environment to make CuSiBEA active in the SCR of NO by ethanol or propane? A spectroscopy and catalysis study. Appl. Catal. B 2009, 85, 131–138. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Janas, J.; Mizera, J.; Gurgul, J.; Socha, R.P.; Che, M. Incorporation of Copper in SiBEA Zeolite as Isolated Lattice Mononuclear Cu(II) Species and its Role in Selective Catalytic Reduction of NO by Ethanol. Catal. Lett. 2008, 126, 36–42. [Google Scholar] [CrossRef]
- Li, C.; Xiong, G.; Liu, J.; Ying, P.; Xin, Q.; Feng, Z. Identifying Framework Titanium in TS-1 Zeolite by UV Resonance Raman Spectroscopy. J. Phys. Chem. B 2001, 105, 2993–2997. [Google Scholar] [CrossRef]
- Nogier, J.P.; Millot, Y.; Man, P.P.; Méthivier, C.; Che, M.; Dzwigaj, S. Nature, Environment and Quantification of Titanium Species in TiSiBEA Zeolites Investigated by XRD, NMR, DR UV–Vis and XPS. Catal. Lett. 2009, 130, 588–592. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Maronna, M.M.; Kruissink, E.C.; Parton, R.F.; Soulimani, F.; Weckhuysen, B.M.; Hoelderich, W.F. Spectroscopic study on the active site of a SiO2 supported niobia catalyst used for the gas-phase Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. Phys. Chem. Chem. Phys. 2016, 18, 22636–22646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z. Processing pathway dependence of amorphous silica nanoparticle toxicity: Colloidal vs pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, T.; Chaikittisilp, W.; Liu, Z.; Iida, T.; Yanaba, Y.; Yoshikawa, T.; Kohara, S.; Wakihara, T.; Okubo, T. Structure-directing behaviors of tetraethylammonium cations toward zeolite Beta revealed by the evolution of aluminosilicate species formed during the crystallization process. J. Am. Chem. Soc. 2015, 137, 14533–14544. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Mitchell, S.; Pérezramírez, J. Surface and pore structure assessment of hierarchical MFI zeolites by advanced water and argon sorption studies. J. Phys. Chem. C 2012, 116, 18816–18823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).