Abstract
The global climate crisis, driven by unchecked industrialization and ecological negligence, compels humanity to seek alternative ways to either avert or mitigate the disastrous environmental phenomena encountered, particularly in recent years. The significant quantities of biomass generated by human activities may serve as important resources for technological applications, and biomass valorization offers dual benefits. This review emphasizes the potential of starch and lignin as adaptable materials for the advancement of sustainable and eco-friendly technologies. By investigating catalytic alterations, we may advance a more sustainable future and tackle the escalating issues of environmental pollution and sustainability. Catalytic alterations of lignin and starch have become essential techniques for their valorization. Biopolymers can be changed into useful chemicals and materials, like levulinic acid, lactic acid, 5-HMF and modified starch, which are used in the paper, textile, and coatings industries. Besides transforming into chemicals, lignin and starch can produce reactive carbon compounds that find application in both classical chemistry and photocatalysis. Additionally, we can use their highly functionalized polymeric matrices as catalysts. We can change the polymeric matrices’ chemical backbone to make them better at speeding up reactions like cross-coupling and multicomponent reactions.
1. Introduction
The current global climate crisis and the growing request for energy and material sources urge us to find alternatives to oil- and fossil-derived ones. Materials deriving from the oil industry and hydrocarbon-derived chemicals produce an alarming pollution capable of the potential destruction of several ecosystems. In 2010, 9 Mt of plastics were delivered into the oceans by human activities, although the current amount of plastic detected in the oceans represents less than 1% of the total released in Earth’s hydrosphere [1]. The top 10 most relevant incidents related to the fossil-fuel industry caused, over the 1960–2000 period, spills of about 20,000 tons of oil [2]. In this context, the valorization of biopolymers from biomasses is of critical importance, as they are abundant and typically highly functionalized. Due to their biological origin, they are part of an eco-friendly family of compounds, the so-called environmentally degradable polymers (EDP), naturally occurring or bio-derived polymers which can be biodegraded under mild conditions such as microorganisms, sunlight, oxygen, temperature and moisture. Moreover, being produced naturally, they represent a sustainable feedstock, without the environmental drawbacks related to their extraction, such as the aforementioned ones [3].
Among these vast sets of polymers, lignin and starch can play an important role. Starch is strongly released in the environment due to food processing techniques such as starch wastewater (SWW). SWW is a starch-containing aqueous by-product that is typically generated by the food industry. Due to the high quantity of organic compounds and nutrients they contain, they cannot be released in the environment, and for this reason, a pretreatment is necessary [4]. Lignin is one of the most abundant natural biopolymers on the planet but has been exploited very little for technological applications thus far [5]. This review will be focused on the valorization of starch and lignin biomasses, with a particular emphasis on both their catalytic valorization and the catalysts that can be prepared starting from these materials.
2. Origins and Classification
Biomasses are usually defined as vegetable-derived energetic resources that can be present in the environment as solid or liquid materials [6], and they can be industrially produced. Therefore, starch and lignin waste biomasses can be classified as starting from the industrial pathways they belong to.
2.1. Starch
Starch is the major energetic storage polysaccharide found in plants [7] and it is mainly produced through the processing of vegetable resources, such as wheat, potatoes, corn and cassava. Industrial agri-food production leads to the release of high amounts of starch wastewater (SWW), starch-rich water suspensions that represent a serious environmental threat. Their high carbon content, when delivered in water bodies, leads to the depletion of dissolved oxygen due to the high COD (chemical oxygen demand) they are associated with [8,9,10].
Because of the extended contaminations caused by SWW, starch represents an important target compound for green methodologies. These elaborations allow its removal from the environment and at the same time its exploitation as a renewable starting material. Moreover, it is also important to convert starch because it constitutes nearly 15% of the total worldwide generated food waste. Starch is a renewable, highly abundant reservoir of chemicals for both biofuel and plastic materials preparation [8,9,10,11,12,13,14]. In this way, the waste-contained starch, being employed for the production of high value-added materials, acquires high economic significance [9,11,12,13,14]. Therefore, chemical modifications of starch for both catalyst and material preparation have been deeply explored in the recent literature.
Starch is composed of two polymers, amylose and amylopectin (Figure 1). The first one has a lower molecular weight (1.03–4.89 × 105 Da) and it is constituted by linear chains of D-glucose, with monomers that are bound to each other via α-1,4-glycosidic bonds. Amylopectin (MW 7.08–9.88 × 107 Da) is constituted by short and highly branched chains of D-glucose connected together through α-1,4 and α-1,6-glycosidic bonds: these two polymers are then associated together macroscopically, forming granules whose diameters range from 5 to 15 µm [7]. The granules are structurally built through the association of layers around their core, named hilum, where each layer is constituted by a couple of amorphous and crystalline shells. Each shell is composed of the intertwined chains of amylose and amylopectin, which contribute to structurally different entities. In particular, the layers are formed by crystalline and amorphous lamellae. The first are constituted of long-chain amylopectin clusters, while the latter by short-chain amylopectin and amylose molecules. Due to the different spatial arrangements of these molecules, the nature of the layers strongly affects starch crystallinity and thermal properties. In general, normal starch is composed of 70–85% amylopectin and 15–30% amylose [7].
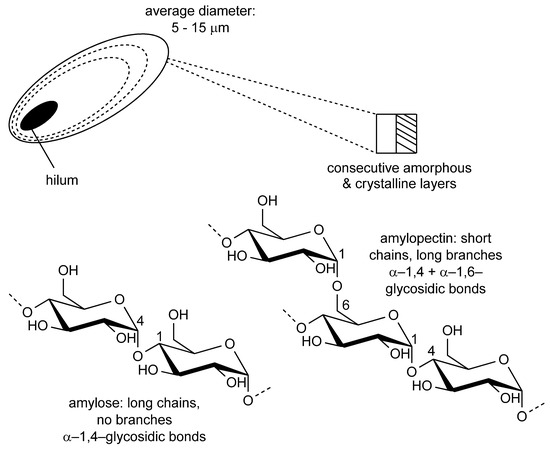
Figure 1.
Macroscopic structure of starch granule and of amylose and amylopectin polymers.
2.2. Lignin
Lignin, one of the most abundant biological polymers present on Earth, is produced as a by-product of the paper and biofuel industries [15,16]. Lignin worldwide production is estimated to be around 100 million tons/year, for a total value of USD 732.7 in 2015 [15,16,17]. This polymer is formed by three 4-hydroxypropanoid monomers, named monolignols, which are characterized by the presence of an aromatic ring and that differ one from the other depending on the hydroxylation degree (Figure 2). They are present in lignin in three main forms, namely p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) alcohol, which are biochemically derived from L-phenylalanine and L-tyrosine. There are also other uncommon monolignols that do not occur as often as the aforementioned ones but that contribute to the high chemical diversity that is proper of lignin [18]. The carbon composition of lignin is 56–60 and 60–65% depending on the provenance, being hardwood lignin (coming from deciduous trees that lose their leaves annually), which is poorer in carbon with respect to the softwood one (that comes from evergreen conifers). This percentage also changes as a result of the different quantities of H, G and S units, a result of the gene expression in the plant producing the lignin [19]. It is still not possible to exactly determine the precise sequence of a given lignin sample, but in general, it is held together by ether linkages between the aromatic rings of the monolignol units. These occur in a statistical rather than specific fashion, depending on the units that are bound, and the resulting connections are better known as lignin-linking motifs or substructures. The most abundant crosslinks are aryl ether linkages engaging the carbon atom in the β position of the propanoid fragment of the monolignols: they are the β-O-4, the β-β (resinol) and the phenylcoumaran (β-5) motifs. Some other rarer connections can also be found in different lignin samples, such as biphenyl (5-5), diphenyl ether (4-O-5) and spirodienone (β-1) [20].
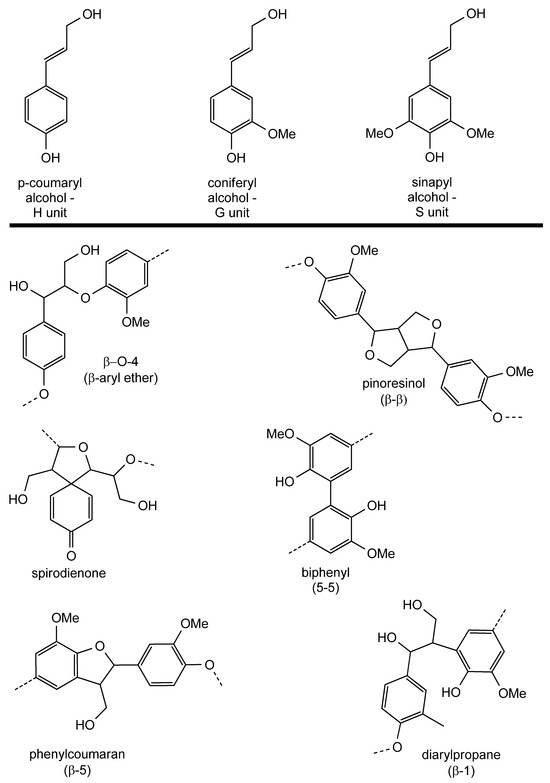
Figure 2.
Chemical structure of monolignols and most abundant lignin substructures.
The distribution of the substructures depends on the process by which it is obtained; therefore, lignins are classified starting from their extraction strategy. Generally speaking, two categories, native and technical lignins, can be found. Native lignin is the biopolymer that is naturally occurring in plants and that conserves the structural features found in plants. Technical lignins are obtained as by-products of industrial treatments in which wood is fragmented into its lignin, hemicellulose and cellulose components, and during which, native lignin undergoes massive chemical changes [21].
The most important treatments by which technical lignins are produced are the Kraft sulfite process, together with organosolv and steam-explosion/cellulolytic enzyme extraction techniques. Kraft lignin is obtained as a by-product of the pulping process, in which wood chips are treated at high temperatures with an aqueous solution of NaOH and Na2S [22]. The sulfite process consists of an acid hydrolysis that lignocellulosic material undergoes in the presence of sulfurous acid together with a calcium, magnesium or ammonium sulfite salt at high temperatures (165–170 °C) leading to the production of lignosulfonates which are negatively charged and water-soluble [23,24]. In the organosolv process, an organic solvent (typically alcohols, ketones and glycols) is employed to remove lignin from the lignocellulosic material [25,26,27].
Steam-explosion treatment is generally carried out before enzymatic hydrolysis of the cellulose bound to lignin, and is a protocol principally employed in the biorefinery sector since 1980 to produce useful chemicals, phenols and other biodiesel products [28]. It consists of a high temperature compression of lignocellulosic biomass caused by the injection of water vapor heated by a boiler tank followed by depressurization that causes the separation of lignin from the other components. Finally, cellulolytic enzyme lignin (CEL) is obtained either from ball-milled wood powder of a steam-exploded biomass through an enzymatic treatment, during which carbohydrate residues linked to lignin polymeric matrices are cleaved, with a final product that is recovered after resolubilization with solvent mixtures such as water/dioxane and precipitation [29].
3. Catalysts for Lignin and Starch Valorization
3.1. Catalytic Modifications of Starch
Starch catalytic modifications are mainly related to its valorization for valuable chemicals, biofuel production and material sciences. In the first case, hydrolysis and subsequent chemical transformations must occur, while in order to employ starch for material science applications, oxidation is one of the most common treatments.
Starch hydrolytic valorization is very important because precious platform chemicals can be produced with this method, especially levulinic and lactic acids, together with 5-hydroxymethyl furfural (5-HMF) [30]. Previously employed methods required the use of homogeneous catalysts such as sulfuric, nitric and phosphoric acids, but recently, alternative methods based of nano-supported systems have been preferred, due to their reusability, ease of separation and simpler purification of the obtained product which is also advantageous from an economic point of view. Different technologies have recently been proposed to this aim, both organic and inorganic: in the first case, sulfonated/triflated multiwalled carbon nanotubes (CNT) and graphene oxides (GO) obtained thorough treatment with HNO3 and sulfuric and triflic acids, while in the second, mesoporous silica/sulfated zirconia composites [31,32].
Graphene oxide and carbon nanotube-oxide have been demonstrated to be highly efficient in the hydrolysis of starch with marked selectivity towards the formation of specific biochemicals, such as levulinic and lactic acids. First, the treatment of carbon nanotubes and graphene with HNO3 provided hydroxyl, epoxy and carboxylic groups on their surface [33], while the reactivity and overall properties were further improved through the addition of niobia (NbO2/Nb2O5) and magnetite (Fe3O4) nanoparticles to increase the reactivity and the ease of separation. This preliminary treatment does not bring about deep modifications in their physicochemical properties, for example, the specific surface area is maintained. In both the cases of CNTs and GO, the oxygenated functional groups reacted with sulfuric and triflic acids, bringing about the formation of sulfonic (-SO3H) and triflic (-CF3SO3) moieties. Sulfonic acid moieties and protons bring about, on the one hand, the hydrolysis of starch (breaking of glycosidic bonds), while on the other hand, the heterogeneous catalyst promotes the transformation of hydrolytic intermediates such as 5-HMF into more stable products like levulinic, glycolic and lactic acids. Starch chemical conversion proceeds first by hydrolysis of the glycosidic bonds, followed by acid-promoted isomerization of the produced glucose into fructose. Fructose then either undergoes a retro aldol reaction furnishing lactic acid, or dehydration, which yields 5-HMF and subsequently levulinic acid. CNT-SO3H (sulfonated carbon nanotubes) are equipped with Brønsted acid sites, which promote the dehydration and selective production of levulinic acid (49% selectivity after treatment at 180 °C for 6 h). On the other hand, with GO-SO3CF3 (triflated graphene oxide), Lewis acids are prevailing and this leads to a lowering of the activation energy related to the retro aldol pathway, leading to the preferred formation of lactic acid (62.9% selectivity after 4 h at 150 °C) (Figure 3).
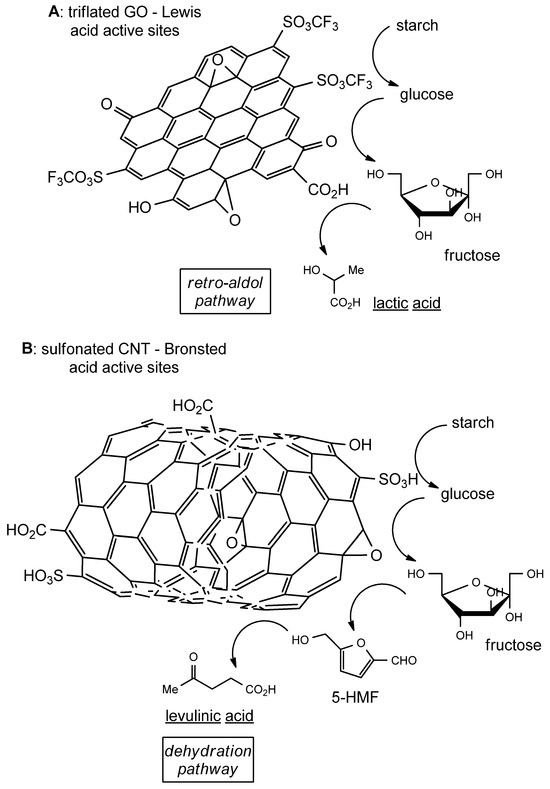
Figure 3.
(A): triflated graphene oxide (GO) promotes retro-aldol post hydrolytic decomposition of glucose; (B): sulfonated carbon nanotubes (CNT) catalyse post hydrolytic dehydration of glucose to furnish levulinic acid.
On the other hand, inorganic catalysts have also been reported to be highly efficient in the catalytic valorization of starch. Silico-tungstic acid and sulfonated ash have been employed for the production of alkyl levulinates, compounds that are employed as synthetic fuel and fuel additives [34,35,36]. Recently, a mesoporous composite formed by KIT-5 silica zeolites and sulfated zirconia (SO4/ZrO2) has been employed for the production of hexyl levulinate by exploiting the synergistic effects of acidic sites on both of these solid matrices [32]. The mixing of sulfated zirconia with KIT-5 occurred with the conservation of the morphological properties (specific surface area and total pore size and volume) and acidic site density was strongly increased with respect to pristine KIT-5. In order to produce hexyl levulinate, starch decomposition was carried out in the presence of 20 wt% catalyst at 190 °C for 6 h in n-hexanol. This solvent selectively brought about the formation of the desired product, preventing the formation of humins by-products.
As mentioned above, starch is also employed for material sciences applications. In particular, oxidized starch is one of the most employed derivatives. It is used in the paper, textile and drilling industries, together with binding materials production, and it has been observed that oxidized starch improves the printability and adhesion of paper.
Starch oxidation takes place most generally at the C2, C3, and C6 positions of the α-anhydroglucose units, with the formation of keto and carboxylic groups depending on the strength of the oxidizing system. The oxidation of starch brings about the disruption of the hydrogen bonds that hold together amylose and amylopectin filaments, thus promoting a decrease in the viscosity that improves the mechanical and rheological properties of this modified polymer. In general, it is possible to oxidize starch with a variety of systems, in which sodium hypochlorite and hydrogen peroxide are used as oxidants. Hydrogen peroxide has been preferred in the last years due to its eco-friendly nature, in low amounts (0.12–4.0 wt%), at both acidic and alkaline pH and at temperatures ranging from 20 to 90 °C [37,38,39]. Iron- and copper-based catalysts, together with tungstate, have been employed in order to oxidize starch, with copper being the most efficient one [37,40,41].
In the most recent advances in the field of starch oxidation that have been reported, iron-containing phthalocyanine and binuclear bridged manganese complexes were employed. This approach is advantageous if compared with the use of simple catalytic metal salts such as CuSO4, since difficult product purification and residual pollution is prevented [42].
Iron-phthalocyanine complexes contain FeIII ions which are able to undergo more efficient, Fenton-like chemistry with the formation of nucleophilic Fe-O-O-H and electrophilic Fe=O species (Figure 4A). The general mechanism in this case is very complex, but it is thought to involve H2O2 decomposition with a generation of hydroxyl anions and radicals, which interact with the hydroxyl groups on starch bringing about the formation of keto groups from secondary alcohols, with iron complexes that act as electron transfer agents. Phthalocyanine complexes are very efficient in the oxidation of starch (Figure 4B). They have been used at a 0.05 wt% concentration, bringing 55 °C and pH 8.4 to the formation of 5.28 CO and 1.02 CO2H groups per 100 anhydroglucose units, with high efficiency when compared with the low pH and high temperature conditions required with tunstate-based systems [41].
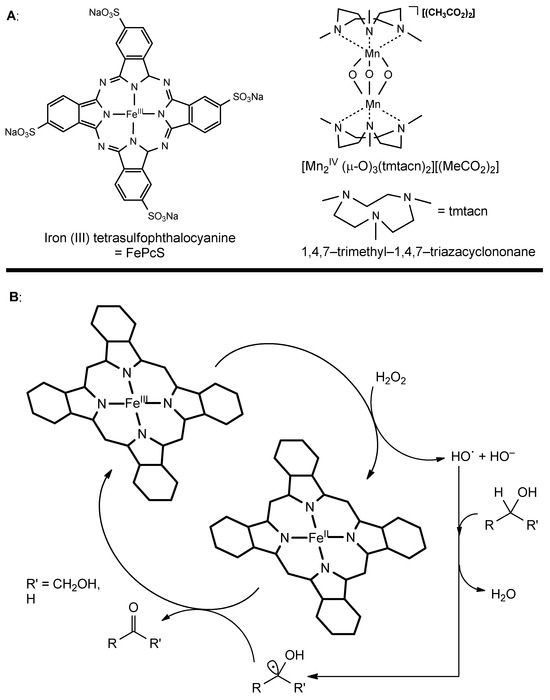
Figure 4.
(A): general structure of iron-phthalocyanine (left) and bridged-binuclear manganese complex (right); (B): representative hypothetical mechanism for first and secondary alcohol oxidation of iron-phthalocyanine complex.
The second latest catalytic approach reported in the field of starch oxidation is represented by Mn-containing compounds. It has been demonstrated that molybdenum and vanadium polyoxometalates can be efficient in the oxidation of starch [43], and Mn-containing bridged-binuclear complexes have shown similar reactivity and catalytic efficiency [44].
An Mn-containing catalytic employed species, [MnIV2(μ-O)3(tmtacn)2][(MeCO2)2], proved to be highly efficient in low amounts (0.0021 mol%) at 25 °C and pH 11 using 30 wt% H2O2 as the oxidant (Figure 4A). In this case, a 97% yield, higher than the one observed with iron-phthalocyanine, was observed, with the oxidation of starch granules that principally took place on the surface, bringing to the improved workability of starch for its applications.
3.2. Catalytic Modifications of Lignin
In the vast scientific literature of the last 20 years, different procedures relating to the catalytic modifications of lignin have been reported. In this paper, techniques involving catalysts for lignin valorization will be discussed. Indeed, lignin can be seen as a huge reservoir of platform and valuable chemicals, and therefore catalysts represent a fundamental tool for the chemical valorization of this biopolymer. From this point of view, this type of transformation, because of their correlation with environmental issues, also represent an important aspect in green chemistry and can be used for both energetic and synthetic applications. Techniques for catalytic modifications of lignin can be roughly divided into three classes, namely oxidative, reductive and redox valorization. In both methods, lignin undergoes a more or less extended depolymerization, ideally leading to a total conversion in aromatic or aliphatic compounds. Depolymerization is an essential step for lignin valorization, and, for example, with pyrolytic treatment (e.g., fast pyrolysis), it is possible to obtain a lignin-derived bio-oil, which is a promising liquid fuel that could replace the current fossil-based fuels [45].
Reductive methods are mainly employed for the preparation of bio-fuel. This approach is advantageous in this application because it is able to strongly decrease the content of oxygen, thus leading to aromatic and aliphatic hydrocarbons which are constituents of diesel and gasoline. Most generally, reductive valorization is carried out by employing so-called cracking methods, which are performed under neutral to reducing atmospheres (containing nitrogen and hydrogen). They can be divided into hydrocracking, hydrotreating and electrocatalytic hydrogenolysis (ECH) [46].
In hydrocracking, lignin is treated at 400–600 °C at atmospheric pressure, using acidic catalysts such as ZSM-5, Beta, MCM-41 and SBA-15 zeolites. Acidic functionality is fundamental because, in high temperature conditions, it brings about the breaking of C-O bonds, leading to efficient depolymerization and low-molecular-weight hydrocarbon production. Furthermore, if transition metals such as Ni, Fe and Co are employed, an enhanced performance is observed [47]. In general, aromatic hydrocarbons and C2-C6 olefins are formed upon the breaking of C-O bonds and the permeation of monolignols inside zeolite pores, which undergo dehydration, hydrogenation, cyclization and aromatization reactions. For this reason, crystal size, zeolite dimensions and acidity are key-factors in the process. Zeolites must have pores that allow molecule penetration, because otherwise low-molecular-weight recondensation reactions can take place on the external surface and oligomers, instead of hydrocarbons, are generated, thus diminishing the yield. On the other hand, acidity plays a key-role in the depolymerization event. By substituting a tetravalent Si atom in the zeolite silicate crystal with a trivalent Al one, a negative charge is created which can be compensated with a proton. In this way, an extremely strong Brønsted acid is formed. Therefore, through the protonation of OH groups on aliphatic fragments and β-elimination, olefination and cleavage of interunits linkage is promoted [48,49,50]. Therefore, Si/Al ratio is particularly important, as it modulates zeolite’s acidity. For example, if a low Si/Al ratio zeolite such as H-USY is employed, mostly monocyclic aromatic hydrocarbons are formed, such as benzene, toluene and xylene. On the contrary, if low acidity zeolites are used, the concentration of carboxylic acids in the produced oil is higher. In general, zeolite structure (surface area, acidic sites density) and composition (Si/Al ratio) affect the ease with which it is possible to obtain aromatic and polyaromatic hydrocarbons (in the case of Beta type and silica-based HSB-960 zeolites) [51,52]. Hydrotreating is a technique in which lignin undergoes hydro-depolymerization under an H2 atmosphere at variable pressures at a temperature of 150–250 °C. Most generally, metals, such as Ni, are employed over acidic supports (Al2O3, ZrO2 and CeO2 zeolites) [53]. A hydrogen atmosphere brings about the stabilization of the reaction intermediates and the removal of oxygen that induces depolymerization through metal-catalyzed deoxygenation and acid-site catalytic processes. This eventually leads to the terminal deoxygenation of monophenols with the production of monomeric aromatics [54].
The aforementioned reductive methods are characterized by the high-energy barrier associated with hydrogen transfer to lignin, which slows down the process, decreasing the overall efficiency. Electrocatalytic hydrogenolysis (ECH) represents a valuable alternative strategy with respect to hydrocracking and hydrotreating, because the same hydrogenolysis-based depolymerization strategy can be employed, bypassing the harsh conditions (of temperature and pressure) needed with the methods that have been shown before. ECH leads to the breaking of α and β-aryl ethers, thus destroying the cross-linking network and releasing monomers from the polymeric matrix (Figure 5A).
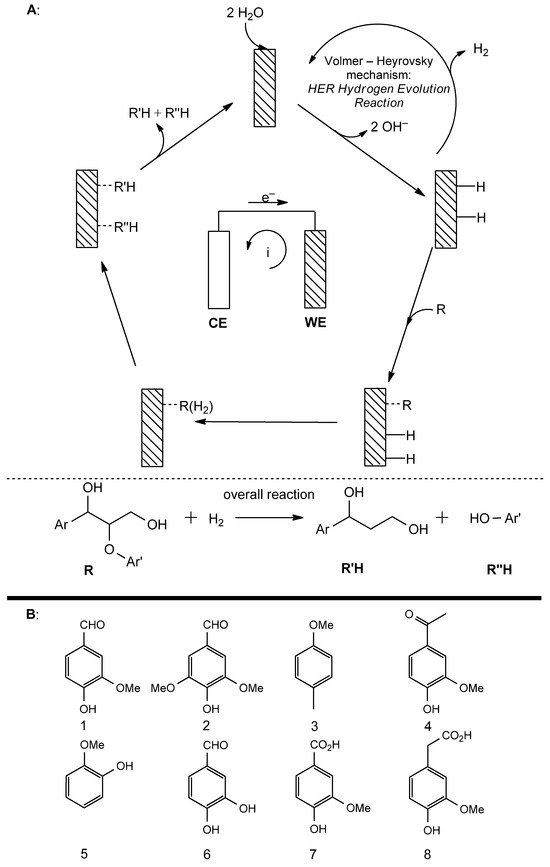
Figure 5.
(A): general configuration of ECH apparatus and representative mechanism and reaction on model lignin substructure (β-O-4 linkage). CE: counter-electrode, WE: working electrode, i: external circuit current; (B): products isolated through ECH of lignin. 1: vanillin, 2: syringaldehyde, 3: 4-methylanisole, 4: apocynin, 5: guaiacol, 6: 3,4-dihydroxybenzaldehyde, 7: vanillic acid, 8: homovanillic acid.
The general process involves the hydrogenolysis of C-O bonds and it is also possible to observe the hydrogenation of aromatic rings, which yields monocyclic aliphatic hydrocarbons. Most generally, the efficiency of reductive depolymerization processes, in terms of aromatic (and aliphatic) monomer production, depends on the selectivity of the process towards hydrogenolysis over hydrogenation. The rate of the hydrogenolysis of ethers follows the order (benzyl)-aryl > aryl-alkyl > alkyl-alkyl ether; therefore, if hydrogenation takes place first, a polymeric, recalcitrant high-molecular weight intermediate product is generated that prevents monomers isolation. ECH allows chemoselectivity towards hydrogenolysis, and for this, and the aforementioned reasons, it is a very promising method for monomer production from lignin [55]. Advantageous aspects of ECH can be better understood by inspecting the general mechanism. The starting point is water electrolysis at low overpotential on the electrode’s surface, with an in situ generation of a couple of metal-bound hydrogen atoms. At the same time, the substrate is adsorbed onto the surface of the electrode, and the exchange of hydrogen atoms leads to the release of hydrogenolysis products. A parallel process that can take place together with hydrogenolysis is the so-called hydrogen evolution reaction (HER), caused by the formation and release of molecular hydrogen through the reaction of metal-absorbed hydrogen atoms with water (Figure 5A). In both cases (hydrogenolysis and HER), the reactions cause the formation of OH− ions, which lead to an increase in pH value, which is beneficial for the reaction since lignin is soluble in alkaline media. Moreover, since hydrogenolysis takes place starting from metal-bound hydrogen atoms, and not molecular H2 in the presence of a metal catalyst, low H2 solubility and the high energetic barrier associated with dissociative absorption, common problems associated with other types of reductive depolymerization, are completely bypassed.
Globally, the yield of monomeric hydrocarbons relies on the predominance of hy-drogenolysis on HER [56]. Furthermore, the ECH-based production of monomers is favored by the predominance of hydrogenolysis on hydrogenation. In ECH, the lower C-O bond energy, if compared with the aromatic carbo-carbon double bonds energy, together with a lower applied current density that produces a less concentrated chemisorbed hydrogen on the electrode’s surface, brings about the complete selectivity of hydrogenolysis over hydrogenation [57,58].
The mechanistic and performance aspects of ECH were investigated by using benzyl phenyl ether (BPE) as a lignin model compound. Optimal conditions were found at a 26 mM concentration of BPE, in EtOH/H2O 75/25 v/v as the solvent, with a current density of 20 mAdm−2 at 40 °C and with a pH ranging from 6.9 to 11.5 (increased due to water reduction), using 0.1 M NaCl as the electrolyte. It was possible to obtain 70% of conversion, with a process that resulted in being selective on hydrogenolysis over hydrogenation (100%) and a performance also maintained with both o- and m-methoxylated aryl ethers. As mentioned above, the current density modulation represents a key-factor for ECH efficiency. During these experiments, it was demonstrated that current density decrease (from 160 to 20 mAdm−2) is associated with a marked predominance of hydrogenolysis on HER. This occurs because in the case of a minor current density, a lower local concentration of hydrogen is achieved, thus allowing more extended substrate’s chemisorption and less hydrogen evolution. In fact, competitive hydrogen evolution takes place through the Volmer–Heyrovsky mechanism, in which in situ-generated, metal-bound hydrogen atoms are combined with water, releasing molecular H2 in the medium. On the other hand, hydrogenolysis results from substrate chemisorption and the combination with hydrogen atoms. Therefore, the efficiency of ECH processes can be increased by current density modulation because the two competitive reactions that take place are distinct chemical and electrochemical phenomena that depend differently on this parameter [55].
In ECH methods, different working and control electrodes can be employed, such as RuO2-IrO2/Ti, Co-Pt/Pt ring, Ni and RVC (reticulated vitreous carbon), which have proved to be efficient in the hydrogenolysis of aspen, Kraft and alkali lignin at concentrations ranging from 5 to 40 g/L and under alkaline conditions. Current densities ranging from 2–10 to 20–90 mAcm−2, at temperatures from 20 to 90 °C, were employed, leading, after a reaction time from 1 to 24 h, to different products such as aldehydes, phenols and carboxylic acids together with vanillin, syringaldehyde, 4-methylanisole, apocynin, guaiacol, 3,4-dihydroxybenzaldehyde, vanillic and homovanillic acids and 2-hydroxycyclopentadecanone with conversions up to over 70% (Figure 5B) [59,60,61,62].
The second and third classes of catalytic valorization of lignin, oxidative and redox processes are employed to produce valuable chemicals that can be used as synthetic building blocks for synthetic applications in organic and medicinal chemistry. These methods are characterized by mild conditions, such as temperatures below 250 °C. These requirements must be met as it is important to avoid overoxidation and collateral repolymerization due to the formation of radical intermediates. In some occasions, oxidative and redox methodologies are advantageous with respect to reductive ones because it is not necessary to employ particular atmospheres and there are not competitive side-reactions such as hydrogenation that can hinder the formation of the products [46,63].
Oxidative valorization methodologies are based on the oxygenation of crosslinking structures, and for this purpose, solid-state reactive systems have been highly effective. In this context, metal-alumina catalysts and doped perovskites will be discussed.
Alumina, in the form of γ-Al2O3, can be combined together with SiO2 and metal catalysts such as Pt, Pd, Co, Mo, Ni and W to produce chemicals from lignin [46]. Alumina acts as a support for the metal catalysts, and it plays an important role since the proximity of the substrate to the crystal lattice is fundamental for both the reactivity and regioselectivity. Pd-enriched γ-Al2O3 crystals have been employed for lignin valorization and production of aldehydes, acids and other low molecular weight molecules [64]. The catalyst was prepared starting from and PdCl3·3H2O, and particles with a specific area and volume of 3.29 × 102 m2/g and 0.42 m3/g, respectively, were obtained. The presence of Pd was confirmed through XRD and atomic absorption spectroscopy analyses. The performance and efficiency of this heterogeneous catalyst (2.85 wt%) was investigated by reaction, in a continuous fluidized-bed reactor, with lignin solubilized in an NaOH 2 mol/L solution under an O2/N2 atmosphere (total pressure 20 bar, pO2 ranging from 2 to 10 bar). The temperature was modulated from 373 to 413 K. Under these conditions, on 0.50 L of 30 g/L lignin solution, at a flow rate of 5 L/h, 5 bar pressure and 393 K, and after 2 h, it was possible to obtain 65.10 × 10−1 g of vanillin and 114.84 × 10−1 g of syringaldehyde. On the catalyst’s surface, the oxidation of aryl ether substructures led to the formation of vicinal diols, which underwent oxidative cleavage, finally furnishing aldehydes and acids as products. Crystal lattice supports led to effective catalysis in lignin’s substructure oxidation by metals, and Cu-doped silica and γ-Al2O3 led to the formation of more than 20 monomeric products such as guaiacol, guaiacyl guaiacol, guaiacyl dimers and acids [65].
Perovskites, crystals with the general formula ABO3, are employed in the catalysis of the wet aerobic oxidation of lignin. Wet aerobic oxidation is a method in which in the liquid phase, a high-pressure O2 atmosphere is applied to oxidize the substrates. For this purpose, perovskites are advantageous because they are not correlated with the secondary pollution usually associated with homogeneous catalysts, with no recycling costs, and are not expensive, like noble metals, while nevertheless maintaining efficiency.
Different crystals can be prepared, and for their application, other than the classical structure, MA1−xBxO3 crystals are also employed, in which the elementary unit is always made up of five atoms and M is a fixed metal counterion. These metal-containing catalysts are prepared through the sol-gel method, starting from metal nitrates and citric acid aqueous solution and working at temperatures ranging from 80 to 100 °C in the first phase, followed by calcination at 700 °C. La-containing perovskites are very efficient for monomeric phenol production from lignin; in this case, LaA1−xBxO3 crystals A and B are represented by metals such as Sr, Ce and Cu and Co, Mn and Fe, respectively [66].
Crystal lattices represent an important support for catalytic activity, because on its surface, the absorption and desorption phenomena of metal ions occur, therefore allowing the transport of the reactants on the active sites. Fe and Cu, employed in LaFe1−xCuxO3 perovskite-type oxide, provide an example of these catalytic systems’ activity. It is possible to characterize this type of crystal through techniques such as XRP, which in this case, was used to confirm the presence, the oxidation states and the distribution of Fe3+ and Cu2+ ions. Lignin depolymerization into valuable monomeric species was evaluated by carrying out wet aerobic oxidation in a high-pressure slurry reactor in the presence of 2 mol/L NaOH aqueous solution, under a N2/O2 atmosphere (20 bar total pressure, pN2 of 15 bar) with the continuous flushing of oxygen. The average reaction time was 30 min, and the concentrations of lignin and catalyst were 60 and 3 g/L, respectively.
Using this catalytic system and varying the reaction time up to 3 h, it was possible to carry out the depolymerization of lignin, bringing about the production of p-hydroxybenzaldehyde, vanillaldehyde and syringaldehyde with up to 70 wt% conversion of the starting material. The reaction mechanism (Figure 6) shows that the catalytic efficiency relies on the cooperation of Fe3+ and Cu2+ ions.
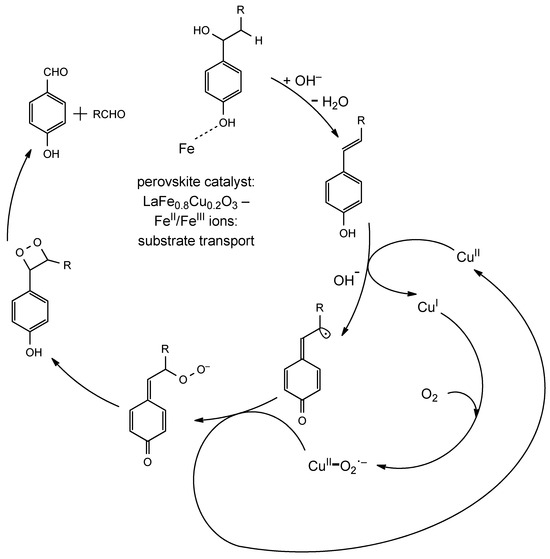
Figure 6.
General mechanism for lignin oxidative cleavage catalyzed by iron-copper perovskites.
Three factors are important: alkaline environment, the mass transport of the substrate on the crystal particles and the redox activity of the counterion. In the presence of NaOH, the elimination of water from the Cα-Cβ fragment leads to the formation of a double bond which represents the starting species taking part in the catalytic cycle. This phenomenon occurs together with the mass transport of phenolic moieties complexed by Fe3+ ions on the surface of perovskite nanocrystals. Iron ions are therefore fundamental in the process, because they allow the overcoming of the energetic barrier needed for lignin absorption. It must be remembered that iron species are also obviously redox active, because electron transfer is provided by the FeIII/FeII couple, but copper ions are usually employed in perovskite catalysts due to their higher reactivity. The best results were indeed observed with LaFe0.8Cu0.2O3 perovskite [67,68]. Cu2+ ions are the fundamental redox active species in the catalytic system and interact with both monolignols and oxygen. In the first step, a single electron transfer (SET) from a α-β unsaturated monolignol to CuII occurs, yielding a radical quinone intermediate. Successively, Cu+ is oxidized back to Cu2+ by O2, yielding CuII-O2.− which undergoes addition to the quinone radical intermediate leading to a dioxetane in two steps. Cycloreversion finally brings about the oxidative Cα-Cβ bond cleavage and the formation of oxidized products (aldehydes after one cycle of catalytic oxidation). In the last step of the catalytic cycle, Cα-Cβ bond breaking occurs with the concurrent reduction of Cu2+ to Cu+, which finally is regenerated at the end of the cycle by O2 (Figure 6). An oxygen-containing atmosphere is therefore important not only because it contains the oxidant in the system but also because it is employed to regenerate the catalytic species. The cooperation between iron and copper ions that takes place in this example is nevertheless representative of the reactive properties of metal-containing perovskites [69].
The third method by which it is possible to carry out lignin’s valorization with the production of aromatic monomeric substances is represented by redox procedures. In general, these techniques are characterized by a two-step sequence, where in the first, an oxidation is carried out, while during the second, a reductive cleavage is performed. They are particularly effective in the breaking of the β-O-4 linkage, which is the most abundant substructure in lignin, reaching up to 85% of all the crosslinking moieties. In redox methods, the benzylic alcohol function is the target of the first oxidative step, which brings about the formation of an acyloin (β-hydroxyarylketone). The formation of this intermediate (commonly referred to as oxidized lignin, which is lignin enriched in oxidized β-O-4 linkages) is a key step in the process, because on the one hand, it allows the cleavage of the substructure by decreasing the C-O bond dissociation energy [70], and on the other hand, acyloin allows the scission of the β-O-4 linkage through either a retro-aldol reaction or hydrolysis reactions. The elaboration of β-O-4 linkages is advantageous because the selective redox cleavage of this substructure preserves the other ones that are already present, thus possibly also bringing about the formation of oligomers functionalized with saturated heterocyclic moieties, which are the residual substructures originally present in lignin’s polymer backbone. This characteristic aspect enables redox methods to be directly used on native lignin, thus avoiding possibly expensive pretreatments and speeding up the valorization process. The production of soluble aromatics prevents the formation of coke and char from lignin and consequently improves the conversion of the starting material. Finally, molecules produced with these methods, such as vanillin and syringaldehyde, can be directly sold on the market.
The first oxidative step of redox techniques is generally carried out under an atmosphere containing oxygen (Figure 7).
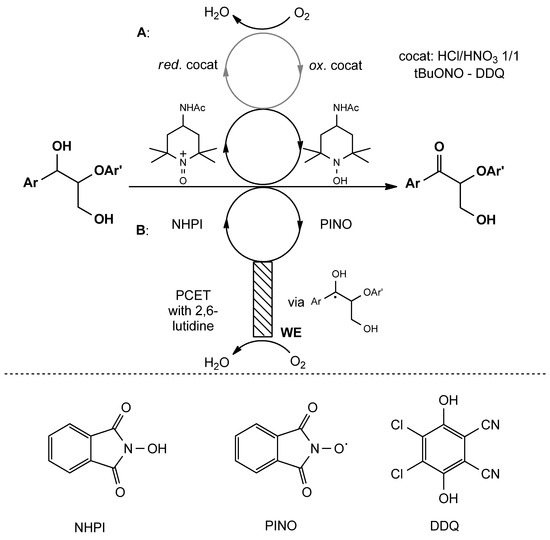
Figure 7.
Preoxidation step of redox cleavage of β-O-4 linkage. (A): oxidation of benzyl alcohol function with organic electrons carriers under aerobic atmosphere; (B): electro-oxidation of benzyl alcohol with assistance of redox mediator. WE: working electrode, NHPI: N-hydroxyphthalimide, PINO: phthalimide N-oxyl, DDQ: 2,3-dichloro-5,6-dicyano-1,4-benzoquinone.
Classified as completely metal-free procedures, there are methods in which a metal-dependent reduction is employed and techniques in which electrocatalytic cycles are used [63]. In metal-free and metal-dependent redox processes, the first oxidation of the benzyl alcohol moieties takes place by employing nitroso-compounds and inorganic systems such as tBuONO and HNO3/HCl 1/1, which act as synthetic equivalents of NO2. NO2 is the co-catalyst that can regenerate the redox catalysts, which actually bring about the oxidation of benzyl alcohol and are commonly represented by TEMPO ((2,2,6,6-Tetramethylpiperidin-1-yl)oxyl), AcNH-TEMPO and DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone). This electron transfer chain that relies on redox interaction between NO2 and the catalyst is continuously regenerated by O2, and therefore an aerobic/oxygen-containing atmosphere is mandatory, and both AcNH-TEMPO and DDQ-based catalytic systems proved to be totally selective towards benzyl alcohol oxidation both in lignin model compounds and pristine lignin (Figure 7A) [71,72]. As already mentioned above, alternative strategies are available, and electrooxidation represents an important example. The direct electrooxidation of lignin is generally correlated with scarce efficiency, generally resulting from the extended oxidation of lignin and the generation of radical cations and intermediates that lead to the formation of several compounds with scarce efficiency and selectivity. To apply this approach to lignin’s oxidative valorization, organic intermediates can be employed. In particular, N-oxyl species, such as N-hydroxyphthalimide (NHPI), has been employed, together with RVC working electrodes under an oxygen-containing atmosphere, to perform benzyl alcohol oxidation in β-O-4 linkage model compounds and pristine pine lignin [73]. NHPI is oxidized, in the presence of oxygen, by an RVD electrode to PINO (phthalimide N-oxyl). PINO successively brings about the abstraction of benzyl hydrogen atoms from the β-O-4 linkage (back-reduction to NHPI), and the complete oxidation of the radical intermediate to the corresponding acyloin is performed through electron transfer with the working electrode with an oxygen-dependent regeneration of the catalytic species. The overall efficiency in the process is increased by the presence of 2,6-lutidine, which, acting as a base, interacts with both NHPI and benzyl alcohol protons, increasing the speed of the oxidation through PCET (proton-coupled electron transfer) events (Figure 7B).
The second reductive step of redox methods can be carried out with different strategies, in which organic compounds, metal and photocatalysts can be employed (Figure 8). The target of this second step is the reductive cleavage of the acyloin, and it may be performed either separately or with an in-flow approach. Acyloin reduction takes place in metal-free strategies with acidic buffers such as HCO2Na/HCO2H containing water (17 wt%). With this approach, formylation, the acid-catalyzed elimination of formic acid and hydrolysis, occurs, leading to the release of phenolic products, such as syringyl and guaiacyl aromatics, p-hydroxy phenyl aldehydes and acids, together with dimers and trimers, with a total recovery of products of over 60% (Figure 8B) [74].
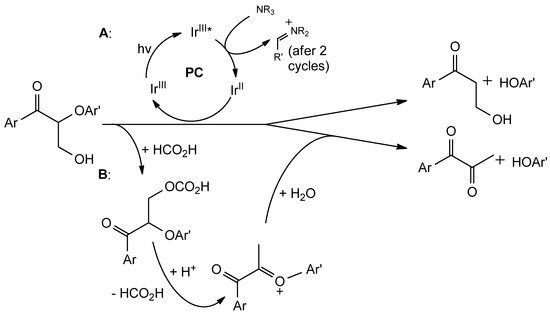
Figure 8.
Reductive cleavage of oxidized lignin. (A): photocatalytic approach with iridium-containing complexes (PC: photocatalyst); (B): formate-based method exploiting hydrolysis.
Finally, a reduction of oxidied lignin can be carried out with metals that act as simple redox catalysts and photocatalysts. In the first case, zinc powder can be employed, together with NH4Cl which acts as an effective reducing agent [71]. Alternatively, photo-reduction through metal-centred photocatalysts such as [Ir(ppy)2(dtbbpy)]PF6 can be carried out, using amines (DIPEA, for example) as electron donors under blue LED irradiation (Figure 8A) [73].
4. Starch and Lignin-Derived Catalysts
4.1. Starch-Based Catalysts
In the last few years, different catalysts and catalytic systems have been developed. They can be roughly divided into two categories: catalysts prepared from starch with conservation of the polymeric structure and catalysts prepared from starch by physical treatments that cause the extensive modifications of the core structure, with polymeric properties that are not or are only partially conserved.
Catalysts prepared from starch through physical treatments have found interesting applications in two important fields, biodiesel preparation and the photochemical removal of pollutants from wastewater. Biodiesel, also known as fatty acid methyl esters (FAME), and more generally referred to as a mixture of long chain fatty acid aliphatic esters, is produced by esterification from fatty acids such as oleic and alcohols (mostly methanol) in the presence of an acid catalyst. Among them, carbon-based solid acid catalysts (CBSAC) are gaining growing importance in this field. CBSAC are composed of amorphous carbon structural domains organized in sheets, coupled together and functionalized with different acidic moieties. Typically, sulfonic -SO3H groups are the most present ones, due to their acidic strength, which provides efficient catalysis.
CBSAC are preferred to liquid acid since they can be separated from the reaction mixture in a better way without using solvents or long experimental procedures. In the field of biodiesel production, they are appreciated because they can tolerate high concentrations of free fatty acids (FFA); also, in the presence of water, they have a high affinity for both esterification and transesterification, and show abundant Brønsted and Lewis acid sites with a high surface-to-volume ratio. These characteristics are especially important from an industrial point of view because CBSAC are also prone to the exploitation of low quality feedstocks (waste cooking oil from alimentary industry), providing a simplification of experimental procedures, which is a crucial step towards industrial scale-up and implementation.
Different starting materials can be used to produce CBSAC. Among these, starch has gained increasing importance due to its availability, low cost and eco-friendly nature. In general, CBSAC are prepared through an initial high-temperature carbonization step in which bonds between carbon and heteroatoms (oxygen) are broken down. In this way, the formation of polycyclic aromatic hydrocarbons (PAH) is provided with the generation of large and reactive conjugated aromatic structures that undergo sulfonation in the second step. In this way, starch can be converted into sulfonated CBSAC [75]. Starch brings about the formation of a CBSAC through carbonization at 400 °C followed by sulfonation with concentrated H2SO4 for 15 h at 150 °C. The obtained starch-based CBSAC shows better characteristics when compared with other polymeric acid catalysts and inorganic compounds such as Amberlyst 15, Niobic Acid and Sulphated Zirconia. Due to the high content of sulfonic groups, large pore size and high surface-to-volume ratio, starch-based CBSAC (loading of 10 wt%) is associated with high catalytic activity, bringing about a high yield (92 wt%) of methyl oleate using waste cooking oil and methanol (1:20) as substrates at 80 °C for 8 h [76]. These findings are not only important for the catalytic efficiency of the proposed systems but also because of the capability of starch-based CBSAC to promote transesterification in addition to direct esterification. This is fundamental from the environmental point of view because it implies that with such an efficient and convergent conversion procedure of substrates in FAME, there is no need for the preparatory cleavage of mono-, di- and triglycerides to obtain the reactive free fatty acids, and the direct use of available fats is made possible.
The other field in which starch-based catalysts are employed is the synthesis of carbon quantum dots for the photocatalytic degradation of pollutants in wastewater. Carbon quantum dots (CQD) are nanometric, quasi 0-dimensional particles enriched with graphitic C sp2 domains that exhibit interesting optical and photophysical properties caused by their semiconductor characteristics.
Recently, CQD preparation from starch has been reported [77]. In this case, the procedure adopted for the preparation was the hydrothermal treatment of starch: this technique is similar in that the output is carbonization but is different from an operational point of view. An aqueous solution of starch (0.3 g/mL) was treated at 200 °C in a sealed autoclave, subsequently washed with ethanol, centrifuged and filtered. Under these conditions, starch underwent structural transformations leading to the formation of nanometric particles whose photocatalytic properties were subsequently investigated.
The analyses that were performed showed that under hydrothermal conditions, amylose and amylopectin chains in starch sustain deoxygenation and conjugation. FT-IR and Raman spectroscopic investigations demonstrated that oxygen from OH groups is removed, and that ordered C sp2 graphitic domains are generated. In particular, Raman analysis showed that after the treatment of starch, the stretching and bending modes of aromatic structures are present, thus implying that high temperature and pressure induce the disconnection of glucose units and the formation of small structures with polyconjugated aromatic moieties, as also confirmed by XRD analysis. Moreover, a Raman band intensity comparison indicated that these structures are highly ordered.
In general, the chemical process associated with the formation of CQD from starch involves the breaking of carbon–oxygen bonds (which however does not lead to the complete loss of oxygen, 50/50 C/O from EDS elemental analysis). Hydrothermal treatment leads to the formation of particles that are much smaller than starting starch granules (micrometric dimensions), with TEM analysis demonstrating that CQD are spherical and have a diameter of about 4 nm. Finally, zeta potential and BET (Brunauer–Emmett–Teller) measurements unveil the chemical and superficial properties of these objects. Zeta potential values of −20/−30 mV indicate the presence of hydroxy and carboxy groups that undergo ionization, which provide negative charges that prevent aggregation and promote dispersion in the solvent. On the other side, BET measurements show an increased surface area and particles characterized by a flat profile without pores.
Starch-based CQDs analysis by DRS (diffuse reflectance spectroscopy) allowed the measurement of the band gap energy that corresponds to 4.1 eV. This value indicates that CQD behaves like photoactive semiconductors, and obtained particles showed fluorescence at 600 nm upon excitation at 300 nm (orange–red coloration). Optical properties (band gap energy) of starch-based CQD can be modified by changing the hydrothermal conditions to modify both dimensions and superficial chemical properties.
The catalytic, photo-oxidative behavior of starch-based CQD has been demonstrated in photodegradation experiments conducted on Acid Dye Blue 21, Reactive Blue 94 and Reactive TB 133, industrial dyes employed in the textile production. The photo-dependent degradation of these model pollutants was carried out using aqueous solutions of dyes under mercury lamp irradiation, and it can be observed that the reactive system relies on different factors. The reactivity of starch-based CQD depends on solution pH, the mass of employed CQD, dye concentration and reaction time. CQDs prepared from starch exhibit a reactivity that is similar to the enzymes’, with substrates that, upon contact with their photoexcited surface, undergo oxidation and finally degradation. Solution pH plays a crucial role in the system, because at low values (under pH 6), the CQD surface is positively charged. This is important since negatively charged dyes can interact electrostatically with CQD and this association provides reactive events: when pH is increased, acidic groups on the CQD surface become negatively charged (deprotonated) and superficial positive charge density decreases. As a result, starch-based CQD-driven photodegradation is possible only at low pH. CQD concentration has to not be too high (>1.2 mg/mL), because despite the increased availability of catalysts in the system, aggregation events take place that hinder the interaction of the substrate with the catalysts’ surface. Also, the starting model pollutants’ concentration influences the dye degradation. This has to not be too high, since excessive dye concentration causes light absorbance competitive with CQDs’, thus preventing the efficient excitation of the photocatalyst. Moreover, if dye solution is too concentrated, there is an overwhelming occupancy of the reactive sites, with substrate molecules that exert the competitive inhibition of the photoactive surface. As a consequence, photodegradation takes place with a diluted dye pollutant solution (Figure 9).
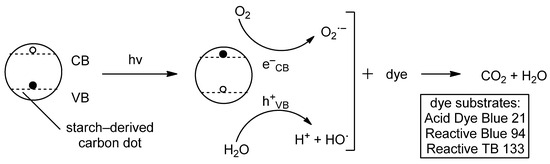
Figure 9.
General mechanism of starch-based photodegradation of pollutants. VB: valence band, CB: conduction band. Oxygen is reduced to superoxide radical by excited electrons in the conduction band, while water is oxidized by holes in the valence band with formation of hydroxyl radicals and protons.
The time dependence of dye degradation reveals insights into the mechanism of the photooxidation. Acid Dye Blue 21 (single azo dye) and Reactive Blue 94 (anthraquinone-based dye), respectively, do not show increments of conversion and increase the conversion upon prolonging the reaction time (30 to 90 min). On the contrary, Reactive TB 133 (Cu-containing phthalocyanine-based dye) is associated with a lower conversion if the reaction time is increased. These data can be explained on a kinetics basis. The photodegradation of dyes on CQDs’ surfaces either follows a pseudo-first order or a pseudo-second order kinetic behavior, with the first only depending on substrate absorption, and the second on both CQDs and dye concentrations. In the case of Acid Dye Blue 21 and Reactive Blue 94, a pseudo-second order kinetics is followed, while Reactive TB 133 (following pseudo-first order kinetic) undergoes lower conversion over longer reaction times due to the passivation of the quantum dots’ surfaces.
This work provides an interesting example of the scope and efficiency of starch-based quantum dot catalysts, whose relatively oxygen-rich, aromatic and polyconjugated structures demonstrate themselves to be highly reactive. Moreover, starch granules are well-suited precursors for carbon dot preparation, with the residual chemical structures coming from glucose units that provide crucial functional groups for catalytic efficiency. The developed starch-derived carbon quantum dots (CQDs) were used as sustainable materials with the potential for metal-free semiconductor photocatalysts for wastewater treatment applications. In the future, using these abundant bio-sourced nanomaterials in environmental cleanup is an important demand because it can provide an economical, environmentally sustainable answer to urgent water purification issues [77].
As was mentioned before, it is also possible to prepare materials with catalytic properties with procedures that do not alter their polymeric properties, which, on the contrary with respect to the previous works, have a crucial role. In this case, supramolecular association and chemical modifications are employed. In general, starch-based catalysts prepared with retention of the polymeric structure have nanometric dimensions, and can be divided into two classes: catalysts formed starting from pristine starch and catalysts in which there is the association with magnetite nanoparticles. Starch-based catalysts are effective because, on the one hand, the carbohydrate-derived structure provides the activation and correct positioning of the reactants, and on the other hand, especially in the case of Fe3O4-associated nanoparticles, the removal of the heterogeneous catalyst is made possible by simple separation with a magnet.
To produce the required catalytic activity, chemical modifications are required. Starting from pristine starch, it is possible to prepare a catalyst by first producing nanoparticles through the treatment of starch powder with malic acid and potassium persulfate, which are then sulfonated using chlorosulfonic acid [78]. On the other hand, starch can be associated with magnetite, in the form of superparamagnetic iron oxide nanoparticles (SPION), which are produced either directly in the presence of starch from an aqueous solution through reaction with aqueous ammonia, or by using a single inorganic salt solution such as aqueous FeSO4·7H2O or FeCl3·6H2O [79,80]. Magnetite nanoparticles, which are formed in the presence of starch, seize amylose and amylopectin chains that, as a consequence of the supramolecular complexation of Fe3O4 crystals, gain a cyclodextrin-like conformation. In some works, the preliminary covering of core magnetite nanoparticles with silica has also been reported. The SiO2-based covering of iron nanoparticles can be exploited in different ways. OH groups provide reactive sites which are used to build crosslinking moieties through reaction with (3-Aminopropyl)triethoxysilane (APTES) or thionyl chloride. This allows the covalent connection of starch-covered magnetite nanoparticles with 2,2′-furil cobalt complexes and PdII ions, with the latter directly interacting with lone pairs on glycosidic bonds’ oxygen atoms [81,82]. A particularly interesting example is provided by the preparation of β-cyclodextrin-associated starch nanoparticles functionalized with ionic liquids [83]. 6-O-tosylated β-cyclodextrins were reacted with methylimidazole to insert a positive charge on the oligomers that provides, with the tosylate counter-anion, the ionic liquid (IL) functionality. IL-functionalized β-cyclodextrins were therefore connected to Fe3O4-associated starch nanoparticles through reaction with hexamethylene diisocyanate that acted as the crosslinker.
The physical and morphological properties of starch-based nanoparticles (SNPs) obtained through chemisorption can be characterized using different techniques. As in the case of the previously described materials, FT-IR spectroscopy, SEM/TEM microscopy and XRD analysis are the most commonly used ones. Chemisorption is confirmed by the presence of stretching signals referred to as both Fe-O bond vibration and methylene and OH stretching modes. The general morphology of starch-based nanoparticles is characterized by a spherical shape with varying dimensions, from 7–19 to 50–80 nm, and in the case of β-cyclodextrin-IL-functionalized SNPs, it is also possible to observe a layered structure of polymer surrounding the crystalline Fe3O4 core. In some cases, BET analysis is employed to measure the surface available, reaching up to 10 m2/g [79,80]. The spherical SNPs’ aspect is retained in the case of the presence of a magnetite core, while with sulfonated SNPs (HO3S-SNPs), a rougher surface is observed if compared with pristine SNPs [78].
Amylose and amylopectin polymer chains, together with carbohydrate residues (glucose units) present in SNPs, are fundamental for the catalytic performances. The catalytic portion is either covalently linked/complexed by starch or corresponds to the anhydroglucose unit itself. A cascade of positive effects from the catalysis point of view derive from this fact. The association of catalytic functions with SNPs increase their stability into the reaction medium, increasing their efficiency. Moreover, catalytic sites are closer to each other, and the concentration of reactant on nanoparticle surfaces contributes to the overall catalytic effectiveness. This effect can also be coupled with solvent exclusion from catalytic pockets which promotes the polarization of reactive groups, which are otherwise difficult to induce. Rigid polymeric filaments stacked together in SNPs or chemisorbed onto Fe3O4 particles cause an easier geometric distortion of reactant geometry that brings about the lowering of the transition state and activation barrier energies. Stereoelectronic activation is also provided by anhydroglucose units OH polarization caused by electrostatic interactions with crystalline SPIONs. Finally, heterocatalysis provided by SNPs is advantageous from both an experimental and environmental point of view, because it makes the separation and product purification steps easier, avoiding the loss of a catalyst and the use of high quantities of toxic solvents.
HO3S-SNPs and Fe3O4-associated SNPs have proven to be efficient catalysts in the organic synthesis field. They can be employed to catalyze multicomponent reactions (MCRs) and reactions promoted by organometallic catalysis. HO3S-SNPs are efficient catalysts in the solventless synthesis of dibenzo-xanthenes starting from different aromatic aldehydes and 2-naphthol (Figure 10A), being employed at a concentration of 400 mg/mL in a closed reaction tube at 110 °C. SO3H groups protonate the aldehyde’s carbonyl group, which successively undergoes nucleophilic attack by the 2-naphthol aromatic ring (SEAr). The benzyl alcohol intermediate is further activated by a loss of water, carbocation generation, with the product that is formed through a second SEAr reaction, and a final condensation. HO3S-SNPs lead to the formation of the product with a yield of 92%, with aldehydes bearing electron-donating groups which result in being more reactive than electron-poor ones, as a result of the first step in the mechanism (aldehyde protonation), which is faster in the case of aldehydes with electron-withdrawing substituents, which proved to be more efficient or equivalent to other, more expensive polymeric or inorganic heterogeneous catalysts such as Dowex 50 W, H5PW10V2O40 and SiO2/H2SO4. Moreover, starch-based nanoparticles generally require lower temperatures and reaction times if compared with the previously mentioned catalysts [78].
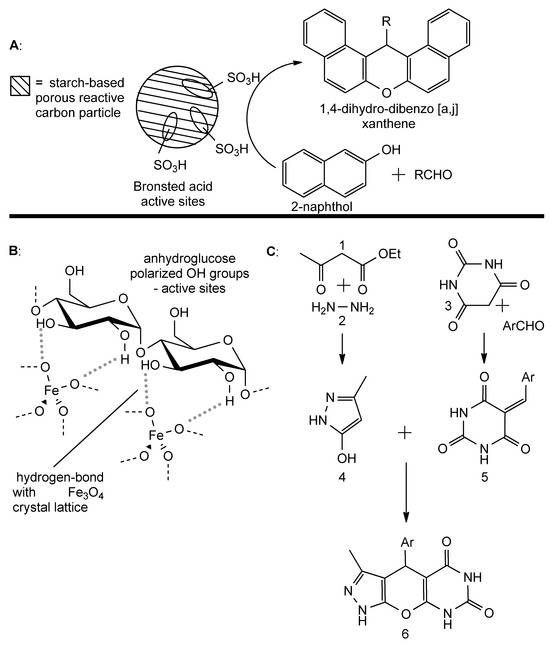
Figure 10.
(A): starch-based sulfonated reactive carbon catalyzes the formation of dibenzoxanthenes from 2-naphthol and aromatic aldehyde. (B): general structure of magnetite-bound starch magnetic nanoparticles. (C): multicomponent reaction bringing to the formation of pyrazolopyranopyrimidine. 1: acetoacetate, 2: hydrazine, 3: barbituric acid, 4: pyrazolol, 5: Knoevenagel adduct, 6: pyrazolopyranopyrimidine.
In the multicomponent assembly of dibenzo-xanthenes, sulfonyl groups on starch polymeric chains play a key role in the acid-base catalysis of the reaction. On the other hand, it is possible to directly exploit the amylose and amylopectin chains themselves. This has been demonstrated in the one-pot multicomponent synthesis of pyrazolopyranopyrimidine derivatives starting from acetoacetate, hydrazine, an aromatic aldehyde and barbituric acid. The reaction was performed in water, at room temperature, for 10 to 25 min and at a catalyst concentration of 1.3 mg/mL. The generally observed yield was 84% [80]. Anhydroglucose units of Fe3O4-associated starch filaments polarized OH groups which provided efficient activation of the reactive functional groups of the substrates in all the steps of the reaction mechanism (Figure 10B). The induced polarization brought about the formation of non-permanent partial charges that facilitate the movements of the electrons through a combination of the molecular orbitals. In particular, this effect is a key factor for both the creation and rupture of chemical bonds and the exchange of protons. Reaction mechanisms start with the parallel formation of a Knoevenagel-like adduct from an aromatic aldehyde and barbituric acid on one side and of pyrazolol from hydrazine and acetoacetate. The Michael-addition of pyrazolol on a Knoevenagel adduct (an SeAR reaction) followed by a condensation brings about the formation of a cycle and the final product (Figure 10C). The polarization that represents the driving force of the reaction was further confirmed by the fact that changing the solvent (from water to ethanol) brought about a lower yield, with ultrasonic irradiation that further promoted the formation of the product.
Starch can also be used as a catalyst when it is bound to reactive functional groups that act as the catalytic sites. This approach has been demonstrated to be effective in different applications, such as the multicomponent synthesis of heterocycles and palladium(II)-catalyzed oxidative Heck coupling. Starch-based catalysts can be covalently functionalized with reactive moieties, such as the aforementioned ionic liquid-bound β-cyclodextrin oligomers (Figure 11A). They can catalyze the formation of imidazothiadiazolamines, which are structural scaffolds often employed in medicinal chemistry. In particular, these products are obtained starting from an aldehyde and a semicarbazide, which, in the presence of FeCl3, furnish a thiadiazolamine intermediate [84]. In the second step, β-cyclodextrin-IL-bound SNPs promote the addition of a second aldehyde molecule and an isocyanide to thiadiazolamine intermediate to give the final product, through both reactant positioning and electronic activation (Figure 11B). In this case, the catalytically active portion is the IL-bound β-cyclodextrin, but the presence of Fe3O4-associated SNPs allows for the easy separation of the catalyst and guarantees its reusability [83].
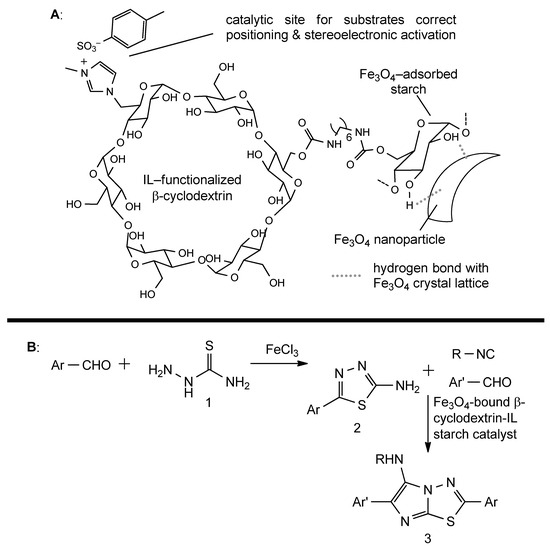
Figure 11.
(A): general schematic structure of β-cyclodextrin-IL functionalized magnetic starch-nanoparticles. (B): Synthetic sequence for the preparation of imidazothiadiazolamines. 1: thiosemicarbazide, 2: thiadiazolamine, 3: imidazothiadiazolamine.
In the case of the starch-based catalysis of oxidative Heck coupling and Tsuji–Trost allylation, Fe3O4-bound SNPs host sites where PdII ions are complexed.
Heck oxidative coupling and Tsuji–Trost allylation employ as starting materials olefin and allylic acetates, respectively. They are characterized by a mechanism in which PdII ions play a key role. In these reactions, silicon and boron-based reactants, instead of aryl and vinyl halides, are employed and are advantageous with respect to the former ones because they are tolerant to air. The driving force is represented by an electron couple shift triggered by a PdII ion, in which a Pd-X bond is substituted with Pd-R, with the R residue that is transferred to the olefin in the final reductive elimination event. Benzoquinone (BQ) is usually employed to regenerate PdII from Pd0 after each catalytic cycle [85].
PdII-functionalized Fe3O4-bound SNPs have been proven to be effective in the catalysis of these reactions with a plethora of substrates (structural variations operated on allylic acetates and olefins). It is possible to carry out the procedure at 50 to 60 °C, using THF as the reaction solvent, in the presence of BQ and tertbutyl ammonium fluoride (TBAF) under air atmosphere, with a general yield above 80% for Heck oxidative coupling (reaction time 12 h) and up to 89% for Tsuji–Trost allylation (reaction time from 4 to 6 h). PdII-functionalized Fe3O4-bound SNPs can catalyze the reaction up to four times without a significant loss of activity (Figure 12) [79].
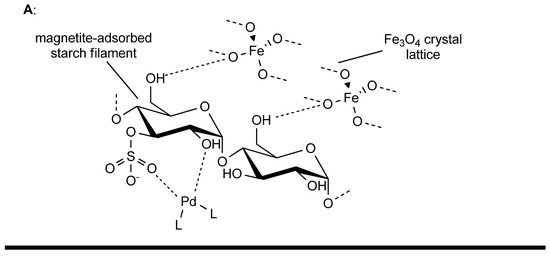
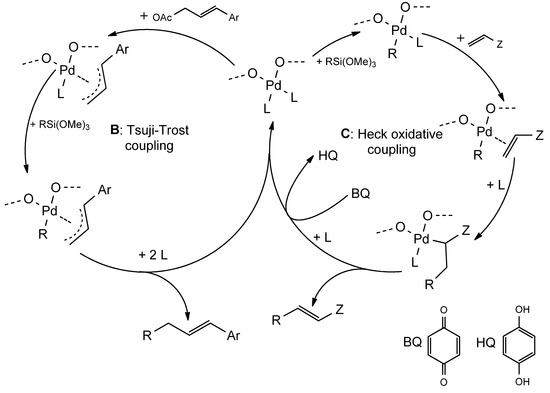
Figure 12.
(A): general structure of magnetite-adsorbed PdII-functionalized starch nanoparticles. (B): Tsuji–Trost allylation mechanism. (C): oxidative Heck coupling mechanism, BQ: benzoquinone, HQ: hydroquinone.
4.2. Lignin-Based Catalysts
Similarly to starch, lignin represents a starting material from which it is possible to realize catalysts. They can be used for both solar and electric energy exploitation and be employed in the field of green chemistry and organic synthesis. In general, catalysts derived from lignin can share a structure similar to that of starting lignin or undergo extensive, high-energy transformations during their preparation.
The first strategy by which it is possible to use lignin for the chemical conversion of energetic inputs is represented by lignin-based materials. Lignin-based materials have found a very interesting application in the field of photovoltaic cells. In recent years, solar cells have been modified by using more eco-friendly materials for their realization, and lignin provides different advantages. In particular, it is possible to exploit its polymeric and resistant structure to produce cross-linked materials that are demonstrably very effective from an operational point of view. One important application field is represented by dye-sensitized solar cells (DSSCs) [86]. DSSCs are constituted by a TiO2-based photoelectrode coupled with a Pt counter-electrode, separated by an electrolyte solution that provides electron transfer and overall neutrality. DSSC mechanisms rely on photon absorption by dye-sensitized mesoporous TiO2, connected to the external circuit through conductive glass (FTO, F-doped SnO2). The photoionization of dye molecules allows electron transport through the TiO2 layer to the external circuit, and photo-oxidized dye molecules are back-reduced by a redox shuttle (usually I3−/I−), which restores the TiO2-electrode’s charge. At the same time, photo-emitted electrons, through the external circuit, reach the Pt-counter-electrode, bringing to the regeneration of the redox shuttle and closing the cycle (Figure 13A) [87,88].
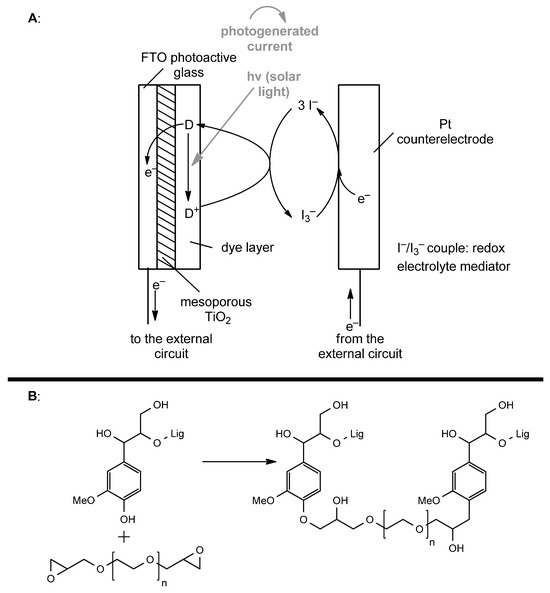
Figure 13.
(A): structure and general mechanism of a DSSC device. (B): cross-linked structure of lignin-based quasi-solid electrolyte using PEGDGE (poly (ethylene glycol) diglycidyl ether).
Kraft lignin, after preliminary oxidation with Fenton’s reagent, was employed together with poly(ethylene glycol) diglycidyl ether (PEGDGE) as a cross-linker to create lignin-based DSSCs [89]. Oxidised, highly reactive lignin was reacted with PEGDGE and epoxide ring-opening polymerization was carried out to obtain a highly functionalized lignin 3D network. In this way, by swelling with an aqueous iodide/triiodide redox shuttle (NaI/I2-H2O), it was possible to prepare a quasi-solid (QS) electrolyte (Figure 13B). The coupling of photo- and counter-electrodes allowed for the fabrication of aqueous DSSCs. Lignin’s role in DSSCs relies on its cooperation with PEGDGE filaments. In particular, it is possible to correlate the ratio between PEGDGE and lignin amounts (w/w ratio) to the performances. A 1:1 mass to mass ratio of PEGDGE and lignin allows both the handleability of QS electrolyte and the effective uptake of aqueous electrolyte. Lignin interrupts strongly interconnected PEGDGE filaments, improving the ionic conductivity. Moreover, UV absorbance by aromatic moieties in lignin and redox activity lead to the increased photostability of dye molecules. Indeed, harmful wavelengths are blocked by lignin, which furthermore acts as a radical scavenger. Lignin-based materials in the field of solar cells open up a new path towards the so-called third-generation photovoltaic devices.
The other interesting application in the field of energetic conversion where lignin is employed is the design of lithium and sodium rechargeable batteries. Indeed, high carbon content and the aromatic structure endow lignin with ideal characteristics to be employed as a carbon material for electrochemical applications. The vast abundance of functional groups present on this polymer allows different strategies for the preparation of carbon materials, although, on the contrary, with respect to what occurs with solar cells, in this case, lignin undergoes deep structural modifications through high temperature procedures [90]. In particular, lignin is employed as a precursor to prepare hierarchical lignin porous carbon (HLPC). This material is characterized by the presence of a three-dimensional network of pores, ranging from micro (<2 nm) to macro (>50 nm) dimensions through intermediate sizes (mesopores, 2–50 nm). This structure provides short pathways for the ions due to better diffusion kinetics, and as a result, the observed conductivity is strongly increased.
HLPC particles are prepared using different methods. The choice of the method is important because during the preparation the starting material is modified in such a way that influences its final performance. For this reason, in the first step of HLPC production, a lignin precursor is treated most generally through carbonization in the presence of a chemical activator, such as KOH and ZnCO3 [91]. In particular, zinc carbonate is used in a two-step strategy. By first mixing lignin and ZnCO3, lignin deposition on carbonate particles occurs. The high temperature treatment of ZnCO3 causes the release of CO2 which is beneficial, because it leads to the expansion of lignin particles through exfoliation favoring the formation of a porous structure in the subsequent step. Alongside carbon dioxide, zinc carbonate decomposition brings the formation of ZnO particles that act as templates for pores formation during the carbonization, which, after the final removal of zinc oxide through acid etching, brings the formation of porous carbon [92,93]. Another strategy by which it is possible to modify and enhance the performances of HLPC is heteroatom-doping, which, for example, can be carried out by exposing, before carbonization, lignosulfonates to poly(2-ethylaniline) [94]. This treatment is advantageous because it induces defects in the structure, modulates both the electronic and chemical properties and increases the available active sites, finally leading to enhanced performances.
HLPC materials prepared with these different approaches have shown good performances that can be correlated with their chemical composition and the structural properties, such as a high surface area and high volume, which cooperate to provide efficient ion transportation during the electrochemical process. In lithium-ion batteries, porous carbon was prepared using ZnCO3 brought to a capacity of 550 mAhg−1, while N-doped ones were associated with 764 mAhg−1 capacity [93,94].
Because of their ability to provide a conductive framework, HLPC materials are often employed to increase the conductivity in anodes starting from metals with the general formula MaXb, where M = Mn, Fe, Co, Ni, Cu and X = O, S, Se, F, N or P [95]. In this context, it has been reported to use both Mo and Ni for this purpose. The first has been used in the realization of MoS2-coated porous carbon nanospheres which are able to promote reversible electrochemical reactions through enhanced ion transportation. They can be prepared starting from a lignosulfonate precursor by carbonization at 800 °C, during which, porous carbon nanospheres are formed, followed by treatment with thiourea and sodium molybdate hexahydrate at 800 °C under a nitrogen atmosphere. With similar methods, it is also possible to prepare NiO-embedded porous carbon materials [96,97]. Using this strategy, with MoS2-associated porous carbon, it was possible to achieve a capacity of 519 mAhg−1 at 0.1 Ag−1 with high recyclability (50 cycles).
Lignin’s structure, with its abundance of aromatic and oxygenated functional groups, provides multiple advantages for the preparation of efficient catalysts. Similarly to carbon materials produced for the exploitation of solar and electric energy, lignin can also be used for the preparation of photocatalysts. In particular, lignin-based photocatalysts are used for the photocatalytic redox degradation of dye pollutants. Degradation takes place at the photoactive interface of a semiconductor catalyst, where photogenerated electrons and holes (lying in the conduction and valence bands, respectively) interact with the substrates leading to its decomposition. Lignin is used in this field because with thermal treatments, carbon-rich structures such as graphene and similar derivatives can be obtained [98]. Carbon-rich structures such as nanosheets, nanotubes and heteroatom-containing conjugated sp2-domains increase the lifetime of excited states of photoactive semiconductors such as TiO2, because they provide conjugated molecular orbitals in which photogenerated electrons can diffuse, preventing electron-hole recombination. In the case of lignin, heteroatom presence is guaranteed by the oxygen-containing moieties originally present in the monolignols residues [99,100,101].
Therefore, lignin has been used for the preparation of photocatalysts whose high efficiency has been demonstrated, and different strategies can be employed to prepare the catalyst. Most generally, high-temperature protocols are required for the formation of conjugated structures, and they can be divided into pyrolytic and hydrothermal treatments. In both these cases, lignin polymeric structure underwent deep structural modifications, with losses in the native structure and extensive deoxygenation, which leads to the formation of conjugated sp2-domains. High vacuum and nitrogen-atmosphere pyrolytic approaches for lignin-based photocatalyst preparation have been reported, in which lignin-based materials have been directly prepared or mixed with different semiconductor photocatalysts, such as TiO2 and ZnO, as well as silica-like insulators [102,103,104,105,106,107], a carbonaceous substance for the solid-phase extraction (SPE) of fluoroquinolones from aqueous solutions. The material was acquired by the pyrolytic deposition of Kraft lignin (LG) onto silica particles. Marbofloxacin (MAR) and enrofloxacin (ENR) serve as a model. Samples treated with MAR and ENR at concentrations between 10 and 1000 ng·L−1 exhibited recoveries between 70 and 116% [103]. Lignin can be either directly mixed with the catalyst in an aqueous phase [108] or undergo chemical pretreatment, such as quaternization with agents like 3-chloro-2-hydroxypropyl trimethylammonium chloride and complexation with zinc oxalate [109], before pyrolytic treatments leading to the production of TiO2- and ZnO-containing lignin-based photocatalysts, which can be carried out both under high vacuum and nitrogen atmospheres. On the other hand, the hydrothermal treatment of lignin can also be used for this purpose. For example, TiO2-containing lignin-based photocatalysts have been prepared in a two-steps process in which in the first one, hydrothermal treatment under high temperatures and pressure conditions of alkali lignin (260 °C, stainless steel autoclave, 4 h), brought about the formation of lignin carbon particles. These were subsequently mixed with TiO2 powder in a water/ethanol 1/1 mixture under UV-A light irradiation to obtain a lignin-TiO2 photocatalyst [110]. Lignin can be loaded in variable mass percentages ranging from 0.9 to 13 wt%, when combined with titanium and zinc oxide.
Lignin-containing photocatalysts have shown a high photocatalytic efficiency in the degradation of different pollutants, such as Methyl Orange, Methylene Blue, Rhodamine-B, phenol and Enrofloxacin with concentrations ranging from 1 μM to 120 ppm and higher efficiency when compared with pristine semiconductor photocatalysts, at concentrations ranging from 0.003 to 0.1% m/v. With semiconductor photoactive materials such as TiO2 and ZnO, the presence of lignin-derived carbon material is fundamental, because of the different features it provides. The polyconjugated carbon-rich matrix in carbon/semiconductor composite materials causes a red-shift in the UV absorption spectrum with respect to pristine crystals, thus also allowing efficient photoredox degradation in the presence of visible light irradiation, and exploiting the complete range of naturally available photoactive wavelengths. The observed red-shift occurs due to the lowering of the energy gap between valence and conduction bands (from 3.2 to 3.0 and 2.63 eV, with lignin-derived carbon/ZnO and TiO2 composites, respectively). Moreover, lignin-derived sp2-carbon rich, aromatic and polyconjugated structures play an important role in the mechanism because, on the one hand, the delocalization of photogenerated electrons brings about longer and more efficient excited states, preventing electron-hole recombination, while on the other hand, a red-shift increased absorption spectrum brings about the increased efficiency of the photocatalyst. Chemical features of lignin-derived photocatalysts include the coordination of metal ions by electronegative groups such as carboxylic ones, which enhance a charge transfer process that actively takes part in the reaction mechanism [111,112].
Photocatalytic redox reaction mechanisms proceed through the interaction of both water and oxygen, active chemical species, with a photoexcited catalyst. The redox process proceeds through an exchange of photogenerated electrons that, alongside with hydroxyl radicals, bring about the formation of superoxide O2.− and holes (h+ species). These are the mainly active species that act as reducing and oxidizing agents, respectively, and the general mechanism is similar to the one described for starch-based CQDs (see also Figure 9) [108,110].
Finally, lignin represents an ideal substrate from which it is possible to prepare catalysts that can be employed in the organic synthesis field and related chemical transformations. Due to its structure, lignin is characterized by high thermal resistance and the possibility to share its functional groups for the catalytic activation of the substrates [113,114]. Most importantly, heterogeneous catalysts can be prepared starting from lignin, thus avoiding collateral environmental pollution and separation issues. As a consequence, lignin-based catalysts are solids bearing different types of functionalizations. They can be classified both on chemical and structural features, and they are generally prepared through techniques that cause deep transformations of the native polymeric structure and others which preserve the substructures and the cross-linkings between monolignols.
Lignin-based solid catalysts are divided into acid, base and neutral. The provided catalysis is involved in hydrogenation, (de)hydration, hydrolysis, condensation and (trans)esterification reactions. An important group of catalysts prepared from lignin is constituted by additives obtained through pyrolysis of lignin feedstock, where sulfonation is the dominating activation procedure of the pyrolysis carbon matrix. In this general technique, lignin is first converted in porous biochar through carbonization, while in the second phase, it undergoes activation with sulfuric acid to obtain an amorphous phenyl propane polymer enriched with -SO3H groups [115,116]. Examples of this type of catalyst are provided by sulfonated carbon materials obtained by lignin through treatment at 400 and 900 °C, respectively [115,117]. In the first reported work, it was possible to produce starting from cellulose, glucose and cellulose nanofibrils with 64 and 8.1% conversion, respectively. In the second one, cellulose was efficiently converted into a mixture of valuable products such as glucose, fructose, 5-HMF, levulinic and formic acids. Sulfonate particles obtained from the lignin-derived pyrolytic carbon have dimensions ranging from 1 to 500 μm, with a size decreasing that is caused by the treatment with H2SO4. Observed starch hydrolysis is caused by the activation of the β-1,4-glycosidic linkage through the formation of -CH hydrogen bonds between hydrophobic methylene groups in cellulose and the aromatic rings in HSO3-enriched carbon, in which the sulfonyl groups further activate, together with hydroxyl and carboxyl groups, the alcoholic moieties present in sugar residues [118].
Alongside acid catalysts, base lignin-derived matrices can also be prepared with the same general strategy seen so far. Indeed, pyrolysis is generally also employed for the preparation of alkaline carbon derived from lignin but using a different strategy. On the contrary, with respect to acid ones, a one-pot procedure is generally followed in which base impregnation occurs before high-temperature treatment. The presence of inorganic activators, such as KOH, NaOH, K2CO3, Na2CO3 and Ba(OH)2, not only is connected to the alkaline behavior but also to the formation of a fine dispersion of pores which are responsible for the catalytic activity. Temperature is a key factor that can be used to finely tune the morphological properties, since it has been demonstrated that turning from 800 to 900 °C in the pyrolytic step can increase the superficial area from 41 to 441 m2/g. Lignin-based alkaline catalysts prepared with this strategy have shown high efficiency in biodiesel production (transesterification of fatty acid) inducing a reaction yield ranging from 91 to 97% with a reactive mixture of 1/15 MeOH/oil, at a concentration of 3 wt% at 65 °C after 2 h [119,120].
To increase their reactivity, carbonaceous matrices prepared with pyrolysis of lignin can be doped with heteroatoms such as nitrogen. Eucalyptus lignin, after carbonization at 1200 °C in the presence of g-C3N4 for 2 h, afforded an efficient catalyst for the reduction of nitrobenzene into aniline with NaBH4. Nitrogen atoms induce a high positive charge density that activates carbon atoms providing a metal-like catalytic behavior [121].
A lignin aromatic-rich structure is an excellent starting material for the preparation of reactive carbon; nevertheless, it can also be used as a catalyst, preserving its moieties. Indeed, functional groups attached to the polymer matrix and substructures themselves can play a key-role in the catalytic activation of the substrates, through involvement of hydroxyl and carboxylic groups. Hydrogen bonding and Van der Waals interactions are fundamental for the electronic activation of the reactants, together with the rigid backbone that induces the correct positioning of the reactive groups. Lignin-derived catalysts in which polymeric structure is preserved are mostly prepared starting from lignosulfonates, which are salts formed upon the exposure of native lignin to sodium hydrogen sulfite, in which α hydroxyls in the propanoid fragment are substituted by -SO3H groups. They are industrially produced in large quantities, and can act as catalysts upon different types of pretreatments. Lignosulfonates have been employed in the catalytic production of monosaccharides from polysaccharides such as cellulose through preliminary hydroxymethylation with formaldehyde in the presence of HCl. Hydroxymethyl groups, together with -SO3H ones, are fundamental for the activation of glycosidic bonds, and the selective production of monosaccharides took place with respect to other products such as levulinic acid. Selectivity for monosaccharides over other by-products is much higher with lignosulfonate catalysis than with other inorganic catalysts such as H-ZSM-5 zeolites and sulfonated zirconia. Lignosulfonate-derived solid catalysts are also associated with the total conversion of polysaccharides such as cellobiose after four cycles of reuse and glucose yields always above 80%. This indicates that these catalysts are characterized by high reusability and stability under the reaction conditions. The overall observed catalytic activity is due to the lowering of the activation energy (up to −20 kcal/mol ΔΔG#) of hydrolysis over other dehydration and retro aldol reactions (Figure 14) [122].
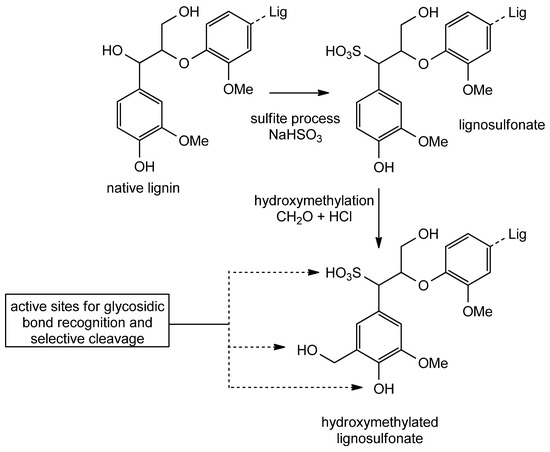
Figure 14.
Hydroxymethylated lignosulfonates for the selective and catalytic conversion of starch into monosaccharides.
Room temperature-modified lignosulfonates are employed in a variety of synthetic transformations, in which both metal complexes and organic reactive functional groups are involved. Due to the presence of strong acid functions (-SO3H), lignosulfonates are used in ion exchange reactions to coordinate both metal and organic cations. In the first case, reactive complexes are generated, while in the second, ionic liquids are obtained. Scandium (ScIII) lignosulfonates have been involved in the catalysis of tandem Michael addition-dehydration and electrophilic ring-opening reactions for the synthesis of substituted 4H-chromenes and using dihydropyran, with 91 and 89% yields, respectively, at temperatures ranging from 80 to 100 °C and polar solvents such as EtOH and MeNO2. On the other side, copper (CuII) lignosulfonates proved to be highly efficient in the catalysis of click 3-components reactions between substituted benzyl halides and phenylacetylene in the presence of sodium azide for the synthesis of triazine heterocycles, with the yield reaching 96%. In these cases, the catalytic activation is provided by both metal centres and functional groups present in the polymeric structure of lignin (-SO3H and -OH), but in the case of ionic liquid functionalized lignin, an organocatalytic approach is exploited. This eventuality has been reported in the case of Knoevenagel condensation of 4-chlorobenzaldehyde and malononitrile, bringing to the formation of the product a 97% yield at room temperature in EtOH. This reaction also represents an example of how lignosulfonates functionalized with ionic liquids provide both acid-base and nucleophilic catalysis. In Knoevenagel condensation, SO3H- groups participate only in proton exchanges, while amino groups from ILs residues are involved in both acid-base equilibria and nucleophilic catalysis through the formation of carbon–nitrogen bonds. Lignosulfonate-based catalysts are employed in low amounts (1 to 5 mol%) at milder conditions with respect to industrial ones (Figure 15) [123].
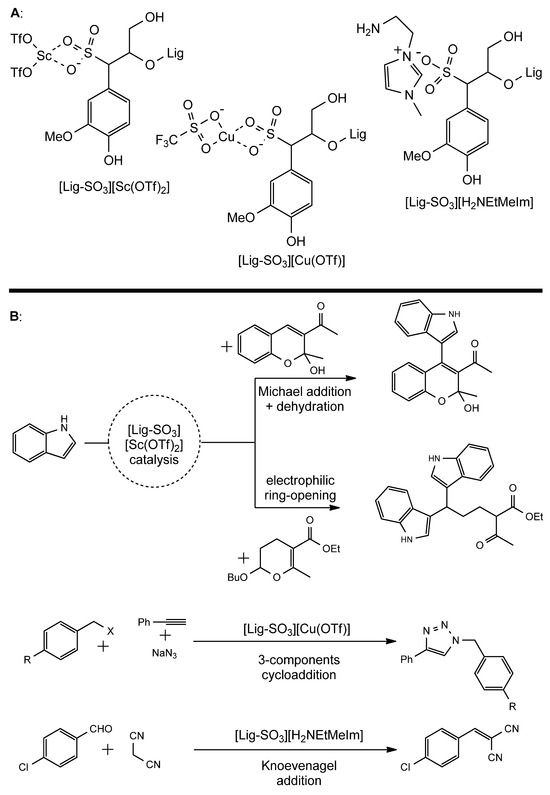
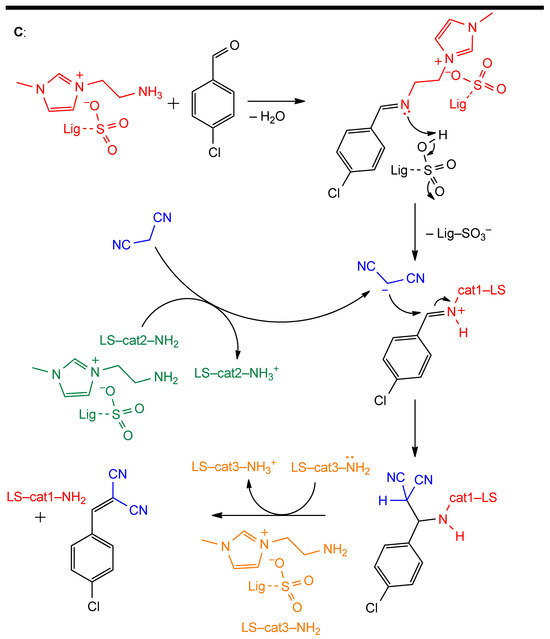
Figure 15.
(A): representative structures of lignosulfonates scandium and copper complexes together with IL-functionalized lignosulfonates. (B): synthetic applications of lignosulfonate derivatives. (C): LS-catn-NH2 (n = 1, 2, 3 depending on the order in which it enters the mechanism) indicates the lignosulfonate-based catalyst that supports the ionic liquid entering the Knoevenagel mechanism.
5. Conclusions
Global climate warming challenges caused by uncontrolled industrialization and ecological misconduct that occurs all over the planet forces humankind to find alternative solutions, to either prevent or slow down the catastrophic environmental phenomena we have been facing, especially in recent years. Vast amounts of biomasses, produced as a result of human activities, can be used as valuable resources for technological applications, and biomass valorization incorporates a double advantage. On the one hand, large deposits of cheap starting materials can be found easily without additional extraction operations, while on the other hand, their conversion into valuable chemicals or materials reduces their environmental impact, thus contributing to a circular economy and an eco-friendlier industrial production.
Starch and lignin, among the most abundant biomasses produced as a result of industrial activities, can be employed both as substrates and precursors for catalytic applications. The main problem is that an abundance of lignin is required, but the lignin that is being used is polluted and comes from sources in the industries [124]. However, because of the chemical diversity and the richness of functional groups observed in lignin, and also the regular structure and the large abundance of hydroxyl groups on starch filaments, this can be exploited. Such characteristics enable both these polymers to efficiently interact with organic and inorganic species.
In this review, it has been shown that catalysts are fundamental for the modifications of lignin and starch. In the case of starch, catalytic modifications are required for both its valorization as a biomass and a material, since it is possible to exploit it for the extraction of chemicals and the preparation of additives that can be used in the industry. Levulinic and lactic acid, together with 5-HMF, are notable examples of valuable molecules that can be obtained with this approach, while oxidation provides an upgraded material for the coating, textile and paper industries. On the other side, it has been seen that lignin is mainly subjected to catalytic transformations for the production and the extraction of chemicals that can also be used in the organic synthesis and medicinal chemistry fields, thus avoiding its thermal conversion, which is associated with massive carbon dioxide emissions.
Furthermore, different types of catalysts can be prepared starting from lignin and starch. Both can undergo thermal and thermochemical transformations to produce reactive carbon materials, with classical chemistry and photocatalysis applications. In addition to this, the highly functionalized polymeric matrices that starch and lignin share can be directly employed for the preparation of catalysts in which the polymeric backbone not only is conserved but is also chemically modified and exploited to enhance the overall catalytic efficiency, for example, in the case of multicomponent and cross-coupling reactions.
In conclusion, the works reported in this paper demonstrate that starch and lignin are materials that will open up new methods in the chemistry and catalysis landscape, and that will contribute to the development of a more environmentally compatible technological advancement, hopefully counteracting the currently growing environmental pollution and unsustainability.
Author Contributions
Conceptualization, D.V. and D.D.; methodology, D.D.; investigation, D.V. and F.F.; resources, F.F. and D.V.; data curation, F.F.; writing—original draft preparation, F.F.; writing—review and editing, D.V. and D.D.; supervision, D.D. and D.V.; project administration, D.D. and D.V.; funding acquisition, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is part of the project NODES which has received funding from the MUR—M4C2 1.5 of PNRR funded by the European Union—NextGenerationEU (Grant agreement no. ECS00000036).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
D.V. and D.D. acknowledge support from the Ministry of University and Research (MUR) and the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Aboobacker, V.M.; Seegobin, V.O.; Al Khayat, J.A.; Rangel-Buitrago, N.; Al-Kuwari, H.A.-S.; Sadooni, F.N.; Vethamony, P. History of a Disaster: A Baseline Assessment of the Wakashio Oil Spill on the Coast of Mauritius, Indian Ocean. Mar. Pollut. Bull. 2022, 175, 113330. [Google Scholar] [CrossRef] [PubMed]
- Cazacu, G.; Pascu, M.C.; Profire, L.; Kowarski, A.I.; Mihaes, M.; Vasile, C. Lignin Role in a Complex Polyolefin Blend. Ind. Crops Prod. 2004, 20, 261–273. [Google Scholar] [CrossRef]
- Wang, S.-K.; Yang, K.-X.; Zhu, Y.-R.; Zhu, X.-Y.; Nie, D.-F.; Jiao, N.; Angelidaki, I. One-Step Co-Cultivation and Flocculation of Microalgae with Filamentous Fungi to Valorize Starch Wastewater into High-Value Biomass. Bioresour. Technol. 2022, 361, 127625. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and Challenges in Biological Lignin Valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef]
- Burritt, R.L.; Schaltegger, S. Measuring the (Un-)Sustainability of Industrial Biomass Production and Use. Sustain. Account. Manag. Policy J. 2012, 3, 109–133. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The Structural Characteristics of Starches and Their Functional Properties. CyTA J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Avancini, S.R.P.; Faccin, G.L.; Vieira, M.A.; Rovaris, A.A.; Podestá, R.; Tramonte, R.; De Souza, N.M.A.; Amante, E.R. Cassava Starch Fermentation Wastewater: Characterization and Preliminary Toxicological Studies. Food Chem. Toxicol. 2007, 45, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.P.; Pugazhenthi, G. Treatment of Starch-rich Wastewater Using Fly Ash-based Low-cost Tubular Ceramic Membrane. Environ. Prog. Sustain. Energy 2022, 41, e13906. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.D.R.; Gandra, E.A. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch Stärke 2020, 72, 1900200. [Google Scholar] [CrossRef]
- Devereux, S.; Shuttleworth, P.S.; Macquarrie, D.J.; Paradisi, F. Isolation and Characterization of Recovered Starch from Industrial Wastewater. J. Polym. Environ. 2011, 19, 971–979. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of Bioplastics Production from Starch and Lignocellulosic Components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, E. Rethinking Sustainability: A Research on Starch Based Bioplastic. J. Sustain. Constr. Mater. Technol. 2018, 3, 249–260. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, Y.; Wu, Q. Simultaneous Saccharification of Cassava Starch and Fermentation of Algae for Biodiesel Production. J. Appl. Phycol. 2011, 23, 115–121. [Google Scholar] [CrossRef]
- Welker, C.; Balasubramanian, V.; Petti, C.; Rai, K.; DeBolt, S.; Mendu, V. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef]
- Santos, R.B.; Hart, P.; Jameel, H.; Chang, H. Wood Based Lignin Reactions Important to the Biorefinery and Pulp and Paper Industries. BioResources 2013, 8, 1456–1477. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Sun, R. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef]
- Ariyanta, H.A.; Sari, F.P.; Sohail, A.; Restu, W.K.; Septiyanti, M.; Aryana, N.; Fatriasari, W.; Kumar, A. Current Roles of Lignin for the Agroindustry: Applications, Challenges, and Opportunities. Int. J. Biol. Macromol. 2023, 240, 124523. [Google Scholar] [CrossRef]
- Wang, Z.; Deuss, P.J. The Isolation of Lignin with Native-like Structure. Biotechnol. Adv. 2023, 68, 108230. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting Lignin: A Tour through Its Structural Features, Characterization Methods and Applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of Current and Future Softwood Kraft Lignin Process Chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Zhou, H.; Lou, H.; Yang, D.; Zhu, J.Y.; Qiu, X. Lignosulfonate To Enhance Enzymatic Saccharification of Lignocelluloses: Role of Molecular Weight and Substrate Lignin. Ind. Eng. Chem. Res. 2013, 52, 8464–8470. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- José Borges Gomes, F.; De Souza, R.E.; Brito, E.O.; Costa Lelis, R.C. A Review on Lignin Sources and Uses. J. Appl. Biotechnol. Bioeng. 2020, 7, 100–105. [Google Scholar] [CrossRef]
- De La Torre, M.J.; Moral, A.; Hernández, M.D.; Cabeza, E.; Tijero, A. Organosolv Lignin for Biofuel. Ind. Crops Prod. 2013, 45, 58–63. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Nakasu, P.Y.S.; Scopel, E.; Araújo, M.F.; Cardoso, L.H.; Costa, A.C.D. Organosolv Pretreatment for Biorefineries: Current Status, Perspectives, and Challenges. Bioresour. Technol. 2023, 369, 128331. [Google Scholar] [CrossRef]
- Li, J.; Gellerstedt, G.; Toven, K. Steam Explosion Lignins; Their Extraction, Structure and Potential as Feedstock for Biodiesel and Chemicals. Bioresour. Technol. 2009, 100, 2556–2561. [Google Scholar] [CrossRef]
- Hu, Z.; Yeh, T.-F.; Chang, H.; Matsumoto, Y.; Kadla, J.F. Elucidation of the Structure of Cellulolytic Enzyme Lignin. Holzforschung 2006, 60, 389–397. [Google Scholar] [CrossRef]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; Da Rosa Zavareze, E. Molecular Structure, Functionality and Applications of Oxidized Starches: A Review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Podolean, I.; Anita, F.; García, H.; Parvulescu, V.I.; Coman, S.M. Efficient Magnetic Recoverable Acid-Functionalized-Carbon Catalysts for Starch Valorization to Multiple Bio-Chemicals. Catal. Today 2017, 279, 45–55. [Google Scholar] [CrossRef]
- Dookheh, M.; Najafi Chermahini, A. Starch Valorization: Direct Conversion of Starch to Hexyl Levulinate over SO4/ZrO2-KIT5 Composite. Int. J. Biol. Macromol. 2024, 262, 130093. [Google Scholar] [CrossRef] [PubMed]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Guo, H.; Bian, K.; Ding, S.; Cai, H.; Zhang, H.; Chen, X.; Wang, C.; Yao, S.; Chen, X. Efficient Utilization of Biomass Hydrolysis Residues in Preparing a Metal/Acid Bifunctional Catalyst for Butyl Levulinate Hydrogenation to γ-Valerolactone. Ind. Eng. Chem. Res. 2023, 62, 5502–5514. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Dong, M.; Wu, Y.; Zheng, G.; Huang, J.; Guan, X.; Zheng, X. MCM-41 Immobilized 12-Silicotungstic Acid Mesoporous Materials: Structural and Catalytic Properties for Esterification of Levulinic Acid and Oleic Acid. J. Taiwan Inst. Chem. Eng. 2016, 61, 147–155. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Selvaraj, M.; Rajabathar, J.R.; Khoerunnisa, F.; Rigolet, S.; Daou, T.J.; Maireles-Torres, P.; El-Bahy, S.M.; El-Bahy, Z.M.; Ng, E.-P. Highly Efficient Non-Microwave Instant Heating Synthesis of Hexyl Levulinate Fuel Additive Enhanced by Sulfated Nanosilica Catalyst. Microporous Mesoporous Mater. 2022, 331, 111645. [Google Scholar] [CrossRef]
- Parovuori, P.; Hamunen, A.; Forssell, P.; Autio, K.; Poutanen, K. Oxidation of Potato Starch by Hydrogen Peroxide. Starch Stärke 1995, 47, 19–23. [Google Scholar] [CrossRef]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of Physicochemical Properties of Hypochlorite- and Peroxide-Oxidized Cassava Starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch Modification through Environmentally Friendly Alternatives: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Wang, X.-L.; Zhao, G.-M.; Wang, Y.-Z. Preparation and Properties of Oxidized Starch with High Degree of Oxidation. Carbohydr. Polym. 2012, 87, 2554–2562. [Google Scholar] [CrossRef]
- Floor, M.; Schenk, K.M.; Kieboom, A.P.G.; Van Bekkum, H. Oxidation of Maltodextrins and Starch by the System Tungstate-Hydrogen Peroxide. Starch Stärke 1989, 41, 303–309. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Kachkarova-Sorokina, S.L.; Donzé, C.; Pinel, C.; Gallezot, P. From Native Starch to Hydrophilic and Hydrophobic Products: A Catalytic Approach. Top. Catal. 2004, 27, 67–76. [Google Scholar] [CrossRef]
- Wang, H.; Poya, Y.; Chen, X.; Jia, T.; Wang, X.; Shi, J. Hydrogen Peroxide as an Oxidant in Starch Oxidation Using Molybdovanadophosphate for Producing a High Carboxylic Content. RSC Adv. 2015, 5, 45725–45730. [Google Scholar] [CrossRef]
- Broekman, J.O.P.; Genuino, H.C.; Heeres, H.J.; Brinksma, J.; Wielema, T.; Deuss, P.J. Benign Catalytic Oxidation of Potato Starch Using a Homogeneous Binuclear Manganese Catalyst and Hydrogen Peroxide. Catal. Sci. Technol. 2023, 13, 1233–1243. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, L.; Liao, B.; Fu, Y.; Guo, Q.-X. Aromatics Production via Catalytic Pyrolysis of Pyrolytic Lignins from Bio-Oil. Energy Fuels 2010, 24, 5735–5740. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic Fast Pyrolysis of Biomass over Zeolites for High Quality Bio-Oil—A Review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Carlson, T.R.; Jae, J.; Lin, Y.-C.; Tompsett, G.A.; Huber, G.W. Catalytic Fast Pyrolysis of Glucose with HZSM-5: The Combined Homogeneous and Heterogeneous Reactions. J. Catal. 2010, 270, 110–124. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic Pyrolysis of Woody Biomass in a Fluidized Bed Reactor: Influence of the Zeolite Structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Jeon, J.-K.; Kim, J.; Ryoo, R.; Jeong, K.-E.; Park, Y.-K. Highly Valuable Chemicals Production from Catalytic Upgrading of Radiata Pine Sawdust-Derived Pyrolytic Vapors over Mesoporous MFI Zeolites. Appl. Catal. B Environ. 2010, 95, 365–373. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Influence of Si/Al Ratio of ZSM-5 Zeolite on the Properties of Lignin Pyrolysis Products. ACS Sustain. Chem. Eng. 2013, 1, 316–324. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Du, X.; Hao, X.; Zhang, Z.; Abudula, A. Selective Catalytic Conversion of Bio-Oil over High-Silica Zeolites. Bioresour. Technol. 2015, 179, 518–523. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.H.; Lin, Y.-C.; Lee, H.V. A Review on Catalytic Hydrodeoxygenation of Lignin to Transportation Fuels by Using Nickel-Based Catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Bjelić, A.; Likozar, B.; Grilc, M. Scaling of Lignin Monomer Hydrogenation, Hydrodeoxygenation and Hydrocracking Reaction Micro-Kinetics over Solid Metal/Acid Catalysts to Aromatic Oligomers. Chem. Eng. J. 2020, 399, 125712. [Google Scholar] [CrossRef]
- Mahdavi, B.; Lafrance, A.; Martel, A.; Lessard, J.; Me’Nard, H.; Brossard, L. Electrocatalytic hydrogenolysis of lignin model dimers at Raney nickel electrodes. J. Appl. Electrochem. 1997, 27, 605–611. [Google Scholar] [CrossRef]
- Robin, D.; Comtois, M.; Martel, A.; Lemieux, R.; Cheong, A.K.; Belot, G.; Lessard, J. The Electrocatalytic Hydrogenation of Fused PolyCyclic Aromatic Compounds at Raney Nickel Electrodes: The Influence of Catalyst Activation and Electrolysis Conditions. Can. J. Chem. 1990, 68, 1218–1227. [Google Scholar] [CrossRef]
- Mahdavi, B.; Chambrion, P.; Binette, J.; Martel, E.; Lessard, J. Electrocatalytic Hydrogenation of Conjugated Enones on Nickel Boride, Nickel, and Raney Nickel Electrodes. Can. J. Chem. 1995, 73, 846–852. [Google Scholar] [CrossRef]
- Mahdavi, B.; Chapuzet, J.M.; Lessard, J. The Electrocatalytic Hydrogenation of Phenanthrene at Raney Nickel Electrodes: The Effect of Periodic Current Control. Electrochim. Acta 1993, 38, 1377–1380. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Smith, K.J.; Kim, C.S.; Gyenge, E.L. Electrocatalytic Hydrogenation and Depolymerization Pathways for Lignin Valorization: Toward Mild Synthesis of Chemicals and Fuels from Biomass. Green Chem. 2020, 22, 7233–7264. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Chen, Y.; Li, G.; Li, H.; Tang, Y.; Wan, P. Electrochemical Depolymerization of Lignin into Renewable Aromatic Compounds in a Non-Diaphragm Electrolytic Cell. RSC Adv. 2014, 4, 29917. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Liu, Z.; Yuan, L.; Li, G. Electrocatalytic Degradation of Aspen Lignin over Pb/PbO2 Electrode in Alkali Solution. Catal. Commun. 2015, 67, 49–53. [Google Scholar] [CrossRef]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical Depolymerisation of Lignin in a Deep Eutectic Solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.A.; Filho, N.M.L.; Abreu, C.A.M. Experimental Evaluation and Continuous Catalytic Process for Fine Aldehyde Production from Lignin. Chem. Eng. Sci. 2007, 62, 5386–5391. [Google Scholar] [CrossRef]
- Pourjafar, S.; Kreft, J.; Bilek, H.; Kozliak, E.; Seames, W. Exploring Large Pore Size Alumina and Silica-Alumina Based Catalysts for Decomposition of Lignin. AIMS Energy 2018, 6, 993–1008. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.; Lin, L. Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-Type Oxide: LaFe1−xCuxO3 (X = 0, 0.1, 0.2). Molecules 2009, 14, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xu, A.; Du, H.; Sun, C.; Li, C. Removal of Salicylic Acid on Perovskite-Type Oxide LaFeO3 Catalyst in Catalytic Wet Air Oxidation Process. J. Hazard. Mater. 2007, 139, 86–92. [Google Scholar] [CrossRef]
- Royer, S.; Levasseur, B.; Alamdari, H.; Barbier, J., Jr.; Duprez, D.; Kaliaguine, S. Mechanism of Stearic Acid Oxidation over Nanocrystalline La1−xA′xBO3La1−xA′xBO3 (A′ = Sr, Ce; B = Co, Mn): The Role of Oxygen Mobility. Appl. Catal. B Environ. 2008, 80, 51–61. [Google Scholar] [CrossRef]
- Fortuny, A. Bimetallic Catalysts for Continuous Catalytic Wet Air Oxidation of Phenol. J. Hazard. Mater. 1999, 64, 181–193. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Matsuura, B.S.; Stephenson, C.R.J. A Photochemical Strategy for Lignin Degradation at Room Temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C-O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. 2015, 127, 260–264. [Google Scholar] [CrossRef]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective Metal-Free Aerobic Alcohol Oxidation in Lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef] [PubMed]
- Bosque, I.; Magallanes, G.; Rigoulet, M.; Kärkäs, M.D.; Stephenson, C.R.J. Redox Catalysis Facilitates Lignin Depolymerization. ACS Cent. Sci. 2017, 3, 621–628. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-Acid-Induced Depolymerization of Oxidized Lignin to Aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.T.; Jusoh, M.; Zakaria, Z.Y. Starch-Derived Solid Acid Catalyst for Biodiesel Production: A Mini Review. Chem. Eng. Trans. 2021, 89, 487–492. [Google Scholar] [CrossRef]
- Lou, W.-Y.; Zong, M.-H.; Duan, Z.-Q. Efficient Production of Biodiesel from High Free Fatty Acid-Containing Waste Oils Using Various Carbohydrate-Derived Solid Acid Catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Sheshmani, S.; Mardali, M.; Shokrollahzadeh, S.; Bide, Y. Starch-Derived Carbon Quantum Dots: Unveiling Structural Insights and Photocatalytic Potential as a Bio-Sourced Metal-Free Semiconductor. Int. J. Biol. Macromol. 2024, 271, 132535. [Google Scholar] [CrossRef] [PubMed]
- Safari, J.; Aftabi, P.; Ahmadzadeh, M.; Sadeghi, M.; Zarnegar, Z. Sulfonated Starch Nanoparticles: An Effective, Heterogeneous and Bio-Based Catalyst for Synthesis of 14-Aryl-14-H-Dibenzo[a,j]Xanthenes. J. Mol. Struct. 2017, 1142, 33–39. [Google Scholar] [CrossRef]
- Patra, D.; Panja, S.; Saha, A. C–C Cross-Coupling Reactions of Organosilanes with Terminal Alkenes and Allylic Acetates Using PdII Catalyst Supported on Starch Coated Magnetic Nanoparticles. Eur. J. Org. Chem. 2020, 2020, 878–883. [Google Scholar] [CrossRef]
- Dharmendra, D.; Chundawat, P.; Vyas, Y.; Ameta, C. Ultrasound-Assisted Efficient Synthesis and Antimicrobial Evaluation of Pyrazolopyranopyrimidine Derivatives Using Starch Functionalized Magnetite Nanoparticles as a Green Biocatalyst in Water. J. Chem. Sci. 2022, 134, 47. [Google Scholar] [CrossRef]
- Dohendou, M.; Pakzad, K.; Nezafat, Z.; Nasrollahzadeh, M.; Dekamin, M.G. Progresses in Chitin, Chitosan, Starch, Cellulose, Pectin, Alginate, Gelatin and Gum Based (Nano)Catalysts for the Heck Coupling Reactions: A Review. Int. J. Biol. Macromol. 2021, 192, 771–819. [Google Scholar] [CrossRef] [PubMed]
- Arghan, M.; Koukabi, N.; Kolvari, E. Magnetic Apple Seed Starch Functionalized with 2,2′-furil as a Green Host for Cobalt Nanoparticles: Highly Active and Reusable Catalyst for Mizoroki–Heck and the Suzuki–Miyaura Reactions. Appl. Organomet. Chem. 2019, 33, e5075. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Ansari, S.; Hamedifar, H.; Mahdavi, M. The Use of Magnetic Starch as a Support for an Ionic Liquid-β-Cyclodextrin Based Catalyst for the Synthesis of Imidazothiadiazolamine Derivatives. Int. J. Biol. Macromol. 2019, 135, 453–461. [Google Scholar] [CrossRef]
- Pandit, N.; Shah, K.; Agrawal, N.; Upmanyu, N.; Shrivastava, S.K.; Mishra, P. Synthesis, Characterization and Biological Evaluation of Some Novel Fluoroquinolones. Med. Chem. Res. 2016, 25, 843–851. [Google Scholar] [CrossRef]
- Lee, A.-L. Enantioselective Oxidative Boron Heck Reactions. Org. Biomol. Chem. 2016, 14, 5357–5366. [Google Scholar] [CrossRef]
- Ma, L.; Zhan, X. Dye-Sensitized Solar Cells (DSSCs). In Organic Optoelectronics; Hu, W., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 437–465. ISBN 978-3-527-32968-7. [Google Scholar]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent Advances in Eco-Friendly and Cost-Effective Materials towards Sustainable Dye-Sensitized Solar Cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef]
- De Haro, J.C.; Tatsi, E.; Fagiolari, L.; Bonomo, M.; Barolo, C.; Turri, S.; Bella, F.; Griffini, G. Lignin-Based Polymer Electrolyte Membranes for Sustainable Aqueous Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 8550–8560. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhao, C.-X.; Li, B.-Q.; Yuan, T.-Q.; Zhang, Q. Lignin-Derived Materials and Their Applications in Rechargeable Batteries. Green Chem. 2022, 24, 565–584. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Lin, Z.; Lin, H.; Lu, H.; Wang, Y.; Huang, W. Facile Preparation of 3D Hierarchical Porous Carbon from Lignin for the Anode Material in Lithium Ion Battery with High Rate Performance. Electrochim. Acta 2015, 176, 1136–1142. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, Y.; Yang, D.; Zhang, Z.; Liu, W.; Li, Q.; Qiu, X. K2CO3 Activation Enhancing the Graphitization of Porous Lignin Carbon Derived from Enzymatic Hydrolysis Lignin for High Performance Lithium-Ion Storage. J. Alloys Compd. 2019, 785, 706–714. [Google Scholar] [CrossRef]
- Xi, Y.; Huang, S.; Yang, D.; Qiu, X.; Su, H.; Yi, C.; Li, Q. Hierarchical Porous Carbon Derived from the Gas-Exfoliation Activation of Lignin for High-Energy Lithium-Ion Batteries. Green Chem. 2020, 22, 4321–4330. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, L.; Lou, X.W. (David) Nanostructured Conversion-Type Anode Materials for Advanced Lithium-Ion Batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef]
- Chen, F.; Wu, L.; Zhou, Z.; Ju, J.; Zhao, Z.; Zhong, M.; Kuang, T. MoS2 Decorated Lignin-Derived Hierarchical Mesoporous Carbon Hybrid Nanospheres with Exceptional Li-Ion Battery Cycle Stability. Chin. Chem. Lett. 2019, 30, 197–202. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, F.; Kuang, T.; Chang, L.; Yang, J.; Fan, P.; Zhao, Z.; Zhong, M. Lignin-Derived Hierarchical Mesoporous Carbon and NiO Hybrid Nanospheres with Exceptional Li-Ion Battery and Pseudocapacitive Properties. Electrochim. Acta 2018, 274, 288–297. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, M.; He, C.; Liu, C.; Li, Z.; Liu, Y. Preparation of Graphene by Catalytic Pyrolysis of Lignin and Its Electrochemical Properties. Mater. Lett. 2020, 274, 128047. [Google Scholar] [CrossRef]
- Reddy, K.R.; Jyothi, M.S.; Raghu, A.V.; Sadhu, V.; Naveen, S.; Aminabhavi, T.M. Nanocarbons-Supported and Polymers-Supported Titanium Dioxide Nanostructures as Efficient Photocatalysts for Remediation of Contaminated Wastewater and Hydrogen Production. In Nanophotocatalysis and Environmental Applications; Inamuddin, Asiri, A.M., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2020; Volume 30, pp. 139–169. ISBN 978-3-030-12618-6. [Google Scholar]
- Ramesh Reddy, N.; Bhargav, U.; Mamatha Kumari, M.; Cheralathan, K.K.; Sakar, M. Review on the Interface Engineering in the Carbonaceous Titania for the Improved Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 7584–7615. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, N.; Wang, X.; Hu, X.; Miao, X.; Chaker, M.; Ma, D. Advanced Fabrication of Chemically Bonded Graphene/TiO2 Continuous Fibers with Enhanced Broadband Photocatalytic Properties and Involved Mechanisms Exploration. Sci. Rep. 2016, 6, 38066. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, D.; Branciforti, D.S.; Speltini, A.; Sturini, M.; Bellani, V.; Malaichamy, I.; Dondi, D. Pyrolytic Formation of TiO2/Carbon Nanocomposite from Kraft Lignin: Characterization and Photoactivities. Catalysts 2020, 10, 270. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Maraschi, F.; Mandelli, E.; Vadivel, D.; Dondi, D.; Profumo, A. Preparation of Silica-Supported Carbon by Kraft Lignin Pyrolysis, and Its Use in Solid-Phase Extraction of Fluoroquinolones from Environmental Waters. Microchim. Acta 2016, 183, 2241–2249. [Google Scholar] [CrossRef]
- Dondi, D.; Zeffiro, A.; Speltini, A.; Tomasi, C.; Vadivel, D.; Buttafava, A. The Role of Inorganic Sulfur Compounds in the Pyrolysis of Kraft Lignin. J. Anal. Appl. Pyrolysis 2014, 107, 53–58. [Google Scholar] [CrossRef]
- Vadivel, D.; Suryakumar, S.; Casella, C.; Speltini, A.; Dondi, D. Advancements in Materials Science and Photocatalysts for Sustainable Development. Catalysts 2024, 14, 378. [Google Scholar] [CrossRef]
- Dondi, D.; Vadivel, D. Preparation of Catalysts from Renewable and Waste Materials. Catalysts 2020, 10, 662. [Google Scholar] [CrossRef]
- Vadivel, D.; Malaichamy, I. Pyrolytic Formation and Photoactivity of Reactive Oxygen Species in a SiO2/Carbon Nanocomposite from Kraft Lignin. F1000Research 2018, 7, 1574. [Google Scholar] [CrossRef]
- Vadivel, D.; Speltini, A.; Zeffiro, A.; Bellani, V.; Pezzini, S.; Buttafava, A.; Dondi, D. Reactive Carbons from Kraft Lignin Pyrolysis: Stabilization of Peroxyl Radicals at Carbon/Silica Interface. J. Anal. Appl. Pyrolysis 2017, 128, 346–352. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Zhong, R.; Fu, F.; Qian, Y.; Yang, D. One-Pot in-Situ Preparation of a Lignin-Based Carbon/ZnO Nanocomposite with Excellent Photocatalytic Performance. Mater. Chem. Phys. 2017, 199, 193–202. [Google Scholar] [CrossRef]
- Donar, Y.O.; Bilge, S.; Sinağ, A. Utilisation of Lignin as a Model Biomass Component for Preparing a Highly Active Photocatalyst under UV and Visible Light. Mater. Sci. Semicond. Process. 2020, 118, 105151. [Google Scholar] [CrossRef]
- Mabuti, L.A.; Manding, I.K.S.; Mercado, C.C. Photovoltaic and Photocatalytic Properties of Bismuth Oxyiodide–Graphene Nanocomposites. RSC Adv. 2018, 8, 42254–42261. [Google Scholar] [CrossRef]
- Matos, J.; García, A.; Zhao, L.; Titirici, M.M. Solvothermal Carbon-Doped TiO2 Photocatalyst for the Enhanced Methylene Blue Degradation under Visible Light. Appl. Catal. A Gen. 2010, 390, 175–182. [Google Scholar] [CrossRef]
- Mennani, M.; Kasbaji, M.; Benhamou, A.A.; Boussetta, A.; Ablouh, E.-H.; Bayousfi, O.; Grimi, N.; Moubarik, A. Effects of Direct Sulfonation on the Catalytic Activity and Recyclability of Novel Lignin-Based Solid Acid Catalysts from Agri-Food Waste. Int. J. Biol. Macromol. 2023, 230, 123242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Z.; Chen, J. Applications of Lignin-Derived Catalysts for Green Synthesis. Green Energy Environ. 2019, 4, 210–244. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, F.; Hsieh, Y.-L. 1D Lignin-Based Solid Acid Catalysts for Cellulose Hydrolysis to Glucose and Nanocellulose. ACS Sustain. Chem. Eng. 2015, 3, 2566–2574. [Google Scholar] [CrossRef]
- Liang, F.; Song, Y.; Huang, C.; Zhang, J.; Chen, B. Preparation and Performance Evaluation of a Lignin-Based Solid Acid from Acid Hydrolysis Lignin. Catal. Commun. 2013, 40, 93–97. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Cheng, Z.; Kuang, Y.; Wu, Q.; Wang, B.; Gao, W.; Zeng, J.; Li, J.; Chen, K. Catalytic Transformation of Cellulose into Short Rod-like Cellulose Nanofibers and Platform Chemicals over Lignin-Based Solid Acid. Appl. Catal. B Environ. 2020, 268, 118732. [Google Scholar] [CrossRef]
- Mennani, M.; Kasbaji, M.; Ait Benhamou, A.; Boussetta, A.; Mekkaoui, A.A.; Grimi, N.; Moubarik, A. Current Approaches, Emerging Developments and Functional Prospects for Lignin-Based Catalysts—A Review. Green Chem. 2023, 25, 2896–2929. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of Activated Carbon from Lignin by Chemical Activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Activated Carbon from Hydrolysis Lignin: Effect of Activation Method on Carbon Properties. Biomass Bioenergy 2022, 159, 106387. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Yu, H.; Yang, H.; Chen, T. Synthesis of Lignin-Derived Nitrogen-Doped Carbon as a Novel Catalyst for 4-NP Reduction Evaluation. Sci. Rep. 2020, 10, 20075. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, F.; Wang, Y.; Song, Q.; Xu, J. Lignosulfonate-Based Heterogeneous Sulfonic Acid Catalyst for Hydrolyzing Glycosidic Bonds of Polysaccharides. J. Mol. Catal. A Chem. 2013, 377, 102–107. [Google Scholar] [CrossRef]
- Sun, S.; Bai, R.; Gu, Y. From Waste Biomass to Solid Support: Lignosulfonate as a Cost-Effective and Renewable Supporting Material for Catalysis. Chem. A Eur. J 2014, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, J.; Shan, R.; Yuan, H.; Chen, Y. Advances in Thermochemical Valorization of Biomass towards Carbon Neutrality. Resour. Conserv. Recycl. 2025, 212, 107905. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).