Catalytic Degradation of Bisphenol A in Water by Non-Thermal Plasma Coupled with Persulfate
Abstract
1. Introduction
2. Result and Discussion
2.1. Influence of Various Experimental Parameters
2.1.1. Effects of Discharge Voltage and Pulse Frequency
2.1.2. Effects of Initial BPA Concentration and Solution pH
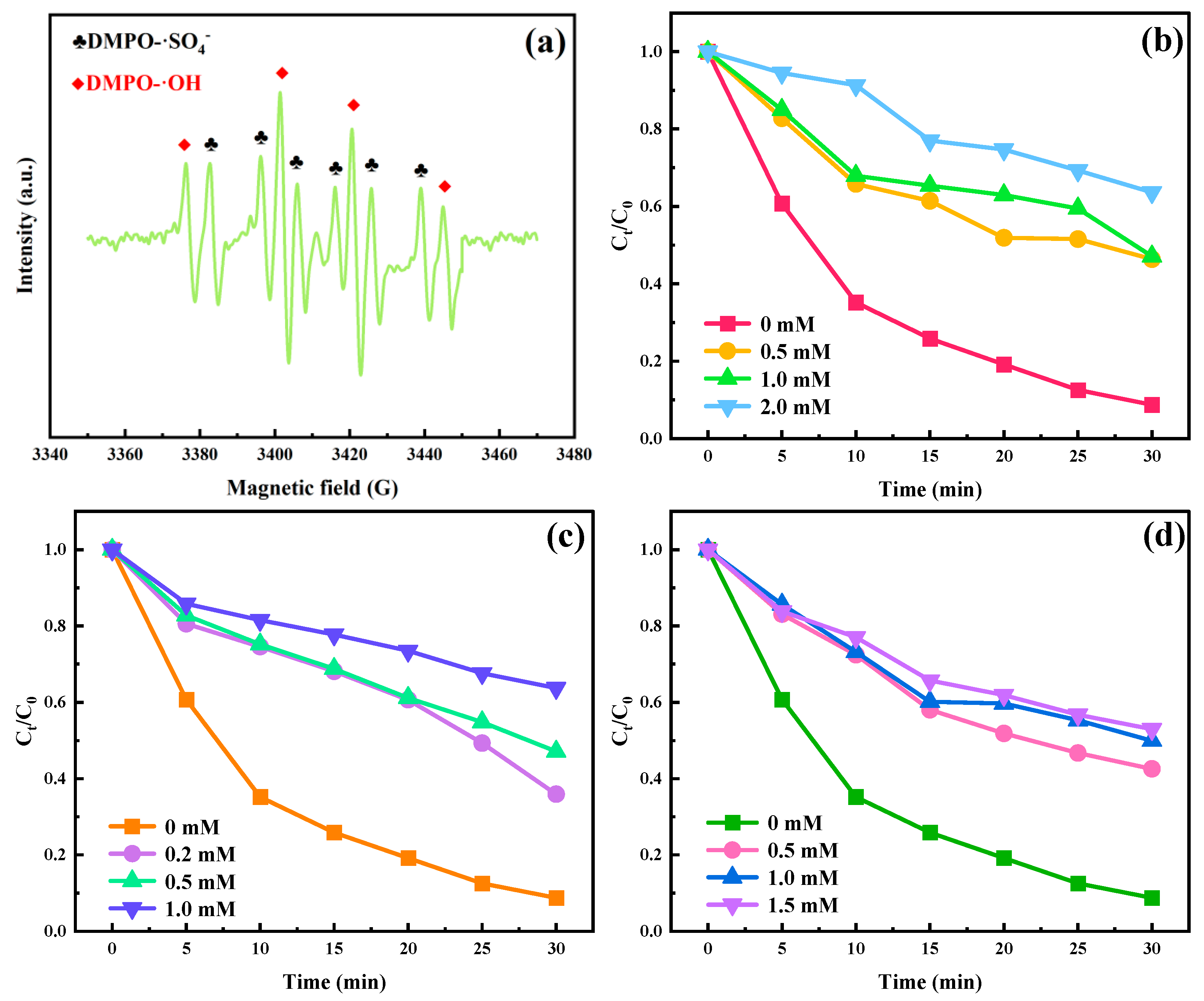
2.1.3. Effects of PS Addition
2.2. Roles of Active Substances
2.3. Analysis of BPA Degradation Process
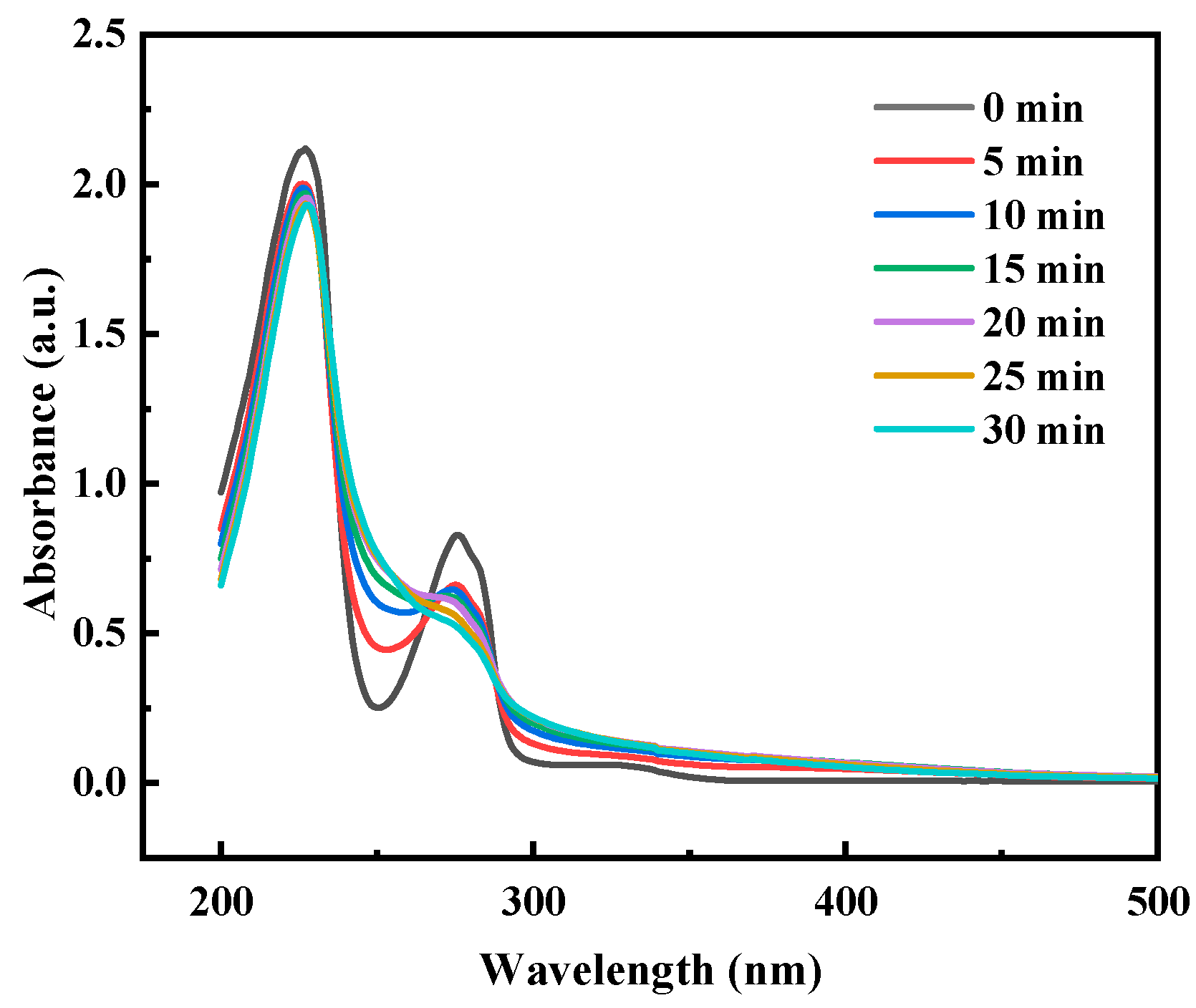
2.3.1. Changes in pH, UV-Vis and Three-Dimensional Fluorescence Spectra
2.3.2. Analysis of Intermediate Products by LC-MS
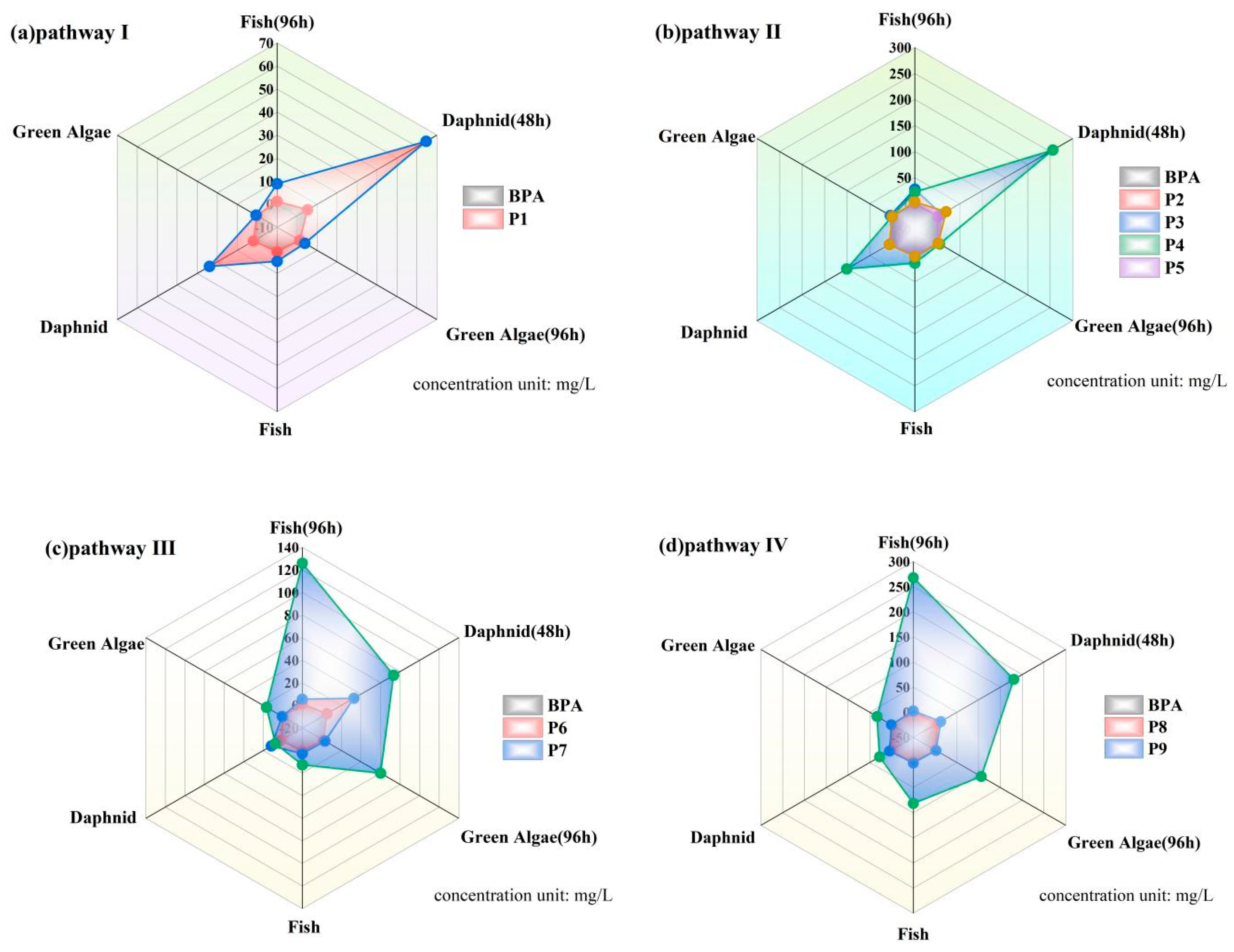
2.4. Toxicity Analysis of Intermediate Products
2.5. Changes in Actual Wastewater COD and Ammonia Nitrogen
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Setup
3.3. Analysis Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.P.; Zhang, M. Adsorption Characteristics and Mechanism of Bisphenol A by Magnetic Biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, J.L. Biological Strategies for Bisphenol A Degradation: Mechanisms and Pathways. Rev. Environ. Sci. Bio/Technol. 2024, 23, 601–632. [Google Scholar] [CrossRef]
- Liu, B.C.; Qiao, M.; Wang, Y.B.; Wang, L.J.; Gong, Y.; Guo, T.; Zhao, X. Persulfate Enhanced Photocatalytic Degradation of Bisphenol A by G-C3N4 Nanosheets under Visible Light Irradiation. Chemosphere 2017, 189, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Yazid, M.D.; Bahari, H.; Keong, Y.Y.; Rajandram, R.; Embong, H.; Teoh, S.H.; Halim, S.; Othman, F. Bisphenol A (BPA) Leading to Obesity and Cardiovascular Complications: A Compilation of Current In Vivo Study. Int. J. Mol. Sci. 2022, 23, 2969. [Google Scholar] [CrossRef]
- Xiao, C.Y.; Wang, L.H.; Zhou, Q.; Huang, X.H. Hazards of Bisphenol A (BPA) Exposure: A Systematic Review of Plant Toxicology Studies. J. Hazard. Mater. 2020, 384, 121488. [Google Scholar] [CrossRef]
- Wang, B.W.; Wang, Y. A Comprehensive Review on Persulfate Activation Treatment of Wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef]
- Xavier, S.; Gandhimathi, R.; Nidheesh, P.V.; Ramesh, S.T. Comparison of Homogeneous and Heterogeneous Fenton Processes for the Removal of Reactive Dye Magenta MB from Aqueous Solution. Desalin. Water Treat. 2015, 53, 109–118. [Google Scholar] [CrossRef]
- Lee, D.-K.; Cho, I.-C.; Lee, G.-S.; Kim, S.-C.; Kim, D.-S.; Yang, Y.-K. Catalytic Wet Oxidation of Reactive Dyes with H2/O2 Mixture on Pd–Pt/Al2O3 Catalysts. Sep. Purif. Technol. 2004, 34, 43–50. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, W.; Zhang, Y.; Fan, Q.; Zhang, Y.; Ling, C.; Pan, Y. Boron/Fe2+/H2O2 combined with resins process cost-effectively remove Ni(II) in wastewater containing Ni-EDTA: Performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 114112. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Liu, J.; Gao, B.; Hao, J.L. Ozone Direct Oxidation Pretreatment and Catalytic Oxidation Post-Treatment Coupled with ABMBR for Landfill Leachate Treatment. Sci. Total Environ. 2021, 794, 148557. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic Ozonation for Water and Wastewater Treatment: Recent Advances and Perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Ren, X.; Duan, X.; Sarmah, A.K.; Zhao, X.S. Remediation of Environmentally Persistent Organic Pollutants (POPs) by Persulfates Oxidation System (PS): A Review. Sci. Total Environ. 2023, 863, 160818. [Google Scholar] [CrossRef] [PubMed]
- Puyang, C.D.; Han, J.G.; Guo, H. Degradation of Emerging Contaminants in Water by a Novel Non-Thermal Plasma/Periodate Advanced Oxidation Process: Performance and Mechanisms. Chem. Eng. J. 2024, 483, 149194. [Google Scholar] [CrossRef]
- Liu, Y.N.; Duan, J.P.; Zhou, Q.; Zhu, L.X.; Liu, N.; Sun, Z.Y. Effective Degradation of Lindane and Its Isomers by Dielectric Barrier Discharge (DBD) Plasma: Synergistic Effects of Various Reactive Species. Chemosphere 2023, 338, 139607. [Google Scholar] [CrossRef]
- Belkessa, N.; Assadi, A.A.; Bouzaza, A.; Nguyen-Tri, P.; Amrane, A.; Khezami, L. A Review of Non-Thermal Plasma -Catalysis: The Mutual Influence and Sources of Synergetic Effect for Boosting Volatile Organic Compounds Removal. Environ. Res. 2024, 257, 119333. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; He, J.; Luo, Y.; Miruka, A.C.; Zhang, A.; Liu, Y. Synergistic Performance and Mechanisms of Plasma and Peracetic Acid for Antibiotic Degradation in Water. Chem. Eng. J. 2024, 498, 155096. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Yu, Z.B.; Wang, L.; Xiang, G.L.; Wan, S.G. Synergistic Degradation Performance and Mechanism of 17β-Estradiol by Dielectric Barrier Discharge Non-Thermal Plasma Combined with Pt–TiO2. Sep. Purif. Technol. 2015, 152, 46–54. [Google Scholar] [CrossRef]
- Liu, S.; Kang, Y. Underwater Bubbling Plasma Assisted with Persulfate Activation for the Synergistic Degradation of Tetracycline Hydrochloride. Environ. Res. 2024, 240, 117539. [Google Scholar] [CrossRef]
- You, C.-S.; Jung, S.-C. Decomposition of the Antibiotic Sulfamethoxazole by the Liquid-Phase Plasma Process Enhanced by Peroxymonosulfate. J. Ind. Eng. Chem. 2024, 131, 422–431. [Google Scholar] [CrossRef]
- Cheng, J.S.; Fan, Y.Y.; Pei, X.Y.; Tian, D.; Liu, Z.W.; Yang, L.Z.; Feng, E.; Ji, H.F.; Chen, Q. An Energy Efficient Process for Degrading Perfluorooctanoic Acid (PFOA) Using Strip Fountain Dielectric Barrier Discharge Plasma. Water 2022, 14, 2420. [Google Scholar] [CrossRef]
- Wang, B.W.; Zhang, Y.J.; Wang, Y. Synergistic Degradation Levofloxacin through Dielectric Barrier Discharge and Sodium Persulfate. J. Environ. Chem. Eng. 2023, 11, 111158. [Google Scholar] [CrossRef]
- Yu, W.L.; Wang, Y.; Wan, S.G.; Sun, L.; Yu, Z. Ultrahigh-Efficient BiOBr-x%La@y%CNQDs Nanocomposites with Enhanced Generation and Separation of Photogenerated Carriers towards Bisphenol A Degradation and Toxicity Reduction. Chemosphere 2022, 308, 136390. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.Q.; Hassan, M.; Ma, Z.B.; Dong, L.Q.; Xie, Y.; He, Y.L. Efficient Degradation of Bisphenol A by Dielectric Barrier Discharge Non-Thermal Plasma: Performance, Degradation Pathways and Mechanistic Consideration. Chemosphere 2022, 286, 131627. [Google Scholar] [CrossRef]
- Liang, C.J.; Su, H.-W. Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Shang, K.F.; Li, W.F.; Wang, X.J.; Lu, N.; Jiang, N.; Li, J.; Wu, Y. Degradation of P-Nitrophenol by DBD Plasma/Fe2+/Persulfate Oxidation Process. Sep. Purif. Technol. 2019, 218, 106–112. [Google Scholar] [CrossRef]
- Wang, B.W.; Li, X.Y.; Wang, Y. Degradation of Metronidazole in Water Using Dielectric Barrier Discharge Synergistic with Sodium Persulfate. Sep. Purif. Technol. 2022, 303, 122173. [Google Scholar] [CrossRef]
- Wu, Y.; Song, C.; Yu, X.; Shen, X.; Xu, L.; Zhang, Y.; Gong, H.; Xia, C.; Gan, L. Waste polypropylene filter induced synthesis of pure phase Fe3O4 and ZVI incorporated carbon composite for non-radical peroxymonosulfate activation dominated sulfameth-oxazole degradation: Singlet oxygen versus electron transfer process. Chem. Eng. J. 2024, 480, 147984. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Shen, X.; Wu, Y.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. New insight into the S and N co-doped poplar biochar for efficient BPA removal via peroxymonosulfate activation: S for adsorptive removal and N for catalytic removal. Sep. Purif. Technol. 2024, 354, 128809. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.; Shen, X.; Wu, Y.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. Lignin regulating the active sites and peroxymonosulfate activation capacities of iron incorporated 1D carbon nanofiber for efficient organic pollutant degradation. Sep. Purif. Technol. 2025, 354, 129156. [Google Scholar] [CrossRef]
- Huo, Y.; Yuan, S.; Zhang, N.; Pei, C.; Pan, Y.; Zhang, Y.; Mei, X.; Qiao, W.; Xu, L.; Gan, L. Important role of cellulose and lignin in controlling the crystal structure of iron-carbon composite: Fe3C surpassing Fe0 in activating peroxymonosulfate. Sep. Purif. Technol. 2025, 355, 129752. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhang, A.; Li, P.; Héroux, P.; Zhang, H.; Yu, X.; Liu, Y.N. Degradation of Aqueous Atrazine Using Persulfate Activated by Electrochemical Plasma Coupling with Microbubbles: Removal Mechanisms and Potential Applications. J. Hazard. Mater. 2021, 403, 124087. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, W.X.; Huang, J.W.; Wang, Y.W.; Guo, H. ZnO Promoted Persulfate Activation in Discharge Plasma System for Ofloxacin Degradation. Catalysts 2023, 13, 847. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.J.; Wu, Q.; Li, J. Degradation and Mechanism Analysis of Bisphenol A in Aqueous Solutions by Pulsed Discharge Plasma Combined with Activated Carbon. Sep. Purif. Technol. 2018, 190, 288–296. [Google Scholar] [CrossRef]
- Song, S.; Huang, Y.; Du, Y.; Xiao, S.; Han, S.; Hu, K.; Zhang, H.; Wang, H.; Wu, C.; Qiong, A. Oxidation of Ciprofloxacin by the Synergistic Effect of DBD Plasma and Persulfate: Reactive Species and Influencing Factors Analysis. Plasma Sci. Technol. 2023, 25, 025505. [Google Scholar] [CrossRef]
- Pei, X.Y.; Ren, H.Y.; Liu, G.S.; Cao, G.L.; Xie, G.J.; Xing, D.F.; Ren, N.Q.; Liu, B.F. Non-Radical Mechanism and Toxicity Analysis of β-Cyclodextrin Functionalized Biochar Catalyzing the Degradation of Bisphenol A and Its Analogs by Peroxydisulfate. J. Hazard. Mater. 2022, 424, 127254. [Google Scholar] [CrossRef]
- Roy, D.; Neogi, S.; De, S. Mechanistic Investigation of Photocatalytic Degradation of Bisphenol-A Using MIL-88A(Fe)/MoS2 Z-Scheme Heterojunction Composite Assisted Peroxymonosulfate Activation. Chem. Eng. J. 2022, 428, 131028. [Google Scholar] [CrossRef]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant. State Environmental Protection Administration: Beijing, China, 2002.
- Su, Y.; Yang, Y.; Jiang, W.; Han, J.; Guo, H. A novel strategy of peracetic acid activation by dielectric barrier discharge plasma for bisphenol a degradation: Feasibility, mechanism and active species dominant to degradation pathway. Chem. Eng. J. 2023, 476, 146469. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, A.; Héroux, P.; Guo, Y.; Sun, Z.; Li, Z.; Zhao, J.; Liu, Y. Remediation of perfluorooctanoic acid (PFOA) polluted soil using pulsed corona discharge plasma. J. Hazard. Mater. 2019, 387, 121688. [Google Scholar] [CrossRef]







| Products | m/z | Compound | Molecular |
|---|---|---|---|
| P0 | 227 | 4,4′-(propane-2,2-diyl)diphenol |  |
| P1 | 215 | bis(4-hydroxyphenyl)methanone |  |
| P2 | 94 | phenol |  |
| P3 | 110 | hydroquinone |  |
| P4 | 108 | benzoquinone | 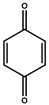 |
| P5 | 186 | [1,1′-biphenyl]-4,4′-diol |  |
| P6 | 259 | 4,4′-(propane-2,2-diyl)bis(benzene-1,2-diol) | 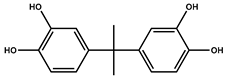 |
| P7 | 255 | 4,4′-(propane-2,2-diyl)bis (cyclohexa-3,5-diene-1,2-dione) |  |
| P8 | 243 | 4-(2-(4-hydroxyphenyl)propan-2-yl) 5-benzene-1,2-diol | 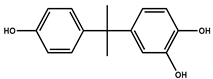 |
| P9 | 241 | 2-hydroxy-4-(2-(4-oxocyclohexa-2,5-dien-1-yl) propan-2-3-yl)cyclohexa-2,5-dien-1-one | 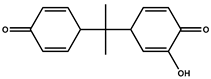 |
| Name of Reagent | Formula | Manufacturer |
|---|---|---|
| BPA | C15H16O2 | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| Sodium persulfate | NaS2O4 | Sinopharm Chemical Reagent Co., Ltd. |
| Phosphoric acid | H3PO4 | Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China) |
| P-benzoquinone | C6H4O2 | Sinopharm Chemical Reagent Co., Ltd. |
| Methanol | CH3OH | Anhui Tedia High Purity Solvents Co., Ltd. (Shanghai, China) |
| Triethylenediamine | C6H12N2 | Shanghai Macklin Biochemical Technology Co., Ltd. |
| Sodium hydroxide | NaOH | Nanjing Chemical Reagent Co., Ltd. (Nanjing, China) |
| Isopropanol | C3H8O | Sinopharm Chemical Reagent Co., Ltd. |
| Acetonitrile | C2H3N | Sinopharm Chemical Reagent Co., Ltd. |
| Sulfuric acid | H2SO4 | Sinopharm Chemical Reagent Co., Ltd. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, S.; Cui, J.; Guo, H. Catalytic Degradation of Bisphenol A in Water by Non-Thermal Plasma Coupled with Persulfate. Catalysts 2024, 14, 750. https://doi.org/10.3390/catal14110750
Zhang H, Yang S, Cui J, Guo H. Catalytic Degradation of Bisphenol A in Water by Non-Thermal Plasma Coupled with Persulfate. Catalysts. 2024; 14(11):750. https://doi.org/10.3390/catal14110750
Chicago/Turabian StyleZhang, Han, Shuang Yang, Jiayu Cui, and He Guo. 2024. "Catalytic Degradation of Bisphenol A in Water by Non-Thermal Plasma Coupled with Persulfate" Catalysts 14, no. 11: 750. https://doi.org/10.3390/catal14110750
APA StyleZhang, H., Yang, S., Cui, J., & Guo, H. (2024). Catalytic Degradation of Bisphenol A in Water by Non-Thermal Plasma Coupled with Persulfate. Catalysts, 14(11), 750. https://doi.org/10.3390/catal14110750







