Comparison of the Effects of NaOH and Deep Eutectic Solvent Catalyzed Tobacco Stock Lignin Isolation: Chemical Structure and Thermal Characteristics
Abstract
1. Introduction
2. Results and Discussion
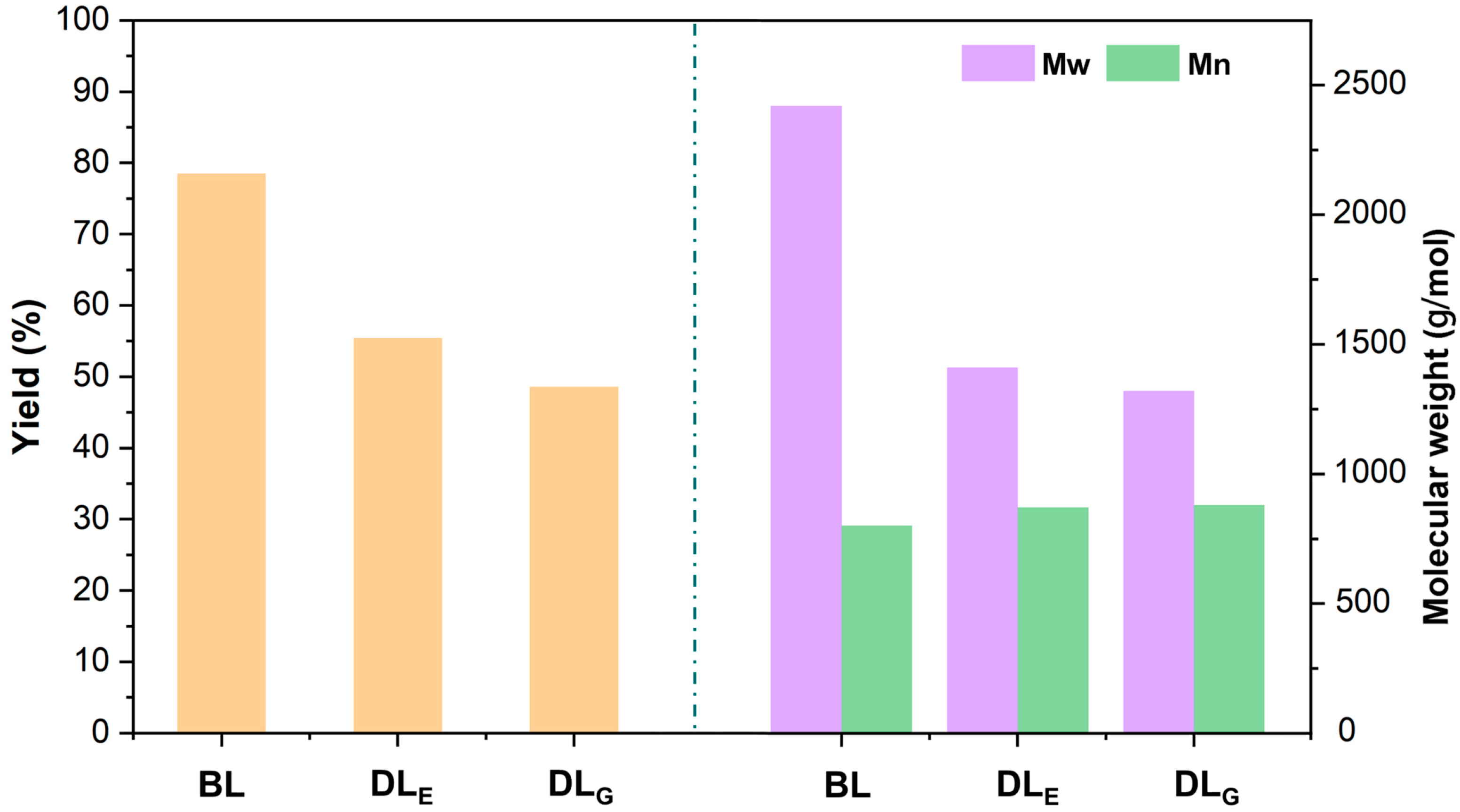
2.1. Yields and Molecular Weight of Lignin Samples
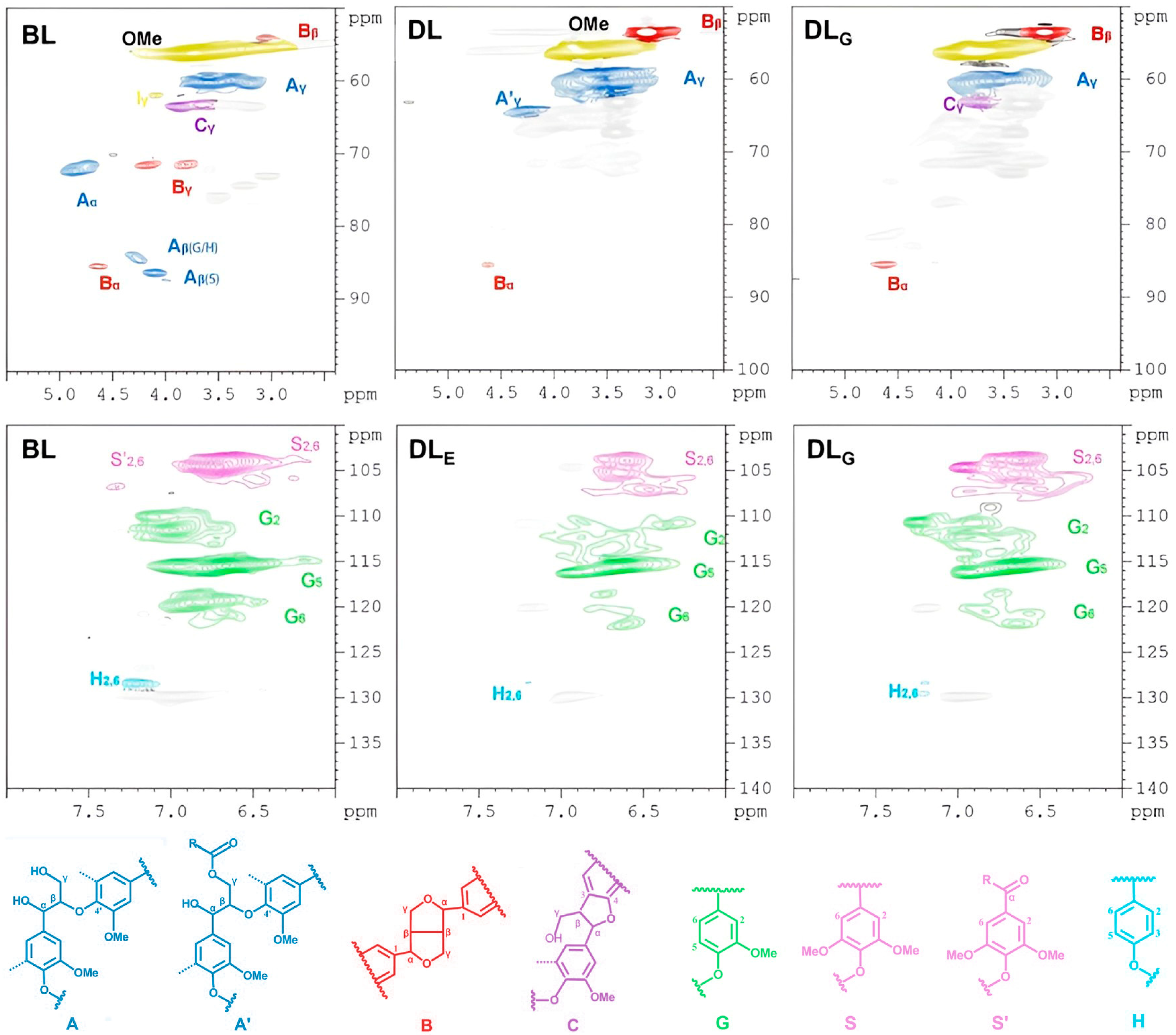
D-HSQC NMR Spectra of Lignin Samples
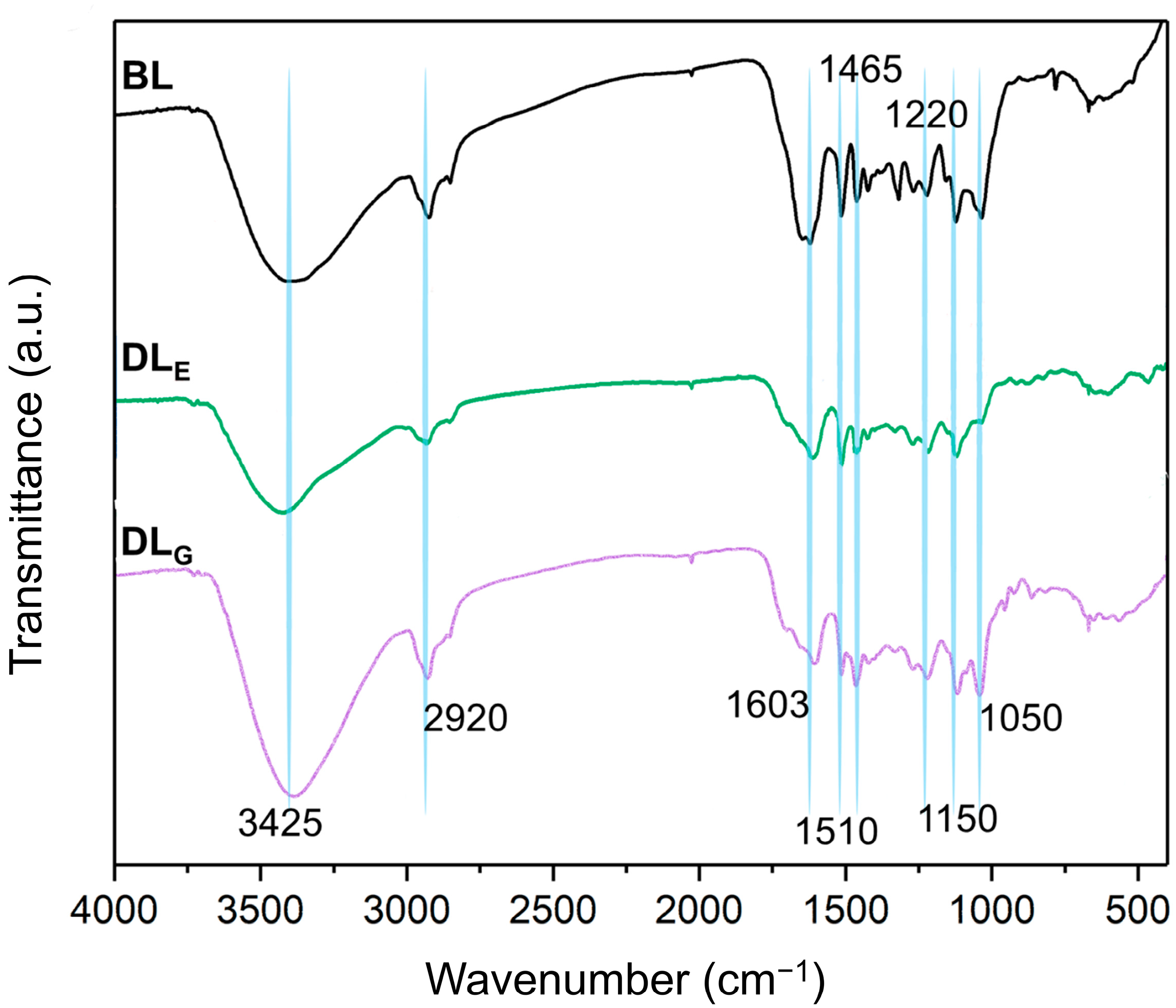
2.2. FT-IR Spectra Element Analysis of Lignin Samples
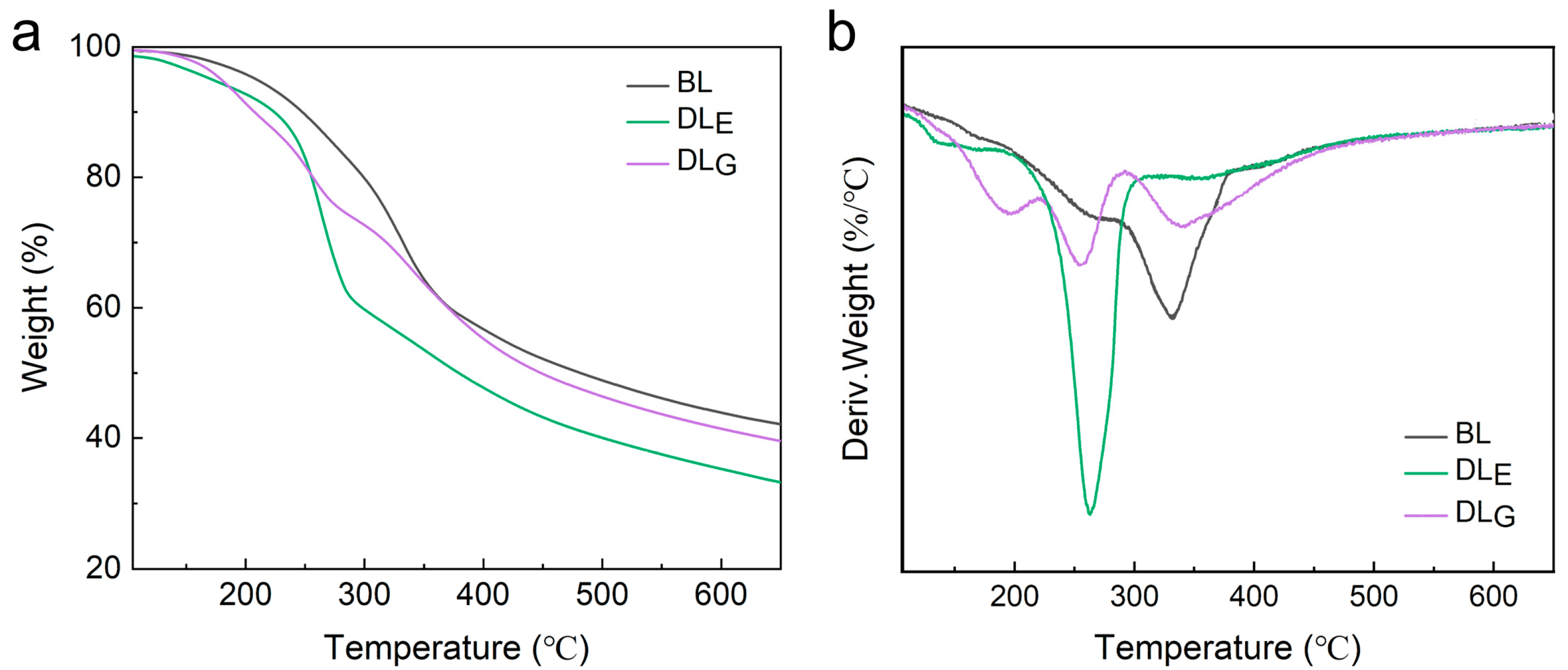
2.3. Thermal Stability of Lignin Samples
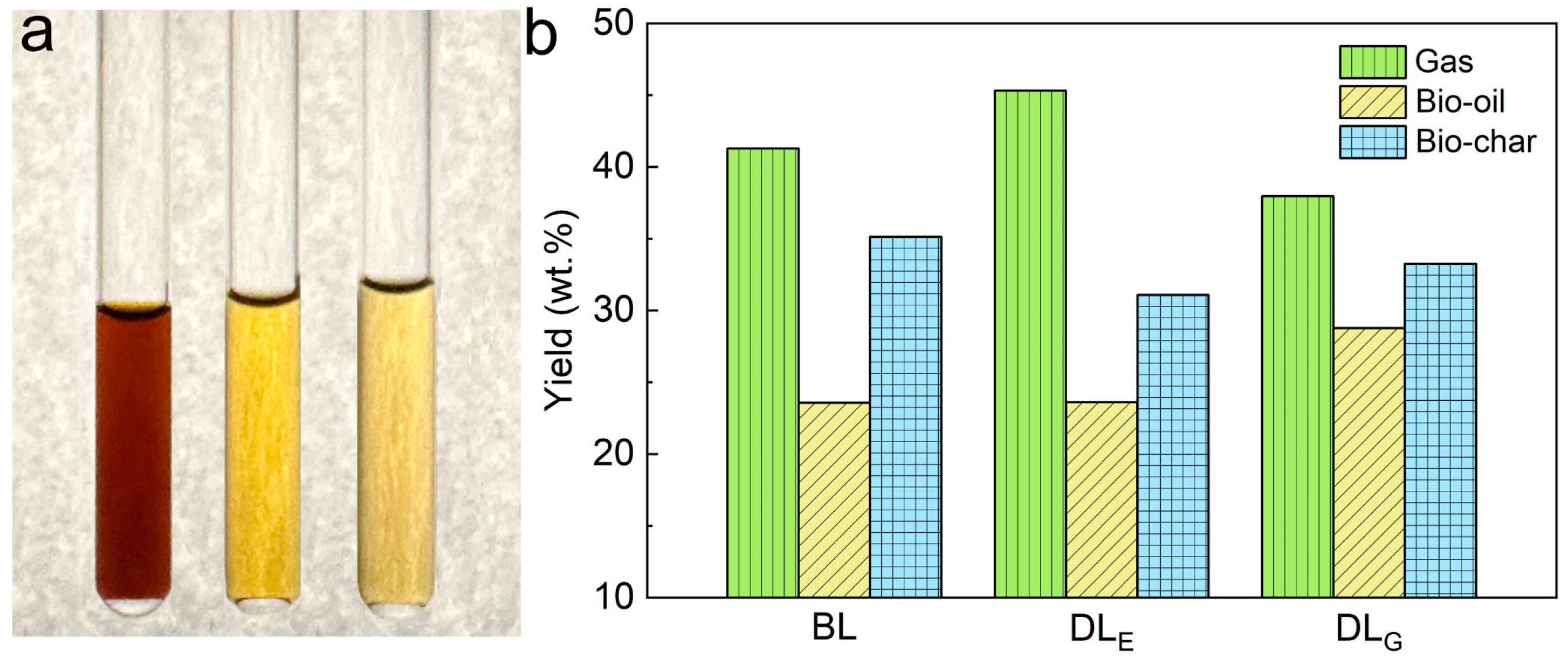
2.4. Pyrolytic Products from Biorefinery Lignin
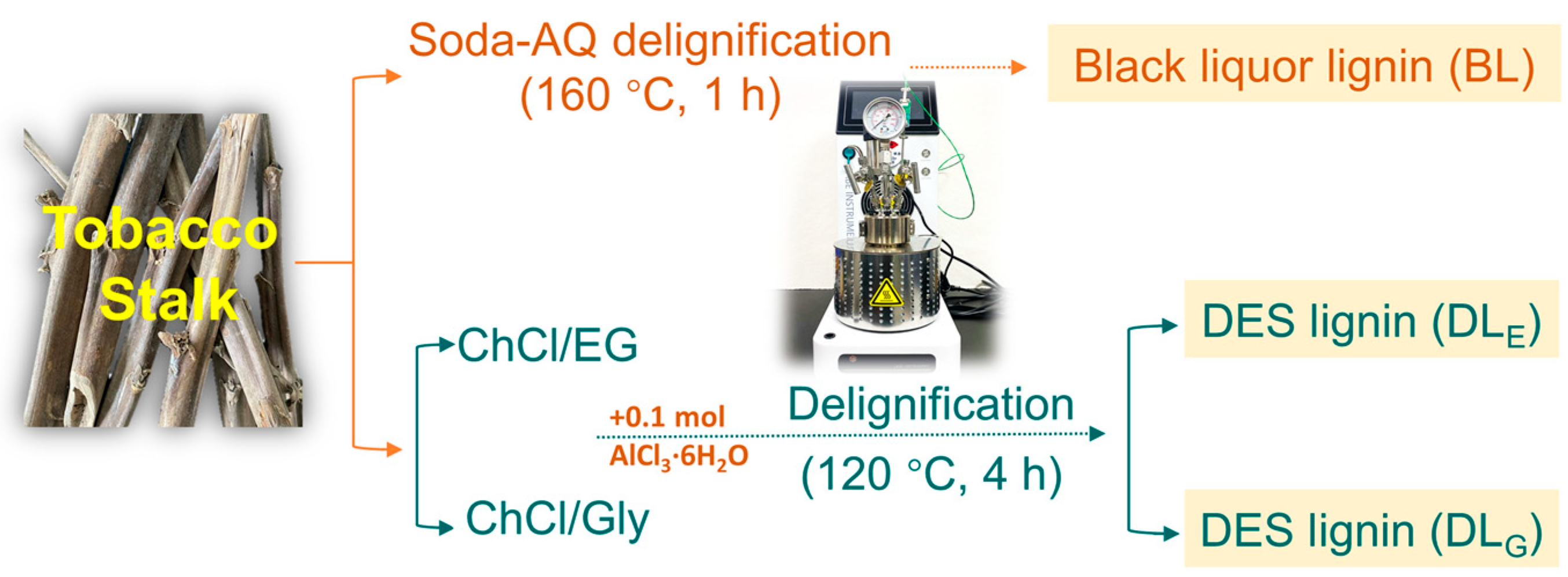
3. Materials and Method
3.1. Materials
3.2. Deep Eutectic Solvents (DESs) Preparation
3.3. Lignin Isolation by Alkali or DES Extraction
3.4. Structural Characterization
3.5. Fixed-Bed Pyrolysis of Lignin Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fu, J.; An, N.; Zhang, Y.; Chen, X.; Wang, L. Rapid and effective molten oxalic acid dihydrate pretreatment to enhance enzymatic saccharification for biohydrogen production by efficient coextraction of lignin and hemicellulose in wheat straw. Chem. Eng. J. 2023, 475, 146422. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick Jr, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, S.-F.; Lam, S.S.; Sonne, C.; Yuan, T.-Q.; Song, G.-Y.; Sun, R.-C. A review on production of lignin-based flocculants: Sustainable feedstock and low carbon footprint applications. Renew. Sustain. Energy Rev. 2020, 134, 110384. [Google Scholar] [CrossRef]
- Alonso, D.M.; Hakim, S.H.; Zhou, S.; Won, W.; Hosseinaei, O.; Tao, J.; Garcia-Negron, V.; Motagamwala, A.H.; Mellmer, M.A.; Huang, K. Increasing the revenue from lignocellulosic biomass: Maximizing feedstock utilization. Sci. Adv. 2017, 3, e1603301. [Google Scholar] [CrossRef]
- Cui, H.; Jiang, W.; Wang, C.; Ji, X.; Liu, Y.; Yang, G.; Chen, J.; Lyu, G.; Ni, Y. Lignin nanofiller-reinforced composites hydrogels with long-lasting adhesiveness, toughness, excellent self-healing, conducting, ultraviolet-blocking and antibacterial properties. Compos. Part B 2021, 225, 109316. [Google Scholar] [CrossRef]
- Wang, H.M.; Yuan, T.Q.; Song, G.Y.; Sun, R.C. Advanced and versatile lignin-derived biodegradable composite film materials toward a sustainable world. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Hu, Z.; Zhu, A.-L.; Shen, X.; Cao, X.; Wen, J.-L.; Yuan, T.-Q. Harnessing Renewable Lignocellulosic Potential for Sustainable Wastewater Purification. Research 2024, 7, 0347. [Google Scholar] [CrossRef]
- Shen, X.; Wen, J.-L.; Huang, C.; Ragauskas, A.J.; Zhang, C. Genetic engineering, pretreatment, thermochemical, and biochemconversion for lignocellulose valorization. Front. Bioeng. Biotechnol. 2023, 11, 1265271. [Google Scholar] [CrossRef]
- FAOSTAT. 2021. Available online: https://www.who.int/campaigns/world-no-tobacco-day/2023/top-50-tobacco-growing-countries (accessed on 25 May 2023).
- Hu, N.; Liu, X.; Wei, S.; Yao, J.; Wang, W.; Liu, B.; Tang, T.; Jiang, J.; Wang, L. Current status and future prospects of pretreatment for tobacco stalk lignocellulose. Front. Bioeng. Biotechnol. 2024, 12, 1465419. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Zargar, S.; Wu, J.; Oguzlu, H.; Baldelli, A.; Yu, Z.; Saddler, J.; Sun, R.; Tu, Q.; et al. Rapid, high-yield production of lignin-containing cellulose nanocrystals using recyclable oxalic acid dihydrate. Ind. Crops Prod. 2021, 173, 114148. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Wan, C. High-solid lignocellulose processing enabled by natural deep eutectic solvent for lignin extraction and industrially relevant production of renewable chemicals. ACS Sustain. Chem. Eng. 2018, 6, 12205–12216. [Google Scholar] [CrossRef]
- Ma, C.Y.; Peng, X.P.; Sun, S.; Wen, J.L.; Yuan, T.Q. Short-time deep eutectic solvents pretreatment enhanced production of fermentable sugars and tailored lignin nanoparticles from abaca. Int. J. Biol. Macromol. 2021, 192, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, S.; Liu, Y. Structural changes of poplar lignin during the ternary deep eutectic solvent (DES) treatment and synergetic alkali-DES treatment. Ind. Crops Prod. 2024, 208, 117782. [Google Scholar] [CrossRef]
- Li, T.; Yin, Y.; Wu, S.; Du, X. Effect of deep eutectic solvents-regulated lignin structure on subsequent pyrolysis products selectivity. Bioresour. Technol. 2022, 343, 126120. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Jiang, J.; Zhang, Y.; Bi, S.; Wang, H.-M. Revealing structural and functional specificity of lignin from tobacco stalk during deep eutectic solvents deconstruction aiming to targeted valorization. Ind. Crops Prod. 2022, 180, 114696. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, C.; Zhan, Y.; Han, S.; Wang, J.; Meng, X.; Yoo, C.G.; Fang, G.; Ragauskas, A.J. Effective biomass fractionation and lignin stabilization using a diol DES system. Chem. Eng. J. 2022, 443, 136395. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, M.; Liu, X.; Qiu, S.; Zeng, W.; Jiang, Z.; Liu, R.; Xiao, Z.; Li, C.; Zhang, Y. Structural characterization of lignin fractionated by acidic deep eutectic solvents and fabrication of lignin nanoparticles from Camellia Oleifera shell. Ind. Crops Prod. 2024, 210, 118018. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; Jiaqiang, E.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wang, C.-W.; Ong, H.C.; Show, P.L.; Hsieh, T.-H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Hu, J.; Wu, S.; Jiang, X.; Xiao, R. Structure-reactivity relationship in fast pyrolysis of lignin into monomeric phenolic compounds. Energy Fuels 2018, 32, 1843–1850. [Google Scholar] [CrossRef]
- Wang, H.M.; Sun, Y.C.; Wang, B.; Sun, D.; Shi, Q.; Zheng, L.; Wang, S.F.; Liu, S.-J.; Xia, R.R.; Sun, R.C. Insights into the structural changes and potentials of lignin from bagasse during the integrated delignification process. ACS Sustain. Chem. Eng. 2019, 7, 13886–13897. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, J.W. Effect of molecular size of lignin on the formation of aromatic hydrocarbon during zeolite catalyzed pyrolysis. Fuel 2019, 240, 92–100. [Google Scholar] [CrossRef]
- Xu, X.; Chen, R.; Pan, R.; Zhang, D. Pyrolysis kinetics, thermodynamics, and volatiles of representative pine wood with thermogravimetry-Fourier transform infrared analysis. Energy Fuels 2020, 34, 1859–1869. [Google Scholar] [CrossRef]
- Park, S.; Jae, J.; Farooq, A.; Kwon, E.E.; Park, E.D.; Ha, J.-M.; Jung, S.-C.; Park, Y.-K. Continuous pyrolysis of organosolv lignin and application of biochar on gasification of high density polyethylene. Appl. Energy 2019, 255, 113801. [Google Scholar] [CrossRef]
- Yang, H.; Gong, M.; Chen, W.; Fang, Y.; Chen, Y.; Wang, X.; Chen, H. Lignin pyrolysis under NH3 atmosphere for 4-vinylphenol product: An experimental and theoretical study. Fuel 2021, 297, 120776. [Google Scholar] [CrossRef]
- Yuan, J.M.; Li, H.; Xiao, L.P.; Wang, T.P.; Ren, W.F.; Lu, Q.; Sun, R.C. Valorization of lignin into phenolic compounds via fast pyrolysis: Impact of lignin structure. Fuel 2022, 319, 123758. [Google Scholar] [CrossRef]
- Li, T.; Ma, H.; Wu, S.; Yin, Y. Effect of highly selective oxypropylation of phenolic hydroxyl groups on subsequent lignin pyrolysis: Toward the lignin valorization. Energy Convers. Manage. 2020, 207, 112551. [Google Scholar] [CrossRef]
- Li, T.; Yin, Y.; Wu, S.; Ma, H.; Zhang, F. Effect of pre-acetylation of hydroxyl functional groups by choline chloride/acetic anhydride on subsequent lignin pyrolysis. Bioresour. Technol. 2020, 317, 124034. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Heo, S.; Choi, J.W. Effects of phenolic hydroxyl functionality on lignin pyrolysis over zeolite catalyst. Fuel 2018, 232, 81–89. [Google Scholar] [CrossRef]
- Wang, Z.K.; Hong, S.; Wen, J.L.; Ma, C.Y.; Tang, L.; Jiang, H.; Chen, J.J.; Li, S.; Shen, X.J.; Yuan, T.Q. Lewis acid-facilitated deep eutectic solvent (DES) pretreatment for producing high-purity and antioxidative lignin. ACS Sustain. Chem. Eng. 2020, 8, 1050–1057. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Sun, R.C. Structural elucidation of whole lignin from Eucalyptus based on preswelling and enzymatic hydrolysis. Green Chem. 2015, 17, 1589–1596. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, A.T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Svinterikos, E.; Zuburtikudis, I.; Al-Marzouqi, M. Electrospun lignin-derived carbon micro-and nanofibers: A review on precursors, properties, and applications. ACS Sustain. Chem. Eng. 2020, 8, 13868–13893. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Sun, D.; Wang, B.; Wang, H.M.; Li, M.F.; Shi, Q.; Zheng, L.; Wang, S.F.; Liu, S.J.; Sun, R.C. Structural elucidation of tobacco stalk lignin isolated by different integrated processes. Ind. Crops Prod. 2019, 140, 111631. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, X.; Xu, Y.; Tang, T.; Wei, S.; Liu, B.; Wang, D.; Wang, H.-M.; Jiang, J. Macromolecular structural characteristics and functional potential of tobacco stalk lignin from the phosphotungstic acid-assisted delignification process. Biomass Bioenergy 2023, 170, 106706. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Liu, M.; Wen, J.; Yuan, T.-Q. Uncovering the structure of lignin from moso bamboo with different tissues and growing ages for efficient ambient-pressure lignin depolymerization. ACS Sustain. Chem. Eng. 2023, 11, 13778–13786. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, L.; Shen, F.; Hu, J.; Huang, M.; He, J.; Zhang, Y.; Deng, S.; Li, Q.; Tian, D. Insights into lignocellulosic waste fractionation for lignin nanospheres fabrication using acidic/alkaline deep eutectic solvents. Chemosphere 2022, 286, 131798. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, C.; Yao, Y.; Li, J.; He, S.; Zhou, Y.; Li, T.; Pan, X.; Yao, Y.; Hu, L. A strong, biodegradable and recyclable lignocellulosic bioplastic. Nat. Sustain. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Si, H.; Hu, S.; Chen, L.; Liao, Y.; Wang, L.; Zhang, Y.; Jiang, J. Comparative investigating the structural characteristics of tobacco stalk lignin during the DES and alkaline deconstruction toward sustainable materials. Front. Bioeng. Biotech. 2022, 10, 994760. [Google Scholar]
- Jiang, J.; An, N.; Fu, J.; Wan, C.; Zhang, K.; Zhang, Y.; Chen, X.; Wang, L. Ethylene glycol inhibited oxalic acid non-derivatization pretreatment for enhanced wheat straw rapid saccharification and with high efficiency. Ind. Crops Prod. 2024, 216, 118751. [Google Scholar] [CrossRef]
- Taverna, M.E.; Bressan, L.B.; Busatto, C.A.; Lescano, M.R.; Estenoz, D.A. Preparation of Lignin/Poly (Lactic Acid) Composite Microspheres as Potential Carriers for Biopesticides Delivery. J. Polym. Environ. 2024, 32, 1811–1820. [Google Scholar] [CrossRef]
- Zhang, R.; Du, Q.; Wang, L.; Zheng, Z.; Guo, L.; Zhang, X.; Yang, X.; Yu, H. Unlocking the response of lignin structure for improved carbon fiber production and mechanical strength. Green Chem. 2019, 21, 4981–4987. [Google Scholar] [CrossRef]
- Chen, H.; Wang, A.; Yan, C.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Liu, Y.; Nie, G.; Nie, S.; et al. Study on the solubility of industrial lignin in choline chloride-based deep eutectic solvents. Sustainability 2023, 15, 7118. [Google Scholar] [CrossRef]
- Guo, G.; Liu, X.; Li, R.; Li, Q.; Yu, H.-B.; Li, M.-J. Characterization of tobacco stalk lignin using nuclear magnetic resonance spectrometry and its pyrolysis behavior at different temperatures. J. Anal. Appl. Pyrolysis 2019, 142, 104665. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, T.; Wang, S.; Liu, S.; Shi, X.; Zheng, L.; Sun, R. Upgrading Traditional Pulp Mill into Biorefinery Platform: Wheat Straw as a Feedstock. ACS Sustain. Chem. Eng. 2018, 6, 15284–15291. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
| Samples | S/G | β-O-4 | β-β |
|---|---|---|---|
| DEL a | 0.4 | 55.8 b | 10.2 |
| BL | 0.8 | 46.1 | 6.6 |
| DLE | 1.0 | ND c | 2.0 |
| DLG | 1.0 | ND | 4.6 |
| Samples | BL | DLE | DLG |
|---|---|---|---|
| Tmax (°C) | 330.1 | 262.2 | 255.3 |
| Residual char at 650 °C (wt.%) | 42.1 | 33.2 | 39.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Z.; Li, Y.; Wang, W.; Liu, X.; Shu, H.; Jiang, J. Comparison of the Effects of NaOH and Deep Eutectic Solvent Catalyzed Tobacco Stock Lignin Isolation: Chemical Structure and Thermal Characteristics. Catalysts 2024, 14, 744. https://doi.org/10.3390/catal14110744
Liu Z, Wang Z, Li Y, Wang W, Liu X, Shu H, Jiang J. Comparison of the Effects of NaOH and Deep Eutectic Solvent Catalyzed Tobacco Stock Lignin Isolation: Chemical Structure and Thermal Characteristics. Catalysts. 2024; 14(11):744. https://doi.org/10.3390/catal14110744
Chicago/Turabian StyleLiu, Zhichang, Ziwei Wang, Yichen Li, Wanxia Wang, Xiongbin Liu, Hao Shu, and Jungang Jiang. 2024. "Comparison of the Effects of NaOH and Deep Eutectic Solvent Catalyzed Tobacco Stock Lignin Isolation: Chemical Structure and Thermal Characteristics" Catalysts 14, no. 11: 744. https://doi.org/10.3390/catal14110744
APA StyleLiu, Z., Wang, Z., Li, Y., Wang, W., Liu, X., Shu, H., & Jiang, J. (2024). Comparison of the Effects of NaOH and Deep Eutectic Solvent Catalyzed Tobacco Stock Lignin Isolation: Chemical Structure and Thermal Characteristics. Catalysts, 14(11), 744. https://doi.org/10.3390/catal14110744







