Sulfite Pretreatment Enhances Tobacco Stalk Deconstruction for Cellulose Saccharification and Lignin Pyrolysis
Abstract
1. Introduction
2. Results and Discussion
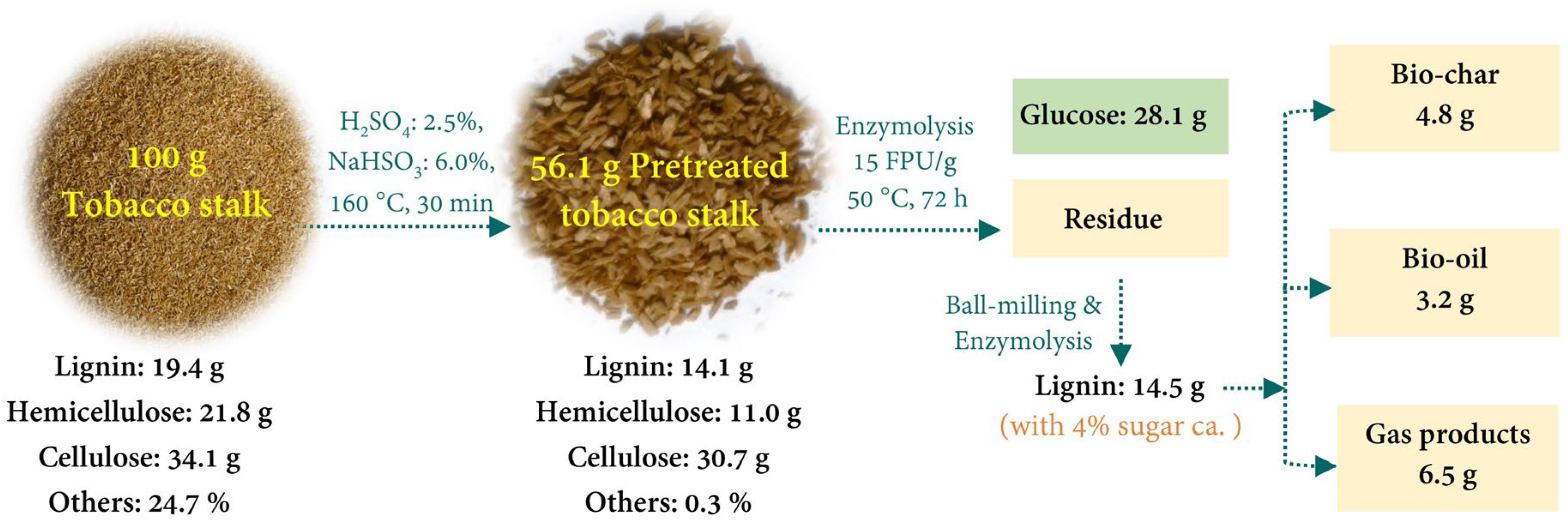
2.1. Composition Analysis
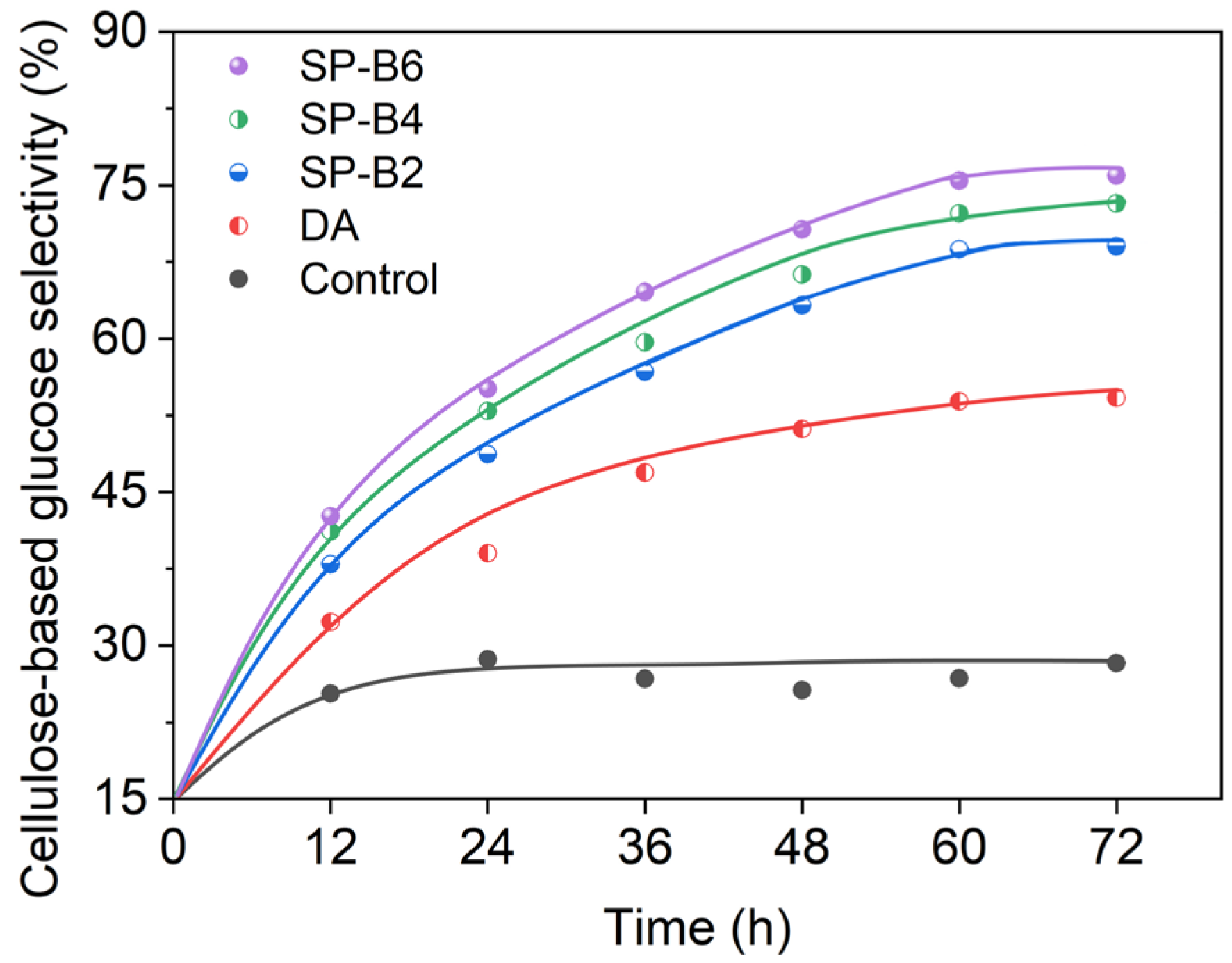
2.2. Enzymolysis of the Pretreated Tobacco Stalk
2.3. Lignin Structure Analysis
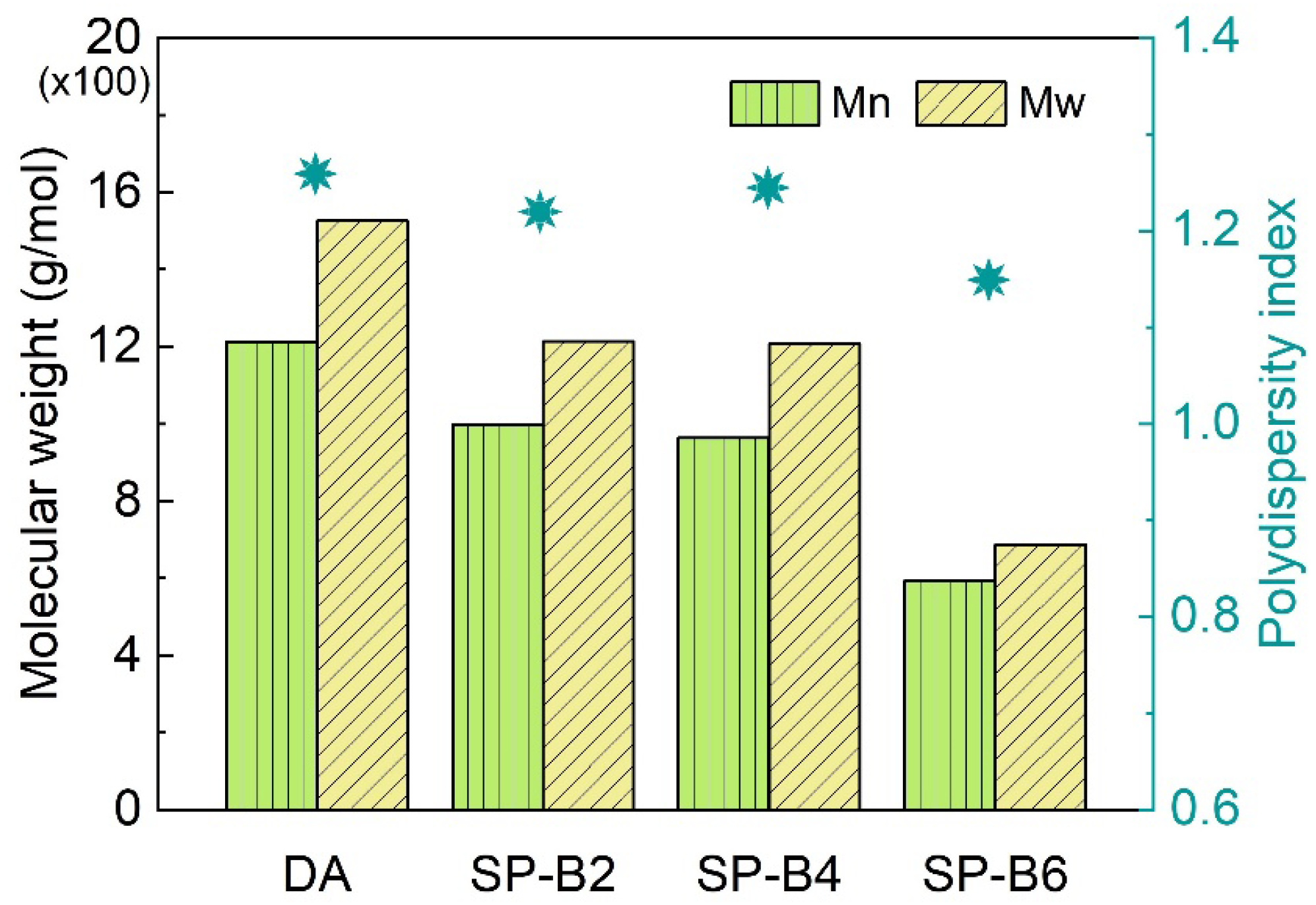
2.3.1. Molecular Analysis
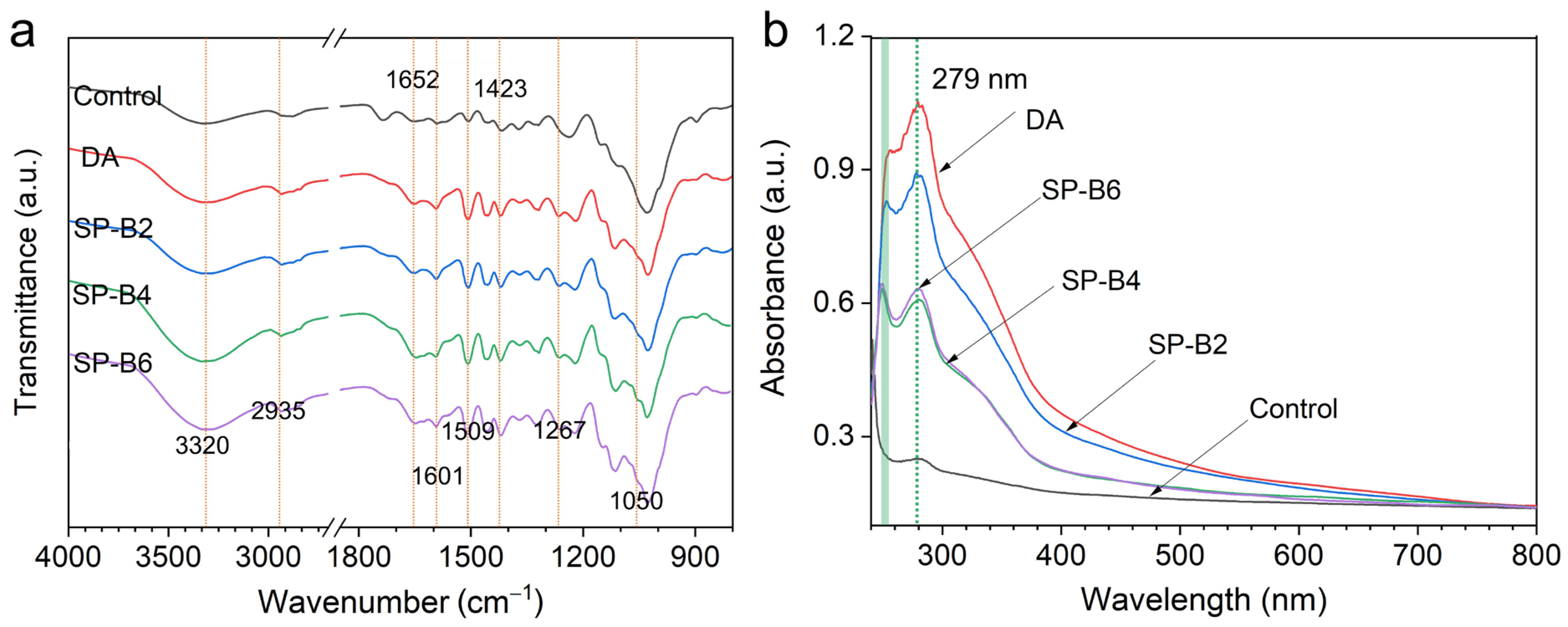
2.3.2. FT-IR and UV-Vis Spectra Analysis
2.4. Lignin Thermostability Analysis
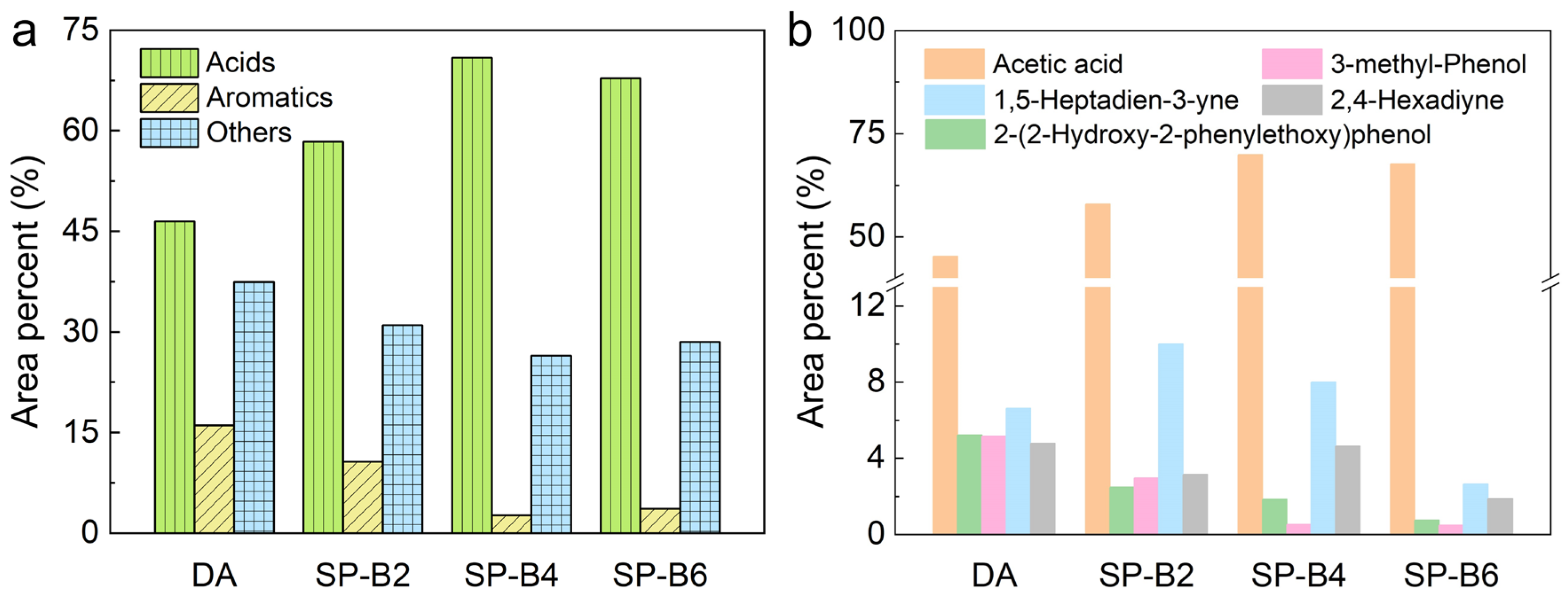
2.5. Lignin Pyrolysis Properties and Pyrolysis Oil Analysis
3. Materials and Methods
3.1. Materials
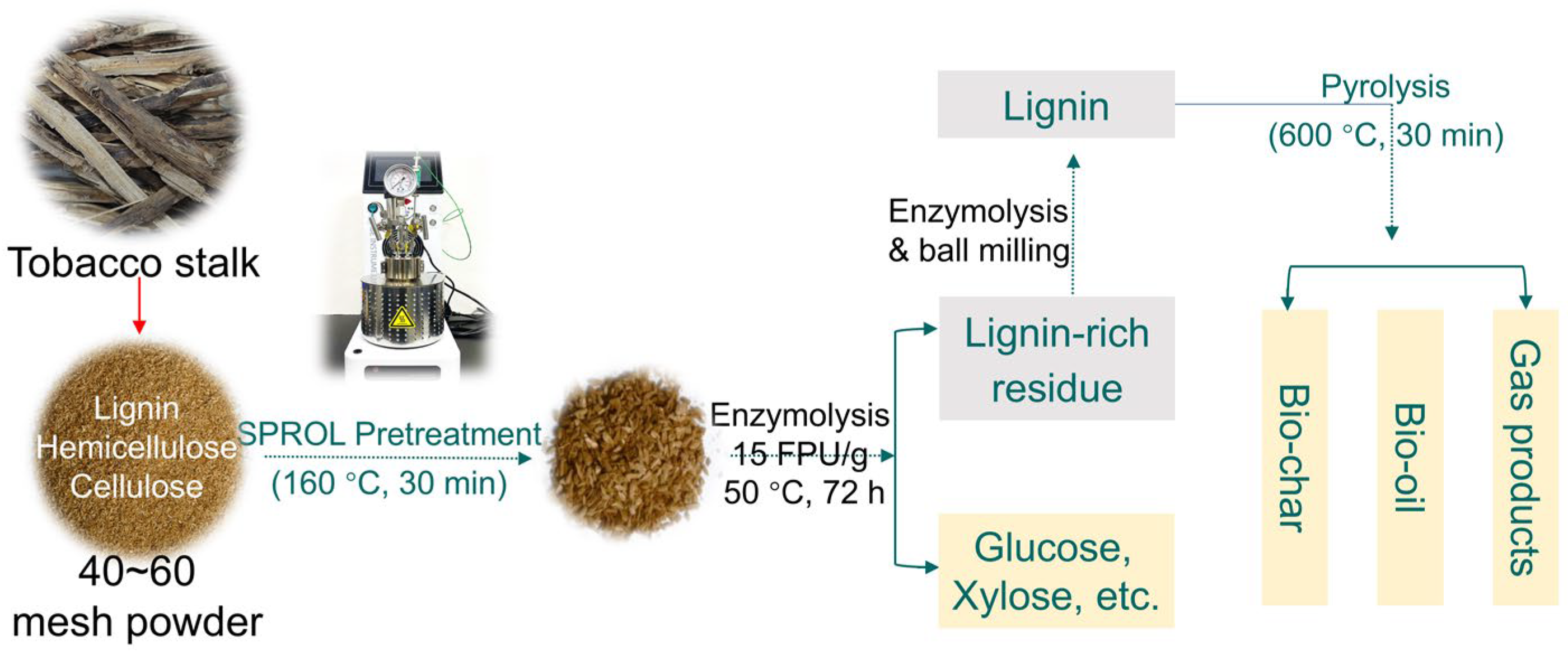
3.2. Tobacco Stalk Pretreatment
3.3. Enzymatic Hydrolysis
3.4. Lignin Isolation
3.5. Lab-Scale Fixed-Bed Lignin Pyrolysis
3.6. Structural and Composition Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sudalai, S.; Prabakaran, S.; Varalakksmi, V.; Sai Kireeti, I.; Upasana, B.; Yuvasri, A.; Arumugam, A. A review on oilcake biomass waste into biofuels: Current conversion techniques, sustainable applications, and challenges: Waste to energy approach (WtE). Energy Convers. Manag. 2024, 314, 118724. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, T.; Wang, J.; Li, B.; Zhai, Y.; Li, R. A review on the overall process of lignin to phenolic compounds for chemicals and fuels: From separation and extraction of lignin to transformation. J. Anal. Appl. Pyrolysis 2024, 181, 106663. [Google Scholar] [CrossRef]
- Hu, N.; Liu, X.; Wei, S.; Yao, J.; Wang, W.; Liu, B.; Tang, T.; Jiang, J.; Wang, L. Current status and future prospects of pretreatment for tobacco stalk lignocellulose. Front. Bioeng. Biotechnol. 2024, 12, 1465419. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Kumar, R.; Chakrabortty, S.; Saha, S.; Chattaraj, S.; Roy, S.; Banerjee, A.; Tripathy, S.K.; Kumar Ghosh, A.; Jeon, B.-H. Technological advancements in the pretreatment of lignocellulosic biomass for effective valorization: A review of challenges and prospects. J. Ind. Eng. Chem. 2024, 137, 29–60. [Google Scholar] [CrossRef]
- Jiang, J.; Fu, J.; An, N.; Zhang, Y.; Chen, X.; Wang, L. Rapid and effective molten oxalic acid dihydrate pretreatment to enhance enzymatic saccharification for biohydrogen production by efficient coextraction of lignin and hemicellulose in wheat straw. Chem. Eng. J. 2023, 475, 146422. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C.; et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, T.; Wang, S.; Liu, S.; Shi, X.; Zheng, L.; Sun, R. Upgrading Traditional Pulp Mill into Biorefinery Platform: Wheat Straw as a Feedstock. ACS Sustain. Chem. Eng. 2018, 6, 15284–15291. [Google Scholar] [CrossRef]
- Tang, W.; Ling, Z.; Huang, C.; He, Y.-C. Utilizing cetyltrimethylammonium bromide assisted alkali pretreatment to improve monosaccharide production of wheat straw by intensifying lignin extraction. Fuel 2023, 352, 129002. [Google Scholar] [CrossRef]
- Jiang, J.; An, N.; Fu, J.; Wan, C.; Zhang, K.; Zhang, Y.; Chen, X.; Wang, L. Ethylene glycol inhibited oxalic acid non-derivatization pretreatment for enhanced wheat straw rapid saccharification and with high efficiency. Ind. Crops Prod. 2024, 216, 118751. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, X.; Xu, Y.; Tang, T.; Wei, S.; Liu, B.; Wang, D.; Wang, H.-M.; Jiang, J. Macromolecular structural characteristics and functional potential of tobacco stalk lignin from the phosphotungstic acid-assisted delignification process. Biomass Bioenergy 2023, 170, 106706. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Li, X.; Wang, Z.; Mu, L.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Mannitol assisted oxalic acid pretreatment of poplar for the deconstruction and separation of hemicellulose. Ind. Crops Prod. 2023, 200, 116811. [Google Scholar] [CrossRef]
- Nair, L.G.; Agrawal, K.; Verma, P. Organosolv pretreatment: An in-depth purview of mechanics of the system. Bioresour. Bioprocess. 2023, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zhu, J.Y.; Lan, T.Q.; Lai, H.; Qiu, X. pH-Induced Lignin Surface Modification to Reduce Nonspecific Cellulase Binding and Enhance Enzymatic Saccharification of Lignocelluloses. ChemSusChem 2013, 6, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Qin, Y.; Zhuang, X.; Xiao, H.; Liu, C.; Fu, X.; Lin, Q.; Lan, T. Recycling enzymatic hydrolysis lignin residues saved cellulase in enzymatic hydrolysis of lignocellulose: An insight from cellulase adsorption mechanism. Ind. Crops Prod. 2024, 208, 117884. [Google Scholar] [CrossRef]
- Ding, K.; Liu, D.; Chen, X.; Zhang, H.; Shi, S.; Guo, X.; Zhou, L.; Han, L.; Xiao, W. Scalable lignocellulosic biorefineries: Technoeconomic review for efficient fermentable sugars production. Renew. Sustain. Energy Rev. 2024, 202, 114692. [Google Scholar] [CrossRef]
- Wu, W.; Li, P.; Huang, L.; Wei, Y.; Li, J.; Zhang, L.; Jin, Y. The Role of Lignin Structure on Cellulase Adsorption and Enzymatic Hydrolysis. Biomass 2023, 3, 96–107. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Meng, X.; Ragauskas, A.J. Toward a Fundamental Understanding of the Role of Lignin in the Biorefinery Process. Front. Energy Res. 2022, 9, 804086. [Google Scholar] [CrossRef]
- Tan, Z.; Li, Y.; Chen, F.; Liu, J.; Zhong, J.; Guo, L.; Zhang, R.; Chen, R. Challenges and Perspectives of the Conversion of Lignin Waste to High-Value Chemicals by Pyrolysis. Processes 2024, 12, 589. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review on the Comparison Between Slow Pyrolysis and Fast Pyrolysis on the Quality of Lignocellulosic and Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Pyrolytic lignin: A promising biorefinery feedstock for the production of fuels and valuable chemicals. Green Chem. 2022, 24, 4680–4702. [Google Scholar] [CrossRef]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Shen, K.; Xia, L.; Jiao, K.; Pan, F.; Xiang, B.; Zhou, W.; Shou, Y.; Gao, X.; Hu, S.; Fang, H.; et al. Characterization techniques for tobacco and its derivatives: A systematic review. Front. Chem. 2024, 12, 1402502. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, X.; Wang, Z.; Liu, B. Catalytic hydrothermal liquefaction of lignin for production of aromatic hydrocarbon over metal supported mesoporous catalyst. Bioresour. Technol. 2021, 323, 124569. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Rueda, C.; Calvo, P.A.; Moncalián, G.; Ruiz, G.; Coz, A. Biorefinery options to valorize the spent liquor from sulfite pulping. J. Chem. Technol. Biotechnol. 2015, 90, 2218–2226. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J.; Wang, G.S.; Gleisner, R. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; Li, K.; Zhu, J.Y.; Fougere, J.D.; Clarke, K. Effects of SPORL and Dilute Acid Pretreatment on Substrate Morphology, Cell Physical and Chemical Wall Structures, and Subsequent Enzymatic Hydrolysis of Lodgepole Pine. Appl. Biochem. Biotechnol. 2012, 168, 1556–1567. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhu, J.Y.; Zalesny, R.S.; Chen, K.F. Ethanol production from poplar wood through enzymatic saccharification and fermentation by dilute acid and SPORL pretreatments. Fuel 2012, 95, 606–614. [Google Scholar] [CrossRef]
- Wang, G.S.; Yu, M.H.; Zhu, J. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of corn stalks. Adv. Mater. Res. 2011, 236, 173–177. [Google Scholar] [CrossRef]
- Sun, D.; Sun, S.-C.; Wang, B.; Sun, S.-F.; Shi, Q.; Zheng, L.; Wang, S.-F.; Liu, S.-J.; Li, M.-F.; Cao, X.-F.; et al. Effect of various pretreatments on improving cellulose enzymatic digestibility of tobacco stalk and the structural features of co-produced hemicelluloses. Bioresour. Technol. 2020, 297, 122471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, Y.; Zargar, S.; Wu, J.; Oguzlu, H.; Baldelli, A.; Yu, Z.; Saddler, J.; Sun, R.; Tu, Q.; et al. Rapid, high-yield production of lignin-containing cellulose nanocrystals using recyclable oxalic acid dihydrate. Ind. Crops Prod. 2021, 173, 114148. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, T.; Zhu, M.-F. A comparison of the surface properties of lignin and sulfonated lignins by FTIR spectroscopy and wicking technique. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 57–60. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Chen, X.; Zhang, Y.; Yang, H.; Li, Q.; Jiang, J. Isolation and characteristics of nanocellulose from hardwood pulp via phytic acid pretreatment. Ind. Crops Prod. 2022, 182, 114921. [Google Scholar] [CrossRef]
- Lepilova, O.; Spigno, G.; Aleeva, S.; Koksharov, S. Study of the ability of reducing saccharides to chemically transform lignin. Eurasian Chem. Technol. J. 2017, 19, 31–40. [Google Scholar] [CrossRef]
- Hu, J.; Wu, S.; Jiang, X.; Xiao, R. Structure-reactivity relationship in fast pyrolysis of lignin into monomeric phenolic compounds. Energy Fuels 2018, 32, 1843–1850. [Google Scholar] [CrossRef]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R.-C. Formic acid based organosolv pulping of bamboo (Phyllostachys acuta): Comparative characterization of the dissolved lignins with milled wood lignin. Chem. Eng. J. 2012, 179, 80–89. [Google Scholar] [CrossRef]
- Lisperguer, J.; Perez, P.; Urizar, S. Structure and thermal properties of lignins: Characterization by infrared spectroscopy and differential scanning calorimetry. J. Chil. Chem. Soc. 2009, 54, 460–463. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Sun, S.L.; Sun, R.C. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1671. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Singh-Morgan, A.; Puente-Urbina, A.; van Bokhoven, J.A. Technology Overview of Fast Pyrolysis of Lignin: Current State and Potential for Scale-Up. ChemSusChem 2022, 15, e202200343. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Smith, R.L., Jr.; Xu, L. Production of Biofuels and Chemicals with Pyrolysis; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Sun, H.; Feng, D.; Sun, S.; Zhao, Y.; Zhang, L.; Chang, G.; Guo, Q.; Wu, J.; Qin, Y. Thermal evolution of gas-liquid-solid products and migration regulation of C/H/O elements during biomass pyrolysis. J. Anal. Appl. Pyrolysis 2021, 156, 105128. [Google Scholar] [CrossRef]
- Chu, S.; Subrahmanyam, A.V.; Huber, G.W. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound. Green Chem. 2013, 15, 125–136. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, D.; Fu, P.; Wang, A.; Sun, Y.; Li, Z.; Fan, Q. Insight into the fast pyrolysis of lignin: Unraveling the role of volatile evolving and char structural evolution. Chem. Eng. J. 2022, 437, 135316. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Sun, L. The pyrolysis of lignin: Pathway and interaction studies. Fuel 2021, 290, 120078. [Google Scholar] [CrossRef]
- Jia, X.; Che, Y.; Li, J.; Yan, B.; Cheng, Z.; Chen, G.; Zhao, J. From cellulose to tar: Analysis of tar formation pathway with distinguishing the primary and secondary reactions. Bioresour. Technol. 2023, 390, 129846. [Google Scholar] [CrossRef]
- Cheng, J.; Geng, Z.-C.; Zheng, J.-L.; Qiu, L.; Jiao, F. Characterization of the pyroligneous acids generated from the pyrolysis of four types mulberry branches. Ind. Crops Prod. 2022, 183, 114949. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Zhang, N.; Liu, Z.; Bao, L.; Xia, Y. Wood fiber biomass pyrolysis solution as a potential tool for plant disease management: A review. Heliyon 2024, 10, e25509. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]

| Sample Label | a Yield (%) | b Cellulose (%) | b Hemicellulose (%) | b Lignin (%) |
|---|---|---|---|---|
| Raw material | - | 34.1 | 21.8 | 19.4 |
| DA | 52.2 (67.4) | 51.4 (78.5) | 16.4 (39.2) | 31.5 (84.5) |
| SP-B2 | 53.2 (67.7) | 52.7 (82.1) | 14.8 (36.2) | 31.0 (84.8) |
| SP-B4 | 55.2 (70.4) | 53.4 (86.3) | 17.8 (45.0) | 28.3 (80.2) |
| SP-B6 | 56.1 (71.0) | 54.8 (90.0) | 19.7 (50.6) | 25.1 (72.4) |
| Sample ID | Bio-Char (%) | Bio-Oil (%) | Gas Products (%) |
|---|---|---|---|
| DA | 34.26 | 23.60 | 42.14 |
| SP-B2 | 33.08 | 22.07 | 44.85 |
| SP-B4 | 33.00 | 22.38 | 44.62 |
| SP-B6 | 33.32 | 21.95 | 44.73 |
| Sample Label | Chemical Dosage on Tobacco Stalk [wt.%] |
|---|---|
| DA | H2SO4: 2.5 |
| SP-B2 | H2SO4: 2.5, NaHSO3: 2.0 |
| SP-B4 | H2SO4: 2.5, NaHSO3: 4.0 |
| SP-B6 | H2SO4: 2.5, NaHSO3: 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wu, R.; Zhang, S.; Liu, Z.; Wei, P.; Hu, X.; Huang, L.; Shen, X.; Jiang, J.; Wang, L. Sulfite Pretreatment Enhances Tobacco Stalk Deconstruction for Cellulose Saccharification and Lignin Pyrolysis. Catalysts 2024, 14, 889. https://doi.org/10.3390/catal14120889
Li D, Wu R, Zhang S, Liu Z, Wei P, Hu X, Huang L, Shen X, Jiang J, Wang L. Sulfite Pretreatment Enhances Tobacco Stalk Deconstruction for Cellulose Saccharification and Lignin Pyrolysis. Catalysts. 2024; 14(12):889. https://doi.org/10.3390/catal14120889
Chicago/Turabian StyleLi, Dong, Rui Wu, Sheng Zhang, Zhichang Liu, Pei Wei, Xin Hu, Lianfeng Huang, Xiaojun Shen, Jungang Jiang, and Lei Wang. 2024. "Sulfite Pretreatment Enhances Tobacco Stalk Deconstruction for Cellulose Saccharification and Lignin Pyrolysis" Catalysts 14, no. 12: 889. https://doi.org/10.3390/catal14120889
APA StyleLi, D., Wu, R., Zhang, S., Liu, Z., Wei, P., Hu, X., Huang, L., Shen, X., Jiang, J., & Wang, L. (2024). Sulfite Pretreatment Enhances Tobacco Stalk Deconstruction for Cellulose Saccharification and Lignin Pyrolysis. Catalysts, 14(12), 889. https://doi.org/10.3390/catal14120889







