Abstract
Photocatalysis can reduce CO2 to available energy by means of light energy, which is considered to be an effective solution to alleviate energy and environmental problems. In this paper, an MPS@Bi2WO6 composite photocatalyst was prepared by in situ hydrothermal method. BWO grew on the surface of MPS, which increased the CO2 absorption capacity of the photocatalyst and improved the microstructure. Under the synergistic effect of the two aspects, BWS achieves the enhancement of light energy absorption capacity and can effectively excite electron-hole pairs. The transition electrons with high reduction ability migrate to the surface and contact with high concentrations of CO2, achieving efficient CO2 reduction under visible light. Among the photocatalysts in this paper, BWS-1 (BWO: MPS = 1:1) has efficient CO2 gas phase reduction ability under visible light, and the CO yield reaches 29.51 μmol/g. The MPS@BWO photocatalyst is a low-cost and efficient CO2 photoreduction catalyst with broad application prospects.
1. Introduction
The extensive use of fossil fuels has created a series of environmental problems. Researchers have paid more and more attention to the problem of excessive CO2 emissions [1,2,3]. The conversion and utilization of CO2 is undoubtedly an effective way to solve environmental problems. Among various conversion methods, photocatalytic reduction of CO2 has become a research hotspot [4,5,6]. Photocatalysis is also known as artificial photosynthesis. Photocatalytic reduction of CO2 is based on renewable light energy, which converts CO2 into high-value products and can be used as chemical raw materials or for direct use [7,8]. This can effectively alleviate the greenhouse effect and energy problems [9,10]. Photocatalysis is the use of the characteristics of semiconductors [11]. After absorbing light energy, electron-hole pairs can be excited, and further redox reactions can occur to achieve the reduction of CO2. In the existing photocatalysts, metal oxides and sulfides occupy the main position [12,13,14,15,16,17,18].
Bismuth is considered to be a green element and is widely used in the field of photocatalysis. Yan et al. [19] successfully replaced In in In2O3 with Bi, which improved the light absorption ability and further promoted the photocatalytic reduction of CO2. Bimetallic oxides have attracted more researchers’ attention due to their strong light response ability [20,21,22,23]. Bismuth tungstate (BWO), an Aurivillius oxide semiconductor, has attracted much attention due to its non-toxicity and high quantum yield under visible light [24].
However, at the same time, higher electron recombination efficiency and lower photocatalytic area also limit the photocatalytic ability of BWO. By constructing a composite photocatalyst, the recombination efficiency of electron-hole pairs can be effectively reduced, and the electron lifetime can be increased [25,26]. Zhang et al. [27] successfully grew flower-like BWO on MoS2 nanosheets to form heterostructures. This allowed photogenerated electrons and holes to be effectively separated and transferred, enhancing the photocatalytic activity under visible light. Ullah et al. [28] used the double tungsten bond to form an interface between BWO and CoPc and guided electrons from BWO to CoPc. This changed the electron transfer mode, optimized the charge separation, and improved the photocatalytic activity. Yang et al. [29] constructed a three-dimensional spatial structure of BI2WO6/ZnIn2S4, providing abundant active sites and effectively promoting charge transport and migration. On the other hand, by finding a suitable carrier, the growth mode can be improved, thereby increasing the photocatalytic area. Zheng et al. [30] successfully synthesized a ZnIn2S4/g-C3N4 heterojunction photocatalyst with a 2D/1D structure. The ZnIn2S4 with lamellar structure was coated on the surface of nanotubular g-C3N4. The crystal growth mode was optimized, the specific surface area was increased, and the photocatalytic activity was enhanced.
The synthesis of SiO2 is simple and low cost., and its raw materials are widely available and can be extracted from industrial and agricultural wastes such as fly ash and rice husk ash [31,32]. As a carrier, SiO2 also has many applications in the field of catalysis [33]. Crystal growth occurs on the surface of SiO2, which can optimize the microstructure. Zhang et al. [34] prepared a micro/nano hierarchical structure. SiO2 was used as the substrate, and TiO2 was loaded on the surface to form a wrapped structure. The photocatalytic activity was improved under ultraviolet light irradiation, which effectively improved the explanation rate of methyl orange. Bera et al. [35] prepared a SiO2-based photocatalyst by loading Au/Pt on the surface and regulating its particle size. In this approach, the surface plasmon resonance (LSPR) effect of the photocatalyst is enhanced, which increases the activity of CO2 reduction under LED lights. These results show that the microstructure of SiO2 can be used as a substrate, and its high specific surface area can provide an effective growth surface. In addition, the crystals can be uniformly dispersed when growing on the surface. More importantly, SiO2 has been widely considered as a CO2 adsorbent. Mesoporous silica (MPS), with a large specific surface area, is widely used in the field of CO2 adsorption [36,37]. Using MPS as the substrate of photocatalysts can greatly increase the adsorption capacity of CO2, which can increase the concentration of CO2 on the surface of the photocatalyst. The increase in reactant concentration is undoubtedly helpful for photocatalytic reactions.
Based on the above discussion, the substrate adsorption enhancement strategy was successfully applied in this paper. The MPS@BWO composite photocatalyst was successfully prepared. MPS has a high specific surface area and can be used as a substrate to load BWO. While MPS and BWO form a composite structure, the microstructure is optimized, and the specific surface area is increased. Through a series of characterizations, the crystal structure of the photocatalyst, the absorption and conversion of light energy, the photoelectrochemical properties, and the energy band structure were analyzed, and the photocatalytic mechanism was explained. Thanks to the construction of the composite structure and the improvement of the surface microstructure, the photocatalytic activity of the photocatalyst was enhanced, the energy absorption and conversion process were improved, and the photogenerated electron reduction was also improved. Due to the addition of MPS, the CO2 adsorption capacity was significantly improved. The photocatalyst was tested for gas phase reduction of CO2, and it had a high CO2 reduction ability under visible light.
2. Results and Discussion
2.1. Structure and Morphology
Figure 1a shows the X-ray diffractometer (XRD) patterns of the photocatalysts. According to the results, the obvious strong characteristic peaks of BWO located at 28.3°, 32.8°, 47.0°, 55.7°, and 58.4°, corresponding to (131), (200), (202), (133), and (262) crystal planes, respectively [38]. As for MPS, there is an obvious broad diffraction peak near 23.3°, which corresponds to amorphous silicon. It is consistent with previous literature reports [39]. Figure 1b shows the Fourier transform infrared (FTIR) spectra of the photocatalysts. The strong absorption peaks of pure BWO are centered at 589 cm−1, 730 cm−1, and 1382 cm−1, corresponding to the bridging stretching modes of Bi-O, W-O, and W-O-W groups, respectively [40]. As for MPS, the absorption peak at 464 cm−1,related to the bending vibration of SiO2, is detected and the absorption peak at 1090 cm−1 is attributed to the SiO2 stretching vibration [41]. In the composite photocatalyst with the increase of MPS content, the absorption peak at 1090 cm−1 shows a significant increase trend. This also proves the gradual increase of Si-O in the composite photocatalyst. XRD and FTIR results prove that the crystal structure of the composite photocatalyst is complete, and the composite amount is the same as expected.
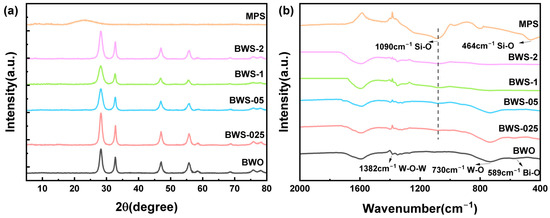
Figure 1.
(a) XRD spectrum of photocatalysts, (b) FTIR spectrum of photocatalysts.
Figure 2 shows the SEM images and TEM images of photocatalysts. According to the results of Figure 2a, BWO shows a flower ball accumulated by a lamellar structure, with a diameter of about 800 nm and a uniform size [42]. According to the results of Figure 2g, the lattice spacing of 0.272 nm can be seen under the transmission electron microscopy, which corresponds to the (200) crystal plane of BWO [43]. At the same time, a single-layer structure can be observed, and its single-layer thickness is about 1.54 nm. Figure 2f shows the microstructure of MPS. Its overall performance is a large-sized block structure, and its surface presents a small particle structure. At the same time, it can be seen that there are abundant pore structures [44]. This is more conducive to the adsorption of gas on its surface, which is conducive to increasing the concentration of surrounding gas. It can be seen from Figure 2i that MPS is composed of tiny sheets with a diameter of about 15–20 nm [45]. As for the composite photocatalyst, MPS placed in the hydrothermal environment in advance has become the basis for the growth of BWO. The original flower spherical structure also changed. When growing on the surface of MPS, it showed incomplete growth of small particles, and with the change in the MPS amount, the package form was also different. Figure 2b shows the microstructure of BWS-025. Due to the small amount of MPS, the lamellar structure grows on the surface and is completely wrapped. After that, it continues to grow on the surface to form the original flower ball shape, which gathers a large amount of accumulation on the surface. In Figure 2c, the surface accumulation is also obvious, and the obvious bulk structure can be observed. A more uniform wrapping structure can be observed in Figure 2d. By magnifying its surface, small particles and a little of the small sheet can be observed. The surface roughness of particles is different from that of MPS. The uniform dispersion structure on the surface is more conducive to the photocatalyst receiving light and surface reaction. The contact between the two structures can be observed in Figure 2h, which also proves that the structure did not change during the synthesis process. With the increase of MPS, different accumulations are observed in Figure 2e. The block structure is not completely wrapped, but the surface also grows to form a flower ball structure.
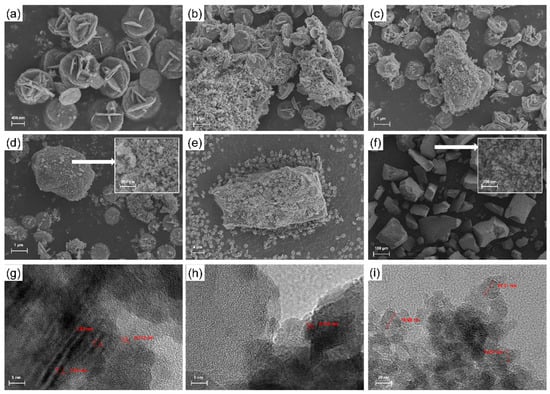
Figure 2.
SEM images of (a) BWO, (b) BWS-025, (c) BWS-05, (d) BWS-1, (e) BWS-2, (f) MPS and TEM images of (g) BWO, (h) BWS-1 and (i) MPS.
Figure 3a shows the N2 adsorption–desorption isotherm of the photocatalysts. It can be seen from the isotherm that due to the capillary absorption phenomenon [46], the adsorption capacity increased significantly after the adsorption pressure increased, and a hysteresis loop appeared during the desorption process. All the isotherms are type IV isotherms [47], which indicates that there are mesoporous structures with a pore size range of 2–50 nm inside the photocatalyst [48]. In the photocatalytic process, electrons move to the surface to undergo a redox reaction, which means that the specific surface area plays an important role in the photocatalytic process. Due to the unique structure of MPS, it has a high specific surface area of 1136.42 m2/g. Using MPS as the substrate of the photocatalysts is beneficial to the increase of the specific surface area of the photocatalysts. It can be seen that with the increase in the amount of MPS added, the specific surface area of the composite photocatalysts increased significantly. Figure 3b shows the CO2 absorption capacity of the photocatalysts. It can be seen from the diagram that with the increase of MPS content, the CO2 adsorption capacity of the photocatalysts increases significantly. Based on the above, it can also be seen that in the hydrothermal synthesis process, MPS as a base synthetic photocatalyst, with its high specific surface area of the micro-framework, does not collapse. At the same time, the CO2 adsorption sites contained in the interior are not destroyed, making the composite photocatalysts have a larger specific surface area and stronger CO2 adsorption capacity. This makes more reaction surfaces contact with reactants and then redox reactions occur in the photocatalytic process. In addition, the concentration of reactants around the photocatalyst surface is increased to promote the photocatalytic reaction. Some relevant data are shown in Table 1. In the table, SBET, VCO2, VTotal and DAverage represent bet surface area, CO2 adsorption quantity, total pore volume and average pore size respectively. The pore structures of photocatalysts are shown in Figure S1.
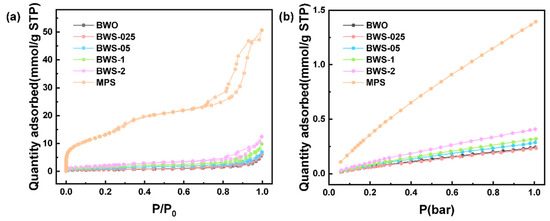
Figure 3.
(a) N2 adsorption–desorption isotherm at 77 K of photocatalysts, (b) CO2 adsorption isotherm at 278 K of photocatalysts.

Table 1.
Texture properties of photocatalysts.
Figure 4 shows the X-ray photoelectron spectroscopy (XPS) of photocatalysts. It shows the chemical states of the elements in photocatalysts. In the BWO photocatalyst, the binding energy peak of W 4f can be estimated to be 35.4 eV and 37.5 eV, corresponding to W 4f7/2 and W 4f5/2, respectively [49]. The Bi 4f spectrum shows two peaks at 159.2 eV and 164.5 eV. This is due to the presence of Bi3+ in BWO [50]. The peaks of the O element can be fitted to two sites at 530.2 eV and 531.8 eV. These two peaks are due to the two states of O 1s7/2 and O 1s5/2 in BWO. This is affected by the two chemical bonds of W-O and Bi-O in BWO [51]. In the MPS, the binding energy peak of O 1s can be estimated to be 533.1 eV, corresponding to the Si-O-Si chemical bonds in MPS [52]. The Si 2p spectrum shows a peak at 103.8 eV. This indicates that the Si element exists in the form of Si4+ in MPS, and there is no other chemical state [41]. As for BWS composite photocatalysts, it can be seen from Figure 4c that with the addition of MPS, a new characteristic peak of O 1s appears near 533 eV, and the peak intensity gradually increases with the increase of MPS content, which also indicates that the MPS input was the same as expected. By observing the change of the binding energy of the composite photocatalyst BWS-1 compared with the single BWO and MPS, the binding energy of O 1s near 530 eV increases, while the binding energy of O 1s near 533 eV decreases. At the same time, the binding energy of Si 2p also decreases. Due to the electron shielding effect, this may be because the electrons on the surface of BWO move to the surface of MPS after the formation of the composite structure, which makes the change of element-binding energy. MPS has a high specific surface area and a porous structure, and the adsorption capacity of CO2 is also strong. Electron transfer to its surface will undoubtedly play a significant role in promoting the photocatalytic reaction. The XPS survey spectra of photocatalysts are shown in Figure S2.
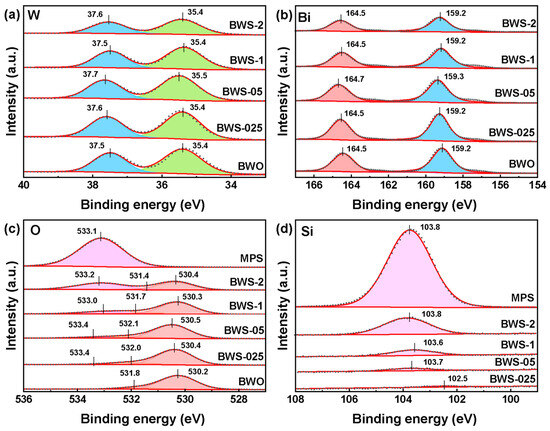
Figure 4.
XPS survey spectra (a) W 4f, (b) Bi 4f, (c) O 1s and (d) Si 2p.
2.2. Photocatalytic Activity
Photocatalytic reduction of CO2 activity was measured under visible light. The photocatalytic reduction product was detected as CO. The detection equipment is a gas chromatograph equipped with FID and TCD. And the CO yield varied with time as shown in Figure 5a. The reaction process is described as follows [53]:
BWS + hv → h+ + e−
H2O + 2h+ → 1/2Q2 + 2H+
CO2 + 2H+ + 2e− → CO + H2O
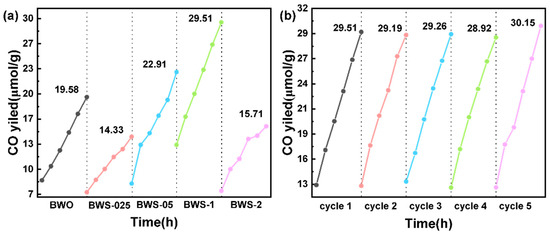
Figure 5.
CO2 reduction performance of photocatalysts: (a) Production rate, (b) Cycling stability.
According to the results of photocatalytic reduction of CO2, the CO yields of the photocatalyst after 6 h of reaction under light were 19.58 μmol/g, 14.33 μmol/g, 22.91 μmol/g, 29.51 μmol/g, and 15.71 μmol/g. The photocatalytic efficiency of BWS-1 reached 4.92 μmol/g/h in 6 h. The results of GC analysis and CO production calculation are shown in Figure S3. The variation of yield with time is shown in Table 2 [54,55,56,57,58,59,60]. Among them, BWS-1 has the highest photocatalytic yield, which may be related to its uniform coating structure and high CO2 adsorption capacity. BWS-025 contains the smallest amount of MPS, but after being stacked and wrapped, the advantages of the porous structure are limited, making the photocatalytic performance lower than that of BWO. Although there is lamellar growth in BWS-05, the overall photocatalytic performance is also slightly improved. The excessive MPS in BWS-2 significantly reduced the proportion of surface photocatalytic active substances, resulting in a decrease in the overall photocatalytic capacity.

Table 2.
Photocatalytic production of CO (μmol/g).
Figure 5b shows the photocatalytic performance of BWS-1 in five-cycle tests. It can be seen that the performance of the photocatalyst is not affected during the cycle. After each test, the gas in the reactor is replaced three times, and the water is replaced to ensure that the reaction system is consistent. This may be because the reactants are gaseous, and the reaction is carried out at room temperature without high-temperature treatment. This does not damage the photocatalyst and makes the performance stable in the cycle test. Cyclic stability is the premise of promoting the use of photocatalysts.
2.3. Photocatalysis Mechanism
The light absorption and electron excitation ability of the photocatalyst reflects the reception and conversion efficiency of the photocatalyst [61]. Figure 6a shows the UV-visible absorption spectrum of the photocatalyst. According to the results, the light absorption ability of composite photocatalysts BWS-025, BWS-05, and BWS-1 has been significantly improved compared with pure BWO. This may be due to the emergence of finer particles, and under the load of MPS, light can be reflected inside the photocatalyst to achieve multiple absorption. Compared with the absorption of the lamellar surface, the unique microstructure effectively promotes the light absorption ability of the photocatalyst. As for BWS-2, the light absorption capacity decreased significantly compared with BWO. This may be because a large number of MPS surfaces are exposed and occupy the position of receiving light. According to the results, the light absorption capacity of MPS is very low. This leads to a significant decrease in the overall light absorption capacity. Such differences have led to a significant gap in the energy-receiving stage of the photocatalysts. The improvement of the absorption capacity means the high utilization of sunlight, which is conducive to the photocatalytic reaction [62].
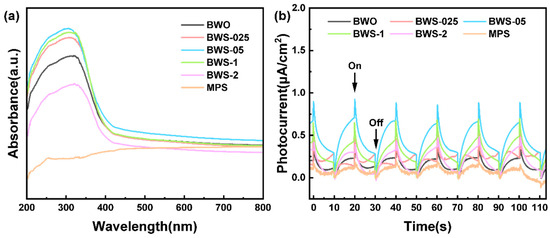
Figure 6.
(a) UV–vis absorption spectra, (b) photocurrent response of photocatalysts.
After receiving light energy, electron-hole pairs are excited inside the photocatalysts. Figure 6b shows the photocurrent response ability of the photocatalysts. According to the results, although BWS-05 has the largest current in the conventional state, BWS-05 and BWS-1 have similar photocurrent response capabilities at the moment of receiving light. The photocurrent response ability of BWS-2 is also higher than that of BWO, but the gap is not very large. This may be because BWO is dispersed on the surface, but there are a large number of MPS with poor light response. This makes the photocurrent response of the composite photocatalyst not significantly improved. In particular, the photocurrent response ability of BWS-025 is very low, even lower than that of BWO. This may be due to the local excessive accumulation growth, which is not conducive to the generation of photogenerated electrons. Compared with BWO with uniform lamellar structure, BWS-025 shows worse response ability. In the case of the same amount of received light energy, the strong light response ability means that more electron-hole pairs can be generated [63]. This will allow more electrons to have the opportunity to migrate to the surface of the photocatalysts and undergo redox reactions [64].
Figure 7 shows the electrochemical impedance spectroscopy and photoluminescence spectra of photocatalysts. Figure 7a shows the AC impedance of the photocatalyst in the state of light on and off [65]. The results show that the photocatalyst exhibits a lower AC impedance value under illumination. MPS shows a high AC impedance value, and the BWS-1 composite photocatalyst formed by growing BWO on its surface shows a very low AC impedance value, even lower than that of BWO. This may be due to the tight wrapping structure of the surface, which does not cut off the electron transfer channel, and the highly dispersed microstructure promotes the transfer of electrons on the surface. Compared with the lamellar structure, BWO shows stronger electron transfer ability. The Nyquist plots of other photocatalysts are shown in Figure S4.
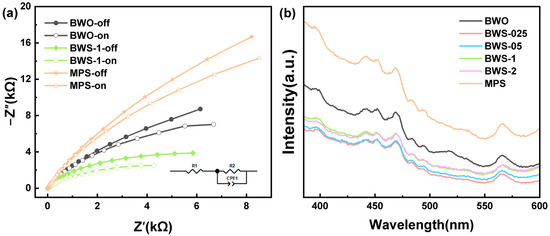
Figure 7.
(a) the Nyquist plot of electrochemical impedance spectroscopy of photocatalysts, (b) photoluminescence spectra of photocatalysts.
Figure 7b shows the photoluminescence spectra of photocatalysts. Photocatalytic efficiency is closely related to the lifetime of photogenerated electrons [66]. The results show that the electron-hole pair recombination efficiency of BWS composite photocatalyst is lower than that of BWO and MPS. This may be because the tiny particles formed on the surface can make the excited electron-hole pairs have longer lifetimes than the lamellar structure. In the process of electron migration, there are fewer electron-hole pairs in the BWS composite photocatalyst, which means that less energy is lost internally. The BWS-1 composite photocatalyst has a low AC impedance during the electron transfer process, which effectively promotes the movement of electrons to the surface of the photocatalyst [67]. At the same time, in the process of electron transfer, there is a small amount of electron-hole pair recombination, which reduces the ability to consume inside the photocatalyst and effectively promotes the photocatalytic reaction [68].
To further analyze electron migration, the band structure of the catalyst was examined. Figure 8a,b shows the Mott–Schottky diagrams of BWO and BWS-1. It can be seen that both the BWO photocatalyst and the BWS composite photocatalyst are n-type semiconductors. The flat band potential position can be obtained by linear fitting of the Mott–Schottky data. The calculated flat band potentials of BWO and BWS-1 are −0.55 V and −0.65 V vs. Ag/AgCl [69]. The measured potentials can be converted to the NHE scale via the Nernst equation: E (NHE, pH = 7) = E (Ag/AgCl) − Eθ + 0.059 × pH, where Eθ is the standard potential of Ag/AgCl at 298 K (0.197 V) [70]. For undoped n-type semiconductors, Efb is about 0.3 eV below the conduction band edge [71]. Therefore, the conduction band edges (ECB) of BWO and BWS-1 are −0.63 eV and −0.73 eV (vs. NHE). BWS-1 has a higher conduction band position, which means that the electrons received and excited by the light have a stronger reduction ability, which is more conducive to the photocatalytic reaction [72]. Mott–Schottky diagrams of other photocatalysts are shown in Figure S5.
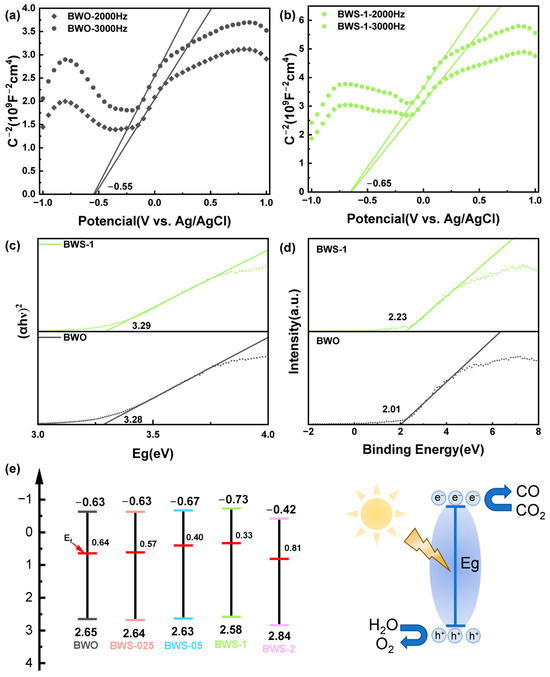
Figure 8.
Mott–Schottky plots of (a) BWO, (b) BWS-1, (c) the band gap, (d) VB-XPS spectra, and (e) energy band structure of photocatalysts.
Based on the UV-vis DRS results, the band position of the photocatalyst can be estimated by using the Kubelka–Munk transformation method [73]. Figure 8c shows the band gap of photocatalysts. According to the calculation method of the semiconductor band gap, plot Eg-(ahν)2 calculates the band gap of the photocatalysts [74]. The calculated band gaps of BWO and BWS-1 are 3.28 eV and 3.29 eV, respectively. It can be seen that the addition of MPS has no obvious effect on the band gap width of the photocatalyst. In the light absorption stage, the absorption of light is obviously different, but there is no obvious shift in the spectral absorption edge. The valence band positions (EVB) of BWO and BWS-1 can be calculated to be 2.65 V and 2.56 V (EVB = ECB + Eg) [75]. The band gap of other photocatalysts is shown in Figure S6.
To explore the Fermi level position of the photocatalysts, the distance between the Fermi level and the valence band position was obtained by the VB-XPS test. As shown in Figure 8d, the distance between the Fermi level and the valence band position of BWO and BWS-1 is 2.01 V and 2.23 V, respectively. According to the results, the Fermi levels of BWO and BWS can be calculated to be 0.64 V and 0.33 V, respectively (EF = EVB − EVB-XPS) [76]. The VB-XPS spectra of other photocatalysts are shown in Figure S7. In summary, the complete band structure of the photocatalysts was calculated, and the results are shown in Figure 8e. BWS-1 has a higher conduction band position when the band gap does not change significantly. This makes the electrons excited after receiving the same energy of light with a higher reduction ability and more effectively promotes the photocatalytic reaction. By calculating the Fermi level, we can see that the Fermi level of BWS-1 is closer to the conduction band position, which makes it easier to generate electron transitions inside the photocatalyst, and more electron holes have separation conditions to further promote photocatalysis.
3. Materials and Methods
3.1. Materials
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, ≥99.0%), sodium tungstate dihydrate (Na2WO4·2H2O, ≥99.5%), ethylene glycol (C2H6O2, ≥99.0%), PEG 400 (HO(CH2CH2O)nH), tetraethyl orthosilicate (C8H20O4Si, SiO2 ≥ 28.4%), CTAB (C19H42BrN, ≥99.0%), and aqueous ammonia (NH3·H2O, 25–28%) were obtained from Sinopharm Chemical Reagent. The water used in the experiment was all self-made ultrapure water from the laboratory, while other chemicals were analytically pure and did not undergo secondary purification treatment before use.
3.2. Preparation of Photocatalysts
Synthesis of mesoporous silica (MPS): First, 15 mL of PEG was added to 60 mL of water at 40 °C and stirred for 10 min. Then, 5 mL of TEOS was added and stirred for another 10 min. Afterward, 1 g of CTAB was added and stirred until fully dissolved. Finally, 0.5 mL of ammonia was added dropwise, and the mixture was sealed and stirred for 12 h. The water bath temperature was maintained at 40 °C throughout the experiment. The resulting gel was washed with water and ethanol. The obtained white powder was then calcined at 550 °C for 6 h. The solid product was dried at 60 °C for 12 h to obtain MPS.
Synthesis of Bi2WO6 (BWO): First, 4 mmol of Bi(NO3)3·5H2O was added to 60 mL of ethylene glycol and stirred continuously until completely dissolved. Then, 2 mmol of Na2WO4·2H2O was dissolved in 20 mL of water, and the resulting solution was added dropwise to the Bi(NO3)3 solution while stirring for 10 min. The mixture was transferred to a polytetrafluoroethylene-lined autoclave and heated to 160 °C for 6 h. The resulting product was washed and dried at 60 °C for 12 h to obtain BWO.
Synthesis of BWO/SiO2 composite photocatalyst (BWS): The BWS composite photocatalyst was synthesized using an in situ hydrothermal method. First, 4 mmol of Bi(NO3)3·5H2O was added to 60 mL of ethylene glycol and stirred until completely dissolved. MPS powder was then dispersed into this solution. Subsequently, the previously prepared Na2WO4·2H2O solution was added and dispersed. The mixture was transferred to a polytetrafluoroethylene-lined autoclave. The remaining experimental steps followed the same procedure as the BWO synthesis. Based on the molar ratios of BWO to SiO2 in the composite photocatalyst: 1:0.25, 1:0.5, 1:1, and 1:2, the photocatalysts were labeled BWS-025, BWS-05, BWS-1, and BWS-2, respectively. The scheme of the photocatalyst synthesis is shown in Figure S8.
3.3. Characterization Methods
X-ray diffractometer (D8 ADVANCE, Bruker, Allentown, PA, USA) was used to measure the crystal phase of the photocatalysts, and the measuring range was from 5° to 80° with a step size of 0.02°. Fourier transform infrared spectroscopy (Cary 660 FTIR, Agilent, Santa Clara, CA, USA) was used to measure functional groups of photocatalysts. The measurement range was 400~2500 cm−1. Scanning electron microscopy (Ultra Plus, ZEISS, Göttingen, Germany) was used to observe the microscopic morphology of the photocatalysts. Elemental mapping was conducted using energy-dispersive X-ray spectroscopy (EDS) to describe the elemental distributions of the heterostructure photocatalyst. Transmission electron microscopy (F200S, FEI Talos, Waltham, MA, USA) was used to observe the crystal lattice structure of the photocatalysts. A physical adsorption instrument (BSD-PS1/2/4, Beishide, Beijing, China) was used to perform low-temperature nitrogen adsorption at 77 K and CO2 adsorption tests at 273 K on photocatalysts. X-ray photoelectron spectroscopy (XPS) data was obtained using a spectrometer (K-Alpha, Thermo Scientific, Waltham, MA, USA). The signal at 284.8 eV of the C (1s) region was employed to adjust the binding energies for all of the XPS spectra. The UV-visible-near-infrared spectrophotometer (Lambda 750s, Perkin-Elmer, Bridgeport Avenue, Shelton, CT, USA) was used to test the light absorption capacity of the photocatalysts. The wavelength range was 200–800 nm. A fluorescence spectrometer (FluoroMax-4, HORIBA, Tokyo, Japan) was used to test the fluorescence spectrum characteristics of the photocatalysts with an excitation wavelength of 325 nm.
The electrochemical analysis was performed on an electrochemical analyzer (Squidstat Plus, Admiral, Fort Lauderdale, FL, USA) with a three-electrode system, using a saturated Ag/AgCl electrode as the reference electrode, platinum wire as the counter electrode, and FTO as the working electrode. Preparation of working electrode: 10 mg of material was dispersed into a mixture of ethanol, water, and Nefen. Then, 25 μL of the suspension was dropped onto an FTO sheet with an area of 1 × 1 cm2 and spread flat. It was then placed at 333 K to dry for 8 h. An Xe lamp (Microsolar300, PerfectLight, Beijing, China) was used as the light source, and the electrolyte was 0.1 mol/L Na2SO4 solution. The photocurrent response of the photocatalysts, the Mott–Schottky results under dark conditions, and the electrochemical impedance spectroscopy under dark and light conditions, were tested respectively. The electrode preparation methods for the three tests were the same.
3.4. Photocatalytic Reduction of CO2
The photocatalytic reaction took place in a glass automatic online trace gas analysis system (Labsolar6A, Perfectlight, Beijing, China), and the reaction temperature was controlled by a chiller (YKKY, Beijing, China). A 300 W Xe lamp (Microsolar300, PerfectLight, China) equipped with a cutoff filter (λ ≥ 420 nm) was used as the visible light source to illuminate the reactant through a quartz window above the reactor. The 20 mg photocatalyst was dispersed in 5 mL H2O, and the dispersion was uniformly sprayed onto the FTO sheet with a diameter of 5 cm by a spray gun and dried. The reaction sheet was mounted with a triangular bracket in the reactor. The temperature of the reaction system was controlled at 15 °C by circulating water. Before the reaction, the reaction system was vacuumed with a vacuum pump, and then CO2 was filled to a system pressure of 80 kPa, repeated three times. Turning on the light is regarded as the beginning of the reaction, and the system peristaltic pump is turned on at the same time. The system automatically samples every 1 h and sends 6 µL of the product to GC (GC-2014C, Shimadzu, Kyoto, Japan) for detection, and the yield is calculated according to the GC display peak area. The product was analyzed by GC with an FID hydrogen flame detector. The analytical column type used was PN. The scheme of the photocatalytic reduction is shown in Figure S9.
As for the cycle test, the reactor was dried, and 5 mL H2O was added. The reaction system was further vacuumed and filled with CO2 to maintain the pressure at 80 kPa, and the light was turned on for the experiment.
4. Conclusions
In conclusion, BWS series composite photocatalysts were successfully prepared by using the in situ hydrothermal method. MPS as a substrate provides a higher specific surface area and stronger CO2 adsorption capacity. At the same time, BWO grows into small particles on the surface and wraps on the surface of MPS. This makes the surface CO2 concentration of the photocatalyst increase during the reaction and promotes the photocatalytic reaction. Such a special structure enhances the light absorption ability and photoelectric conversion ability. It promotes the light absorption ability of the photocatalyst surface. After absorbing light energy, the electrons are excited to the conduction band position with high reduction ability. CO2 is efficiently reduced to CO by contacting with a high concentration of CO2 on the surface. In an under 80 kPa CO2 atmosphere and 300 W Xe lamp irradiation, the yield of BWS-1 photocatalytic reduction of CO2 to CO was 29.51 μmol/g within 6 h. In summary, the BWS composite photocatalyst significantly improves photocatalytic efficiency and provides a new solution for the design of photocatalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14110745/s1.
Author Contributions
Conceptualization, P.C., T.D. and Y.L.; methodology, P.C., Y.L. and H.J.; validation, H.J. and G.C.; formal analysis, H.J., G.C. and J.Z.; writing—original draft preparation, P.C. and Y.L.; writing—review and editing, H.J., G.C. and J.Z.; visualization, P.C. and H.J.; supervision, T.D. and Y.W.; funding acquisition, T.D. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 52270177), the Natural Science Foundation of Shenyang (No. 22-315-6-13), the Liaoning Province Science and Technology Plan Joint Program (Key Research and Development Program Project) (No. 2023JH2/101800058), the Chunhui Project from the Ministry of Education of China (No. HZKY20220436), the Natural Science Foundation of Liaoning Province (No. 2023-MSBA-111), and the Fundamental Research Funds for the Central Universities (No. N2325018).
Data Availability Statement
Our data can only be obtained by contacting us by email.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lei, T.; Wang, D.; Yu, X.; Ma, S.; Zhao, W.; Cui, C.; Meng, J.; Tao, S.; Guan, D. Global Iron and Steel Plant CO2 Emissions and Carbon-Neutrality Pathways. Nature 2023, 622, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ciais, P.; Wu, M.; Padrón, R.S.; Friedlingstein, P.; Schwaab, J.; Gudmundsson, L.; Seneviratne, S.I. Increasingly Negative Tropical Water–Interannual CO2 Growth Rate Coupling. Nature 2023, 618, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.Z.-Q.; Rosentreter, J.A.; Oakes, J.M.; Schulz, K.G.; Eyre, B.D. High Carbon Dioxide Emissions from Australian Estuaries Driven by Geomorphology and Climate. Nat. Commun. 2024, 15, 3967. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Feng, S.; Liang, X.; Wang, C. Light-Driven Thermocatalytic CO2 Reduction over Surface-Passivated b-Mo2C Nanowires: Enhanced Catalytic Stability by Light. Chem. Commun. 2019, 32, 4651–4654. [Google Scholar] [CrossRef]
- Giusi, D.; Ampelli, C.; Genovese, C.; Perathoner, S.; Centi, G. A Novel Gas Flow-through Photocatalytic Reactor Based on Copper-Functionalized Nanomembranes for the Photoreduction of CO2 to C1-C2 Carboxylic Acids and C1-C3 Alcohols. Chem. Eng. J. 2021, 408, 127250. [Google Scholar] [CrossRef]
- He, Y.; Rao, H.; Song, K.; Li, J.; Yu, Y.; Lou, Y.; Li, C.; Han, Y.; Shi, Z.; Feng, S. 3D Hierarchical ZnIn2S4 Nanosheets with Rich Zn Vacancies Boosting Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2019, 29, 1905153. [Google Scholar] [CrossRef]
- Wang, J.; Sheng, R.; Xiao, J.; Lu, L.; Peng, Y.; Gu, D.; Xiao, W. Matched Redox Kinetics on Triazine-Based Carbon Nitride/Ni(OH)2 for Stoichiometric Overall Photocatalytic CO2 Conversion. Small 2024, 20, e2309707. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhang, L. Photobreeding Oxygen Vacancy Facilitates Phtocatalytic Reduction of CO2. Sep. Purif. Technol. 2024, 340, 126842. [Google Scholar] [CrossRef]
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Li, C.-Q.; Yi, S.-S.; Liu, Y.; Niu, Z.-L.; Yue, X.-Z.; Liu, Z.-Y. In-Situ Constructing S-Scheme/Schottky Junction and Oxygen Vacancy on SrTiO3 to Steer Charge Transfer for Boosted Photocatalytic H2 Evolution. Chem. Eng. J. 2021, 417, 129231. [Google Scholar] [CrossRef]
- Mohammad, A. Sulfur-Doped Graphitic Carbon Nitride: Tailored Nanostructures for Photocatalytic, Sensing, and Energy Storage Applications. Adv. Colloid Interface Sci. 2023, 322, 103048. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, H.; Yekeh, D.; Deng, B.; Chen, Z.; Duan, S.; Yang, L. Rapidly Photocatalytic Degradation of Toluene Boosted by Plasmonic Effect and Schottky Junction on Pt Nanoparticles Engineered Self-Supporting Cu2O Nanowires. Sep. Purif. Technol. 2025, 353, 128559. [Google Scholar] [CrossRef]
- Yu, X.; Huang, K.; Zhang, Y.; Jin, Y.; Chen, Y.; Chen, F.; Zhang, X. Photodynamically Functionalized CoP/MoS2 Nanocomposites with Antibacterial Activity. ACS Appl. Nano Mater. 2024, 7, 3260–3268. [Google Scholar] [CrossRef]
- Cao, X.; Tan, B.; Zhang, B.; Huang, G.; Xu, H.; Song, Z.; Yan, J. Integration of Silver Nanoparticles into Carbon-Encapsulated Tungsten Oxide Promoting Visible-Light-Driven Photocatalytic Degradation Efficiency. Appl. Surf. Sci. 2024, 678, 161112. [Google Scholar] [CrossRef]
- Zhao, W. Insights into Photocatalytic Mechanism over a Novel Cu2WS4/MoS2 S-Scheme Heterojunction. Rare Met. 2024, 43, 3118–3133. [Google Scholar] [CrossRef]
- Ge, M.; Yin, H.; Tian, W.; Zhang, H.; Li, S.; Wang, S.; Chen, Z. Electrostatically Induced Furfural-Derived Carbon Dots-CdS Hybrid for Solar Light-Driven Hydrogen Production. J. Colloid Interface Sci. 2024, 660, 147–156. [Google Scholar] [CrossRef]
- Wang, Z.; Min, S.; Li, R.; Lin, W.; Li, K.; Wang, S.; Kang, L. Constructing Cuprous Oxide-Modified Zinc Tetraphenylporphyrin Ultrathin Nanosheets Heterojunction for Enhanced Photocatalytic Carbon Dioxide Reduction to Methane. J. Colloid Interface Sci. 2024, 667, 212–222. [Google Scholar] [CrossRef]
- Kanwal, A.; Shahzadi, T.; Riaz, T.; Zaib, M.; Khan, S.; Habila, M.A.; Sillanpaa, M. Photocatalytic Degradation Studies of Organic Dyes over Novel Cu/Ni Loaded Reduced Graphene Oxide Hybrid Nanocomposite: Adsorption, Kinetics and Thermodynamic Studies. Molecules 2023, 28, 6474. [Google Scholar] [CrossRef]
- Yan, T.; Li, N.; Wang, L.; Ran, W.; Duchesne, P.N.; Wan, L.; Nguyen, N.T.; Wang, L.; Xia, M.; Ozin, G.A. Bismuth Atom Tailoring of Indium Oxide Surface Frustrated Lewis Pairs Boosts Heterogeneous CO2 Photocatalytic Hydrogenation. Nat. Commun. 2020, 11, 6095. [Google Scholar] [CrossRef]
- Bafaqeer, A.; Tahir, M.; Amin, N.A.S. Synergistic Effects of 2D/2D ZnV2O6/RGO Nanosheets Heterojunction for Stable and High Performance Photo-Induced CO2 Reduction to Solar Fuels. Chem. Eng. J. 2018, 334, 2142–2153. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Xu, H.; Hu, X.; Luo, X.; Yang, L.; Tu, X. Synthesis of Hierarchical Flower-like Bi2MoO6 Microspheres as Efficient Photocatalyst for Photoreduction of CO2 into Solar Fuels under Visible Light. CrystEngComm 2016, 18, 3472–3480. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, D.; Zhang, Q.; Zou, H.; Liu, Z.; Peng, F. Z-Scheme Bi2WO6/CuBi2O4 Heterojunction Mediated by Interfacial Electric Field for Efficient Visible-Light Photocatalytic Degradation of Tetracycline. Chem. Eng. J. 2019, 369, 292–301. [Google Scholar] [CrossRef]
- Guo, S.; Di, J.; Chen, C.; Zhu, C.; Duan, M.; Lian, C.; Ji, M.; Zhou, W.; Xu, M.; Song, P.; et al. Oxygen Vacancy Mediated Bismuth Stannate Ultra-Small Nanoparticle towards Photocatalytic CO2-to-CO Conversion. Appl. Catal. B Environ. 2020, 276, 119156. [Google Scholar] [CrossRef]
- Ribeiro, C.S.; Lansarin, M.A. Enhanced Photocatalytic Activity of Bi2WO6 with PVP Addition for CO2 Reduction into Ethanol under Visible Light. Environ. Sci. Pollut. Res. 2020, 28, 23667–23674. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Du, T.; Jia, H.; Zhou, L.; Yue, Q.; Wang, H.; Wang, Y. A Novel Bi2WO6/Si Heterostructure Photocatalyst with Fermi Level Shift in Valence Band Realizes Efficient Reduction of CO2 under Visible Light. Appl. Surf. Sci. 2022, 585, 152665. [Google Scholar] [CrossRef]
- Jiang, P.; Yu, Y.; Wang, K.; Liu, W. Efficient Electron Transfer in G-C3N4/TiO2 Heterojunction for Enhanced Photocatalytic CO2 Reduction. Catalysts 2024, 14, 335. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, P.; Hao, L.; Zhai, X.; Jiang, F.; Sun, C. Novel Z-Scheme MoS2/Bi2WO6 Heterojunction with Highly Enhanced Photocatalytic Activity under Visible Light Irradiation. J. Alloys Compd. 2021, 854, 157224. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, H.; Liu, M.; Zahid, M.; Ahmad, M.; Zeb, J.; Khan, I.; Ismail, A.; Hayat, S.; Bououdina, M.; et al. Precision Engineering of Z-Scheme Interfacial Charge Transfer in CoPc/Bi2WO6 through W-Based Bonds and Internal Electric Field for Efficient CO2 Photoreduction. Sep. Purif. Technol. 2024, 338, 126578. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, F.; Sun, N.; Wu, J.; Qi, Y.; Zhang, Y.; Song, J.; Sun, Y.; Liu, Q.; Wang, X.; et al. Constructing a 3D Bi2WO6/ZnIn2S4 Direct Z-Scheme Heterostructure for Improved Photocatalytic CO2 Reduction Performance. J. Colloid Interface Sci. 2024, 662, 695–706. [Google Scholar] [CrossRef]
- Zheng, Z. 2D/1D Nested Hollow Porous ZnIn2S4/g-C3N4 Heterojunction Based on Morphology Modulation for Photocatalytic CO2 Reduction. J. Environ. Chem. Eng. 2024, 12, 112971. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Chen, P.; Fang, X.; Du, T. Synthesis of La and Ce Modified X Zeolite from Rice Husk Ash for Carbon Dioxide Capture. J. Mater. Res. Technol. 2020, 9, 4368–4378. [Google Scholar] [CrossRef]
- Che, S.; Fang, X.; Li, S.; Chen, X.; Du, T. Modification of Potassium Chabazites Derived from Fly Ash by Dosing Extra Cations: Promoted CO2 Adsorption Capacities and Fine-Tuned Frameworks. Z. Anorg Allge Chem. 2019, 645, 1365–1371. [Google Scholar] [CrossRef]
- Sun, L.; Ouyang, X.; Li, Z.; Yuan, Z.; Gong, W.; Chen, Z.; Mei, S.; Liu, Y.; Zhou, Q. Preparation of Fe3O4@SiO2@N-TiO2 and Its Application for Photocatalytic Degradation of Methyl Orange in Na2SO4 Solution. Appl. Sci. 2024, 14, 5205. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Ding, H.; Chen, D.; Wang, X. Preparation of a Self-Cleaning TiO2-SiO2/PFDTS Coating with Superamphiphobicity and Photocatalytic Performance. Prog. Org. Coat. 2024, 197, 108767. [Google Scholar] [CrossRef]
- Bera, S.; Lee, J.E.; Rawal, S.B.; Lee, W.I. Size-Dependent Plasmonic Effects of Au and Au@SiO2 Nanoparticles in Photocatalytic CO2 Conversion Reaction of Pt/TiO2. Appl. Catal. B Environ. 2016, 199, 55–63. [Google Scholar] [CrossRef]
- Guo, A. Enhanced High Temperature Cyclic CO2 Capture on Li4SiO4 Sorbent from Two-Dimensional SiO2 Nanomeshes. Chem. Eng. J. 2024, 485, 149943. [Google Scholar] [CrossRef]
- Sui, H.; Zhang, F.; Zhang, L.; Wang, D.; Wang, Y.; Yang, Y.; Yao, J. Competitive Sorption of CO2/CH4 and CO2 Capture on Modified Silica Surfaces: A Molecular Simulation. Sci. Total Environ. 2024, 908, 168356. [Google Scholar] [CrossRef]
- Yi, H.; Yan, M.; Huang, D.; Zeng, G.; Lai, C.; Li, M.; Huo, X.; Qin, L.; Liu, S.; Liu, X.; et al. Synergistic Effect of Artificial Enzyme and 2D Nano-Structured Bi2WO6 for Eco-Friendly and Efficient Biomimetic Photocatalysis. Appl. Catal. B Environ. 2019, 250, 52–62. [Google Scholar] [CrossRef]
- Samangsri, S.; Areerob, T.; Chiarakorn, S. Core/Shell Nitrogen-Doped TiO2@SiO2 Nano-Catalyst as an Additive in Photocatalytic Paint for Gaseous Acetaldehyde Decomposition. Catalysts 2023, 13, 351. [Google Scholar] [CrossRef]
- Qian, X.; Yue, D.; Tian, Z.; Reng, M.; Zhu, Y.; Kan, M.; Zhang, T.; Zhao, Y. Carbon Quantum Dots Decorated Bi2WO6 Nanocomposite with Enhanced Photocatalytic Oxidation Activity for VOCs. Appl. Catal. B Environ. 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Sun, H.; Chen, J.; Liu, S.; Agrawal, D.K.; Zhao, Y.; Wang, D.; Mao, Z. Photocatalytic H2 Evolution of Porous Silicon Derived from Magnesiothermic Reduction of Mesoporous SiO2. Int. J. Hydrogen Energy 2019, 44, 7216–7221. [Google Scholar] [CrossRef]
- Liu, J. Construction of Bi2WO6/g-C3N4/Cu Foam as 3D Z-Scheme Photocatalyst for Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2024, 664, 160274. [Google Scholar] [CrossRef]
- Sun, C.; Lu, J.; Rao, F.; Sun, Y.; Ye, J.; Gong, S.; Hassan, Q.-U.; Zubairu, S.M.; Zhu, L.; An, Y.; et al. Highly Efficient and Selective NO Photocatalytic Abatement by Tuning Charge Separation in Multi-Walled Carbon Nanotubes and Bi2WO6 Microsphere Heterojunction. Surf. Interfaces 2024, 51, 104806. [Google Scholar] [CrossRef]
- Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Bakir, E.M. Photodegradation of Congo Red by Modified P25-Titanium Dioxide with Cobalt-Carbon Supported on SiO2 Matrix, DFT Studies of Chemical Reactivity. Catalysts 2022, 12, 248. [Google Scholar] [CrossRef]
- Pal, S.; Taurino, A.; Catalano, M.; Licciulli, A. Block Copolymer and Cellulose Templated Mesoporous TiO2-SiO2 Nanocomposite as Superior Photocatalyst. Catalysts 2022, 12, 770. [Google Scholar] [CrossRef]
- Jia, H.; Du, T.; Fang, X.; Gong, H.; Qiu, Z.; Li, Y.; Wang, Y. Synthesis of Template-Free ZSM-5 from Rice Husk Ash at Low Temperatures and Its CO2 Adsorption Performance. ACS Omega 2021, 6, 3961–3972. [Google Scholar] [CrossRef]
- Song, H.; Liu, D.; Yang, J.; Wang, L.; Xu, H.; Xiong, Y. Highly Crystalline Mesoporous Silicon Spheres for Efficient Visible Photocatalytic Hydrogen Evolution. ChemNanoMat 2017, 3, 22–26. [Google Scholar] [CrossRef]
- Jiang, M.; Gao, Y.; Wang, Z.; Ding, Z. Photocatalytic CO2 Reduction Promoted by a CuCo2O4 Cocatalyst with Homogeneous and Heterogeneous Light Harvesters. Appl. Catal. B Environ. 2016, 198, 180–188. [Google Scholar] [CrossRef]
- Xing, Z.; Hu, J.; Ma, M.; Lin, H.; An, Y.; Liu, Z.; Zhang, Y.; Li, J.; Yang, S. From One to Two: In Situ Construction of an Ultrathin 2D-2D Closely Bonded Heterojunction from a Single-Phase Monolayer Nanosheet. J. Am. Chem. Soc. 2019, 141, 19715–19727. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chen, J.-K.; Lin, K.-S.; Wei, Y.-H.; Chao, P.-Y.; Huang, C.-Y. Enhanced Visible-Light-Driven Photocatalytic Degradation by Metal Wire-Mesh Supported Ag/Flower-like Bi2WO6 Photocatalysts. J. Alloys Compd. 2020, 813, 152186. [Google Scholar] [CrossRef]
- Qiang, Z.; Liu, X.; Li, F.; Li, T.; Zhang, M.; Singh, H.; Huttula, M.; Cao, W. Iodine Doped Z-Scheme Bi2O2CO3/Bi2WO6 Photocatalysts: Facile Synthesis, Efficient Visible Light Photocatalysis, and Photocatalytic Mechanism. Chem. Eng. J. 2021, 403, 126327. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, N.Y.; Yoo, E.; Kim, J.; Joo, J.B. Enhanced Coke Resistant Ni/SiO2@SiO2 Core–Shell Nanostructured Catalysts for Dry Reforming of Methane: Effect of Metal-Support Interaction and SiO2 Shell. Chem. Eng. Sci. 2024, 299, 120480. [Google Scholar] [CrossRef]
- Li, K. A Critical Review of CO2 Photoconversion: Catalysts and Reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef]
- Ahmadi, M.; Alavi, S.M.; Larimi, A. UV–Vis Light Responsive Bi2WO6 Nanosheet/TiO2 Nanobelt Heterojunction Photo-Catalyst for CO2 Reduction. Catal. Commun. 2023, 179, 106681. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, H.; Wang, F.; Li, Y.; Zhu, L.; Xia, D. 2D/2D Bi2WO6/C3N5 S-Scheme Heterojunction for Highly Selective Production of CH4 by Photocatalytic CO2 Reduction under Visible Light. Appl. Catal. A Gen. 2024, 686, 119914. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Wang, D.; He, W.; Fang, X.; Zhao, C.; Pan, J.; Liu, D.; Liu, S.; Chen, T.; et al. Nanoengineering Construction of G-C3N4/Bi2WO6 S-Scheme Heterojunctions for Cooperative Enhanced Photocatalytic CO2 Reduction and Pollutant Degradation. Sep. Purif. Technol. 2025, 354, 128893. [Google Scholar] [CrossRef]
- Jiang, H.; An, Q.; Zang, S. Ultrathin RuO2/Bi2WO6 and PtO2-Pt/Bi2WO6 Heterojunction Nanosheets toward Efficient Photocatalytic CO2 Reduction under Visible Light Irradiation. J. Environ. Chem. Eng. 2023, 11, 110960. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Wang, Y.; Di, Y.; Liu, J.; Li, D.; Wang, Y.; Zhu, Z.; Liu, H.; Wei, M. Withered Magnolia-Derived BCDs onto 3D Flower-like Bi2WO6 for Efficient Photocatalytic TC Degradation and CO2 Reduction. J. Alloys Compd. 2023, 965, 171520. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Wei, D.; Li, Z. Ultrasonic-Assisted Fabrication of Cs2AgBiBr6/Bi2WO6 S-Scheme Heterojunction for Photocatalytic CO2 Reduction under Visible Light. Chin. J. Catal. 2022, 43, 2606–2614. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, R.; Wang, J.; Cui, C.; Wang, J.; Li, Z. A Novel Hollow-Hierarchical Structured Bi2WO6 with Enhanced Photocatalytic Activity for CO2 Photoreduction. J. Colloid Interface Sci. 2018, 523, 151–158. [Google Scholar] [CrossRef]
- Cirena, Z. Fe Doped G-C3N4 Composited ZnIn2S4 Promoting Cr(VI) Photoreduction. Chin. Chem. Lett. 2023, 34, 107726. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lu, Y.; Lou, X.W. “David” Formation of Hierarchical In 2S3 –CdIn2S4 Heterostructured Nanotubes for Efficient and Stable Visible Light CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 17305–17308. [Google Scholar] [CrossRef]
- Huang, L. Fabrication of Hierarchical Co3O4@CdIn2S4 p–n Heterojunction Photocatalysts for Improved CO2 Reduction with Visible Light. J. Mater. Chem. A 2020, 8, 7177–7183. [Google Scholar] [CrossRef]
- Song, M.; Qi, K.; Wen, Y.; Zhang, X.; Yuan, Y.; Xie, X.; Wang, Z. Rational Design of Novel Three-Dimensional Reticulated Ag2O/ZnO Z-Scheme Heterojunction on Ni Foam for Promising Practical Photocatalysis. Sci. Total Environ. 2021, 793, 148519. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Z.; Sun, M.; Liu, Y.; Liu, L.; Liu, H.; Huang, C.-P.; Qu, J.; Li, J. Formation of Bi2WO6 Bipyramids with Vacancy Pairs for Enhanced Solar-Driven Photoactivity. Adv. Funct. Mater. 2015, 25, 3726–3734. [Google Scholar] [CrossRef]
- Jin, P.; Wang, L.; Ma, X.; Lian, R.; Huang, J.; She, H.; Zhang, M.; Wang, Q. Construction of Hierarchical ZnIn2S4@PCN-224 Heterojunction for Boosting Photocatalytic Performance in Hydrogen Production and Degradation of Tetracycline Hydrochloride. Appl. Catal. B Environ. 2021, 284, 119762. [Google Scholar] [CrossRef]
- Su, B.; Huang, L.; Xiong, Z.; Yang, Y.; Hou, Y.; Ding, Z.; Wang, S. Branch-like ZnS–DETA/CdS Hierarchical Heterostructures as an Efficient Photocatalyst for Visible Light CO2 Reduction. J. Mater. Chem. A 2019, 7, 26877–26883. [Google Scholar] [CrossRef]
- Yang, R.; Zhong, S.; Zhang, L.; Liu, B. PW12/CN@Bi2WO6 Composite Photocatalyst Prepared Based on Organic-Inorganic Hybrid System for Removing Pollutants in Water. Sep. Purif. Technol. 2020, 235, 116270. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4–In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Xiong, H.; Wu, L.; Liu, Y.; Gao, T.; Li, K.; Long, Y.; Zhang, R.; Zhang, L.; Qiao, Z.; Huo, Q.; et al. Controllable Synthesis of Mesoporous TiO2 Polymorphs with Tunable Crystal Structure for Enhanced Photocatalytic H2 Production. Adv. Energy Mater. 2019, 9, 1901634. [Google Scholar] [CrossRef]
- Chang, X. CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc Plot Method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, S.; Casals, G.; Tang, J.; Lin, Y.; Li, X.; Liang, L.; Jia, Z.; Zeng, M.; Casals, E. Facile Aqueous Synthesis and Comparative Evaluation of TiO2-Semiconductor and TiO2-Metal Nanohybrid Photocatalysts in Antibiotics Degradation under Visible Light. RSC Adv. 2023, 13, 33187–33203. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Zhu, Q.; Gu, X.; Ma, M.; Li, D.; Huang, K.; Fu, X.; Zhu, Y.; Zhang, Y. Understanding the Mechanism of Saturated and Mono-/Tri-Lacunary Keggin SiWx Doped in Bi2WO6 and BiOBr for Efficient Photocatalytic CO2 Reduction. Sep. Purif. Technol. 2023, 321, 124228. [Google Scholar] [CrossRef]
- Yang, F.; Ba, G.; Wang, Z.; Li, H. Surface Modification Induced Construction of Core-Shell Homojunction of Polymeric Carbon Nitride for Boosted Photocatalytic Performance. J. Colloid Interface Sci. 2021, 594, 64–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).