Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting
Abstract
1. Introduction
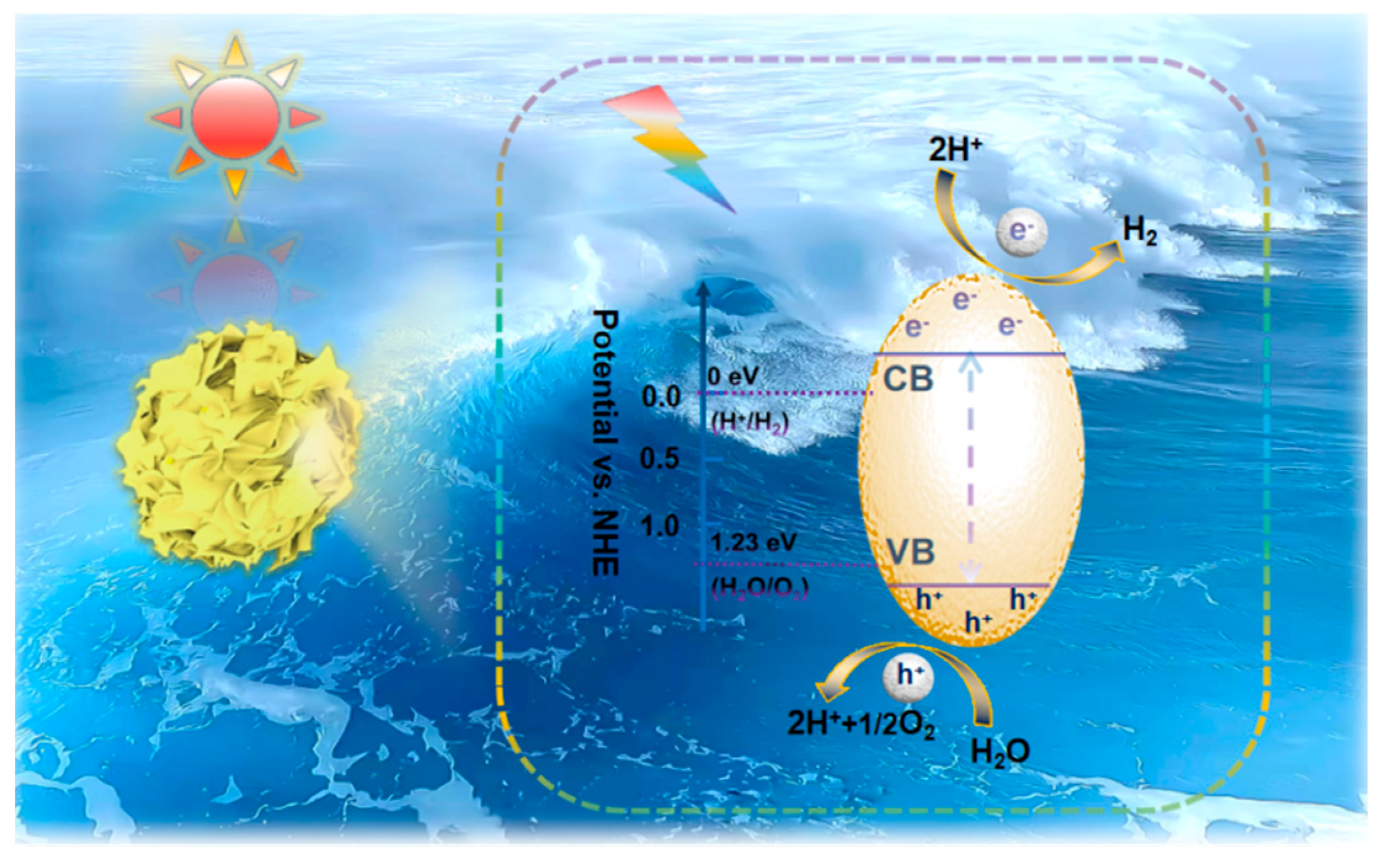
2. Mechanisms of Photocatalytic Overall Water Splitting
3. Introduction of ZnIn2S4
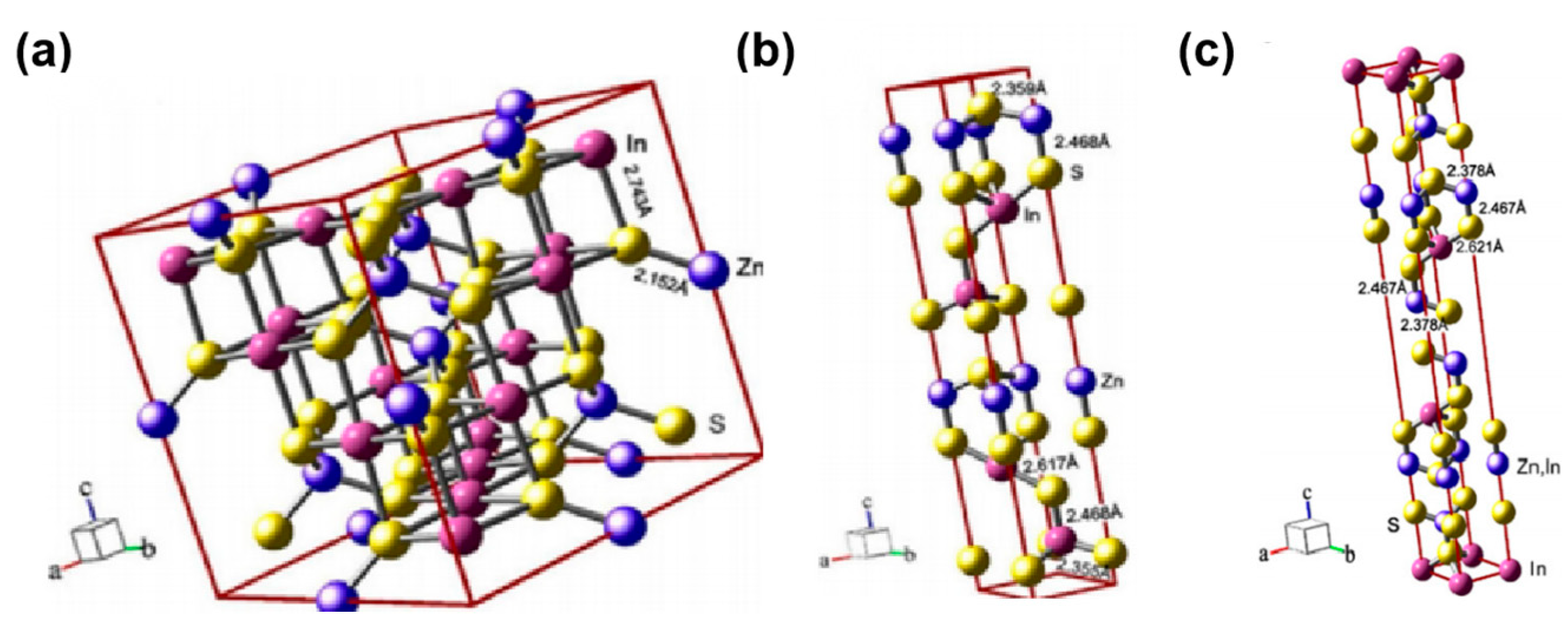
3.1. Crystal and Energy Band Structures
3.2. Synthetic Methods and Morphology of ZnIn2S4
3.2.1. Morphology of ZnIn2S4
3.2.2. Synthetic Methods of ZnIn2S4
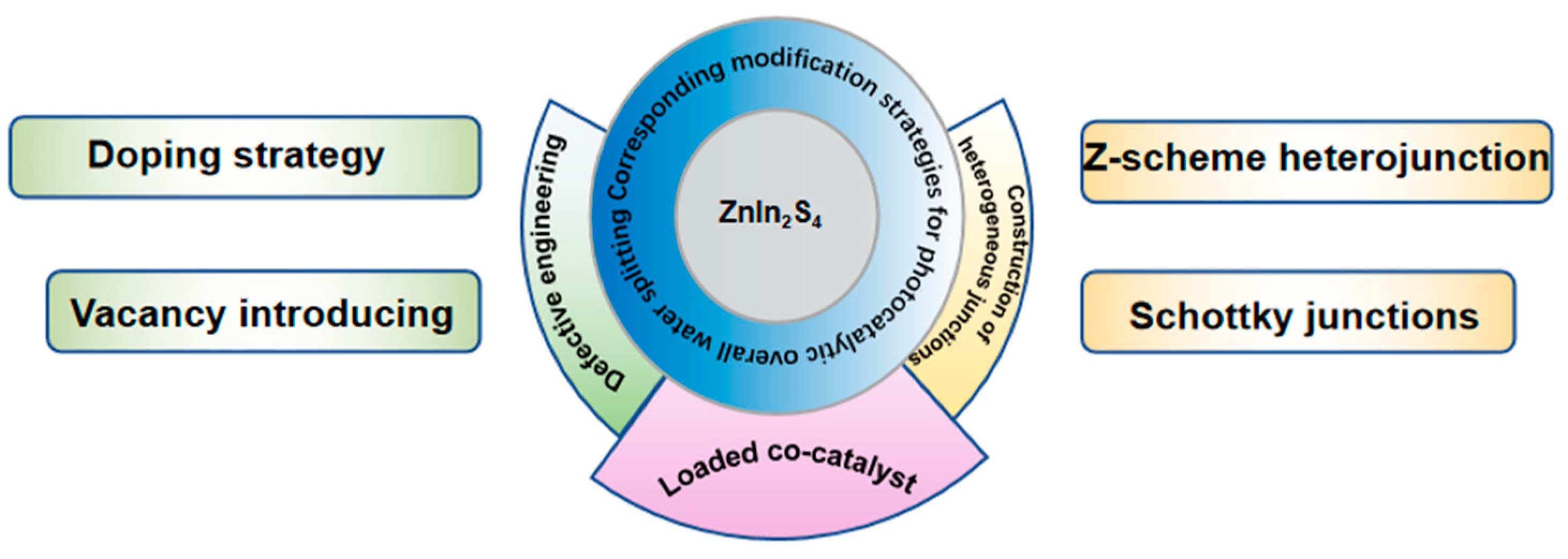
4. Photocatalytic Overall Water Splitting Modification Based on ZnIn2S4 Catalyst
4.1. Defect Engineering
4.1.1. Doping Strategy
Metal Doping
Non-Metal Doping
4.1.2. Vacancy Introduction
4.2. Construction of Heterogeneous Junctions
4.2.1. Z-Scheme Heterojunction
Indirect Z-Scheme Heterojunction
Direct Z-Scheme Heterojunction
4.2.2. Schottky Junctions
4.3. Loaded Co-Catalyst
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, C.; Zheng, Y.; Zhang, G.; Li, C.M. Spatially Separating Redox Centers on Zlog cheme ZnIn2S4/BiVO4 Hierarchical Heterostructure for Highly Efficient Photocatalytic Hydrogen Evolution. Small 2020, 16, 2002988. [Google Scholar] [CrossRef]
- Su, T.; Men, C.; Chen, L.; Chu, B.; Luo, X.; Ji, H.; Chen, J.; Qin, Z. Sulfur Vacancy and Ti3C2Tx Cocatalyst Synergistically Boosting Interfacial Charge Transfer in 2D/2D Ti3C2Tx/ZnIn2S4 Heterostructure for Enhanced Photocatalytic Hydrogen Evolution. Adv. Sci. 2022, 9, 2103715. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.; Yan, B.; Xia, C.; Yang, G. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Zhang, J.; Lin, W.; Wang, J.; Xu, Y.; Xiang, Y.; Hisatomi, T.; Domen, K.; Ma, G. Synthesis of Narrow-Band-Gap GaN:ZnO Solid Solution for Photocatalytic Overall Water Splitting. ACS Catal. 2022, 12, 14637–14646. [Google Scholar] [CrossRef]
- Zhang, G.G.; Wang, X.C. Oxysulfide Semiconductors for Photocatalytic Overall Water Splitting with Visible Light. Angew. Chem.-Int. Ed. 2019, 58, 15580–15582. [Google Scholar] [CrossRef]
- Gogoi, D.; Shah, A.; Rambabu, P.; Qureshi, M.; Golder, A.; Peela, N. Step-Scheme Heterojunction between CdS Nanowires and Facet-Selective Assembly of MnOx-BiVO4 for an Efficient Visible-Light-Driven Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 45475–45487. [Google Scholar] [CrossRef]
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-schematic water splitting into H2 and O2 using metal sulfide as a hydrogen-evolving photocatalyst and reduced graphene oxide as a solid-state electron mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Anwar, U.; Taha, T.A.; El-Nasser, K.S.; Alenad, A.M.; Al-Sehemi, A.G.; Alghamdi, N.A.; Al-Hartomy, O.A.; Amin, M.A.; et al. Enhanced photocatalytic overall water splitting from an assembly of donor-π-acceptor conjugated polymeric carbon nitride. J. Colloid Interface Sci. 2022, 624, 411–422. [Google Scholar] [CrossRef]
- Sun, S.; Gao, R.; Liu, X.; Pan, L.; Shi, C.; Jiang, Z.; Zhang, X.; Zou, J.J. Engineering interfacial band bending over bismuth vanadate/carbon nitride by work function regulation for efficient solar-driven water splitting. Sci. Bull. 2021, 67, 389–397. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, X.; Jiang, L.; Yu, H.; Zhang, J.; Wang, H.; Guan, R.; Zeng, G. Recent advances in synthesis, modification and photocatalytic applications of micro/nano-structured zinc indium sulfide. Chem. Eng. J. 2018, 354, 407–431. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q.; Du, M.; Zeng, X.; Zhong, G.; Qiu, B.; Zhang, J. Recent Progress of Metal Sulfide Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, e2202929. [Google Scholar] [CrossRef]
- Hao, X.Q.; Zhou, J.; Cui, Z.W.; Wang, Y.C.; Wang, Y.; Zou, Z. Zn-vacancy mediated electron-hole separation in ZnS/g-C3N4 heterojunction for efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 229, 41–51. [Google Scholar] [CrossRef]
- Xue, S.; Huang, W.; Lin, W.; Xing, W.; Shen, M.; Ye, X.; Liang, X.; Yang, C.; Hou, Y.; Yu, Z. Interfacial engineering of lattice coherency at ZnO-ZnS photocatalytic heterojunctions—ScienceDirect. Chem Catal. 2022, 2, 125–139. [Google Scholar] [CrossRef]
- Yu, J.; Yu, Y.; Peng, Z.; Wei, X.; Bei, C. Morphology-dependent photocatalytic H2-production activity of CdS. Appl. Catal. B Environ. 2014, 156–157, 184–191. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Chen, D.; Yu, Z.-T.; Zou, Z.-G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Ren, Y.; Foo, J.J.; Zeng, D.; Ong, W.J. ZnIn2S4-Based Nanostructures in Artificial Photosynthesis: Insights into Photocatalytic Reduction toward Sustainable Energy Production. Small Struct. 2022, 3, 2200017. [Google Scholar] [CrossRef]
- Chen, K.; Shi, Y.; Shu, P.; Luo, Z.; Shi, W.; Guo, F. Construction of core–shell FeS2@ZnIn2S4 hollow hierarchical structure S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2023, 454, 140053. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Shi, Y.; Shi, W.; Bai, S.; Yang, S.; Guo, F. A one-photon excitation pathway in 0D/3D CoS2/ZnIn2S4 composite with nanoparticles on micro-flowers structure for boosted visible-light-driven photocatalytic hydrogen evolution. Compos. Part B Eng. 2022, 238, 109955. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Fu, Y.; Guo, F.; Tang, Y.; Yan, X. Enhancement of synergistic effect photocatalytic/persulfate activation for degradation of antibiotics by the combination of photo-induced electrons and carbon dots. Chem. Eng. J. 2022, 433, 133741. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent advances in designing ZnIn2S4-based heterostructured photocatalysts for hydrogen evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. Recent progress on synthesis and modifications of ZnIn2S4 based novel hybrid materials for potential applications. Mater. Sci. Eng. B 2023, 292, 116418. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- He, R.; Ran, J. Dilemma faced by photocatalytic overall water splitting. J. Mater. Sci. Technol. 2023, 157, 107–109. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Ferrer, B.; Garcia, H. Metal-Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef]
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Feng, S.; Jia, Y.; Guo, S.; Wu, X.; Chen, M.; Shi, W. Pt Atoms/Clusters on Ni-phytate-sensitized Carbon Nitride for Enhanced NIR-light-driven Overall Water Splitting beyond 800 nm. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212234. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Hu, L.; Huang, J.; Wang, J.; Jiang, S.; Sun, C.; Song, S. Efficiently photocatalytic H2O overall splitting within the strengthened polarized field by reassembling surface single atoms. Appl. Catal. B Environ. 2023, 320, 121945. [Google Scholar] [CrossRef]
- Chen, J.; Xin, F.; Yin, X.; Xiang, T.; Wang, Y. Synthesis of hexagonal and cubic ZnIn2S4 nanosheets for the photocatalytic reduction of CO2 with methanol. RSC Adv. 2015, 5, 3833–3839. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Lee, T.; Jang, W.; Lee, K.H.; Soon, A. Revisiting Polytypism in Hexagonal Ternary Sulfide ZnIn2S4 for Photocatalytic Hydrogen Production within the Z-Scheme. Chem. Mater. 2019, 31, 9148–9155. [Google Scholar] [CrossRef]
- Yue, Y.; Zou, J. One-step hydrothermal synthesis of rhombohedral ZnIn2S4 with high visible photocatalytic activity for aqueous pollutants removal. J. Colloid Interface Sci. 2023, 640, 270–280. [Google Scholar] [CrossRef]
- Shen, S.; Guo, P.; Zhao, L.; Du, Y.; Guo, L. Insights into photoluminescence property and photocatalytic activity of cubic and rhombohedral ZnIn2S4. J. Solid State Chem. 2011, 184, 2250–2256. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Zhou, R.; Li, Y.; He, Z.; Ding, H.; Chen, D.; Ao, W. A review: Synthesis, modification and photocatalytic applications of ZnIn2S4. J. Mater. Sci. Technol. 2021, 78, 1–19. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Wang, M.; Yan, J.; Chen, Y.; Liu, S. Unveiling the Origin of Co3O4 Quantum Dots for Photocatalytic Overall Water Splitting. Small 2023, 19, e2206695. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, Y.; Jia, L.; Tian, L.; Srinivasan, M.; Ramakrishna, S.; Yan, Q.; Mhaisalkar, S.G. Size- and shape-controlled synthesis of ZnIn2S4 nanocrystals with high photocatalytic performance. CrystEngComm 2013, 15, 1922–1930. [Google Scholar] [CrossRef]
- Shi, L.; Yin, P.; Dai, Y. Synthesis and photocatalytic performance of ZnIn2S4 nanotubes and nanowires. Langmuir 2013, 29, 12818–12822. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. Templated Electrochemical Fabrication of Hollow Molybdenum Sulfide Microstructures and Nanostructures with Catalytic Properties for Hydrogen Production. ACS Catal. 2016, 6, 3985–3993. [Google Scholar] [CrossRef]
- Luo, R.-B.; Liu, Q.-J.; Fan, D.-H.; Liu, Z.-T. First-principles calculations to investigate structural, electronic and optical properties of two-dimensional ZnIn2S4. Appl. Surf. Sci. 2022, 605, 154739. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zai, J.; Yuan, Y.; Qian, X. 3D hierarchical ZnIn2S4: The preparation and photocatalytic properties on water splitting. Int. J. Hydrogen Energy 2012, 37, 16986–16993. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C.; Gong, J.; Li, Q. Rapid Mass Production of Hierarchically Porous ZnIn2S4 Submicrospheres via a Microwave-Solvothermal Process. Cryst. Growth Des. 2007, 7, 2444–2448. [Google Scholar] [CrossRef]
- Fang, F.; Chen, L.; Chen, Y.B.; Wu, L.M. Synthesis and Photocatalysis of ZnIn2S4 Nano/Micropeony. J. Phys. Chem. C 2010, 114, 2393–2397. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, Q.; Huang, J.; Liu, J.; Tan, G.; Sun, C.; Li, T.; Liu, S.; Li, Y.; Wang, H.; et al. Visually resolving the direct Z-scheme heterojunction in CdS@ZnIn2S4 hollow cubes for photocatalytic evolution of H2 and H2O2 from pure water. Appl. Catal. B Environ. 2021, 293, 120213. [Google Scholar] [CrossRef]
- Chaudhari, N.S.; Warule, S.S.; Kale, B.B. Architecture of rose and hollow marigold-like ZnIn2S4 flowers: Structural, optical and photocatalytic study. RSC Adv. 2014, 4, 12182–12187. [Google Scholar] [CrossRef]
- Chen, Z.X.; Li, D.Z.; Zhang, W.J.; Chen, C.; Li, W.J.; Sun, M.; He, Y.H.; Fu, X.Z. Low-Temperature and Template-Free Synthesis of ZnIn2S4 Microspheres. Inorg. Chem. 2010, 40, 9766–9772. [Google Scholar] [CrossRef]
- Ding, S.; Liu, X.; Shi, Y.; Liu, Y.; Zhou, T.; Guo, Z.; Hu, J. Generalized Synthesis of Ternary Sulfide Hollow Structures with Enhanced Photocatalytic Performance for Degradation and Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 17911–17922. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Cheng, F.; Shi, Y.; Zhang, L.; Shen, P. Shape-controlled synthesis of ternary chalcogenide ZnIn2S4 and CuIn(S,Se)2 nano-/microstructures via facile solution route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, R.; Chen, D.; Wang, Y.; Liu, W.; Li, X.; Li, Z. Exploring the different photocatalytic performance for dye degradations over hexagonal ZnIn2S4 microspheres and cubic ZnIn2S4 nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lv, B.; Kang, Y.; Xu, X.; Lei, X.; Li, L.; Wang, H.; Xi, H.; Yang, J.; Yang, Z. Solvent-Induced ZnIn2S4 Nanosheets Self-assembled Micro-Flowers to Boosting the Photocatalytic Semi-dehydrogenation of 1,2,3,4-Tetrahydroisoquinoline. Catal. Lett. 2022, 153, 570–583. [Google Scholar] [CrossRef]
- Zou, P.; Su, G.; Li, Z.; Li, Y.; Zhou, T.; Kang, Y. Oxalic acid modified hexagonal ZnIn2S4 combined with bismuth oxychloride to fabricate a hierarchical dual Z-scheme heterojunction: Accelerating charge transfer to improve photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127835. [Google Scholar] [CrossRef]
- Su, L.; Ye, X.; Meng, S.; Fu, X.; Chen, S. Effect of different solvent on the photocatalytic activity of ZnIn2S4 for selective oxidation of aromatic alcohols to aromatic aldehydes under visible light irradiation. Appl. Surf. Sci. 2016, 384, 161–174. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Morphology, structure and photocatalytic performance of ZnIn2S4 synthesized via a solvothermal/hydrothermal route in different solvents. J. Phys. Chem. Solids 2008, 69, 2426–2432. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Ranjbar, M.; Sabet, M. Synthesis and Characterization of ZnIn2S4 Nanoparticles by a Facile Microwave Approach. J. Inorg. Organomet. Polym. Mater. 2012, 23, 452–457. [Google Scholar] [CrossRef]
- Yang, W.; Liu, B.; Fang, T.; Jennifer, W.A.; Christophe, L.; Li, Z.; Zhang, X.; Jiang, X. Layered crystalline ZnIn2S4 nanosheets: CVD synthesis and photo-electrochemical properties. Nanoscale 2016, 8, 18197–18203. [Google Scholar] [CrossRef]
- Hemalatha, S.; Illakkiya, J.T.; Oommen, R.; Rajalakshmi, P.U. Marigold microstructure of zinc thioindate (ZnIn2S4) thin film—Characteristics. Optik 2016, 127, 3858–3861. [Google Scholar] [CrossRef]
- Huang, J.; Cheuk, W.; Wu, Y.; Lee, F.; Ho, W. Template-free synthesis of ternary sulfides submicrospheres as visible light photocatalysts by ultrasonic spray pyrolysis. Catal. Sci. Technol. 2012, 2, 1825. [Google Scholar] [CrossRef]
- Cadiş, A.I.; Mureșan, L.E.; Perhaița, I.; Pop, L.C.; Saszet, K.; Barbu-Tudoran, L.; Borodi, G. Peculiarities on methyl orange adsorption by porous ZnIn2S4 prepared in different conditions. J. Nanoparticle Res. 2022, 24, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Afzal, N.; Pan, L.; Zhang, X.; Zou, J.J. Structure-Activity Relationship of Defective Metal-Based Photocatalysts for Water Splitting: Experimental and Theoretical Perspectives. Adv. Sci. 2019, 6, 1900053. [Google Scholar] [CrossRef]
- Su, Z.; Fang, F.; Li, X.; Han, W.; Liu, X.; Chang, K. Synergistic surface oxygen defect and bulk Ti3+ defect engineering on SrTiO3 for enhancing photocatalytic overall water splitting. J. Colloid Interface Sci. 2022, 626, 662–673. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Ma, J.; Long, R.; Liu, D.; Low, J.; Xiong, Y. Defect Engineering in Photocatalytic Methane Conversion. Small Struct. 2021, 3, 2100147. [Google Scholar] [CrossRef]
- Patnaik, P. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Yang, R.; Mei, L.; Fan, Y.; Zhang, Q.; Zhu, R.; Amal, R.; Yin, Z.; Zeng, Z. ZnIn2S4-Based Photocatalysts for Energy and Environmental Applications. Small Methods 2021, 5, e2100887. [Google Scholar] [CrossRef]
- Pan, R.; Hu, M.; Liu, J.; Li, D.; Wan, X.; Wang, H.; Li, Y.; Zhang, X.; Wang, X.; Jiang, J.; et al. Two-Dimensional All-in-One Sulfide Monolayers Driving Photocatalytic Overall Water Splitting. Nano Lett. 2021, 21, 6228–6236. [Google Scholar] [CrossRef]
- Sun, B.; Bu, J.; Chen, X.; Fan, D.; Li, S.; Li, Z.; Zhou, W.; Du, Y. In-situ interstitial zinc doping-mediated efficient charge separation for ZnIn2S4 nanosheets visible-light photocatalysts towards optimized overall water splitting. Chem. Eng. J. 2022, 435, 135074. [Google Scholar] [CrossRef]
- Sun, B.; Fan, D.; Chen, X.; Li, Z.; Zhou, W.; Du, Y. Heteroatom-induced domain electrostatic potential difference in ZnIn2S4 nanosheets for efficient charge separation and boosted photocatalytic overall water splitting. Mater. Chem. Front. 2022, 6, 1795–1802. [Google Scholar] [CrossRef]
- Jing, H.; Ren, J.; Yue, J.; Liu, S.; Liang, Q.; Wu, R.; Wang, Y.; Fang, Z.; Li, H.; Wei, S. ZnIn2S4 with oxygen atom doping and surface sulfur vacancies for overall water splitting under visible light irradiation. Catal. Sci. Technol. 2023, 13, 226–232. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, X.; Hu, G.; Chen, M.; Shen, H.; Li, B.; Yang, L.; Dai, W.; Zou, J.; Luo, S. Configuration regulation of active sites by accurate doping inducing self-adapting defect for enhanced photocatalytic applications: A review. Coord. Chem. Rev. 2023, 478, 214970. [Google Scholar] [CrossRef]
- He, J.; Hu, L.; Shao, C.; Jiang, S.; Sun, C.; Song, S. Photocatalytic H2O Overall Splitting into H2 Bubbles by Single Atomic Sulfur Vacancy CdS with Spin Polarization Electric Field. ACS Nano 2021, 15, 18006–18013. [Google Scholar] [CrossRef]
- Jing, H.; Xu, G.; Yao, B.; Ren, J.; Wang, Y.; Fang, Z.; Liang, Q.; Wu, R.; Wei, S. Sulfur Vacancy-Enriched Rhombohedral ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Overall Water Splitting under Visible Light Irradiation. ACS Appl. Energy Mater. 2022, 5, 10187–10195. [Google Scholar] [CrossRef]
- Abdul Nasir, J.; Munir, A.; Ahmad, N.; Haq, T.U.; Khan, Z.; Rehman, Z. Photocatalytic Z-Scheme Overall Water Splitting: Recent Advances in Theory and Experiments. Adv. Mater. 2021, 33, 2105195. [Google Scholar] [CrossRef]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Guo, F.; Shi, W. Construction of full solar-spectrum available S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2023, 459, 151976. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Sun, H.; Shi, W.; Guo, F. Fabrication of Bi4Ti3O12/ZnIn2S4 S-scheme heterojunction for achieving efficient photocatalytic hydrogen production. J. Alloys Compd. 2023, 930, 167450. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Li, M.; Shi, Y.; Shi, M.; Yan, C. Constructing 3D sub-micrometer CoO octahedrons packed with layered MoS2 shell for boosting photocatalytic overall water splitting activity. Appl. Surf. Sci. 2019, 473, 928–933. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Sun, H.; Chen, L. Anchoring CoP nanoparticles on the octahedral CoO by self-phosphating for enhanced photocatalytic overall water splitting activity under visible light. Chin. J. Chem. Eng. 2021, 40, 114–123. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Yan, C.; Guo, F. Facile synthesis of 2D/2D Co3(PO4)2/g-C3N4 heterojunction for highly photocatalytic overall water splitting under visible light. Chem. Eng. J. 2020, 382, 122960. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, F.; Sun, H.; Shi, Y.; Shi, W. Well-designed three-dimensional hierarchical hollow tubular g-C3N4/ZnIn2S4 nanosheets heterostructure for achieving efficient visible-light photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 607, 1391–1401. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Shi, Y.; Sun, H.; Cheng, X.; Shi, W.; Chen, L. Formation of unique hollow ZnSnO3@ZnIn2S4 core-shell heterojunction to boost visible-light-driven photocatalytic water splitting for hydrogen production. J. Colloid Interface Sci. 2021, 602, 889–897. [Google Scholar] [CrossRef]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on Metal Sulphide-based Z-scheme Photocatalysts. ChemCatChem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Shi, Y.; Guo, F.; Tang, Y. Effect of different carbon dots positions on the transfer of photo-induced charges in type I heterojunction for significantly enhanced photocatalytic activity. Sep. Purif. Technol. 2023, 304, 122337. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.; Shi, Y.; Sun, H.; Li, L.; Guo, F.; Wen, H. Carbon dots as solid-state electron mediator and electron acceptor in S-scheme heterojunction for boosted photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 595, 153482. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Zhang, S.; Song, F. Construction of Z-scheme photocatalytic systems using ZnIn2S4, CoOx-loaded Bi2MoO6 and reduced graphene oxide electron mediator and its efficient nonsacrificial water splitting under visible light. Chem. Eng. J. 2017, 325, 690–699. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Feng, J.; Hao, Q.; Zhu, Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 234, 260–267. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, D.; He, R.; Yuan, R.; Xie, T.; Li, Z. Rational design of Z-scheme PtS-ZnIn2S4/WO3-MnO2 for overall photo-catalytic water splitting under visible light. Appl. Catal. B Environ. 2019, 258, 117948. [Google Scholar] [CrossRef]
- Ou, M.; Li, J.; Geng, M.; Wang, J.; Wan, S.; Zhong, Q. Construction of Z-scheme Photocatalyst Containing ZnIn2S4, Co3O4-Photodeposited BiVO4 (110) Facets and rGO Electron Mediator for Overall Water Splitting into H2 and O2. Catal. Lett. 2021, 151, 2570–2582. [Google Scholar] [CrossRef]
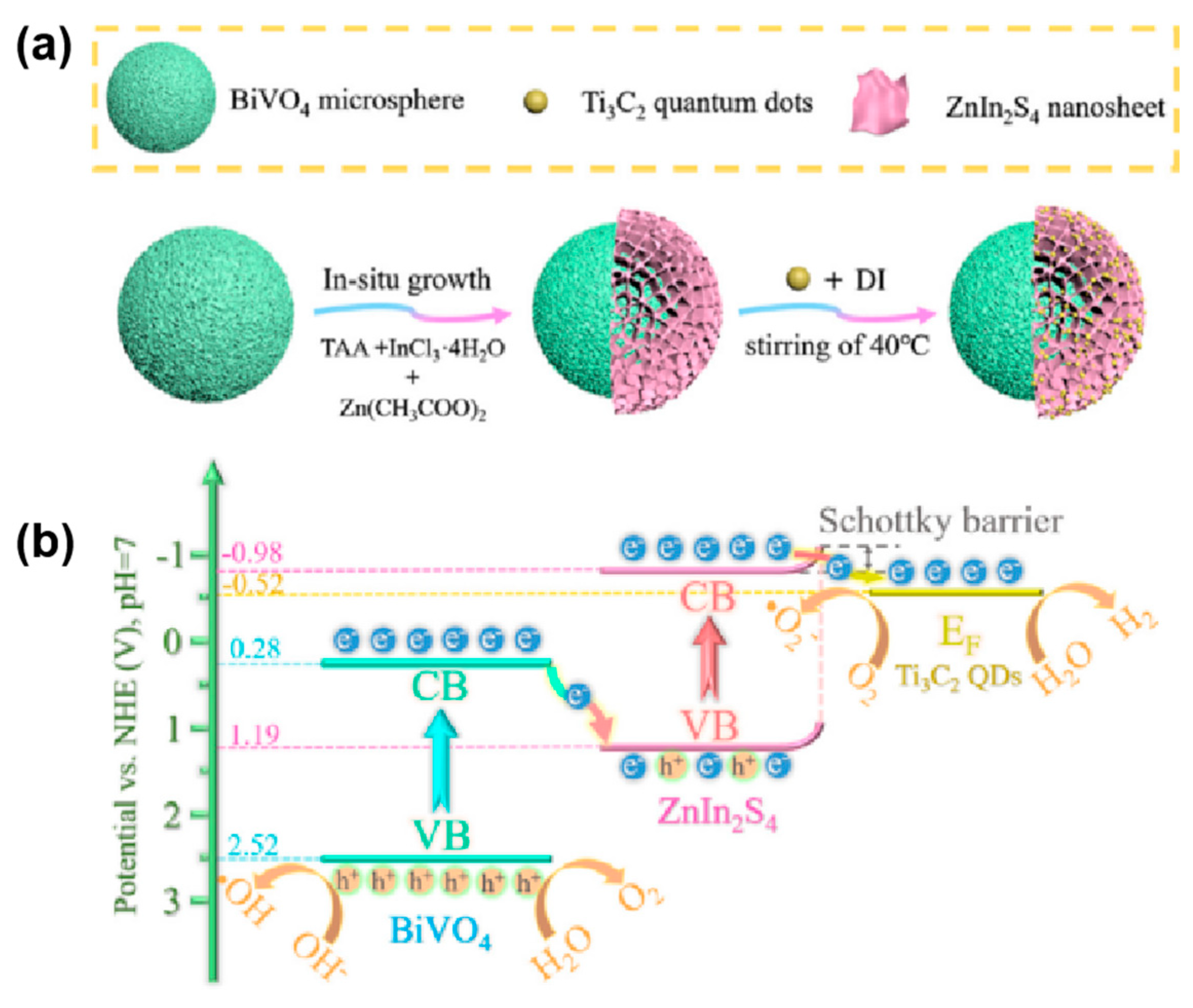
- Du, X.; Zhao, T.; Xiu, Z.; Xing, Z.; Li, Z.; Pan, K.; Yang, S.; Zhou, W. BiVO4@ZnIn2S4/Ti3C2 MXene quantum dots assembly all-solid-state direct Z-Scheme photocatalysts for efficient visible-light-driven overall water splitting. Appl. Mater. Today 2020, 20, 100719. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Guo, S.; Xin, X.; Zhang, Y.; Guo, P.; Tang, S.; Li, X. Sulfur-Deficient ZnIn2S4/Oxygen-Deficient WO3 Hybrids with Carbon Layer Bridges as a Novel Photothermal/Photocatalytic Integrated System for Z-Scheme Overall Water Splitting. Adv. Energy Mater. 2021, 11, 2102452. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xian, Q.; Zhao, Y. Direct Z-scheme TiO2–ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl. Catal. B Environ. 2021, 291, 120126. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, L.; Yan, W.; Wang, H.; Mao, W.; Cui, Y.; Li, Y.; Zhu, X. Synergizing the internal electric field and ferroelectric polarization of the BiFeO3/ZnIn2S4 Z-scheme heterojunction for photocatalytic overall water splitting. J. Mater. Chem. A 2023, 11, 434–446. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Z.; Liu, C.; Wang, J.; Qiu, M.; Yan, G.; Zhang, K. Boosting Photocatalytic Overall Water Splitting on Direct Z-Scheme BiOBr/ZnIn2S4 Heterostructure by Atomic-Level Interfacial Charge Transport Modulation. ACS Appl. Energy Mater. 2022, 5, 15559–15565. [Google Scholar] [CrossRef]
- Zuo, G.; Chen, W.; Yin, Z.; Ma, S.; Wang, Y.; Ji, Q.; Xian, Q.; Yang, S.; He, H. Covalency dominating Z-scheme perylene-dicarboximide@ZnIn2S4 organic-inorganic hybrids for overall water splitting. Chem. Eng. J. 2023, 456, 141096. [Google Scholar] [CrossRef]
- Zuo, G.; Ma, S.; Yin, Z.; Chen, W.; Wang, Y.; Ji, Q.; Xian, Q.; Yang, S.; He, H. Z-Scheme Modulated Charge Transfer on InVO4@ZnIn2S4 for Durable Overall Water Splitting. Small 2023, 19, e2207031. [Google Scholar] [CrossRef]
- Li, X.H.; Antonietti, M. Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: Functional Mott-Schottky heterojunctions for catalysis. Chem. Soc. Rev. 2014, 44, 6593–6604. [Google Scholar] [CrossRef]
- Chu, J.H.; Han, K.; Yu, Q.Y.; Wang, H.L.; Xi, K.; Lai, F.L.; Zhang, J.G.; Bao, Y.P. Schottky junction and multiheterostructure synergistically enhance rate performance and cycling stability. Chem. Eng. J. 2022, 430, 11. [Google Scholar] [CrossRef]
- Cai, X.; Zeng, Z.; Liu, Y.; Li, Z.; Gu, X.; Zhao, Y.; Mao, L.; Zhang, J. Visible-light-driven water splitting by yolk-shelled ZnIn2S4-based heterostructure without noble-metal co-catalyst and sacrificial agent. Appl. Catal. B Environ. 2021, 297, 120391. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Xu, J.; Li, Y.; Du, Y.; Jiang, Y.; Lin, K. Hydroxyl-modified Nb4C3T(x) MXene@ZnIn2S4 sandwich structure for photocatalytic overall water splitting. J. Colloid Interface Sci. 2023, 633, 992–1001. [Google Scholar] [CrossRef]
- An, H.; Lv, Z.; Zhang, K.; Deng, C.; Yin, Z. Plasmonic coupling enhancement of core-shell Au@Pt assemblies on ZnIn2S4 nanosheets towards photocatalytic H2 production. Appl. Surf. Sci. 2020, 536, 147934. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Qin, X.; Cai, Y.; Zhang, W.; Shi, W.; Du, X.; Guo, F. Coupled internal electric field with hydrogen release kinetics for promoted photocatalytic hydrogen production through employing carbon coated transition metal as co-catalyst. J. Colloid Interface Sci. 2023, 630, 274–285. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Liu, X.; Pan, L.; Zhang, X.; Zou, J. Design and Construction of Cocatalysts for Photocatalytic Water Splitting. Acta Phys.-Chim. Sin. 2020, 36, 1905007–1905000. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Vequizo, J.J.M.; Hisatomi, T.; Nakabayashi, M.; Pan, Z.; Shibata, N.; Yamakata, A.; Takata, T.; Domen, K. One-Step Excitation Overall Water Splitting over a Modified Mg-Doped BaTaO2N Photocatalyst. ACS Catal. 2022, 12, 10179–10185. [Google Scholar] [CrossRef]
- Ma, B.; Dang, Y.; Li, D.; Wang, X.; Lin, K.; Wang, W.; Zhou, X.; Chen, Y.; Xie, T.; Zhang, X. A Yin-Yang hybrid co-catalyst (CoOx-Mo2N) for photocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 298, 120491. [Google Scholar] [CrossRef]
- Jing, H.; Li, H.; Yue, J.; Fan, S.; Yao, B.; Liu, S.; Fang, Z.; Wu, R.; Yang, L.; Wei, S. Synergistic effects of the Rh-S bond and spatially separated dual cocatalysts on photocatalytic overall water splitting activity of ZnIn2S4 nanosheets under visible light irradiation. Dalton Trans. 2023, 52, 2924–2927. [Google Scholar] [CrossRef]



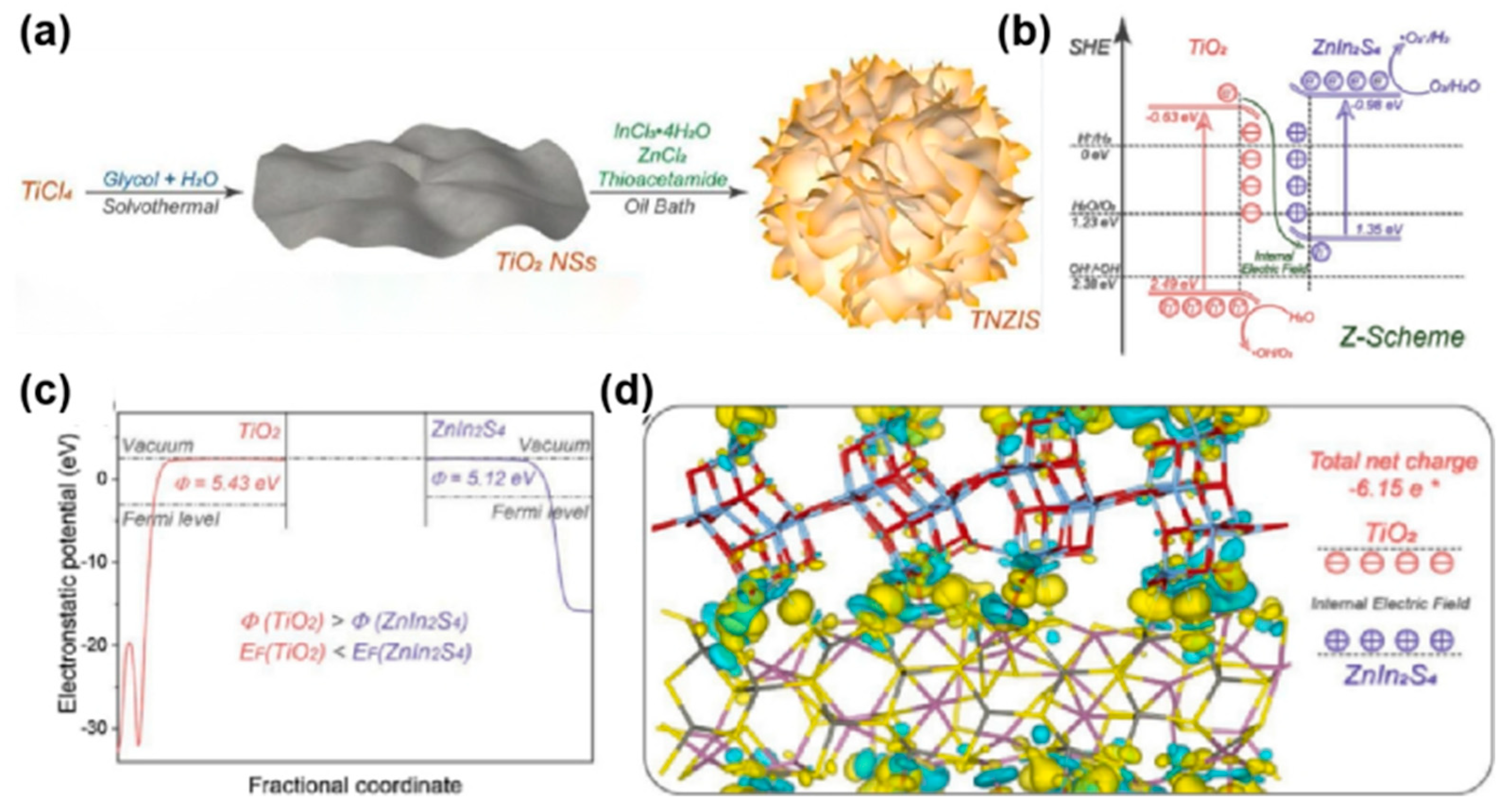

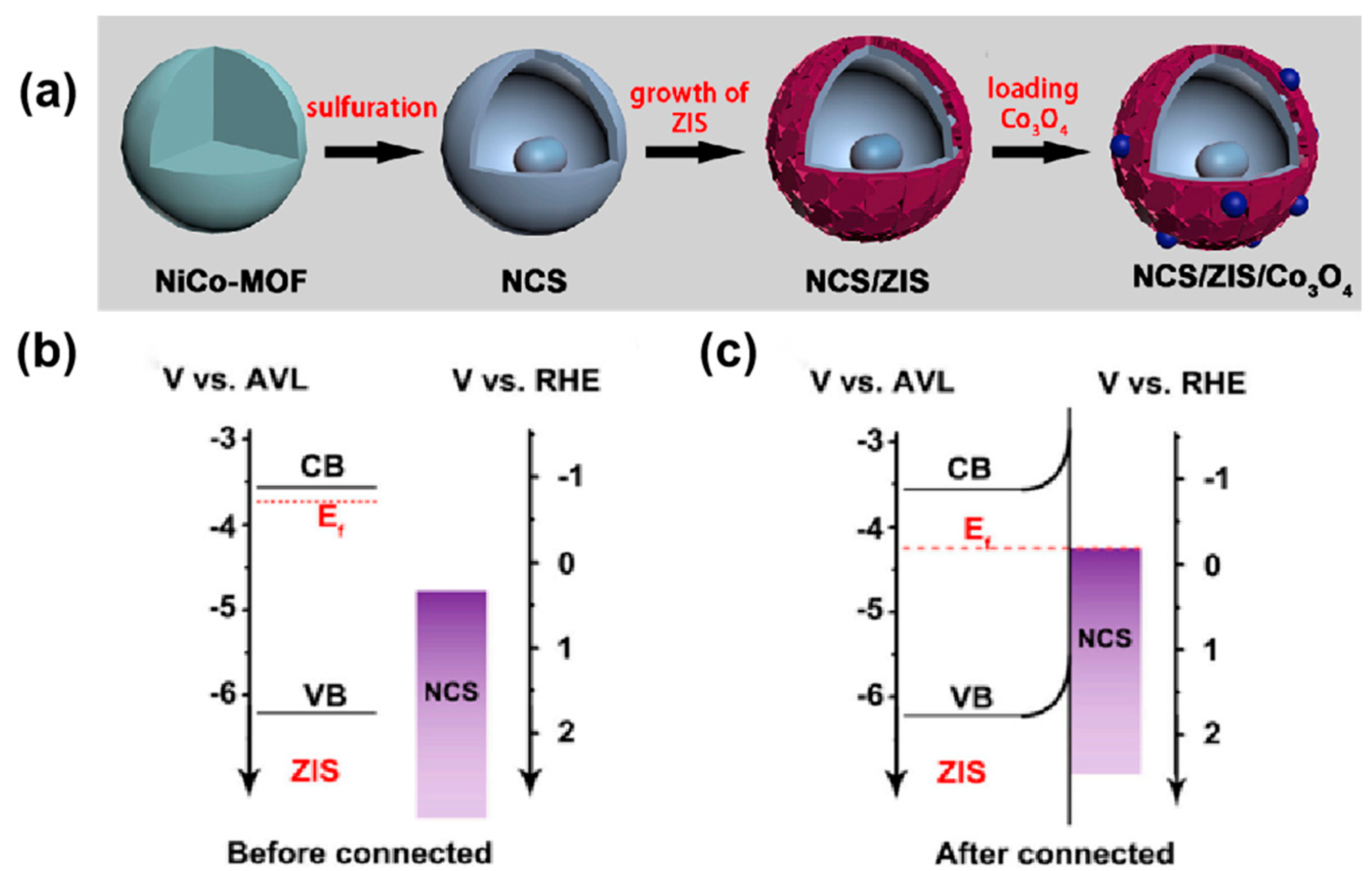
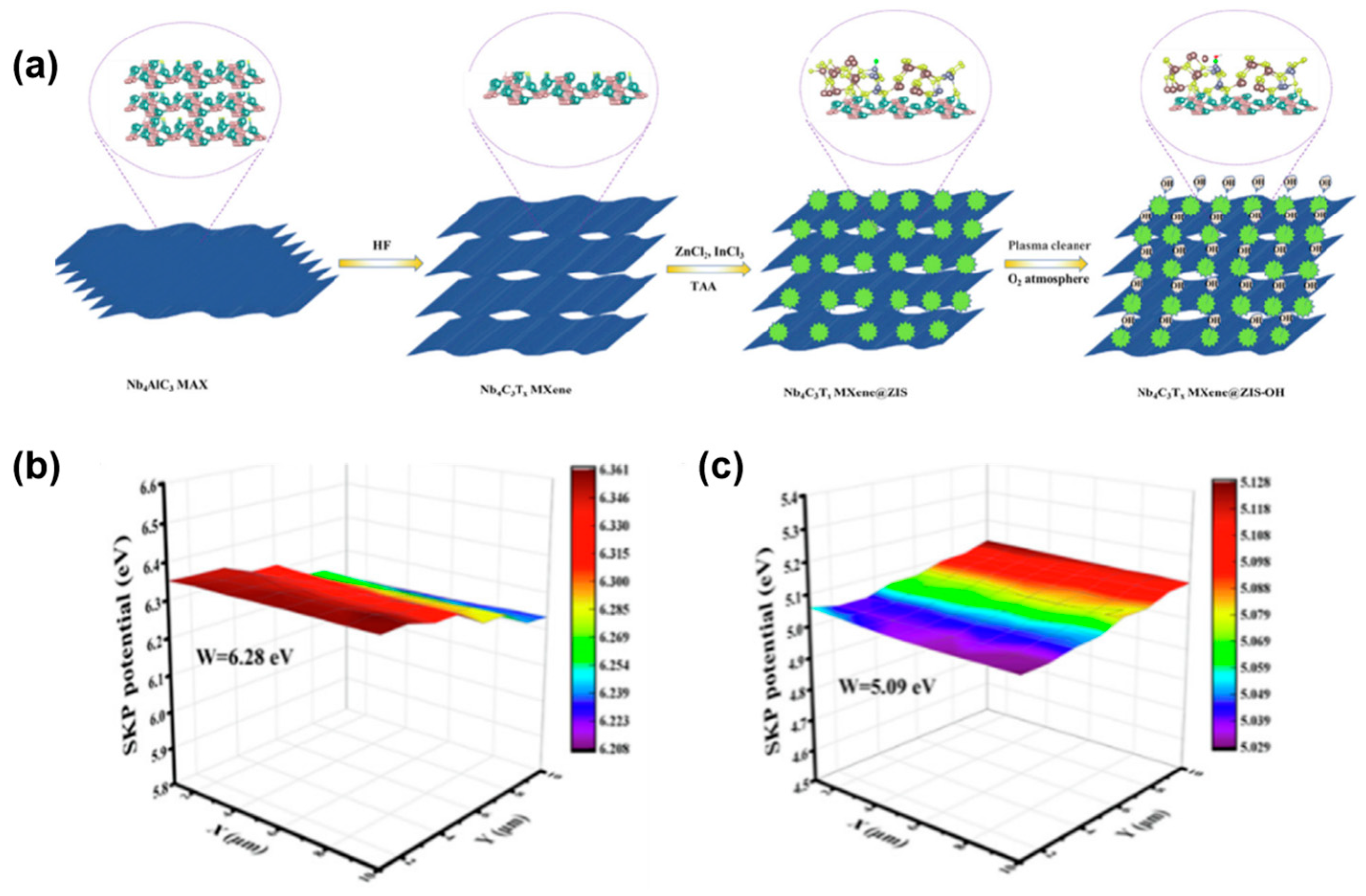
| Type | Morphology | Photocatalyst | Synthetic Method | Sulfur Source | Solvent | Light Source | Application | Ref |
|---|---|---|---|---|---|---|---|---|
| 0D | quantum dots | ZnIn2S4 | solvothermal | sulfur powder | octadecene | 500 W Xe lamp | degradation | [40] |
| 1D | nanowires | ZnIn2S4 | wet-chemical | thioacetamide (TAA) | H2O | 500 W Xe lamp | degradation | [41] |
| 1D | nanotubes | ZnIn2S4 | wet-chemical | TAA | H2O | 500 W Xe lamp | degradation | [41] |
| 2D | ultrathin nanosheet | Vs-M-ZnIn2S4 | lithium intercalation | TAA | N,N-Dimethylformamide, ethylene glycol | 300 W Xe lamp | hydrogen generation | [44] |
| 3D | persimmon-like shape | ZnIn2S4 | solvothermal | CS2 | tetrahydrofuran (THF) | 300 W Xe lamp | hydrogen generation | [45] |
| 3D | porous ZnIn2S4 submicrospheres | ZnIn2S4 | microwave-solvothermal | excess thiourea | ethylene glycol | 300 W tungsten–halogen | degradation | [46] |
| 3D | peony-flower-like | ZnIn2S4 | solventhermal | dioctyldithiocarbamic acid sodium (OTC) | CH3OH | 300 W tungsten-halogen | degradation | [47] |
| 3D | rose-flower-like microsphere | ZnIn2S4 | hydrothermal | thiourea | H2O, diethyl amine (DEA) | 300 W Xe lamp | hydrogen generation | [49] |
| 3D | hollow marigold-like flowers | ZnIn2S4 | hydrothermal | thiourea | H2O, polyvinyl pyrrolidone (PVP) | 300 W Xe lamp | hydrogen generation | [49] |
| 3D | porous microspheres | ZnIn2S4 | microwave-sol vothermal | excessive TAA | H2O | 500 W tungsten–halogen lamp | degradation | [50] |
| 3D | hollow Structure | ZnIn2S4 | hydrothermal | glutathione (GSH) | H2O | 300 W Xe lamp | hydrogen generation | [51] |
| Photocatalyst | Cocatalyst | Reaction Systems | Light Source | H2 (μmol g−1 h−1) | O2 (μmol g−1 h−1) | AQE | Ref. |
|---|---|---|---|---|---|---|---|
| Ag-ZnIn2S4 | / | 12 mg (100 mL H2O) | 300 W Xe lamp (λ > 420 nm) | 56.6 | 29.1 | 0.70% (405 nm) 0.57% (420 nm) 0.20% (450 nm) | [69] |
| dZni-ZnIn2S4 | / | 50 mg (120 mL H2O) | 300 W Xe lamp (λ > 420 nm) | 42.8 | 19.1 | 1.51% (420 nm) | [70] |
| Al-ZnIn2S4 | / | 50 mg (100 mL H2O) | 300 W Xe lamp (λ ≥ 420 nm) | 77.2 | 35.3 | 1.61% (420 nm) | [71] |
| Sv-ZnIn2S4-O (ZnIn2S4-350 °C-4 h) | Pt/Cr | 50 mg (100 mL H2O) | 300 W Xe lamp (λ ≥ 420 nm) | 270.2 | 130.0 | 0.21% (420 nm) | [72] |
| Sv-ZnIn2S4 (ZnIn2S4-800) | Pt/Cr | 50 mg (100 mL H2O) | 300 W Xe lamp (λ ≥ 420 nm) | 68.0 | 31.0 | 0.041% (420 nm) 0.016% (450 nm) 0.004% (500 nm) | [75] |
| ZnIn2S4/RGO/BMO | Pt/CoOx | 100 mg (100 mL H2O) | 200 W Xe lamp (λ > 420 nm) | 31.4 | 15.8 | / | [89] |
| ZnIn2S4-Au-TiO2 | / | 50 mg (100 mL H2O) | 300 W Xe lamp | 186.3 | 66.3 | / | [90] |
| Pt-ZnIn2S4/RGO/Co3O4-BiVO4(110) | Pt/Co3O4 | 50 mg (100 mL H2O) | 300 W Xe lamp(λ > 420 nm) | 24.5 | 11.9 | / | [92] |
| PtS-ZnIn2S4/WO3-MnO2 | Pt/MnO2 | 50 mg (100 mL H2O) | 300 W Xe lamp (λ > 420 nm) | 38.8 | 15.7 | / | [91] |
| BiVO4@ZnIn2S4/Ti3C2 MXene QDs | Ti3C2 MXene QDs | 60 mg (H2O) | 300 W Xe lamp (λ > 400 nm) | 102.67 | 50.83 | 2.40% (410 nm) 2.90% (460 nm) 1.40% (510 nm) 0.20% (560 nm) | [93] |
| ZIS-WO/C-wood (Sv-ZnIn2S4-Ov-WO3/C-wood) | Pt/CoOx | floated at the water–air interface | 300 W Xe lamp (AM 1.5G) | 169.2 | 82.5 | / | [94] |
| TiO2-ZnIn2S4 | / | 20 mg (50 mL H2O) | 300 W Xe lamp | 214.9 | 81.7 | / | [95] |
| BiFeO3/ZnIn2S4 | / | 12 mg (100 mL H2O) | 300 W Xe lamp (λ > 420 nm) | 87.3 | 42.3 | 1.12% (420 nm) | [96] |
| BiOBr/ZnIn2S4 | Pt | 100 mg (100 mL H2O) | 300 W Xe lamp (λ > 420 nm) | 628 | 304 | / | [97] |
| HC-PDI@ZnIn2S4 OIHs | / | 5 mg (50 mL H2O) | 300 W Xe lamp (λ ≥ 400 nm) | 275.4 | 138.4 | 16.14% (400 nm) | [98] |
| InVO4@ZnIn2S4 | / | 5 mg (50 mL H2O) | 300 W Xe lamp | 153.3 | 76.9 | 24.28% (365 nm) 19.31% (380 nm) 15.29% (400 nm) 9.75% (420 nm) 6.93% (460 nm) | [99] |
| NiCo2S4/ZnIn2S4/Co3O4 | NiCo2S4/Co3O4 | 10 mg (15 mL H2O) | / | 103.3 | 26.7 | / | [102] |
| Nb4C3Tx MXene@ZnIn2S4-OH | Nb4C3Tx MXene/OH | 20 mg (100 mL H2O) | 300 W Xe lamp (λ > 420 nm) | 53.8 | 26.7 | / | [103] |
| ZnIn2S4-Rh-Cr | Rh-Cr | 50 mg (100 mL H2O) | 300 W Xe lamp (AM 1.5G) | 118 | 58 | 0.084% (420 nm) 0.028% (450 nm) 0.017% (500 nm) | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Chen, Z.; Cheng, X.; Shi, W. Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting. Catalysts 2023, 13, 967. https://doi.org/10.3390/catal13060967
Yan Y, Chen Z, Cheng X, Shi W. Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting. Catalysts. 2023; 13(6):967. https://doi.org/10.3390/catal13060967
Chicago/Turabian StyleYan, Yujie, Zhouze Chen, Xiaofang Cheng, and Weilong Shi. 2023. "Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting" Catalysts 13, no. 6: 967. https://doi.org/10.3390/catal13060967
APA StyleYan, Y., Chen, Z., Cheng, X., & Shi, W. (2023). Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting. Catalysts, 13(6), 967. https://doi.org/10.3390/catal13060967







