Mesoporous Copper-Cerium Mixed Oxide Catalysts for Aerobic Oxidation of Vanillyl Alcohol
Abstract
1. Introduction
2. Results and Discussion
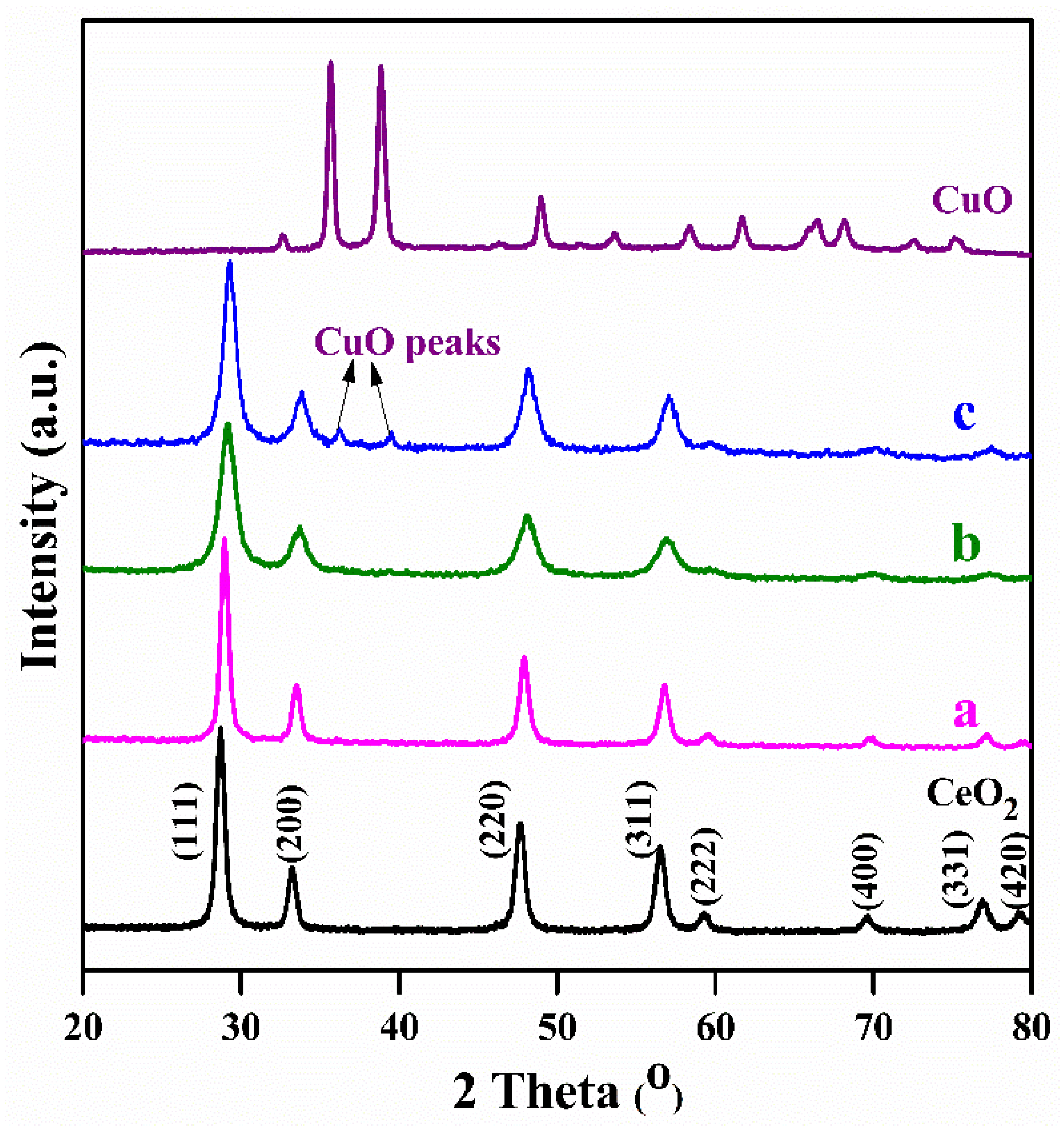
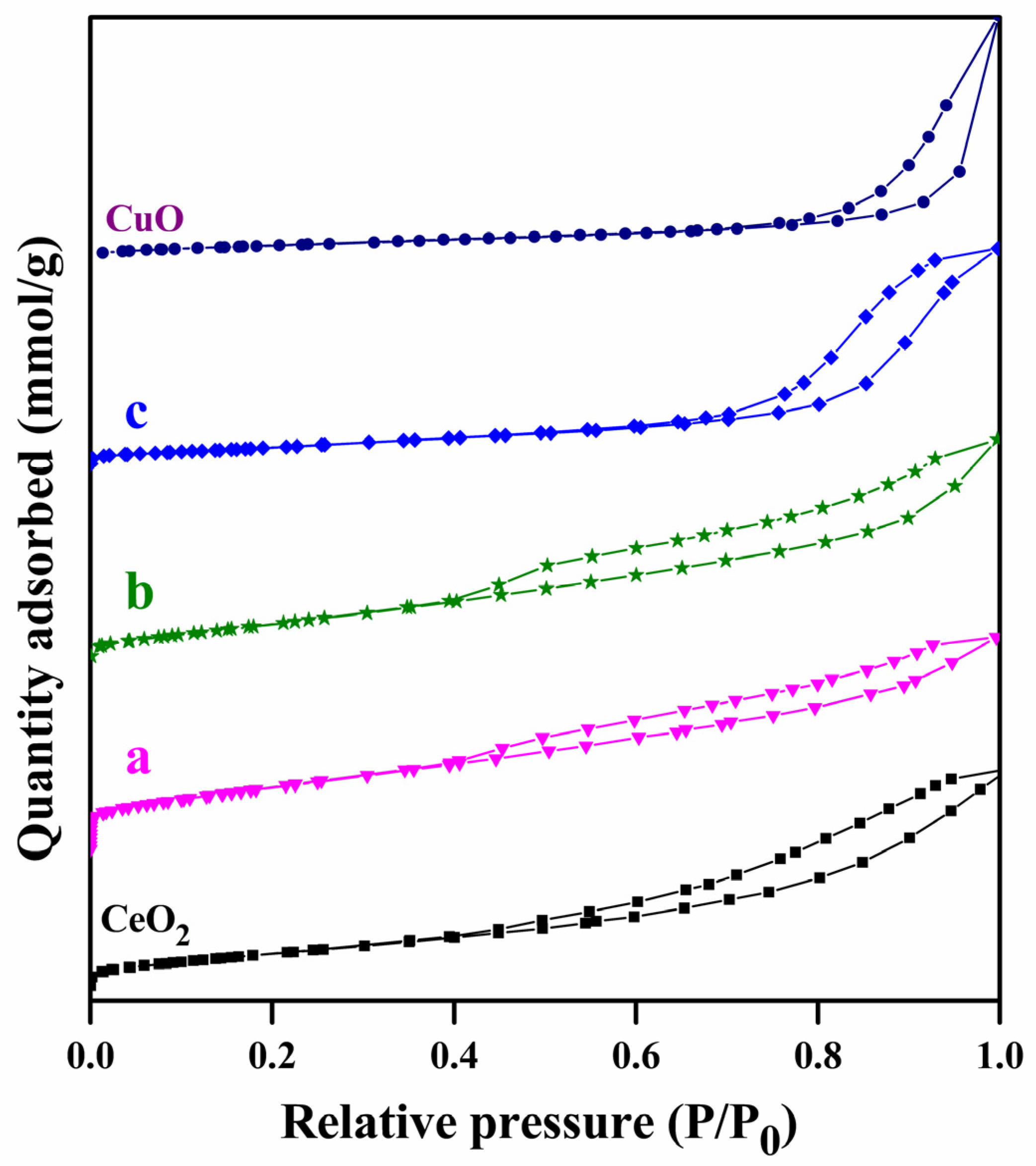
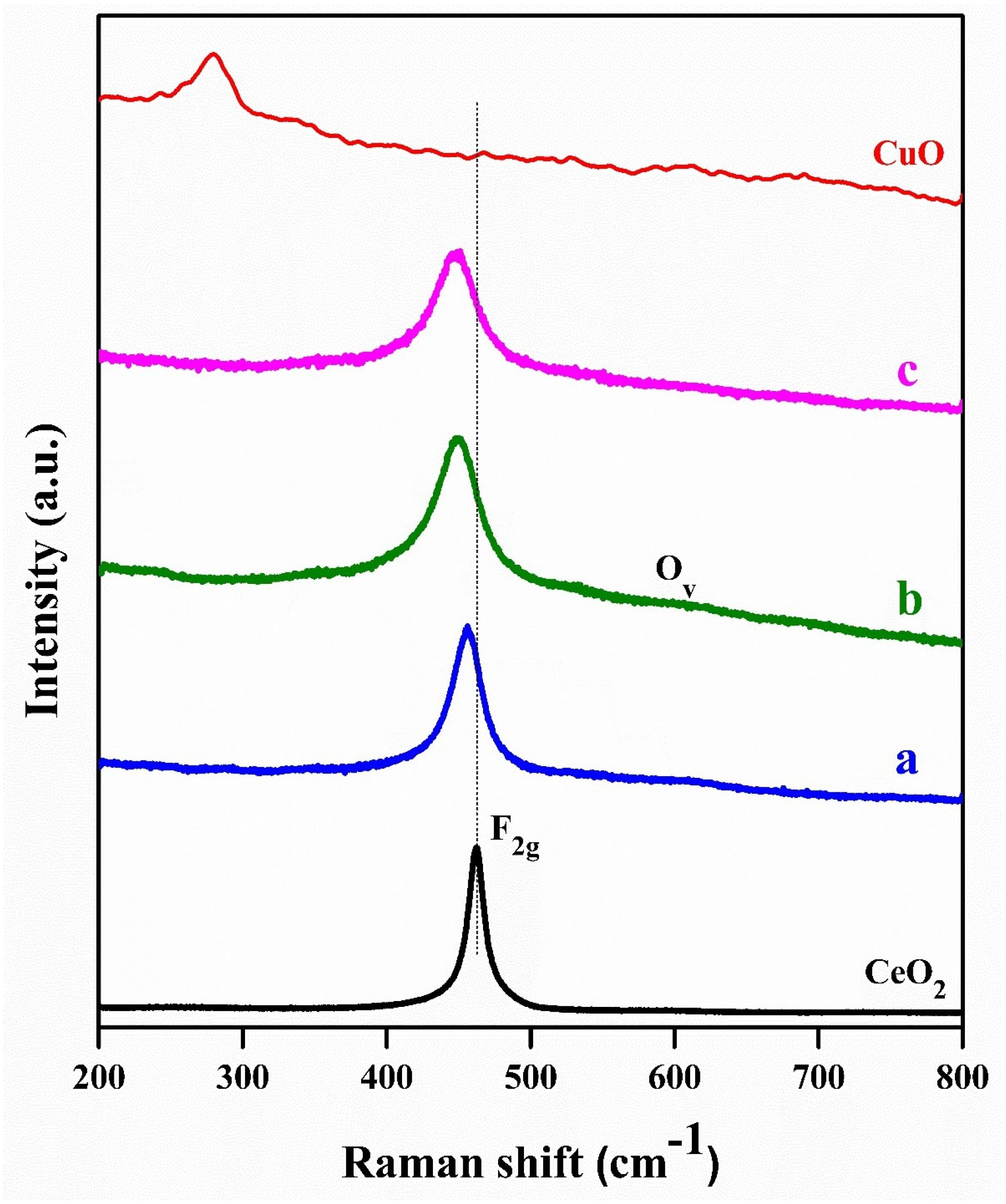
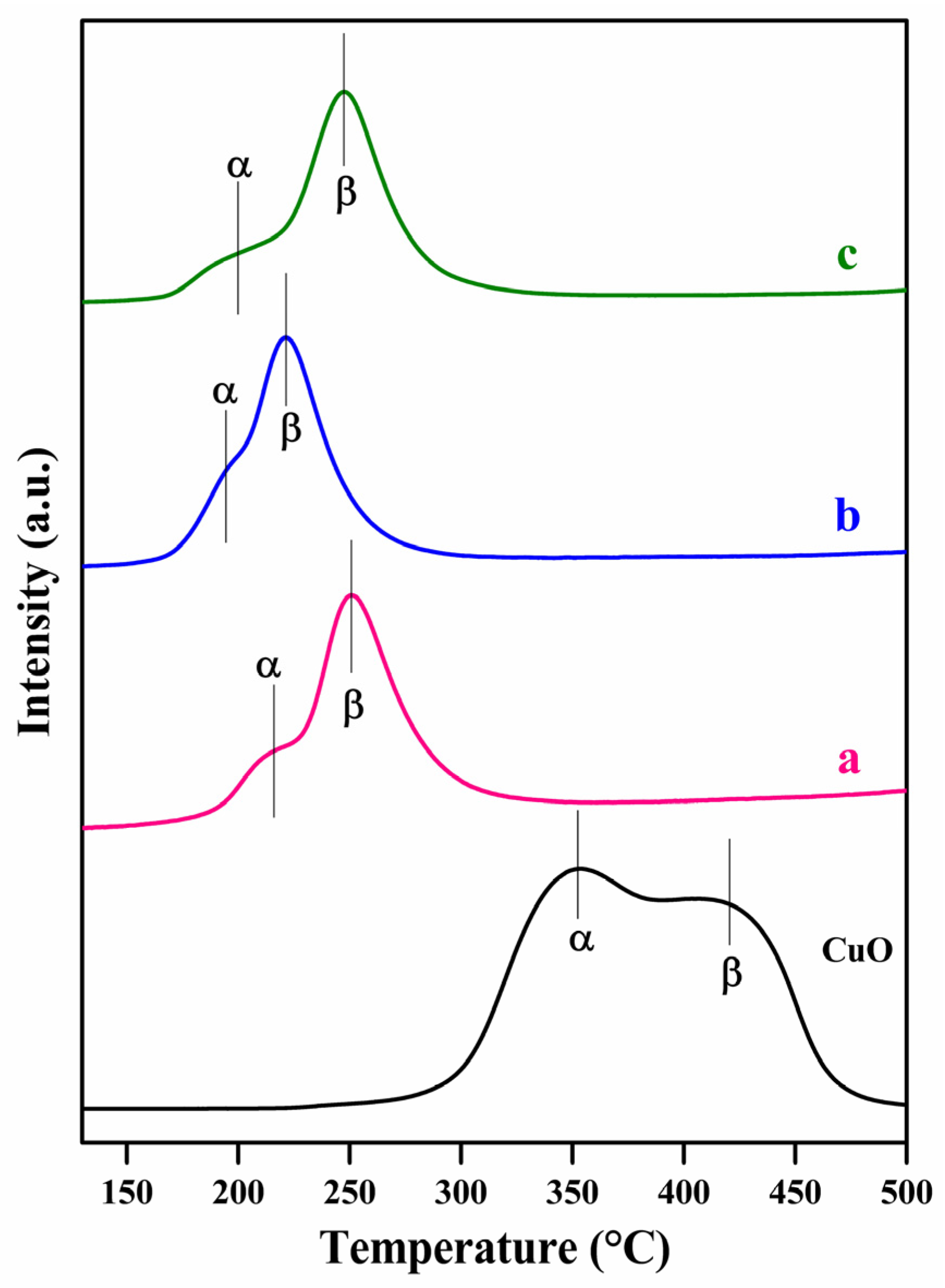
2.1. Characterization Studies
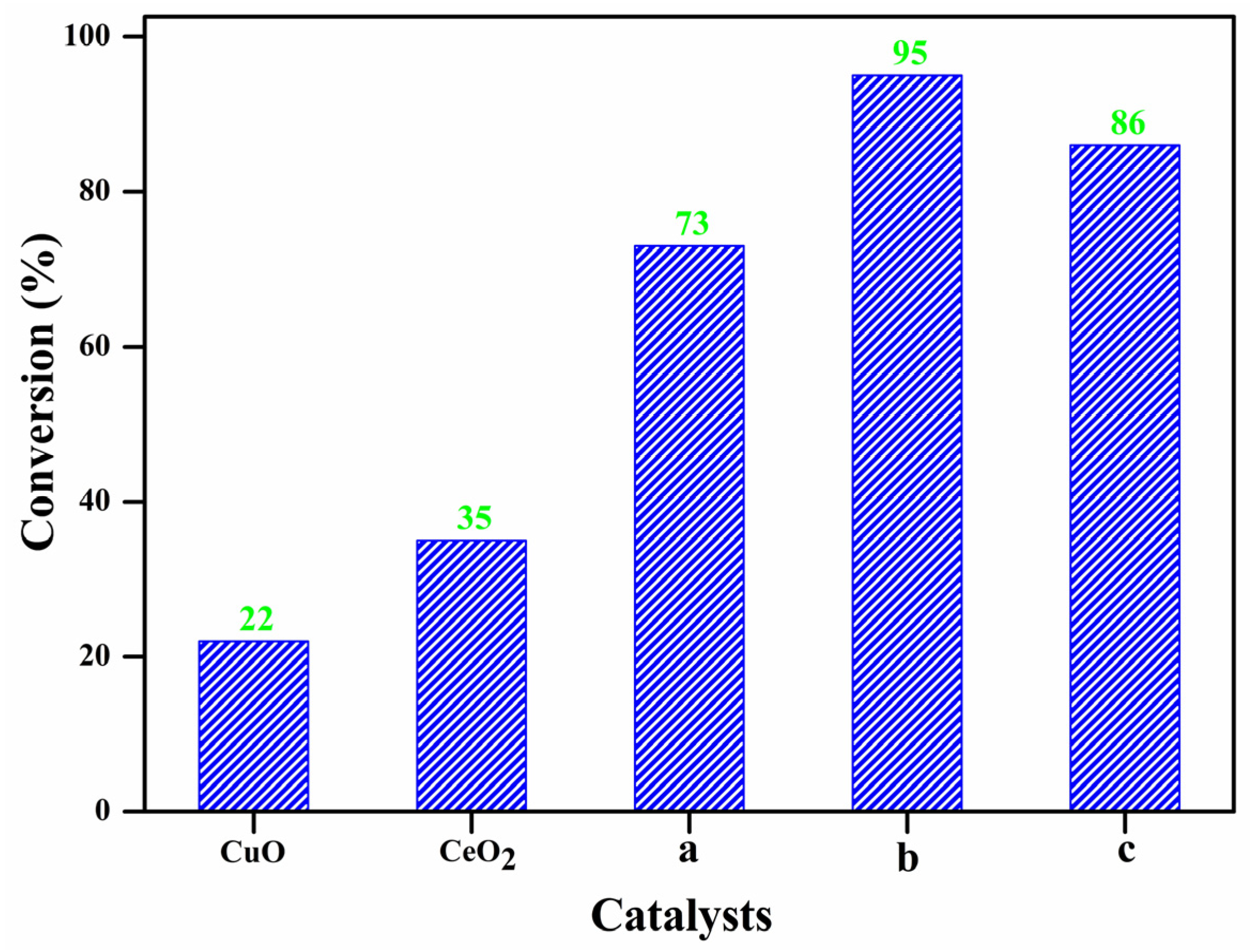
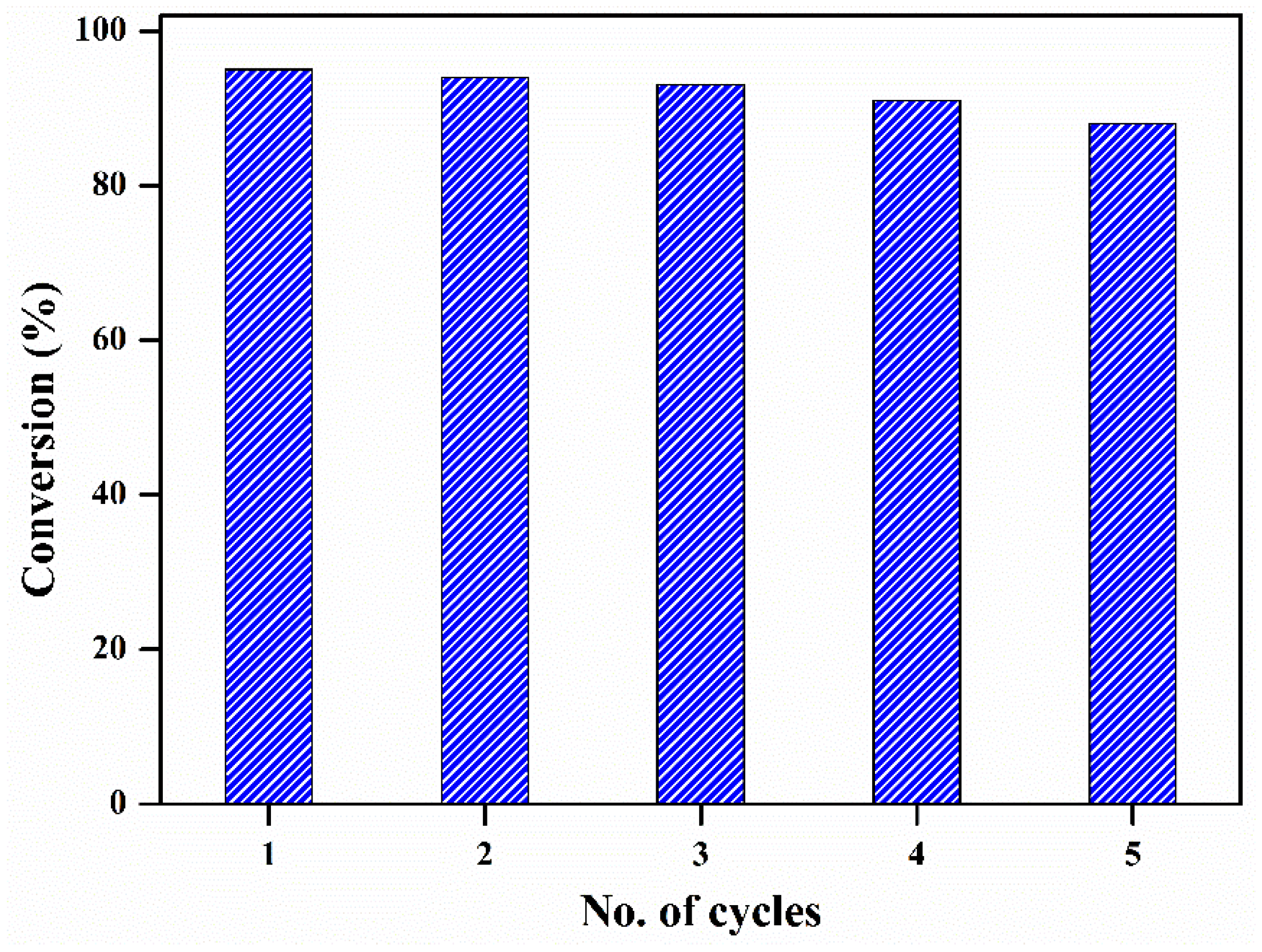
2.2. Catalytic Activity
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, M.; Pang, J.H.; Song, P.P.; Peng, J.J.; Xu, F.; Li, Q.; Zhang, X.M. Visible light-driven oxidation of vanillyl alcohol in air with Au–Pd bimetallic nanoparticles on phosphorylated hydrotalcite. New J. Chem. 2019, 43, 1964–1971. [Google Scholar] [CrossRef]
- Elamathi, P.; Kolli, M.K.; Chandrasekar, G. Catalytic oxidation of vanillyl alcohol using FeMCM-41 nanoporous tubular reactor. Int. J. Nanosci. 2018, 17, 1760010. [Google Scholar] [CrossRef]
- Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2008, 59, 299–326. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, S.C.; Saxena, N.; Chadda, N.; Singh, N.P.; Sinha, A.K. Microwave- and ultrasound-assisted extraction of vanillin and its quantification by high-performance liquid chromatography in vanilla planifolia. J. Sep. Sci. 2006, 29, 613–619. [Google Scholar] [CrossRef]
- Valdez-Flores, C.; Cañizares-Macías, M.P. On-line dilution and detection of vainillin in vanilla extracts obtained by ultrasound. Food Chem. 2007, 105, 1201–1208. [Google Scholar] [CrossRef]
- Jadhav, D.; Rekha, B.N.; Gogate, P.R.; Rathod, V.K. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J. Food Eng. 2009, 93, 421–426. [Google Scholar] [CrossRef]
- De Jong, E.D.; Van Berkel, W.J.; Van Der Zwan, R.P.; De Bont, J.A. Purification and characterization of vanillyl-alcohol oxidase from Penicillium simplicissimum: A novel aromatic alcohol oxidase containing covalently bound FAD. Eur. J. Biochem. 1992, 208, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, S.; Liu, B. Nitrogen-doped reduced graphene oxide-supported Mn3O4: An efficient heterogeneous catalyst for the oxidation of vanillyl alcohol to vanillin. J. Mater. Sci. 2016, 52, 164–172. [Google Scholar] [CrossRef]
- Saha, S.; Abd Hamid, S.B.; Ali, T.H. Catalytic evaluation on liquid phase oxidation of vanillyl alcohol using air and H2O2 over mesoporous Cu-Ti composite oxide. Appl. Surf. Sci. 2017, 394, 205–218. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Zheng, M.W.; Lai, H.K.; Lin, K.Y.A. Valorization of vanillyl alcohol by pigments: Prussian blue analogue as a highly-effective heterogeneous catalyst for aerobic oxidation of vanillyl alcohol to vanillin. Waste Biomass Valorization 2019, 10, 2933–2942. [Google Scholar] [CrossRef]
- Lahtinen, M.; Heinonen, P.; Oivanen, M.; Karhunen, P.; Kruus, K.; Sipila, J. On the factors affecting product distribution in laccase-catalyzed oxidation of a lignin model compound vanillyl alcohol: Experimental and computational evaluation. Org. Biomol. Chem. 2013, 11, 5454–5464. [Google Scholar] [CrossRef]
- Hu, J.; Hu, Y.; Mao, J.; Yao, J.; Chen, Z.; Li, H. A cobalt Schiff base with ionic substituents on the ligand as an efficient catalyst for the oxidation of 4-methyl guaiacol to vanillin. Green Chem. 2012, 14, 2894–2898. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Cao, X.; Zhao, J. Liquid-phase oxidation of 2-methoxy-p-cresol to vanillin with oxygen catalyzed by a combination of CoCl2 and N-hydroxyphthalimide. Res. Chem. Intermed. 2014, 40, 1303–1311. [Google Scholar] [CrossRef]
- Garade, A.C.; Biradar, N.S.; Joshi, S.M.; Kshirsagar, V.S.; Jha, R.K.; Rode, C.V. Liquid phase oxidation of p-vanillyl alcohol over synthetic Co-saponite catalyst. Appl. Clay Sci. 2011, 53, 157–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef]
- Martín-Hernández, M.; Carrera, J.; Suárez-Ojeda, M.E.; Besson, M.; Descorme, C. Catalytic wet air oxidation of a high strength p-nitrophenol wastewater over Ru and Pt catalysts: Influence of the reaction conditions on biodegradability enhancement. Appl. Catal. B Environ. 2012, 123–124, 141–150. [Google Scholar] [CrossRef]
- Shilpy, M.; Ali Ehsan, M.; Hussein Ali, T.; Abd Hamid, S.B.; Alia, M.E. Performance of cobalt titanate towards H2O2 based catalytic oxidation of lignin model compound. RSC Adv. 2015, 5, 79644–79653. [Google Scholar] [CrossRef]
- Jha, A.; Rode, C.V. Highly selective liquid-phase aerobic oxidation of vanillyl alcohol to vanillin on cobalt oxide (Co3O4) nanoparticles. New J. Chem. 2013, 37, 2669–2674. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by doped oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Abd Hamid, S.B. Nanosized spinel Cu–Mn mixed oxide catalyst prepared via solvent evaporation for liquid phase oxidation of vanillyl alcohol using air and H2O2. RSC Adv. 2016, 6, 96314–96326. [Google Scholar] [CrossRef]
- Jha, A.; Patil, K.R.; Rode, C.V. Mixed Co–Mn Oxide-Catalysed Selective Aerobic Oxidation of Vanillyl Alcohol to Vanillin in Base-Free Conditions. ChemPlusChem 2013, 78, 1384–1392. [Google Scholar] [CrossRef]
- Jha, A.; Mhamane, D.; Suryawanshi, A.; Joshi, S.M.; Shaikh, P.; Biradar, N.; Rode, C.V. Triple nano-composites of CoMn2O4, Co3O4 and reduced graphene oxide for oxidation of aromatic alcohols. Catal. Sci. Technol. 2014, 4, 1771–1778. [Google Scholar] [CrossRef]
- Lin, P.; Shang, R.; Zhang, Q.; Gu, B.; Tang, Q.; Jing, F.; Cao, Q.; Fang, W. One-pot synthesis of finely-dispersed Au nanoparticles on ZnO hexagonal sheets for base-free aerobic oxidation of vanillyl alcohol. Catal. Sci. Technol. 2022, 12, 4613–4623. [Google Scholar] [CrossRef]
- Jana, N.C.; Sethi, S.; Saha, R.; Bagh, B. Aerobic oxidation of vanillyl alcohol to vanillin catalyzed by air-stable and recyclable copper complex and TEMPO under base-free conditions. Green Chem. 2022, 24, 2542–2556. [Google Scholar] [CrossRef]
- Katta, L.; Sudarsanam, P.; Thrimurthulu, G.; Reddy, B.M. Doped nanosized ceria solid solutions for low temperature soot oxidation: Zirconium versus lanthanum promoters. Appl. Catal. B Environ. 2010, 101, 101–108. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, Y.; Zhang, Y.; Mei, X.; Wu, Q.; Zhao, Z.; Li, J. Facile synthesis of 3D ordered macro-mesoporous Ce1-xZrxO2 catalysts with enhanced catalytic activity for soot oxidation. Catal. Today 2020, 355, 587–595. [Google Scholar] [CrossRef]
- Palli, S.; Kamma, Y.; Silligandla, N.; Reddy, B.M.; Tumula, V.R. Aerobic oxidation of ethylbenzene to acetophenone over mesoporous ceria–cobalt mixed oxide catalyst. Res. Chem. Intermed. 2022, 48, 471–490. [Google Scholar] [CrossRef]
- Mandal, S.; Santra, C.; Bando, K.K.; James, O.O.; Maity, S.; Mehta, D.; Chowdhury, B. Aerobic oxidation of benzyl alcohol over mesoporous Mn-doped ceria supported Au nanoparticle catalyst. J. Mol. Catal. A: Chem. 2013, 378, 47–56. [Google Scholar] [CrossRef]
- Rao, B.G.; Sudarsanam, P.; Rangaswamy, A.; Reddy, B.M. Highly efficient CeO2–MoO3/SiO2 catalyst for solvent-free oxidative coupling of benzylamines into N-benzylbenzaldimines with O2 as the oxidant. Catal. Lett. 2015, 145, 1436–1445. [Google Scholar] [CrossRef]
- Rao, B.G.; Sudarsanam, P.; Nallappareddy, P.R.G.; Reddy, M.Y.; Rao, T.V.; Reddy, B.M. Selective allylic oxidation of cyclohexene catalyzed by nanostructured Ce-Sm-Si materials. Catal. Commun. 2017, 101, 57–61. [Google Scholar] [CrossRef]
- Reddy, P.N.; Rao, B.G.; Rao, T.V.; Reddy, B.M. Mesoporous Ce–Zr mixed oxides for selective oxidation of styrene in liquid phase. Appl. Petrochem. Res. 2020, 10, 67–76. [Google Scholar] [CrossRef]
- Luo, J.Y.; Meng, M.; Yao, J.S.; Li, X.G.; Zha, Y.Q.; Wang, X.; Zhang, T.Y. One-step synthesis of nanostructured Pd-doped mixed oxides MOx-CeO2 (M = Mn, Fe, Co, Ni, Cu) for efficient CO and C3H8 total oxidation. Appl. Catal. B Environ. 2009, 87, 92–103. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Zhou, M.; Huang, Z.; Gao, J.; Ma, Z.; Tang, X. Enhanced performance of ceria-based NOx reduction catalysts by optimal support effect. Environ. Sci. Technol. 2017, 51, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, D.; Zhang, L.; Huang, L.; Li, H.; Gao, R.; Zhang, J. Comparative study of 3D ordered macroporous Ce0.75Zr0.2M0.05O2−δ (M = Fe, Cu, Mn, Co) for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2014, 4, 93–101. [Google Scholar] [CrossRef]
- Su, Y.; Tang, Z.; Han, W.; Song, Y.; Lu, G. Enhanced catalytic performance of three-dimensional ordered mesoporous transition metal (Co, Cu, Fe)-doped CeO2 catalysts for CO catalytic oxidation. Catal. Surv. Asia 2015, 19, 68–77. [Google Scholar] [CrossRef]
- Ramana, S.; Rao, B.G.; Swamy, P.V.; Rangaswamy, A.; Reddy, B.M. Nanostructured Mn-doped ceria solid solutions for efficient oxidation of vanillyl alcohol. J. Mol. Catal. A Chem. 2016, 415, 113–121. [Google Scholar] [CrossRef]
- Reddy, P.N.; Rao, B.G.; Rao, T.V.; Reddy, B.M. Selective aerobic oxidation of vanillyl alcohol to vanillin catalysed by nanostructured Ce-Zr-O solid solutions. Catal. Lett. 2019, 149, 533–543. [Google Scholar] [CrossRef]
- Palli, S.; Yogendra, K.; Silligandla, N.; Reddy, B.M.; Tumula, V.R. Oxidation of vanillyl alcohol to vanillin over nanostructured cerium–iron mixed oxide catalyst with molecular oxygen. Res. Chem. Intermed. 2022, 48, 4579–4599. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, Q.; Jiang, Z.; Chen, L.; Wu, R. Catalytic combustion of dilute acetone over Cu-doped ceria catalysts. J. Chem. Eng. 2009, 152, 583–590. [Google Scholar] [CrossRef]
- Aranda, A.; Aylon, E.; Solsona, B.; Murillo, R.; Mastral, A.M.; Sellick, D.R.; Taylor, S.H. High activity mesoporous copper doped cerium oxide catalysts for the total oxidation of polyaromatic hydrocarbon pollutants. Chem. Commun. 2012, 48, 4704–4706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Zhao, J. Transition-metal doped ceria microspheres with nanoporous structures for CO oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, Y.; Gao, Y.; Liu, W.; Wang, Z.; Cui, C.; Wang, L. Catalytic oxidation of CO on mesoporous codoped ceria catalysts: Insights into correlation of physicochemical property and catalytic activity. J. Rare Earth. 2019, 37, 961–969. [Google Scholar] [CrossRef]
- Challa, P.; Enumula, S.S.; Kondeboina, M.; Burri, D.R.; Rao, K.S.R. Catalytic conversion of benzyl alcohol to benzaldehyde over a Cu-CeO2 catalyst: Utilization of CO2 for enhancement of selectivity and activity. Ind. Eng. Chem. Res. 2020, 59, 17720–17728. [Google Scholar] [CrossRef]
- Mužina, K.; Kurajica, S.; Dražić, G.; Guggenberger, P.; Matijašić, G. True doping levels in hydrothermally derived copper-doped ceria. J. Nanopart. Res. 2021, 23, 149. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, G.; Jang, M.G.; Noh, K.J.; Han, J.W. Rational design of transition metal Co-doped ceria catalysts for low-temperature CO oxidation. ChemCatChem 2019, 11, 2288–2296. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Wang, Z.; Liu, W.; Wang, L. Catalytic oxidation of CO over mesoporous copper-doped ceria catalysts via a facile CTAB-assisted synthesis. RSC Adv. 2018, 8, 14888–14897. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Liu, W.; Wang, S.; Xie, A.; Liu, X.; Yang, Y. Facile preparation of Mn+-doped (M = Cu, Co, Ni, Mn) hierarchically mesoporous CeO2 nanoparticles with enhanced catalytic activity for CO oxidation. Euro. J. Inorg. Chem. 2015, 6, 969–976. [Google Scholar] [CrossRef]
- Curran, C.D.; Lu, L.; Kiely, C.J.; McIntosh, S. Ambient temperature aqueous synthesis of ultra-small copper doped ceria nanocrystals for the water gas shift and carbon monoxide oxidation reactions. J. Mater. Chem. A 2018, 6, 244–255. [Google Scholar] [CrossRef]
- Li, L.; Han, W.; Tang, Z.; Zhang, J.; Lu, G. Hard-template synthesis of three-dimensional mesoporous Cu–Ce based catalysts with tunable architectures and their application in the CO catalytic oxidation. RSC Adv. 2016, 6, 64247–64257. [Google Scholar] [CrossRef]
- Jampa, S.; Wangkawee, K.; Tantisriyanurak, S.; Changpradit, J.; Jamieson, A.M.; Chaisuwan, T.; Wongkasemjit, S. Sujitra Wongkasemjit, High performance and stability of copper loading on mesoporous ceria catalyst for preferential oxidation of CO in presence of excess of hydrogen. Int. J. Hydrogen Energy 2017, 42, 5537–5548. [Google Scholar] [CrossRef]
- Yang, F.; Wei, J.; Liu, W.; Guo, J.; Yang, Y. Copper doped ceria nanospheres: Surface defects promoted catalytic activity and a versatile approach. J. Mater. Chem. A 2014, 2, 5662–5667. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Kuo, C.H.; Biswas, S.; Kingondu, C.K.; Suib, S.L. A general approach to crystalline and monomodal pore size mesoporous materials. Nat. Commun. 2013, 4, 2952. [Google Scholar] [CrossRef] [PubMed]

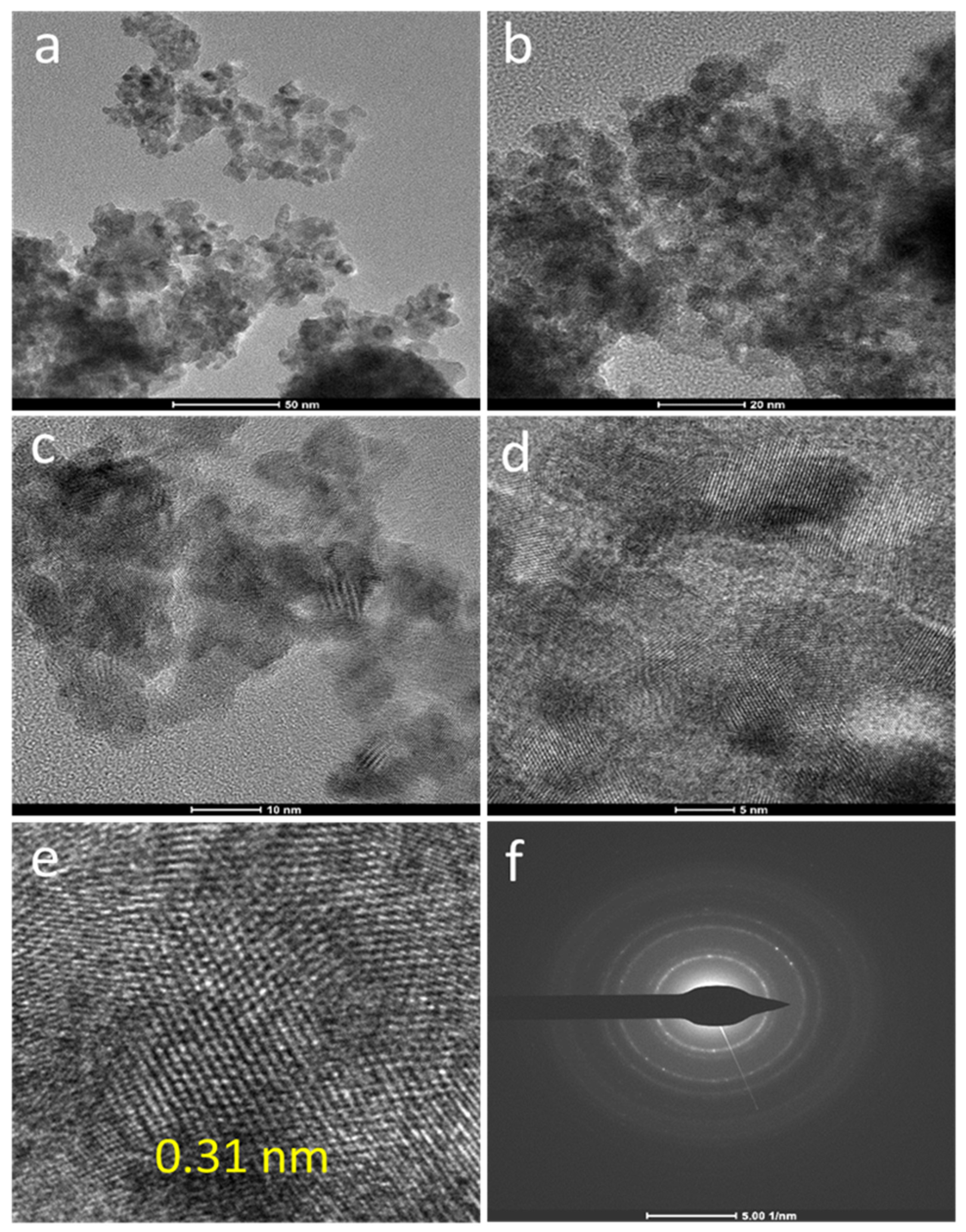
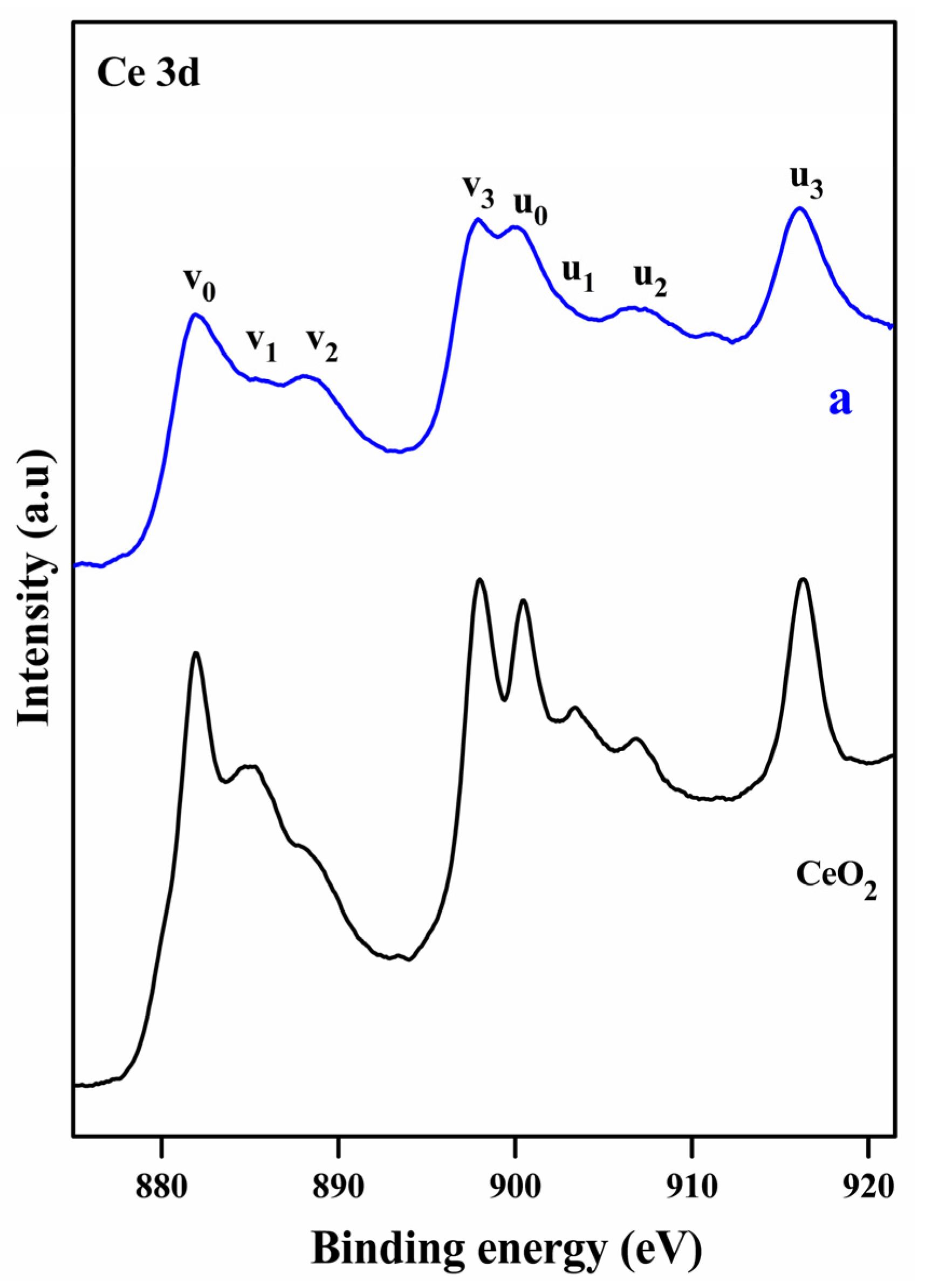
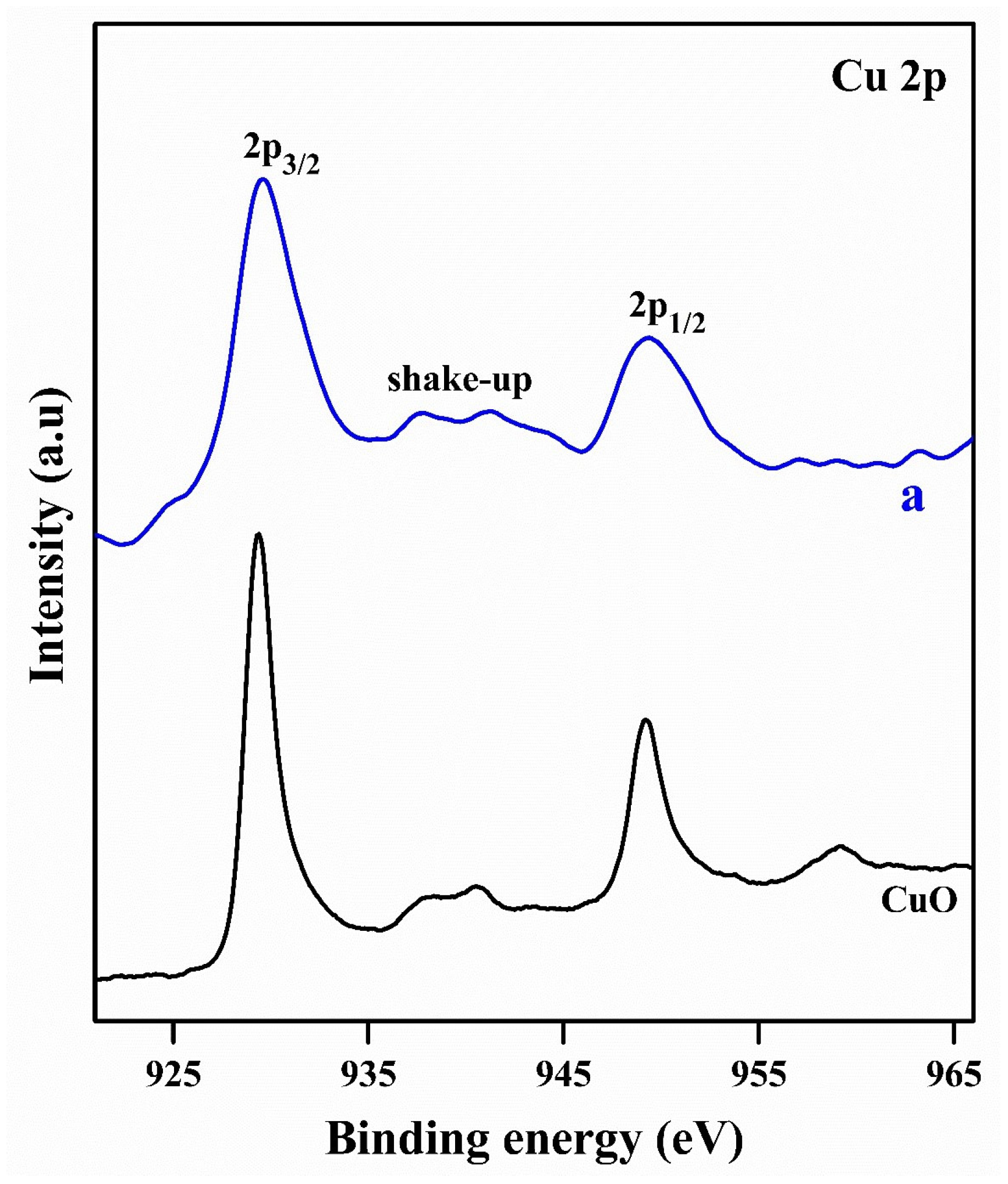
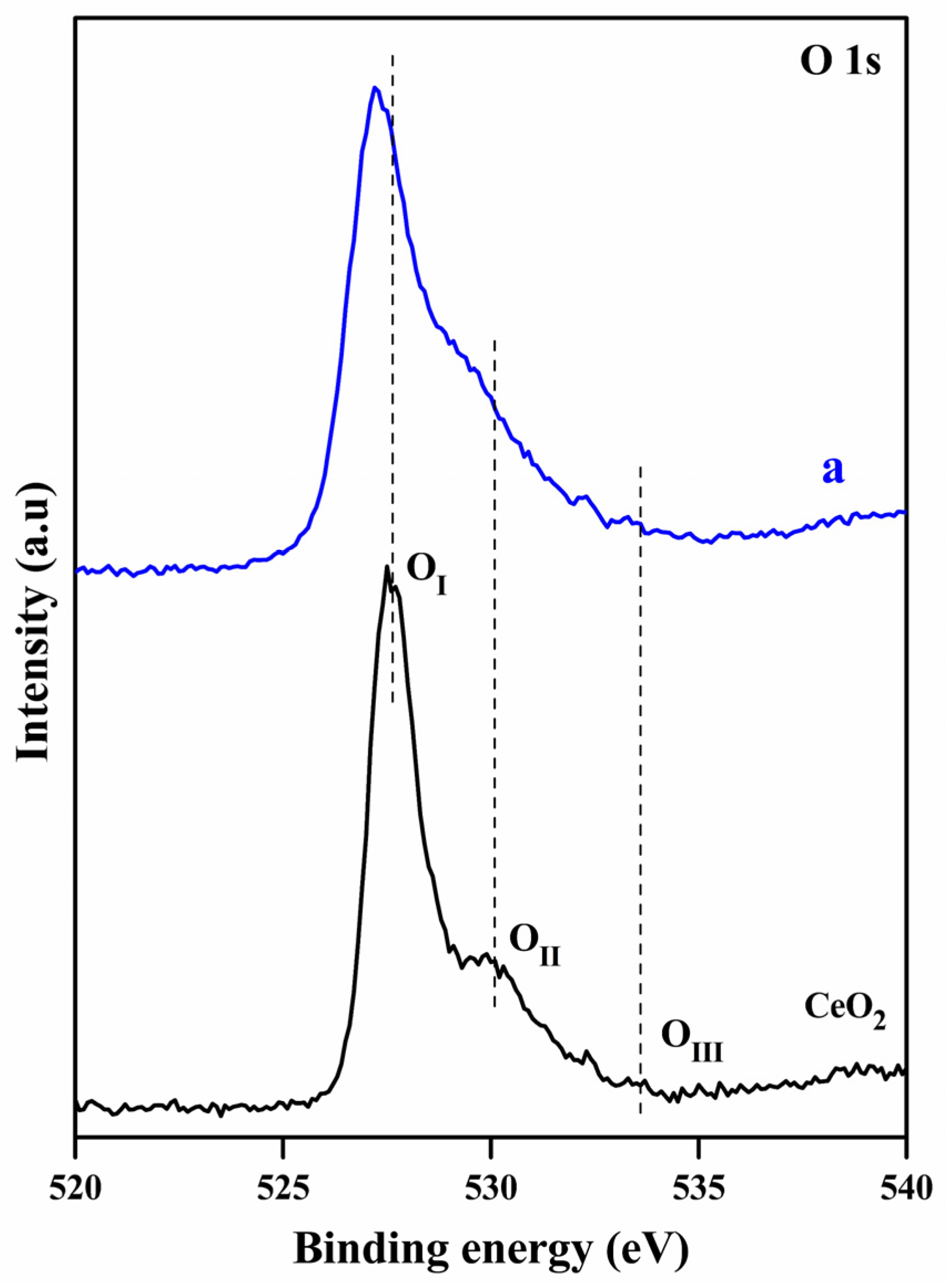

| Entry | Sample | S (m2g−1) a | D (nm) b |
|---|---|---|---|
| 1 | CeO2 | 51 | 9.74 |
| 2 | CuO | 14 | 12.9 |
| 3 | Cu0.05Ce0.95O2−δ | 75 | 9.64 |
| 4 | Cu0.1Ce0.9O2−δ | 81 | 6.35 |
| 5 | Cu0.15Ce0.85O2−δ | 69 | 7.85 |
| Entry | Sample | Nominal Values | ICP-OES Analysis Values | ||
|---|---|---|---|---|---|
| Ce | Cu | Ce | Cu | ||
| 1 | Cu0.05Ce0.95O2−δ | 0.95 | 0.05 | 0.93 | 0.07 |
| 2 | Cu0.1Ce0.9O2−δ | 0.90 | 0.10 | 0.87 | 0.13 |
| 3 | Cu0.15Ce0.85O2−δ | 0.85 | 0.15 | 0.84 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazeer, S.; Sitaramulu, P.; Yogendra, K.; Kumar, P.M.; Reddy, B.M.; Venkateshwar Rao, T. Mesoporous Copper-Cerium Mixed Oxide Catalysts for Aerobic Oxidation of Vanillyl Alcohol. Catalysts 2023, 13, 1058. https://doi.org/10.3390/catal13071058
Nazeer S, Sitaramulu P, Yogendra K, Kumar PM, Reddy BM, Venkateshwar Rao T. Mesoporous Copper-Cerium Mixed Oxide Catalysts for Aerobic Oxidation of Vanillyl Alcohol. Catalysts. 2023; 13(7):1058. https://doi.org/10.3390/catal13071058
Chicago/Turabian StyleNazeer, Silligandla, Palli Sitaramulu, Kamma Yogendra, Palnati Manoj Kumar, Benjaram M. Reddy, and Tumula Venkateshwar Rao. 2023. "Mesoporous Copper-Cerium Mixed Oxide Catalysts for Aerobic Oxidation of Vanillyl Alcohol" Catalysts 13, no. 7: 1058. https://doi.org/10.3390/catal13071058
APA StyleNazeer, S., Sitaramulu, P., Yogendra, K., Kumar, P. M., Reddy, B. M., & Venkateshwar Rao, T. (2023). Mesoporous Copper-Cerium Mixed Oxide Catalysts for Aerobic Oxidation of Vanillyl Alcohol. Catalysts, 13(7), 1058. https://doi.org/10.3390/catal13071058