Abstract
Heterogeneous catalysts have been widely used for peroxymonosulfate (PMS) activation to remove persistent contaminants in water. This study successfully prepared cobalt-doped TiO2 using a simple two-step approach for activating PMS to remove tetracycline (TC). The batch experiments showed complete TC degradation within 25 min caused by Co-TiO2 (0.1 g/L) activation of PMS (1 mM) under visible light. The system also demonstrated excellent catalytic efficiency in various water environments, such as artificial seawater, tap water, and wastewater. According to the radical capture tests and electron spin resonance analysis, the contribution of active species involved in the degradation of TC with the Vis/Co-TiO2/PMS system were in the following order: 1O2> SO4•−> O2•−> •OH. The possible TC degradation pathway was proposed using intermediate identification and Fukui function calculation. This study provides a promising method toward organic pollutants degradation and provides a novel perspective on the rational design of competent and stable catalysts.
1. Introduction
Over the last few decades, the concentration of antibiotics in surface water has risen above the standard due to the rapid growth of the pharmaceutical industry and agriculture [1]. Tetracycline (TC), one of the most widely used broad-spectrum antibiotics in the world, is commonly used in medicines, feed additives, and antibacterial agents [2,3]. However, the excessive use of TC poses a potential threat to ecosystems and human health [4]. The excessive use of TC causes resistance in microorganisms and also affects the immunity of animals [5]. Due to its stable structure and antibacterial activity, it is difficult to remove TC using conventional techniques such as physical adsorption, biodegradation, and chemical reactions [6,7]. Therefore, there is an urgent need to develop efficient, cost-effective, and environmentally sustainable technologies for removing TC.
In recent years, advanced oxidation processes (AOP) based on sulfate radical (SO4•−) have received significant attention in treating organic contaminants in wastewater. Peroxymonosulfate (PMS), as a stable and non-toxic oxidant, is usually activated to form SO4•− and hydroxyl radical (·OH) [8,9]. Compared with ·OH, SO4•− possesses a stronger oxidation potential, broader pH range, and longer half-life [10]. The peroxide O-O bond of PMS could be activated via thermal treatment, ultraviolet irradiation, electrochemistry, and metal or metal-free catalysts [11,12]. However, the practical applications of the above methods were reduced due to their excessive energy consumption and low efficiency [13]. Among these, transition metal ions (Mn, Ce, Ni, Co, Fe, etc.) or metallic oxides, especially Co2+ ions, were widely used as an efficient catalysts for PMS activation due to their high activation efficiency and low energy consumption [14,15]. However, the leaching process releases Co2+ ions, thus leading to secondary contamination and severely limiting the application of AOPs [16,17].
Multiple efforts have been made to prepare heterogeneous catalysts to activate PMS [18,19]. These include highly active nanocatalysts and cobalt nano-oxides/hydroxides, such as CoO, Co2O3, CoO (OH), and Co3O4 [20,21]. Unlike homogeneous Co(II) ions, heterogeneous cobalt nanocatalysts have the advantages of low released ion concentration, excellent separability, and high reusability [22]. However, their catalytic capacity can be considerably reduced due to the tendency of cobalt nanoparticles to aggregate in aqueous environments [23]. To overcome this shortcoming, different porous substrates (such as carbon materials, Al2O3, and TiO2) are used as carriers of cobalt nanoparticles, which can effectively improve the catalytic performance [24,25,26,27]. Compared with conventional porous support, TiO2 has been widely investigated for environmental applications due to its advantages of excellent chemical stability, non-toxicity, simple preparation, and low material cost [28,29,30]. Based on these advantages, these materials were tried as carriers of cobalt nanoparticles, leading to the fabrication of various catalysts with excellent PMS activation [31,32,33]. However, multiple steps and complex reagents are involved in synthesizing these catalysts, thus hindering their practical application.
Herein, the preparation of novel Co(II)-doped TiO2 (Co-TiO2) materials via a facile two-step methodology have been reported. The as-prepared Co-TiO2 catalyst was utilized for PMS activation to remove TC under visible light. The effect of the catalyst concentration, PMS doses, the initial solution pH, and inorganic anionic on TC removal in the Vis/Co-TiO2/PMS system was explored. Additionally, the efficiency of Co-TiO2 in different TC-contaminated water, such as artificial seawater, tap water, and wastewater, was examined to determine the potential of this material. The stability of the Co-TiO2 and the contribution of reactive oxygen species (ROS) to the reaction were also investigated. Further, the efficiency of the Vis/Co-TiO2/PMS system in the removal of other contaminants such as methyl blue, metronidazole, and rhodamine B was investigated. Finally, the degeneration mechanism of the TC removal process in the optimal system was determined by identifying the intermediates and performing Fukui function calculations. This study reports a simple method to design green and efficient catalysts for PMS activation to remove refractory organic contaminants.
2. Results and Discussion
2.1. Characterization
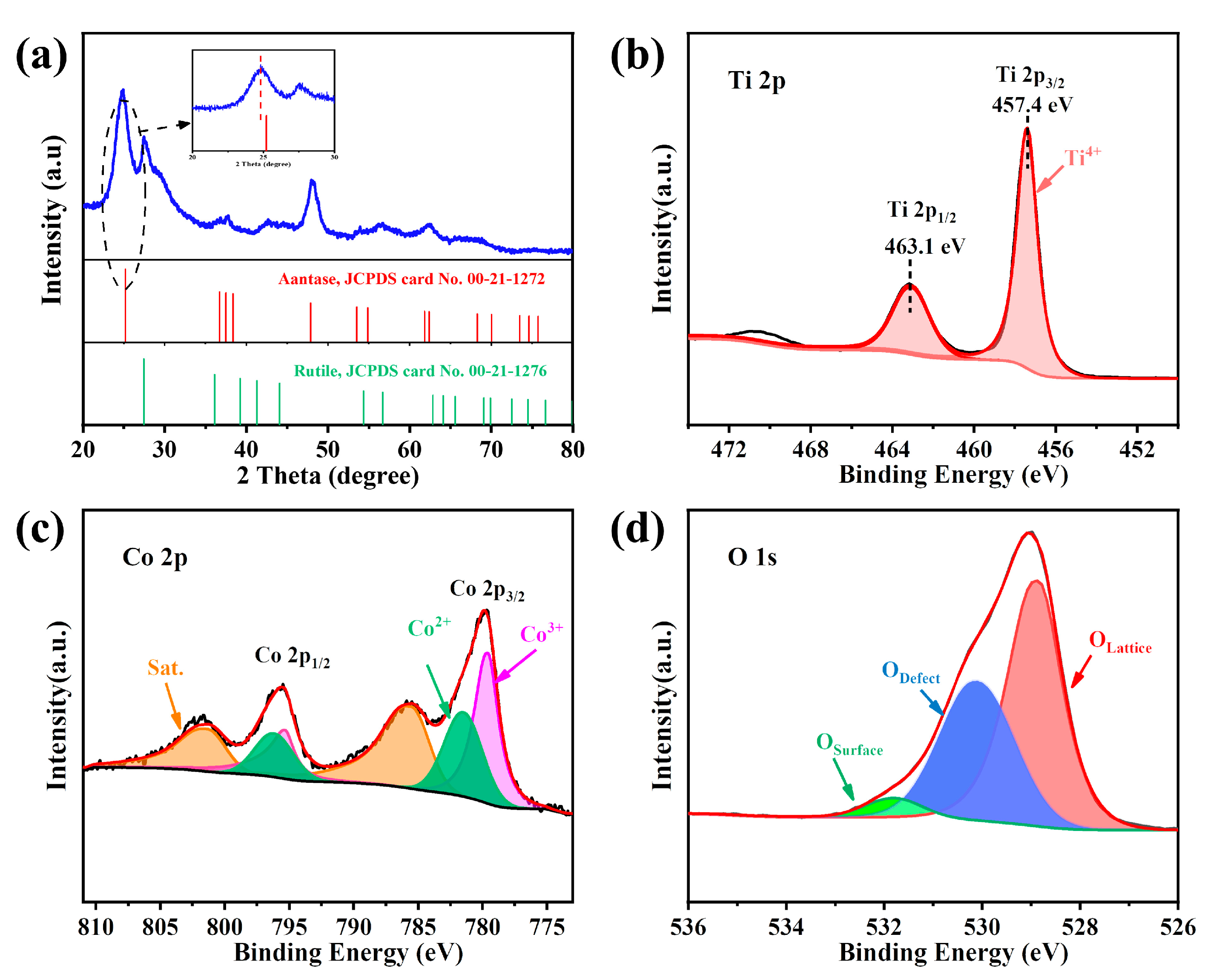
In the XRD analysis results (Figure 1a), some diffraction peaks at 2θ at 25.21°, 37.53°, 48.87°, 53.53°, 54.86°, 62.36°, 70.02°, and 74.62° were assigned to (101), (004), (200), (105), (211), (204), (220), and (215) crystal facets of anatase (JCPDS No. 00-21-1272), respectively [34]. In addition to the anatase peak, Co-TiO2 exhibited rutile peaks of 2θ at 27.5° (110 plane), 36.1° (101 plane), 41.3° (111 plane), and 56.7° (220 plane) (JCPDS No. 00-21-1276) [25]. No characteristic peaks of cobalt oxides and/or hydroxides were found in the XRD data, indicating that the Co(II) ions were implanted into the TiO2 lattice [35,36]. Additionally, the Figure 1a inset suggests a slight shift to a lower angle at 2θ = 25.21° for the diffraction peak relative to the standard card (JCPDS card No. 00-21-1272). This phenomenon can be explained by the smaller size of Ti4+ (0.745 Å) being replaced by the larger size of Co2+ (0.792 Å), which increased the d value of Co-TiO2. [37].
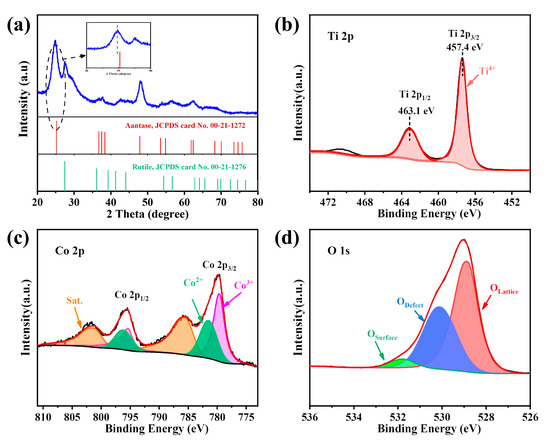
Figure 1.
(a) XRD spectra of Co-TiO2; (b) Ti 2p spectra, (c) Co 2p spectra, and (d) O 1s spectra of Co-TiO2.
The XPS survey spectra revealed the strong diffraction peaks of Ti, Co, O, and C (Figure S1). The carbon peaks might be attributed to contamination during the powder sample being transferred to the UHV chamber [38]. The element survey revealed oxygen to be the most abundant element in the Co-TiO2 catalyst and confirmed the incorporation of Co (Table S1). The ratio of Ti/Co/O elements measured using XPS was consistent with the results of the SEM mapping (Table S1). In the Ti 2p spectrum, two distinct peaks were observed around 457.4 and 463.1 eV (Figure 1b), corresponding to Ti 2p1/2 and Ti 2p3/2 of the Ti4+ in Co-TiO2. Moreover, the distance between the two peaks was 5.7 eV, indicating that Ti existed in the +4 oxidation state in Co-TiO2 [39]. The shoulder peak appearing in the Ti 2p spectrum near 471 eV was the satellite peak of Ti 2p. The Co2p core level spectrum of Co-TiO2 revealed that Co mainly exists in the form of Co(II) and Co(III) (see Figure 1c). Four broad peaks were observed. The 796.2 eV and 781.5 eV peaks were ascribed to Co2+ 2p3/2 and Co2+ 2p1/2, while 779.6 eV and 795.3 eV were attributed to Co3+ 2p1/2 and Co3+ 2p3/2, respectively [40]. The O 1s spectrum of Co-TiO2 exhibited three peaks at 528.9 eV, 530.1 eV, and 531.8 eV (Figure 1d), which corresponded to the lattice oxygen (such as Ti-O and Co-O), oxygen defects, and surface-adsorbed oxygen, respectively.
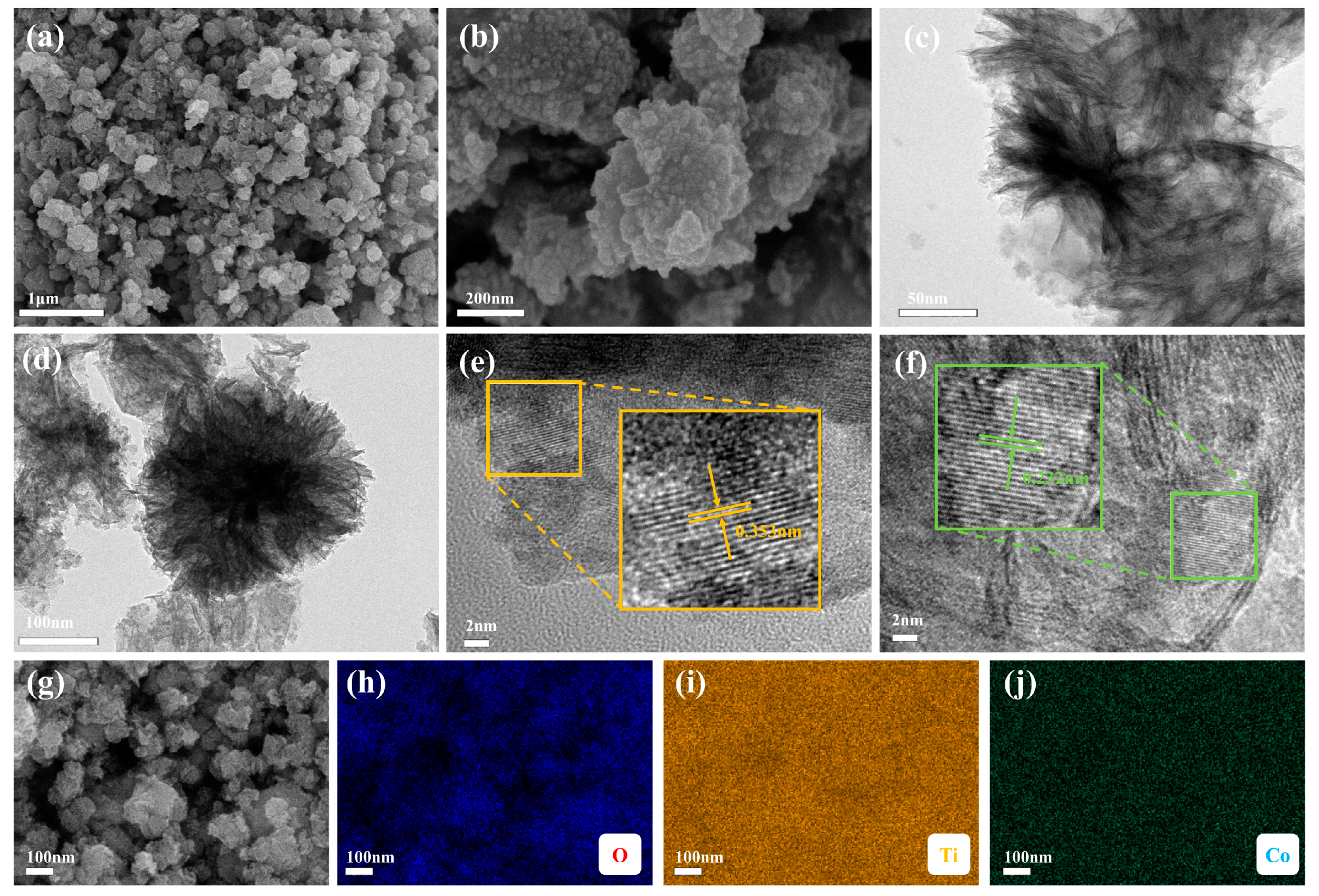
The SEM of Co-TiO2 (Figure 2a,b) revealed a nano-spherical structure. The average size of a single Co-TiO2 nanoparticle was about 20–30 nm. The TEM imaging (Figure 2c,d) further verified the nanosphere structure of Co-TiO2. The surface area and the pore characteristics of the as-prepared catalysts were shown in Table S2. The Co-TiO2 catalysts have higher specific surface areas compared to pure TiO2. Moreover, the catalyst exhibited typical IV-type isotherms (Figure S2), which suggested that the catalyst had mesoporous characteristics [41]. The greater surface area and pore volume of the catalyst might provide more active sites available for PMS activation, and, at last, accelerated the contaminant removal. During the activation of PMS, the HRTEM imaging (Figure 2e,f) revealed the lattice spacing of 0.35 nm, which was consistent with the (101) plane of anatase TiO2, while the lattice spacing of 0.23 nm was consistent with the (200) plane of rutile TiO2, which was consistent with the XRD analysis results. Further, the SEM mapping results (Figure 2g–j) demonstrated that the distribution of the Co, O, and Ti elements on the Co-TiO2 catalyst was uniform.
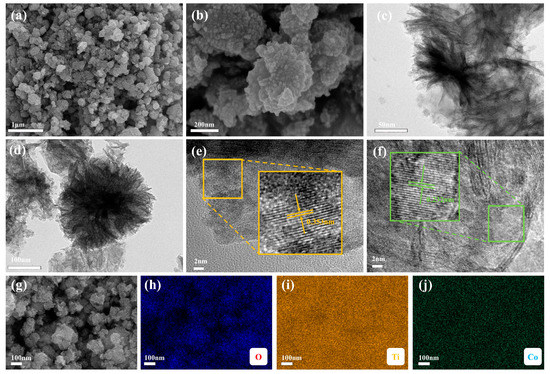
Figure 2.
SEM image (a,b) and TEM image (c,d) of Co-TiO2; (e,f) HRTEM image of Co-TiO2; and (g–j) SEM mapping image of Co-TiO2.
2.2. Catalytic Degradation Performance and Kinetics Study
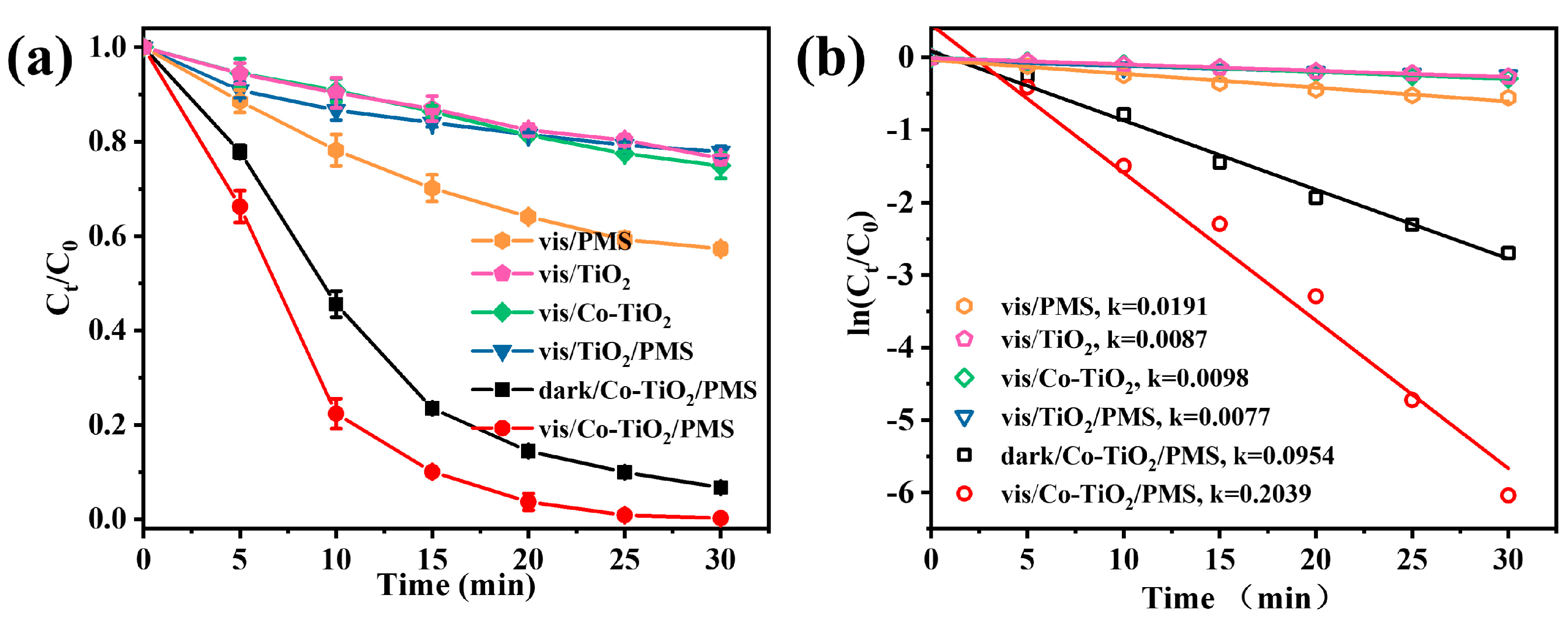
Batch control tests were performed to compare the degradation performance in different reaction systems (Figure 3a). The single catalysts achieved minor TC decomposition efficiency within 30 min, including the Vis/TiO2 (23.5%) and Vis/Co-TiO2 (25.1%). After the addition of PMS, the Vis/PMS degradation efficiency reached 42.6%. The decomposition of PMS might be accelerated under light, and formed more additional free radicals (Equation (1)) for pollutant removal [42]. In addition, the removal of TC by the Vis/TiO2/PMS system was insignificant, and only about 22.2% of the TC was removed in this system. This result suggested that the photogenerated electrons from the pure TiO2 could not promote PMS decomposition [43]. Once pure TiO2 is irradiated with visible light, the PMS molecules can only be slowly activated to produce active radicals, which severely affects the degradation rate. Surprisingly, the Vis/Co-TiO2/PMS system was able to degrade TC almost completely within 25 min. The degradation rate of TC caused by the dark/Co-TiO2/PMS system was about 93.3% within 30 min. This may be due to the rapid activation of PMS (as an electron acceptor) by Co-TiO2 with the assistance of visible light, thus resulting in the generation of more active substances, such as •OH and SO4•−, and the realization of the elevated degradation efficiency [44]. It should be noted that the combined application of pure TiO2 and PMS under visible light did not result in rapid TC degradation. Therefore, the efficient degradation of TC could be attributed to catalysis caused by Co. The photogenerated electrons generated on TiO2 promote the synergistic effect between Co and PMS. Briefly, the photogenerated electrons can reduce the Co3+ to Co2+, and the formation of Co2+ have a positive effect on PMS activation. In addition, Figure 3b showed that the degradation process in the Vis/Co-TiO2/PMS system was fitted using the pseudo first-order kinetic equation. The k value in the Vis/Co-TiO2/PMS system was 0.2039 min−1, which was 26.5 and 10.7 times than that of Vis/TiO2/PMS (k = 0.0077 min−1) and Vis/PMS (k = 0.0191 min−1) systems, respectively. The increased performances were attributed to the mutual coupling of Co-TiO2 and PMS under visible light, promoting the charge migration via a synergistic effect, and prolonging the service life of the charge carriers.
HSO5− + hv→ SO42− + •OH
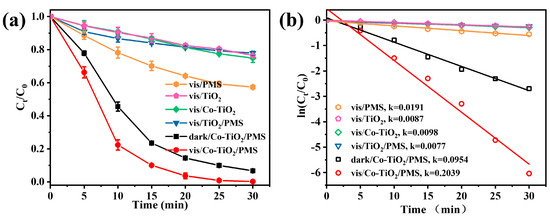
Figure 3.
(a) TC degradation in different systems and (b) the kinetic rate constant kobs value under different conditions (Conditions: catalyst concentration = 0.1 g/L, PMS doses = 0.5 mM, and pH = 4.8).
The adsorption of TiO2 and Co-TiO2 on TC was investigated under dark conditions to better understand the effect of photoelectrons on the systems (Figure S3a). The preliminary results showed that the removal efficiency of TC in dark/TiO2 and dark/Co-TiO2 systems were 11.8% and 19.9%, respectively, within 30 min. This result indicated that pure TiO2 has only a small part of TC adsorption. After doping metal Co, the adsorption performance of TiO2 was slightly improved due to the enhanced surface area of Co-TiO2 (Table S2). Despite their relatively low adsorption capacity, TiO2 and Co-TiO2 play an important role in heterogeneous catalytic oxidation processes [45]. In the dark/PMS system, the TC concentration decreased by 28.1% within 30 min, which was consistent with previous reports [41]. The catalytic rate was slightly reduced when pure TiO2 was added to the dark/PMS system due to the adsorption effect of pure TiO2 on PMS, slowing down the direct reaction between PMS and TC. The rate constant was similar to the experimental result (Figure S3b). The excellent performance of the Vis/Co-TiO2/PMS system was attributed to the following three aspects: (i) The presence of Co could accelerate PMS activation and lead to good catalytic performance. (ii) Photogenerated electrons generated by TiO2 under the action of visible light promoted the synergistic effect of metal Co and PMS, providing more electrons to activate PMS and accelerate pollutant removal in water.
2.3. Different Influence Factors
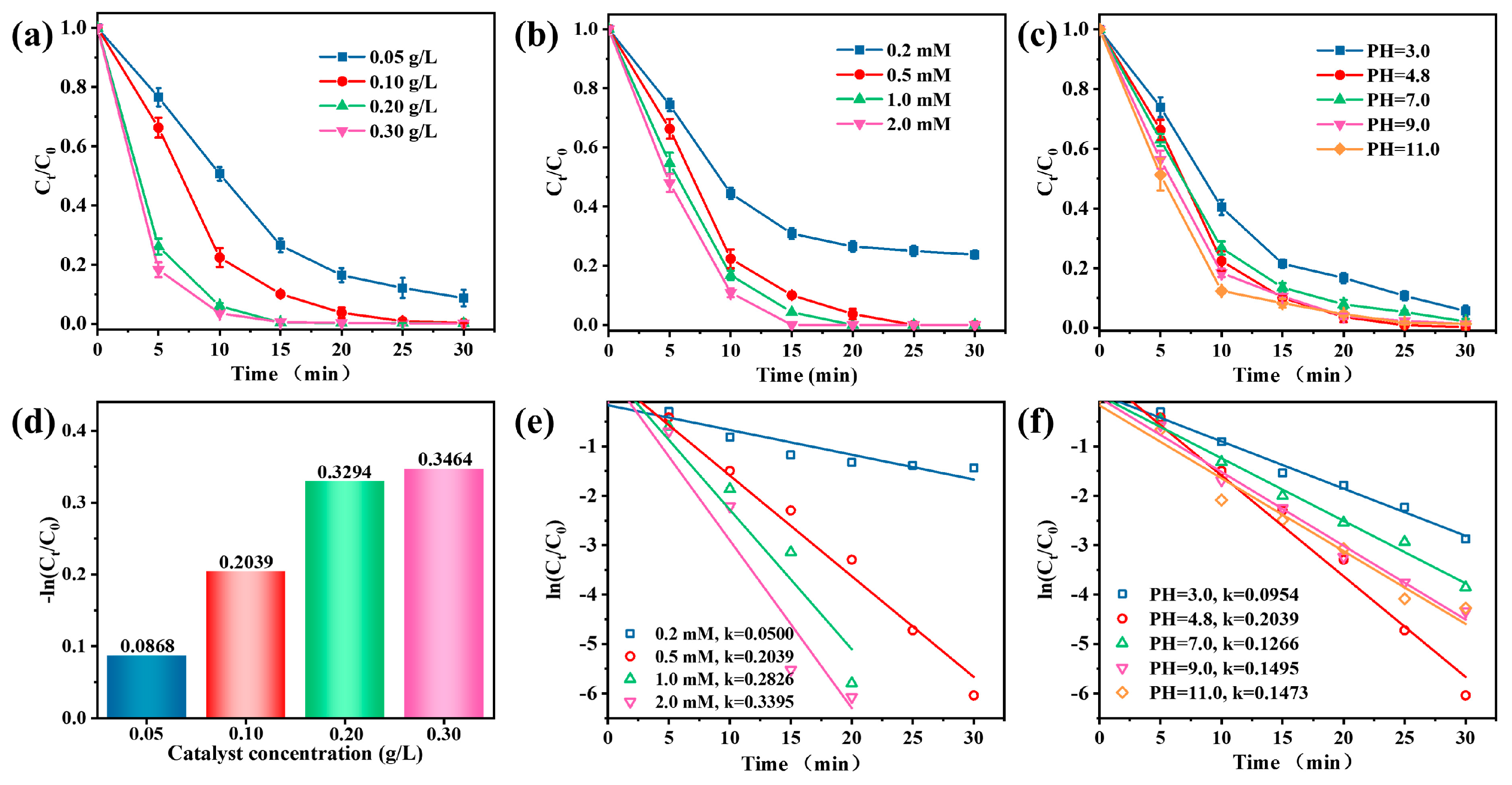
Figure 4a showed that TC degradation increased with the increasing catalyst concentration (0.05–0.30 g/L). The kinetic constants of TC degradation increased from 0.0868 to 0.3464 min−1 (Figure 4d). An increase in the catalyst concentration increases the number of available sites to activate PMS, thus promoting TC degradation. When the amount of catalyst was 0.10 g/L, the TC was almost degraded within 25 min. Further increasing the catalyst concentration did not significantly improve the degradation rate, indicating that, from an economic point of view, a small dose can achieve the goal of complete TC degradation.
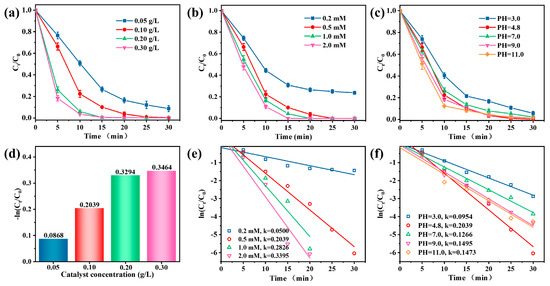
Figure 4.
Effects of (a) catalyst concentration, (b) PMS doses, and (c) initial pH on TC removal in the Vis/Co–TiO2/PMS system. Pseudo–first–order kinetics of different factors, (d) catalyst concentration, (e) PMS doses, and (f) pH values (Conditions: catalyst concentration = 0.1 g/L, PMS doses = 0.5 mM, and pH = 4.8).
The oxidant concentration also positively affected the degradation efficiency (Figure 4b). As the PMS dosage increased from 0.2 mM to 0.5 mM, the removal rate increased from 76.3% to 100% within 30 min since more free radicals were formed at higher concentrations of PMS. When the oxidant concentration is greater than 0.5 mM, the TC was completely degraded within 20 min. The degradation rate constant also showed the same trend, increasing from 0.0500 to 0.3395⋅min−1 (Figure 4e). However, the TC removal efficiency did not increase substantially when the PMS addition was greater than 0.5 mM because the excess PMS acts as a quencher for SO4•− and •OH at the same time, forming less reactive SO5•− (Equations (2)–(4)) [46].
SO4•− + SO4•−→ S2O82−
SO4•− + HSO5− → SO5•− + SO4− + H+
•OH + HSO5− → SO5•− + H2O
As shown in Figure 4c, the efficiency of the Vis/Co-TiO2/PMS system remained outstanding over a broad pH range of from 4.8 to 11.0, exhibiting removal rates between 98.2% and 100%. Thus, the Vis/Co-TiO2/PMS system demonstrated excellent pH adaptability. At pH = 3.0, the removal efficiency of TC was slightly suppressed, possibly because PMS is difficult to activate in acidic conditions [47]. Figure 4f shows that the k value varied between 0.0954 and 0.2039. Therefore, it is reasonable to assume that the highly active oxidation species produced in large quantities at the active sites of Co-TiO2 have little dependence on the pH value.
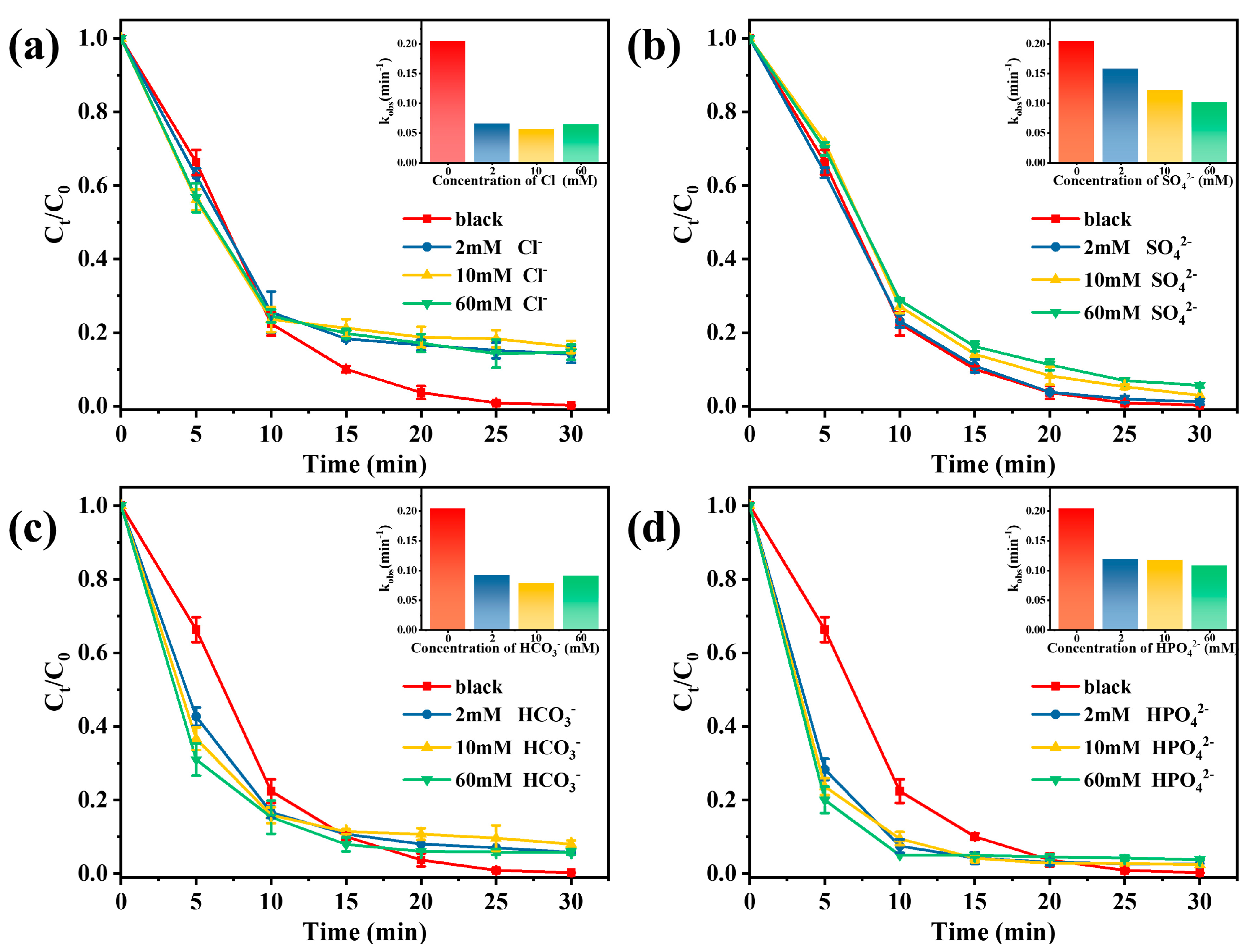
In practical applications, different anions in wastewater affect the performance of AOPs, which is attributed to the fact that inorganic anions can affect the activation process of PMS by interacting with different species (such as SO4•− and •OH) to produce different kinds of new free radicals [48]. In this study, different inorganic anion concentrations (2 mM, 10 mM, and 60 mM) were added to the Vis/Co-TiO2/PMS system to study possible problems in practical applications. As shown in Figure 5a, the TC removal efficiency is affected by the presence of Cl- at concentrations of 2–60 mM. The inhibition effect can be concluded that Cl- could react with SO4•− and •OH to form low oxidation species (Cl• and ClOH•−) (Equations (5)–(7)) [49].
SO4•− + Cl−→Cl− + SO42− k = 3.1 × 108 M−1S−1
Cl− + •OH →ClOH•− k = 6.1 × 109 M−1S−1
ClOH•− + H+ →Cl• + H2O k = 4.3 × 1010 M−1S−1
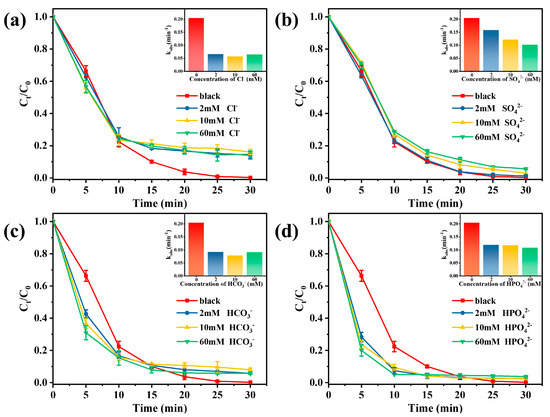
Figure 5.
Effects of inorganic anions on TC removal in Vis/Co-TiO2/PMS system: (a) Cl−, (b) SO42−, (c) HCO3−, and (d) HPO42− (Conditions: catalyst concentration = 0.1 g/L, PMS doses = 0.5 mM, and pH = 4.8).
Figure 5b revealed that the presence of SO42− restrained the degradation of TC. Sulfate ions do not react with sulfate radicals, but the suppression of TC degradation can be found in the presence of sulfate radicals. This is attributed to the effect of sulfate ions on the sulfate radical reduction potential [50]. In the Vis/Co-TiO2/PMS system, two other typical anions (HCO3− and HPO42−) have positive effects on pollutant removal (Figure 5c,d). For PMS, both HCO3− and HPO42− can directly activate it to produce more active species (Equations (8)–(11)) [51,52].
•OH + HCO3−→OH− + HCO3•− k = 8.5 × 106 M−1s−1
SO4•− + HCO3−→ SO42− + HCO3•− k = 9.1 × 106 M−1s−1
•OH + HPO42−→HPO4•− + OH− k = 8 × 105 M−1s−1
SO4•− + HPO42−→HPO4•− + SO42− k = 1.2 × 106 M−1s−1
2.4. Universality and Recyclability of Catalysts
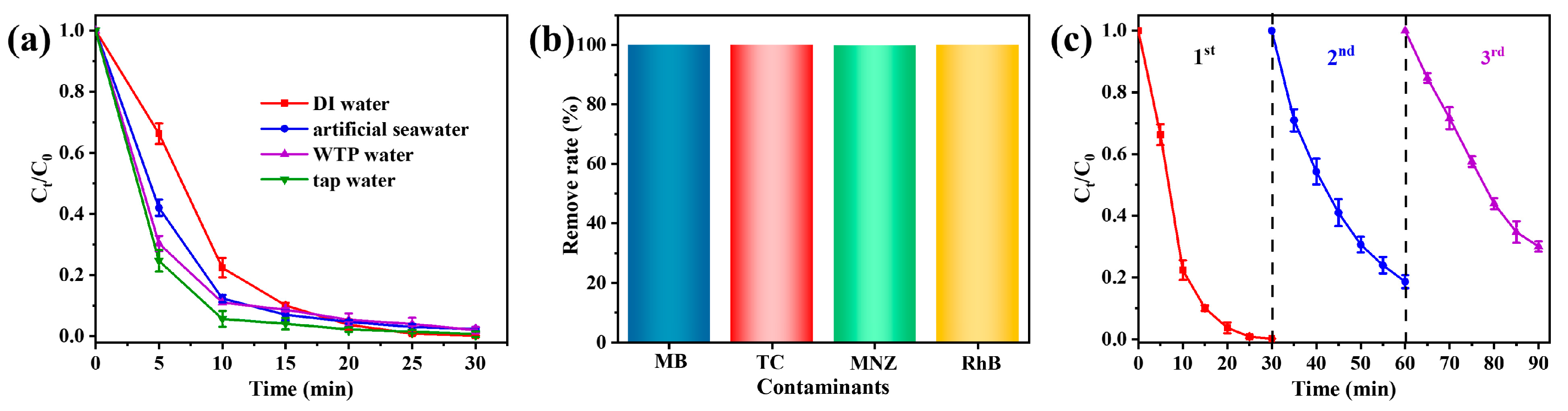
To explore the feasibility of the prepared Vis/Co-TiO2/PMS system for TC degradation in an actual wastewater system, we simulated a variety of water quality environments for the degradation reactions, including artificial seawater, tap water, and wastewater treatment plant (WTP) effluents. The preparation of artificial seawater can be found in our previous research [41]. As shown in Figure 6a, the TC can be completely degraded within 30 min in the tap water system, thus indicating that tap water does not have a significant negative effect on pollutant removal. The removal rate of the TC was about 93.4% in the artificial seawater system and 90.1% in the WTP water system, suggesting that Co-TiO2 can keep excellent feasibility. In addition, we selected a variety of typical pollutants, including methyl blue (MB, 20 mg/L), metronidazole (MNZ, 20 mg/L), and Rhodamine (RhB, 20 mg/L), to further evaluate the versatility of the Vis/Co-TiO2/PMS system for a variety of pollutants (Figure 6b). The results revealed that the optimal system demonstrated excellent degradation efficiency for the contaminants, with the removal of almost 100% of the contaminants within 30 min. Therefore, it can be concluded that the Vis/Co-TiO2/PMS system has great potential for practical application. To study the stability of Co-TiO2, three reaction cycles were carried out consecutively for a cycle test (Figure 6c). Even though no treatment was applied to the collected Co-TiO2, the TC degradation rate of 70.1% was obtained in the third cycle, proving that Co-TiO2 has good recycling performance in the Vis/Co-TiO2/PMS system. The used Co-TiO2 sample is also examined using XRD and XPS (Figure S4). The XRD and XPS peaks of the used Co-TiO2 show almost no change, indicating that the chemical structure of Co-TiO2 remains stable after use. The decline of the performance in the third cycle may be attributed to the increased by-products that occupy some active sites of the catalyst.

Figure 6.
(a) The removal efficiency of TC by the Vis/Co-TiO2/PMS system in various water systems, and (b) degradation of different pollutants by the Vis/Co-TiO2/PMS system and (c) cycling runs of Co-TiO2 composite.
2.5. Identification of the Main Active Species
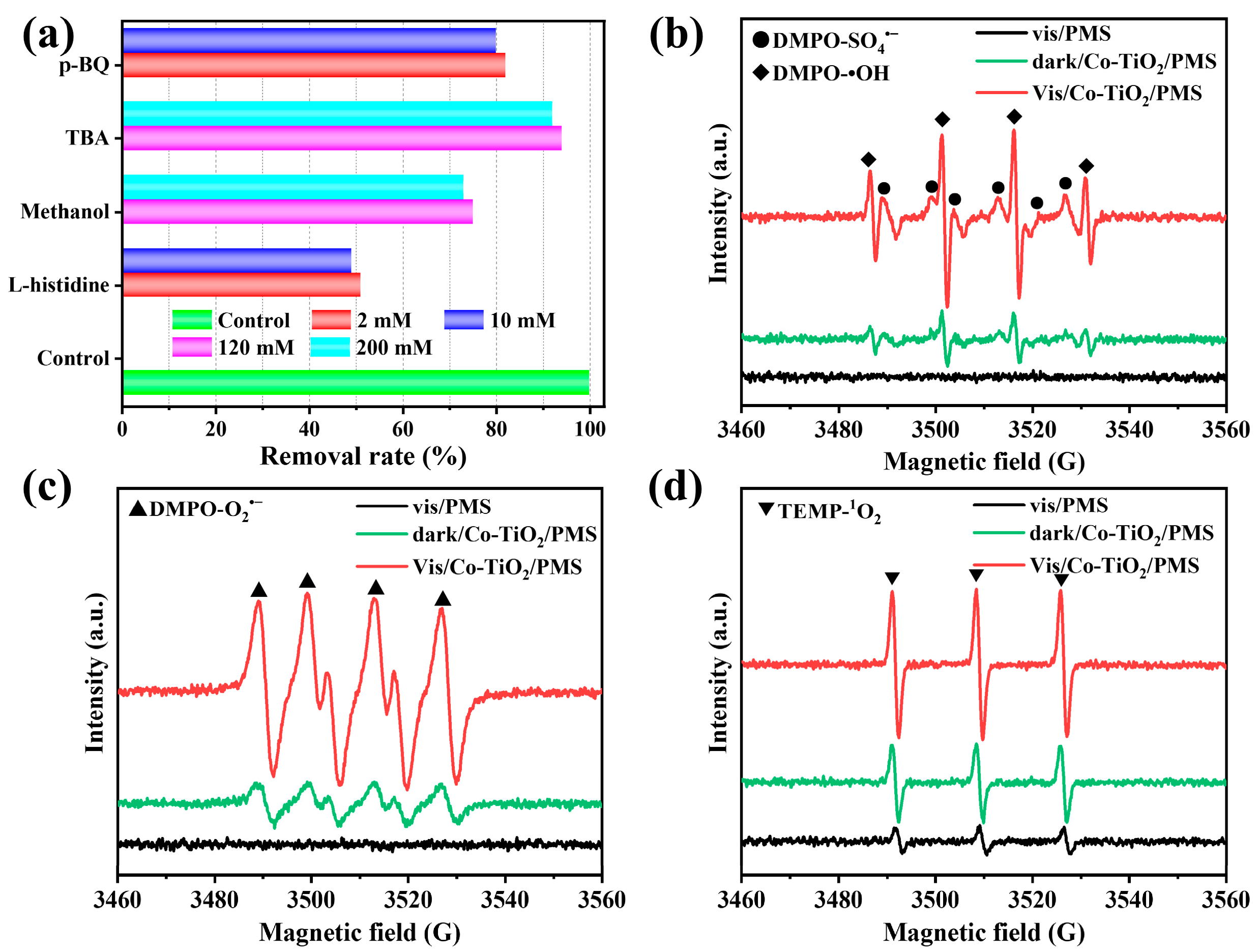
Quenching experiments and ESR analysis were conducted to investigate ROS generation in the optimal system. Typically, methanol was applied as a scavenger of •OH and SO4•−, while TBA was mainly applied as a quencher of •OH. Methanol can react with either •OH (k = 9.7 × 108 M−1s−1) or SO4•− (k = 2.5 × 107 M−1s−1), whereas the reaction between TBA •OH (k = (3.8–7.6) × 108 M−1s−1) was faster than that with SO4•− (k = (4.0–9.1) × 105 M−1s−1). Moreover, L-histidine and p-benzoquinone (p-BQ) were added as the scavengers of 1O2 (3 × 107 M−1s−1) and O2•− (9.6 × 108 M−1s−1), respectively. The rate constants of the quenchers reacting with different ROS are summarized in Table S3. As displayed in Figure 7a, the negative effect of the TBA on the TC removal was low (about 6%), indicating that •OH has a limited contribution to TC removal in the Vis/Co-TiO2/PMS system. Moreover, 10 mM of p-BQ was added to the solution (inhibition of about 20%), indicating that O2•− was involved in the TC removal. In addition, the removal efficiency was obviously reduced to 65.9% (methanol) and 49.6% (L-histidine) in the Vis/Co-TiO2/PMS system, respectively, demonstrating that SO4•− and 1O2 generated and played pivotal roles in TC removal. Based on the above results, the impact of various active species in the Vis/Co-TiO2/PMS + TC system was as follows: 1O2> SO4•−> O2•−> •OH.

Figure 7.
(a) The effect of various concentrations of the scavengers on the degradation of TC. ESR spectra of DMPO−•OH/DMPO-SO4•− (b), DMPO-O2•− (c), and TEMP-1O2 (d) after 10 min under different systems.
To further study the free radicals produced, ESR spectroscopy was conducted, and the results are shown in Figure 7b–d. The ESR analysis was performed using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trapping agent for radicals (•OH, SO4•−, and O2•−), and 2,2,6,6-tetramethyl-4-piperidone (TEMP) was used as a spin trapping agent for the non-radical singlet oxygen (1O2). As displayed in Figure 7b, the signal intensities of DMPO-•OH and DMPO-SO4•− were greatly enhanced in the Vis/Co-TiO2/PMS system, indicating that more •OH and SO4•− were formed in this optimal system. Moreover, the signal intensities of DMPO-O2•− and TEMP-1O2 in the Vis/Co-TiO2/PMS system were also much stronger than those in the vis/PMS and dark/Co-TiO2/PMS systems. Thus, the ESR and free radical quenching experiment results were consistent and confirmed that •OH, SO4•−, O2•−, and 1O2 were formed in the optimal system and played a key role in removing TC.
2.6. Degradation Mechanism
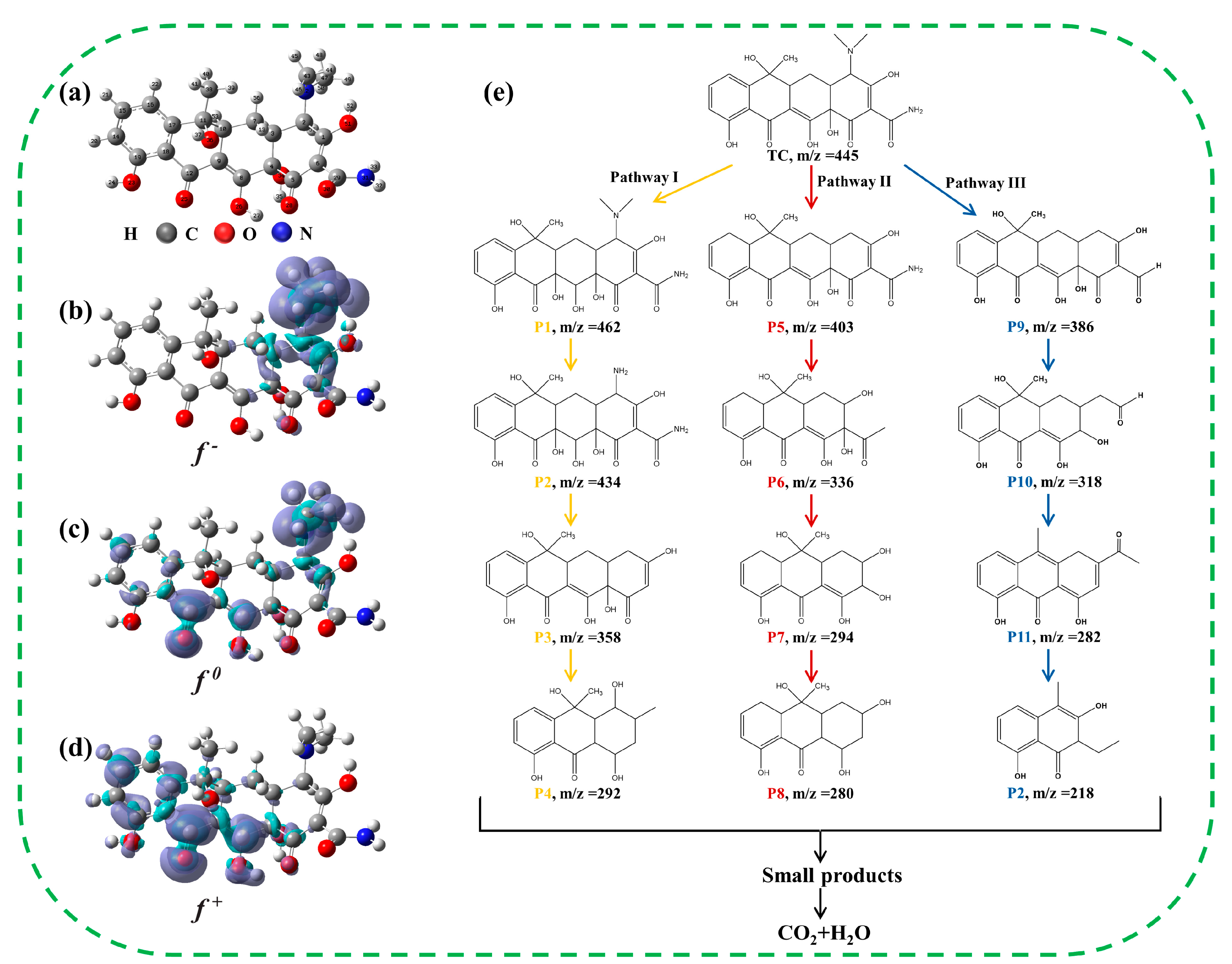
As shown in Table S4, twelve types of intermediates were found in the Vis/Co-TiO2/PMS system during TC degradation. The structure of the TC molecular after geometric optimization was shown in Figure 8a. To further quantitatively analyze the types of chemical reactions that may occur among the atoms on TC molecules, the nucleophilic (ƒ+), the electrophilic (ƒ-), and the general free radical (ƒ0) attacks of atoms on the TC molecules were analyzed using the concept of condensed Fukui functions (CFF) [53,54,55], and the results are shown in Table S5 and Figure 8b–d. Figure 8e illustrates the potential degradation pathways of TC. In pathway I, the C8 (0.0436) atom and N42 atom (0.1388) with a high ƒ0 value were susceptible to ROS (SO4•−, •OH, O2•−, or 1O2) attack, leading to the generation of P1 and P2 [56]. The formation of intermediate (P3) was related to the loss of acetamide (N31) and amino group (N42) in P2 [57]. Due to the SO4•− attack, the carbon ring cracking and demethylation directly cleft P3 molecules into small molecules (P4) [58]. In pathway II, the C2 site with a high ƒ- value (0.0268) was easily hit by electrophilic groups and dealkylation, forming P5 intermediates [59]. P6 is formed when P5 was hydroxylated, deaminated by 1O2, and then further oxidized to form P7 and P8 [60,61]. In pathway III, C2 and N31 were more susceptible to attack by electrophilic groups, and then underwent dealkylation and deamidation to form P9 [62], followed by a C=C bond and carbonyl cleavage at the α- position to form P10 [63]. Subsequently, P10 was further dehydrated and ring opened to form P11 and P12 [64]. Finally, the intermediates were broken down into small by-products or fully mineralized into CO2 and H2O.

Figure 8.
(a) TC molecular structure; (b) ƒ+, (c) ƒ−, and (d) ƒ0 isosurface map (isovalue = 0.015 a.u.); (e) detail of removal pathway of TC.
3. Experimental Section
3.1. Chemicals
All materials used in this study were displayed in the Supplementary Information (Text S1).
3.2. Preparation of TiO2 Nanospheres
First, an amount of 50 mL of ethylene glycol was added to the beaker. Next, 1 mL of TiCl4 was dispersed into the solution, followed by slowly adding 5 mL of H2O with continuous stirring for 10 min until the white fumes disappeared. After that, the above suspension was transferred to the autoclave and kept at 170 °C for 5 h. The precipitate was centrifuged, washed, and dried in a vacuum oven at 70 °C to prepare the TiO2 nanospheres.
3.3. Preparation of Co-TiO2 Nanospheres
To prepare the Co-TiO2 nanospheres, 0.10 g of TiO2 nanospheres were mixed with 60 mL H2O. Next, 0.05 g sodium dodecyl sulfate was added to the above solution, and stirred for 10 min. After stirring, 0.1 g CoCl2·6H2O was added, followed by 0.05 g NaBH4. Then, the product was washed with DI water and dried.
3.4. Characterization
The characterization applied in this work is shown in the Supplementary Information (Text S2).
3.5. Experimental Procedures
A 300 W xenon lamp with a cutoff filter (k > 420 nm) was used as a light source. As in a typical test, 10 mg of sample was added to a 100 mL TC solution (20 mg/L). Then, a selected amount of PMS was added to the above solution for TC degradation; 5 mL of aqueous suspension was filtered at intervals through a 0.22 μm syringe filter. The TC concentration was analyzed using high-performance liquid chromatography (HPLC). The detailed contents are shown in Text S3. The stability of the catalyst was evaluated by washing it with distilled water three times after a degradation cycle and drying it in a vacuum oven at 70 °C for subsequent recycling experiments. The degradation performances of the system in the three successive cycles were recorded, and the average was calculated. The initial pH of the solution was adjusted using 0.1 M of HCl or NaOH. The catalytic activity of the Co-TiO2 nanospheres was investigated to determine the degradation percentage (η%) (See Equation (12)). The apparent rate constant of the TC degradation was calculated by fitting the pseudo first-order equation (Equation (13)).
where η% represents the removal rate; C0 and Ct denote the concentration of the contamination at the initial and t time, respectively; and kobs is the pseudo first-order rate constant.
3.6. Theoretical Calculation
In this study, the theoretical calculations of the TC degradation mechanism, such as geometric structure optimization and single-point energy calculation, were carried out in Gaussian 16 software using the M06-2X method with the 6-31G(d, p) basis set and the solvated SMD model to simulate an aqueous solution environment [65]. To determine the reaction sites of the TC molecules, the Condensed Fukui Function (CFF) method was employed based on Density Functional Theory (DFT). The Hirshfeld charge was obtained using Multiwfn [66]. Finally, Gaussview was used to visualize the contour surface diagram of the Fukui function. More details of the Fukui function are described in Text S4.
4. Conclusions
In this study, a novel Co-TiO2 catalyst was synthesized using a simple two-step method and tested for PMS activation to remove TC. We verified its potential applications in realistic water environments. The as-prepared Co-TiO2 catalyst showed excellent catalytic activity in PMS activation and was able to achieve rapid degradation of the target pollutant TC. In addition, the Vis/Co-TiO2/PMS system has also shown excellent TC removal rates in WTP water, artificial seawater, and tap water, indicating its practical applications. Moreover, the Vis/Co-TiO2/PMS system performed the degradation of MB, MNZ, and RhB at a high removal rate. The catalytic system could be applied to a wide range of pHs, different water quality conditions, and a variety of organic pollutants, demonstrating its potential for practical applications. According to the results of the ROS quenching test and ESR analyses, SO4•− and 1O2 were the main active radicals involved in PMS activation. Further, the possible degradation pathways of TC have been illustrated based on theoretical calculations and the identification of the intermediates and products involved in the reaction. This study presents a fresh idea on the removal of various antibiotics in a water environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13050836/s1, Text S1: Chemicals; Text S2: Characterization; Text S3: TC concentration detection method by HPLC; Text S4: Theoretical calculations; Figure S1: XPS survey spectra of the Co-TiO2; Figure S2: N2 sorption and desorption isotherms of TiO2 and Co-TiO2; Figure S3: (a) The degradation efficiency in different systems and (b) pseudo-second -order kinetics of TC degradation under dark condition. (Condition: catalyst doses =0.1 g/L, PMS=0.5 mM, pH = 4.8); Table S1: Atomic percent of elements measured by different characterization techniques; Table S2:Textural properties of TiO2, Co-TiO2; Table S3: Rate constants for reactions of quenching agents with different ROS; Table S4: Rate constants for reactions of quenching agents with different ROS; Table S5: Natural population analysis (NPA) charges and calculated Fukui index (ƒ−, ƒ+, and ƒ0) of TC for Vis/Co-TiO2/PMS system.
Author Contributions
Conceptualization, X.J. and W.X.; methodology, J.L. (Jiesen Li); validation, W.X., Z.L., and J.L. (Jianghong Li); formal analysis, J.L. (Jianghong Li); investigation, X.J.; resources, W.X., and X.J.; data curation, J.L. (Jianghong Li); writing—original draft preparation, J.L. (Jianghong Li) and W.X.; writing—review and editing, Z.L. and W.X.; visualization, X.J. and J.L. (Jiesen Li); supervision, W.X. and Z.L.; project administration, W.X.; funding acquisition, W.X., and X.J.; All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (No. 52000031, W.C. Xu), Open Foundation of State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences (No. SKLECRA2022OFP02, X.D. Jiang).
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate Xiukui Li for supporting and providing suggestions for our work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Q.; Jiang, L.; Wang, J.; Zhu, Y.; Pu, Y.; Dai, W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B Environ. 2020, 277, 119122. [Google Scholar] [CrossRef]
- Baumschlager, A.; Rullan, M.; Khammash, M. Exploiting natural chemical photosensitivity of anhydrotetracycline and tetracycline for dynamic and setpoint chemo-optogenetic control. Nat. Commun. 2020, 11, 3834. [Google Scholar] [CrossRef] [PubMed]
- Zuber, J.; McJunkin, K.; Fellmann, C.; Dow, L.E.; Taylor, M.J.; Hannon, G.J.; Lowe, S.W. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat. Biotechnol. 2011, 29, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.C.; Lai, S.F.; Pillai, S.C.; Chu, W.; Hu, Y.; Jiang, X.D.; Fu, M.L.; Wu, X.L.; Li, F.H.; Wang, H.L. Visible light photocatalytic degradation of tetracycline with porous Ag/graphite carbon nitride plasmonic composite: Degradation pathways and mechanism. J. Colloid Interface Sci. 2020, 574, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, H.; Cai, L.; Yu, Y. Prevalence of antibiotic resistance genes and bacterial pathogens in long-term manured greenhouse soils as revealed by metagenomic survey. Environ. Sci. Technol. 2015, 49, 1095–1104. [Google Scholar] [CrossRef]
- Han, C.H.; Park, H.D.; Kim, S.B.; Yargeau, V.; Choi, J.W.; Lee, S.H.; Park, J.A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Leng, Y.; Bao, J.; Song, D.; Li, J.; Ye, M.; Li, X. Background Nutrients Affect the Biotransformation of Tetracycline by Stenotrophomonas maltophilia as Revealed by Genomics and Proteomics. Environ. Sci. Technol. 2017, 51, 10476–10484. [Google Scholar] [CrossRef]
- Fan, J.H.; Qin, H.H.; Jiang, S.M. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Jia, J.; Liu, D.; Wang, S.; Li, H.; Ni, J.; Li, X.; Tian, J.; Wang, Q. Visible-light-induced activation of peroxymonosulfate by TiO2 nano-tubes arrays for enhanced degradation of bisphenol A. Sep. Purif. Technol. 2020, 253, 117510. [Google Scholar] [CrossRef]
- Li, X.; Ao, Z.; Liu, J.; Sun, H.; Rykov, A.I.; Wang, J. Topotactic Transformation of Metal–Organic Frameworks to Graphene Encapsulated Transition Metal Nitrides as Efficient Fenton-Like Catalysts. ACS Nano 2016, 10, 11532–11540. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, P.; Nie, G.; Cheng, C.; Duan, X.; Zhang, H.; Wang, S. Hydroxyl radical dominated elimination of plasticizers by peroxymonosulfate on metal-free boron: Kinetics and mechanisms. Water Res. 2020, 186, 116361. [Google Scholar] [CrossRef]
- Zhu, M.P.; Yang, J.-C.E.; Duan, X.G.; Zhang, D.D.; Wang, S.B.; Yuan, B.L.; Fu, M.L. Interfacial CoAl2O4 from ZIF-67@γ-Al2O3 pellets toward catalytic activation of peroxymonosulfate for metronidazole removal. Chem. Eng. J. 2020, 397, 125339. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.; Gao, Y.; Zhou, Y.; Cao, Y.; Jiang, J. Relative contribution of ferryl ion species (Fe(IV)) and sulfate radical formed in nanoscale zero valent iron activated peroxydisulfate and peroxymonosulfate processes. Water Res. 2020, 172, 115504. [Google Scholar] [CrossRef]
- Kim, J.; Coulibaly, G.N.; Yoon, S.; Assadi, A.A.; Hanna, K.; Bae, S. Red mud-activated peroxymonosulfate process for the removal of fluoroquinolones in hospital wastewater. Water Res. 2020, 184, 116171. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Li, H.; Han, B.; Xia, K.; Zhou, C. One-pot synthesis of a novel hierarchical Co(II)-doped TiO2 nanostructure: Toward highly active and durable catalyst of peroxymonosulfate activation for degradation of antibiotics and other organic pollutants. Chem. Eng. J. 2019, 368, 377–389. [Google Scholar] [CrossRef]
- Xie, R.; Lei, D.; Zhan, Y.; Liu, B.; Tsang CH, A.; Zeng, Y.; Li, K.; Leung DY, C.; Huang, H. Efficient photocatalytic oxidation of gaseous toluene over F-doped TiO2 in a wet scrubbing process. Chem. Eng. J. 2020, 386, 121025. [Google Scholar] [CrossRef]
- Ding, M.; Ao, W.; Xu, H.; Chen, W.; Tao, L.; Shen, Z.; Liu, H.; Lu, C.; Xie, Z. Facile construction of dual heterojunction CoO@TiO2/MXene hybrid with efficient and stable catalytic activity for phenol degradation with peroxymonosulfate under visible light irradiation. J. Hazard. Mater. 2021, 420, 126686. [Google Scholar] [CrossRef]
- Cai, P.C.; Zhao, J.; Zhang, X.H.; Zhang, T.Y.; Yin, G.M.; Chen, S.; Dong, C.L.; Huang, Y.C.; Sun, Y.Y.; Yang, D.J.; et al. Synergy between cobalt and nickel on NiCo2O4 nanosheets promotes peroxymonosulfate activation for efficient norfloxacin degradation. Appl. Catal. B Environ. 2022, 306, 121091. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, X.; Zhou, L.; Huang, Y.; Yang, S.; Cai, T.; Ding, D. Efficient heterogeneous activation of peroxymonosulfate by facilely prepared Co/Fe bimetallic oxides: Kinetics and mechanism. Chem. Eng. J. 2018, 345, 364–374. [Google Scholar] [CrossRef]
- Lin, K.A.; Lai, H.K.; Tong, S. One-step prepared cobalt-based nanosheet as an efficient heterogeneous catalyst for activating peroxymonosulfate to degrade caffeine in water. J. Colloid Interface Sci. 2018, 514, 272–280. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Feng, Q.; Liu, G.; Zhan, Y.; Wu, M.; Lu, H.; Shu, Y.; Leung, D.Y.C. Catalytic oxidation of benzene over Mn modified TiO2/ZSM-5 under vacuum UV irradiation. Appl. Catal. B Environ. 2017, 203, 870–878. [Google Scholar] [CrossRef]
- Basnet, P.; Anderson, E.; Zhao, Y. Hybrid CuxO–TiO2 Nanopowders Prepared by Ball Milling for Solar Energy Conversion and Visible-Light-Induced Wastewater Treatment. ACS Appl. Nano Mater. 2019, 2, 2446–2455. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Lai, L.; Yan, J.; Li, J.; Lai, B. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism. Chem. Eng. J. 2018, 343, 676–688. [Google Scholar] [CrossRef]
- Choi, M.; Lim, J.; Baek, M.; Choi; Kim, W.; Yong, K. Investigating the Unrevealed Photocatalytic Activity and Stability of Nanostructured Brookite TiO2 Film as an Environmental Photocatalyst. ACS Appl. Mater. 2017, 9, 16252–16260. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.-H.; Lim, J.; Lee, J.H.; Choi, W.; Jang, H.M. Enhanced photocatalytic activity of {101}-oriented bipyramidal TiO2 agglomerates through interparticle charge transfer. Appl. Catal. B Environ. 2015, 176–177, 76–82. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of Peroxymonosulfate by Oxygen Vacancies-Enriched Cobalt-Doped Black TiO2 Nanotubes for the Removal of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Pham, G.H.; Tade, M.; Phan, C.; Vagnoni, R.; Liu, S. Microwave-Assisted Dry and Bi-reforming of Methane over M–Mo/TiO2 (M = Co, Cu) Bimetallic Catalysts. Energy Fuels 2020, 34, 7284–7294. [Google Scholar] [CrossRef]
- Ullah, S.; Lovell, E.C.; Tan, T.H.; Xie, B.; Kumar, P.V.; Amal, R.; Scott, J. Photoenhanced CO2 methanation over La2O3 promoted Co/TiO2 catalysts. Appl. Catal. B Environ. 2021, 294, 120248. [Google Scholar] [CrossRef]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, M.A.; Cadús, L.E.; Morales, M.R. Tailoring materials by high-energy ball milling: TiO2 mixtures for catalyst support application. Mater. Today Chem. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W.; Xu, B. Modeling the heterogeneous peroxymonosulfate/Co-MCM41 process for the degradation of caffeine and the study of influence of cobalt sources. Chem. Eng. J. 2014, 235, 10–18. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W.; Xu, B. Catalytic degradation of caffeine in aqueous solutions by cobalt-MCM41 activation of peroxymonosulfate. Appl. Catal. B Environ. 2013, 134–135, 324–332. [Google Scholar] [CrossRef]
- Anbalagan, K. UV-Sensitized Generation of Phasepure Cobalt-Doped Anatase: CoxTi1−xO2−δ Nanocrystals with Ferromagnetic Behavior Using Nano-TiO2/cis-[CoIII(en)2(MeNH2)Cl]2+. J. Phys. Chem. C 2011, 115, 3821–3832. [Google Scholar] [CrossRef]
- Li, T.; Yuan, G.; Li, W. Particle Filter with Novel Nonlinear Error Model for Miniature Gyroscope-Based Measurement While Drilling Navigation. Sensors 2016, 16, 371. [Google Scholar] [CrossRef]
- Lalitha, K.; Reddy, J.K.; Phanikrishna Sharma, M.V.; Kumari, V.D.; Subrahmanyam, M. Continuous hydrogen production activity over finely dispersed Ag2O/TiO2 catalysts from methanol:water mixtures under solar irradiation: A structure–activity correlation. Int. J. Hydrogen Energy 2010, 35, 3991–4001. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Z.; Huang, C.; Wang, P.; Huang, S.; Yang, X.; Cheng, H.; Zheng, X.; Tian, H.; Liu, Z. A novel 3D Co/Mo co-catalyzed graphene sponge-mediated peroxymonosulfate activation for the highly efficient pollutants degradation. Sep. Purif. Technol. 2022, 301, 122035. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, K.; Liu, Z.; Xu, W.; Liang, F.; Mo, S.; Wu, X.; Beiyuan, J. Novel 0D-1D-2D nanostructured MCN/NCDs recyclable composite for boosted peroxymonosulfate activation under visible light toward tetracycline degradation. Sep. Purif. Technol. 2022, 296, 120532. [Google Scholar] [CrossRef]
- Ghanbari, F.; Ahmadi, M.; Gohari, F. Heterogeneous activation of peroxymonosulfate via nanocomposite CeO2-Fe3O4 for organic pollutants removal: The effect of UV and US irradiation and application for real wastewater. Sep. Purif. Technol. 2019, 228, 115732. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A. Visible and UV photocatalysis of aqueous perfluorooctanoic acid by TiO2 and peroxymonosulfate: Process kinetics and mechanistic insights. Chemosphere 2020, 243, 125366. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.X.; Yao, F.B.; Yang, Q.; Zheng, Y.M.; Zhao, Q.B.; Yu, H.Q. Catalytic degradation of ciprofloxacin by a visible-light-assisted peroxymonosulfate activation system: Performance and mechanism. Water Res. 2020, 173, 115559. [Google Scholar] [CrossRef]
- Zhong, Q.; Liu, J.; Wang, J.; Li, Y.; Li, J.; Zhang, G. Efficient degradation of organic pollutants by activated peroxymonosulfate over TiO2@C decorated Mg-Fe layered double oxides: Degradation pathways and mechanism. Chemosphere 2022, 300, 134564. [Google Scholar] [CrossRef]
- Takdastan, A.; Kakavandi, B.; Azizi, M.; Golshan, M. Efficient activation of peroxymonosulfate by using ferroferric oxide supported on carbon/UV/US system: A new approach into catalytic degradation of bisphenol A. Chem. Eng. J. 2018, 331, 729–743. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Daisuke, M.; Divya, K. MS Mechanistic Insight into the Reactivity of Chlorine-derived Radicals in the Aqueous-phase UV/chlorine Advanced Oxidation Process: Quantum Mechanical Calculations. Environ. Sci. Technol. 2017, 51, 6918–6926. [Google Scholar] [CrossRef]
- Wu, X.; Gu, X.; Lu, S.; Qiu, Z.; Sui, Q.; Zang, X.; Miao, Z.; Xu, M. Strong enhancement of trichloroethylene degradation in ferrous ion activated persulfate system by promoting ferric and ferrous ion cycles with hydroxylamine. Sep. Purif. Technol. 2015, 147, 186–193. [Google Scholar] [CrossRef]
- Duan, P.; Liu, X.; Liu, B.; Akram, M.; Li, Y.; Pan, J.; Yue, Q.; Gao, B.; Xu, X. Effect of phosphate on peroxymonosulfate activation: Accelerating generation of sulfate radical and underlying mechanism. Appl. Catal. B Environ. 2021, 298, 120532. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Cheng, Y.; Cao, L.; Bai, F.; Yue, S.; Xie, P.; Ma, J. Molybdenum disulfide (MoS2): A novel activator of peracetic acid for the degradation of sulfonamide antibiotics. Water Res. 2021, 201, 117291. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.D.; Lim, T.T. Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Mao, S.; Liu, C.; Wu, Y.; Xia, M.; Wang, F. Porous P, Fe-doped g-C3N4 nanostructure with enhanced photo-Fenton activity for removal of tetracycline hydrochloride: Mechanism insight, DFT calculation and degradation pathways. Chemosphere 2022, 291, 133039. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xiong, Z.; Lai, B. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: Adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, Y.; Zhang, L.; Jiang, W.; Xie, R. Catalytic degradation of tetracycline hydrochloride by persulfate activated with nano Fe0 immobilized mesoporous carbon. Chem. Eng. J. 2018, 341, 392–401. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Luo, T.; Feng, H.; Tang, L.; Lu, Y.; Tang, W.; Chen, S.; Yu, J.; Xie, Q.; Ouyang, X.; Chen, Z. Efficient degradation of tetracycline by heterogeneous electro-Fenton process using Cu-doped Fe@Fe2O3: Mechanism and degradation pathway. Chem. Eng. J. 2020, 382, 122970. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Z.; Luo, X.; Ma, Y.; Chen, C.; Chen, X.; Cui, L. Activation of persulfate for tetracycline degradation using the catalyst regenerated from Fenton sludge containing heavy metal: Synergistic effect of Cu for catalysis. Chem. Eng. J. 2020, 396, 125238. [Google Scholar] [CrossRef]
- Gao, X.; Ma, C.; Liu, Y.; Xing, L.; Yan, Y. Self-induced Fenton reaction constructed by Fe(III) grafted BiVO4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Surf. Sci. 2019, 467–468, 673–683. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Wan, D.; Huang, J.; Liu, Y. Peroxymonosulfate-enhanced photocatalysis by carbonyl-modified g-C3N4 for effective degradation of the tetracycline hydrochloride. Sci. Total Environ. 2020, 749, 142313. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, C.; Lyu, J.; Hu, Z.; Ge, M. Tetracycline degradation by persulfate activated with magnetic Cu/CuFe2O4 composite: Efficiency, stability, mechanism and degradation pathway. J. Hazard. Mater. 2019, 373, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, G.; Qin, Y.; Ji, Y.; Mai, B.; An, T. New theoretical insight into indirect photochemical transformation of fragrance nitro-musks: Mechanisms, eco-toxicity and health effects. Environ. Int. 2019, 129, 68–75. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).