Controlled Synthesis of Ag-SnO2/α-Fe2O3 Nanocomposites for Improving Visible-Light Catalytic Activities of Pollutant Degradation and CO2 Reduction
Abstract
:1. Introduction
2. Results
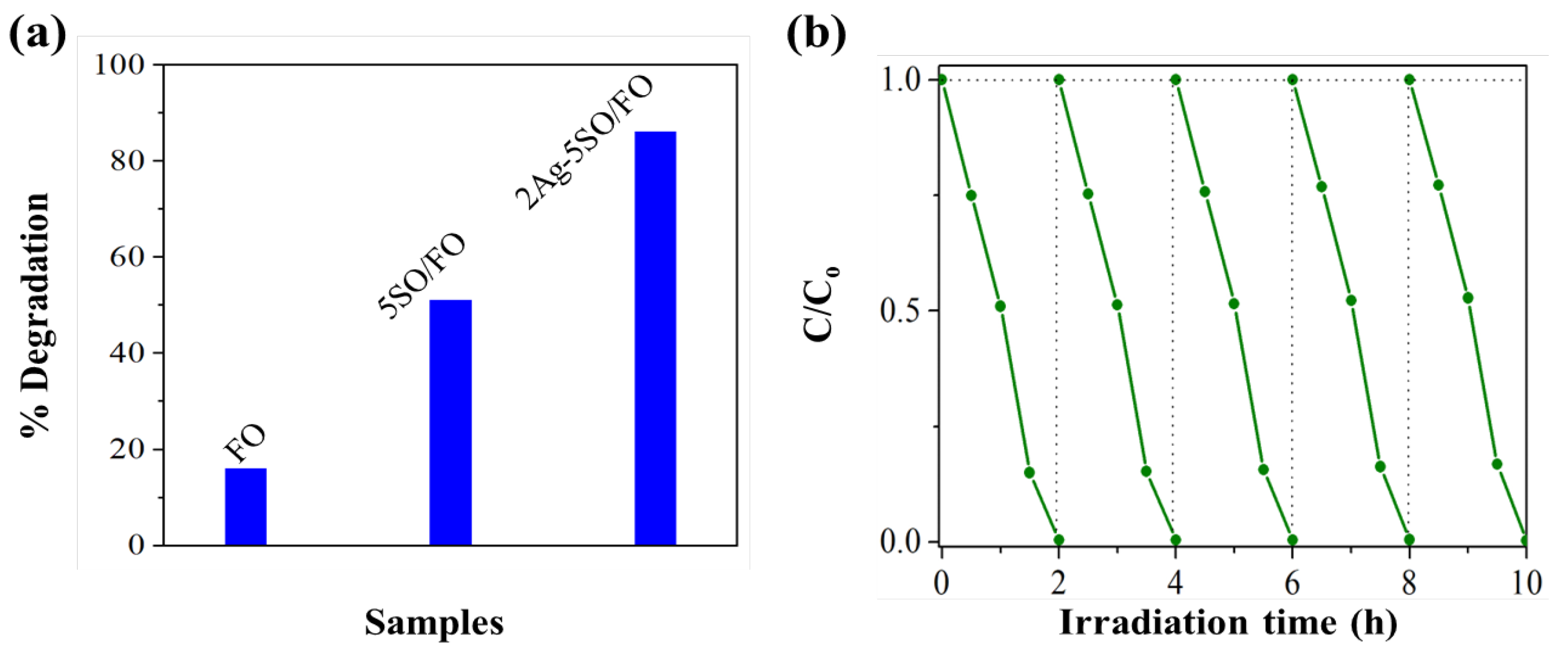
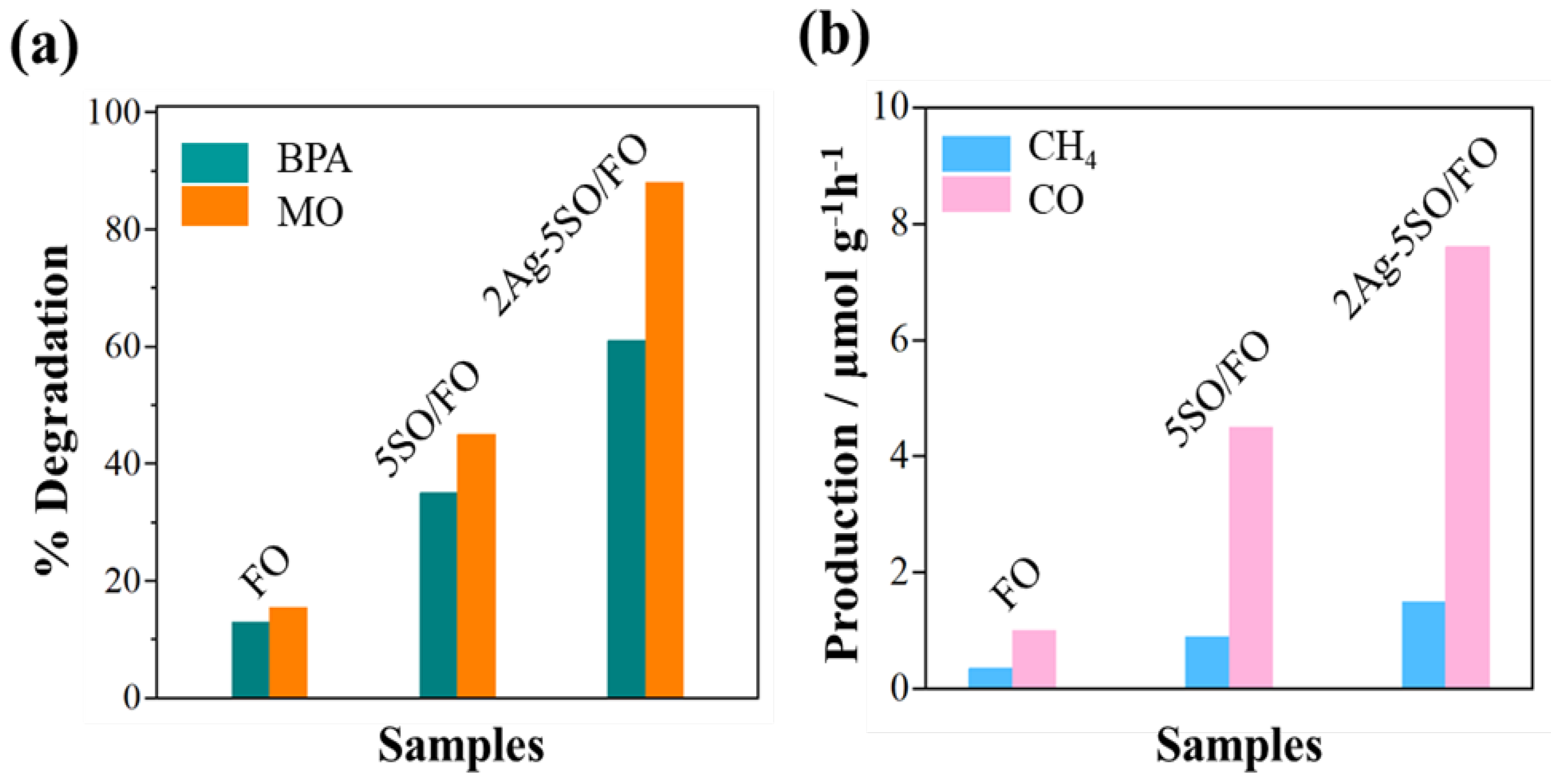
2.1. The Effect of Co-Modification by SO and Nanosized Ag on Photocatalytic Performance
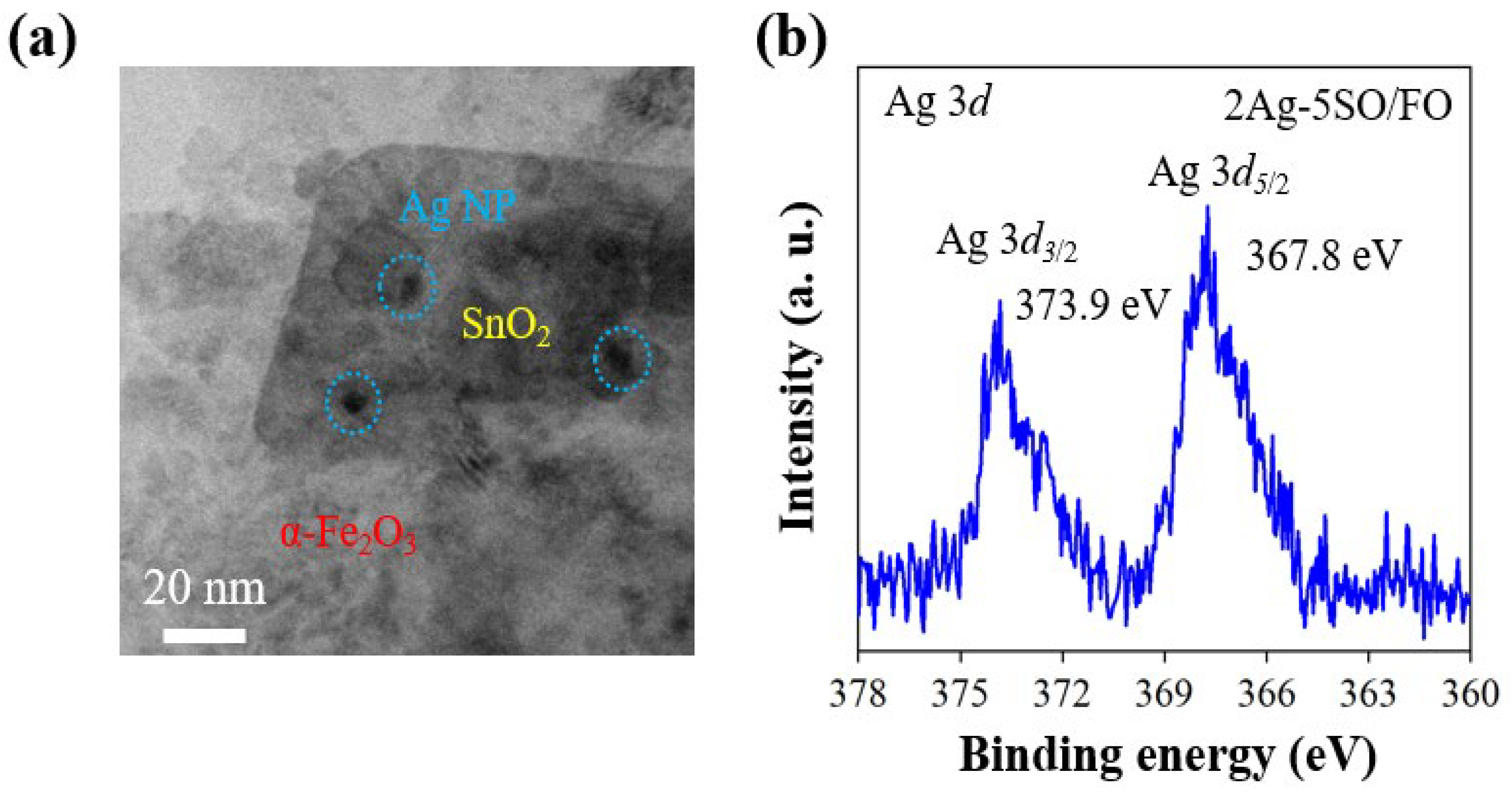
2.2. Structural and Surface Characterization
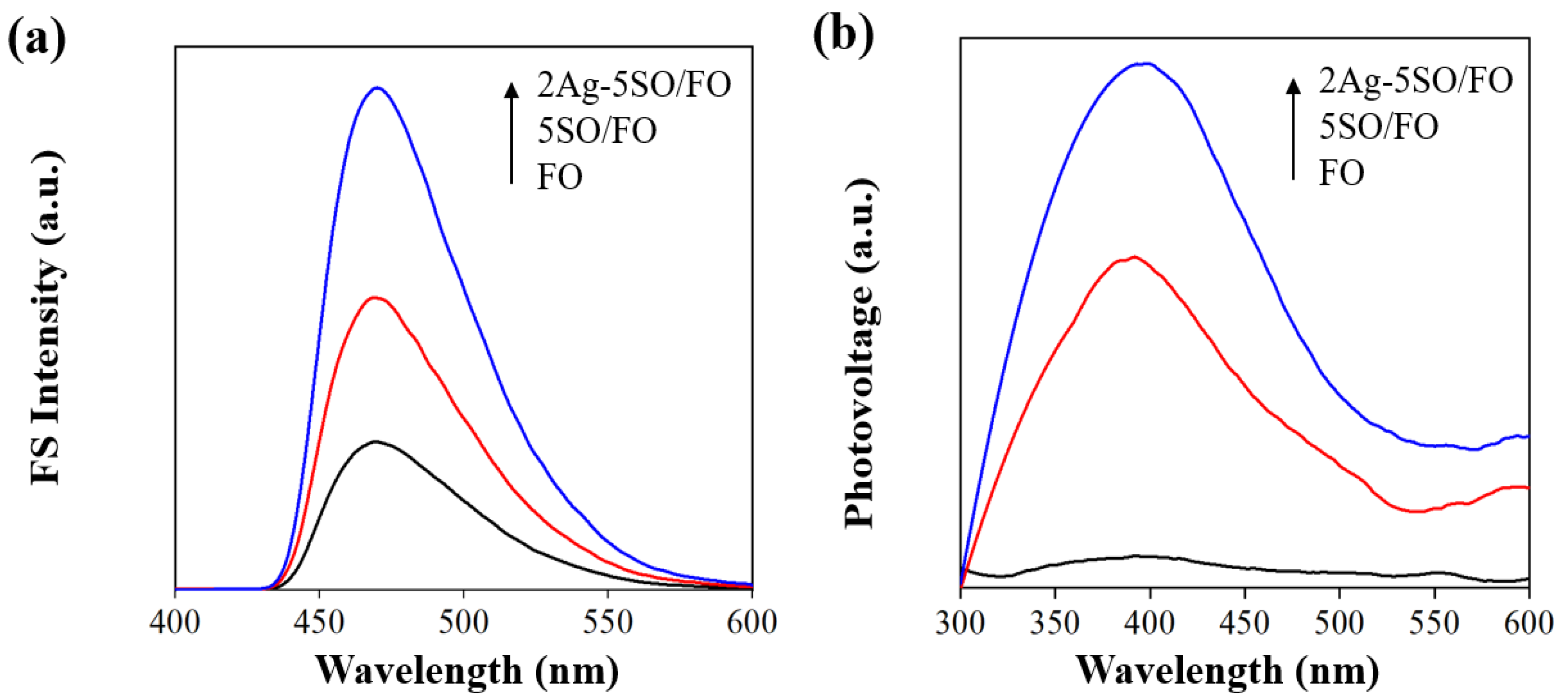
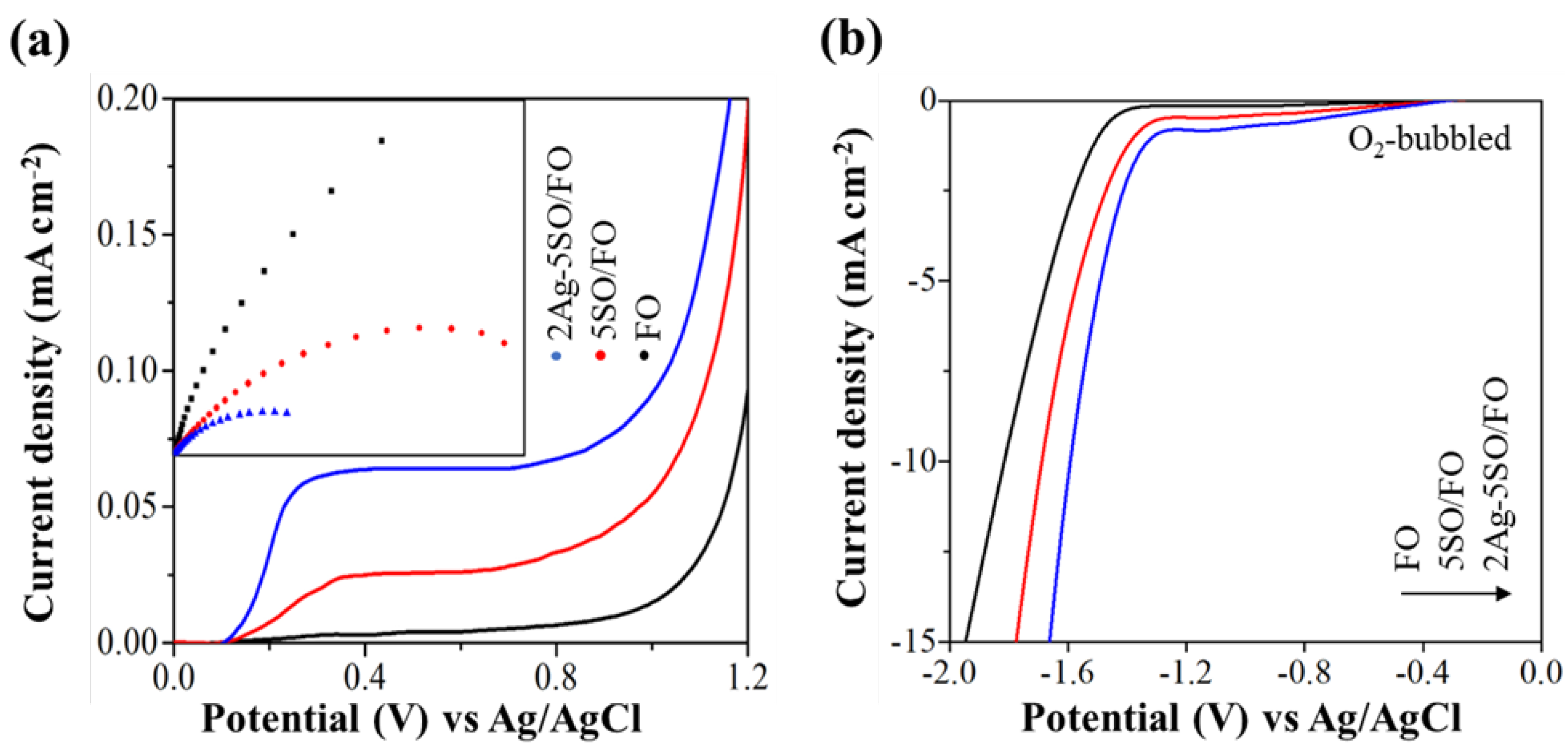
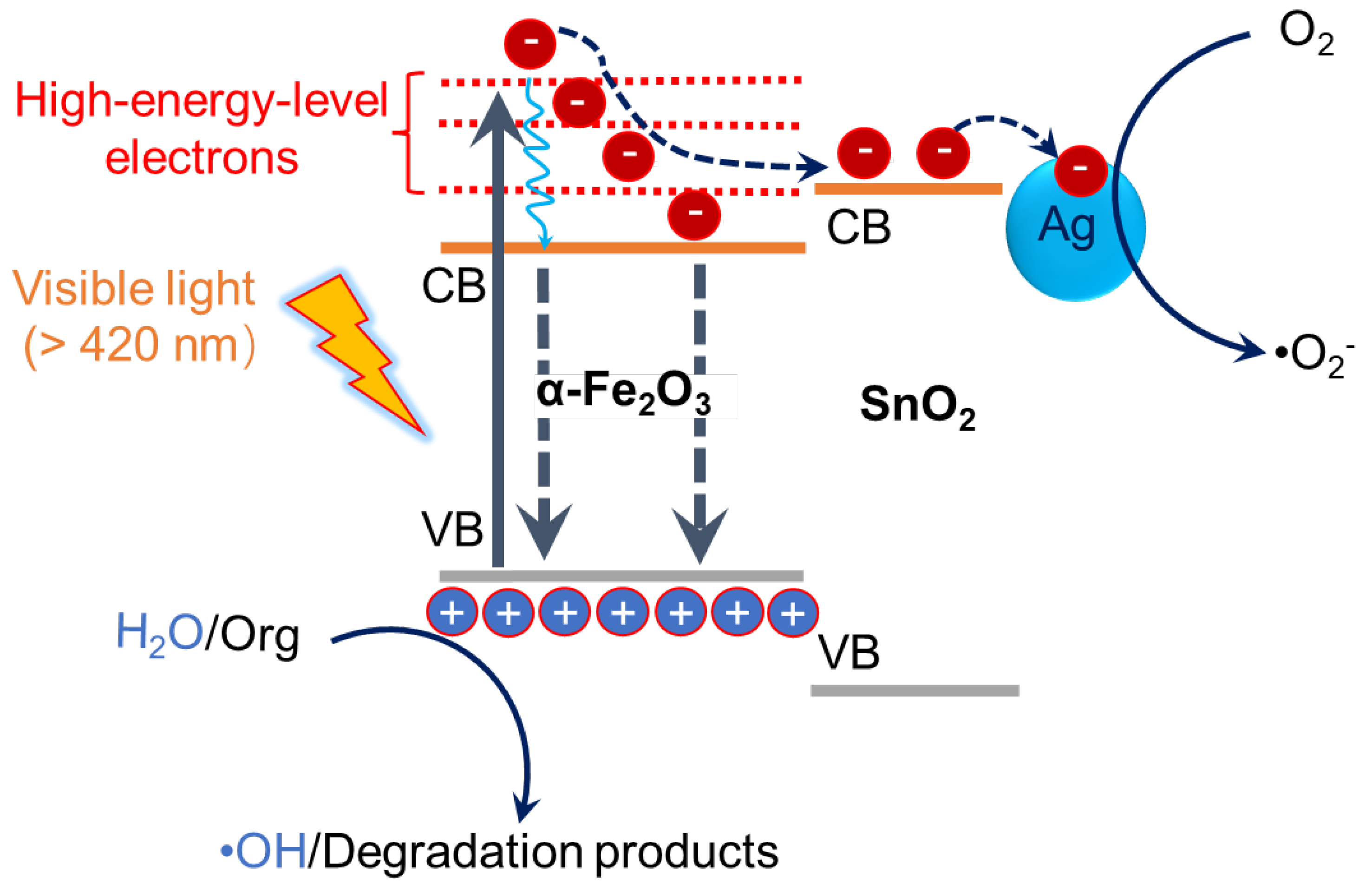
2.3. Photogenerated Charge Separation and Catalytic Function
2.4. Discussion
3. Materials and Methods
3.1. Synthesis of FO
3.2. Synthesis of SO
3.3. Synthesis of SO/FO
3.4. Synthesis of Ag-SO/FO
3.5. Photocatalytic Activity for Pollutants Degradation
3.6. Photocatalytic Activity for CO2 Conversion
3.7. Scavenger Experiments
3.8. Recycle Experiment
3.9. Fluorescence Spectra Measurement
3.10. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van, K.N.; Huu, H.T.; Nguyen Thi, V.N.; Le Thi, T.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Tran, D.L.; Vasseghian, Y. Facile construction of S-scheme SnO2/g-C3N4 photocatalyst for improved photoactivity. Chemosphere 2022, 289, 133120. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Hu, K.; Wang, J.; Liu, Y.; Ali, S.; Qin, C.; Jing, L. Synthesis of g-C3N4-based photocatalysts with recyclable feature for efficient 2,4-dichlorophenol degradation and mechanisms. Appl. Catal. B Environ. 2019, 243, 57–65. [Google Scholar] [CrossRef]
- Yu, X.; Cabooter, D.; Dewil, R. Effects of process variables and kinetics on the degradation of 2,4-dichlorophenol using advanced reduction processes (ARP). J. Hazard. Mater. 2018, 357, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ullah, H.; Zada, A.; Muhammad, W.; Ali, S.; Shaheen, S.; Alamgir, M.K.; Ansar, M.Z.; Khan, Z.U.; Bilal, H.; et al. Synthesis of TiO2 modified self-assembled honeycomb ZnO/SnO2 nanocomposites for exceptional photocatalytic degradation of 2,4-dichlorophenol and bisphenol A. Sci. Total Environ. 2020, 746, 141291. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Hu, Z.; Khan, A.; Cheng, W.; Yuan, Y.; Zheng, Z.; Fu, Q.; Luo, W. Highly efficient degradation of 2,4-dichlorophenol over CeO2/g-C3N4 Composites under visible-light irradiation: Detailed reaction pathway and mechanism. J. Hazard. Mater. 2019, 364, 635–644. [Google Scholar] [CrossRef]
- Guo, L.; Yang, J.; Zhang, H.; Wang, R.; Xu, J.; Wang, J. Highly Enhanced Visible-Light Photocatalytic Activity via a Novel Surface Structure of CeO2/g−C3N4 toward Removal of 2,4-Dichlorophenol and Cr(VI). ChemCatChem 2021, 13, 2034–2044. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, G.; Zeng, G.; Guo, Z.; He, K.; Hu, L.; Wu, J.; Zhang, L.; Zhu, Y.; Song, Z. Toxicity mechanisms and synergies of silver nanoparticles in 2,4-dichlorophenol degradation by phanerochaete chrysosporium. J. Hazard. Mater. 2017, 321, 37–46. [Google Scholar] [CrossRef]
- Raziq, F.; Humayun, M.; Ali, A.; Wang, T.; Khan, A.; Fu, Q.; Luo, W.; Zeng, H.; Zheng, Z.; Khan, B.; et al. Synthesis of S-doped porous g-C3N4 by using ionic liquids and subsequently coupled with au-tio2 for Exceptional Cocatalyst-Free Visible-Light Catalytic Activities. Appl. Catal. B Environ. 2018, 237, 1082–1090. [Google Scholar] [CrossRef]
- Li, X.; Anwer, S.; Guan, Q.; Anjum, D.H.; Palmisano, G.; Zheng, L. Coupling Long-Range Facet Junction and Interfacial Heterojunction via Edge-Selective Deposition for High-Performance Z-Scheme Photocatalyst. Adv. Sci. 2022, 9, 2200346. [Google Scholar] [CrossRef]
- Gong, Y.; Quan, X.; Yu, H.; Chen, S.; Zhao, H. Enhanced photocatalytic performance of a two-dimensional BiOIO3/g-C3N4 heterostructured composite with a Z-scheme configuration. Appl. Catal. B Environ. 2018, 237, 947–956. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D Photocatalysts: Electronic-Structure Tailoring, Hybridization, and Applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Z-Scheme 2D/2D α-Fe2O3/g-C3N4 heterojunction for photocatalytic oxidation of nitric oxide. Appl. Catal. B Environ. 2021, 280, 119409. [Google Scholar] [CrossRef]
- Yan, S.; Shi, Y.; Tao, Y.; Zhang, H. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4/Fe2O3 heterojunction. Chem. Eng. J. 2019, 359, 933–943. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D α-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Mu, Z.; Chen, S.; Wang, Y.; Zhang, Z.; Li, Z.; Xin, B.; Jing, L. Controlled Construction of Copper Phthalocyanine/α-Fe2O3 Ultrathin S-Scheme Heterojunctions for Efficient Photocatalytic CO2 Reduction under Wide Visible-Light Irradiation. Small Sci. 2021, 1, 2100050. [Google Scholar] [CrossRef]
- Jin, Y.; Dang, L.; Zhang, H.; Song, C.; Lu, Q.; Gao, F. Synthesis of unit-cell-thick α-Fe2O3 nanosheets and their transformation to γ-Fe2O3 nanosheets with enhanced LIB performances. Chem. Eng. J. 2017, 326, 292–297. [Google Scholar] [CrossRef]
- Bian, J.; Qu, Y.; Fazal, R.; Li, X.; Sun, N.; Jing, L. Accepting Excited High-Energy-Level Electrons and Catalyzing H2 Evolution of Dual-Functional Ag-TiO2 Modifier for Promoting Visible-Light Photocatalytic Activities of Nanosized Oxides. J. Phys. Chem. C 2016, 120, 11831–11836. [Google Scholar] [CrossRef]
- Li, Z.; Luan, P.; Zhang, X.; Qu, Y.; Raziq, F.; Wang, J.; Jing, L. Prolonged lifetime and enhanced separation of photogenerated charges of nanosized α-Fe2O3 by coupling SnO2 for efficient visible-light photocatalysis to convert CO2 and degrade acetaldehyde. Nano Res. 2017, 10, 2321–2331. [Google Scholar] [CrossRef]
- Qu, Y.; Li, Z.; Sun, N.; Zhang, X.; Chen, S.; Jing, L. Visible-light induced electron modulation to improve photoactivities of coral-like Bi2WO6 by coupling SnO2 as a Proper energy platform. Catal. Today 2019, 327, 288–294. [Google Scholar] [CrossRef]
- Chen, S.; Yan, R.; Zhang, X.; Hu, K.; Li, Z.; Humayun, M.; Qu, Y.; Jing, L. Photogenerated electron modulation to dominantly induce efficient 2,4-dichlorophenol degradation on BiOBr nanoplates with different phosphate modification. Appl. Catal. B Environ. 2017, 209, 320–328. [Google Scholar] [CrossRef]
- Humayun, M.; Sun, N.; Raziq, F.; Zhang, X.; Yan, R.; Li, Z.; Qu, Y.; Jing, L. Synthesis of ZnO/Bi-doped porous LaFeO3 nanocomposites as highly efficient nano-photocatalysts dependent on the enhanced utilization of visible-light-excited electrons. Appl. Catal. B Environ. 2018, 231, 23–33. [Google Scholar] [CrossRef]
- Sun, N.; Qu, Y.; Yang, C.; Yang, Z.; Yan, R.E.W.; Zhang, Z.; Li, Z.; Li, H.; Khan, I.S.R.; Jing, L.; et al. Efficiently photocatalytic degradation of monochlorophenol on In-Situ fabricated BiPO4/β-Bi2O3 heterojunction microspheres and O2-Free Hole-induced selective dechloridation conversion with H2 Evolution. Appl. Catal. B Environ. 2020, 263, 118313. [Google Scholar] [CrossRef]
- Yang, J.; Sun, N.; Zhang, Z.; Bian, J.; Qu, Y.; Li, Z.; Xie, M.; Han, W.; Jing, L. Ultrafine SnO2/010 facet-exposed BiVO4 nanocomposites as efficient photoanodes for controllable conversion of 2,4-dichlorophenol via a preferential dechlorination path. ACS Appl. Mater. Interfaces 2020, 12, 28264–28272. [Google Scholar] [CrossRef]
- Chu, X.; Qu, Y.; Zada, A.; Bai, L.; Li, Z.; Yang, F.; Zhao, L.; Zhang, G.; Sun, X.; Yang, Z.D.; et al. Ultrathin Phosphate-Modulated Co Phthalocyanine/g-C3N4 Heterojunction Photocatalysts with Single Co–N4 (II) Sites for Efficient O2 Activation. Adv. Sci. 2020, 7, 2001543. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Wang, D.; Tang, X.; Yan, Y.; Shi, W.; Wang, Y.; Gao, N.; Yao, X.; Dong, H. Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity. Appl. Catal. B Environ. 2016, 182, 115–122. [Google Scholar] [CrossRef]
- Raziq, F.; He, J.; Gan, J.; Humayun, M.; Faheem, M.B.; Iqbal, A.; Hayat, A.; Fazal, S.; Yi, J.; Zhao, Y.; et al. Promoting visible-light photocatalytic activities for carbon nitride based 0D/2D/2D hybrid system: Beyond the conventional 4-electron mechanism. Appl. Catal. B Environ. 2020, 270, 118870. [Google Scholar] [CrossRef]
- Em, S.; Yedigenov, M.; Khamkhash, L.; Atabaev, S.; Molkenova, A.; Poulopoulos, S.G.; Atabaev, T.S. Uncovering the role of surface-attached Ag nanoparticles in photodegradation improvement of rhodamine B by ZnO-Ag nanorods. Nanomaterials 2022, 12, 2882. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, Z.; Feng, J.; Thangamuthu, M.; Yang, F.; Sun, L.; Li, Z.; Qu, Y.; Tang, D.; Lin, Z.; et al. Energy Platform for Directed Charge Transfer in the Cascade Z-Scheme Heterojunction: CO2 Photoreduction without a Cocatalyst. Angew. Chemie—Int. Ed. 2021, 60, 20906–20914. [Google Scholar] [CrossRef]
- Xiao, Y.; Yao, B.; Wang, Z.; Chen, T.; Xiao, X.; Wang, Y. Plasma Ag-modified α-Fe2O3/g-C3N4 self-assembled S-scheme heterojunctions with enhanced photothermal-photocatalytic-fenton performances. Nanomaterials 2022, 12, 4212. [Google Scholar] [CrossRef]
- Khan, I.; Chu, X.; Khan, I.; Liu, H.; Li, W.; Bai, L.; Jing, L. Synthesis of Nanosized Ag-Modified 2D/2D Hydroxylated g-C3N4/TS-1 Z-Scheme Nanocomposites for Efficient Photocatalytic CO2 Reduction. Mater. Res. Bull. 2020, 130, 110926. [Google Scholar] [CrossRef]
- Zha, R.; Shi, T.; He, L.; Zhang, M. Synergetic Excitonic and Defective Effects in Confined SnO2/α-Fe2O3 Nanoheterojunctions for Efficient Photocatalytic Molecular Oxygen Activation. Chem. Eng. J. 2021, 421, 129883. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-Induced Defect Formation and Pt Single Atoms Synergistically Boost Photocatalytic H2 Production in 2D TiO2-Bronze Nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, W.; Li, Z.; Bai, L.; Ansar, M.Z.; Zada, A.; Qu, Y.; Shaheen, S.; Jing, L. Controlled Synthesis of Ag-SnO2/α-Fe2O3 Nanocomposites for Improving Visible-Light Catalytic Activities of Pollutant Degradation and CO2 Reduction. Catalysts 2023, 13, 696. https://doi.org/10.3390/catal13040696
Ali W, Li Z, Bai L, Ansar MZ, Zada A, Qu Y, Shaheen S, Jing L. Controlled Synthesis of Ag-SnO2/α-Fe2O3 Nanocomposites for Improving Visible-Light Catalytic Activities of Pollutant Degradation and CO2 Reduction. Catalysts. 2023; 13(4):696. https://doi.org/10.3390/catal13040696
Chicago/Turabian StyleAli, Wajid, Zhijun Li, Linlu Bai, Muhammad Zaka Ansar, Amir Zada, Yang Qu, Shabana Shaheen, and Liqiang Jing. 2023. "Controlled Synthesis of Ag-SnO2/α-Fe2O3 Nanocomposites for Improving Visible-Light Catalytic Activities of Pollutant Degradation and CO2 Reduction" Catalysts 13, no. 4: 696. https://doi.org/10.3390/catal13040696
APA StyleAli, W., Li, Z., Bai, L., Ansar, M. Z., Zada, A., Qu, Y., Shaheen, S., & Jing, L. (2023). Controlled Synthesis of Ag-SnO2/α-Fe2O3 Nanocomposites for Improving Visible-Light Catalytic Activities of Pollutant Degradation and CO2 Reduction. Catalysts, 13(4), 696. https://doi.org/10.3390/catal13040696






