Hydrodeoxygenation of Bio-Oil over an Enhanced Interfacial Catalysis of Microemulsions Stabilized by Amphiphilic Solid Particles
Abstract
1. Introduction
2. Results and Discussion
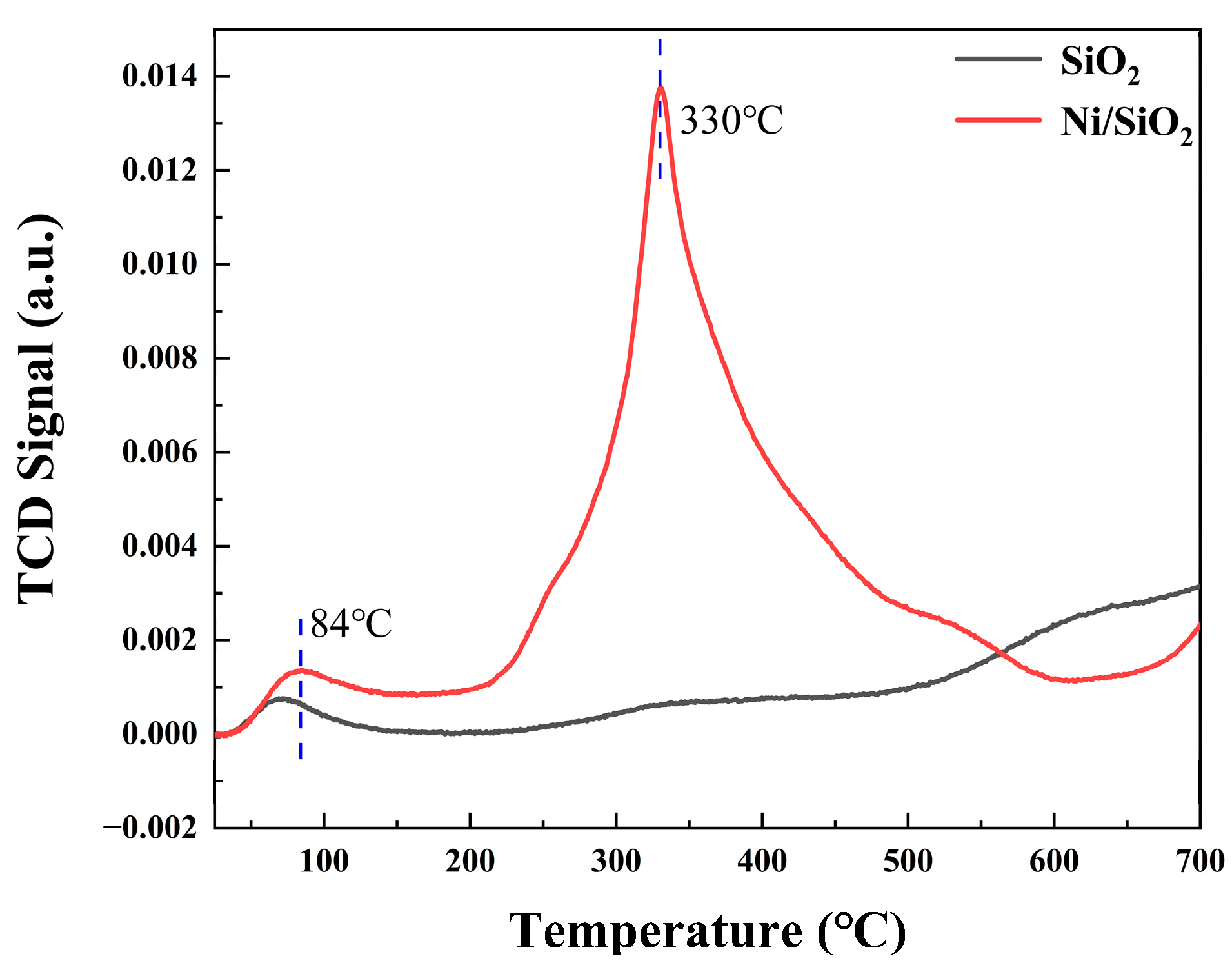
2.1. Characteristics of Solid Particles and Catalyst
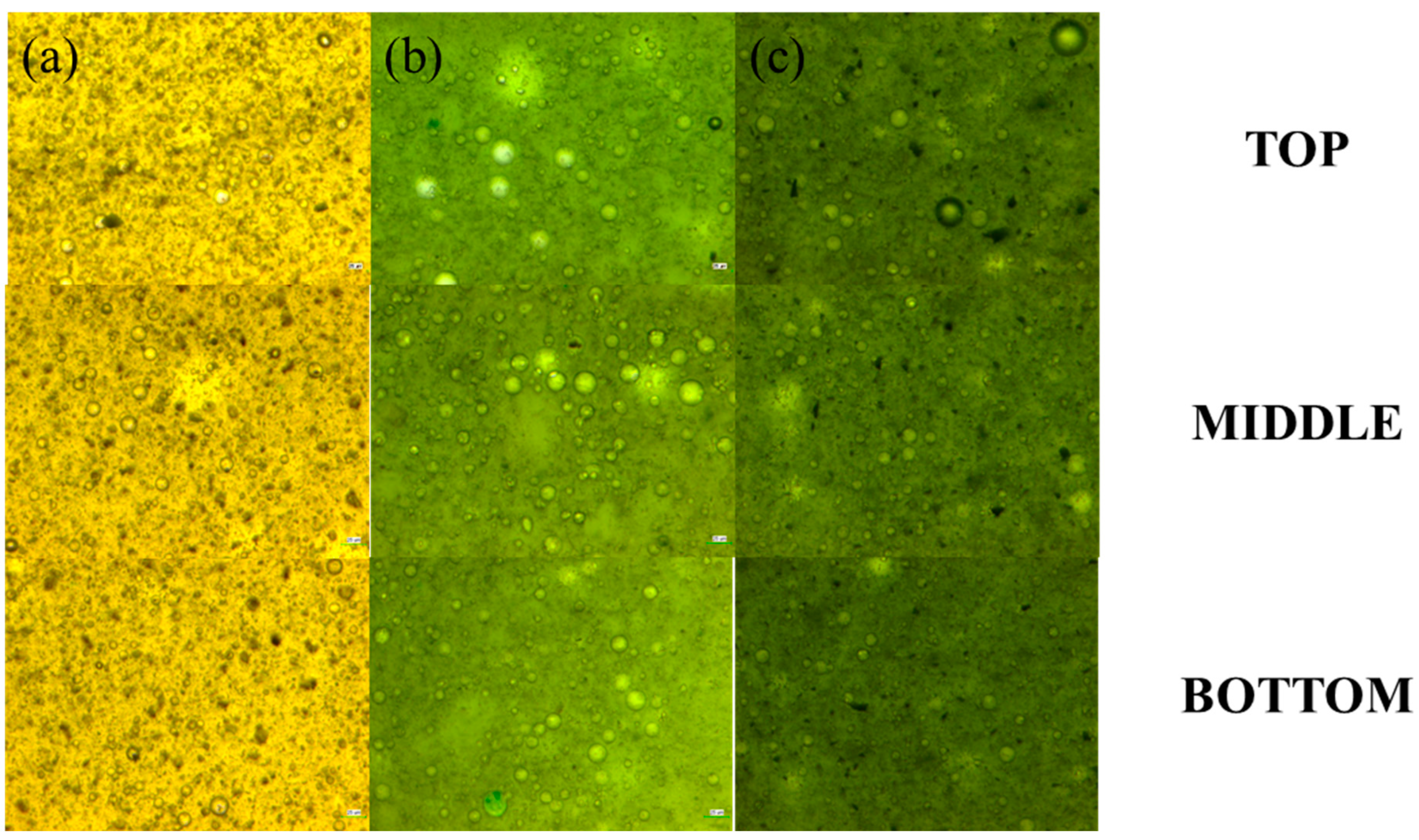
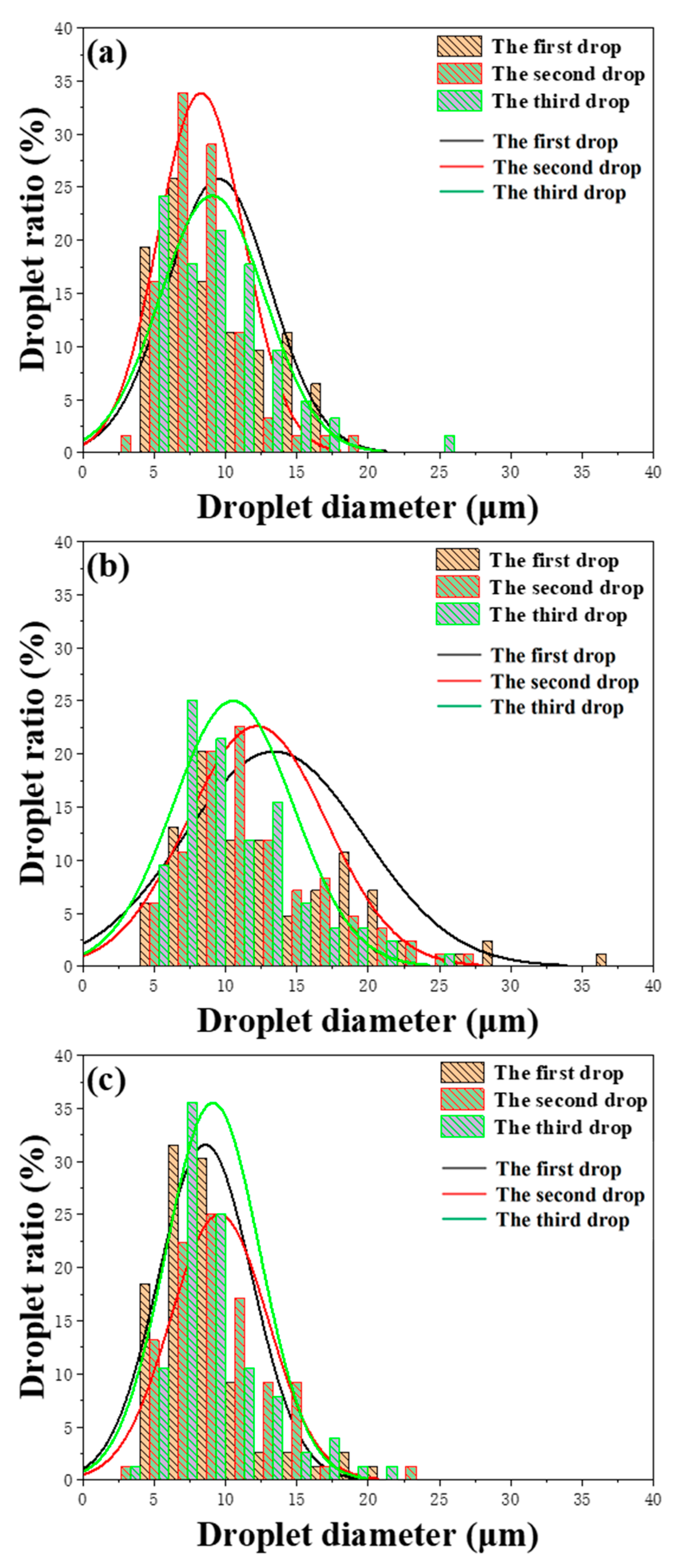
2.2. Stability of Bio-Oil Pickering Emulsion
2.3. Hydrodeoxygenation of Bio-Oil
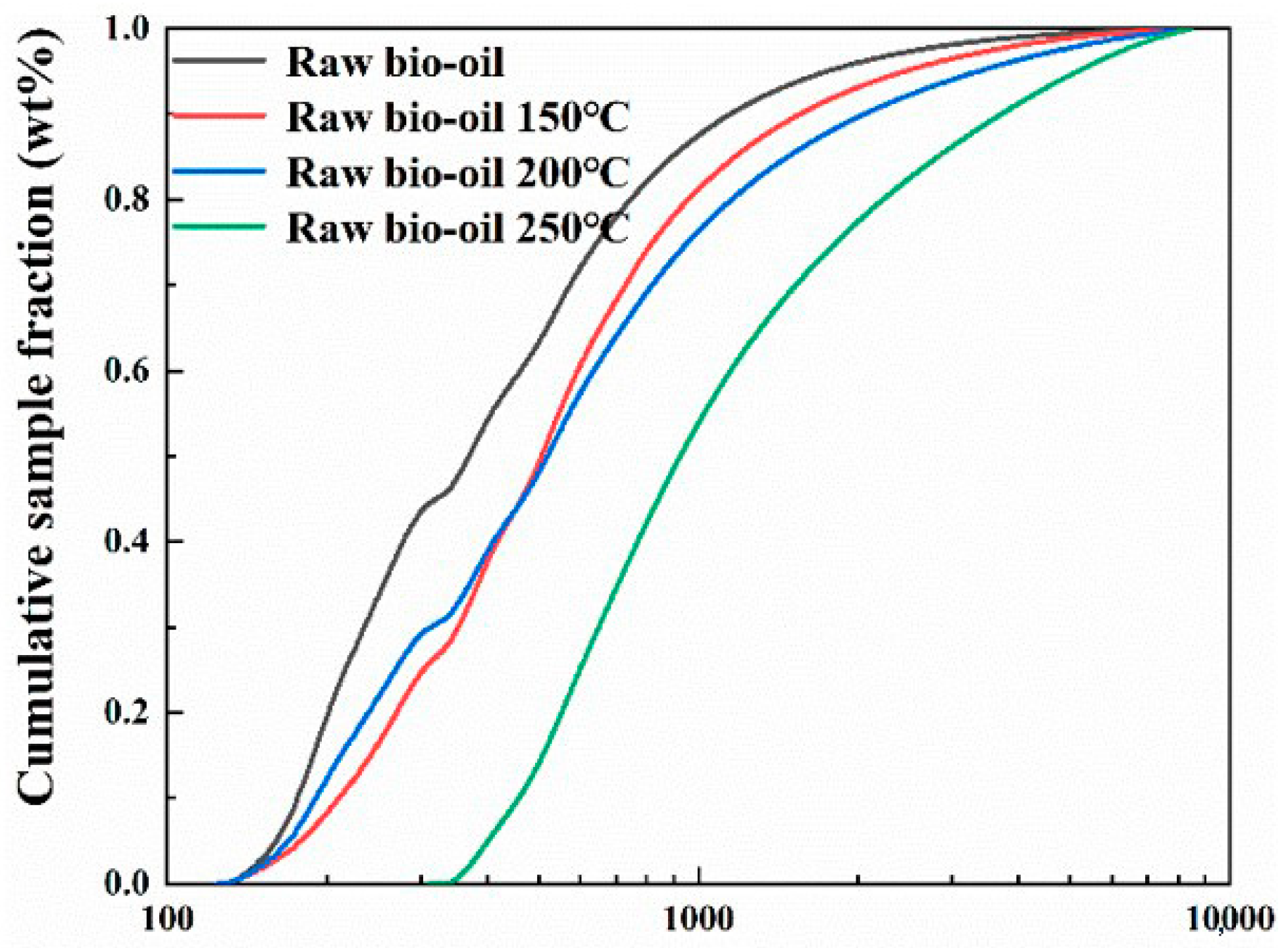
2.3.1. Hydrodeoxygenation of Raw Bio-Oil
2.3.2. Hydrodeoxygenation of Bio-Oil under Pickering Emulsion System
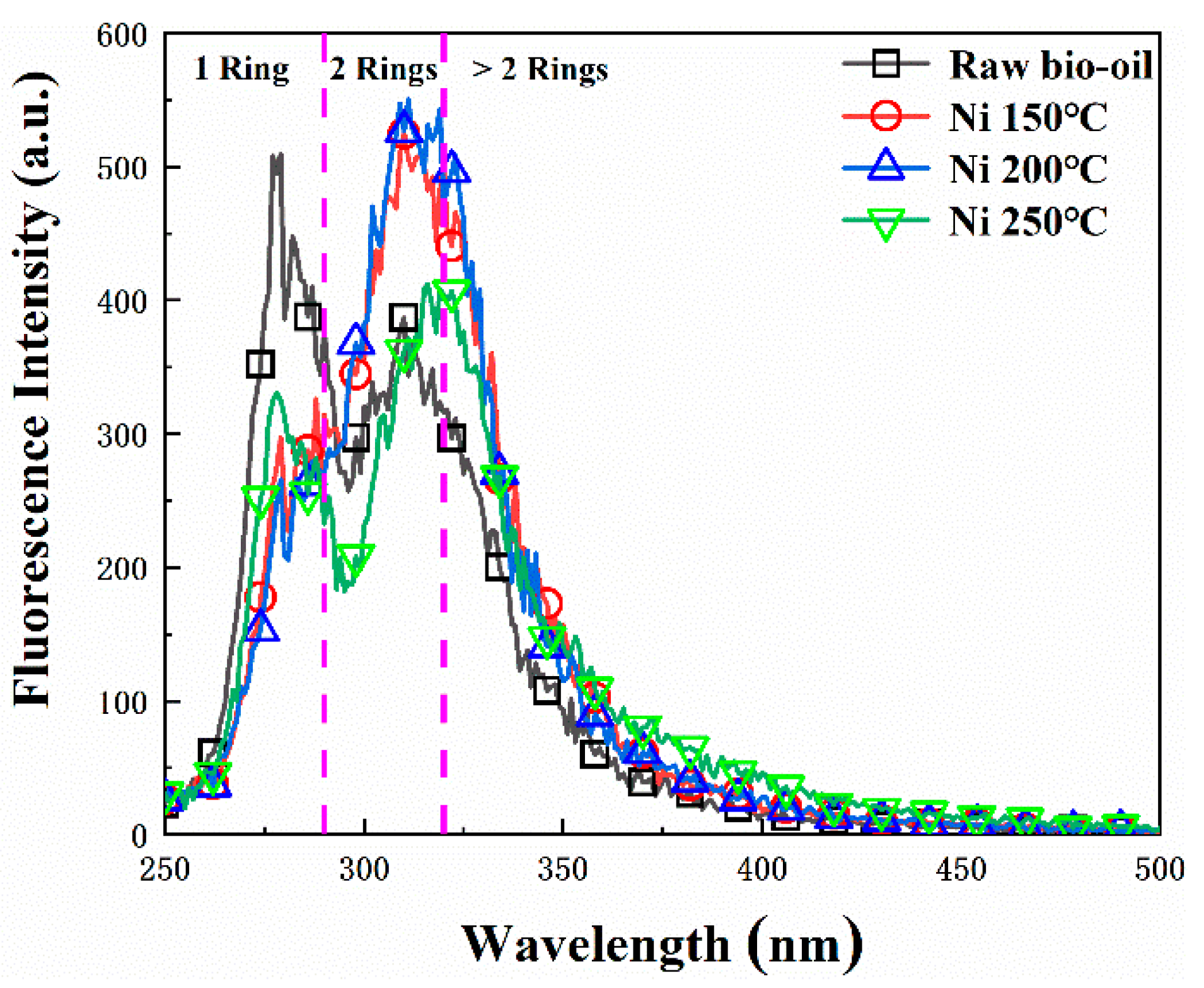
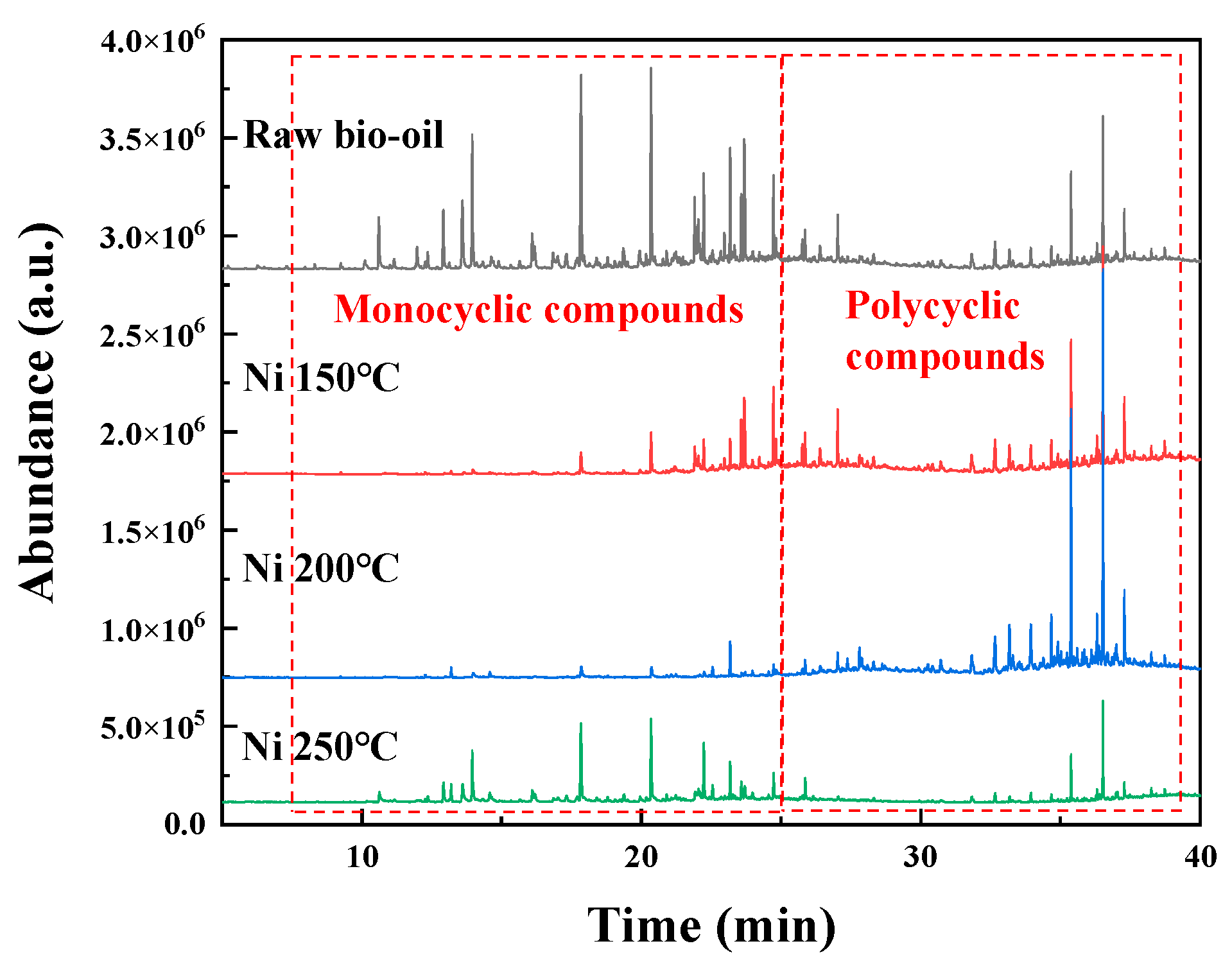
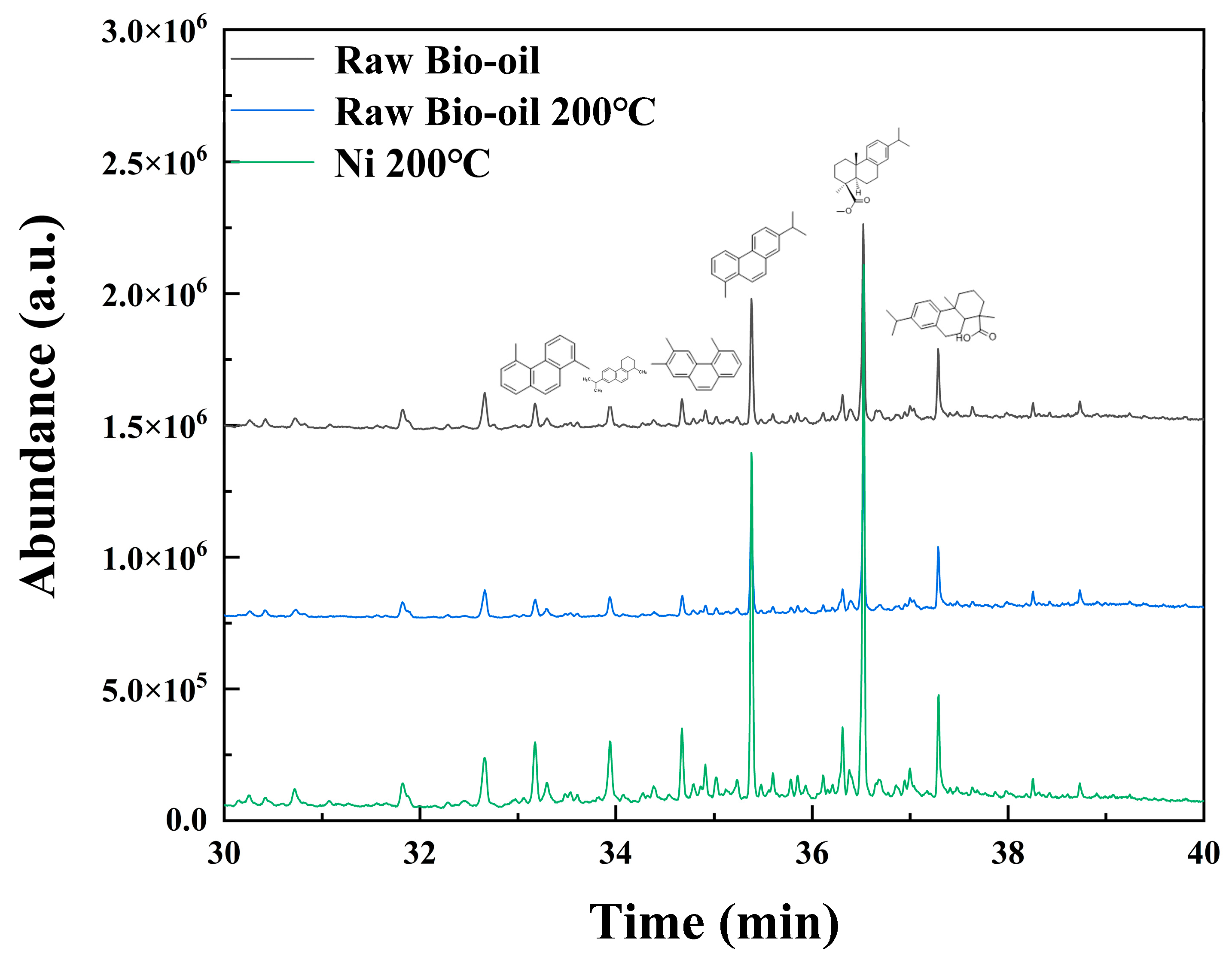
2.3.3. Effect of Pickering Emulsion System on Bio-Oil Hydrodeoxygenation
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation and Characterization
3.3. Emulsion Preparation and Stability Test
3.4. Hydrodeoxygenation Reactions
3.5. Characterization of the Bio-Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sebestyén, V. Renewable and Sustainable Energy Reviews: Environmental impact networks of renewable energy power plants. Renew. Sustain. Energy Rev. 2021, 151, 111626. [Google Scholar] [CrossRef]
- He, Q.; Cheng, C.; Raheem, A.; Lam, S.S. Effect of hydrothermal carbonization on woody biomass: From structure to reactivity. Fuel 2022, 330, 125586. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Gong, Z.; Han, K.; Wang, Y.; Lu, C. Wollastonite decorated with calcium oxide as heterogeneous transesterification catalyst for biodiesel production: Optimized by response surface methodology. Renew. Energy 2020, 159, 873–884. [Google Scholar] [CrossRef]
- Yang, K.; Wu, K.; Zhang, H. Machine learning prediction of the yield and oxygen content of bio-oil via biomass characteristics and pyrolysis conditions. Energy 2022, 254, 124320. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Malek, N.H.; Alias, R.; Syed Hassan, S.S.A. Low-tar, highly burnable gas production from the gasification of pelletized palm empty fruit bunch. Can. J. Chem. Eng. 2023, 101, 1207–1218. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, J.; Hwang, H.T.; Choi, J.H.; Woo, H.C.; Kim, S.S. Catalytic Hydrodeoxygenation of Fast Pyrolysis Bio-Oil from Saccharina japonica Alga for Bio-Oil Upgrading. Catalysts 2019, 9, 1043. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Ma, L.; Zhang, Q.; Chen, P.; Wang, T. Synergistic effects of highly active Ni and acid site on the hydrodeoxygenation of syringol. Catal. Commun. 2017, 91, 1–5. [Google Scholar] [CrossRef]
- Yan, N.; Yuan, Y.; Dykeman, R.; Kou, Y.; Dyson, P.J. Hydrodeoxygenation of Lignin-Derived Phenols into Alkanes by Using Nanoparticle Catalysts Combined with Brønsted Acidic Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 5549–5553. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Pighinelli, A.L.M.T.; Boateng, A.A. Hydrodeoxygenation of fast-pyrolysis bio-oils from various feedstocks using carbon-supported catalysts. Fuel Process. Technol. 2014, 123, 11–18. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Palos, R.; Arandes, J.M.; Castano, P.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Stability of an acid activated carbon based bifunctional catalyst for the raw bio-oil hydrodeoxygenation. Appl. Catal. B Environ. 2017, 203, 389–399. [Google Scholar] [CrossRef]
- Kim, J. Production, separation and applications of phenolic-rich bio-oil—A review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Lin, B.; Zhang, J.; Castano, P.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Efficient catalytic hydrodeoxygenation of phenolic compounds and bio-oil over highly dispersed Ru/TiO2. Fuel Process. Technol. 2019, 184, 12–18. [Google Scholar] [CrossRef]
- Saisu, M.; Sato, T.; Watanabe, M.; Adschiri, T.; Arai, K. Conversion of Lignin with Supercritical Water−Phenol Mixtures. Energy Fuels 2003, 17, 922–928. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, Z.; Syed-Hassan, S.S.A.; Deng, Z.; Zhao, X.; Su, S.; Hu, S. Effect of the pre-reforming by Fe/bio-char catalyst on a two-stage catalytic steam reforming of bio-oil. Fuel 2019, 239, 282–289. [Google Scholar] [CrossRef]
- Jia, L.; Cao, C.; Cheng, Z.; Wang, J.; Huang, J.; Yang, J.; Pan, Y.; Xu, M.; Wang, Y. Ex Situ Catalytic Pyrolysis of Algal Biomass in a Double Microfixed-Bed Reactor: Catalyst Deactivation and Its Coking Behavior. Energy Fuels 2020, 34, 1918–1928. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Y.; Zhao, X.; Songa, Y.; Hu, L.; Wang, X.; Wanga, Z.; Qiu, J. Au/CNTs catalyst for highly selective hydrodeoxygenation of vanillin at the water/oil interface. RSC Adv. 2014, 4, 31932–31936. [Google Scholar] [CrossRef]
- Leng, L.; Li, H.; Yuan, X.; Zhou, W.; Huang, H. Bio-oil upgrading by emulsification/microemulsification: A review. Energy 2018, 161, 214–232. [Google Scholar] [CrossRef]
- Attia, M.; Farag, S.; Chaouki, J. Upgrading of Oils from Biomass and Waste: Catalytic Hydrodeoxygenation. Catalysts 2020, 10, 1381. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Ni, L.; Yu, C.; Wei, Q.; Liu, D.; Qiu, J. Pickering Emulsion Catalysis: Interfacial Chemistry, Catalyst Design, Challenges, and Perspectives. Angew. Chem. 2022, 134, e202115885. [Google Scholar] [CrossRef]
- Zhu, Z.; Tan, H.; Wang, J.; Yu, S.; Zhou, K. Hydrodeoxygenation of vanillin as a bio-oil model over carbonaceous microspheres-supported Pd catalysts in the aqueous phase and Pickering emulsions. Green Chem. 2014, 16, 2636–2643. [Google Scholar] [CrossRef]
- Yang, S.; Chen, G.; Guan, Q.; Xu, H.; Wang, Z.; Liu, B.; Lin, L. An efficient Pd/carbon-silica-alumina catalyst for the hydrodeoxygenation of bio-oil model compound phenol. Mol. Catal. 2021, 510, 111681. [Google Scholar] [CrossRef]
- Rekha, M.; Manjunath, H.R.; Nagaraju, N. Mn/Al2O3 and Mn/ZrO2 as selective catalysts for the synthesis of bis(indolyl)methanes: The role of surface acidity and particle morphology. J. Ind. Eng. Chem. 2013, 19, 337–346. [Google Scholar] [CrossRef]
- Shafaghat, H.; Tsang, Y.F.; Jeon, J.; Kim, J.M.; Kim, Y.; Kim, S.; Park, Y.K. In-situ hydrogenation of bio-oil/bio-oil phenolic compounds with secondary alcohols over a synthesized mesoporous Ni/CeO2 catalyst. Chem. Eng. J. 2020, 382, 122912. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, P.; Wang, K.; Xu, J.; Jiang, J. Catalytic hydrogenation and one step hydrogenation-esterification to remove acetic acid for bio-oil upgrading: Model reaction study. Catal. Sci. Technol. 2016, 6, 7783–7792. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; He, Y.; Feng, J.; Wu, T.; Li, D. Highly efficient PdAg catalyst using a reducible Mg-Ti mixed oxide for selective hydrogenation of acetylene: Role of acidic and basic sites. J. Catal. 2017, 348, 135–145. [Google Scholar] [CrossRef]
- Deng, W.; Xu, K.; Xiong, Z.; Chaiwat, W.; Wang, X.; Su, S.; Xiang, J. Evolution of Aromatic Structures during the Low-Temperature Electrochemical Upgrading of Bio-oil. Energy Fuels 2019, 33, 11292–11301. [Google Scholar] [CrossRef]
- Zhang, M.; Ettelaie, R.; Dong, L.; Li, X.; Li, T.; Zhang, X.; Yang, H. Pickering emulsion droplet-based biomimetic microreactors for continuous flow cascade reactions. Nat. Commun. 2022, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Syed-Hassan, S.S.A.; Xu, J.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Evolution of coke structures during the pyrolysis of bio-oil at various temperatures and heating rates. J. Anal. Appl. Pyrolysis 2018, 134, 336–342. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Syed-Hassan, S.S.A.; Chen, Y.; Jiang, L.; Xu, J.; Hu, S.; Xiang, J. Effect of Ni/Al2O3 mixing on the coking behavior of bio-oil during its pyrolysis: Further understanding based on the interaction between its components. Fuel 2022, 315, 123136. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, Y.; Azis, M.M.; Hu, X.; Deng, W.; Han, H.; Xiang, J. Roles of furfural during the thermal treatment of bio-oil at low temperatures. J. Energy Chem. 2020, 50, 85–95. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Mourant, D.; Gunawan, R.; Zhang, S.; Li, C.Z. Formation of Aromatic Structures during the Pyrolysis of Bio-oil. Energy Fuels 2012, 26, 241–247. [Google Scholar] [CrossRef]
- Deng, W.; Syed-Hassan, S.S.A.; Lam, C.H.; Hu, X.; Wang, X.; Xiong, Z.; Xiang, J. Polymerization during low-temperature electrochemical upgrading of bio-oil: Multi-technique characterization of bio-oil evolution. Energy Convers. Manag. 2022, 253, 115165. [Google Scholar] [CrossRef]








| Sample | Peak Position (°C) | |
|---|---|---|
| Relatively Low Temperature | Relatively High Temperature | |
| Ni/SiO2 | 285, 325 | 500 |
| Element Analysis (ar, %) | Water Content (%) | HHV (MJ/kg) | ||||
|---|---|---|---|---|---|---|
| C | H | N | S | O * | 20.26 | 20.14 |
| 53.18 | 5.02 | 0.1 | 0.70 | 41.00 | ||
| Solid Particles | Hydrophobic Nano-Fumed Silica | CTAB-GO | YK |
|---|---|---|---|
| Average size (nm) | 197.27 | 108.30 | 639.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, K.; Yu, B.; Xiong, Y.; Jiang, L.; Xu, J.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Hydrodeoxygenation of Bio-Oil over an Enhanced Interfacial Catalysis of Microemulsions Stabilized by Amphiphilic Solid Particles. Catalysts 2023, 13, 573. https://doi.org/10.3390/catal13030573
Du K, Yu B, Xiong Y, Jiang L, Xu J, Wang Y, Su S, Hu S, Xiang J. Hydrodeoxygenation of Bio-Oil over an Enhanced Interfacial Catalysis of Microemulsions Stabilized by Amphiphilic Solid Particles. Catalysts. 2023; 13(3):573. https://doi.org/10.3390/catal13030573
Chicago/Turabian StyleDu, Kuan, Beichen Yu, Yimin Xiong, Long Jiang, Jun Xu, Yi Wang, Sheng Su, Song Hu, and Jun Xiang. 2023. "Hydrodeoxygenation of Bio-Oil over an Enhanced Interfacial Catalysis of Microemulsions Stabilized by Amphiphilic Solid Particles" Catalysts 13, no. 3: 573. https://doi.org/10.3390/catal13030573
APA StyleDu, K., Yu, B., Xiong, Y., Jiang, L., Xu, J., Wang, Y., Su, S., Hu, S., & Xiang, J. (2023). Hydrodeoxygenation of Bio-Oil over an Enhanced Interfacial Catalysis of Microemulsions Stabilized by Amphiphilic Solid Particles. Catalysts, 13(3), 573. https://doi.org/10.3390/catal13030573











