Recent Advances on Furan-Based Visible Light Photoinitiators of Polymerization
Abstract
1. Introduction
2. Furane-Based Photoinitiators of Polymerization
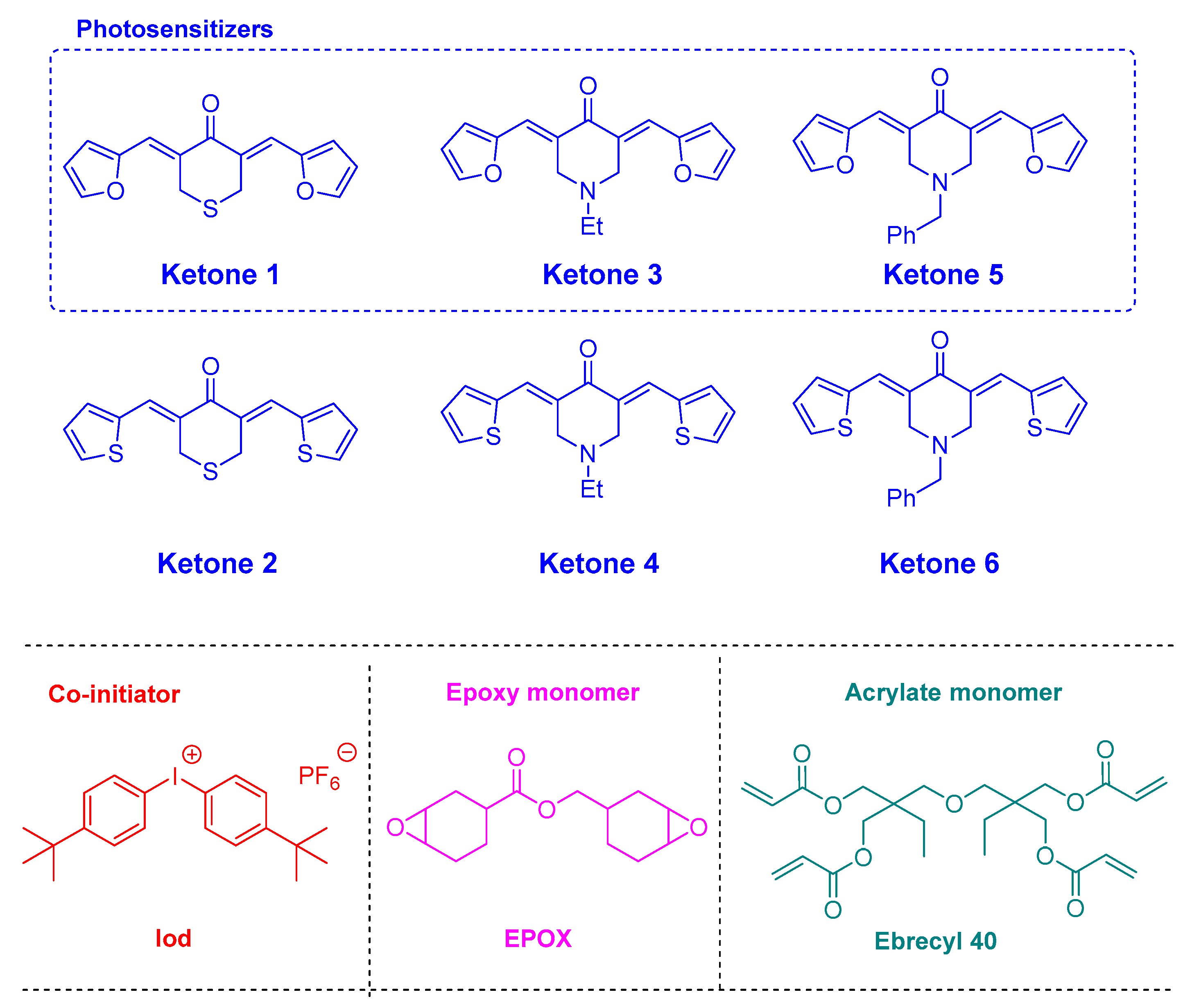
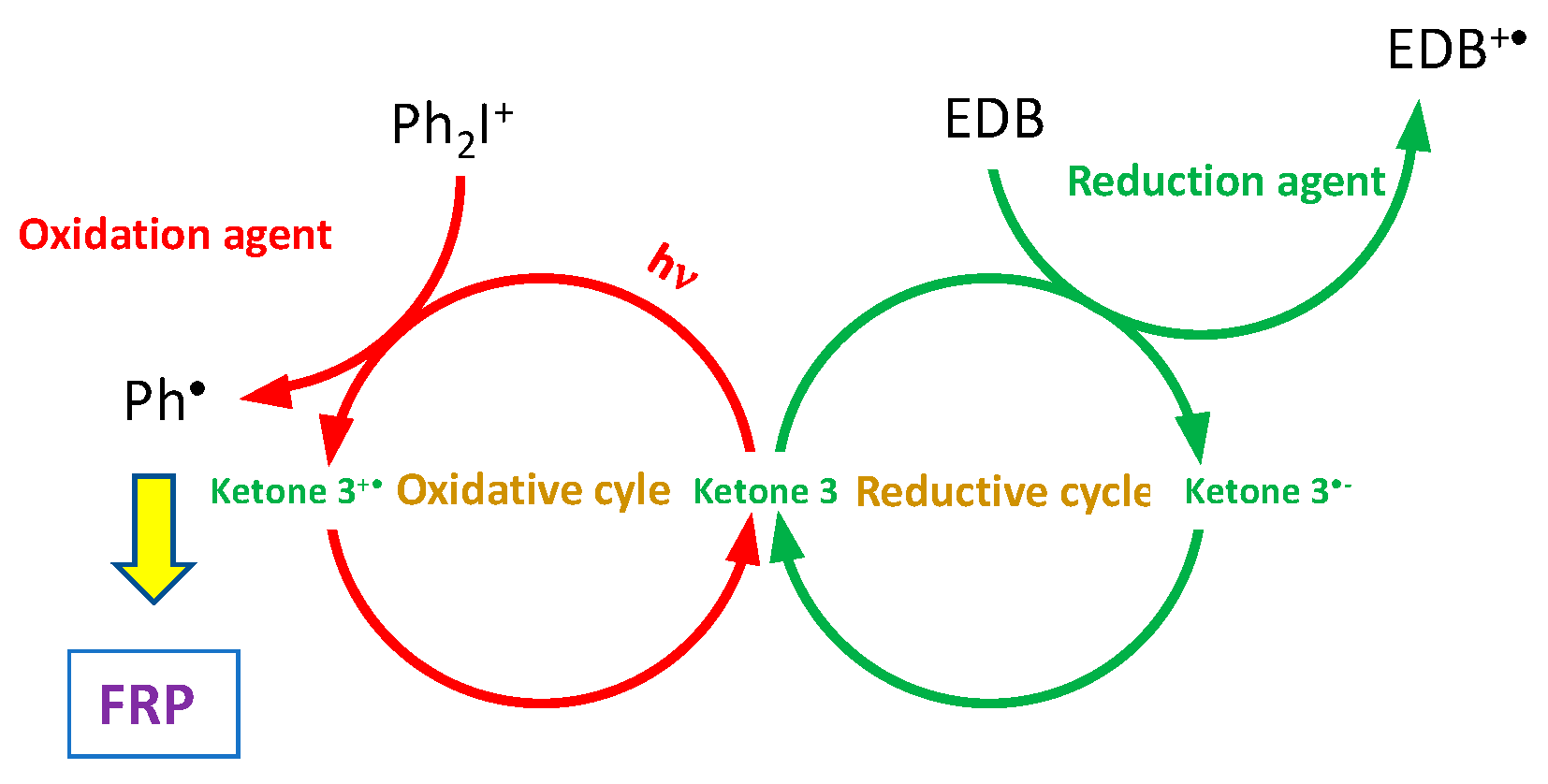
2.1. Benzylidene Ketones
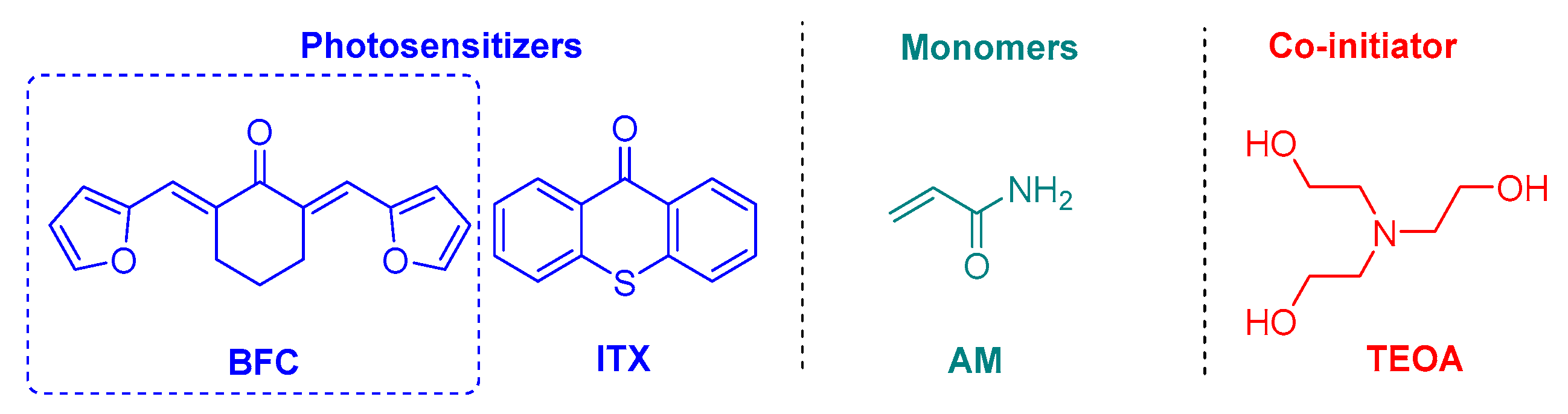
2.2. Charge Transfer Complexes Based on Benzylidene Ketones
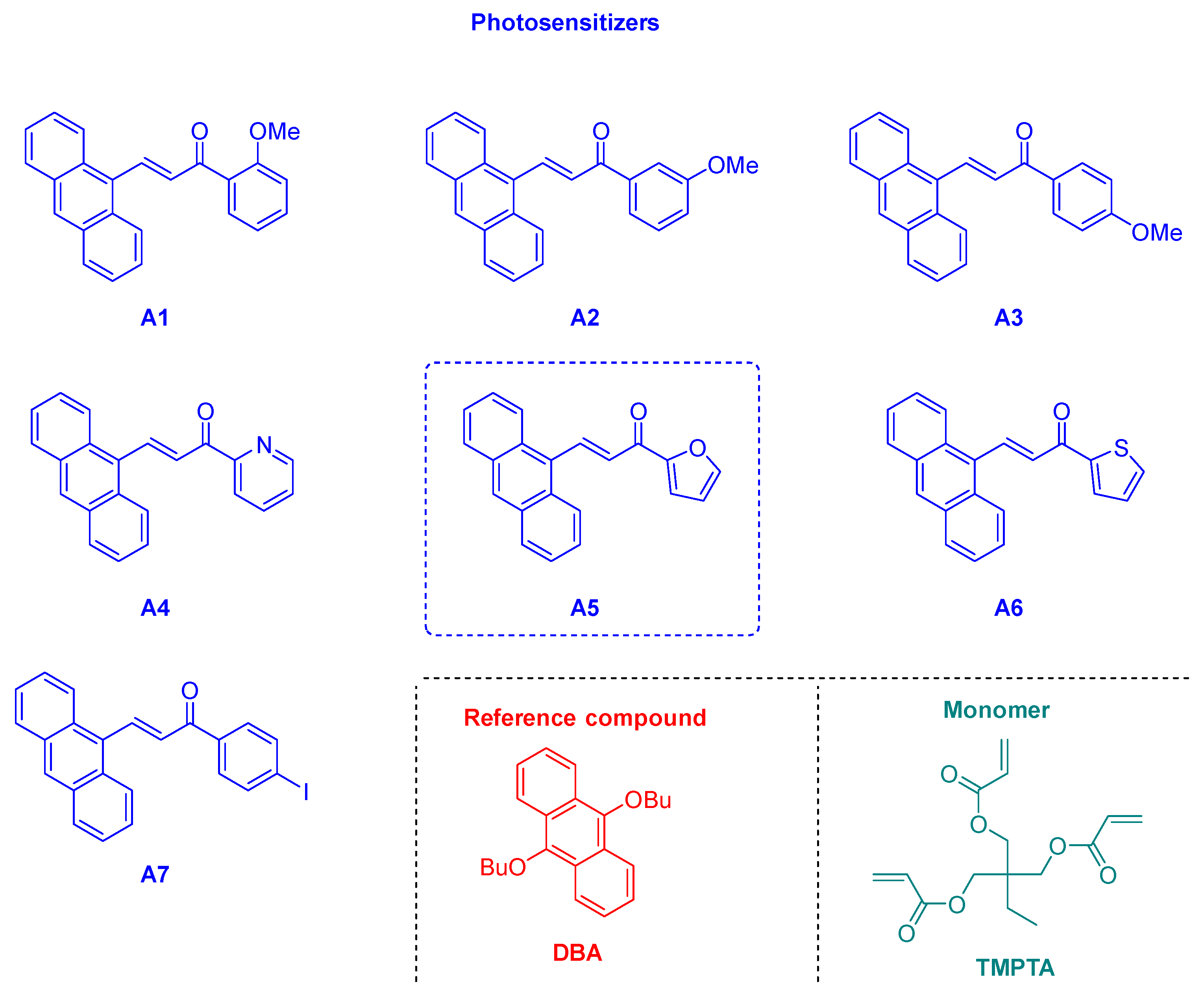
2.3. Chalcones
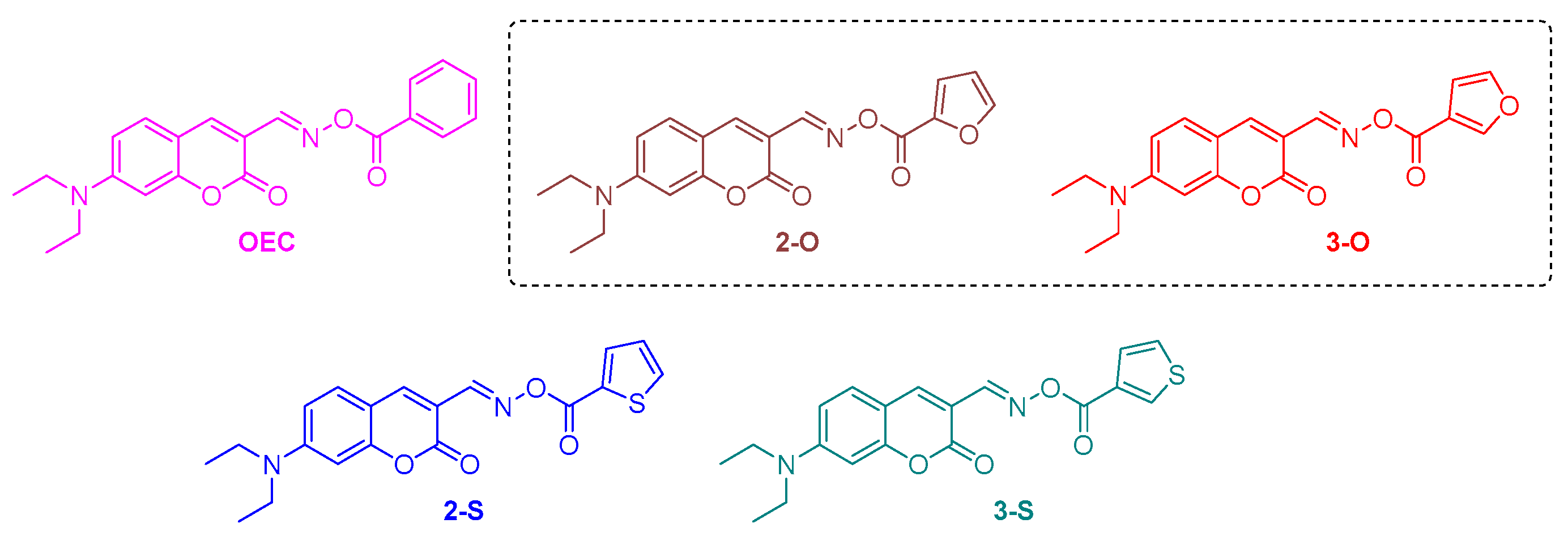
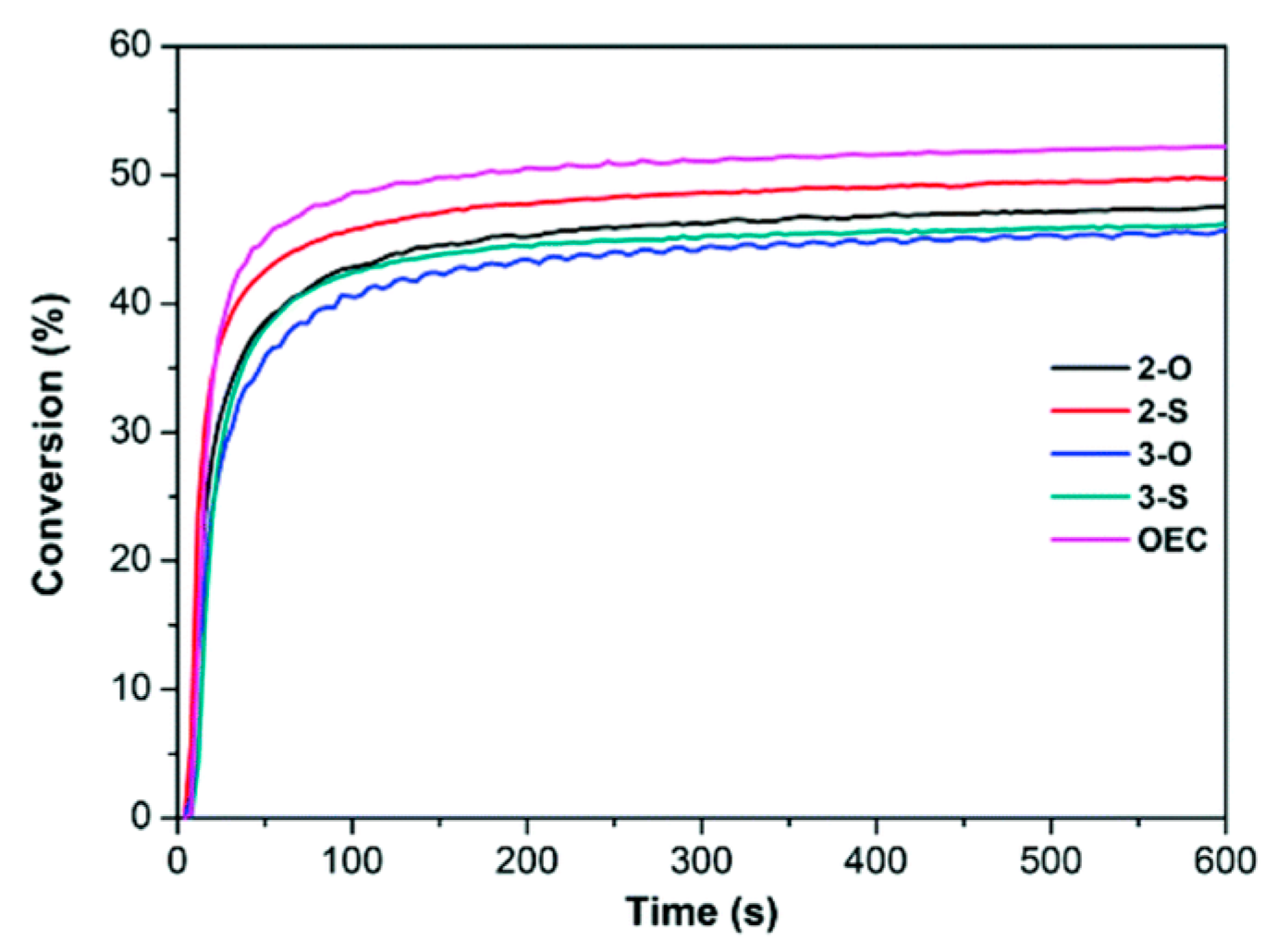
2.4. Coumarins
3. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jasinski, F.; Zetterlund, P.B.; Braun, A.M.; Chemtob, A. Photopolymerization in Dispersed Systems. Prog. Polym. Sci. 2018, 84, 47–88. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, C.; Zhang, R.; Liu, R.; Liu, J. Low Volume Shrinkage Photopolymerization System Using Hydrogen-Bond-Based Monomers. Prog. Org. Coat. 2019, 137, 105308. [Google Scholar] [CrossRef]
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of Photopolymerization of Acrylates with Functionality of 1−6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359. [Google Scholar] [CrossRef]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Maffezzoli, A.; Pietra, A.D.; Rengo, S.; Nicolais, L.; Valletta, G. Photopolymerization of Dental Composite Matrices. Biomaterials 1994, 15, 1221–1228. [Google Scholar] [CrossRef]
- Dikova, T.; Maximov, J.; Todorov, V.; Georgiev, G.; Panov, V. Optimization of Photopolymerization Process of Dental Composites. Processes 2021, 9, 779. [Google Scholar] [CrossRef]
- Andreu, A.; Su, P.-C.; Kim, J.-H.; Ng, C.S.; Kim, S.; Kim, I.; Lee, J.; Noh, J.; Subramanian, A.S.; Yoon, Y.-J. 4D Printing Materials for Vat Photopolymerization. Addit. Manuf. 2021, 44, 102024. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Zhang, Y.; Sun, K.; Liu, S.; Brunel, D.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Xiao, P.; et al. Photopolymerization and 3D/4D Applications Using Newly Developed Dyes: Search around the Natural Chalcone Scaffold in Photoinitiating Systems. Dyes Pigments 2021, 188, 109213. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.-P. Dyes and Chromophores in Polymer Science; ISTE Ltd. and John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; ISBN 978-1-84821-742-3. [Google Scholar]
- Belon, C.; Allonas, X.; Croutxé-barghorn, C.; Lalevée, J. Overcoming the Oxygen Inhibition in the Photopolymerization of Acrylates: A Study of the Beneficial Effect of Triphenylphosphine. J. Polym. Sci. Part Polym. Chem. 2010, 48, 2462–2469. [Google Scholar] [CrossRef]
- Lalevée, J.; Mokbel, H.; Fouassier, J.-P. Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources. Molecules 2015, 20, 7201–7221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dumur, F.; Thirion, D.; Fagour, S.; Vacher, A.; Sallenave, X.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; et al. Multicolor Photoinitiators for Radical and Cationic Polymerization: Monofunctional vs Polyfunctional Thiophene Derivatives. Macromolecules 2013, 46, 6786–6793. [Google Scholar] [CrossRef]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P. Metal and Metal-Free Photocatalysts: Mechanistic Approach and Application as Photoinitiators of Photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Noirbent, G.; Dumur, F. Photoinitiators of Polymerization with Reduced Environmental Impact: Nature as an Unlimited and Renewable Source of Dyes. Eur. Polym. J. 2021, 142, 110109. [Google Scholar] [CrossRef]
- Kim, K.; Sinha, J.; Gao, G.; Childress, K.K.; Sartor, S.M.; Salazar, A.M.; Huang, S.; Musgrave, C.B.; Stansbury, J.W. High-Efficiency Radical Photopolymerization Enhanced by Autonomous Dark Cure. Macromolecules 2020, 53, 5034–5046. [Google Scholar] [CrossRef]
- Shaukat, U.; Sölle, B.; Rossegger, E.; Rana, S.; Schlögl, S. Vat Photopolymerization 3D-Printing of Dynamic Thiol-Acrylate Photopolymers Using Bio-Derived Building Blocks. Polymers 2022, 14, 5377. [Google Scholar] [CrossRef]
- Müller, S.M.; Schlögl, S.; Wiesner, T.; Haas, M.; Griesser, T. Recent Advances in Type I Photoinitiators for Visible Light Induced Photopolymerization. ChemPhotoChem 2022, 6, e202200091. [Google Scholar] [CrossRef]
- Petko, F.; Świeży, A.; Ortyl, J. Photoinitiating Systems and Kinetics of Frontal Photopolymerization Processes—The Prospects for Efficient Preparation of Composites and Thick 3D Structures. Polym. Chem. 2021, 12, 4593–4612. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Awwad, N.; Bui, A.T.; Danilov, E.O.; Castellano, F.N. Visible-Light-Initiated Free-Radical Polymerization by Homomolecular Triplet-Triplet Annihilation. Chem 2020, 6, 3071–3085. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Ortyl, J. Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts 2020, 10, 284. [Google Scholar] [CrossRef]
- Hola, E.; Fiedor, P.; Dzienia, A.; Ortyl, J. Visible-Light Amine Thioxanthone Derivatives as Photoredox Catalysts for Photopolymerization Processes. ACS Appl. Polym. Mater. 2021, 3, 5547–5558. [Google Scholar] [CrossRef]
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, Highly Versatile Bimolecular Photoinitiating Systems for Free-Radical, Cationic and Thiol–Ene Photopolymerization Processes under Low Light Intensity UV and Visible LEDs for 3D Printing Application. RSC Adv. 2020, 10, 7509–7522. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Vidal, L.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structural Effects in the Indanedione Skeleton for the Design of Low Intensity 300–500 Nm Light Sensitive Initiators. Macromolecules 2014, 47, 26–34. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Three-Component Photoinitiating Systems: Towards Innovative Tailor Made High Performance Combinations. RSC Adv. 2012, 2, 2621–2629. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Fries, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. New Thioxanthone and Xanthone Photoinitiators Based on Silyl Radical Chemistry. Polym. Chem. 2011, 2, 1077–1084. [Google Scholar] [CrossRef]
- Lalevée, J.; Telitel, S.; Tehfe, M.A.; Fouassier, J.P.; Curran, D.P.; Lacôte, E. N-Heterocyclic Carbene Boranes Accelerate Type I Radical Photopolymerizations and Overcome Oxygen Inhibition. Angew. Chem. Int. Ed. 2012, 51, 5958–5961. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New Photoinitiating Systems and Strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Liu, S.; Borjigin, T.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers 2023, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D.; Mills, R.W. A Brief History of LED Photopolymerization. Dent. Mater. 2013, 29, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Gallastegui, A.; Dominguez-Alfaro, A.; Lezama, L.; Alegret, N.; Prato, M.; Gómez, M.L.; Mecerreyes, D. Fast Visible-Light Photopolymerization in the Presence of Multiwalled Carbon Nanotubes: Toward 3D Printing Conducting Nanocomposites. ACS Macro Lett. 2022, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, J.; Banaszak Holl, M.M.; Xiao, P. Thiol-Ene Photopolymerization under Blue, Green and Red LED Irradiation. ChemPhotoChem 2021, 5, 571–581. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The Epidemiology of UV Induced Skin Cancer. Consequences Expo. Sunlightelements Assess Prot. 2001, 63, 8–18. [Google Scholar] [CrossRef] [PubMed]
- de Gruijl, F.R. Skin Cancer and Solar UV Radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible Light Initiating Systems for Photopolymerization: Status, Development and Challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High Performance Near-Infrared (NIR) Photoinitiating Systems Operating under Low Light Intensity and in the Presence of Oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Novel Naphthalimide Scaffold Based Iodonium Salt as a One-Component Photoacid/Photoinitiator for Cationic and Radical Polymerization under LED Exposure. Polym. Chem. 2016, 7, 5873–5879. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Zahouily, S.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Ibrahim-Ouali, M.; Gigmes, D.; Hoffmann, N.; Dumur, F.; Lalevée, J. New 1,8-Naphthalimide Derivatives as Photoinitiators for Free-Radical Polymerization Upon Visible Light. Catalysts 2019, 9, 637. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide-Tertiary Amine Derivatives as Blue-Light-Sensitive Photoinitiators. ChemPhotoChem 2018, 2, 481–489. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide Derivatives: Substituent Effects on the Photoinitiating Ability in Polymerizations under Near UV, Purple, White and Blue LEDs (385, 395, 405, 455, or 470 Nm). Macromol. Chem. Phys. 2015, 216, 1782–1790. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide-Phthalimide Derivative Based Photoinitiating Systems for Polymerization Reactions under Blue Lights. J. Polym. Sci. Part Polym. Chem. 2015, 53, 665–674. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Benzophenone-Naphthalimide Derivative as Versatile Photoinitiator of Polymerization under near UV and Visible Lights. J. Polym. Sci. Part Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. N-[2-(Dimethylamino)Ethyl]-1,8-Naphthalimide Derivatives as Photoinitiators under LEDs. Polym. Chem. 2018, 9, 994–1003. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structure Design of Naphthalimide Derivatives: Toward Versatile Photoinitiators for Near-UV/Visible LEDs, 3D Printing, and Water-Soluble Photoinitiating Systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. UV-Violet-Blue LED Induced Polymerizations: Specific Photoinitiating Systems at 365, 385, 395 and 405 Nm. Polymer 2014, 55, 6641–6648. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Blue Light Sensitive Dyes for Various Photopolymerization Reactions: Naphthalimide and Naphthalic Anhydride Derivatives. Macromolecules 2014, 47, 601–608. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Frigoli, M.; Tehfe, M.-A.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide Based Methacrylated Photoinitiators in Radical and Cationic Photopolymerization under Visible Light. Polym. Chem. 2013, 4, 5440–5448. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent Advances on Naphthalic Anhydrides and 1,8-Naphthalimide-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Pertici, V.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Naphthalimide-Based Dyes as Photoinitiators under Visible Light Irradiation and Their Applications: Photocomposite Synthesis, 3D Printing and Polymerization in Water. ChemPhotoChem 2021, 5, 476–490. [Google Scholar] [CrossRef]
- Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Ibrahim-Ouali, M.; Dumur, F.; Lalevée, J. Naphthyl-Naphthalimides as High-Performance Visible Light Photoinitiators for 3D Printing and Photocomposites Synthesis. Catalysts 2021, 11, 1269. [Google Scholar] [CrossRef]
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.-L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel Naphthalimide–Amine Based Photoinitiators Operating under Violet and Blue LEDs and Usable for Various Polymerization Reactions and Synthesis of Hydrogels. Polym. Chem. 2015, 7, 418–429. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Design of High Performance Photoinitiators at 385–405 Nm: Search around the Naphthalene Scaffold. Macromolecules 2014, 47, 973–978. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Amino and Nitro Substituted 2-Amino-1H-Benzo[de]Isoquinoline-1,3(2H)-Diones: As Versatile Photoinitiators of Polymerization from Violet-Blue LED Absorption to a Panchromatic Behavior. Polym. Chem. 2015, 6, 1171–1179. [Google Scholar] [CrossRef]
- Chen, H.; Pieuchot, L.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble/Visible-Light-Sensitive Naphthalimide Derivative-Based Photoinitiating Systems: 3D Printing of Antibacterial Hydrogels. Polym. Chem. 2022, 13, 2918–2932. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Graff, B.; Morlet-Savary, F.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-Naphthalimide Ester Derivatives as Type Ⅰ Photoinitiators for LED Photopolymerization. Mater. Today Chem. 2022, 26, 101137. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Insights into Radical and Cationic Polymerizations upon Visible Light Exposure: Role of Novel Photoinitiator Systems Based on the Pyrene Chromophore. Polym. Chem. 2013, 4, 1625–1634. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Faury, T.; Graff, B.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Core-Pyrene π Structure Organophotocatalysts Usable as Highly Efficient Photoinitiators. Beilstein J. Org. Chem. 2013, 9, 877–890. [Google Scholar] [CrossRef]
- Uchida, N.; Nakano, H.; Igarashi, T.; Sakurai, T. Nonsalt 1-(Arylmethyloxy)Pyrene Photoinitiators Capable of Initiating Cationic Polymerization. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Mishra, A.; Daswal, S. 1-(Bromoacetyl)Pyrene, a Novel Photoinitiator for the Copolymerization of Styrene and Methylmethacrylate. Radiat. Phys. Chem. 2006, 75, 1093–1100. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Design of New Type I and Type II Photoinitiators Possessing Highly Coupled Pyrene–Ketone Moieties. Polym. Chem. 2013, 4, 2313–2324. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Pyrene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Vilà, N.; Graff, B.; Mayer, C.R.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. A Multicolor Photoinitiator for Cationic Polymerization and Interpenetrated Polymer Network Synthesis: 2,7-Di-Tert-Butyldimethyldihydropyrene. Macromol. Rapid Commun. 2013, 34, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Telitel, S.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.P.; Lalevée, J. New Functionalized Aromatic Ketones as Photoinitiating Systems for near Visible and Visible Light Induced Polymerizations. Polymer 2013, 54, 2857–2864. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Telitel, S.; Contal, E.; Dumur, F.; Gigmes, D.; Bertin, D.; Nechab, M.; Graff, B.; Morlet-Savary, F.; et al. Polyaromatic Structures as Organo-Photoinitiator Catalysts for Efficient Visible Light Induced Dual Radical/Cationic Photopolymerization and Interpenetrated Polymer Networks Synthesis. Macromolecules 2012, 45, 4454–4460. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent Photoinitiators Based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Variations on the Benzophenone Skeleton: Novel High Performance Blue Light Sensitive Photoinitiating Systems. Macromolecules 2013, 46, 7661–7667. [Google Scholar] [CrossRef]
- Zhang, J.; Frigoli, M.; Dumur, F.; Xiao, P.; Ronchi, L.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Design of Novel Photoinitiators for Radical and Cationic Photopolymerizations under Near UV and Visible LEDs (385, 395, and 405 Nm). Macromolecules 2014, 47, 2811–2819. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Noirbent, G.; Mau, A.; Chen, H.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Xiao, P.; Dumur, F.; et al. New Multifunctional Benzophenone-Based Photoinitiators with High Migration Stability and Their Applications in 3D Printing. Mater. Chem. Front. 2021, 5, 1982–1994. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Zhang, Y.; Chen, H.; Xiao, P.; Dumur, F.; Lalevée, J. Novel Photoinitiators Based on Benzophenone-Triphenylamine Hybrid Structure for LED Photopolymerization. Macromol. Rapid Commun. 2020, 41, 2000460. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A Monocomponent Bifunctional Benzophenone–Carbazole Type II Photoinitiator for LED Photoinitiating Systems. Polym. Chem. 2020, 11, 3551–3556. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Trifunctional Photoinitiators Based on a Triazine Skeleton for Visible Light Source and UV LED Induced Polymerizations. Macromolecules 2012, 45, 8639–8647. [Google Scholar] [CrossRef]
- Lin, J.-T.; Lalevee, J. Efficacy Modeling of New Multi-Functional Benzophenone-Based System for Free-Radical/Cationic Hybrid Photopolymerization Using 405 Nm LED. 2021, 29, 100. J. Polym. Res. 2021, 29, 100. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. The Carbazole-Bound Ferrocenium Salt as a Specific Cationic Photoinitiator upon near-UV and Visible LEDs (365–405 Nm). Polym. Bull. 2016, 73, 493–507. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Carbazole Scaffold Based Photoinitiator/Photoredox Catalysts: Toward New High Performance Photoinitiating Systems and Application in LED Projector 3D Printing Resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Lara, D.M.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole Derivatives with Thermally Activated Delayed Fluorescence Property as Photoinitiators/Photoredox Catalysts for LED 3D Printing Technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Arar, A.; Ibrahim-Ouali, M.; Duval, S.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; et al. Carbazole-Based Compounds as Photoinitiators for Free Radical and Cationic Polymerization upon near Visible Light Illumination. Photochem. Photobiol. Sci. 2018, 17, 578–585. [Google Scholar] [CrossRef]
- Abdallah, M.; Magaldi, D.; Hijazi, A.; Graff, B.; Dumur, F.; Fouassier, J.-P.; Bui, T.-T.; Goubard, F.; Lalevée, J. Development of New High-Performance Visible Light Photoinitiators Based on Carbazole Scaffold and Their Applications in 3d Printing and Photocomposite Synthesis. J. Polym. Sci. Part Polym. Chem. 2019, 57, 2081–2092. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Carbazole-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Liu, S.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Nitro-Carbazole Based Oxime Esters as Dual Photo/Thermal Initiators for 3D Printing and Composite Preparation. Macromol. Rapid Commun. 2021, 42, 2100207. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, F.; Hijazi, A.; Duval, S.; Lalevée, J.; Dumur, F. 5,12-Dihydroindolo[3,2-a]Carbazole: A Promising Scaffold for the Design of Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2022, 162, 110880. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Schmitt, M.; Nechab, M.; Graff, B.; Morlet-Savary, F.; Xiao, P.; Dumur, F.; Lalevée, J. Effect of Decarboxylation on the Photoinitiation Behavior of Nitrocarbazole-Based Oxime Esters. Macromolecules 2022, 55, 2475–2485. [Google Scholar] [CrossRef]
- Hammoud, F.; Hijazi, A.; Ibrahim-Ouali, M.; Lalevée, J.; Dumur, F. Chemical Engineering around the 5,12-Dihydroindolo[3,2-a]Carbazole Scaffold: Fine Tuning of the Optical Properties of Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2022, 172, 111218. [Google Scholar] [CrossRef]
- Xu, C.; Gong, S.; Wu, X.; Wu, Y.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. High-Efficient Carbazole-Based Photo-Bleachable Dyes as Free Radical Initiators for Visible Light Polymerization. Dyes Pigments 2022, 198, 110039. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Carbazole-Based Oxime Esters as Photoinitiators of Polymerization. Eur. Polym. J. 2022, 175, 111330. [Google Scholar] [CrossRef]
- Hammoud, F.; Giacoletto, N.; Nechab, M.; Graff, B.; Hijazi, A.; Dumur, F.; Lalevée, J. 5,12-Dialkyl-5,12-Dihydroindolo[3,2-a]Carbazole-Based Oxime-Esters for LED Photoinitiating Systems and Application on 3D Printing. Macromol. Mater. Eng. 2022, 307, 2200082. [Google Scholar] [CrossRef]
- Karaca, N.; Ocal, N.; Arsu, N.; Jockusch, S. Thioxanthone-Benzothiophenes as Photoinitiator for Free Radical Polymerization. J. Photochem. Photobiol. Chem. 2016, 331, 22–28. [Google Scholar] [CrossRef]
- Balta, D.K.; Cetiner, N.; Temel, G.; Turgut, Z.; Arsu, N. An Annelated Thioxanthone as a New Type II Initiator. J. Photochem. Photobiol. Chem. 2008, 199, 316–321. [Google Scholar] [CrossRef]
- Balta, D.K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Thioxanthone–Diphenyl Anthracene: Visible Light Photoinitiator. Macromolecules 2012, 45, 119–125. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a Light on an Adaptable Photoinitiator: Advances in Photopolymerizations Initiated by Thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Eren, T.N.; Yasar, N.; Aviyente, V.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevee, J.; Avci, D. Photophysical and Photochemical Studies of Novel Thioxanthone-Functionalized Methacrylates through LED Excitation. Macromol. Chem. Phys. 2016, 217, 1501–1512. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, J. Thioxanthone Photoinitiator Containing Polymerizable N-Aromatic Maleimide for Photopolymerization. J. Polym. Res. 2014, 21, 559. [Google Scholar] [CrossRef]
- Tar, H.; Sevinc Esen, D.; Aydin, M.; Ley, C.; Arsu, N.; Allonas, X. Panchromatic Type II Photoinitiator for Free Radical Polymerization Based on Thioxanthone Derivative. Macromolecules 2013, 46, 3266–3272. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Xiong, Y.; Yang, J.; Tang, H. Thioxanthone Based One-Component Polymerizable Visible Light Photoinitiator for Free Radical Polymerization. RSC Adv. 2016, 6, 66098–66107. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, K.; Xiong, Y.; Wang, X.; Yang, J.; Tang, H. High-Performance and Low Migration One-Component Thioxanthone Visible Light Photoinitiators. Macromol. Chem. Phys. 2017, 218, 1600484. [Google Scholar] [CrossRef]
- Wu, X.; Jin, M.; Malval, J.-P.; Wan, D.; Pu, H. Visible Light-Emitting Diode-Sensitive Thioxanthone Derivatives Used in Versatile Photoinitiating Systems for Photopolymerizations. J. Polym. Sci. Part Polym. Chem. 2017, 55, 4037–4045. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P. Light-Harvesting Organic Photoinitiators of Polymerization. Macromol. Rapid Commun. 2013, 34, 239–245. [Google Scholar] [CrossRef]
- Esen, D.S.; Karasu, F.; Arsu, N. The Investigation of Photoinitiated Polymerization of Multifunctional Acrylates with TX-BT by Photo-DSC and RT-FTIR. Prog. Org. Coat. 2011, 70, 102–107. [Google Scholar] [CrossRef]
- Gencoglu, T.; Eren, T.N.; Lalevée, J.; Avci, D. A Water Soluble, Low Migration, and Visible Light Photoinitiator by Thioxanthone-Functionalization of Poly(Ethylene Glycol)-Containing Poly(β-Amino Ester). Macromol. Chem. Phys. 2022, 223, 2100450. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated Camphorquinone as Visible-Light Photoinitiator for Biomedical Application: Synthesis, Characterization, and Application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Santini, A.; Gallegos, I.T.; Felix, C.M. Photoinitiators in Dentistry: A Review. Prim. Dent. J. 2013, 2, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lalevée, J.; Lu, H.; MacQueen, R.; Kable, S.H.; Schmidt, T.W.; Stenzel, M.H.; Xiao, P. A New Role of Curcumin: As a Multicolor Photoinitiator for Polymer Fabrication under Household UV to Red LED Bulbs. Polym. Chem. 2015, 6, 5053–5061. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Curcumin: A Naturally Occurring Long-Wavelength Photosensitizer for Diaryliodonium Salts. J. Polym. Sci. Part Polym. Chem. 2005, 43, 5217–5231. [Google Scholar] [CrossRef]
- Han, W.; Fu, H.; Xue, T.; Liu, T.; Wang, Y.; Wang, T. Facilely Prepared Blue-Green Light Sensitive Curcuminoids with Excellent Bleaching Properties as High Performance Photosensitizers in Cationic and Free Radical Photopolymerization. Polym. Chem. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Mishra, A.; Daswal, S. Curcumin, A Novel Natural Photoinitiator for the Copolymerization of Styrene and Methylmethacrylate. J. Macromol. Sci. Part A 2005, 42, 1667–1678. [Google Scholar] [CrossRef]
- Zhang, J.; Lalevée, J.; Zhao, J.; Graff, B.; Stenzel, M.H.; Xiao, P. Dihydroxyanthraquinone Derivatives: Natural Dyes as Blue-Light-Sensitive Versatile Photoinitiators of Photopolymerization. Polym. Chem. 2016, 7, 7316–7324. [Google Scholar] [CrossRef]
- Kirschner, J.; Baralle, A.; Paillard, J.; Graff, B.; Becht, J.-M.; Klee, J.E.; Lalevée, J. Silyl Glyoximides: Toward a New Class of Visible Light Photoinitiators. Macromol. Chem. Phys. 2022, 223, 2100500. [Google Scholar] [CrossRef]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.P.; Ortyl, J.; Lalevée, J. Specific Cationic Photoinitiators for near UV and Visible LEDs: Iodonium versus Ferrocenium Structures. J. Appl. Polym. Sci. 2015, 132, 42759. [Google Scholar] [CrossRef]
- Villotte, S.; Gigmes, D.; Dumur, F.; Lalevée, J. Design of Iodonium Salts for UV or Near-UV LEDs for Photoacid Generator and Polymerization Purposes. Molecules 2020, 25, 149. [Google Scholar] [CrossRef] [PubMed]
- Tasdelen, M.A.; Kumbaraci, V.; Jockusch, S.; Turro, N.J.; Talinli, N.; Yagci, Y. Photoacid Generation by Stepwise Two-Photon Absorption: Photoinitiated Cationic Polymerization of Cyclohexene Oxide by Using Benzodioxinone in the Presence of Iodonium Salt. Macromolecules 2008, 41, 295–297. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Wu, F.; Li, M.; Wang, E. Photoreaction and Photopolymerization Studies on Squaraine Dyes/Iodonium Salts Combination. J. Photochem. Photobiol. Chem. 2004, 162, 463–471. [Google Scholar] [CrossRef]
- Jun, L.I.; Miaozhen, L.I.; Huaihai, S.; Yongyuan, Y.; Erjian, W. Photopolymerization Initiated by Dimethylaminochalcone/Diphenyliodonium Salt Combination System Sensitive to Visible Light. Chin. J Polym Sci 1993, 11, 163–170. [Google Scholar]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent Advances and Challenges in the Design of Organic Photoacid and Photobase Generators for Polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Phenothiazine-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 165, 110999. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Novel Chalcone-Based Phenothiazine Derivative Photoinitiators for Visible Light Induced Photopolymerization with Photobleaching and Good Biocompatibility. Prog. Org. Coat. 2022, 167, 106859. [Google Scholar] [CrossRef]
- Rahal, M.; Abdallah, M.; Bui, T.-T.; Goubard, F.; Graff, B.; Dumur, F.; Toufaily, J.; Hamieh, T.; Lalevée, J. Design of New Phenothiazine Derivatives as Visible Light Photoinitiators. Polym. Chem. 2020, 11, 3349–3359. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Zhang, S.; Feng, C.; Zhang, L.; Wang, C.; He, Z.; Zhang, L. Novel A-π-D-π-A Structure Two-Photon Polymerization Initiators Based on Phenothiazine and Carbazole Derivatives. Chem. Pap. 2021, 75, 5249–5256. [Google Scholar] [CrossRef]
- Grishin, D.F.; Lizyakina, O.S.; Vaganova, L.B.; Kaltenberg, A.A.; Grishin, I.D. Radical Polymerization of Methyl Methacrylate in the Presence of Methylene Blue and Organobromides under Visible Light Irradiation. Iran. Polym. J. 2021, 30, 1117–1126. [Google Scholar] [CrossRef]
- Xu, D.; Zou, X.; Zhu, Y.; Yu, Z.; Jin, M.; Liu, R. Phenothiazine-Based Charge-Transfer Complexes as Visible-Light Photoinitiating Systems for Acrylate and Thiol-Ene Photopolymerization. Prog. Org. Coat. 2022, 166, 106772. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, D.; Zhang, Y.; Zhou, Y.; Yagci, Y.; Liu, R. Phenacyl Phenothiazinium Salt as a New Broad-Wavelength-Absorbing Photoinitiator for Cationic and Free Radical Polymerizations. Angew. Chem. Int. Ed. 2021, 60, 16917–16921. [Google Scholar] [CrossRef]
- Chao, P.; Gu, R.; Ma, X.; Wang, T.; Zhao, Y. Thiophene-Substituted Phenothiazine-Based Photosensitisers for Radical and Cationic Photopolymerization Reactions under Visible Laser Beams (405 and 455 Nm). Polym. Chem. 2016, 7, 5147–5156. [Google Scholar] [CrossRef]
- Zhou, T.F.; Ma, X.Y.; Han, W.X.; Guo, X.P.; Gu, R.Q.; Yu, L.J.; Li, J.; Zhao, Y.M.; Wang, T. D–D–A Dyes with Phenothiazine–Carbazole/Triphenylamine as Double Donors in Photopolymerization under 455 Nm and 532 Nm Laser Beams. Polym. Chem. 2016, 7, 5039–5049. [Google Scholar] [CrossRef]
- Wang, M.; Ma, X.; Yu, J.; Jia, X.; Han, D.; Zhou, T.; Yang, J.; Nie, J.; Wang, T. Aromatic Amine–Sulfone/Sulfoxide Conjugated D–π-A–π-D-Type Dyes in Photopolymerization under 405 Nm and 455 Nm Laser Beams. Polym. Chem. 2015, 6, 4424–4435. [Google Scholar] [CrossRef]
- Hao, F.; Liu, Z.; Zhang, M.; Liu, J.; Zhang, S.; Wu, J.; Zhou, H.; Tian, Y. Four New Two-Photon Polymerization Initiators with Varying Donor and Conjugated Bridge: Synthesis and Two-Photon Activity. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 118, 538–542. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Gigmes, D.; Al Mousawi, A.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Copper (Photo)Redox Catalyst for Radical Photopolymerization in Shadowed Areas and Access to Thick and Filled Samples. Macromolecules 2017, 50, 3761–3771. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Kermagoret, A.; Versace, D.-L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper Photoredox Catalysts for Polymerization upon near UV or Visible Light: Structure/Reactivity/Efficiency Relationships and Use in LED Projector 3D Printing Resins. Polym. Chem. 2017, 8, 568–580. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Copper Complexes in Radical Photoinitiating Systems: Applications to Free Radical and Cationic Polymerization upon Visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper and Iron Complexes as Visible-Light-Sensitive Photoinitiators of Polymerization. J. Polym. Sci. Part Polym. Chem. 2015, 53, 2673–2684. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper Complexes: The Effect of Ligands on Their Photoinitiation Efficiencies in Radical Polymerization Reactions under Visible Light. Polym. Chem. 2014, 5, 6350–6357. [Google Scholar] [CrossRef]
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) Complexes as Promising Emitters for Solid–State Light–Emitting Electrochemical Cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395. [Google Scholar] [CrossRef]
- Dumur, F.; Nasr, G.; Wantz, G.; Mayer, C.R.; Dumas, E.; Guerlin, A.; Miomandre, F.; Clavier, G.; Bertin, D.; Gigmes, D. Cationic Iridium Complex for the Design of Soft Salt-Based Phosphorescent OLEDs and Color-Tunable Light-Emitting Electrochemical Cells. Org. Electron. 2011, 12, 1683–1694. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural Effects in the Iridium Complex Series: Photoredox Catalysis and Photoinitiation of Polymerization Reactions under Visible Lights. Macromol. Chem. Phys. 2017, 218, 1700192. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Telitel, S.; Soppera, O.; Lepeltier, M.; Guillaneuf, Y.; Poly, J.; Morlet-Savary, F.; Fioux, P.; Fouassier, J.-P.; et al. Photoredox Catalysis Using a New Iridium Complex as an Efficient Toolbox for Radical, Cationic and Controlled Polymerizations under Soft Blue to Green Lights. Polym. Chem. 2014, 6, 613–624. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.P. Iridium Photocatalysts in Free Radical Photopolymerization under Visible Lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, Y.; Jiang, S.; Nie, J.; Sun, F. Cinnamoylformate Derivatives Photoinitiators with Excellent Photobleaching Ability and Cytocompatibility for Visible LED Photopolymerization. Prog. Org. Coat. 2022, 170, 106969. [Google Scholar] [CrossRef]
- He, X.; Jia, W.; Gao, Y.; Jiang, S.; Nie, J.; Sun, F. Water-Soluble Benzoylformic Acid Photoinitiators for Water-Based LED-Triggered Deep-Layer Photopolymerization. Eur. Polym. J. 2022, 167, 111066. [Google Scholar] [CrossRef]
- He, X.; Gao, Y.; Nie, J.; Sun, F. Methyl Benzoylformate Derivative Norrish Type I Photoinitiators for Deep-Layer Photocuring under Near-UV or Visible LED. Macromolecules 2021, 54, 3854–3864. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators Derived from Natural Product Scaffolds: Monochalcones in Three-Component Photoinitiating Systems and Their Applications in 3D Printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Tang, L.; Nie, J.; Zhu, X. A High Performance Phenyl-Free LED Photoinitiator for Cationic or Hybrid Photopolymerization and Its Application in LED Cationic 3D Printing. Polym. Chem. 2020, 11, 2855–2863. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Allyloxy Ketones as Efficient Photoinitiators with High Migration Stability in Free Radical Polymerization and 3D Printing. Dyes Pigments 2021, 185, 108900. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, Z.; Zhu, H.; Graff, B.; Knopf, S.; Xiao, P.; Dumur, F.; Lalevée, J. Design of Ketone Derivatives as Highly Efficient Photoinitiators for Free Radical and Cationic Photopolymerizations and Application in 3D Printing of Composites. J. Polym. Sci. 2020, 58, 3432–3445. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-Chalcone Derivatives Derived from Natural Products as near-UV/Visible Light Sensitive Photoinitiators for 3D/4D Printing. Mater. Chem. Front. 2021, 5, 901–916. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Sun, K.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Design of Photoinitiating Systems Based on the Chalcone-Anthracene Scaffold for LED Cationic Photopolymerization and Application in 3D Printing. Eur. Polym. J. 2021, 147, 110300. [Google Scholar] [CrossRef]
- Giacoletto, N.; Dumur, F. Recent Advances in Bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules 2021, 26, 3192. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Dumur, F. Recent Advances on Chalcone-Based Photoinitiators of Polymerization. Eur. Polym. J. 2021, 158, 110688. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Gigmes, D.; Xiao, P.; Dumur, F.; Lalevée, J. In Situ Generation of Ag Nanoparticles during Photopolymerization by Using Newly Developed Dyes-Based Three-Component Photoinitiating Systems and the Related 3D Printing Applications and Their Shape Change Behavior. J. Polym. Sci. 2021, 59, 843–859. [Google Scholar] [CrossRef]
- Chen, H.; Vahdati, M.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers 2021, 13, 3195. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Delgove, M.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Chalcone Derivatives as Highly Versatile Photoinitiators for Radical, Cationic, Thiol–Ene and IPN Polymerization Reactions upon Exposure to Visible Light. Polym. Chem. 2014, 5, 382–390. [Google Scholar] [CrossRef]
- Sun, K.; Xu, Y.; Dumur, F.; Morlet-Savary, F.; Chen, H.; Dietlin, C.; Graff, B.; Lalevée, J.; Xiao, P. In Silico Rational Design by Molecular Modeling of New Ketones as Photoinitiators in Three-Component Photoinitiating Systems: Application in 3D Printing. Polym. Chem. 2020, 11, 2230–2242. [Google Scholar] [CrossRef]
- Chen, H.; Regeard, C.; Salmi, H.; Morlet-Savary, F.; Giacoletto, N.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. Interpenetrating Polymer Network Hydrogels Using Natural Based Dyes Initiating Systems: Antibacterial Activity and 3D/4D Performance. Eur. Polym. J. 2022, 166, 111042. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Lu, H.; Li, J.; Yao, L.; Wang, Y.; Zhou, X.; Nie, J.; Zhu, X.; Fu, Z. Study on Pyrrole Chalcone Derivatives Used for Blue LED Free Radical Photopolymerization: Controllable Initiating Activity Achieved through Photoisomerization Property. Eur. Polym. J. 2022, 176, 111393. [Google Scholar] [CrossRef]
- Allegrezza, M.L.; DeMartini, Z.M.; Kloster, A.J.; Digby, Z.A.; Konkolewicz, D. Visible and Sunlight Driven RAFT Photopolymerization Accelerated by Amines: Kinetics and Mechanism. Polym. Chem. 2016, 7, 6626–6636. [Google Scholar] [CrossRef]
- Ciftci, M.; Tasdelen, M.A.; Yagci, Y. Sunlight Induced Atom Transfer Radical Polymerization by Using Dimanganese Decacarbonyl. Polym. Chem. 2014, 5, 600–606. [Google Scholar] [CrossRef]
- Decker, C.; Bendaikha, T. Interpenetrating Polymer Networks. II. Sunlight-Induced Polymerization of Multifunctional Acrylates. J. Appl. Polym. Sci. 1998, 70, 2269–2282. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Schröder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible Light and Sunlight Photoinduced ATRP with Ppm of Cu Catalyst. ACS Macro Lett. 2012, 1, 1219–1223. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. Recent Advances in Sunlight Induced Polymerization: Role of New Photoinitiating Systems Based on the Silyl Radical Chemistry. Polym. Chem. 2011, 2, 1107–1113. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Zheng, H.; Zhou, X.; Nie, J.; Zhu, X. Thermally Activated Pyrrole Chalcone Free Radical Photoinitiator with Excellent Stability to Sunlight. Eur. Polym. J. 2022, 162, 110884. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Zheng, H.; Li, J.; Yao, L.; Wang, Y.; Zhou, X.; Fu, Z.; Nie, J.; Zhu, X. Improvement in the Storage Stability of Free Radical Photocurable Materials under Sunlight Based on the Cis → Trans Photoisomerization of Pyrrole Chalcone Photoinitiator. Prog. Org. Coat. 2022, 171, 107025. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: Sunlight-Induced Cationic Polymerization of Renewable Epoxy Monomers Under Air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Wang, J.; Rivero, M.; Muñoz Bonilla, A.; Sanchez-Marcos, J.; Xue, W.; Chen, G.; Zhang, W.; Zhu, X. Natural RAFT Polymerization: Recyclable-Catalyst-Aided, Opened-to-Air, and Sunlight-Photolyzed RAFT Polymerizations. ACS Macro Lett. 2016, 5, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gao, Y.; Jiang, S.; Sun, F. Naphthalimide Aryl Sulfide Derivative Norrish Type I Photoinitiators with Excellent Stability to Sunlight under Near-UV LED. Macromolecules 2019, 52, 1707–1717. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Nie, J.; Zhu, X. Visible Light and Water-Soluble Photoinitiating System Based on the Charge Transfer Complex for Free Radical Photopolymerization. J. Photochem. Photobiol. Chem. 2020, 402, 112803. [Google Scholar] [CrossRef]
- Allen, N.S.; Chen, W.; Catalina, F.; Green, P.N.; Green, A. Photochemistry of Novel Water-Soluble Para-Substituted Benzophenone Photoinitiators: A Polymerization, Spectroscopic and Flash Photolysis Study. J. Photochem. Photobiol. Chem. 1988, 44, 349–360. [Google Scholar] [CrossRef]
- Alupei, I.C.; Alupei, V.; Ritter, H. Cyclodextrins in Polymer Synthesis: Photoinitiated Free-Radical Polymerization of N-Isopropylacrylamide in Water Initiated by a Methylated β-Cyclodextrin/2-Hydroxy-2-Methyl-1-Phenylpropan-1-One Host/Guest Complex. Macromol. Rapid Commun. 2002, 23, 55–58. [Google Scholar] [CrossRef]
- Balta, D.K.; Temel, G.; Aydin, M.; Arsu, N. Thioxanthone Based Water-Soluble Photoinitiators for Acrylamide Photopolymerization. Eur. Polym. J. 2010, 46, 1374–1379. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly Efficient Water-Soluble Visible Light Photoinitiators. J. Polym. Sci. Part Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Bibaut-Renauld, C.; Burget, D.; Fouassier, J.P.; Varelas, C.G.; Thomatos, J.; Tsagaropoulos, G.; Ryrfors, L.O.; Karlsson, O.J. Use of α-Diketones as Visible Photoinitiators for the Photocrosslinking of Waterborne Latex Paints. J. Polym. Sci. Part Polym. Chem. 2002, 40, 3171–3181. [Google Scholar] [CrossRef]
- Guo, L.; Yang, D.; Xia, L.; Qu, F.; Dou, Y.; Qu, F.; Kong, R.; You, J. A Highly Water-Soluble, Sensitive, Coumarin-Based Fluorescent Probe for Detecting Thiols, and Its Application in Bioimaging. New J. Chem. 2017, 41, 15277–15282. [Google Scholar] [CrossRef]
- Eren, T.N.; Lalevée, J.; Avci, D. Bisphosphonic Acid-Functionalized Water-Soluble Photoinitiators. Macromol. Chem. Phys. 2019, 220, 1900268. [Google Scholar] [CrossRef]
- Eren, T.N.; Lalevée, J.; Avci, D. Water Soluble Polymeric Photoinitiator for Dual-Curing of Acrylates and Methacrylates. J. Photochem. Photobiol. Chem. 2020, 389, 112288. [Google Scholar] [CrossRef]
- Dietliker, K. Chapter 13 Water-Soluble Photoinitiators: Present and Future. In Photopolymerisation Initiating Systems; The Royal Society of Chemistry: London, UK, 2018; pp. 358–430. ISBN 978-1-78262-962-7. [Google Scholar]
- Huang, X.; Wang, X.; Zhao, Y. Study on a Series of Water-Soluble Photoinitiators for Fabrication of 3D Hydrogels by Two-Photon Polymerization. Dyes Pigments 2017, 141, 413–419. [Google Scholar] [CrossRef]
- Li, Z.; Torgersen, J.; Ajami, A.; Mühleder, S.; Qin, X.; Husinsky, W.; Holnthoner, W.; Ovsianikov, A.; Stampfl, J.; Liska, R. Initiation Efficiency and Cytotoxicity of Novel Water-Soluble Two-Photon Photoinitiators for Direct 3D Microfabrication of Hydrogels. RSC Adv. 2013, 3, 15939–15946. [Google Scholar] [CrossRef]
- Knaus, S.; Gruber, H.F. Photoinitiators with Functional Groups. III. Water-Soluble Photoinitiators Containing Carbohydrate Residues. J. Polym. Sci. Part Polym. Chem. 1995, 33, 929–939. [Google Scholar] [CrossRef]
- Kminek, I.; Yagci, Y.; Schnabel, W. A Water-Soluble Poly(Methylphenylsilylene) Derivative as a Photoinitiator of Radical Polymerization of Hydrophilic Vinyl Monomers. Polym. Bull. 1992, 29, 277–282. [Google Scholar] [CrossRef]
- Le, C.M.Q.; Petitory, T.; Wu, X.; Spangenberg, A.; Ortyl, J.; Galek, M.; Infante, L.; Thérien-Aubin, H.; Chemtob, A. Water-Soluble Photoinitiators from Dimethylamino-Substituted Monoacylphosphine Oxide for Hydrogel and Latex Preparation. Macromol. Chem. Phys. 2021, 222, 2100217. [Google Scholar] [CrossRef]
- Akhigbe, J.; Luciano, M.; Zeller, M.; Brückner, C. Mono- and Bisquinoline-Annulated Porphyrins from Porphyrin β,Β′-Dione Oximes. J. Org. Chem. 2015, 80, 499–511. [Google Scholar] [CrossRef]
- Allonas, X.; Lalevée, J.; Fouassier, J.P.; Tachi, H.; Shirai, M.; Tsunooka, M. Triplet State of O-Acyloximes Studied by Time-Resolved Absorption Spectroscopy. Chem. Lett. 2004, 29, 1090–1091. [Google Scholar] [CrossRef]
- Chen, S.; Jin, M.; Malval, J.-P.; Fu, J.; Morlet-Savary, F.; Pan, H.; Wan, D. Substituted Stilbene-Based Oxime Esters Used as Highly Reactive Wavelength-Dependent Photoinitiators for LED Photopolymerization. Polym. Chem. 2019, 10, 6609–6621. [Google Scholar] [CrossRef]
- Fast, D.E.; Lauer, A.; Menzel, J.P.; Kelterer, A.-M.; Gescheidt, G.; Barner-Kowollik, C. Wavelength-Dependent Photochemistry of Oxime Ester Photoinitiators. Macromolecules 2017, 50, 1815–1823. [Google Scholar] [CrossRef]
- Hu, P.; Qiu, W.; Naumov, S.; Scherzer, T.; Hu, Z.; Chen, Q.; Knolle, W.; Li, Z. Conjugated Bifunctional Carbazole-Based Oxime Esters: Efficient and Versatile Photoinitiators for 3D Printing under One- and Two-Photon Excitation. ChemPhotoChem 2020, 4, 224–232. [Google Scholar] [CrossRef]
- Lee, W.J.; Kwak, H.S.; Lee, D.; Oh, C.; Yum, E.K.; An, Y.; Halls, M.D.; Lee, C.-W. Design and Synthesis of Novel Oxime Ester Photoinitiators Augmented by Automated Machine Learning. Chem. Mater. 2022, 34, 116–127. [Google Scholar] [CrossRef]
- Ma, X.; Cao, D.; Fu, H.; You, J.; Gu, R.; Fan, B.; Nie, J.; Wang, T. Multicomponent Photoinitiating Systems Containing Arylamino Oxime Ester for Visible Light Photopolymerization. Prog. Org. Coat. 2019, 135, 517–524. [Google Scholar] [CrossRef]
- Pang, Y.; Fan, S.; Wang, Q.; Oprych, D.; Feilen, A.; Reiner, K.; Keil, D.; Slominsky, Y.L.; Popov, S.; Zou, Y.; et al. NIR-Sensitized Activated Photoreaction between Cyanines and Oxime Esters: Free-Radical Photopolymerization. Angew. Chem. Int. Ed. 2020, 59, 11440–11447. [Google Scholar] [CrossRef]
- Sameshima, K.; Kura, H.; Matsuoka, Y.; Sotome, H.; Miyasaka, H. Improvement of the Photopolymerization and Bottom-Curing Performance of Benzocarbazole Oxime Ester Photoinitiators with Red-Shifted Absorption. Jpn. J. Appl. Phys. 2022, 61, 035504. [Google Scholar] [CrossRef]
- Qiu, W.; Li, M.; Yang, Y.; Li, Z.; Dietliker, K. Cleavable Coumarin-Based Oxime Esters with Terminal Heterocyclic Moieties: Photobleachable Initiators for Deep Photocuring under Visible LED Light Irradiation. Polym. Chem. 2020, 11, 1356–1363. [Google Scholar] [CrossRef]
- Breloy, L.; Negrell, C.; Mora, A.-S.; Li, W.S.J.; Brezová, V.; Caillol, S.; Versace, D.-L. Vanillin Derivative as Performing Type I Photoinitiator. Eur. Polym. J. 2020, 132, 109727. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II Photoinitiators as Activators for Photoinduced Metal-Free Atom Transfer Radical Polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Kuo, Y.-T. Photocuring Kinetic Studies of TMPTMA Monomer by Type II Photoinitiators of Different Weight Ratios of 2-Chlorohexaaryl Biimidazole (o-Cl-HABI) and N-Phenylglycine (NPG). J. Photopolym. Sci. Technol. 2018, 31, 487–492. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Ali, S.; Akram, M.Y.; Nie, J.; Zhu, X. The Effect of Polyethylene Glycoldiacrylate Complexation on Type II Photoinitiator and Promotion for Visible Light Initiation System. J. Photochem. Photobiol. Chem. 2019, 384, 112037. [Google Scholar] [CrossRef]
- Kirschner, J.; Baralle, A.; Graff, B.; Becht, J.-M.; Klee, J.E.; Lalevée, J. 1-Aryl-2-(Triisopropylsilyl)Ethane-1,2-Diones: Toward a New Class of Visible Type I Photoinitiators for Free Radical Polymerization of Methacrylates. Macromol. Rapid Commun. 2019, 40, 1900319. [Google Scholar] [CrossRef]
- Christmann, J.; Allonas, X.; Ley, C.; Ibrahim, A.; Croutxé-Barghorn, C. Triazine-Based Type-II Photoinitiating System for Free Radical Photopolymerization: Mechanism, Efficiency, and Modeling. Macromol. Chem. Phys. 2017, 218, 1600597. [Google Scholar] [CrossRef]
- Kreutzer, J.; Kaya, K.; Yagci, Y. Poly(Propylene Oxide)-Thioxanthone as One-Component Type II Polymeric Photoinitiator for Free Radical Polymerization with Low Migration Behavior. Eur. Polym. J. 2017, 95, 71–81. [Google Scholar] [CrossRef]
- Kabatc, J.; Iwińska, K.; Balcerak, A.; Kwiatkowska, D.; Skotnicka, A.; Czech, Z.; Bartkowiak, M. Onium Salts Improve the Kinetics of Photopolymerization of Acrylate Activated with Visible Light. RSC Adv. 2020, 10, 24817–24829. [Google Scholar] [CrossRef]
- Kocaarslan, A.; Kütahya, C.; Keil, D.; Yagci, Y.; Strehmel, B. Near-IR and UV-LED Sensitized Photopolymerization with Onium Salts Comprising Anions of Different Nucleophilicities. ChemPhotoChem 2019, 3, 1127–1132. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef]
- Kabatc, J.; Ortyl, J.; Kostrzewska, K. New Kinetic and Mechanistic Aspects of Photosensitization of Iodonium Salts in Photopolymerization of Acrylates. RSC Adv. 2017, 7, 41619–41629. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nomura, K.; Inagaki, A. Cu–Pd Dinuclear Complexes with Earth-Abundant Cu Photosensitizer: Synthesis and Photopolymerization. Organometallics 2020, 39, 2464–2469. [Google Scholar] [CrossRef]
- Wang, J.; Mathias, L.J. A Polymerizable Photosensitizer and Its Photopolymerization Kinetics. Polym. Int. 2005, 54, 1537–1542. [Google Scholar] [CrossRef]
- Aydogan, B.; Gunbas, G.E.; Durmus, A.; Toppare, L.; Yagci, Y. Highly Conjugated Thiophene Derivatives as New Visible Light Sensitive Photoinitiators for Cationic Polymerization. Macromolecules 2010, 43, 101–106. [Google Scholar] [CrossRef]
- Aydogan, B.; Gundogan, A.S.; Ozturk, T.; Yagci, Y. A Dithienothiophene Derivative as a Long-Wavelength Photosensitizer for Onium Salt Photoinitiated Cationic Polymerization. Macromolecules 2008, 41, 3468–3471. [Google Scholar] [CrossRef]
- Aydogan, B.; Gundogan, A.S.; Ozturk, T.; Yagci, Y. Polythiophene Derivatives by Step-Growth Polymerizationvia Photoinduced Electron Transfer Reactions. Chem. Commun. 2009, 41, 6300–6302. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, B.; Yagci, Y.; Toppare, L.; Jockusch, S.; Turro, N.J. Photoinduced Electron Transfer Reactions of Highly Conjugated Thiophenes for Initiation of Cationic Polymerization and Conjugated Polymer Formation. Macromolecules 2012, 45, 7829–7834. [Google Scholar] [CrossRef]
- Beyazit, S.; Aydogan, B.; Osken, I.; Ozturk, T.; Yagci, Y. Long Wavelength Photoinitiated Free Radical Polymerization Using Conjugated Thiophene Derivatives in the Presence of Onium Salts. Polym. Chem. 2011, 2, 1185–1189. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Thiophene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 169, 111120. [Google Scholar] [CrossRef]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Tabrizi, F.P.F.; Kamari, N. Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L. Recent Advances of Furan and Its Derivatives Based Semiconductor Materials for Organic Photovoltaics. Small Methods 2021, 5, 2100493. [Google Scholar] [CrossRef]
- Gidron, O.; Dadvand, A.; Wei-Hsin Sun, E.; Chung, I.; Shimon, L.J.W.; Bendikov, M.; Perepichka, D.F. Oligofuran-Containing Molecules for Organic Electronics. J. Mater. Chem. C 2013, 1, 4358–4367. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Sun, J.-P.; McCormick, T.M.; Hill, I.G.; Welch, G.C. Unusual Loss of Electron Mobility upon Furan for Thiophene Substitution in a Molecular Semiconductor. Org. Electron. 2015, 18, 118–125. [Google Scholar] [CrossRef]
- Mulay, S.V.; Bogoslavky, B.; Galanti, I.; Galun, E.; Gidron, O. Bifuran-Imide: A Stable Furan Building Unit for Organic Electronics. J. Mater. Chem. C 2018, 6, 11951–11955. [Google Scholar] [CrossRef]
- Zhao, Z.; Nie, H.; Ge, C.; Cai, Y.; Xiong, Y.; Qi, J.; Wu, W.; Kwok, R.T.K.; Gao, X.; Qin, A.; et al. Furan Is Superior to Thiophene: A Furan-Cored AIEgen with Remarkable Chromism and OLED Performance. Adv. Sci. 2017, 4, 1700005. [Google Scholar] [CrossRef]
- Wheeler, D.; Tannir, S.; Smith, E.; Tomlinson, A.; Jeffries-EL, M. A Computational and Experimental Investigation of Deep-Blue Light-Emitting Tetraaryl-Benzobis[1,2-d:4,5-D′]Oxazoles. Mater. Adv. 2022, 3, 3842–3852. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.K.; Kim, H.J.; Choi, S.; Jung, S.W.; Lee, H.; Kim, J.Y.; Yoon, D.-W.; Han, C.W.; Chae, W.-S.; et al. Asymmetric Host Molecule Bearing Pyridine Core for Highly Efficient Blue Thermally Activated Delayed Fluorescence OLEDs. Chem.–Eur. J. 2020, 26, 16383–16391. [Google Scholar] [CrossRef]
- Bakhiya, N.; Appel, K.E. Toxicity and Carcinogenicity of Furan in Human Diet. Arch. Toxicol. 2010, 84, 563–578. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for Public Health Related to the Presence of Furan and Methylfurans in Food. EFSA J. 2017, 15, e05005. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of Furan Derivative as LED Light Photoinitiator: One-Pot, Low Usage, Photobleaching for Light Color 3D Printing. Dyes Pigments 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Photobleachable Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2023, 186, 111874. [Google Scholar] [CrossRef]
- Duan, C.; Adam, V.; Byrdin, M.; Ridard, J.; Kieffer-Jaquinod, S.; Morlot, C.; Arcizet, D.; Demachy, I.; Bourgeois, D. Structural Evidence for a Two-Regime Photobleaching Mechanism in a Reversibly Switchable Fluorescent Protein. J. Am. Chem. Soc. 2013, 135, 15841–15850. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mao, H.; Bao, C.; Wan, D.; Jin, M. Fused Carbazole–Coumarin–Ketone Dyes: High Performance and Photobleachable Photoinitiators in Free Radical Photopolymerization for Deep Photocuring under Visible LED Light Irradiation. Polym. Chem. 2022, 13, 3367–3376. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, G.; Liu, X.; Liu, R. Coumarin-Based Oxime Esters: Photobleachable and Versatile Unimolecular Initiators for Acrylate and Thiol-Based Click Photopolymerization under Visible Light-Emitting Diode Light Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 16113–16123. [Google Scholar] [CrossRef]
- Liao, W.; Xu, C.; Wu, X.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Photobleachable Cinnamoyl Dyes for Radical Visible Photoinitiators. Dyes Pigments 2020, 178, 108350. [Google Scholar] [CrossRef]
- Liao, W.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Design, Synthesis and Properties of Carbazole-Indenedione Based Photobleachable Photoinitiators for Photopolymerization. J. Photochem. Photobiol. Chem. 2023, 435, 114297. [Google Scholar] [CrossRef]
- Mitterbauer, M.; Knaack, P.; Naumov, S.; Markovic, M.; Ovsianikov, A.; Moszner, N.; Liska, R. Acylstannanes: Cleavable and Highly Reactive Photoinitiators for Radical Photopolymerization at Wavelengths above 500 Nm with Excellent Photobleaching Behavior. Angew. Chem. Int. Ed. 2018, 57, 12146–12150. [Google Scholar] [CrossRef]
- Nan, X.; Huang, Y.; Fan, Q.; Shao, J.; Yao, Y. Different Photoinitiating Ability and Photobleaching Efficiency of Erythrosine B Derivatives in Radical/Cationic Photopolymerization. Fibers Polym. 2017, 18, 1644–1651. [Google Scholar] [CrossRef]
- Rothman, J.H.; Still, W.C. A New Generation of Fluorescent Chemosensors Demonstrate Improved Analyte Detection Sensitivity and Photobleaching Resistance. Bioorg. Med. Chem. Lett. 1999, 9, 509–512. [Google Scholar] [CrossRef]
- Takemura, F. Dye-Sensitized Photopolymerization of Vinyl Monomers. II. Photo-Bleaching of Acridine Yellow in Some Vinyl Monomers. Bull. Chem. Soc. Jpn. 1962, 35, 1078–1086. [Google Scholar] [CrossRef]
- Terrones, G.; Pearlstein, A.J. Effects of Optical Attenuation and Consumption of a Photobleaching Initiator on Local Initiation Rates in Photopolymerizations. Macromolecules 2001, 34, 3195–3204. [Google Scholar] [CrossRef]
- Wu, X.; Gong, S.; Chen, Z.; Hou, J.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Photobleachable Bis-Chalcones-Based Oxime Ester Dyes for Radical Visible Photopolymerization. Dyes Pigments 2022, 205, 110556. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Liu, F.; Gigmes, D.; Sun, K.; Zhang, Y.; Liu, S.; Morlet-Savary, F.; Xiao, P.; et al. Ketone Derivatives as Photoinitiators for Both Radical and Cationic Photopolymerizations under Visible LED and Application in 3D Printing. Eur. Polym. J. 2020, 132, 109737. [Google Scholar] [CrossRef]
- Encinas, M.V.; Rufs, A.M.; Bertolotti, S.G.; Previtali, C.M. Xanthene Dyes/Amine as Photoinitiators of Radical Polymerization: A Comparative and Photochemical Study in Aqueous Medium. Polymer 2009, 50, 2762–2767. [Google Scholar] [CrossRef]
- Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Becht, J.-M.; Fouassier, J.-P.; Lalevée, J. Charge Transfer Complexes as Pan-Scaled Photoinitiating Systems: From 50 Μm 3D Printed Polymers at 405 Nm to Extremely Deep Photopolymerization (31 Cm). Macromolecules 2018, 51, 57–70. [Google Scholar] [CrossRef]
- Garra, P.; Fouassier, J.P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible Light Photoinitiating Systems by Charge Transfer Complexes: Photochemistry without Dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Lenka, S.; Nayak, P.L.; Ray, S. Photopolymerization Initiated by Charge-Transfer Complex: V—Photopolymerization of Methyl Methacrylate with the Use of Isoquinoline-Bromine Charge-Transfer Complex. Polym. Photochem. 1984, 4, 167–177. [Google Scholar] [CrossRef]
- Mishra, M.K.; Lenka, S.; Nayak, P.L. Photopolymerization Initiated by Charge-Transfer Complex. I. Photopolymerization of Methylmethacrylate with the Use of Quinaldine-Bromine and Lutidine–Bromine Charge-Transfer Complexes as Photoinitiator. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 2457–2464. [Google Scholar] [CrossRef]
- Derevyanko, D.I.; Shelkovnikov, V.V.; Kovalskii, V.Y.; Zilberberg, I.L.; Aliev, S.I.; Orlova, N.A.; Ugozhaev, V.D. The Charge Transfer Complex Formed between the Components of Photopolymer Material as an Internal Sensitizer of Spectral Sensitivity. ChemistrySelect 2020, 5, 11939–11947. [Google Scholar] [CrossRef]
- Lenka, S.; Nayak, P.L.; Nayak, S.K. Photopolymerization Initiated by Charge-Transfer Complex. VII. Photopolymerization of Methyl Methacrylate with the Use of α-Picoline Chlorine Charge=Transfer Complex. J. Macromol. Sci. Part-Chem. 1983, 20, 835–845. [Google Scholar] [CrossRef]
- Kurihara, T.; Sato, R.; Takeishi, M. Photopolymerization of Methyl Methacrylate in the Presence of a Charge-Transfer Complex of an Ether with Oxygen. Polym. J. 1991, 23, 1397–1400. [Google Scholar] [CrossRef]
- Sakai, S.; Takahashi, K.; Sakota, N. Photopolymerization of Methyl Methacrylate Initiated by Iodine—Monoethanolamine. Polym. J. 1974, 6, 341–347. [Google Scholar] [CrossRef]
- Kaya, K.; Kreutzer, J.; Yagci, Y. A Charge-Transfer Complex of Thioxanthonephenacyl Sulfonium Salt as a Visible-Light Photoinitiator for Free Radical and Cationic Polymerizations. ChemPhotoChem 2019, 3, 1187–1192. [Google Scholar] [CrossRef]
- Wang, D.; Garra, P.; Lakhdar, S.; Graff, B.; Fouassier, J.P.; Mokbel, H.; Abdallah, M.; Lalevée, J. Charge Transfer Complexes as Dual Thermal and Photochemical Polymerization Initiators for 3D Printing and Composites Synthesis. ACS Appl. Polym. Mater. 2019, 1, 561–570. [Google Scholar] [CrossRef]
- Wang, D.; Kaya, K.; Garra, P.; Fouassier, J.-P.; Graff, B.; Yagci, Y.; Lalevée, J. Sulfonium Salt Based Charge Transfer Complexes as Dual Thermal and Photochemical Polymerization Initiators for Composites and 3D Printing. Polym. Chem. 2019, 10, 4690–4698. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced Free Radical Promoted Cationic Polymerization 40 Years after Its Discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Garra, P.; Caron, A.; Al Mousawi, A.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Yagci, Y.; Fouassier, J.-P.; Lalevée, J. Photochemical, Thermal Free Radical, and Cationic Polymerizations Promoted by Charge Transfer Complexes: Simple Strategy for the Fabrication of Thick Composites. Macromolecules 2018, 51, 7872–7880. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D Printing of Photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Tazuke, S. Initiation of Photopolymerization by Charge Transfer Interactions. Pure Appl. Chem. 1973, 34, 329–352. [Google Scholar] [CrossRef]
- Perdana, F.; Eryanti, Y.; Zamri, A. Synthesis and Toxicity Assessments Some Para-Methoxy Chalcones Derivatives. Int. Symp. Appl. Chem. 2015 2015, 16, 129–133. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Tekale, S.; Mashele, S.; Pooe, O.; Thore, S.; Kendrekar, P. Rajandra Pawar Biological Role of Chalcones in Medicinal Chemistry. In Vector-Borne Diseases; Claborn, D., Bhattacharya, S., Roy, S., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. 9. ISBN 978-1-83880-022-2. [Google Scholar]
- Rozmer, Z.; Perjési, P. Naturally Occurring Chalcones and Their Biological Activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Gou, L.; Opheim, B.; Coretsopoulos, C.N.; Scranton, A.B. Consumption of the Molecular Oxygen in Polymerization Systems Using Photosensitized Oxidation of Dimethylanthracene. Chem. Eng. Commun. 2006, 193, 620–627. [Google Scholar] [CrossRef]
- Sanai, Y.; Kagami, S.; Kubota, K. Initiation and Termination Pathways in the Photopolymerization of Acrylate Using Methyl Phenylglyoxylate as an Initiator. Polym. J. 2020, 52, 375–385. [Google Scholar] [CrossRef]
- Hu, S.; Wu, X.; Neckers, D.C. Methyl Phenylglyoxylate as a Photoinitiator. Macromolecules 2000, 33, 4030–4033. [Google Scholar] [CrossRef]
- Hu, S.; Popielarz, R.; Neckers, D.C. Fluorescence Probe Techniques (FPT) for Measuring the Relative Efficiencies of Free-Radical Photoinitiators. Macromolecules 1998, 31, 4107–4113. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J. Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts. Materials 2020, 13, 4093. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Xiong, D.; Xiong, S.; Su, F.; Idrees, M.; Chen, Z. Photopolymerization-Based Additive Manufacturing of Ceramics: A Systematic Review. J. Adv. Ceram. 2021, 10, 442–471. [Google Scholar] [CrossRef]



















| λmax (nm) | εmax (M−1.cm−1) | ε@405 nm (M−1.cm−1) | |
|---|---|---|---|
| ketone 1 | 368 | 29,230 | 9740 |
| ketone 2 | 365 | 25,020 | 7980 |
| ketone 3 | 370 | 34,920 | 11,690 |
| ketone 4 | 368 | 34,200 | 10,130 |
| ketone 5 | 372 | 31,470 | 11,950 |
| ketone 6 | 370 | 29,750 | 10,280 |
| Ketone 1 | Ketone 2 | Ketone 3 | Ketone 4 | Ketone 5 | Ketone 6 | |
|---|---|---|---|---|---|---|
| FCs (thick films) | 30% | 24% | 94% | 24% | 90% | 25% |
| FCs (thin films) | 55% | 67% | 55% | 81% | 59% | 71% |
| LED@405 nm | LED@470 nm | |||
|---|---|---|---|---|
| Dye 1 | Dye 9 | Dye 1 | Dye 9 | |
| FCs (thick films) | 7% | 14% | - | - |
| FCs (thin films) | 76% | 90% | 40% | 63% |
| Chalcone | CHC-13 | CHC-14 | CHC-15 | CHC-16 | CHC-17 |
|---|---|---|---|---|---|
| FCs | 73% | 74% | 79% | 65% | 69% |
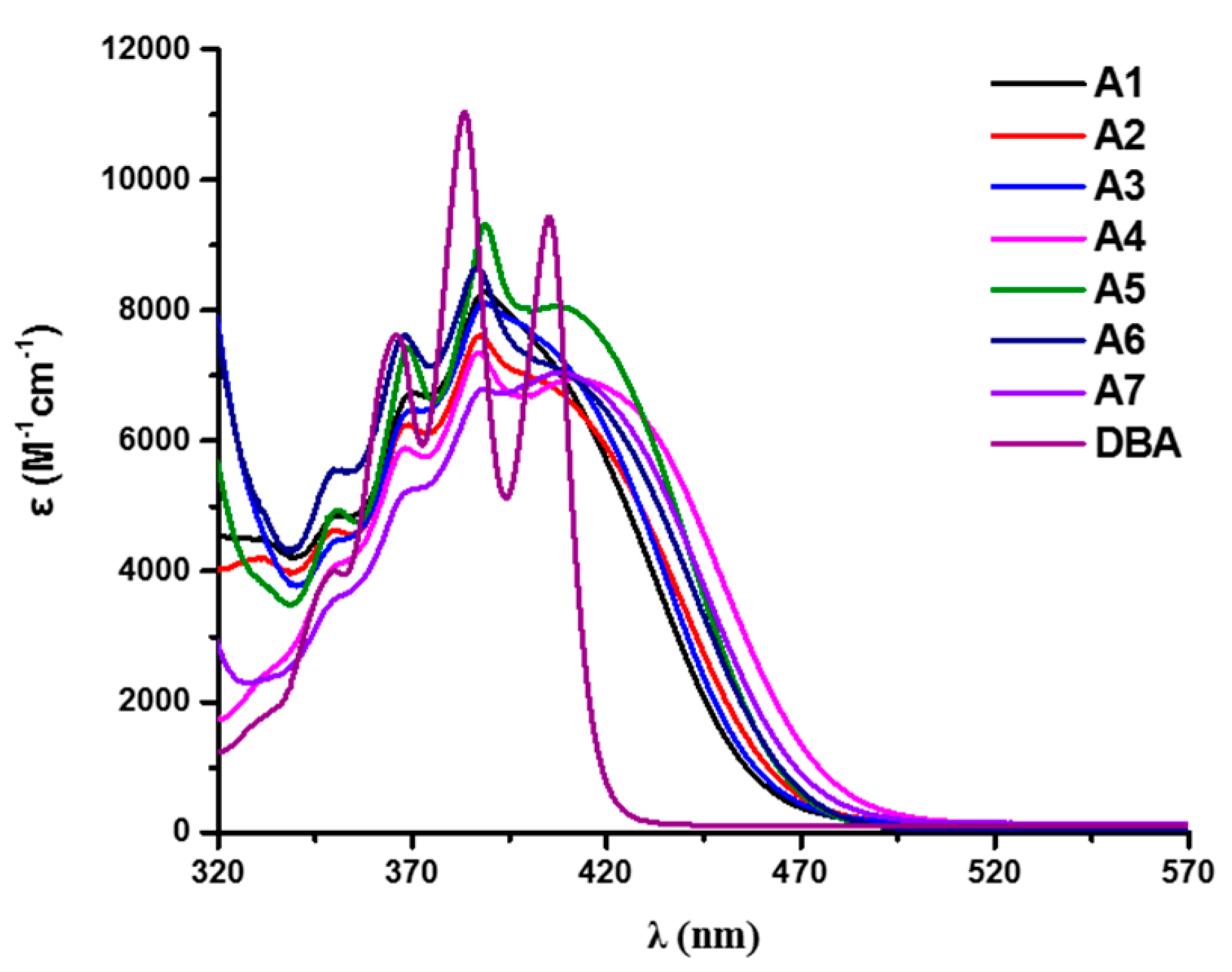
| λmax (nm) | εmax (M−1.cm−1) | ε405 nm (M−1.cm−1) | ε470 nm (M−1.cm−1) | |
|---|---|---|---|---|
| A1 | 389 | 8300 | 7300 | 400 |
| A2 | 388 | 7600 | 6900 | 550 |
| A3 | 388 | 8100 | 7400 | 450 |
| A4 | 387 | 7300 | 6800 | 1350 |
| A5 | 389 | 9300 | 8000 | 600 |
| A6 | 387 | 8700 | 7200 | 650 |
| A7 | 389 | 6800 | 7000 | 900 |
| DBA | 384 | 11,000 | 9400 | 0 |
| PIS | TMPTA (%) | EPOX (%) | |
|---|---|---|---|
| Dyes/Iod/EDB a | Dyes/Iod a | Dyes/Iod b | |
| A1 | 57 | 39 | 21 |
| A2 | 51 | 37 | 24 |
| A3 | 45 | 21 | 15 |
| A4 | 60 | 43 | 33 |
| A5 | 60 | 52 | 36 |
| A6 | 61 | 47 | 27 |
| A7 | 45 | 33 | 19 |
| Blank c | 5 | - | - |
| DBA | - | 38 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumur, F. Recent Advances on Furan-Based Visible Light Photoinitiators of Polymerization. Catalysts 2023, 13, 493. https://doi.org/10.3390/catal13030493
Dumur F. Recent Advances on Furan-Based Visible Light Photoinitiators of Polymerization. Catalysts. 2023; 13(3):493. https://doi.org/10.3390/catal13030493
Chicago/Turabian StyleDumur, Frédéric. 2023. "Recent Advances on Furan-Based Visible Light Photoinitiators of Polymerization" Catalysts 13, no. 3: 493. https://doi.org/10.3390/catal13030493
APA StyleDumur, F. (2023). Recent Advances on Furan-Based Visible Light Photoinitiators of Polymerization. Catalysts, 13(3), 493. https://doi.org/10.3390/catal13030493







