Photocatalytic Degradation of Ciprofloxacin with Supramolecular Materials Consisting of Nitrogenous Organic Cations and Metal Salts
Abstract
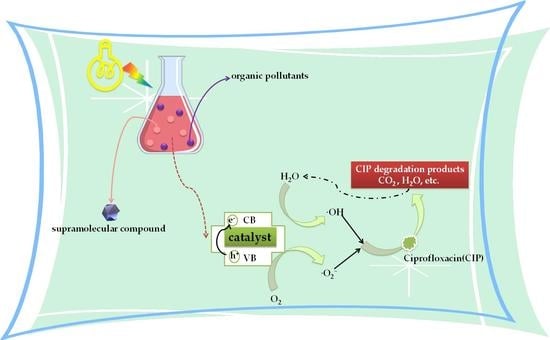
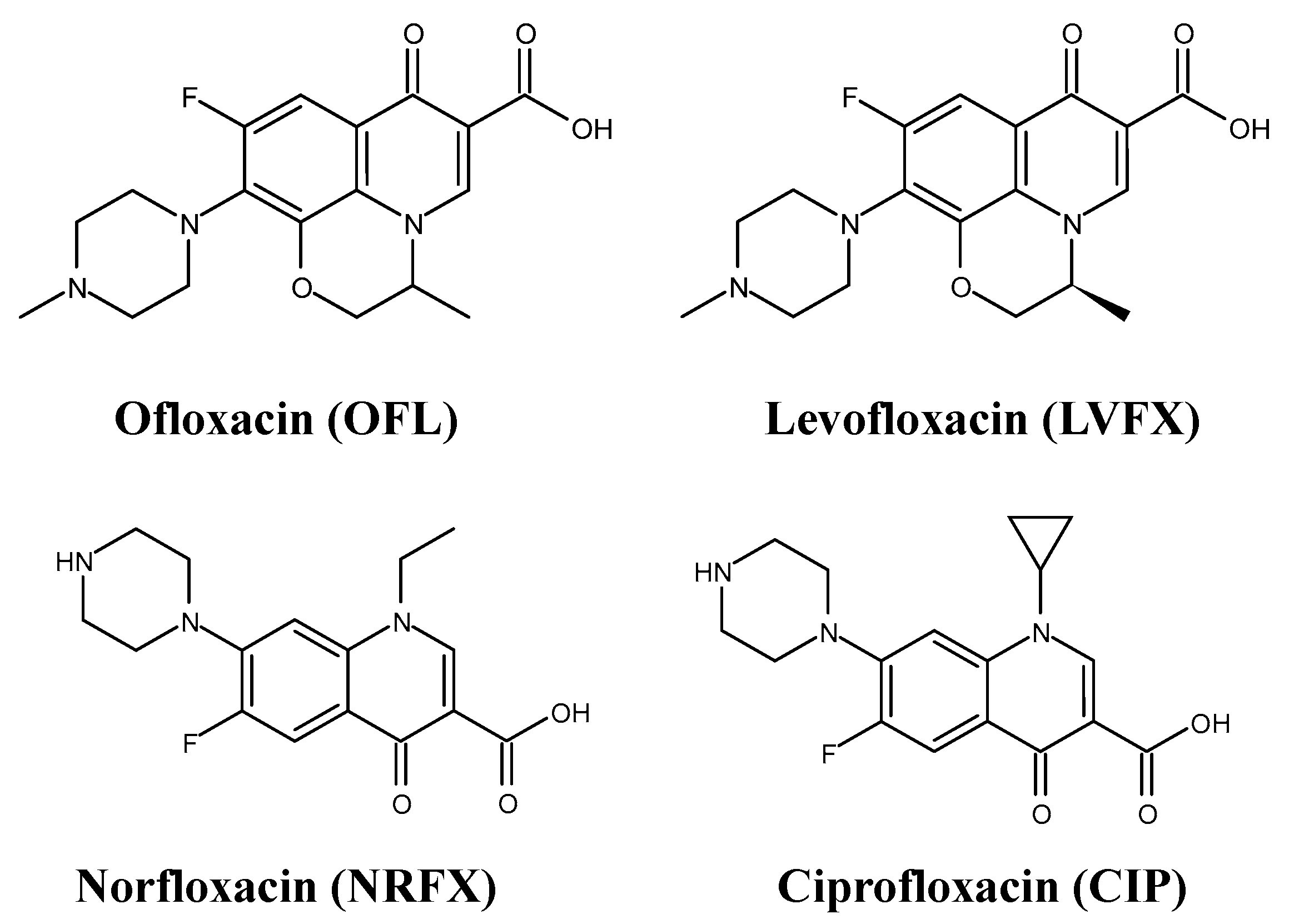
1. Introduction
2. Results and Discussion
2.1. Description of Crystal Structures of {[L1]2·[Cu4I8]} (1) and {[L1]2·[Ag4I8]} (2)
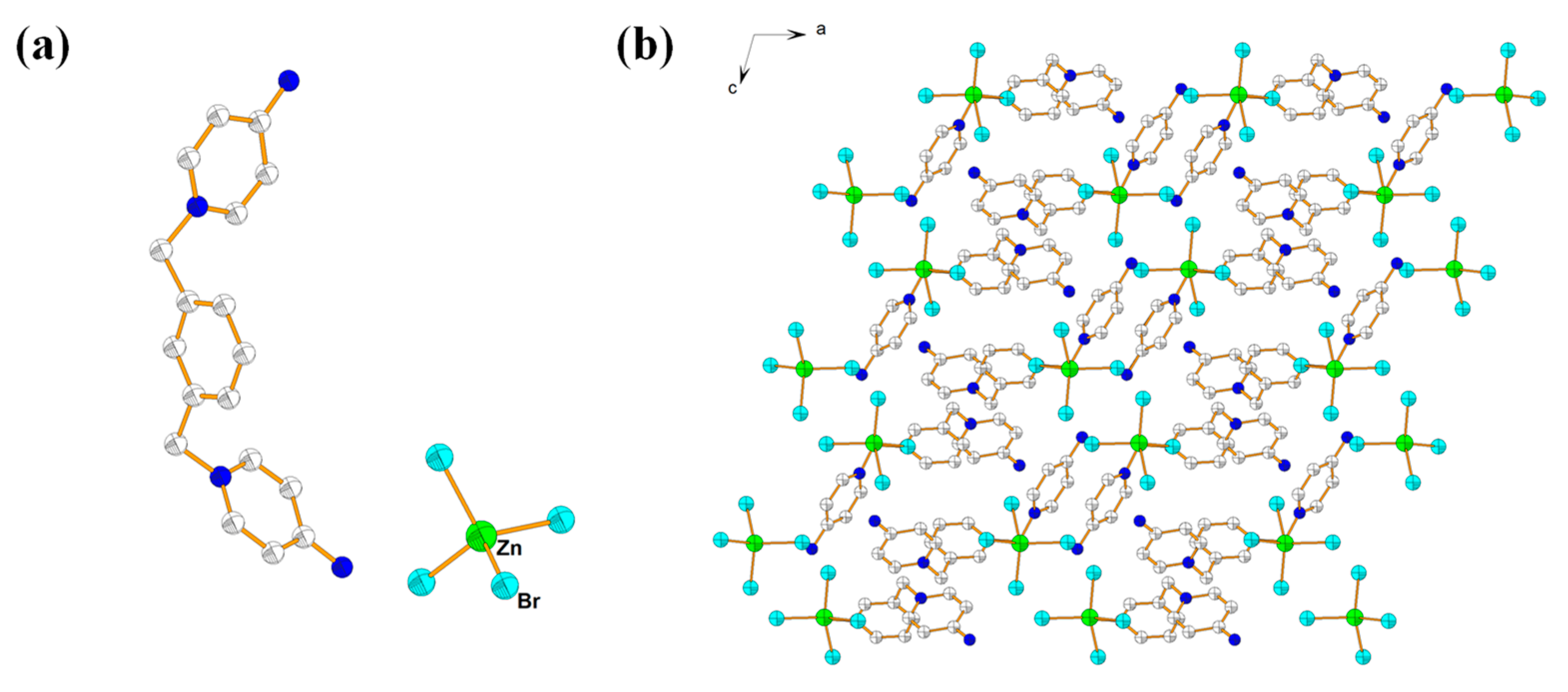
2.2. Description of Crystal Structure of {[L2]·[Zn(Br)4]} (3)
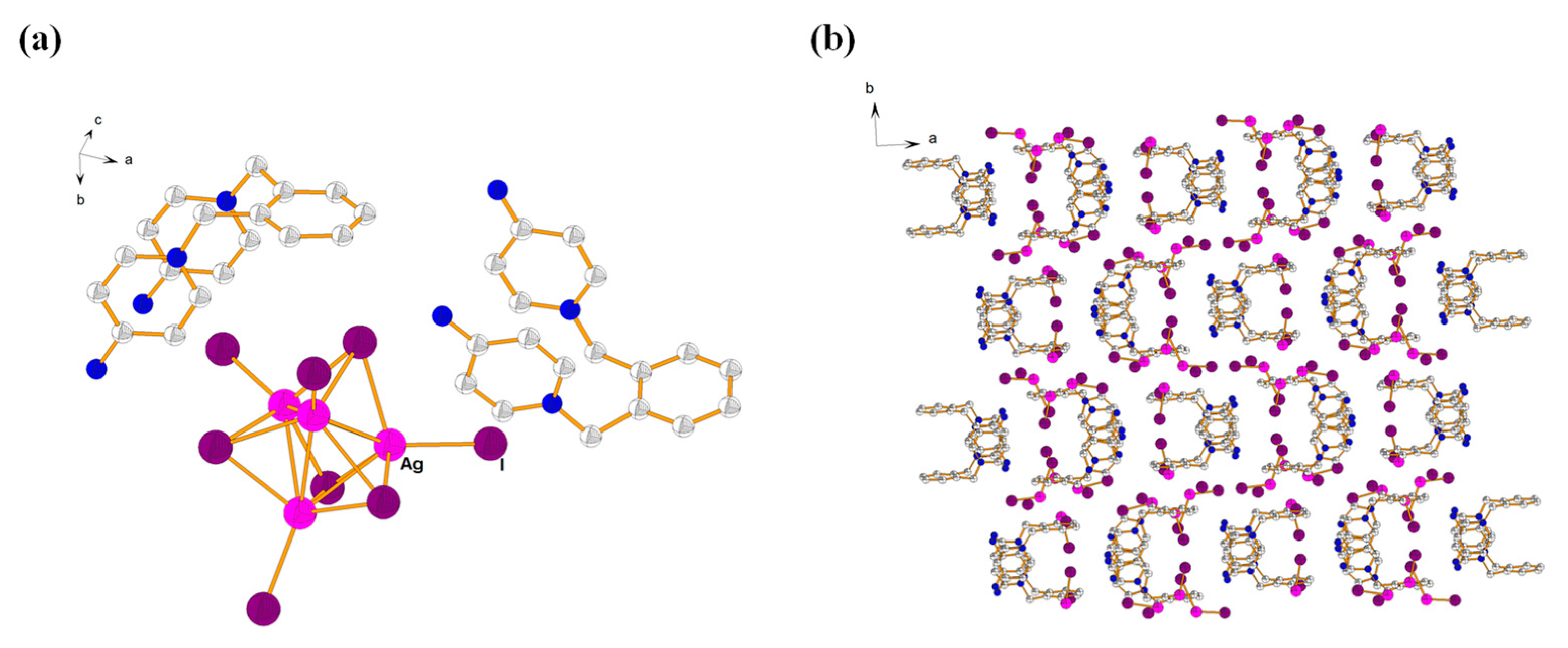
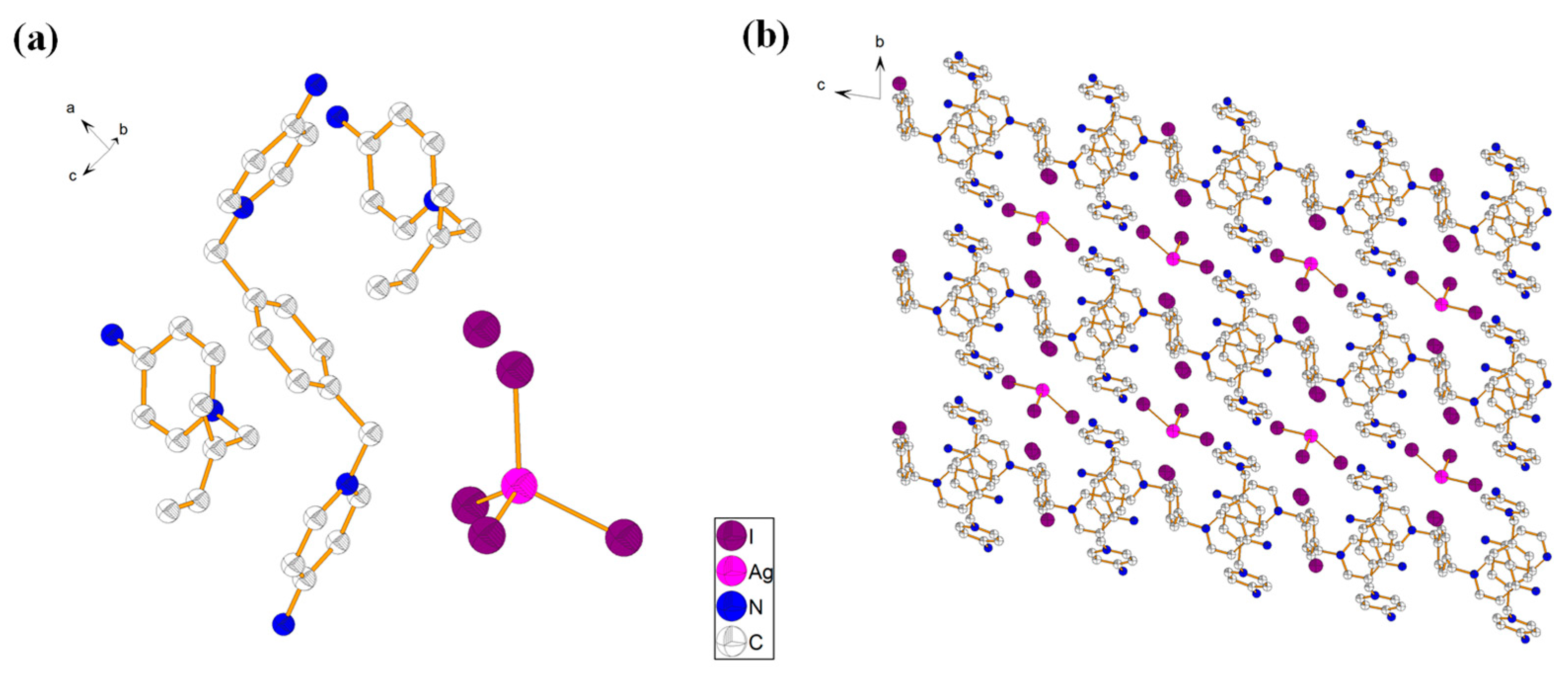
2.3. Description of Crystal Structure of {[L3]2·[AgI5]} (4)
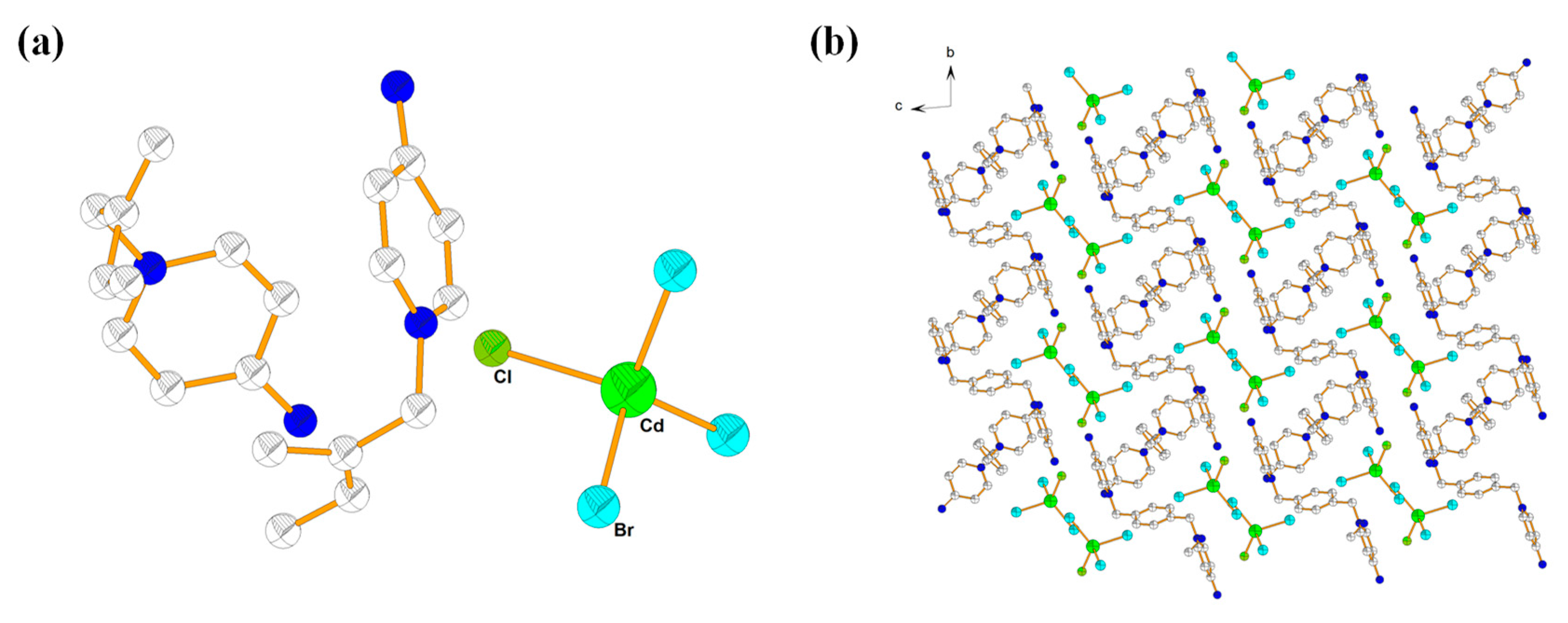
2.4. Description of Crystal Structure of {[L3]·[CdBr3Cl]} (5)
2.5. Photocatalytic Activities of Compounds 1–5
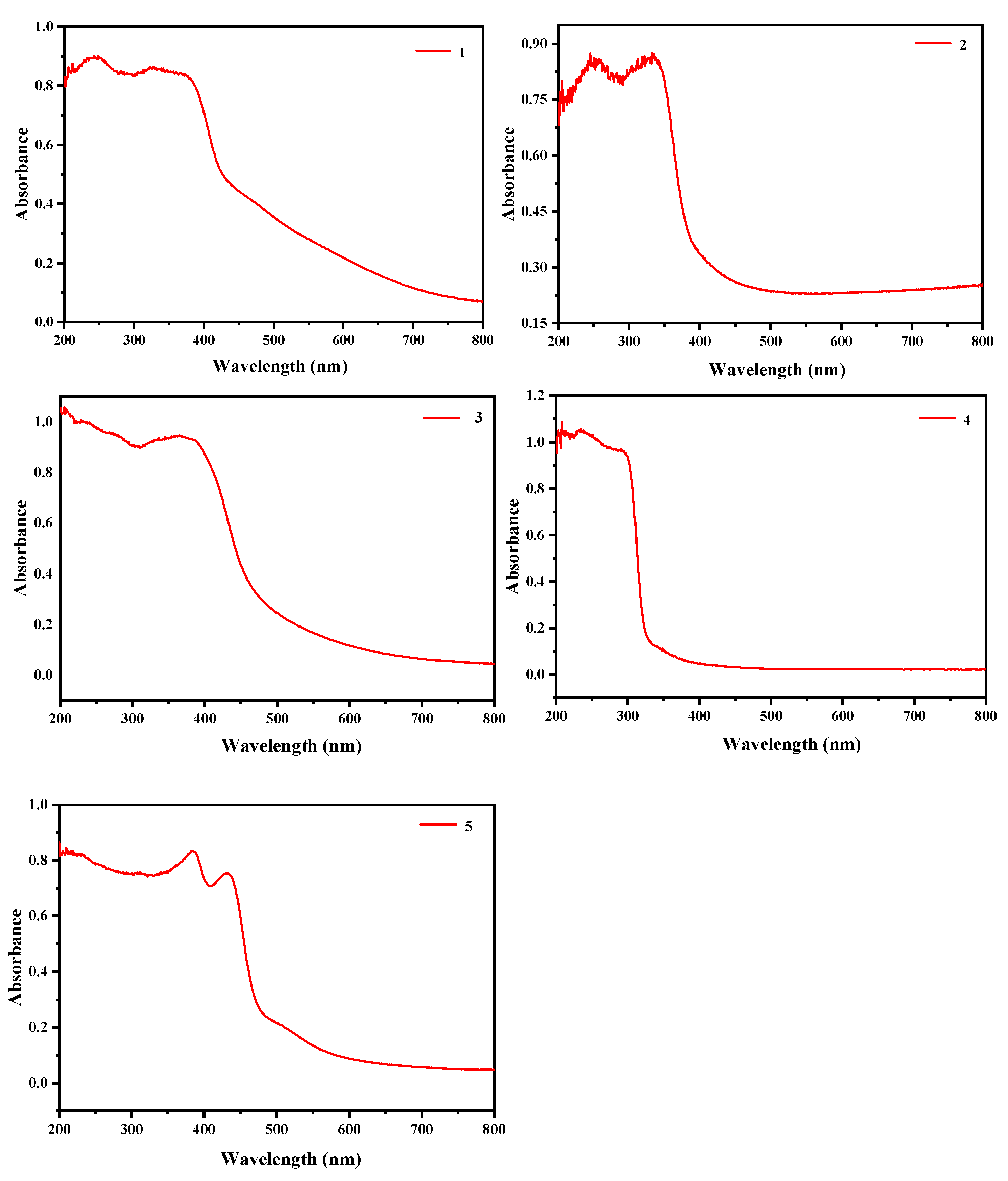
2.5.1. Semiconductor Properties of Compounds 1–5
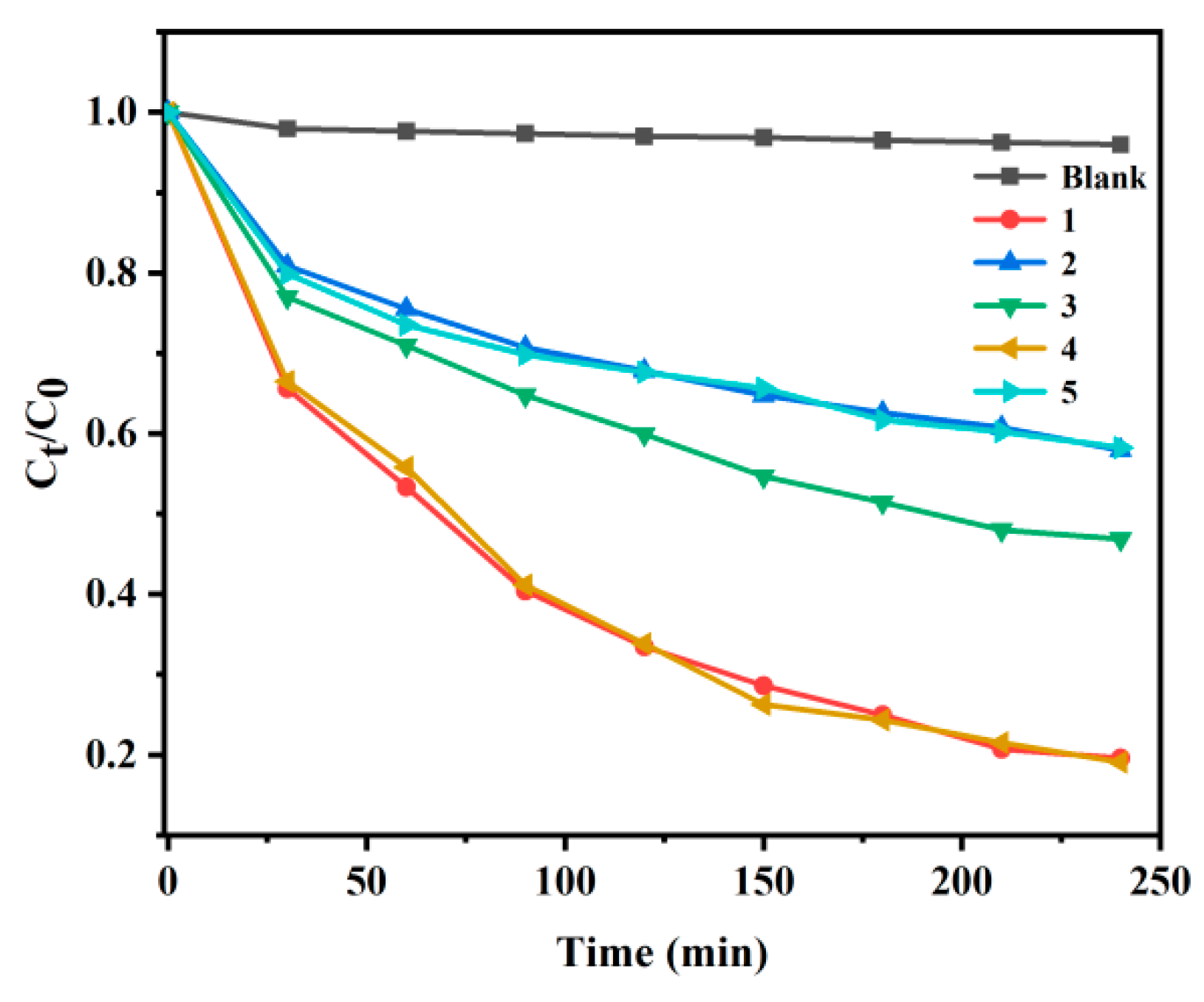
2.5.2. Degradation of CIP by Compounds 1–5
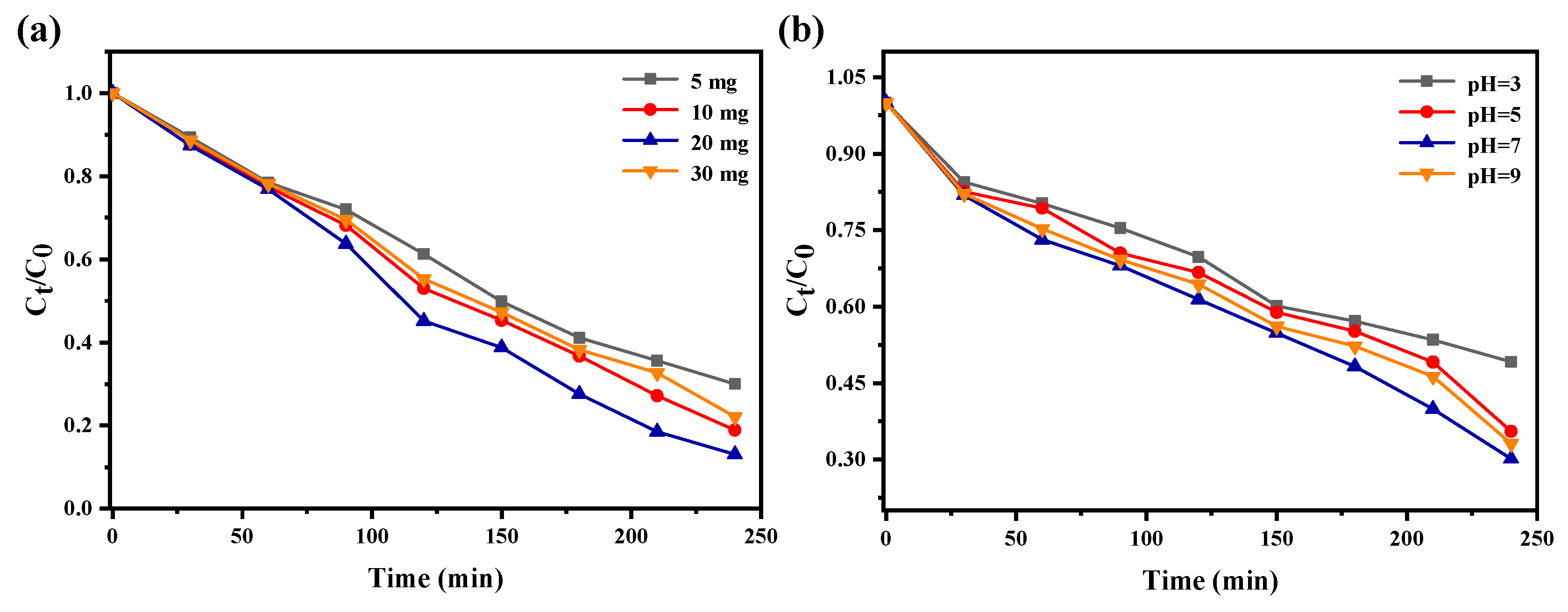
2.5.3. Effect of Dosage and pH Value on Photocatalytic Effect
2.5.4. Degradation of CIP by Compounds 1–5
3. Materials and Methods
3.1. Materials
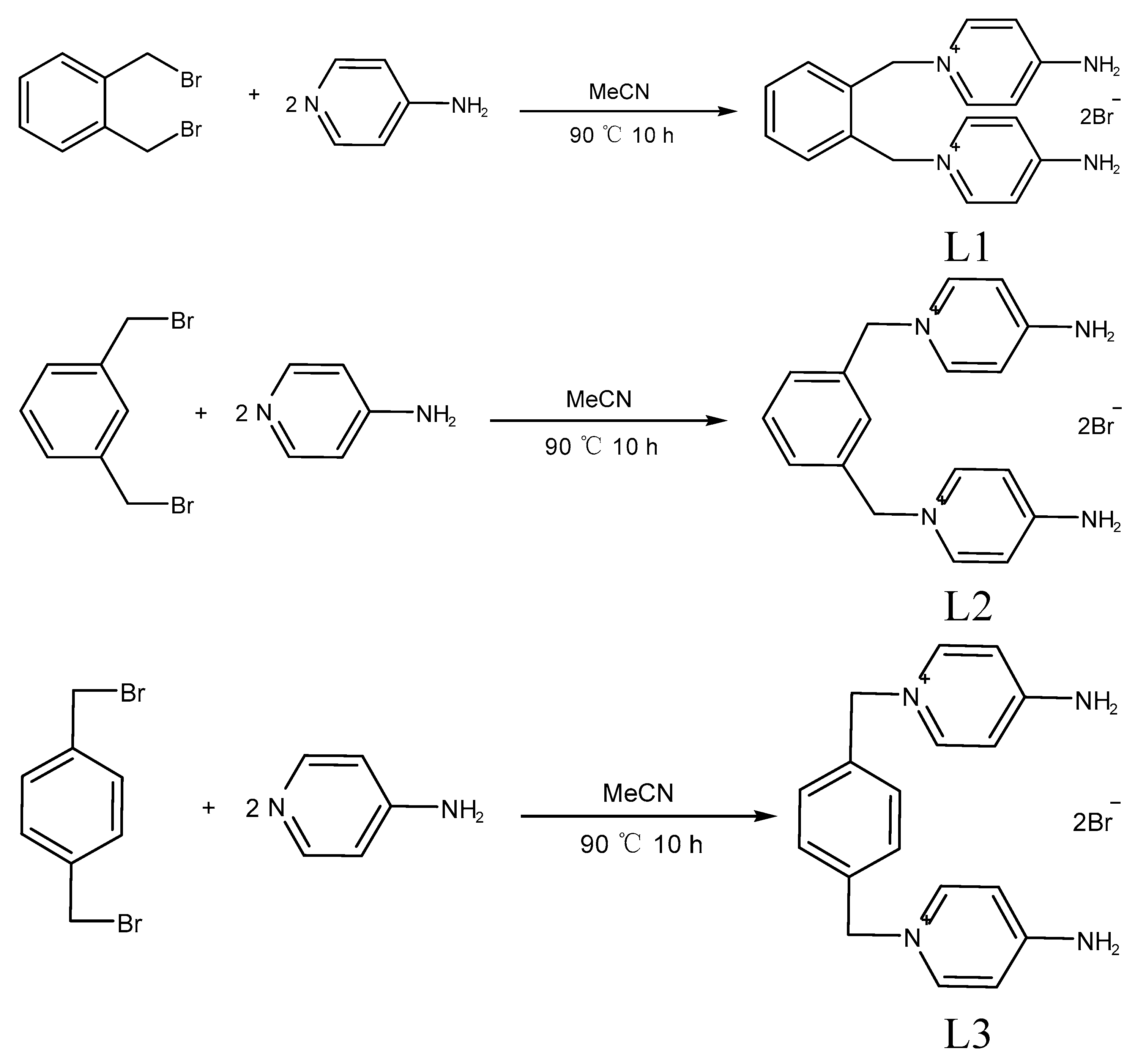
3.2. Synthesis of Compounds
3.2.1. The Synthesis of {[L1]2·[Cu4I8]} (1)
3.2.2. The Synthesis of {[L1]2·[Ag4I8]} (2)
3.2.3. The Synthesis of {[L2]·[ZnBr4]} (3)
3.2.4. The Synthesis of {[L3]2·[AgI5]} (4)
3.2.5. The Synthesis of {[L3]·[CdBr3Cl]} (5)
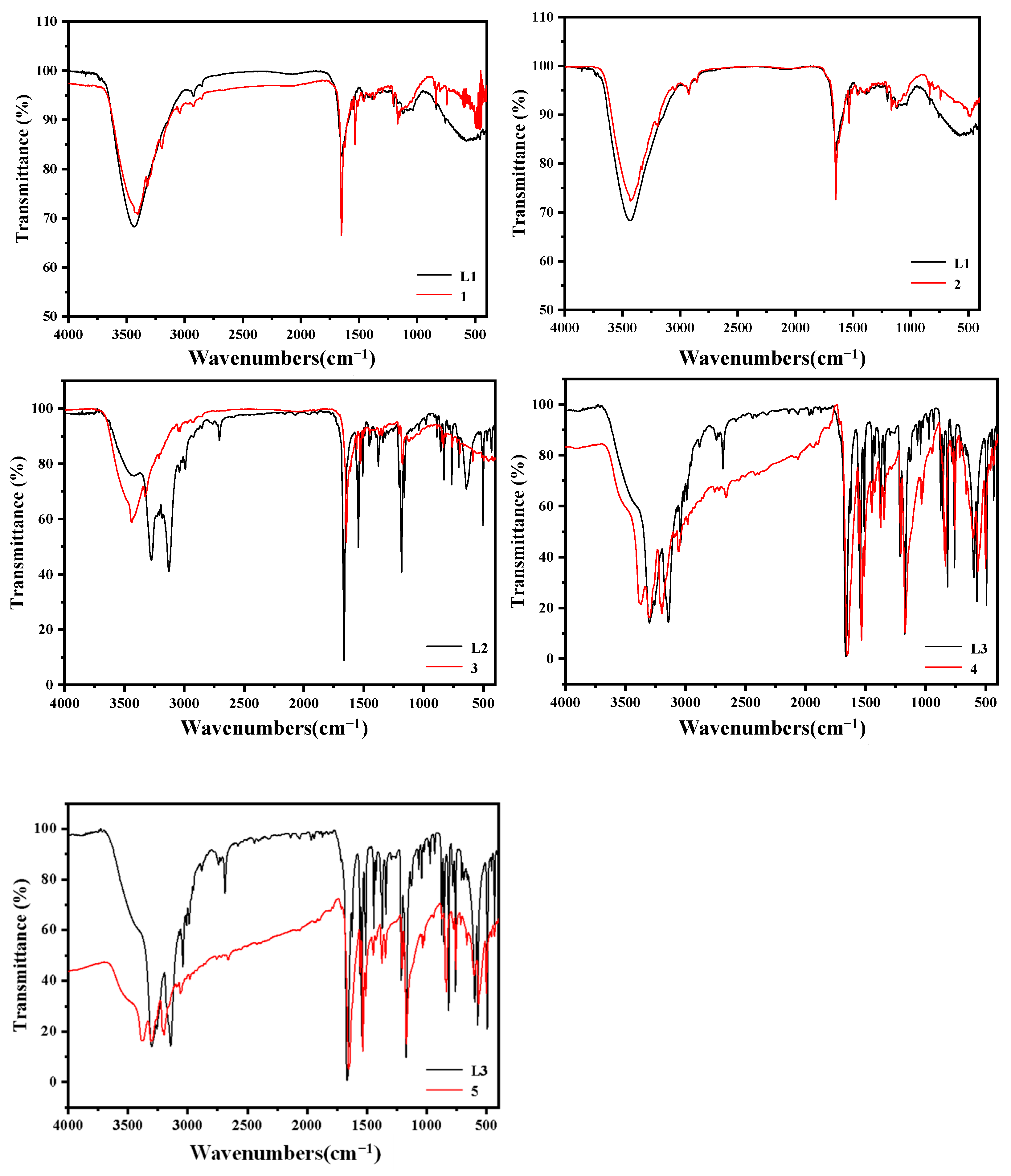
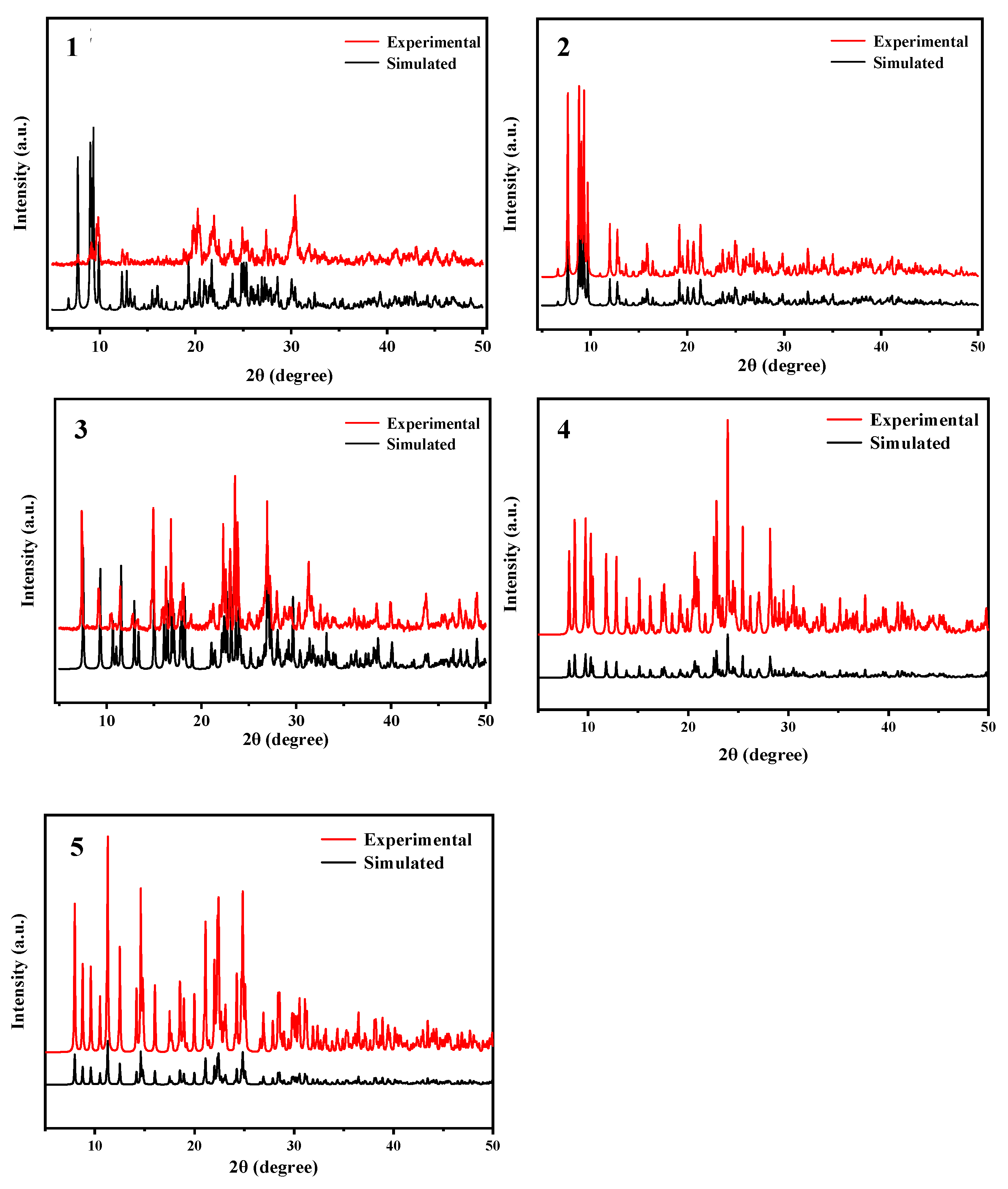
3.3. Methods for Characterizing Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosi-Marshall, E.J.; Kincaid, D.W.; Bechtold, H.A.; Royer, T.V.; Rojas, M.; Kelly, J.J. Pharmaceuticals suppress algal growth and microbial respiration and alter bacterial communities in stream biofilms. Ecol. Appl. 2013, 23, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Yezli, S.; Li, H. Antibiotic resistance amongst healthcare-associated pathogens in China. Int. J. Antimicrob. Agents 2012, 40, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Tong, L.; Li, P.; Wang, Y.; Zhu, K. Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS. Chemosphere 2009, 74, 1090–1097. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, H.T.; Pham, T.D.; Tran, T.D.; Chu, H.T.; Dang, H.T.; Nguyen, V.H.; Nguyen, K.M.; Pham, T.T.; Van der Bruggen, B. UV–Visible Light Driven Photocatalytic Degradation of Ciprofloxacin by N,S Co-doped TiO2: The Effect of Operational Parameters. Top. Catal. 2020, 63, 985–995. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci. Total Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef]
- Liang, Z.; Zhaob, Z.; Sun, T.; Shi, W.; Cui, F. Adsorption of quinolone antibiotics in spherical mesoporous silica: Effects of the retained template and its alkyl chain length. J. Hazard. Mater. 2016, 305, 8–14. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Peres, J.A.; Gil-Álvarez, V.; Ovejero, G.; García, J. Effective adsorption of non-biodegradable pharmaceuticals from hospital wastewater with different carbon materials. Chem. Eng. J. 2017, 320, 319–329. [Google Scholar] [CrossRef]
- Lyu, J.; Shao, J.; Wang, Y.; Qiu, Y.; Li, J.; Li, T.; Peng, Y.; Liu, F. Construction of a porous core-shell homojunction for the photocatalytic degradation of antibiotics. Chem. Eng. J. 2019, 358, 614–620. [Google Scholar] [CrossRef]
- Murugesan, S.; Huda, M.N.; Yan, Y.; Al-Jassim, M.M.; Subramanian, V. Band-Engineered Bismuth Titanate Pyrochlores for Visible Light Photocatalysis. J. Phys. Chem. C 2010, 114, 10598–10605. [Google Scholar] [CrossRef]
- Yu, X.; Huang, L.; Wei, Y.; Zhang, J.; Zhao, Z.; Dai, W.; Yao, B. Controllable preparation, characterization and performance of Cu2O thin film and photocatalytic degradation of methylene blue using response surface methodology. Mater. Res. Bull. 2015, 64, 410–417. [Google Scholar] [CrossRef]
- Hou, X.; Cai, Y.; Mushtaq, M. Deposition of TiO2 Nanoparticles on Porous Polylactic Acid Fibrous Substrates and Its Photocatalytic Capability. J. Nanosci. Nanotechnol. 2018, 18, 5617–5623. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Naushad, M.; Stadler, F.J.; Ghfar, A.A.; Dhiman, P.; Saini, R.V. Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO4 for solar powered degradation of noxious pollutants- Synergism of adsorption, photocatalysis & photo-ozonation. J. Cleaner Prod. 2017, 165, 431–451. [Google Scholar]
- Li, Y.; Xiao, M.; Niu, Y. Preparation and application of two kinds of supramolecular compounds with photodegradation of organic contaminants in wastewater. Main Group Chem. 2019, 18, 43–54. [Google Scholar] [CrossRef]
- Wang, F.R.; Wang, C.H.; Wu, B.L.; Yan, Z.N.; Niu, Y.Y.; Hou, H.W. Synthesis, structure and photocatalytic properties of two hybrid compounds prepared by N-methyl-4,4′-bipyridinium chloride. Main Group Chem. 2018, 17, 211–218. [Google Scholar] [CrossRef]
- Liu, X.J.; Qiao, X.Y.; Niu, Y.Y. Synthesis, structures and properties of Cd(II) supramolecular compound based on nitrogen heterocyclic cation. Main Group Chem. 2020, 19, 199–206. [Google Scholar] [CrossRef]
- Qiao, G.Y.; Li, S.M.; Niu, Y.Y. One new copper iodide coordination polymer directed by 4-pyridyl dithioether ligand: Syntheses, structures, and photocatalysis. Main Group Chem. 2020, 19, 217–225. [Google Scholar] [CrossRef]
- Xing, X.; Du, Z.; Zhuang, J.; Wang, D. Removal of ciprofloxacin from water by nitrogen doped TiO2 immobilized on glass spheres: Rapid screening of degradation products. J. Photochem. Photobiol. A 2018, 359, 23–32. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, K.H.; Chen, Z.; Yu, J.C.; Zhao, J.; Hu, C.; Chan, C.Y.; Wong, P.K. AgBr-Ag-Bi2WO6 nanojunction system: A novel and efficient photocatalyst with double visible-light active components. Appl. Catal. A 2009, 363, 221–229. [Google Scholar] [CrossRef]
- Gan, Y.; Wei, Y.; Xiong, J.; Cheng, G. Impact of post-processing modes of precursor on adsorption and photocatalytic capability of mesoporous TiO2 nanocrystallite aggregates towards ciprofloxacin removal. Chem. Eng. J. 2018, 349, 1–16. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.H.; Pignatello, J.J. Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, Y.; Yang, Z.; Tang, X.; Li, X.; He, G.; Cheng, Y.; Fang, Z.; He, R.; Zhang, Y. Analysis of synergistic effect between graphene and octahedral cuprous oxide in cuprous oxide-graphene composites and their photocatalytic application. J. Alloys Compd. 2017, 712, 704–713. [Google Scholar] [CrossRef]
- Wang, X.J.; Qiao, X.Y.; Niu, Y.Y. Synthesis, characterization, adsorption and catalytic activity of a polyoxometalate supramolecule templated by arylmethylamine. Main Group Chem. 2020, 19, 187–198. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Liu, Y.Y.; Liu, J.; Li, Y.Y.; Qiao, X.Y.; Huang, W.M.; Niu, Y.Y. POM-based Metal–organic Compounds: Assembly, Structures and Properties. Main Group Chem. 2021, 20, 575–592. [Google Scholar] [CrossRef]
- Guo, X.Y.; Wang, X.J.; Liu, Z.; Liu, Y.Y.; Liu, J.; Niu, Y.Y. Two Supramolecular Compounds Constructed by Polyacid Anion Clusters: Synthesis, Characterization and Performance Research. Main Group Chem. 2021, 20, 601–610. [Google Scholar] [CrossRef]
- Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K. Reduced graphene oxide-CdS heterostructure: An efficient fluorescent probe for the sensing of Ag(I) and sunset yellow and a visible-light responsive photocatalyst for the degradation of levofloxacin drug in aqueous phase. Appl. Catal. B 2019, 245, 143–158. [Google Scholar] [CrossRef]
- Kolesnichenko, I.V.; Ansly, E.V. Practical Applications of Supramolecular Chemistry. Chem. Soc. Rev. 2017, 46, 197–238. [Google Scholar] [CrossRef]
- Christopher, B.R.; Joshua, E.M.; Jason, A.B. Supramolecular Guest-Host Interactions for the Preparation of Biomedical Materials. Bioconjugate. Chem. 2015, 26, 2279–2289. [Google Scholar]
- Prins, L.J.; Reinhoudt, D.N.; Timmerman, P. Noncovalent Synthesis using Hydrogen Bonding. Angew. Chem. Int. Ed. 2001, 40, 2382–2426. [Google Scholar] [CrossRef]
- Fyfe, M.C.T.; Stoddart, J.F. Synthetic Supramolecular Chemistry. Acc. Chem. Res. 1997, 30, 393–401. [Google Scholar] [CrossRef]
- Mueller-Dethlefs, K.; Hobza, P. Noncovalent Interactions: A Challenge for Experiment and Theory. Chem. Rev. 2000, 100, 143–167. [Google Scholar] [CrossRef]
- Hamley, I. Nanotechnology with Soft Materials. Angew. Chem. Int. Ed. 2003, 42, 1692–1712. [Google Scholar] [CrossRef]
- Mammen, M.; Chio, S.K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
- Ercolani, G. Assessment of Cooperativity in Self-Assembly. J. Am. Chem. Soc. 2003, 125, 16097–16103. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef]
- Kitov, P.I.; Bundle, D.R. On the Nature of the Multivalency Effect: A Thermodynamic Model. J. Am. Chem. Soc. 2003, 125, 16271–16284. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, X.Y.; Wang, C.M.; Yu, K.; Lv, J.H.; Wang, C.X.; Zhou, B.B. The supercapacitor and photocatalytic supermolecule materials constructed by 4′4-pyridine and {PMo12O40}. J. Solid State Chem. 2022, 312, 123235. [Google Scholar] [CrossRef]
- Sabina, G.I.; Octavian, D.P.; Nicolae, G.; Madalina, T.; Simona, M.C.; Vasile, I.P.; Bogdan, C.; Elisabeth, E.J. Use of Photocatalytically Active Supramolecular Organic–Inorganic Magnetic Composites as Efficient Route to Remove β-Lactam Antibiotics from Water. Catalysts 2022, 12, 1044. [Google Scholar]
- Zhou, X.H.; Chen, Y.Y.; Wang, P.Y.; Xu, C.Y.; Yan, Q.S. Fabrication of AgI/BiPO4 n–n heterojunction photocatalyst for efficient degradation of organic pollutants. J. Mater. Sci. Mater. Electron. 2020, 31, 12638–12648. [Google Scholar] [CrossRef]
- Lu, J.R.; Yue, M.T.; Cui, W.Q.; Sun, C.H.; Liu, L. Supramolecular photocatalyst of perylene bisimide decorated with α-Fe2O3: Efficient photo-Fenton degradation of organic pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130222. [Google Scholar] [CrossRef]
- Bao, S.H.; Wu, S.S.; Huang, L.P.; Xu, X.; Xu, R.; Li, Y.G.; Liang, Y.R.; Yang, M.Y.; Yoon, D.K.; Lee, M.; et al. Supramolecular Nanopumps with Chiral Recognition for Moving Organic Pollutants from Water. ACS Appl. Mater. Interfaces 2019, 11, 31220–31226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wang, X.J.; Niu, Y.-Y. Photocatalytic Degradation of Tetracycline by Supramolecular Materials Constructed with Organic Cations and Silver Iodide. Catalysts 2022, 12, 1581. [Google Scholar] [CrossRef]
- Ren, C.; Li, J.; Zhang, X.; Niu, Y. Synthesis and Photocatalytic Properties of Four Coordination Compounds Constructed from Two Benzimidazole-Based Asymmetric Polyazocyclic Ligands. Molecules 2023, 28, 3841. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, P.F.; Wei, Y.G.; Wang, Y.; Guo, H.Y. Synthesis, Crystal Structure, and Optical Properties of a Polyoxometalate-Based Inorganic-Organic Hybrid Solid, (n-Bu4N)2[Mo6O17(≡NAr)2] (Ar = o-CH3OC6H4). Cryst. Growth Des. 2006, 6, 253–257. [Google Scholar] [CrossRef]
- Liu, H.Y.; Bo, L.; Yang, J.; Liu, Y.Y.; Ma, J.F.; Wu, H. Two novel inorganic-organic hybrid materials constructed from two kinds of octamolybdate clusters and flexible tetradentate ligands. Dalton Trans 2011, 40, 9782-8. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Wang, P.; Wu, T.; Wang, C.; Hou, J.; Qian, J.; Ao, Y. Combining Heterojunction Engineering with Surface Cocatalyst Modification To Synergistically Enhance the Photocatalytic Hydrogen Evolution Performance of Cadmium Sulfide Nanorods. ACS Sustain. Chem. Eng. 2017, 5, 7670–7677. [Google Scholar] [CrossRef]
- Liang, H.; Tai, X.; Du, Z. Photocatalytic degradation of nonylphenol ethoxylate and its degradation mechanism. J. Mol. Liq. 2020, 302, 112567. [Google Scholar]
- Xu, M.M.; Li, Y.; Zheng, L.J.; Niu, Y.Y.; Hou, H.W. Three cation-templated Cu(i) self-assemblies: Synthesis, structures, and photocatalytic properties. New J. Chem. 2016, 40, 6086–6092. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Li, J.; Zhang, X.; Niu, Y. Photocatalytic Degradation of Ciprofloxacin with Supramolecular Materials Consisting of Nitrogenous Organic Cations and Metal Salts. Catalysts 2023, 13, 1134. https://doi.org/10.3390/catal13071134
Ren C, Li J, Zhang X, Niu Y. Photocatalytic Degradation of Ciprofloxacin with Supramolecular Materials Consisting of Nitrogenous Organic Cations and Metal Salts. Catalysts. 2023; 13(7):1134. https://doi.org/10.3390/catal13071134
Chicago/Turabian StyleRen, Chenfei, Jian Li, Xingxing Zhang, and Yunyin Niu. 2023. "Photocatalytic Degradation of Ciprofloxacin with Supramolecular Materials Consisting of Nitrogenous Organic Cations and Metal Salts" Catalysts 13, no. 7: 1134. https://doi.org/10.3390/catal13071134
APA StyleRen, C., Li, J., Zhang, X., & Niu, Y. (2023). Photocatalytic Degradation of Ciprofloxacin with Supramolecular Materials Consisting of Nitrogenous Organic Cations and Metal Salts. Catalysts, 13(7), 1134. https://doi.org/10.3390/catal13071134