Abstract
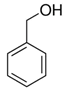
Benzyl alcohol (BnOH) oxidation to benzaldehyde (PhCHO) is a pivotal industrial reaction. The aerobic oxidation of BnOH in solvent-free conditions is highly compatible with the necessity of low environmental impact. In this research work, palladium oxide (PdOx) supported on ceria nanorods (CeO2-NR), was synthesized, and utilized for aerobic solvent-free oxidation of BnOH derivatives to the corresponding aldehydes. The catalyst, PdOx/CeO2-NR, was characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy/energy-dispersive spectroscopy (FE-SEM/EDS), N2 adsorption-desorption analysis, temperature-programmed reduction with hydrogen (H2-TPR), and X-ray Photoelectron Spectroscopy (XPS), proving that the PdOx (x > 1) particles were highly dispersed on CeO2-NR and have a strong interaction with the support. The PdOx/CeO2-NR catalyst permitted the aerobic oxidation of various benzyl alcohol derivatives with good conversion, and high selectivity towards the corresponding aldehydes. The presence of electron donating groups (EDG) on the benzylic ring enhanced the reactivity as opposed to the electron withdrawing groups (EWG) which were detrimental for the catalytic activity. During the reaction a partial reduction of the metal produced a Pd(0)/PdOx/CeO2-NR redox couple stable in the reaction condition, more reactive and recyclable. Some mechanistic hypotheses are presented.
1. Introduction
The selective oxidation of benzyl alcohol (BnOH) to benzaldehyde (PhCHO) is considered a critical organic transformation either for the importance of PhCHO as a fine chemical and for the environmental implications that the reaction can bring about [1,2].
To avoid toxic and pollutant oxidants, various studies have focused the attention on the use of oxygen as an environmentally benign oxidizing agent. This kind of reaction could be effectively catalyzed by noble metals, such as gold, ruthenium, or palladium, that are often nano dispersed on less expensive supporting materials such as ceria, silica, and carbon [3]. Recent literature has evidenced a strong influence on the catalytic activity of metal dispersion [4], support morphology [5,6], and reaction conditions (concentration, temperature, solvent) [2]; thus, the preparation of the support and the method of dispersion of the metal could play a pivotal role in the efficiency and selectivity of the reaction.
In addition, due to the cost and toxicity of many organic solvents, omitting their usage can be a further attractive improvement to the environmental impact of the reaction. This could be done either using water [7] or the alcohol substrate itself as the solvent as proposed by Wang [8] and more recently by Xia [9], Li [10], and others [11,12].
At the same time, in-depth studies have been devoted to elucidating the catalytic mechanism of oxidation of benzyl alcohol by metals supported on nanoparticles in order to avoid secondary products such as toluene and benzoic acid [2,13,14,15,16,17,18]. In the case of Pd(0), various competing reaction pathways were proposed depending on temperature, substrate, and oxygen concentration. The prevalence of one or the other leads to the main product with a different percentage of secondary derivatives [13].
Recently, researchers have analyzed the BnOH oxidation mechanism under solvent free conditions [14]. From a mechanistic point of view, the absence of solvent, i.e., the use of polar protic BnOH alone, may have a massive effect, considering that these reactions are normally carried out in toluene or aromatic aprotic solvents. Xin et al. observed that, using Pd on crystalline nanoporous CeO2 in solvent free conditions, BnOH derivatives with electron-donating groups (EDG) on the aromatic ring were oxidized less than BnOH itself (18.63% for 4-methylbenzyl alcohol, 11.12% for 4-methoxybenzyl alcohol, and 26.60% for BnOH), while a slightly higher percentage of the benzylic alcohol with 4-nitro electron-withdrawing group (EWG) was converted to the aldehyde derivative (27.13% of 4-nitrobenzyl alcohol) [4]. These results contrast with the studies of Xu et al. [19], for the oxidation of similar substrates catalyzed by Pd supported on mesoporous carbon, using toluene as solvent. As a matter of fact, Xu et al. observed that addition of a nitro group decreased the alcohol conversion (54% in 4 h), while EDG resulted in higher conversion of alcohol compared to BnOH (92% and 93% in 4 h for 4-methyl and 4-methoxy derivatives, respectively).
The oxidation state of palladium plays a crucial role in the catalytic activity and the reaction mechanism [2,5]. Many research works focused on Pd(0) catalysts due to high reactivity [20] but the metallic palladium could be oxidized because of the oxygen or an oxidizing environment such as nanostructured ceria support [21], so maintaining the palladium in reduced form on support materials with high oxygen reservoir capacity such as ceria nanomaterials is a difficult task [22]. Therefore, preparing the catalyst with the oxide species of palladium might be easier to control and lead to a more stable catalyst compared to Pd(0) counterparts. In addition, the more reactive Pd(0) species can be produced from the palladium oxide species during the solvent-free oxidation of alcohols because the high concentration of alcohol can initiate the palladium reduction, as observed by Jürgensen et al., for the reduction of PdO supported on TiO2 in presence of propyl alcohol at temperatures higher than 90 °C [23].
In this work, palladium oxide (PdOx) supported on ceria nanorods (CeO2-NR) was prepared by a synthetic procedure slightly different than our previously published method [5], due to a change of palladium precursor (see Section 3. Materials and Methods). The catalyst was characterized and compared with the one previously produced, showing different chemical and physical properties. The new PdOx/CeO2-NR was then tested for solvent-free oxidation of various BnOH derivatives substituted in the aromatic ring with EDG and EWG groups. Some mechanistic hypotheses are proposed based on the findings of catalyst analysis after reaction and the results of oxidation of different BnOH derivatives.
2. Results and Discussion
In this work the palladium precursor used for wet impregnation was a commercial solution of Pd(NO3)2 (see Section 3. Materials and Methods). These conditions probably produced a more homogeneous deposition of the palladium on the ceria surface, facilitating a better oxidation during the calcination process. Only one palladium oxide species was detected in the sample as discussed in the X-ray photoelectron spectroscopy (XPS).
2.1. Characterization
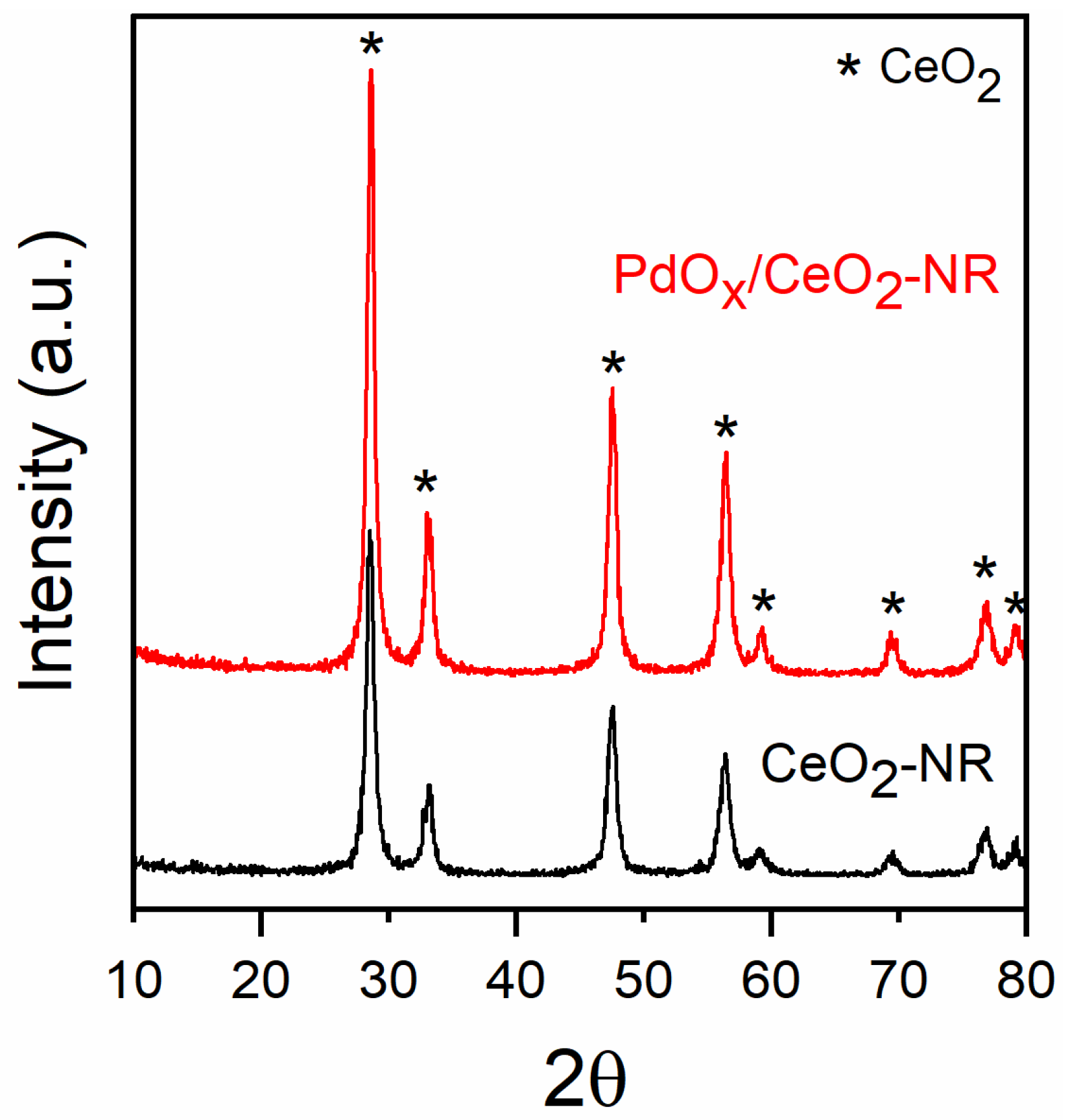
X-ray Diffraction (XRD) pattern of the CeO2-NR support showed the reflections of pure cubic fluorite CeO2 at 2θ about 28.5° (111), 33.1° (200), 47.5° (220), 56.4° (311), 59.2° (222), 69.5° (400), and 79.1° (420), (Joint Committee on Powder Diffraction Standards (JCPDS) card 81-0792) (Figure 1). The cubic cell size, “a” parameter, was 5.408 Å, and the average crystallite size calculated from the Size–Strain plot (Figure S1a) was about 13 nm (Table 1). The XRD pattern of the PdOx/CeO2-NR sample showed only the reflections assigned to CeO2, with the same position and nearly the same full width at half maximum (FWHM) of CeO2-NR support, corresponding to an average crystallite dimension of about 12 nm (Figure S1b); an unvaried size cell of 5.405 Å indicates that the Pd-loading process did not affect the crystallite structure (Figure 1, Table 1), excluding the solution of Pd into CeO2 crystal lattice. The absence of the PdOx diffractions is due to the low content. However, the most intense peak of PdO is expected at 33.8° (101), very close to the (200) reflection of CeO2, and so it might be hardly revealed.

Figure 1.
X-ray diffraction (XRD) patterns of the CeO2-NR and PdOX/CeO2-NR samples.

Table 1.
Crystallite size and cell parameters of CeO2 and textural properties of samples.
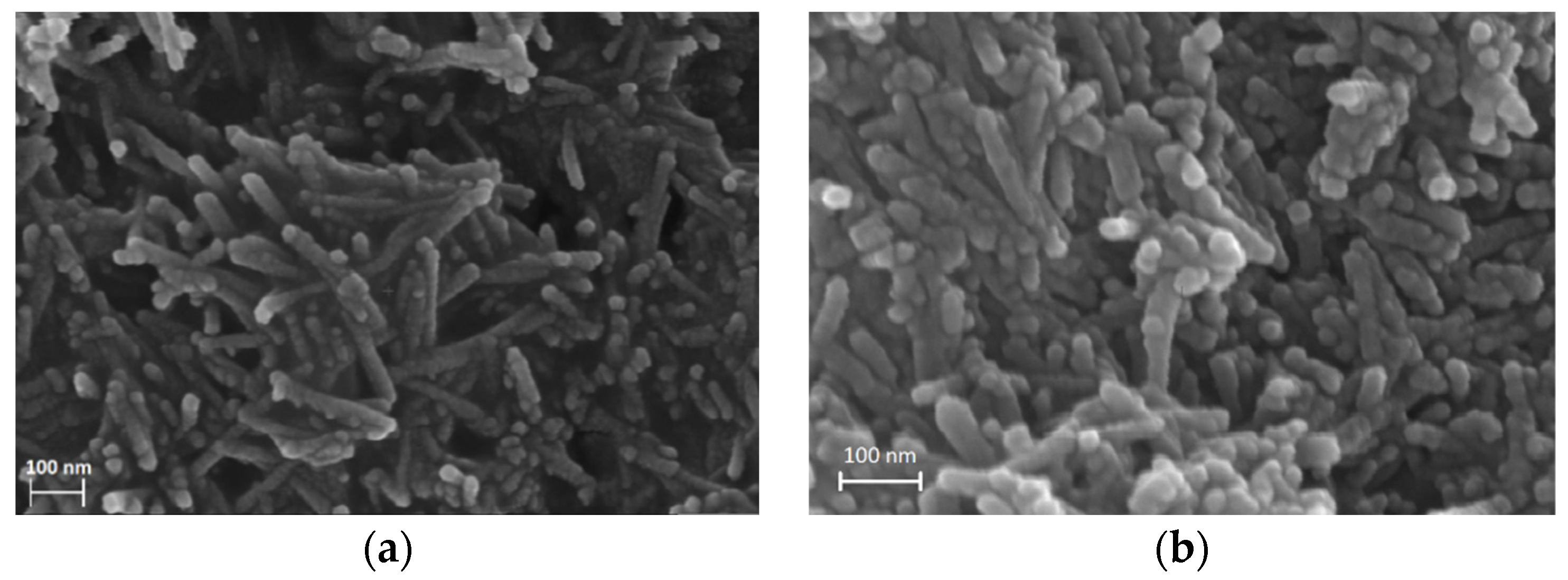
The morphology and chemical composition of the pure ceria support and the PdOx/CeO2-NR catalyst were analyzed by field-emission scanning electron microscopy/energy-dispersive spectroscopy (FE-SEM/EDS). The FE-SEM images of CeO2-NR and PdOx/CeO2-NR showed a rod-like morphology (length to width ratio above 5 with a uniform and well-developed structure (Figure 2)), suggesting that palladium deposition did not affect the morphology of the ceria support. No evidence of palladium particles was observed, suggesting that the metal oxide particles could be well-dispersed. The EDS analysis of PdOx/CeO2-NR measured 2.01 ± 0.07 wt.% of palladium in the sample, in good agreement with the nominal 2.00 wt.% metal content.

Figure 2.
Field-emission scanning electron microscopy (FE-SEM) of (a) CeO2-NR and (b) PdOx/CeO2-NR.
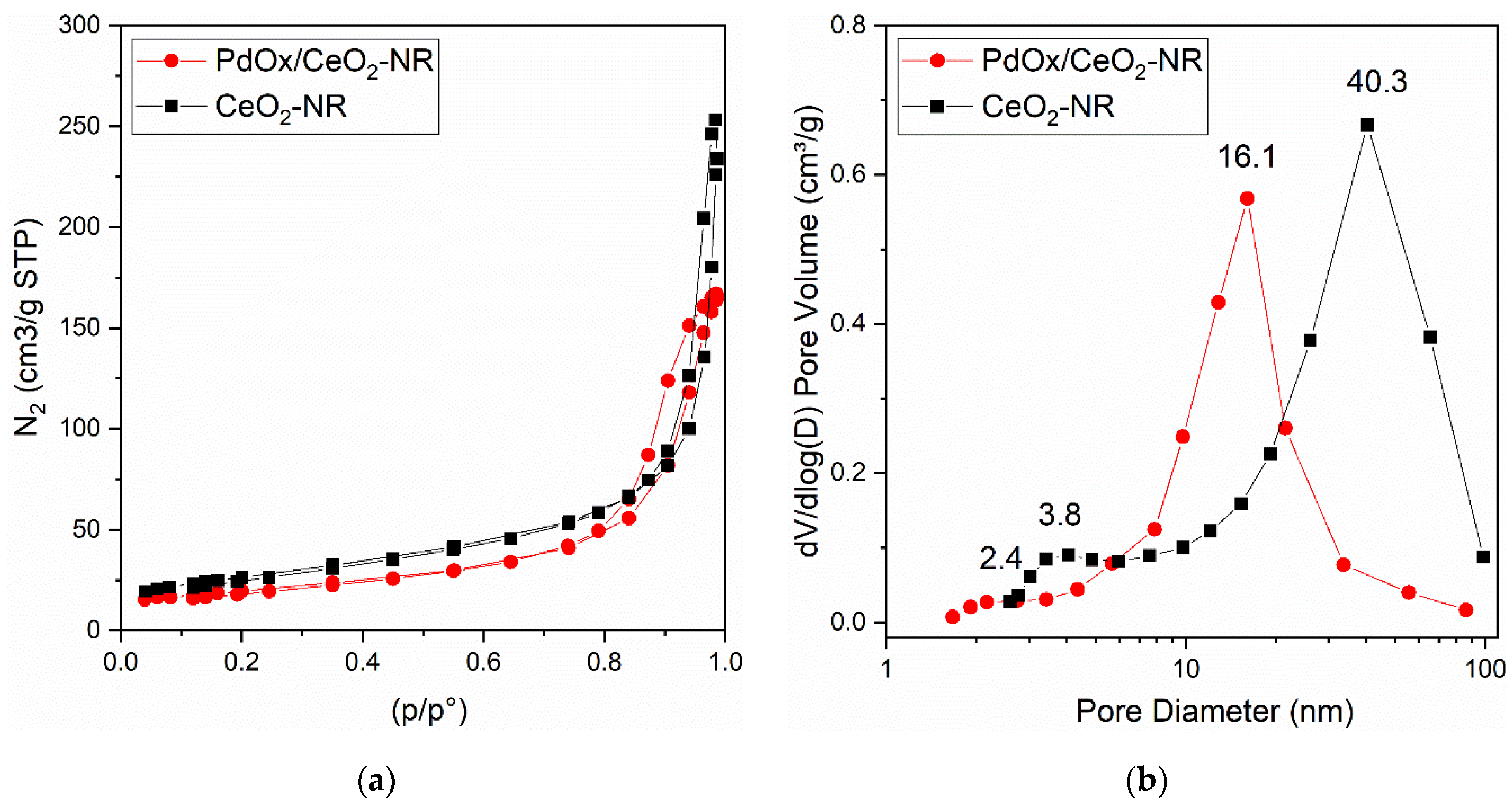
Brunauer–Emmett–Teller (BET) surface area of pure CeO2-NR was 94 m2 g−1. The palladium deposition caused a decrease of the support surface area down to 68 m2 g−1 (Figure 3 and Table 1).

Figure 3.
Brunauer–Emmet–Teller (BET) analysis of the CeO2-NR and PdOx/CeO2-NR samples: (a) nitrogen adsorption–desorption isotherms as a function of the relative pressure p/p°; (b) Barrett–Joyner–Halenda (BHJ) pore size distributions (PSD).
CeO2-NR pure support and PdOx/CeO2-NR catalyst both showed N2 adsorption–desorption curves Type V, with hysteresis loops H1-Type for CeO2-NR and H2(b)-Type for the PdOx/CeO2-NR catalyst by IUPAC classification [24]. Both these features are characteristic of a well-developed and uniform mesoporous morphology (Figure 3a). In addition, the H2(b)-Type shape is associated with the blocking of pores, with a large distribution of neck widths. Barrett–Joyner–Halenda (BJH) pore size distributions (PSD) of both samples were in the range of 1.5–100 nm, centered at 40.3 nm for pure CeO2-NR, and at 16.1 nm for the PdOx/CeO2-NR catalyst (Figure 3b). The corresponding average pore diameter was 19 nm for pure CeO2-NR and decreased down to 16 nm for the PdOx/CeO2-NR sample; in agreement, the corresponding total pore volume decreased from 0.40 down to 0.26 cm3 g−1 (Table 1). As expected, the addition of palladium oxide on the surface causes a decrease of surface area, pore size, and pore volume of the material, suggesting a partial occlusion of ceria pores.
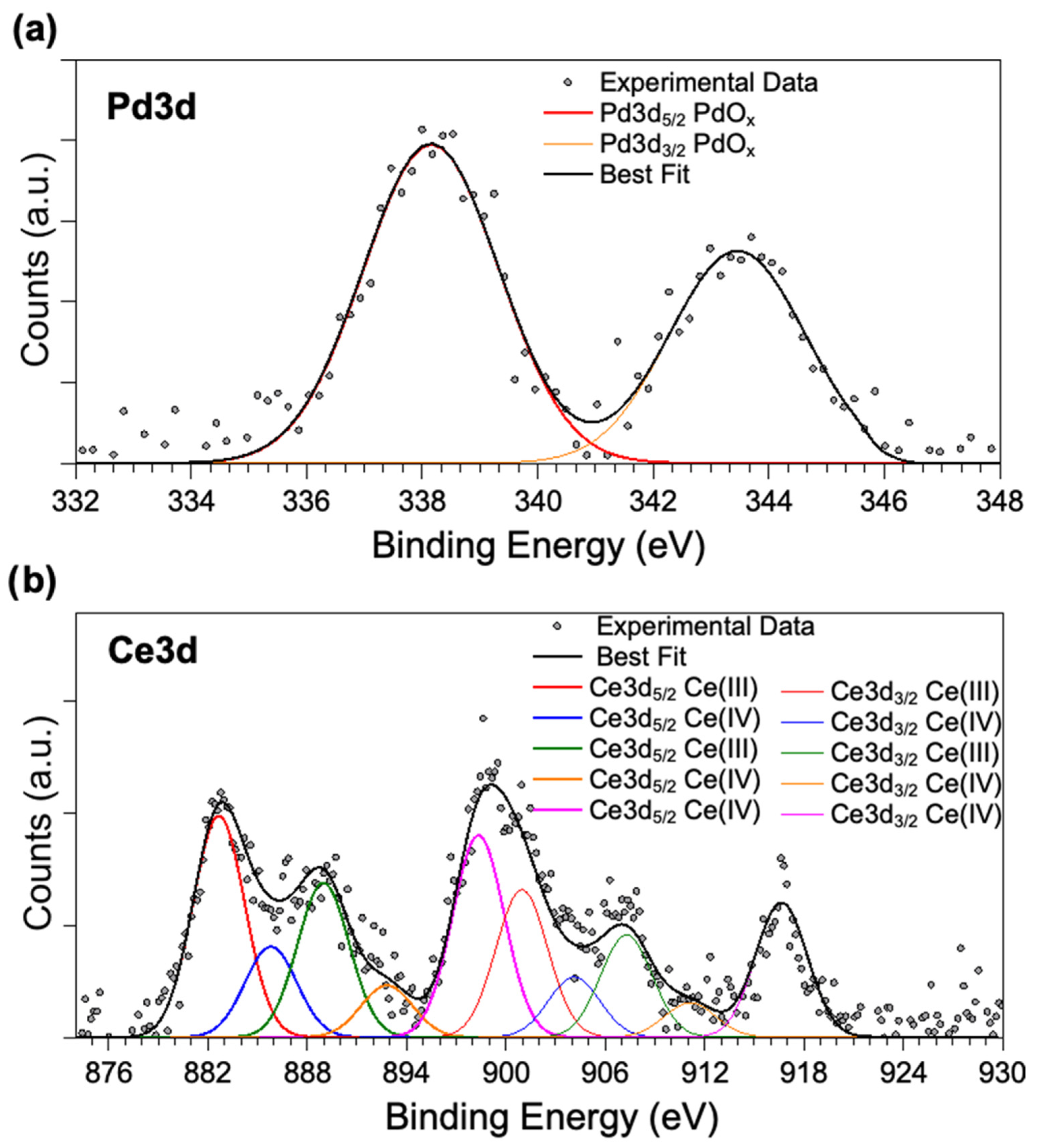
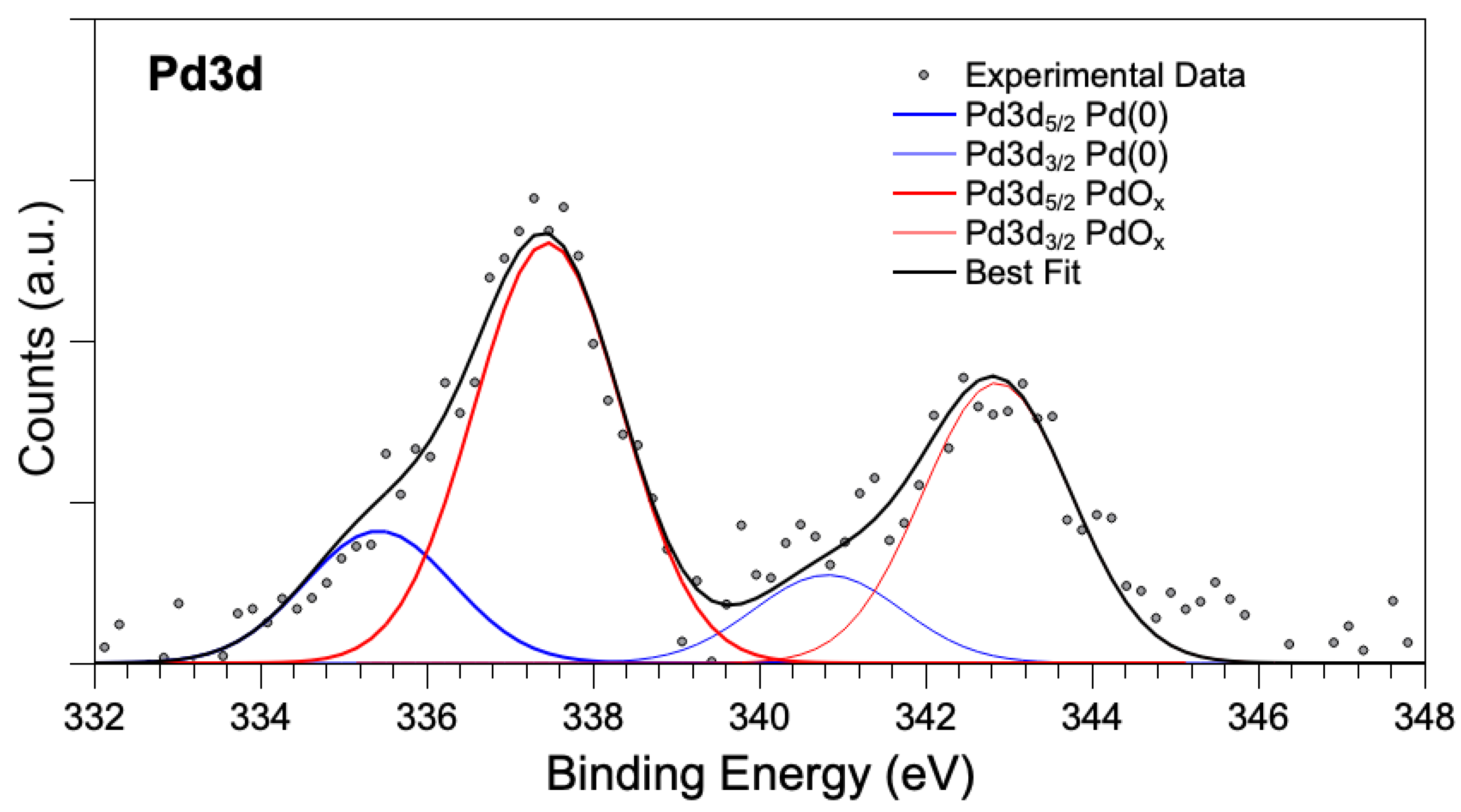
The XPS analysis of PdOx/CeO2-NR showed that there was only one Pd species on the surface of ceria, with Pd 3d5/2 component at almost 338 eV binding energy (BE; Figure 4), a binding energy value indicative of oxidized palladium (PdOx, x > 1), similar to the PdOx component observed in PdOx/CeO2-NR in our previous work [5]. However, here the PdOx is the only palladium species detected on the CeO2-NR, whereas, in the catalyst of our previous work, 10% PdO was present as well. In addition, the palladium atomic ratio i.e., Pd/(Pd + Ce) on the surface of PdOx/CeO2-NR was 10.3%.

Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of (a) Pd 3d region, (b) Ce 3d region for PdOX/CeO2-NR.
Ce3d spectra of the sample is as expected, extremely complicated, due to the different components arising by Ce(III) and Ce(IV) ions. By following a peak-fitting procedure, five spin orbit pairs related to Ce3d were individuated, and the resulting components were associated to the different ions by comparison with literature data [25,26], as reported in detail in Table 2. The Ce(III)/Ce(IV) atomic ratio was estimated at about 1:1 (Ce(III) percentage = 52%, Ce(IV) percentage = 48%), significantly higher than the 24% Ce(III) of the catalyst of our previous study [5], suggesting enhanced oxygen vacancy on the support.

Table 2.
Binding energy (BE) and full width at half maximum (FWHM) values and proposed assignments for the PdOx/CeO2-NR catalyst.
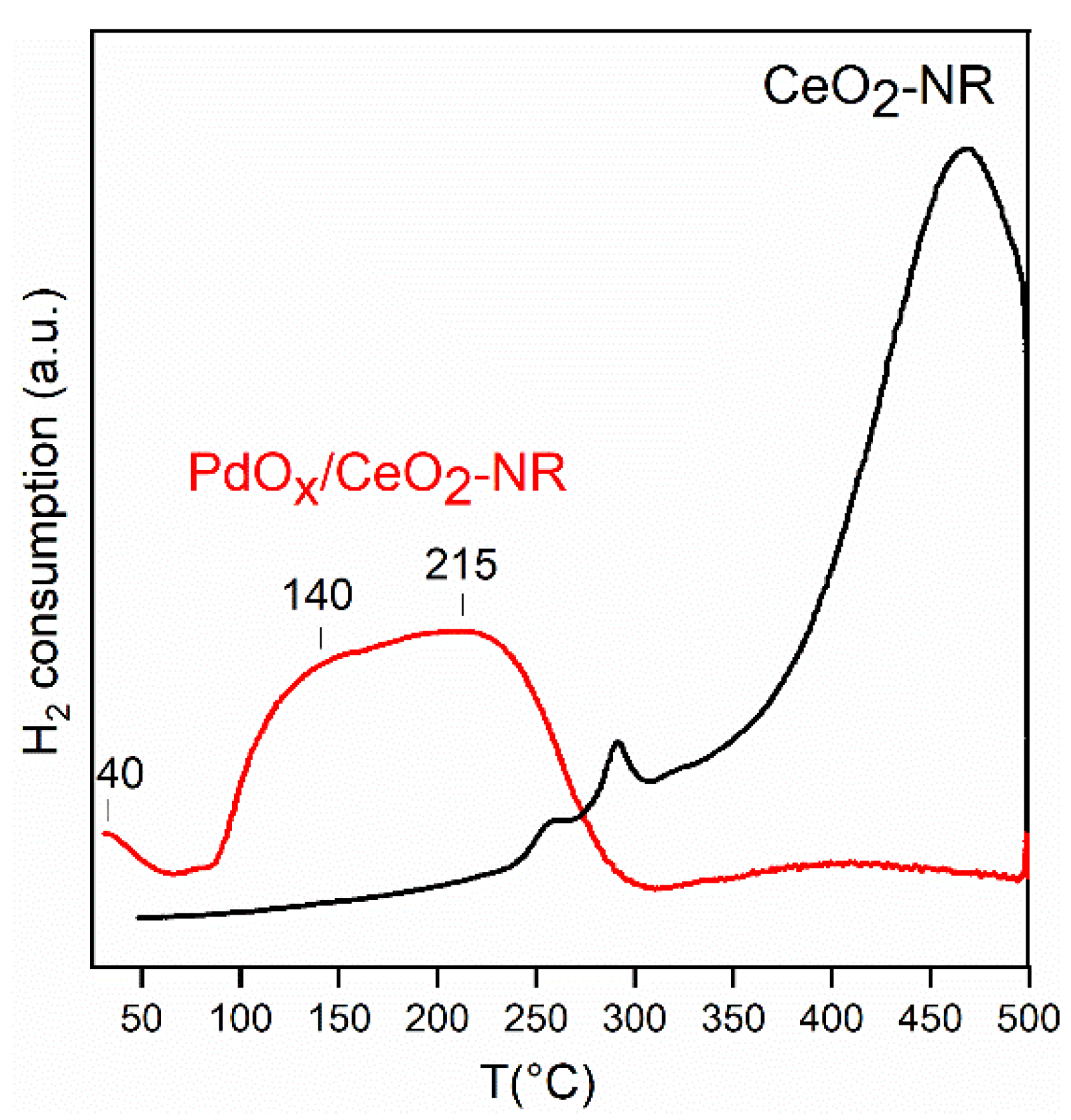
H2-TPR experiment was performed to obtain information about the homogeneity of PdOx species on the surface, and to test the redox properties of Ce(III)/Ce(IV) couple, that determines the oxygen storing capacity of the ceria surface. The reduction was conducted from 40 °C to 500 °C, to limit the sintering of CeO2 and Pd at higher temperatures. The pure support CeO2-NR showed an intense reduction peak, starting at about 250 °C with a maximum at about 450 °C, assigned to the reduction of Ce(IV) to Ce(III) on the surface of ceria (Figure 5). The total consumption of hydrogen corresponded to a ratio of exchanged electrons for Ce atom (e-/Ce) equal to 0.178, related to the reduction of 17.8% of the nominal content of CeO2 (Table 3). The PdOx/CeO2-NR catalyst showed a hydrogen consumption below 60 °C, and an intense broad peak in the range of 100–300 °C, with two components at 140 °C and 215 °C, and a very weak hydrogen consumption in the range of surface ceria reduction (250–500 °C) (Figure 5). The TPR profile of PdOx/CeO2-NR did not show a negative peak, observed by many authors in the temperature range of 50–100 °C due to the decomposition of PdHx species [5,27]. The formation of PdHx species has been related to the presence of larger Pd particles [27,28].

Figure 5.
Temperature programmed reduction (H2-TPR) profiles of the CeO2-NR and PdOx/CeO2-NR samples.

Table 3.
Redox properties by H2-TPR analysis: hydrogen consumption and reduction degree of Pd and Ce.
The reduction temperatures reported in the literature for PdOx/CeO2 catalysts with a similar Pd loading are in a very wide temperature range, depending on the preparation method and on the calcination temperature. PdOx reduction was observed at low temperature: in the range (–13)–(–23) °C [27], 30–64 °C [29,30], 20–160 °C [31], 50–200 °C [32], and 130–257 °C [28,33]. The presence of several peaks at different temperatures was attributed by the authors to the reduction of PdOx species with different support interaction and/or different particle dimensions, but in the latter case there is no total agreement between the authors. Based on the literature data, the three peaks at about T < 60 °C, T = 140 °C, and T = 215 °C, observed for PdOx/CeO2-NR of this study, are attributed to the reduction of PdOx species with increasing dispersion and increasing strength of the interaction with the support [32], and to the reduction of the Ce(IV) to Ce(III) promoted by palladium at lower temperature. The total consumption of hydrogen in the range 40–300 °C corresponds to a ratio of exchanged electron for palladium atom e-/Pd= 1.87. The very weak H2 consumption in the range 300–500 °C, corresponds to the reduction of only about 0.64% of the nominal content of CeO2 to Ce(III) (Table 3). Furthermore, it is important to consider the occurrence of the hydrogen spill-over phenomena, likely present on noble metal supported on ceria, causing difficulty in peak attribution and hydrogen consumption larger than the stoichiometric amount. The e-/Pd value lower than two, together with the profile shape in range 40–60 °C, that evidences a hydrogen consumption at 40 °C, both suggest that a fraction of PdOx was reduced during the initial thermal conductivity detector (TCD) stabilization step at T = 40 °C, consisting of a H2 flow throw the sample for 40 min. The TPR characterization shows that the Pd strongly influences the redox properties of ceria surface, promoting the ceria reduction at very lower temperature, lower than that observed in the previously studied Pd-CeO2-NR sample [5], and reinforcing the evidence that PdOx is highly dispersed and strongly interacting with the surface.
2.2. Catalytic Tests
In the literature, BnOH oxidation in solvent free conditions on various supported Pd(0) nanocatalysts gave different results from the 19.8% conversion (97.8% selectivity) on layered hydroxides [10] to the 89.9% conversion (63.7% selectivity) of functionalized crystalline Pd nanoparticles on nanoporous CeO2 [8].
Different studies have evidenced that Pd(0) is more reactive as a catalyst than PdO [20]. However, in presence of CeO2, Pd(0) can be rapidly oxidized to PdO by oxygen [34]. Considering that in solvent-free reactions the high concentration of alcohol can initiate the reduction of the palladium oxide, as in presence of propyl alcohol at temperatures higher than 90 °C [23], the catalyst prepared in this research was not pre-reduced but used in oxide form. The oxidation reactions (Table 4) were performed in solvent-free conditions, in presence of PdOx/CeO2-NR as catalyst and benzophenone as internal standard, keeping the temperature at 100 °C (unless otherwise specified) and the air flow at 5 mL min−1 for 18 h. The reactants were identified by gas chromatography-mass spectrometry (GC-MS) and were quantified via high-performance liquid chromatography (HPLC). In the blank experiment in which CeO2-NR was used as catalyst, no BnOH conversion occurred after 18 h. The results of the oxidation of BnOH derivatives are reported in Table 4. BnOH conversion was 50% with 93% PhCHO as product. Neither toluene nor benzoic acid were detected, and the only secondary product was benzyl benzoate, probably derived from the acid esterification by concentrated BnOH.

Table 4.
Substrates and reaction results of catalytic oxidation of benzyl alcohol (BnOH) derivatives and 4-(benzyloxy)phenol.
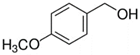
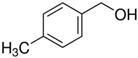
The catalytic activity of PdOx/CeO2-NR was also analyzed in the presence of various BnOH derivatives. In the same reaction conditions, presence of EDG on the para position of the aromatic ring led to an increase in substrate conversion with a slight decrease in the selectivity towards the aldehydic product. In case of 4-methoxybenzyl alcohol and 4-methylbenzyl alcohol (entry 2 and 6, Table 4), the conversion raised up to 70% and 66% respectively, with 86% and 81% selectivity towards the corresponding 4-methoxy- and 4-methylbenzaldehyde. On the contrary, in the oxidation of 4-phenoxybenzyl alcohol, a decrease in conversion and an improvement of selectivity was observed (62% and 94%, respectively, entry 8, Table 4). This last result could be attributable to the presence of a second aromatic ring that could compete with the adsorption of the benzyl ring on the active sites of the catalyst surface.
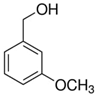
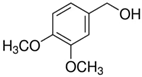
In the case of 3-methoxybenzyl alcohol (entry 3, Table 4) the reactivity decreases in comparison with the reference 4-methoxy isomer. It can be assumed that the stabilizing contribution to the rate-determining transition state is more relevant if the EDG is in a resonance position. With 3,4-dimethoxybenzyl alcohol the two different effects produced a small decrease in conversion respect to 4-methoxyderivative, maintaining the high selectivity towards the aldehydic product (entry 4, Table 4).
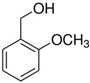
If the substituent is in the ortho position, as in the 2-methoxy- and 2-methyl-derivatives, the steric hinderance of the group seemed more influential than the electron donating effect; therefore, the substrate hardly reacted (<3% conversion, entry 5 and 7, respectively, Table 4). These effects of hinderance have already been highlighted by Kara et al. [35] in the oxidation of 4-methoxy- and 2-methoxybenzyl alcohol using perlite/V2O5 nanospheres catalyst with yields of 92% and 70% respectively.
In the oxidation of highly reactive 4-hydroxybenzyl alcohol (at 130 °C), the fast decrease of the substate concentration did not lead to a concurrent enhancement of the concentration of aldehydic product (entry 9, Table 4). Instead, in the HPLC analyses a big peak appeared at higher retention times. Probably, in solvent-free conditions, a parallel reaction could have occurred between the highly concentrated unreacted substrate and the corresponding aldehyde leading to polymerization products, easily visible after purification as sticky materials, which could result from the acetalization process.
Acidic work-up conditions used to revert the formation of these secondary products permitted the isolation of a derivative that, after partial chromatographic purification and GC-MS analysis, proved to be a mixture of 4-hydroxybenzaldehyde and a dimer.
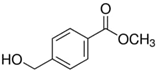
On the contrary, the oxidation of BnOH derivatives containing EWG were unsuccessful by PdOx/CeO2-NR, as observed for 4-nitrobenzyl alcohol and methyl(4-hydroxymethyl)benzoate, with less than 3% conversion (entry 10 and 11, Table 4).
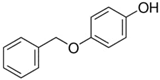
In the end, 4-(benzyloxy)phenol (entry 12, Table 4) as a model compound of lignin structures was tried with PdOx/CeO2-NR in solvent free conditions at 130 °C. The HPLC analysis showed that 31% of the substrate was converted after 18 h. A small amount of PhCHO and bigger amounts of two other oxidation products were detected, suggesting the partial breakage of the C–O bond. This last result is particularly significative because it is generally difficult to break the ether bond at ambient pressure which is extremely useful in lignin degradation and its waste valorization [36]. Further studies are underway to fully identify the products and extend the use of this catalyst in lignin depolymerization.
2.3. Mechanistic Aspects
Even if many research groups have studied the mechanism of BnOH oxidation [2], only a few articles have dealt with the effect of EWG or EDG on the reactivity [4,19,35,37,38]. The reactions performed with different benzyl alcohol derivatives help to hypothesize on the reaction mechanism. In general, the effect of the substituents is more evident in heterolytic reactions where an ion stabilizing effect is more influential. In solvent-free reactions with PdOx/CeO2-NR as catalyst, the use of PdOx and high concentration of alcohol produced an environment more polar than the toluene solvent generally used in similar reactions. BnOH is absorbed on PdOx through the dissociation of its OH bond as already demonstrated by various research groups [2,13]. Then, the oxygen acts directly as hydrogen abstractor producing the oxidation to aldehyde of the alkoxide bound and the concurrent reduction of palladium to a lower oxidation state after the release of the adsorbed hydrogen as water [39]. This is observable during the reaction where the catalyst color changes from light brown of the PdOx/CeO2 to a dark grey due to the presence of Pd(0) (Figure S2). In fact, the massive amount of the benzylic alcohol derivative present in the reaction can facilitate the in situ reduction of PdOx to Pd(0) as previously demonstrated by Jürgensen in BnOH oxidation by PdO/TiO2 catalyst [23]. XPS measurements carried out on the “used” PdOx/CeO2-NR sample, recovered from the catalytic reaction on BnOH, confirmed this hypothesis, showing the presence of approximately 30% reduced Pd(0) atoms, assigned to the low BE signal (Pd 3d5/2 BE = 335.42 eV) in Figure 6. The signal at higher BE is attributed to unperturbed PdOx species [40].

Figure 6.
Pd 3d XPS spectrum of “used” PdOx/CeO2-NR. The catalyst was recovered after BnOH oxidation, washed with acetonitrile, dried under vacuum at lab temperature, and kept in a sealed container before XPS analysis.
The contemporary presence of the more reactive produced Pd(0) then activates a faster catalytic process in which Pd(0) and PdOx can catalyze synergistically the benzyl alcohol oxidation as recently demonstrated by Zhe Wang et al. on a similar ceria-based catalyst [39].
The oxygen flow partially re-oxidizes the Pd(0) to PdOx leading to an equilibrium between the two redox species. It is likely that CeO2-NR facilitates this equilibrium.
The recyclability tests performed on the catalyst (Table 5) not only confirmed the stability of the Pd(0)/PdOx redox couple on CeO2-NR but underlined the increased reactivity of the catalyst used in subsequent runs.

Table 5.
Recyclability test of PdOX/CeO2-NR catalyst in BnOH to benzaldehyde (PhCHO) oxidation.
As regards the different reactivity of the BnOH derivatives with respect to the data of Xin et al. [4], this could be attributed to the presence of PdOx catalyst instead of only metallic palladium.
Furthermore, when compared with other solvent free processes [4,38,41], the higher PdOx/CeO2-NR reactivity difference in substrates containing EDG on the aromatic ring as compared to those with EWG could be attributed to an ionic transition state facilitated by the presence of palladium oxide and the polar environment. In these conditions, groups that can donate electrons during the rate determining hydride abstraction enhance the BnOH conversion while electron withdrawing groups on the aromatic ring strongly slow down the process.
3. Materials and Methods
Benzyl alcohol ≥ 99%, 4-methoxybenzyl alcohol 98%, 2-methoxybenzyl alcohol 99%, 3-methoxybenzyl alcohol 98%, 3,4-dimethoxybenzyl alcohol 96%, 2-methylbenzyl alcohol 98%, 4(benzyloxy)phenol 98%, palladium(II) nitrate solution (10 wt.% in 10 wt.% nitric acid; 99.999% trace metal), cerium(III) nitrate hexahydrate (≥99.0% trace metal basis), acetonitrile (for HPLC, gradient grade, >99.9%), ethanol (for HPLC, ≥99.8%), and Whatman®® quantitative filter paper (ashless, Grade 42) were purchased from Sigma Aldrich (Darmstadt, Germany). 4-methylbenzyl alcohol 99%, 4-phenoxybenzyl alcohol 97%, 4-hydroxybenzyl alcohol 99%, 4-nitrobenzyl alcohol 99%, methyl(4-hydroxymethyl)benzoate 99% were bought from Alfa Aesar (Kandel Germany). Ethyl acetate, diethyl ether, NaOH (for analysis-ACS-ISO) were purchased from Carlo Erba (Cornaredo, Italy). Benzophenone (>99%) was bought from Fluka (Buchs, Switzerland).
The PdOx/CeO2-NR catalyst was prepared according to our previously published work [5] but with changing the palladium precursor from Alfa Aesar solid Pd(NO3)2∙2H2O solubilized in HNO3 solution to Sigma Aldrich Pd(NO3)2 solution. The CeO2-NR was prepared by hydrothermal treatment of Ce(NO3)3·6H2O at high pH and at 100 °C. Cerium nitrate hexahydrate, 1.300 g, was dissolved in 20.0 mL distilled water and then mixed in a Teflon bottle with an alkali solution of 14.400 g sodium hydroxide in 40.0 mL distilled water. After 30 min stirring, the Teflon bottle was inserted in a stainless-steel autoclave vessel and put in an oven at 100 °C for 24 h. Then the obtained precipitate was vacuum filtered, washed with distilled water and ethanol. To fully evaporate the remaining solvent, the solid was left in the oven overnight at 120 °C. Finally, the prepared solid was uniformly milled and then calcined at 400 °C for 5 h, resulting in the light-yellow ceria powder (Figure S2a). The prepared ceria support was then used in a wet impregnation process aiming at the deposition of 2.00 wt.% palladium. For this, 10.0 mL of Pd(NO3)2·2H2O commercial solution was added drop by drop to 1.000 g of CeO2-NR in a 50 mL flask maintaining the temperature at 60 °C for 2 h under stirring. Then, using a rotavapor, the water was removed and the solid was dried in the same drying condition of ceria support. Finally, the dried solid was milled and calcined at 500 °C for 5 h to acquire the PdOx/CeO2-NR with light brown color (Figure S2b).
XRD patterns were recorded using a Scintag X1 diffractometer (Scintag Inc., San Francisco, CA, USA) equipped with a Cu Kα (λ = 1.5406 Å) source and the Bragg–Brentano θ–θ configuration in the 2θ = 10–80 range, with 0.05° step increment and 3 s acquisition time. The CeO2 crystallite size was estimated from the Size–Strain plot, as reported in [42]. The “a” lattice parameter of ceria was calculated from the eight XRD reflections by Equation (1):
The morphologies of CeO2-NR and the PdOx/CeO2-NR were investigated via a Zeiss Sigma 300 VP-FESEM apparatus (20 kV accelerating voltage, 4.0 mm working distance; Carl Zeiss Microscopy, Jena, Germany), equipped with a high-resolution secondary electron in-lens detector, and with EDS. The powder samples were dispersed in isopropyl alcohol followed by 15 min ultrasonication. A thin graphite film was used to place a tiny droplet of sample suspension with approximately 20 μL volume on the sample holder. The EDS analysis was performed in six different areas, and the Pd wt.% was obtained as the mean value.
The N2 adsorption-desorption isotherms at 77 K were obtained using a Micrometrics Gemini V apparatus (Micromeritics Instrument Corporation, Norcross, GA, USA). Before the N2 adsorption, the surface was degassed with flowing He at 350 °C for 2 h. The surface area was calculated by the BET method in equilibrium pressure range p/p° = 0.05–0.20. The PSD was calculated by the BJH method from the desorption isotherm; the total pore volume was calculated from the maximum adsorption point p/p° = 0.98; the average pore diameter was calculated as (diameter = 4 × Pore Volume/Surface Area) assuming a cylindrical shape of pores.
X-ray Photoelectron Spectroscopy measurements were carried out in an X-ray photoelectron spectrometer of our own construction (home-made instrumentation) with a dual-anode Mg/Al X-ray source, at Mg K-alpha Photon Energy. Measurements were carried out in UHV, and typical vacuum pressure in the analysis chamber during measurements was in the 10−9–10−10 Torr range. Photoelectrons emitted by C1s, O1s, Pd3d, and Ce3d core levels were detected. BEs are reported after correction for charging using the aliphatic C1s as a reference (BE 285.0 eV) [43]. Core level spectra were fitted with a polynomial background and Gaussian peak functions [44,45]. The XPS spectra plotted with peak fitting residuals are reported in Figure S3.
The temperature programmed reduction with hydrogen (H2-TPR) was performed by a Thermo Scientific TPDRO1100 (Thermo Fisher Scientific, Waltham, MA, USA) flow apparatus. The H2 consumption was measured by a TCD calibrated by the reduction of a known mass of CuO (purity 99.99%). A soda lime trap removed the H2O produced before flowing into the TCD. The sample (0.100 g) was pre-treated in a flow (20 cm3 min−1) of 10% O2/He mixture at 400 °C for 30 min, and then cooled down. The H2-TPR experiment was conducted flowing 30 cm3 min−1 of 5% H2/Ar mixture. The TCD signal was stabilized flowing the mixture at 40 °C for 40 min and then the temperature was increased up to 500 °C with a heating rate of 10 °C min−1; the final temperature was maintained for 60 min.
Catalytic activity of PdOx/CeO2-NR, for solvent-free aerobic oxidation of BnOH derivatives was assessed in a 5 mL two-neck round bottom flask, equipped with reflux condenser and gas inlet and thermometer. A total of 5 mmol of substrate was added to the reaction flask (pre-heated in a silicon oil bath). After the temperature of the substrate was stabilized at the desired value (130 °C for 4-hydroxybenzyl alcohol and 4-(benzyloxy)phenol due to their high melting point; 100 °C for the rest of the substrates), 5 mg of PdOx/CeO2-NR was added to the flask and 5 mL min−1 air flow was bubbled above the reactants. The reactants were constantly stirred during the reaction (500 rpm) to avoid the mass transfer restrictions. The reactions continued for 18 h. Benzophenone was used as internal standard. The conversion of BnOH derivatives, and the selectivity of desired (aldehydic) products are defined in Equations (2) and (3) below:
Acetonitrile was used to dilute the reactants before being analyzed. The substrates and products were identified by means of GC-MS (Shimadzu VG 70/250S; Kyoto, Japan), using Supelco SLB™ column (30 m, 0.25 mm, and 0.25 µm film thickness; Bellefonte; PA, USA). The program of analysis started at 50 °C for 4 min and then the oven was heated by a 10 °C/min temperature gradient until reaching 250 °C for 10 min. The injector temperature was 250 °C.
A HPLC/autosampler/vacuum degasser system (1260 Infinity II, Agilent, Santa Clara, CA, USA) was used for evaluating the results of reactions. The separation was performed on a Luna C18 100 Å column (4.6 × 150 mm; 5 µm; Phenomenex®, Torrance, CA, USA). The mixture of water and acetonitrile (phase A and B respectively) was utilized as the mobile phase, starting from 30% acetonitrile which was linearly increased to 100% within 20 min (flow rate 1 mL min−1).
The catalyst “used” in BnOH oxidation was recovered after the reaction by vacuum filtration, followed by washing with acetonitrile and drying under vacuum in room temperature. The sample was kept in a sealed container before being analyzed by XPS.
4. Conclusions
PdOx/CeO2-NR was prepared through wet Ce(NO3)3·6H2O impregnation process using commercial Pd(NO3)2 solution, and subsequent calcination. The Pd-loading process does not affect the crystallite structure and size of CeO2-NR support (excluding any solution of Pd into CeO2 lattice). As expected, the addition of palladium oxide on the surface causes a decrease of surface area, pore size, and pore volume of the material, suggesting a partial occlusion of ceria pores by PdOx. The TPR suggests that PdOx is highly dispersed and strongly interacting with the ceria surface, influencing the ceria redox properties. The XPS evidenced that PdOx is the only metal oxide species on the ceria surface.
The PdOx/CeO2-NR, used without pre-reduction, catalyzed the solvent free reactions on benzyl alcohol and its derivatives containing EDG with good to high conversion and selectivity. Derivatives with EWG did not react in these conditions. The more polar environment of the reaction due to the presence of BnOH and the use of PdOx probably facilitates an ionic mechanism that could explain the high difference of reactivity between substrates containing ERG and EWGs.
During the reaction the in situ partial reduction of the palladium oxide produces a Pd(0)/PdOx/CeO2-NR redox couple stable in the oxidizing condition. We hypothesize that ceria facilitates the redox couple generation and stabilization. The newly formed palladium redox couple was stable in the subsequent runs and even more reactive, confirming the synergistic effect of the simultaneous presence of Pd(0) and PdOx in the catalytic process.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal13010005/s1. Figure S1: Evaluation of the average particle dimension of ceria particles by the Size–Strain Plot of XRD diffractions of: (a) CeO2-NR; (b) PdOx/CeO2-NR; Figure S2: Images of (a) calcined CeO2-NR, (b) calcined PdOx/CeO2-NR, (c) used PdOx/CeO2-NR; Figure S3: XPS spectra, with Peak Fitting Residuals (green curve), of pristine PdOx/CeO2-NR (a) Pd3d and (c) Ce3d core levels, and (b) for the “used” PdOx/CeO2-NR Pd3d core level.
Author Contributions
Conceptualization, S.S.M. and D.T.; investigation, S.S.M. and S.T.; resources, D.T. and S.T.; data curation, S.S.M., S.T., C.B. and I.L.; writing—original draft preparation, S.S.M., D.T., S.T. and C.B.; writing—review and editing, S.S.M., S.T. and D.T.; funding acquisition, S.T. and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Ministero dell’Istruzione dell’Università e della Ricerca (MIUR)—Departments of Excellence, 2017—legge 232/2016—art.1, commi 314–337 awarded to Dept. of Science, University Roma Tre, Rome, Italy for 2018–2022.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors express appreciation for the kind and generous contribution of Sergio Lo Mastro, Department of Science, Università Roma Tre, for conducting XRD and SEM characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giang, L.T.K.; Ku, Y. Selective Oxidation of Benzyl Alcohol in Aqueous Phase by TiO2 Based Photocatalysts: A Review. Chem. Eng. Technol. 2021, 14, 2178–2190. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective Benzyl Alcohol Oxidation over Pd Catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef]
- Hu, H.; Xi, J. Single-Atom Catalysis for Organic Reactions. Chin. Chem. Lett. 2022, 2022, 107959. [Google Scholar] [CrossRef]
- Xin, P.; Li, J.; Xiong, Y.; Wu, X.; Dong, J.; Chen, W.; Wang, Y.; Gu, L.; Luo, J.; Rong, H.; et al. Revealing the Active Species for Aerobic Alcohol Oxidation by Using Uniform Supported Palladium Catalysts. Angew. Chem. 2018, 130, 4732–4736. [Google Scholar] [CrossRef]
- Moeini, S.S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized Palladium Supported on Ceria Nanorods for Catalytic Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde in Protic Solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef]
- Moeini, S.S.; Pasqual Laverdura, U.; Marconi, E.; Lisi, N.; Serra, E.; Chierchia, R.; Luisetto, I.; Tuti, S.; Tofani, D. A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol. Catalysts 2022, 12, 20. [Google Scholar] [CrossRef]
- Bourbiaux, D.; Mangematin, S.; Djakovitch, L.; Rataboul, F. Selective Aerobic Oxidation of Benzyl Alcohols with Palladium(0) Nanoparticles Suspension in Water. Catal. Lett. 2021, 151, 3239–3249. [Google Scholar] [CrossRef]
- Wang, H.; Kong, W.; Zhu, W.; Wang, L.; Yang, S.; Liu, F. One-Step Synthesis of Pd Nanoparticles Functionalized Crystalline Nanoporous CeO2 and Their Application for Solvent-Free and Aerobic Oxidation of Alcohols. Catal. Commun. 2014, 50, 87–91. [Google Scholar] [CrossRef]
- Xia, X.; Liu, S.; Long, Z.; Zhu, W.; Chen, G.; Huang, H.; Tong, M. P,N Co-Doped Biomass Carbon as a Remarkable Metal-Free Catalyst for Solvent-Free Oxidation of Benzyl Alcohol with Ambient Air: The Key Promoting Role of N Co-Doping. Appl. Surf. Sci. 2022, 571, 151409. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Yu, S.; Wei, Z.; Zhang, H. Highly Dispersed Pd Nanoclusters on Layered Double Hydroxides with Proper Calcination Improving Solvent-Free Oxidation of Benzyl Alcohol. ACS Sustain. Chem. Eng. 2022, 10, 7223–7233. [Google Scholar] [CrossRef]
- Yi, X.-T.; Li, C.-Y.; Wang, F.; Xu, J.; Xue, B. The Solvent-Free and Aerobic Oxidation of Benzyl Alcohol Catalyzed by Pd Supported on Carbon Nitride/CeO2 Composites. New J. Chem. 2022, 46, 7108–7117. [Google Scholar] [CrossRef]
- Nair, V.R.; Maiyalagan, T.; Shendage, S.S. Halloysite Clay Nanotubes with Fe–Al Deposits for the Oxidation of Benzyl Alcohol. New J. Chem. 2022, 46, 17213–17222. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Rossetti, I.; Villa, A.; Prati, L. Benzyl Alcohol Oxidation on Carbon-Supported Pd Nanoparticles: Elucidating the Reaction Mechanism. ChemCatChem 2014, 6, 3464–3473. [Google Scholar] [CrossRef]
- Galvanin, F.; Sankar, M.; Cattaneo, S.; Bethell, D.; Dua, V.; Hutchings, G.J.; Gavriilidis, A. On the Development of Kinetic Models for Solvent-Free Benzyl Alcohol Oxidation over a Gold-Palladium Catalyst. Chem. Eng. J. 2018, 342, 196–210. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, X.-T.; He, X.-Q.; Ji, H.-B. Mechanism and Kinetics of the Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde Catalyzed by Cobalt Porphyrin in a Membrane Microchannel Reactor. Chem. Eng. Sci. 2021, 245, 116847. [Google Scholar] [CrossRef]
- Takeyama, T.; Kobayashi, M.; Kikuchi, M.; Ogura, T.; Shimazaki, Y.; Iwatsuki, S. Benzyl Alcohol Oxidation Mechanisms by One- and Two-Electron Oxidized Species of Cu(II)-Salen Complexes with Para-R-Substituents, [Cu(R-Salen)]N+ (R = MeO, MeS; n = 1, 2). Inorg. Chim. Acta 2020, 511, 119848. [Google Scholar] [CrossRef]
- Arena, F.; Gumina, B.; Cannilla, C.; Spadaro, L.; Patti, A.; Spiccia, L. Nanostructured MnOx Catalysts in the Liquid Phase Selective Oxidation of Benzyl Alcohol with Oxygen: Part II. Reaction Mechanism, Kinetics and Deactivation Pattern. Appl. Catal. B Environ. 2015, 170–171, 233–240. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Qayyum, A.; Barczak, M.; Colmenares-Quintero, R.F.; Borowski, P.; Triantafyllidis, K.; Colmenares, J.C. Mechanistic and Kinetic Studies of Benzyl Alcohol Photocatalytic Oxidation by Nanostructured Titanium (Hydro) Oxides: Do We Know the Entire Story? Appl. Catal. B Environ. 2023, 320, 121939. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.-K.; Chen, Y.; Wang, Y.; Li, Y.-X. Palladium Nanoparticles Supported on Mesoporous Carbon Nitride for Efficiently Selective Oxidation of Benzyl Alcohol with Molecular Oxygen. Appl. Catal. Gen. 2017, 542, 380–388. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Caravati, M.; Baiker, A. Oxidic or Metallic Palladium: Which Is the Active Phase in Pd-Catalyzed Aerobic Alcohol Oxidation? J. Phys. Chem. B 2006, 110, 25586–25589. [Google Scholar] [CrossRef]
- Wu, P.; Cao, Y.; Zhao, L.; Wang, Y.; He, Z.; Xing, W.; Bai, P.; Mintova, S.; Yan, Z. Formation of PdO on Au–Pd Bimetallic Catalysts and the Effect on Benzyl Alcohol Oxidation. J. Catal. 2019, 375, 32–43. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Jürgensen, A.; Heutz, N.; Raschke, H.; Merz, K.; Hergenröder, R. Behavior of Supported Palladium Oxide Nanoparticles under Reaction Conditions, Studied with near Ambient Pressure XPS. Anal. Chem. 2015, 87, 7848–7856. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Weber, W.H.; Gandhi, H.S. Surface Characterization of Alumina-Supported Ceria. J. Phys. Chem. 1988, 92, 4964–4970. [Google Scholar] [CrossRef]
- Szijjártó, G.P.; Pászti, Z.; Sajó, I.; Erdőhelyi, A.; Radnóczi, G.; Tompos, A. Nature of the Active Sites in Ni/MgAl2O4-Based Catalysts Designed for Steam Reforming of Ethanol. J. Catal. 2013, 305, 290–306. [Google Scholar] [CrossRef]
- Danielis, M.; Betancourt, L.E.; Orozco, I.; Divins, N.J.; Llorca, J.; Rodríguez, J.A.; Senanayake, S.D.; Colussi, S.; Trovarelli, A. Methane Oxidation Activity and Nanoscale Characterization of Pd/CeO2 Catalysts Prepared by Dry Milling Pd Acetate and Ceria. Appl. Catal. B Environ. 2021, 282, 119567. [Google Scholar] [CrossRef]
- Gopinath, R.; Lingaiah, N.; Sreedhar, B.; Suryanarayana, I.; Sai Prasad, P.S.; Obuchi, A. Highly Stable Pd/CeO2 Catalyst for Hydrodechlorination of Chlorobenzene. Appl. Catal. B Environ. 2003, 46, 587–594. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, G.; Lee, H. Continuous Methane to Ethane Conversion Using Gaseous Oxygen on Ceria-Based Pd Catalysts at Low Temperatures. Appl. Catal. Gen. 2021, 623, 118245. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.; Ma, K.; Zhang, Y.; Hu, Y.-M.; Kong, L.; Jia, A.; Zhang, Z.; Huang, W.; Lu, J.-Q. Ceria Morphology-Dependent Pd-CeO2 Interaction and Catalysis in CO2 Hydrogenation into Formate. J. Catal. 2021, 397, 116–127. [Google Scholar] [CrossRef]
- Yan, Z.; Tomer, A.; Perrussel, G.; Ousmane, M.; Katryniok, B.; Dumeignil, F.; Ponchel, A.; Liebens, A.; Pera-Titus, M. A Pd/CeO2 “H2 Pump” for the Direct Amination of Alcohols. ChemCatChem 2016, 8, 3347–3352. [Google Scholar] [CrossRef]
- Luo, M.-F.; Hou, Z.-Y.; Yuan, X.-X.; Zheng, X.-M. Characterization Study of CeO2 Supported Pd Catalyst for Low-Temperature Carbon Monoxide Oxidation. Catal. Lett. 1998, 50, 205–209. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Meng, M.; Xian, H.; Tu, Y.-B.; Li, X.-G.; Ding, T. The Nanomorphology-Controlled Palladium-Support Interaction and the Catalytic Performance of Pd/CeO2 Catalysts. Catal. Lett. 2009, 133, 328–333. [Google Scholar] [CrossRef]
- Neal, L.M.; Everett, M.L.; Hoflund, G.B.; Hagelin-Weaver, H.E. Characterization of Palladium Oxide Catalysts Supported on Nanoparticle Metal Oxides for the Oxidative Coupling of 4-Methylpyridine. J. Mol. Catal. Chem. 2011, 335, 210–221. [Google Scholar] [CrossRef]
- Kara, G.K.; Rahimi, J.; Niksefat, M.; Taheri-Ledari, R.; Rabbani, M.; Maleki, A. Preparation and Characterization of Perlite/V2O5 Nano-Spheres via a Novel Green Method: Applied for Oxidation of Benzyl Alcohol Derivatives. Mater. Chem. Phys. 2020, 250, 122991. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic Oxidation of Lignin in Solvent Systems for Production of Renewable Chemicals: A Review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective Photocatalytic Oxidation of Benzyl Alcohol and Its Derivatives into Corresponding Aldehydes by Molecular Oxygen on Titanium Dioxide under Visible Light Irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Goksu, H.; Sen, F. Handy and Highly Efficient Oxidation of Benzylic Alcohols to the Benzaldehyde Derivatives Using Heterogeneous Pd/AlO(OH) Nanoparticles in Solvent-Free Conditions. Sci. Rep. 2020, 10, 5731. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Yang, S.; Yang, X.; Meng, F.; Zhai, L.; Li, Z.; Zhao, S.; Zhang, G.; Qin, Y. Dual Pd2+ and Pd0 Sites on CeO2 for Benzyl Alcohol Selective Oxidation. J. Catal. 2022, 414, 385–393. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1. Available online: https://srdata.nist.gov/xps/ (accessed on 13 November 2022).
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple Preparation of Nitrogen-Doped TiO2 and Its Performance in Selective Oxidation of Benzyl Alcohol and Benzylamine under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125743. [Google Scholar] [CrossRef]
- Tuti, S.; Luisetto, I.; Pasqual Laverdura, U.; Marconi, E. Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability. Reactions 2022, 3, 333–351. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; ISBN 978-0-9627026-2-4. [Google Scholar]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Hantsche, H. High Resolution XPS of Organic Polymers, the Scienta ESCA300 Database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., Hardcover, ISBN 0-471-93592-1. Adv. Mater. 1993, 5, 778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).