Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles
Abstract
:1. Introduction
| Plant Name | Precursor | Average Size and Morphology | Featured Study | References |
|---|---|---|---|---|
| O. sanctum (Tulsi) | Cu(CH3COO)2 | 50 nm and flower shape | photocatalytic activity with H2O2 oxidant against degradation of methylene blue | [1] |
| Saraca indica | CuCl2⋅H2O | 40–70 nm and spherical | The photoluminescence behaviour | [10] |
| Adiantum lunulatum | CuSO4 | 6–7 nm and quasi-spherical | Investigation of innate immunity, antioxidative enzymatic and oxidative stress of model plants such as Lens | [12] |
| Ocimum basilicum | CuSO4·5H2O | 70 nm and spherical | Antibacterial activity against E. coli and S. aureus | [16] |
| Tilia Tomentosa | Cu(CH3COO)2 | 113 nm and rod shape | Thermal and Antibacterial activity | [17] |
| Calotropis gigantean | Cu(NO3)2 | 20 nm and spherical | Dye-sensitized solar cells | [18] |
| Catharanthus Roseus | CuSO4 | 5–10 nm and spherical | Studied the CuO membrane efficacy for chromium (VI) removal from waste water | [19] |
| Seidlitzia rosmarinus | Cu(CH3COO)2 | 222 nm and Cauliflower shape | Photocatalytic (degradation) and self-cleaning activity (decolouration) of methylene blue | [20] |
| Gundelia tournefortii | CuCl2 | 50–60 nm and spherical | The catalytic activity for the synthesis of N-monosubstituted urea and reduction of 4-nitrophenol | [21] |
| Gloriosa superba | Cu(NO3)2 | 5–10 nm and spherical | Antibacterial studies | [22] |
| Alchemilla vulgaris | Cu(CH3COO)2 | 85 nm and rectangular monoclinic | Thermal and electrochemical studies | Present paper |
2. Experimental
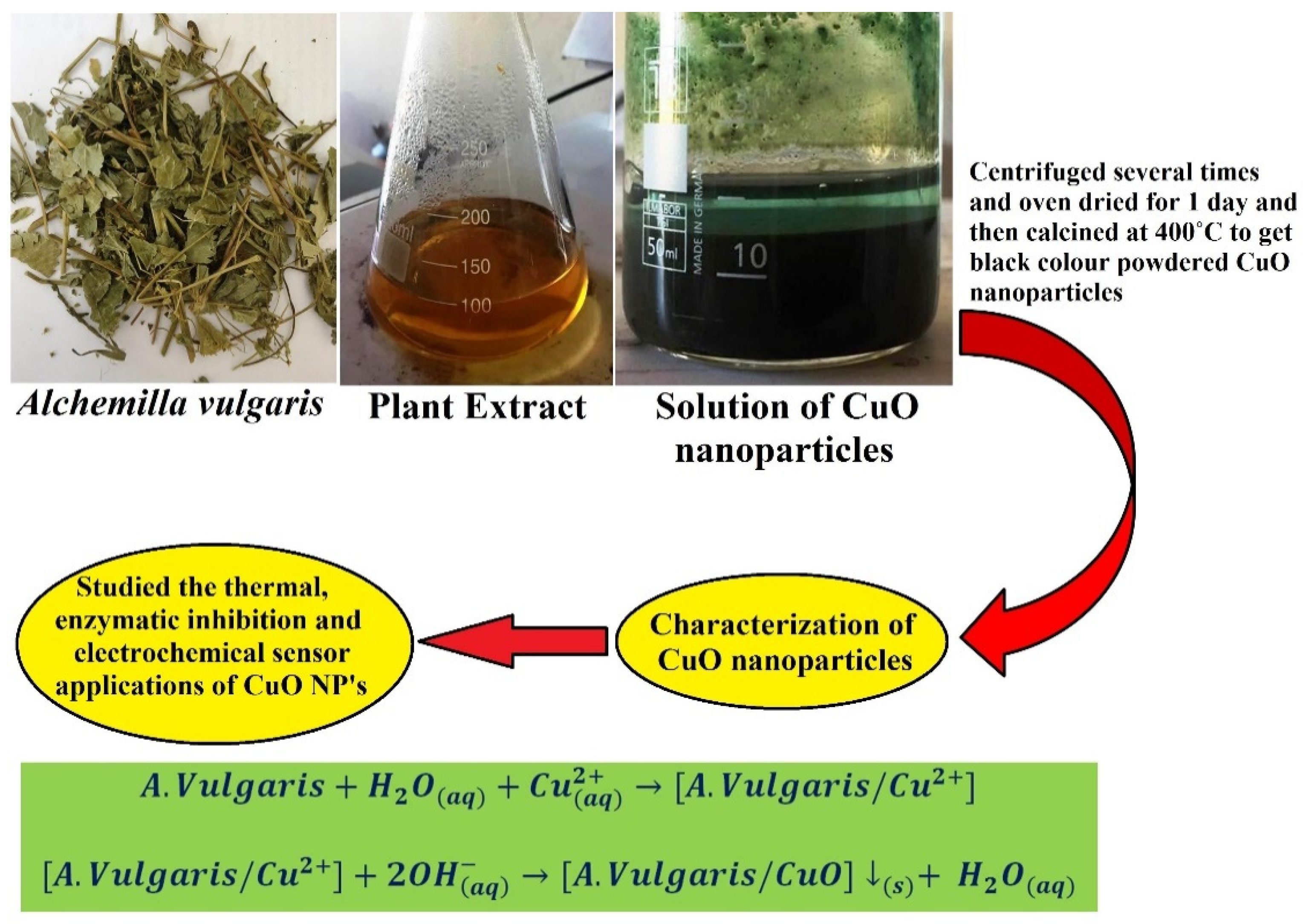
2.1. Preparation of Samples of Alchemilla vulgaris
2.2. Preparation of Rectangular Monoclinic CuO NPs
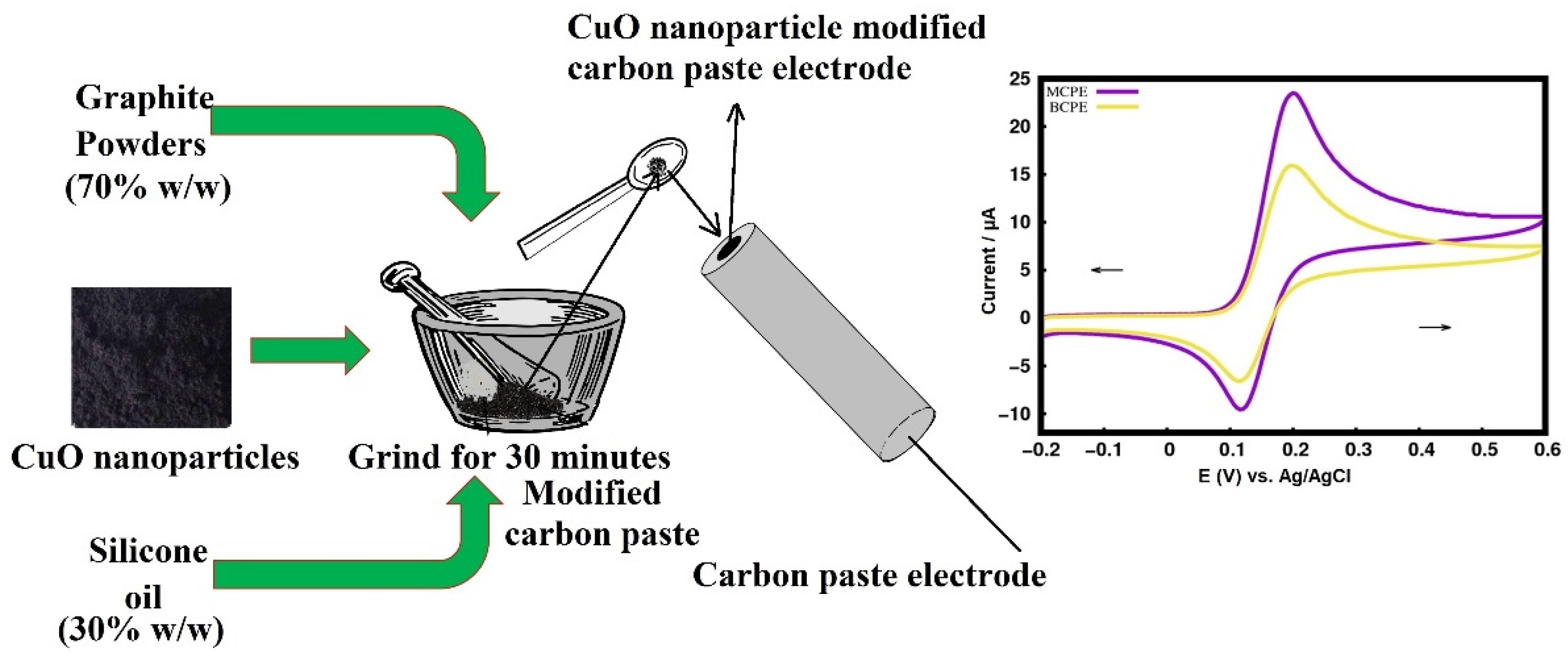
2.3. Fabrication of the Carbon Paste Electrode
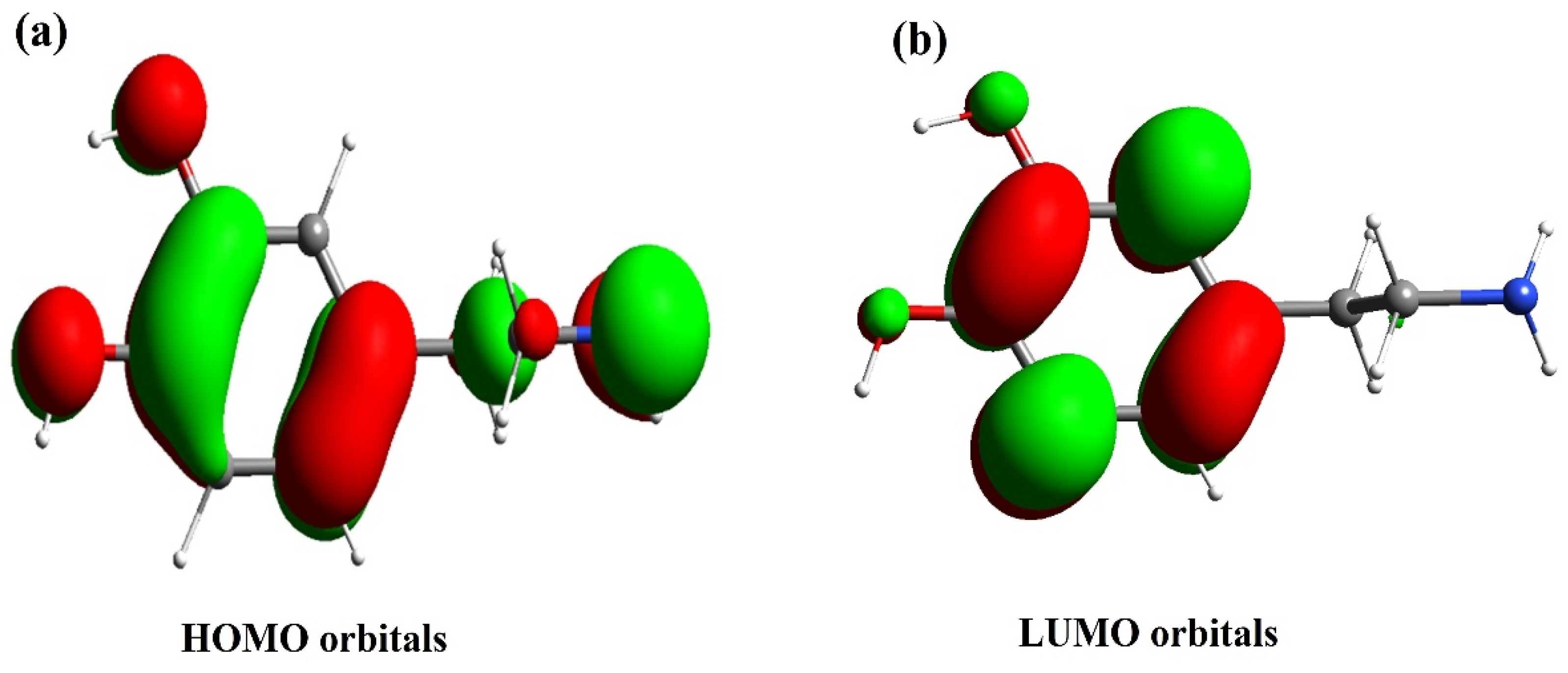
2.4. Computational Methods
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
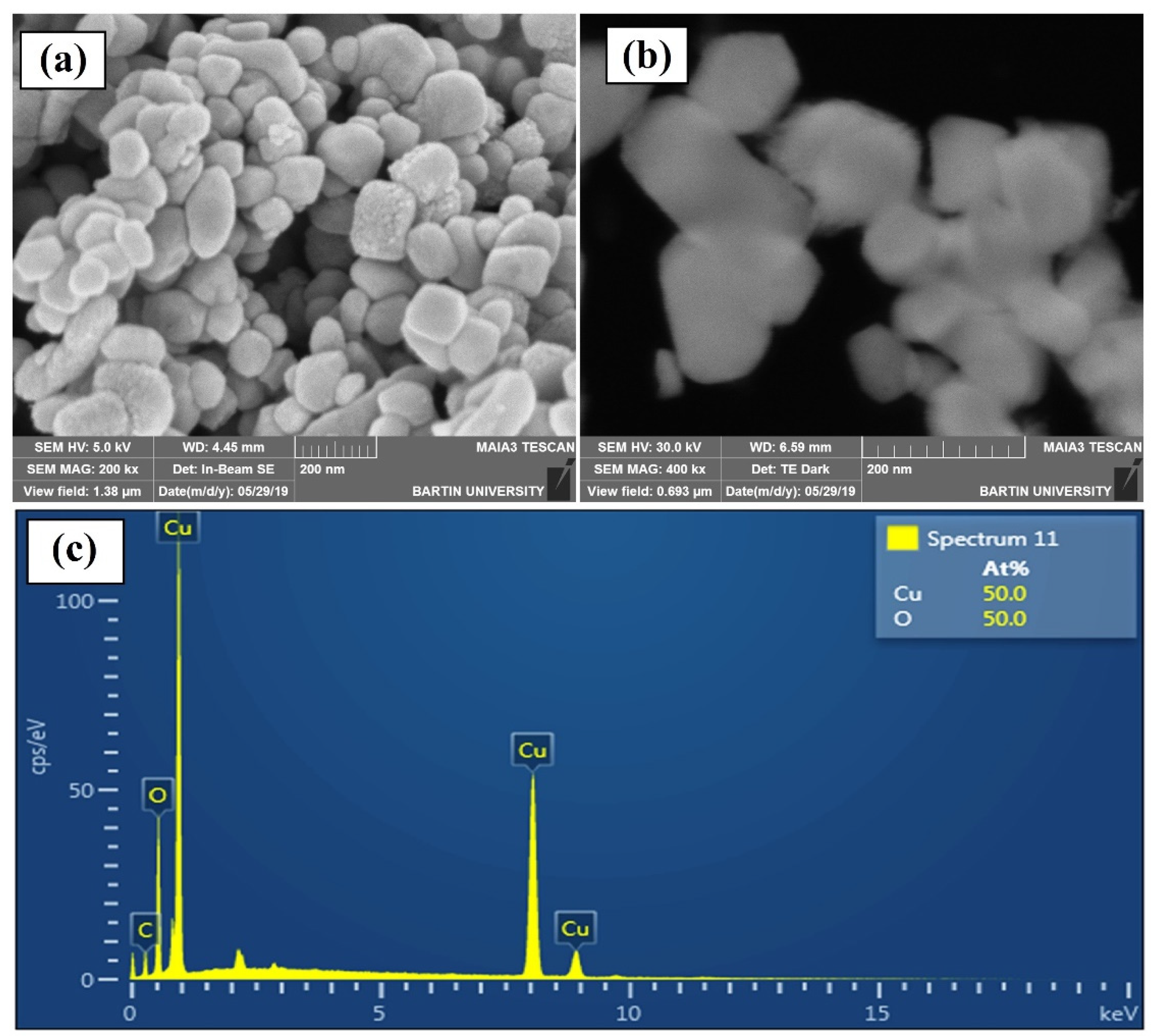
3.2. Scanning Electron Microscopy (SEM)
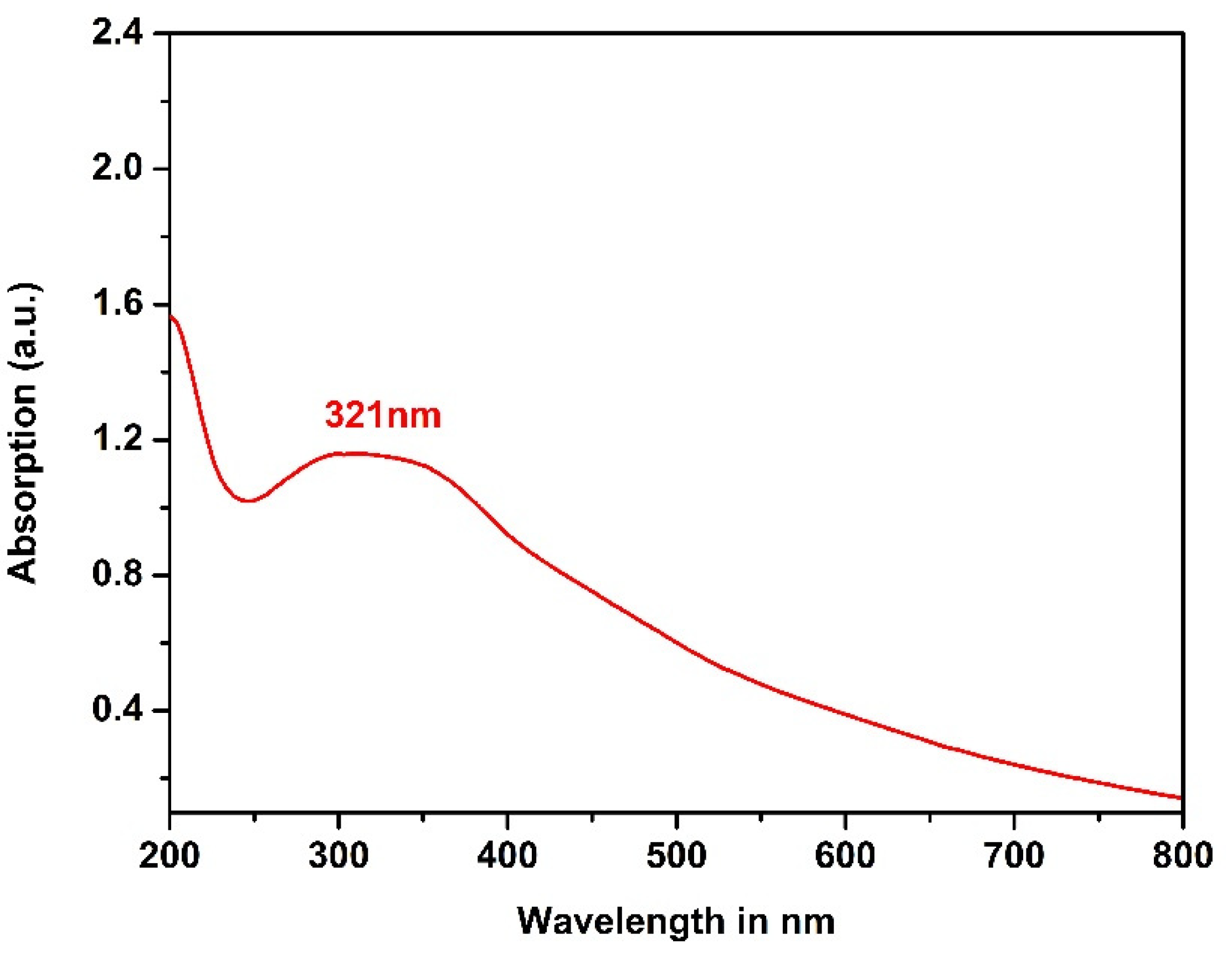
3.3. UV-Visible Spectroscopy
3.4. Thermal Analysis
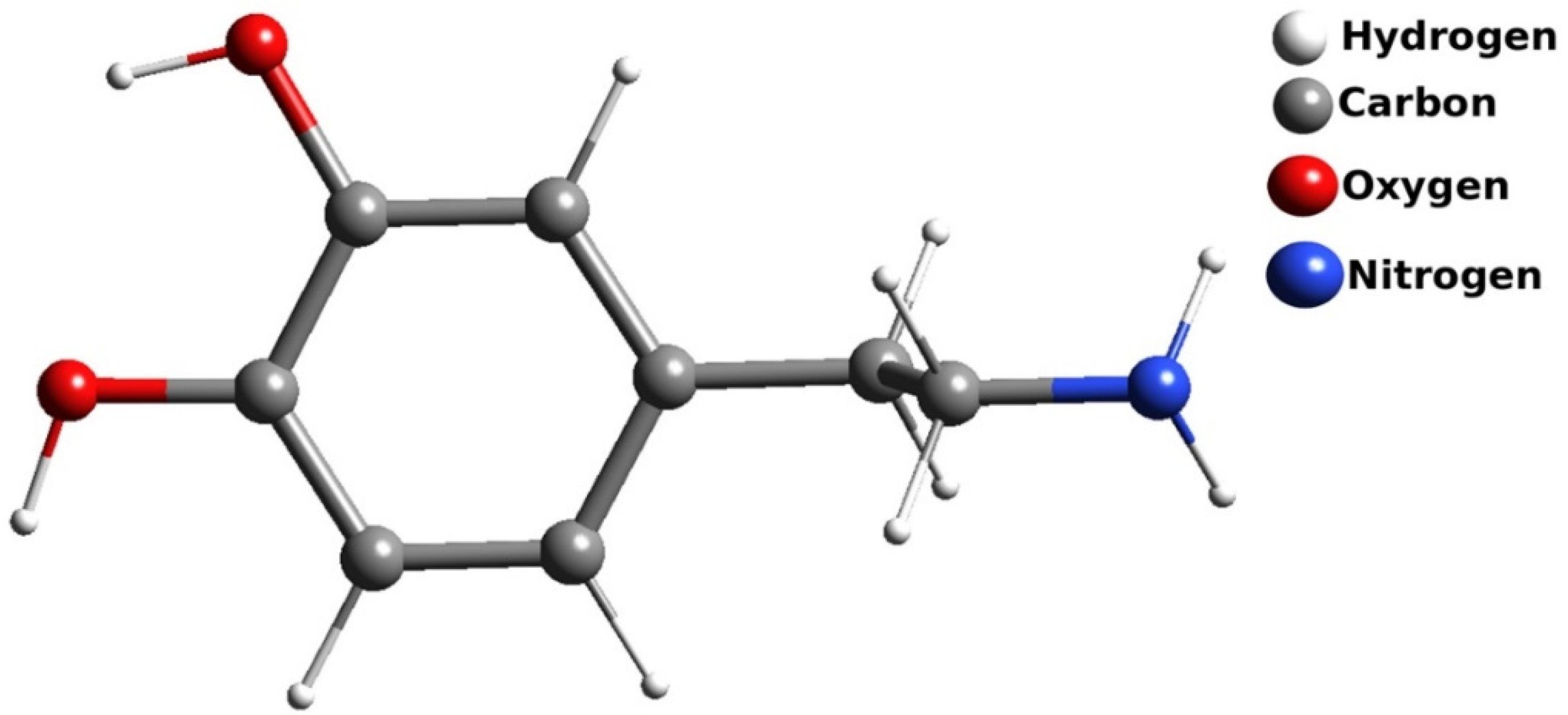
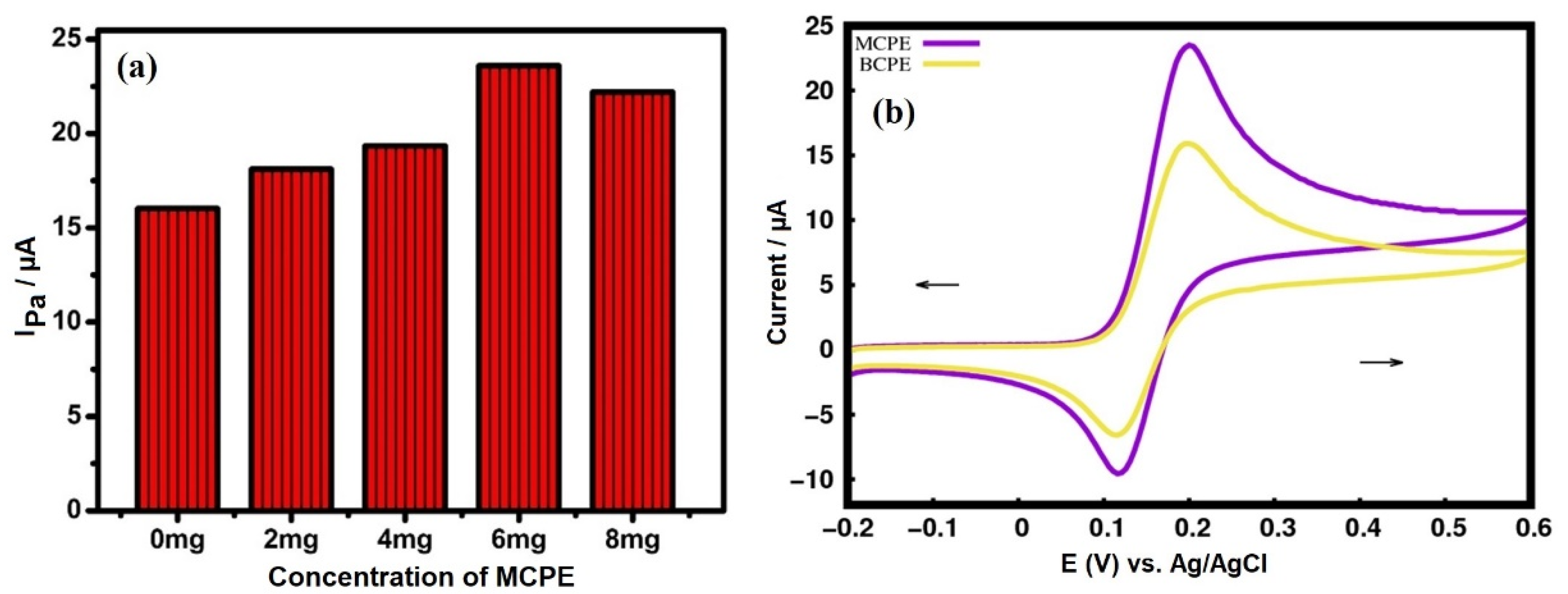
3.5. Electrocatalytic Property of Dopamine (DA) at MCPE
3.5.1. Electrocatalytic Property of CuO for Determining Dopamine (DA)
3.5.2. Effect of Scan Rate
| Type of Electrode | Scan Rate (mV/s) | ∆Ep | k0 (S−1) |
|---|---|---|---|
| CuO MCPE | 100 | 79 | 1.28 |
| CuO MCPE | 150 | 87 | 1.76 |
| CuO MCPE | 250 | 103 | 2.44 |
| CuO MCPE | 350 | 114 | 3.02 |
| CuO MCPE | 400 | 129 | 2.88 |
| BCPE | 50 | 86 | 0.59 |
| Modifier Used | Scan Rate (υ) at Which the Maximum k0 is Recorded (mV/s) | Rate Constant, k0 (s−1) | References |
|---|---|---|---|
| TiO2 Nanorods | 100 | 1.17 | [47] |
| CuO nanoparticles | 100 | 1.023 | [24] |
| Graphene oxide | 100 | 0.99 | [48] |
| Gold nanoparticles | 100 | 4.25 × 10−2 | [49] |
| Tungsten oxide nanoparticles | 100 | 0.604 | [50] |
| CuO nanoparticles | 100 | 1.28 | [Present paper] |
3.5.3. Effect of pH Variation
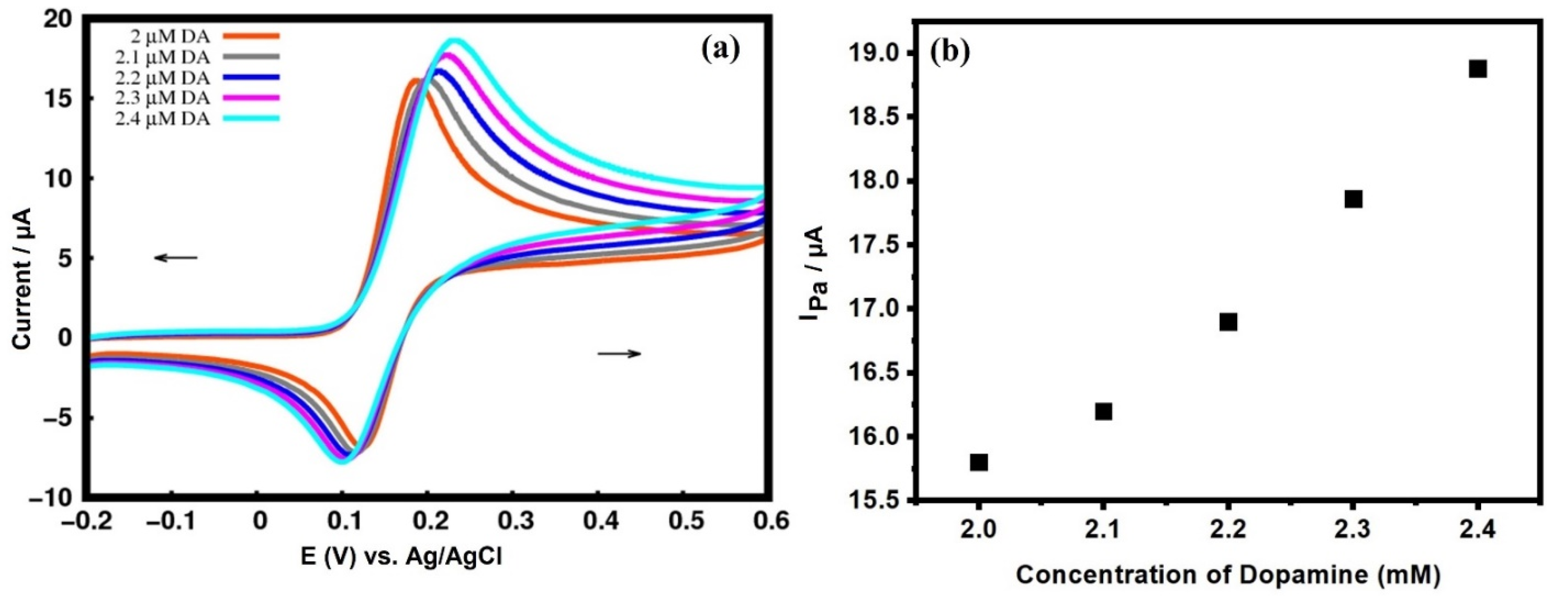
3.5.4. Effect of Variation in DA Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, H.; Qureshi, M.S.; Haque, F.Z. Biosynthesis of Flower-Shaped CuO Nanostructures and Their Photocatalytic and Antibacterial Activities. Nano-Micro Lett. 2020, 12, 29. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.E.K.; Aruna, S.; Kumar, M.; Shashanka, R.; Jayadevappa, H. Preparation of NiO/ZnO hybrid nanoparticles for electrochemical sensing of dopamine and uric acid. Chem. Sens. 2012, 2, 1–7. [Google Scholar]
- Shashanka, R.; Chaira, D.; Swamy, B.E.K. Fabrication of yttria dispersed duplex stainless steel electrode to determine dopamine, ascorbic and uric acid electrochemically by using cyclic voltammetry. Int. J. Sci. Eng. Res. 2016, 7, 1275–1285. [Google Scholar]
- Shashanka, R.; Chaira, D.; Swamy, B.E.K. Electrochemical investigation of duplex stainless steel at carbon paste electrode and its application to the detection of dopamine, ascorbic and uric acid. Int. J. Sci. Eng. Res. 2015, 6, 1863–1871. [Google Scholar]
- Shashanka, R.; Chaira, D.; Swamy, B.E.K. Electrocatalytic Response of Duplex and Yittria Dispersed Duplex Stainless Steel Modified Carbon Paste Electrode in Detecting Folic Acid Using Cyclic Voltammetry. Int. J. Electrochem. Sci. 2015, 10, 5586–5598. [Google Scholar]
- Gupta, S.; Rajendrachari, S.; Chaira, D. Synthesis of nano-structured duplex and ferritic stainless steel powders by planetary milling: An experimental and simulation study. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 012033. [Google Scholar] [CrossRef]
- Nayak, A.K.; Rajendrachari, S.; Chaira, D. Effect of Nanosize Yittria and Tungsten Addition to Duplex Stainless Steel During High Energy Planetary Milling. IOP Conf. Ser. Mater. Sci. Eng. 2016, 115, 012008. [Google Scholar] [CrossRef]
- Rajendrachari, S. Synthesis of nano-structured stainless steel powder by mechanical alloying-an overview. Int. J. Sci. Eng. Res. 2017, 8, 588–594. [Google Scholar]
- Shashanka, R.; Kamaci, Y.; Tas, R.; Ceylan, Y.; Bülbül, A.S.; Uzun, O.; Karaoglanli, A.C. Antimicrobial investigation of CuO and ZnO nanoparticles prepared by a rapid combustion method. Phys. Chem. Res. 2019, 7, 799–812. [Google Scholar]
- Prasad, K.S.; Patra, A.; Shruthi, G.; Chandan, S. Aqueous Extract of Saraca indica Leaves in the Synthesis of Copper Oxide Nanoparticles: Finding a Way towards Going Green. J. Nanotechnol. 2017, 2017, 7502610. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. Green synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Doble, M.; Kruthiventi, A.K. Green Chemistry and Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Rajendrachari, S.; Swamy, B.E.K. Simultaneous electro-generation and electrodeposition of copper oxide nanoparticles on glassy carbon electrode and its sensor application. SN Appl. Sci. 2020, 2, 956. [Google Scholar]
- Tobyn, G.; Denham, A.; Whitelegg, M. Alchemilla vulgaris, lady’s mantle. In Medicinal Herbs; Elsevier: Amsterdam, The Netherlands, 2011; pp. 57–65. [Google Scholar]
- Gültekin, D.D.; Güngör, A.A.; Önem, H.; Babagil, A.; Nadaroğlu, H. Synthesis of Copper Nanoparticles Using a Different Method: Determination of Its Antioxidant and Antimicrobial Activity. J. Turk. Chem. Soc. Sect. A Chem. 2016, 3, 623. [Google Scholar] [CrossRef]
- Shashanka, R.; Yilmaz, V.M.; Karaoglanli, A.C.; Uzun, O. Investigation of activation energy and antibacterial activity of CuO nano-rods prepared by Tilia Tomentosa (Ihlamur) leaves. Moroc. J. Chem. 2020, 8, 497–509. [Google Scholar]
- Sharma, J.K.; Akhtar, M.S.; Ameen, S.; Srivastava, P.; Singh, G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compd. 2015, 632, 321–325. [Google Scholar] [CrossRef]
- Choudhury, P.; Mondal, P.; Majumdar, S.; Saha, S.; Sahoo, G.C. Preparation of ceramic ultrafiltration membrane using green synthesized CuO nanoparticles for chromium (VI) removal and optimization by response surface methodology. J. Clean. Prod. 2018, 203, 511–520. [Google Scholar] [CrossRef]
- Rezaie, A.B.; Montazer, M.; Rad, M.M. Photo and biocatalytic activities along with UV protection properties on polyester fabric through green in-situ synthesis of cauliflower like CuO nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 176, 100–111. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Maham, M.; Sajadi, S.M. Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N-monosubstituted ureas and reduction of 4-nitrophenol. J. Colloid Interface Sci. 2015, 455, 245–253. [Google Scholar] [CrossRef]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2015, 9, 7–12. [Google Scholar] [CrossRef]
- Bhatti, N.K.; Subhani, M.S.; Khan, A.Y.; Qureshi, R.; Rahman, A. Heterogeneous Electron Transfer Rate Constants of Viologen Monocations at a Platinum Disk Electrode. Turk. J. Chem. 2006, 30, 165–180. [Google Scholar]
- Reddy, S.; Swamy, B.E.K.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A.; Shoja, Y.; Najafi, M. Determination of Dopamine in the Presence of Uric Acid and Folic Acid by Carbon Paste Electrode Modified with CuO Nanoparticles/Hemoglobin and Multi-Walled Carbon Nanotube. J. Electrochem. Soc. 2015, 162, B69–B74. [Google Scholar] [CrossRef]
- Flores-Moreno, R.; Urbina, K.P.; Sandoval, Z.G. Sinapsis, Version XII-V; Sinapsis Developers: Guadalajara, Mexico, 2012. [Google Scholar]
- Koster, A.M.; Geudtner, G.; Calaminici, P.; Casida, M.E.; Dominguez, V.D.; Flores-Moreno, R.; Gamboa, G.U.; Goursot, A.; Heine, T.; Ipatov, A.; et al. deMon Developers, deMon2k; Version 3; Cinvestav: Mexico City, Mexico, 2011. [Google Scholar]
- Gerald, G.; Patrizia, C.; Javier, C.; del Campo, J.M.D.D.V.; Flores, M.R.; Ulises, G.G.; Annick, G.; Koster, A.M.; Ulises, R.J.; Tzonka, M.; et al. demon2k. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 548–555. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Salahub, D.R.; Noskov, S.Y.; Lev, B.; Zhang, R.; Ngo, V.; Goursot, A.; Calaminici, P.; Köster, A.M.; Alvarez-Ibarra, A.; Mejía-Rodríguez, D.; et al. QM/MM Calculations with deMon2k. Molecules 2015, 20, 4780–4812. [Google Scholar] [CrossRef] [PubMed]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Swamy, B.E.K.; Reddy, S.; Chaira, D. Synthesis of Silver Nanoparticles and their Applications. Anal. Bioanal. Electrochem. 2013, 5, 455–466. [Google Scholar]
- Rajendrachari, S. Investigation of Electrochemical Pitting Corrosion by Linear Sweep Voltammetry: A Fast and Robust Approach. In Voltammetry; IntechOpen: London, UK, 2019; pp. 77–90. [Google Scholar]
- Shashanka, R.; Esgin, H.; Yilmaz, V.M.; Caglar, Y. Fabrication and characterization of green synthesized ZnO nanoparticle based dye-sensitized solar cells. J. Sci. Adv. Mater. Devices 2020, 5, 185–191. [Google Scholar] [CrossRef]
- Aparna, Y.; Rao, K.V.E.; Subbarao, P.S. Synthesis and Characterization of CuO Nano Particles by Novel Sol-Gel Method. J. Nano-Electron. Phys. 2012, 4, 03005. [Google Scholar]
- Shashanka, R. Effect of Sintering Temperature on the Pitting Corrosion of Ball Milled Duplex Stainless Steel by using Linear Sweep Voltammetry. Anal. Bioanal. Electrochem. 2018, 10, 349–361. [Google Scholar]
- Rajendrachari, S.; Karaoglanli, A.C.; Ceylan, Y.; Uzun, O. A fast and robust approach for the green synthesis of spherical Magnetite (Fe3O4) nanoparticles by Tilia Tomentosa (Ihlamur) leaves and its antibacterial studies. Pharm. Sci. 2020, 26, 175–183. [Google Scholar] [CrossRef]
- Shashanka, R.; Ceylan, K.B. The activation energy and antibacterial investigation of spherical Fe3O4 nanoparticles prepared by Crocus sativus (Saffron) flowers. Biointerface Res. Appl. Chem. 2020, 10, 5951–5959. [Google Scholar]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Dodoo-Arhin, D.; Leoni, M.; Scardi, P. Microemulsion Synthesis of Copper Oxide Nanorod-Like Structures. Mol. Cryst. Liq. Cryst. 2012, 555, 17–31. [Google Scholar] [CrossRef]
- Manjari, G.; Saran, S.; Arun, T.; Rao, A.V.B.; Devipriya, S.P. Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. J. Saudi Chem. Soc. 2017, 21, 610–618. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Umer, M.; Riaz, S.; Naseem, S. Characterization of Copper Oxide Nanoparticles Fabricated by the Sol–Gel Method. J. Electron. Mater. 2015, 44, 3704–3709. [Google Scholar] [CrossRef]
- Gururaj, K.J.; Swamy, B.E.K.; Casillas, N.; Flores-Moreno, R. Analytical Fukui and cyclic voltammetric studies on ferrocene modified carbon electrodes and effect of Triton X-100 by immobilization method. Electrochim. Acta 2017, 258, 1025–1034. [Google Scholar]
- Tender, L.; Carter, M.T.; Murray, R.W. Cyclic Voltammetric Analysis of Ferrocene Alkanethiol Monolayer Electrode Kinetics Based on Marcus Theory. Anal. Chem. 1994, 66, 3173–3181. [Google Scholar] [CrossRef]
- Ingram, R.S.; Murray, R.W. Electron Transfer Kinetics of Ferrocene Alkanethiolate Monolayers in Ether and Poly-ether Solvents. Faraday Trans. Roy. Soc. Chem. 1996, 92, 3946. [Google Scholar]
- Silvia, C.-A.; Georgina, A.-A.; Maria Teresa, R.-S.; Giselle, R.-P.; Mario, R.-R.; Manuel, P.-P. On the electrochemistry of dopamine in aqueous solution. Part I: The role of [SDS] on the voltammetric behavior of dopamine on a carbon paste electrode. J. Electroanal. Chem. 2007, 609, 17–26. [Google Scholar]
- Ashoka, N.B.; Swamy, B.E.K.; Jayadevappa, H. Nanorod TiO2 Sensor for Dopamine: A Voltammetric Study. New J. Chem. 2017, 41, 11817–11827. [Google Scholar] [CrossRef]
- Prinith, N.S.; Manjunatha, J.G.; Hareesha, N. Electrochemical validation of L-tyrosine with dopamine using composite surfactant modifed carbon nanotube electrode. J. Iran. Chem. Soc. 2021, 18, 3493–3503. [Google Scholar] [CrossRef]
- Khudaish, E.A.; Rather, J.A. Electrochemical studies of dopamine under stagnant and convective conditions at a sensor based on gold nanoparticles hosted in poly(triaminopyrimidine). J. Electroanal. Chem. 2016, 776, 206–212. [Google Scholar] [CrossRef]
- Ashoka, N.B.; Swamy, B.E.K.; Jayadevappa, H. Tungsten oxide (WO3) Modified Carbon Paste Electrode for Electrochemical Investigation of Dopamine in Presence of Uric Acid and Folic Acid. Anal. Bioanal. Electrochem. 2017, 9, 1057–1069. [Google Scholar]
- Gururaj, K.J.; Swamy, B.E.K.; Shashanka, R.; Sharma, S.C.; Flores-Moreno, R. Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications. J. Mol. Liq. 2021, 334, 116348. [Google Scholar]
- Shashanka, R.; Gururaj, K.J.; Prakashaiah, B.G.; Kumar, M.; Swamy, B.E.K. Electrocatalytic determination of ascorbic acid using a green synthesised magnetite nano-flake modified carbon paste electrode by cyclic voltammetric method. Mater. Res. Innov. 2021, 26, 229–239. [Google Scholar] [CrossRef]
- Gururaj, K.J.; Swamy, B.E.K.; Chandrashekar, B.N.; Roberto, F.-M. Theoretical and cyclic voltammetric studies on electrocatalysis of benzethonium chloride at carbon paste electrode for detection of dopamine in presence of ascorbic acid. J. Mol. Liq. 2017, 240, 395–401. [Google Scholar]

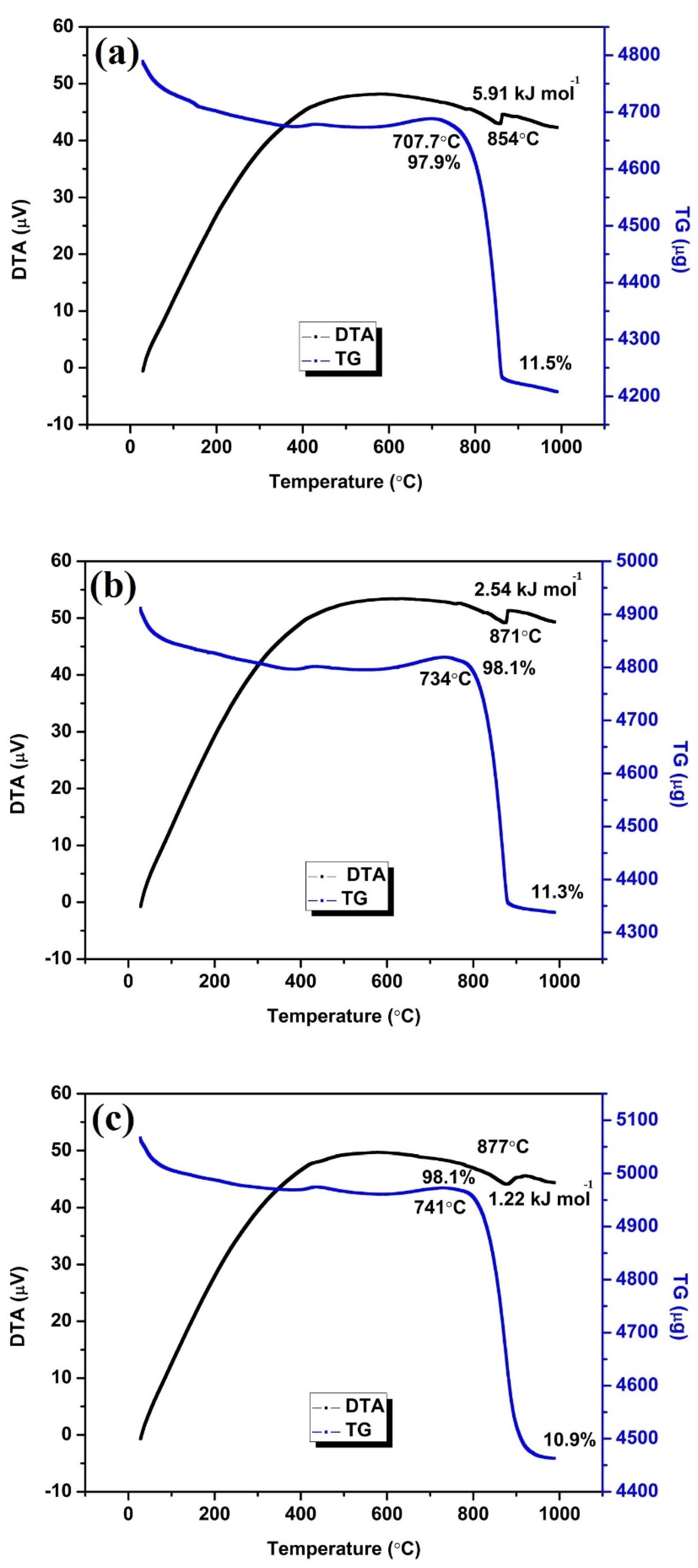


| Type of Nanomaterial | Heating Rate (°C/min) | Decomposition Temperature (°C) | Percentage Weight Loss | * Enthalpy Change (kJ/mol) |
|---|---|---|---|---|
| CuO nanoparticles | 6 | 854 | 11.5 | 5.91 |
| 8 | 871 | 11.3 | 2.54 | |
| 10 | 877 | 10.9 | 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendrachari, S.; Kudur Jayaprakash, G.; Pandith, A.; Karaoglanli, A.C.; Uzun, O. Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles. Catalysts 2022, 12, 994. https://doi.org/10.3390/catal12090994
Rajendrachari S, Kudur Jayaprakash G, Pandith A, Karaoglanli AC, Uzun O. Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles. Catalysts. 2022; 12(9):994. https://doi.org/10.3390/catal12090994
Chicago/Turabian StyleRajendrachari, Shashanka, Gururaj Kudur Jayaprakash, Anup Pandith, Abdullah Cahit Karaoglanli, and Orhan Uzun. 2022. "Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles" Catalysts 12, no. 9: 994. https://doi.org/10.3390/catal12090994
APA StyleRajendrachari, S., Kudur Jayaprakash, G., Pandith, A., Karaoglanli, A. C., & Uzun, O. (2022). Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles. Catalysts, 12(9), 994. https://doi.org/10.3390/catal12090994









