Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework
Abstract
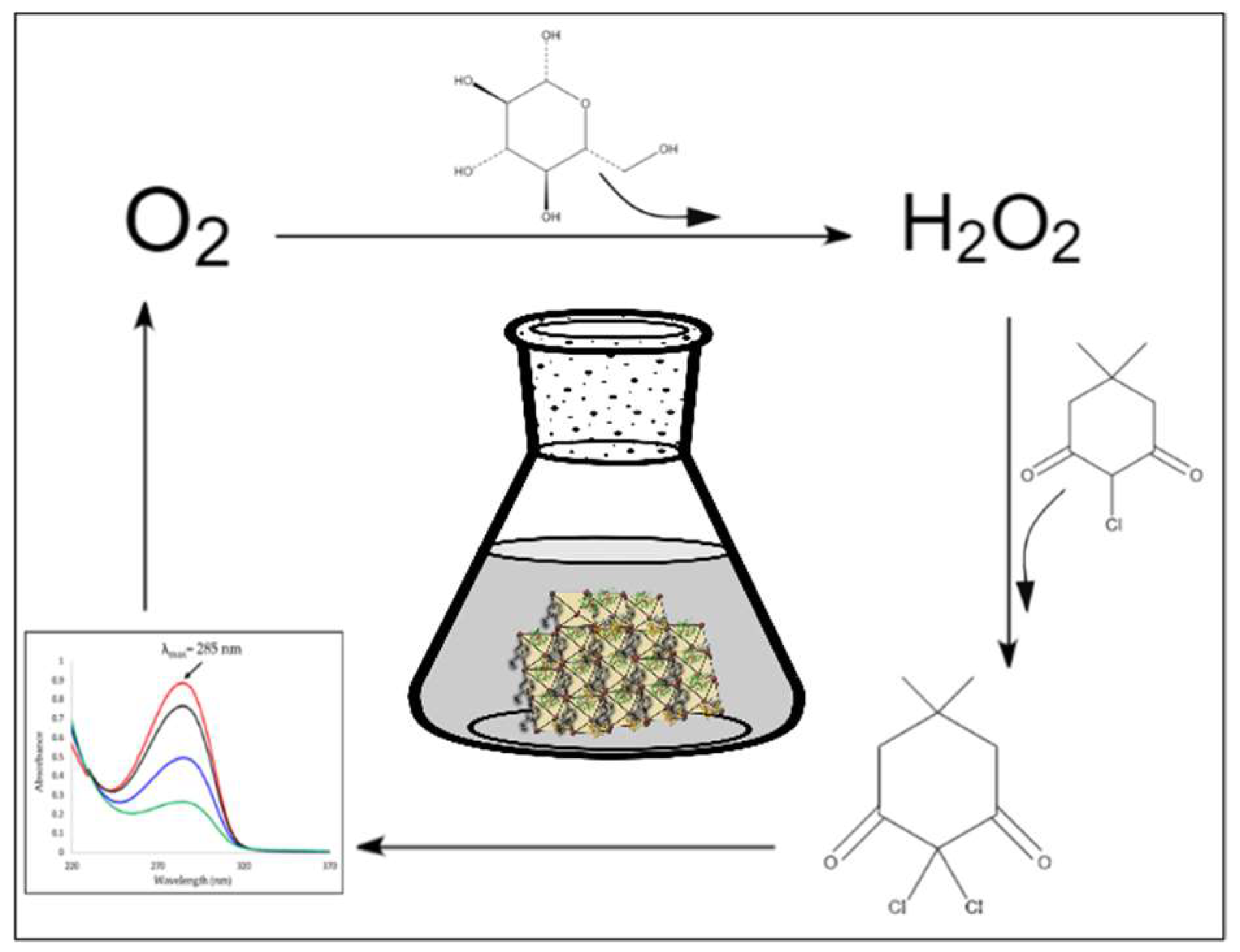
1. Introduction
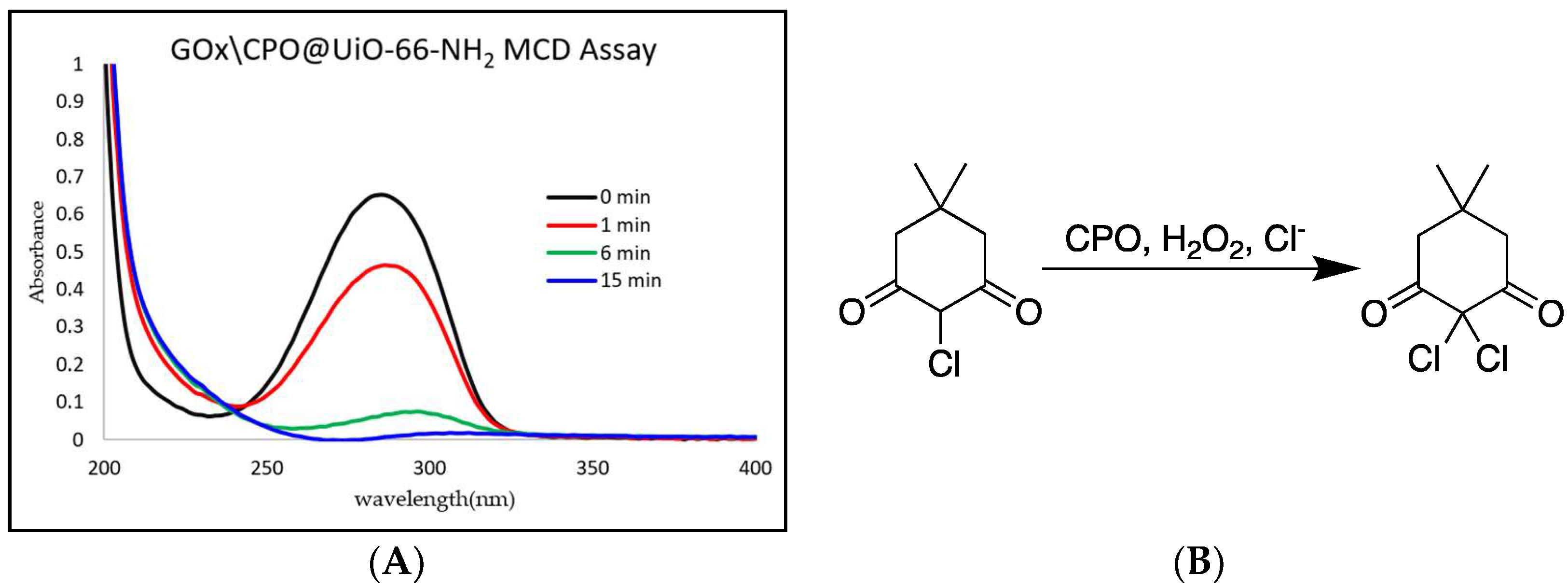
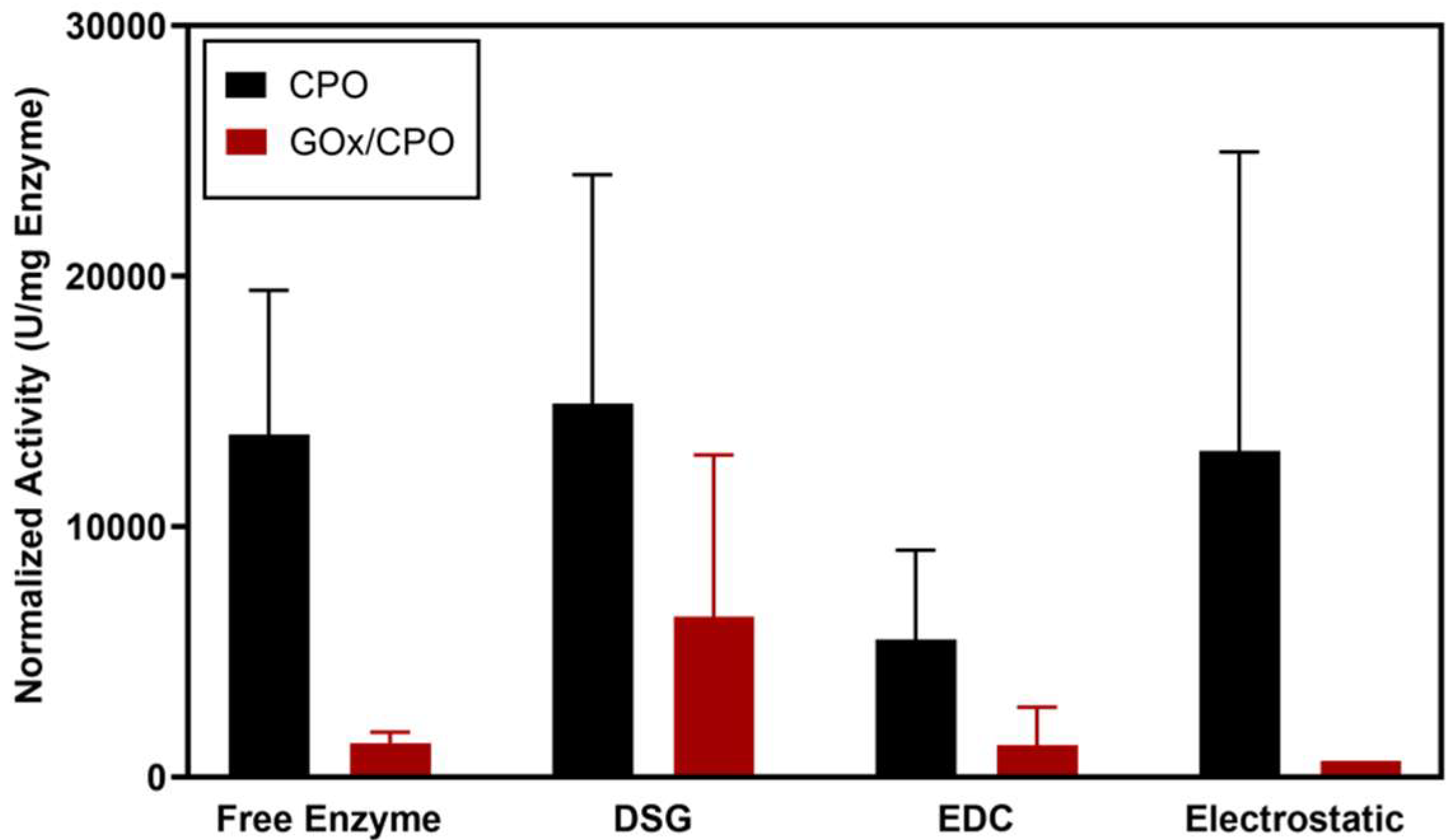
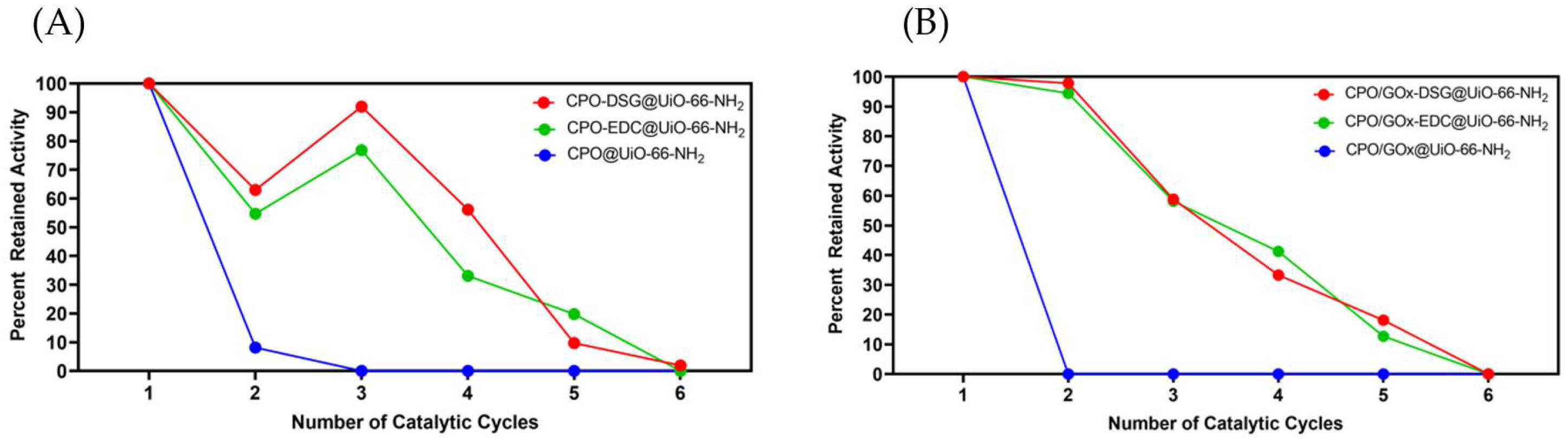
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Production of CPO
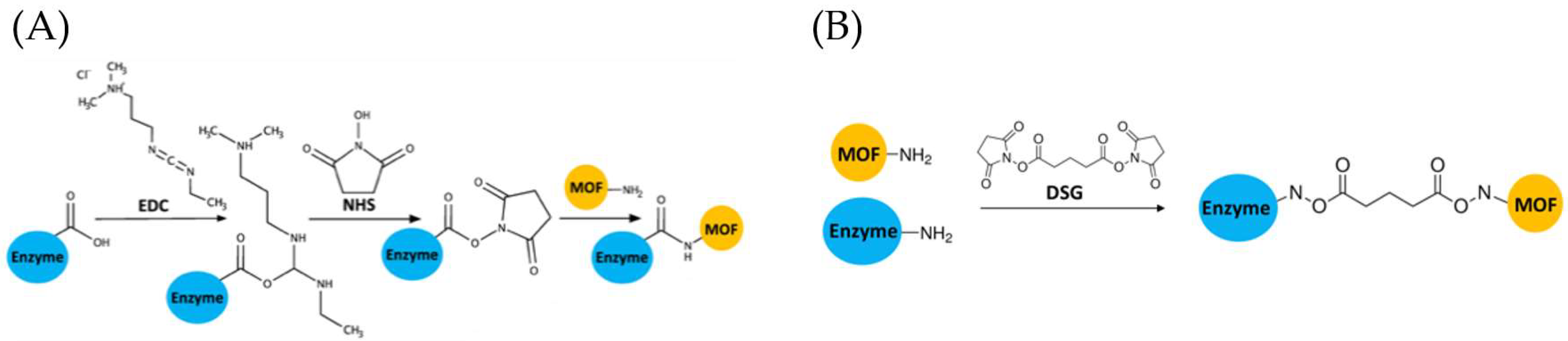
4.3. Crosslinking
4.3.1. EDC/NHS
4.3.2. DSG
4.4. Characterization of MOFs and Enzyme/MOF Composites
4.5. Enzyme Activity
4.6. Recyclability
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Rehm, F.B.H.; Chen, S.; Rehm, B.H.A. Enzyme Engineering for In Situ Immobilization. Molecules 2016, 21, 1370. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q.; Saleemuddin, M. Immobilization of glycoenzymes using crude concanavalin A and glutaraldehyde. Enzym. Microb. Technol. 1986, 8, 686–690. [Google Scholar] [CrossRef]
- Jung, D.; Hartmann, M. Oxidation of Indole with CPO and Gox Immobilized on SBA-15. Stud Surf Sci Catal. 2008, 174, 1045–1050. [Google Scholar]
- Jung, D.; Streb, C.; Hartmann, M. Oxidation of indole using chloroperoxidase and glucose oxidase immobilized on SBA-15 as tandem biocatalyst. Microporous Mesoporous Mater. 2008, 113, 523–529. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Cowana, D.A. Enhancing the Functional Properties of Thermophilic Enzymes by Chemical Modification and Immobilization. Enzyme Microb. Technol. 2011, 49, 326–346. [Google Scholar]
- Pisklak, T.J.; Macías, M.; Coutinho, D.H.; Huang, R.S.; Balkus, K.J. Hybrid materials for immobilization of MP-11 catalyst. Top. Catal. 2006, 38, 269–278. [Google Scholar] [CrossRef]
- Zare, A.; Bordbar, A.-K.; Razmjoub, A.; Jafarian, F. The Immobilization of Candida Rugosa Lipase on the Modified Polyethersulfone with MOF Nanoparticles as an Excellent Performance Bioreactor Membrane. J. Biotechnol. 2019, 289, 55–63. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Wang, T.; Xiao, Y.; Huo, Q.; Liu, Y. Immobilization of Bacillus subtilis lipase on a Cu-BTC based hierarchically porous metal–organic framework material: A biocatalyst for esterification. Dalton Trans. 2016, 45, 6998–7003. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme-MOF (Metal-Organic Framework) Composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Novick, S.J.; Rozzell, J.D. Immobilization of Enzymes by Covalent Attachment. Microb. Enzyme and Biotransfor. 2005, 17, 247–271. [Google Scholar]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme Immobilization by Adsorption: A Review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme encapsulation in metal–organic frameworks for applications in catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.-F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.-P.; Wang, X.; Wang, K.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Yang, K.-L. Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions. Enzym. Microb. Technol. 2017, 100, 52–59. [Google Scholar] [CrossRef]
- Meldal, M.; Schoffelen, S. Recent Advances in Covalent, Site-Specific Protein Immobilization. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Co-ord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Huo, J.; Aguilera-Sigalat, J.; El-Hankari, S.; Bradshaw, D. Magnetic MOF Microreactors for Recyclable Size-Selective Biocatalysis. Chem. Sci. 2015, 6, 1938–1943. [Google Scholar] [CrossRef]
- Gascón, V.; Castro-Miguel, E.; Díaz-García, M.; Blanco, R.M.; Sanchez-Sanchez, M. In situ and post-synthesis immobilization of enzymes on nanocrystalline MOF platforms to yield active biocatalysts. J. Chem. Technol. Biotechnol. 2017, 92, 2583–2593. [Google Scholar] [CrossRef]
- Cao, S.-L.; Yue, D.-M.; Li, X.; Smith, T.; Li, N.; Zong, M.-H.; Wu, H.; Ma, Y.Z.; Lou, W.-Y. Novel Nano-/Micro-Biocatalyst: Soybean Epoxide Hydrolase Immobilized on UiO-66-NH2 MOF for Efficient Biosynthesis of Enantiopure (R)-1, 2-Octanediol in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2016, 4, 3586–3595. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour. Bioprocess. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Chen, Y.-P.; Liu, T.-F.; Zhou, H.-C. Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 2016, 7, 6969–6973. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Marshall, R.J.; Richards, T.; Hobday, C.L.; Murphie, C.F.; Wilson, C.; Moggach, S.A.; Bennett, T.D.; Forgan, R.S. Postsynthetic bromination of UiO-66 analogues: Altering linker flexibility and mechanical compliance. Dalton Trans. 2015, 45, 4132–4135. [Google Scholar] [CrossRef]
- Ahmad, R.; Shanahan, J.; Rizaldo, S.; Kissel, D.S.; Stone, K.L. Co-immobilization of an Enzyme System on a Metal-Organic Framework to Produce a More Effective Biocatalyst. Catalysts 2020, 10, 499. [Google Scholar] [CrossRef]
- Betancor, L.; Luckarift, H.R. Co-immobilized coupled enzyme systems in biotechnology. Biotechnol. Genet. Eng. Rev. 2010, 27, 95–114. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Van Aken, B.; Ledent, P.; Naveau, H.; Agathos, S.N. Co-immobilization of manganese peroxidase from Phlebia radiata and glucose oxidase from Aspergillus niger on porous silica beads. Biotechnol. Lett. 2000, 22, 641–646. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Su, Y.; Ouyang, P.; Ge, J.; Liu, Z. Spatial co-localization of multi-enzymes by inorganic nanocrystal–protein complexes. Chem. Commun. 2014, 50, 12465–12468. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic metal–organic frameworks as scaffolds for spatial co-location and positional assembly of multi-enzyme systems enabling enhanced cascade biocatalysis. RSC Adv. 2017, 7, 21205–21213. [Google Scholar] [CrossRef]
- Zore, O.V.; Pattammattel, A.; Gnanaguru, S.; Kumar, C.V.; Kasi, R.M. Bienzyme-Polymer-Graphene Oxide Quaternary Hybrid Biocatalysts: Efficient Substrate Channeling under Chemically and Thermally Denaturing Conditions. ACS Catal. 2015, 5, 4979–4988. [Google Scholar] [CrossRef]
- Kumar, A.; Malhorta, R.; Malhorta, B.D.; Grover, S.K. Co-Immobilization of Cholesterol Oxidase and Horseradish Peroxidase in a Sol–Gel Film. Anal. Chimi. Acta 2000, 414, 43–50. [Google Scholar] [CrossRef]
- Gustafsso, H.; Kuchler, A.; Holmberg, K.; Walde, P. Co-Immobilization of Enzymes with the Help of a Dendronized Polymer and Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2015, 3, 6174–6184. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, R.; Zhai, J.; Tian, C. Bienzymatic Glucose Biosensor Based on Co-Immobilization of Peroxidase and Glucose Oxidase on a Carbon Nanotubes Electrode. Biosens. Bioelectron. 2007, 23, 528–535. [Google Scholar] [CrossRef]
- Memon, A.H.; Ding, R.; Yuan, Q.; Liang, H.; Wei, Y. Coordination of GMP Ligand with Cu to Enhance the Multiple Enzymes Stability and Substrate Specificity by Co-Immobilization Process. Biochem. Eng. J. 2018, 136, 102–108. [Google Scholar] [CrossRef]
- Manoj, K.M.; Hager, L.P. Chloroperoxidase, a Janus Enzyme. Biochemistry 2008, 47, 2997–3003. [Google Scholar] [CrossRef]
- Morris, D.R.; Hager, L.P. Chloroperoxidase. I. Isolation and properties of the crystalline glycoprotein. J. Biol. Chem. 1966, 241, 1763–1768. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Turner, J.; Poulos, T.L. The Crystal Structure of Chloroperoxidase: A Heme Peroxidase-Cytochrome P450 Functional Hybrid. Structure 1995, 3, 1367–1378. [Google Scholar] [CrossRef]
- García-Zamora, J.L.; León-Aguirre, K.; Quiroz-Morales, R.; Parra-Saldívar, R.; Gómez-Patiño, M.B.; Arrieta-Baez, D.; Rebollar-Pérez, G.; Torres, E. Chloroperoxidase-Mediated Halogenation of Selected Pharmaceutical Micropollutants. Catalysts 2018, 8, 32. [Google Scholar] [CrossRef]
- Miller, V.; Tschirretguth, R.; De Montellano, P.R.O. Chloroperoxidase-Catalyzed Benzylic Hydroxylation. Arch. Biochem. Biophys. 1995, 319, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Corbett, M.D.; Chipdo, B.R.; Baden, D.G. Chloroperoxidase-Catalysed Oxidation of 4-Chloroaniline to 4-Chloronitrosobenzene. Biochem. J. 1978, 175, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Niedan, V.; Pavasars, I.; Oberg, G. Chloroperoxidase-Mediated Chlorination of Aromatic Groups in Fulvic Acid. Chemosphere 2000, 41, 779–785. [Google Scholar] [CrossRef]
- Van de Velde, F.; Bakker, M.; van Rantwijk, F.; Sheldon, R.A. Chloroperoxidase-Catalyzed Enantioselective Oxidations in Hydrophobic Organic Media. Biotech. Bioengin. 2001, 72, 523–529. [Google Scholar] [CrossRef]
- Pickard, M.A.; Kadima, T.A.; Carmichael, R.D. Chloroperoxidase, a Peroxidase with Potential. J. Ind. Microbiol. 1991, 7, 235–241. [Google Scholar] [CrossRef]
- Santhanam, L.; Dordick, J.S. Chloroperoxidase-catalyzed Epoxidation of Styrene in Aqueous and Nonaqueous Media. Biocatal. Biotransformation 2002, 20, 265–274. [Google Scholar] [CrossRef]
- Yamada, Y.; Itoh, N.; Izum, Y. Chloroperoxidase-Catalyzed Halogenation.of Trans-Cinnamic Acid and Its Derivative. J. Biol. Chem. 1985, 260, 11962–11969. [Google Scholar] [CrossRef]
- Torres, E.; Aburto, J. Chloroperoxidase-Catalyzed Oxidation of 4,6-Dimethyldibenzothiophene as Dimer Complexes: Evidence for Kinetic Cooperativity. Arch. Biochem. Biophys. 2005, 437, 224–232. [Google Scholar] [CrossRef]
- Thomas, A.J.; Morris, D.R.; Hager, L.P. Chloroperoxidase. VII. Classical peroxidatic, catalatic, and halogenating forms of the enzyme. J. Biol. Chem. 1970, 245, 3129–3134. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Tan, Y.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Rapid Decolorization of Anthraquinone and Triphenylmethane Dye Using Chloroperoxidase: Catalytic Mechanism, Analysis of Products and Degradation Route. Chem. Eng. J. 2014, 244, 9–18. [Google Scholar] [CrossRef]
- Hu, S.; Hager, L.P. Unusual Propargylic Oxidations Catalyzed by Chloroperoxidase. Biochem. Biophys. Res. Comm. 1998, 253, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Hager, L.P.; Morris, D.R.; Brown, F.S.; Eberwein, H. Chloroperoxidase II. Utilization of Halogen Anions. J. Biol. Chem. 1966, 241, 1769. [Google Scholar] [CrossRef]
- Libby, R.D.; Thomas, J.A.; Kaiser, L.W.; Hager, L.P. Chloroperoxidase Halogenation Reactions: Chemical versus Enzymatic Halogenating Intermediates. J. Biol. Chem. 1982, 257, 5030–5037. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, P. Novel Interface-Binding Chloroperoxidase for Interfacial Epoxidation of Styrene. J. Biotechnol. 2005, 117, 195–202. [Google Scholar] [CrossRef]
- Salcedo, K.; Torres-Ramírez, E.; Haces, I.; Ayala, M. Halogenation of β-Estradiol by a Rationally Designed Mesoporous Biocatalyst Based on Chloroperoxidase. Biocatalysis 2015, 1, 33–43. [Google Scholar] [CrossRef]
- Pereira, P.C.; Arends, I.W.; Sheldon, R.A. Optimizing the chloroperoxidase–glucose oxidase system: The effect of glucose oxidase on activity and enantioselectivity. Process Biochem. 2015, 50, 746–751. [Google Scholar] [CrossRef]
- Gao, F.; Wang, L.; Liu, Y.; Wang, S.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Enzymatic Synthesis of (R)-Modafinil by Chloroperoxidase-Catalyzed Enantioselective Sulfoxidation of 2-(Diphenylmethylthio) Acetamide. Biochem. Eng. J. 2015, 15, 243–249. [Google Scholar] [CrossRef]
- Ayala, M.; Horjales, E.; Pickard, M.A.; Vazquez-Duhalt, R. Cross-Linked Crystals of Chloroperoxidase. Biochem. Biophys. Res. Commun. 2002, 295, 828–831. [Google Scholar] [CrossRef]
- Kadima, T.A.; Pickard, M.A. Immobilization of Chloroperoxidase on Aminopropyl-Glass. Appl. Environ. Microbiol. 1990, 56, 3473–3477. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Hodnett, A.B.K.; Magner, E. Chloroperoxidase on Periodic Mesoporous Organosilanes: Immobilization and Reuse. Chem. Mater. 2007, 19, 2049–2055. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2019, 90, 66–80. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Bai, C.-H.; Wang, Y.-S.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G. Improvement of chloroperoxidase stability by covalent immobilization on chitosan membranes. Biotechnol. Lett. 2009, 31, 1269–1272. [Google Scholar] [CrossRef]
- de Hoog, H.M.; Nallani, M.; Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M.; Arends, I.W.C.E. Biocatalytic oxidation by chloroperoxidase from Caldariomyces fumago in polymersome nanoreactors. Org. Biomol. Chem. 2009, 7, 4604–4610. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Kiralp, S.; Yilmaz, M.; Toppare, L.; Yakup Arıca, M. Covalent Immobilization of Chloroperoxidase onto Magnetic Beads: Catalytic Properties and Stability. Biochem. Eng. J. 2008, 38, 180–188. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Yuan, Q.; Liang, H. Catalytic Activity and Application of Immobilized Chloroperoxidase by Biometric Magnetic Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 3555–3560. [Google Scholar] [CrossRef]
- Petri, A.; Gambicorti, T.; Salvador, P. Covalent Immobilization of Chloroperoxidase on Silica Gel and Properties of the Immobilized Biocatalyst. J. Mol. Catal. B Enzym. 2004, 27, 103–106. [Google Scholar] [CrossRef]
- Montiel, C.; Terrés, E.; Domínguez, J.-M.; Aburto, J. Immobilization of chloroperoxidase on silica-based materials for 4,6-dimethyl dibenzothiophene oxidation. J. Mol. Catal. B Enzym. 2007, 48, 90–98. [Google Scholar] [CrossRef]
- Trevisan, V.; Signoretto, M.; Colonna, S.; Pironti, V.; Strukul, G. Microencapsulated Chloroperoxidase as a Recyclable Catalyst for the Enantioselective Oxidation of Sulfides with Hydrogen Peroxide. Angew. Chem. Int. Ed. 2004, 43, 4097–4099. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Altintas, B.; Yilmaz, M.; Arica, M.Y. Immobilization of chloroperoxidase onto highly hydrophilic polyethylene chains via bio-conjugation: Catalytic properties and stabilities. Bioresour. Technol. 2011, 102, 475–482. [Google Scholar] [CrossRef]
- Gao, F.; Guo, Y.; Fan, X.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y.; Wang, X. Enhancing the Catalytic Performance of Chloroperoxidase by Coimmobilization with Glucose Oxidase on Magnetic Graphene Oxide. Biochem. Eng. J. 2019, 143, 101–109. [Google Scholar] [CrossRef]
- Jung, D.; Streb, C.; Hartmann, M. Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15. Int. J. Mol. Sci. 2010, 11, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Campos-Teran, J.; Inarritu, I.; Aburto, J.; Torres, E. Enhanced Functionality of Peroxidases by its Immobilization at the Solid-Liquid Interface of Mesoporous Materials and Nanoparticles; John Wiley & Sons, Inc.: New York, NY, USA, 2013; Chapter 16; pp. 335–351. [Google Scholar]
- Pešić, M.; Lopez, C.; Álvaro, G.; López-Santín, J. A Novel Immobilized Chloroperoxidase Biocatalyst with Improved Stability for the Oxidation of Amino Alcohols to Amino Aldehydes. J. Mol. Catal. B Enzym. 2012, 84, 144–151. [Google Scholar] [CrossRef]
- Dong, X.; Li, H.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Rapid and Efficient Degradation of Bisphenol A by Chloroperoxidase from Caldariomyces Fumago: Product Analysis and Ecotoxicity Evaluation of the Degraded Solution. Biotechnol. Lett. 2016, 38, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Guerrero, F.A.; Alderete, J.B.; Vazquez-Duhalt, R.; Aguila, S. Enhancement of Operational Stability of Chloroperoxidase from Caldariomyces Fumago by Immobilization onto Mesoporous Supports and the Use of Co-Solvents. J. Mol. Catal. B Enzym. 2015, 116, 1–8. [Google Scholar] [CrossRef]
- Kurdistani, S.K.; Grunstein, M. In Vivo Protein–Protein and Protein–DNA Crosslinking for Genomewide Binding Microarray. Methods 2003, 31, 90–95. [Google Scholar] [CrossRef]
- Gaucher, S.P.; Hadi, M.Z.; Young, M.M. Influence of crosslinker identity and position on gas-phase dissociation of lys-lys crosslinked peptides. J. Am. Soc. Mass Spectrom. 2006, 17, 395–405. [Google Scholar] [CrossRef][Green Version]
- Corbillé, A.-G.; Neunlist, M.; Derkinderen, P. Cross-linking for the analysis of α-synuclein in the enteric nervous system. J. Neurochem. 2016, 139, 839–847. [Google Scholar] [CrossRef]
- Fischer, M.J.E. Amine coupling through EDC/NHS: A practical approach. In Surface Plasmon Resonance; Methods in Molecular Biology; Mol, N.J., Fischer, M.J.E., Eds.; Humana Press: Totowa, NJ, USA, 2010; Volume 627, pp. 55–73. [Google Scholar]
- Kazenwadel, F.; Wagner, H.; Rapp, B.E.; Franzreb, M. Optimization of enzyme immobilization on magnetic microparticles using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as a crosslinking agent. Anal. Methods 2015, 7, 10291–10298. [Google Scholar] [CrossRef]
- Pickard, M.A. A defined growth medium for the production of chloroperoxidase by Caldariomyces fumago. Can. J. Microbiol. 1981, 27, 1298–1305. [Google Scholar] [CrossRef]

| Enzyme@UiO-66-NH2 | Electrostatic (μg/mg) | DSG (μg/mg) | EDC (μg/mg) |
|---|---|---|---|
| GOx/CPO | 0.413 ± 0.18 | 0.458 ± 0.12 | 0.282 ± 0.17 |
| CPO | 0.223 ± 0.22 | 0.358 ± 0.22 | 0.179 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Rizaldo, S.; Shaner, S.E.; Kissel, D.S.; Stone, K.L. Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework. Catalysts 2022, 12, 969. https://doi.org/10.3390/catal12090969
Ahmad R, Rizaldo S, Shaner SE, Kissel DS, Stone KL. Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework. Catalysts. 2022; 12(9):969. https://doi.org/10.3390/catal12090969
Chicago/Turabian StyleAhmad, Raneem, Sydnie Rizaldo, Sarah E. Shaner, Daniel S. Kissel, and Kari L. Stone. 2022. "Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework" Catalysts 12, no. 9: 969. https://doi.org/10.3390/catal12090969
APA StyleAhmad, R., Rizaldo, S., Shaner, S. E., Kissel, D. S., & Stone, K. L. (2022). Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework. Catalysts, 12(9), 969. https://doi.org/10.3390/catal12090969









