Cu2O/CuS/ZnS Nanocomposite Boosts Blue LED-Light-Driven Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
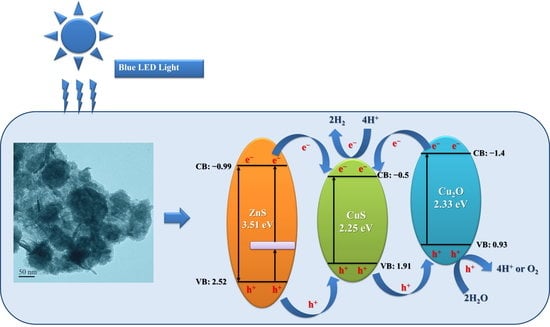
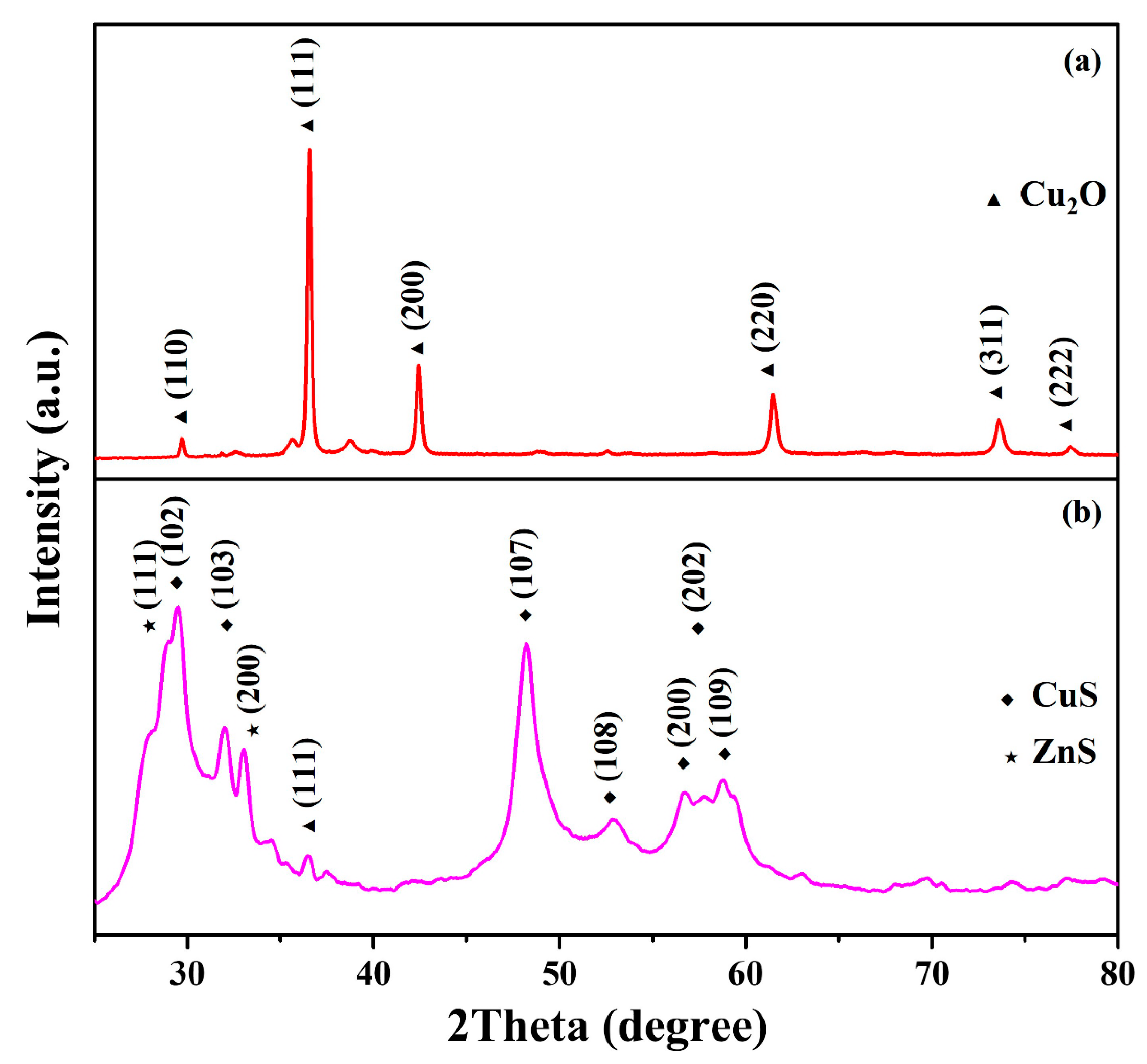
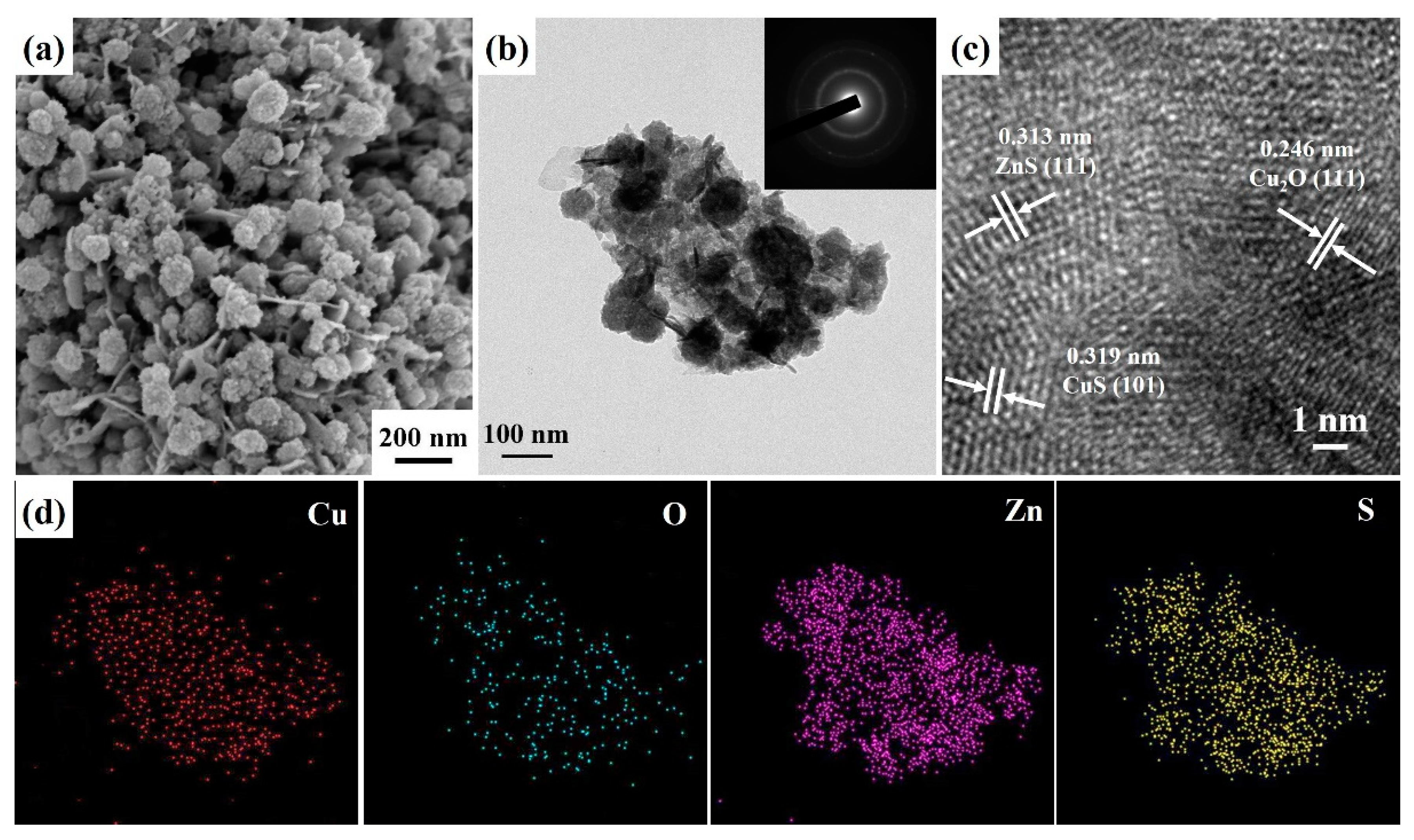
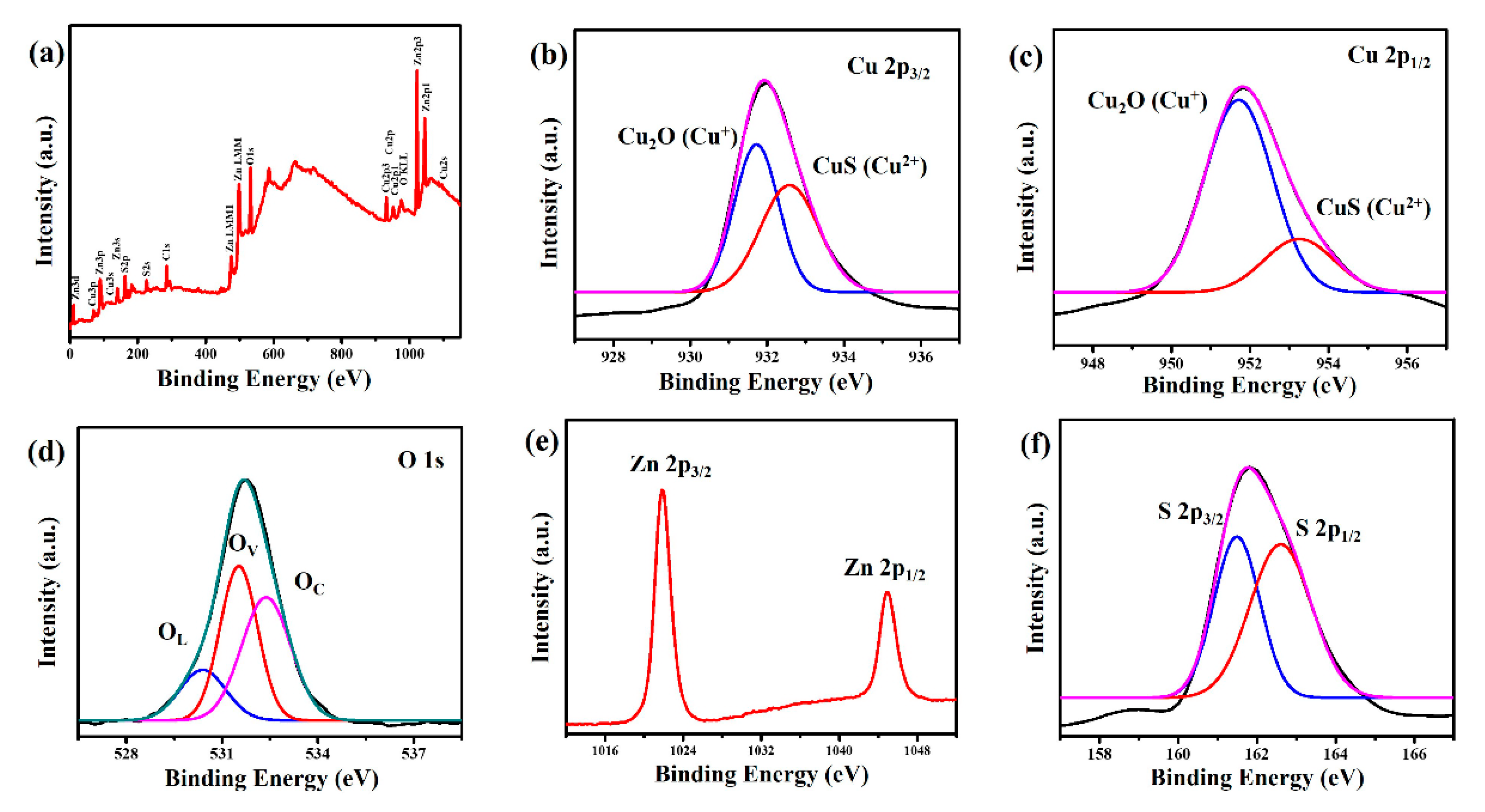
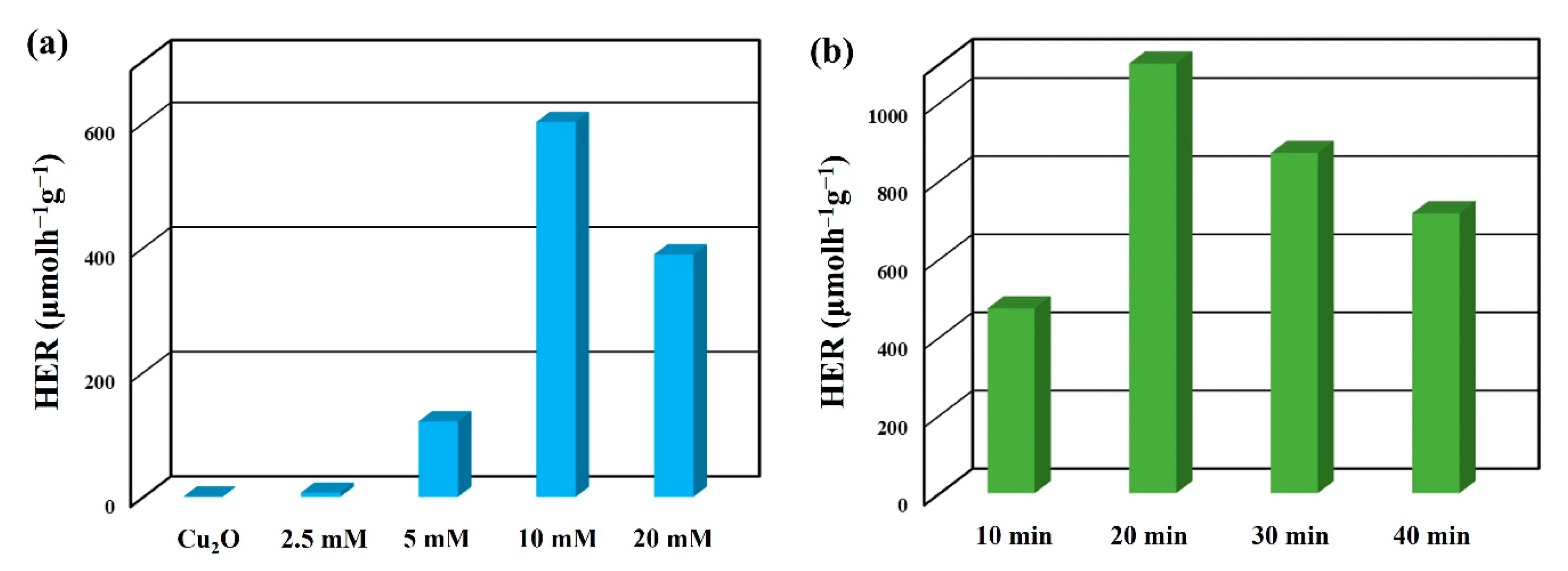
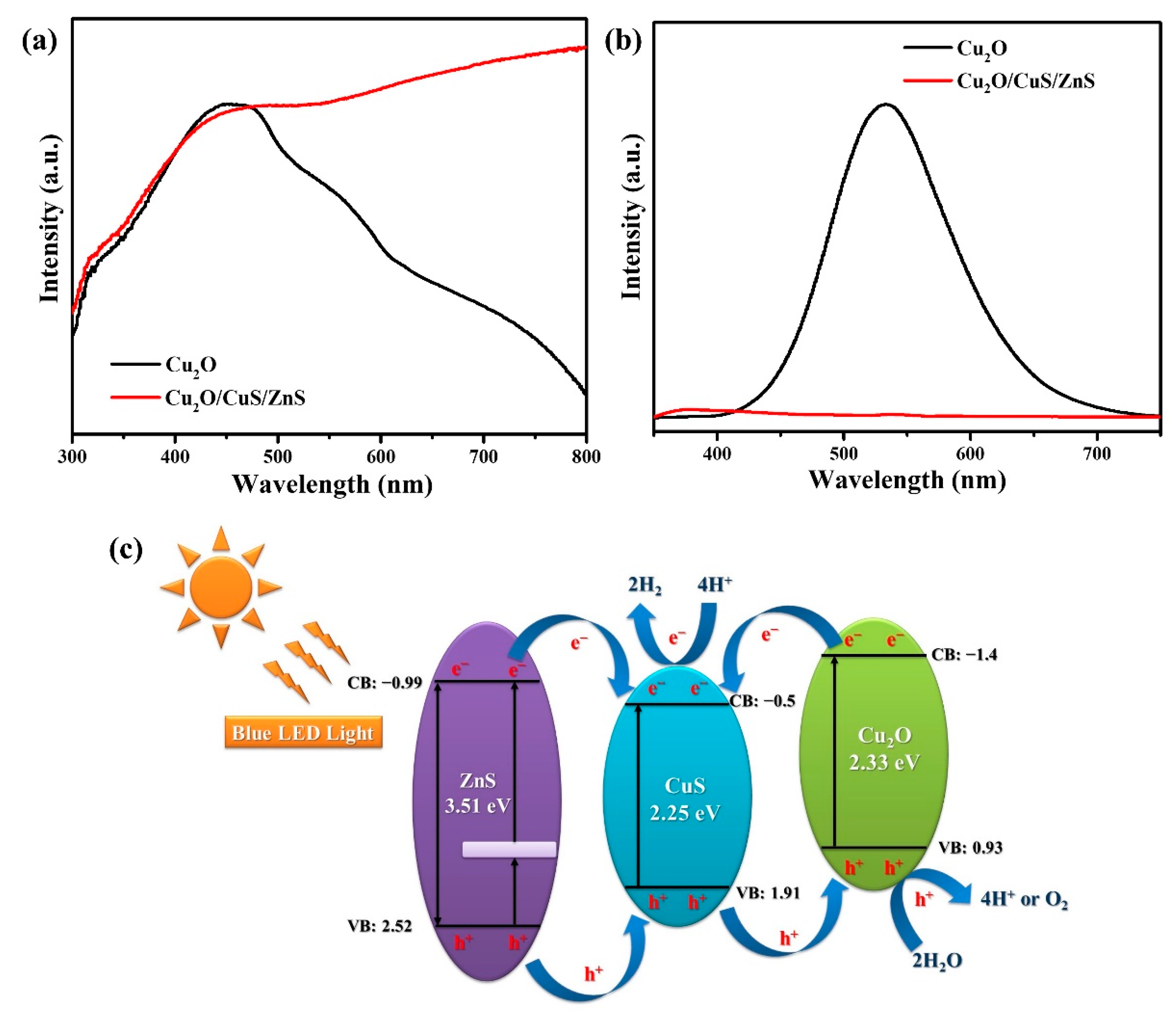
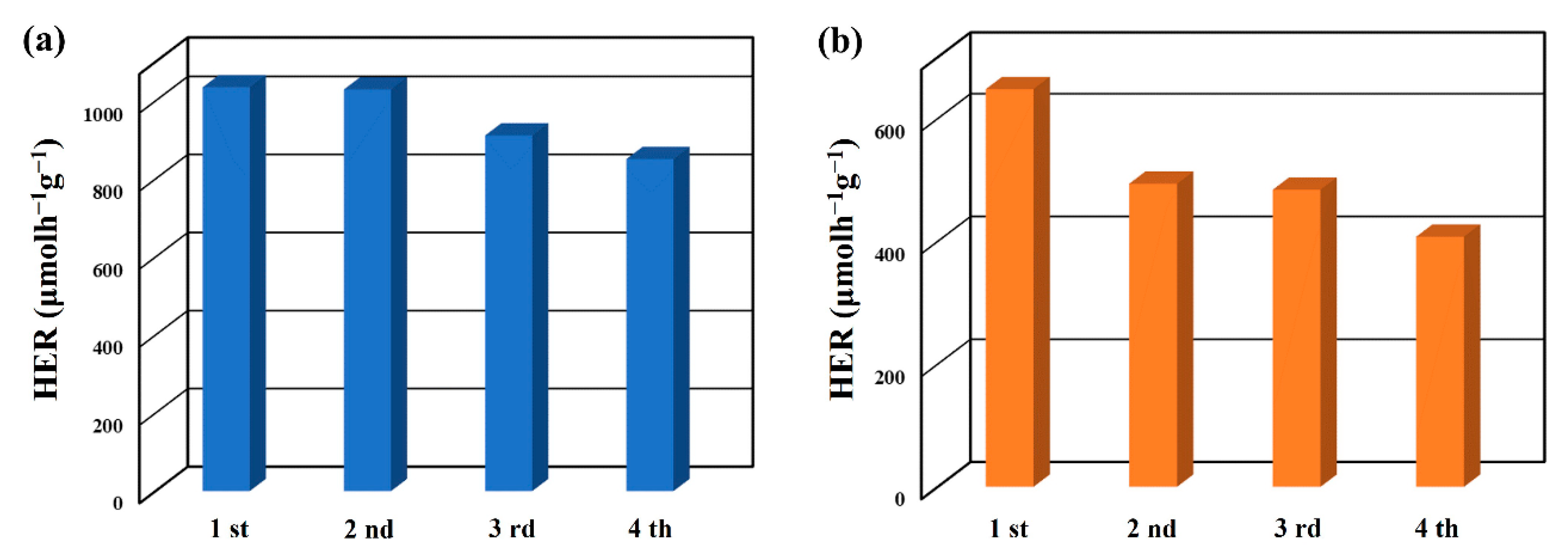
2. Results and Discussion
3. Material and Methods
3.1. Preparation of Cu2O Nanostructures
3.2. Preparation of the Cu2O/CuS/ZnS Nanocomposite
3.3. Characterization
3.4. Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, Y.; Zhou, B.; Li, C.; Cao, Y.; Li, L.; Zeng, L. A review of renewable energy utilization in islands. Renew. Sustain. Energy Rev. 2016, 59, 504–513. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Martin, A.; Agnoletti, M.-F.; Brangier, E. Users in the design of Hydrogen Energy Systems: A systematic review. Int. J. Hydrog. Energy 2020, 45, 11889–11900. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Kumar, V.; Shrivastava, R.L.; Untawale, S.P. Solar Energy: Review of Potential Green & Clean Energy for Coastal and Offshore Applications. Aquat. Procedia 2015, 4, 473–480. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Chou, C.-M.; Chang, T.-T.; Chen, C.-Y.; Chang, Y.-C. Constructing Er-Doped ZnO/CuS/Au Core-Shell Nanowires with Enhanced Photocatalytic and SERS Properties. Catalysts 2021, 11, 1347. [Google Scholar] [CrossRef]
- Kitano, M.; Hara, M. Heterogeneous photocatalytic cleavage of water. J. Mater. Chem. 2010, 20, 627–641. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, Y.-W.; Lu, M.-Y. Construction of MoS2/ZnO heterostructures as highly efficient photocatalysts for enhanced visible-light decomposition of methylene blue and hydrogen evolution. Mater. Chem. Phys. 2021, 266, 124560. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrog. Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Syu, S.-Y.; Wu, Z.-Y. Fabrication of ZnO-In2S3 composite nanofiber as highly efficient hydrogen evolution photocatalyst. Mater. Lett. 2021, 302, 130435. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Lin, Y.-Z.; Wang, K.; Liu, F.-T. Versatile Functional Porous Cobalt–Nickel Phosphide–Carbon Cocatalyst Derived from a Metal–Organic Framework for Boosting the Photocatalytic Activity of Graphitic Carbon Nitride. ACS Appl. Mater. Interfaces 2019, 11, 28918–28927. [Google Scholar] [CrossRef]
- Wu, T.; Zheng, H.; Kou, Y.; Jin, S.; Jiang, Y.; Gao, M.; Chen, L.; Kadasala, N.R.; Liu, Y. Rhombic dodecahedral Cu2O/Ag-3D Fe3O4 micro-flower composites for water purification under visible light irradiation. J. Alloy. Compd. 2021, 858, 157698. [Google Scholar] [CrossRef]
- Zhou, T.; Zang, Z.; Wei, J.; Zheng, J.; Hao, J.; Ling, F.; Tang, X.; Fang, L.; Zhou, M. Efficient charge carrier separation and excellent visible light photoresponse in Cu2O nanowires. Nano Energy 2018, 50, 118–125. [Google Scholar] [CrossRef]
- Wang, N.; Tao, W.; Gong, X.; Zhao, L.; Wang, T.; Zhao, L.; Liu, F.; Liu, X.; Sun, P.; Lu, G. Highly sensitive and selective NO2 gas sensor fabricated from Cu2O-CuO microflowers. Sens. Actuators B Chem. 2022, 362, 131803. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Chen, K.; Wang, T.; Sun, P.; Wang, C.; Chuai, X.; Zhang, S.; Liu, X.; Lu, G. Double shell Cu2O hollow microspheres as sensing material for high performance n-propanol sensor. Sens. Actuators B Chem. 2021, 333, 129540. [Google Scholar] [CrossRef]
- Liu, P.; Qin, K.; Wen, S.; Wang, L.; He, F.; Liu, E.; He, C.; Shi, C.; Li, J.; Li, Q.; et al. In situ fabrication of Ni(OH)2/Cu2O nanosheets on nanoporous NiCu alloy for high performance supercapacitor. Electrochim. Acta 2018, 283, 970–978. [Google Scholar] [CrossRef]
- Purushothaman, S.; Jeyasubramanian, K.; Muthuselvi, M.; Hikku, G.S. Cu2O nanosheets decorated CuMnO2 nanosphere electrodeposited on Cu foil as high-performance supercapacitor electrode. Mater. Sci. Semicond. Processing 2021, 121, 105366. [Google Scholar] [CrossRef]
- Kim, E.-S.; Kim, M.-C.; Moon, S.-H.; Shin, Y.-K.; Lee, J.-E.; Choi, S.; Park, K.-W. Surface modified and size-controlled octahedral Cu2O nanostructured electrodes for lithium-ion batteries. J. Alloy. Compd. 2019, 794, 84–93. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Xue, H.; Pang, H. Fabrication of Cu2O-based Materials for Lithium-Ion Batteries. ChemSusChem 2018, 11, 1581–1599. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Gil Seo, J. Cu2O/CuO Electrocatalyst for Electrochemical Reduction of Carbon Dioxide to Methanol. Electroanalysis 2021, 33, 705–712. [Google Scholar] [CrossRef]
- Xu, H.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Cu2O–Cu Hybrid Foams as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2017, 7, 986–991. [Google Scholar] [CrossRef]
- Mohan, S.; Honnappa, B.; Augustin, A.; Shanmugam, M.; Chuaicham, C.; Sasaki, K.; Ramasamy, B.; Sekar, K. A Critical Study of Cu2O: Synthesis and Its Application in CO2 Reduction by Photochemical and Electrochemical Approaches. Catalysts 2022, 12, 445. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Liu, M.-M.; Chen, J.-L.; Xie, K.-F.; Fang, S.-M. Dendritic branching Z-scheme Cu2O/TiO2 heterostructure photocatalysts for boosting H2 production. J. Phys. Chem. Solids 2021, 152, 109948. [Google Scholar] [CrossRef]
- Ng, B.-J.; Tang, J.-Y.; Ow, L.Y.; Kong, X.Y.; Ng, Y.H.; Putri, L.K.; Chai, S.-P. Nanoscale p-n junction integration via the synergetic hybridization of facet-controlled Cu2O and defect modulated g-C3N4−x atomic layers for enhanced photocatalytic water splitting. Mater. Today Energy 2022, 101102. [Google Scholar] [CrossRef]
- Park, B.H.; Park, H.; Kim, T.; Yoon, S.J.; Kim, Y.; Son, N.; Kang, M. S-scheme assisted Cu2O/ZnO flower-shaped heterojunction catalyst for breakthrough hydrogen evolution by water splitting. Int. J. Hydrog. Energy 2021, 46, 38319–38335. [Google Scholar] [CrossRef]
- Muscetta, M.; Andreozzi, R.; Clarizia, L.; Di Somma, I.; Marotta, R. Hydrogen production through photoreforming processes over Cu2O/TiO2 composite materials: A mini-review. Int. J. Hydrog. Energy 2020, 45, 28531–28552. [Google Scholar] [CrossRef]
- Seo, Y.J.; Arunachalam, M.; Ahn, K.-S.; Kang, S.H. Integrating heteromixtured Cu2O/CuO photocathode interface through a hydrogen treatment for photoelectrochemical hydrogen evolution reaction. Appl. Surf. Sci. 2021, 551, 149375. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Moiseev, N.; Mikhaylov, A.; Yüksel, S. Facile Synthesis of Copper Oxide-Cobalt Oxide/Nitrogen-Doped Carbon (Cu2O-Co3O4/CN) Composite for Efficient Water Splitting. Appl. Sci. 2021, 11, 9974. [Google Scholar] [CrossRef]
- Kumar, U.; Das Chakraborty, S.; Sahu, R.K.; Bhattacharya, P.; Mishra, T. Improved Interfacial Charge Transfer on Noble Metal-Free Biomimetic CdS-Based Tertiary Heterostructure @ 2D MoS2-CdS-Cu2O with Enhanced Photocatalytic Water Splitting. Adv. Mater. Interfaces 2022, 9, 2101680. [Google Scholar] [CrossRef]
- Mahzoon, S.; Haghighi, M.; Nowee, M.; Zeinalzadeh, H. Sonoprecipitation design of novel efficient all-solid Z-Scheme Cu(OH)2/Cu2O/C3N4 nanophotocatalyst applied in water splitting for H2 production: Synergetic effect of Cu-Based cocatalyst (Cu(OH)2) and electron mediator (Cu). Sol. Energy Mater. Sol. Cells 2021, 219, 110772. [Google Scholar] [CrossRef]
- Yoo, H.; Kahng, S.; Hyeun Kim, J. Z-scheme assisted ZnO/Cu2O-CuO photocatalysts to increase photoactive electrons in hydrogen evolution by water splitting. Sol. Energy Mater. Sol. Cells 2020, 204, 110211. [Google Scholar] [CrossRef]
- Cai, L.; Sun, Y.; Li, W.; Zhang, W.; Liu, X.; Ding, D.; Xu, N. CuS hierarchical hollow microcubes with improved visible-light photocatalytic performance. RSC Adv. 2015, 5, 98136–98143. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, R.; Yu, Z.; Huang, W. Crystal-plane effect of Cu2O templates on compositions, structures and catalytic performance of Ag/Cu2O nanocomposites. CrystEngComm 2019, 21, 2002–2008. [Google Scholar] [CrossRef]
- Ji, Q.; Yan, X.; Xu, J.; Wang, C.; Wang, L. Fabrication of hollow type-II and Z-scheme In2O3/TiO2/Cu2O photocatalyst based on In-MIL-68 for efficient catalytic degradation of tetracycline. Sep. Purif. Technol. 2021, 265, 118487. [Google Scholar] [CrossRef]
- Giribabu, K.; Oh, S.Y.; Suresh, R.; Kumar, S.P.; Manigandan, R.; Munusamy, S.; Gnanamoorthy, G.; Kim, J.Y.; Huh, Y.S.; Narayanan, V. Sensing of picric acid with a glassy carbon electrode modified with CuS nanoparticles deposited on nitrogen-doped reduced graphene oxide. Microchim. Acta 2016, 183, 2421–2430. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered Mesoporous Cobalt Oxide as Highly Efficient Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Wang, C.-C. Surface crystal feature-dependent photoactivity of ZnO–ZnS composite rods via hydrothermal sulfidation. RSC Adv. 2018, 8, 5063–5070. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Jiang, L.; Wu, L.; Zhang, M.; Wang, T. The structure control of ZnS/graphene composites and their excellent properties for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 13384–13389. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarkar, D.; Madras, G. Hierarchical Design of CuS Architectures for Visible Light Photocatalysis of 4-Chlorophenol. ACS Omega 2017, 2, 4009–4021. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Z.; Dong, X. Nickel sulfide modified TiO2 nanotubes with highly efficient photocatalytic H2 evolution activity. J. Energy Chem. 2016, 25, 136–140. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, C.-C. Synergetic effect of carbon black as co-catalyst for enhanced visible-light photocatalytic activity and stability on ZnO nanoparticles. Solid State Sci. 2020, 107, 106366. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; Zhang, X.; Wang, W.; Feng, X.; Song, A. Pulsed-laser-deposited, single-crystalline Cu2O films with low resistivity achieved through manipulating the oxygen pressure. Appl. Surf. Sci. 2018, 435, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Sekar, K.; Chuaicham, C.; Vellaichamy, B.; Li, W.; Zhuang, W.; Lu, X.; Ohtani, B.; Sasaki, K. Cubic Cu2O nanoparticles decorated on TiO2 nanofiber heterostructure as an excellent synergistic photocatalyst for H2 production and sulfamethoxazole degradation. Appl. Catal. B Environ. 2021, 294, 120221. [Google Scholar] [CrossRef]
- Ge, H.; Tian, H.; Zhou, Y.; Wu, S.; Liu, D.; Fu, X.; Song, X.-M.; Shi, X.; Wang, X.; Li, N. Influence of Surface States on the Evaluation of the Flat Band Potential of TiO2. ACS Appl. Mater. Interfaces 2014, 6, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, C.; Lee, H.C.; Bard, A.J. Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping. J. Phys. Chem. C 2013, 117, 9633–9640. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Z.; Guo, L.; Wan, Y. ZnO nanoplate-induced phase transformation synthesis of the composite ZnS/In(OH)3/In2S3 with enhanced visible-light photodegradation activity of pollutants. CrystEngComm 2014, 16, 10997–11006. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Hsieh, P.-L.; Naresh, G.; Tsai, H.-Y.; Huang, M.H. Photocatalytic Activity Suppression of CdS Nanoparticle-Decorated Cu2O Octahedra and Rhombic Dodecahedra. J. Phys. Chem. C 2018, 122, 12944–12950. [Google Scholar] [CrossRef]
- Burek, B.O.; Timm, J.; Bahnemann, D.W.; Bloh, J.Z. Kinetic effects and oxidation pathways of sacrificial electron donors on the example of the photocatalytic reduction of molecular oxygen to hydrogen peroxide over illuminated titanium dioxide. Catal. Today 2019, 335, 354–364. [Google Scholar] [CrossRef]
- Schneider, J.; Bahnemann, D.W. Undesired Role of Sacrificial Reagents in Photocatalysis. J. Phys. Chem. Lett. 2013, 4, 3479–3483. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Takata, T.; Domen, K. Self-Templated Synthesis of Nanoporous CdS Nanostructures for Highly Efficient Photocatalytic Hydrogen Production under Visible Light. Chem. Mater. 2008, 20, 110–117. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nano particles with ZnS and CdS. Int. J. Hydrog. Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Strataki, N.; Antoniadou, M.; Dracopoulos, V.; Lianos, P. Visible-light photocatalytic hydrogen production from ethanol–water mixtures using a Pt–CdS–TiO2 photocatalyst. Catal. Today 2010, 151, 53–57. [Google Scholar] [CrossRef]
- Khan, A.U.; Arooj, A.; Tahir, K.; Ibrahim, M.M.; Jevtovic, V.; Al-Abdulkarim, H.A.; Saleh, E.A.M.; Al-Shehri, H.S.; Amin, M.A.; Li, B. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J. Mol. Struct. 2022, 1251, 131991. [Google Scholar] [CrossRef]
- Park, Y.-K.; Kim, B.-J.; Jeong, S.; Jeon, K.-J.; Chung, K.-H.; Jung, S.-C. Characteristics of hydrogen production by photocatalytic water splitting using liquid phase plasma over Ag-doped TiO2 photocatalysts. Environ. Res. 2020, 188, 109630. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Chiao, Y.-C.; Fun, Y.-X. Cu2O/CuS/ZnS Nanocomposite Boosts Blue LED-Light-Driven Photocatalytic Hydrogen Evolution. Catalysts 2022, 12, 1035. https://doi.org/10.3390/catal12091035
Chang Y-C, Chiao Y-C, Fun Y-X. Cu2O/CuS/ZnS Nanocomposite Boosts Blue LED-Light-Driven Photocatalytic Hydrogen Evolution. Catalysts. 2022; 12(9):1035. https://doi.org/10.3390/catal12091035
Chicago/Turabian StyleChang, Yu-Cheng, Yung-Chang Chiao, and Ya-Xiu Fun. 2022. "Cu2O/CuS/ZnS Nanocomposite Boosts Blue LED-Light-Driven Photocatalytic Hydrogen Evolution" Catalysts 12, no. 9: 1035. https://doi.org/10.3390/catal12091035
APA StyleChang, Y.-C., Chiao, Y.-C., & Fun, Y.-X. (2022). Cu2O/CuS/ZnS Nanocomposite Boosts Blue LED-Light-Driven Photocatalytic Hydrogen Evolution. Catalysts, 12(9), 1035. https://doi.org/10.3390/catal12091035