Synthesis of Zinc-Titanium Oxide Nanocomposites by Plasma Jet and Its Application to Photocatalyst
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Description of the Soft Jet Plasma Generator
3.2. Preparation of Zn@Ti Precursors and Synthesis of Zn-Ti Oxide Nanocomposites
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baur, E.; Perret, A. On the action of light on dissolved silver salts in the presence of zinc oxide. Helv. Chim. Acta 1924, 7, 910–915. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Rubin, M.B. The history of ozone. Part VIII. Photochemical formation of ozone. Photochem. Photobiol. Sci. 2011, 10, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Doodeve, C.; Kitchener, J. Photosensitisation by titanium dioxide. Trans. Faraday Soc. 1938, 34, 570–579. [Google Scholar]
- Lotfabadi, P. Analyzing passive solar strategies in the case of high-rise building. Renew. Sust. Energ. Rev. 2015, 52, 1340–1353. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis:Amulti-facetedconceptforgreenchemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Schrauzer, G.; Guth, T. Photolysis of Water and Photoreduction of Nitrogen on Titanium Dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Kudo, A.; Sayama, K.; Tanaka, A.; Asakura, K.; Domen, K.; Maruya, K.; Onishi, T. Nickel-loaded K4Nb6O17 photocatalyst in the decomposition of H2O into H2 and O2: Structure and reaction mechanism. J. Catal. 1989, 120, 337–352. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R.; Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Environmental photochemistry: Is iron oxide (hematite) an active photocatalyst? A comparative study: α-Fe2O3, ZnO, TiO2. J. Photochem. Photobiol. A Chem. 1989, 48, 161–169. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T. Selective removal of organic and inorganic air pollutants by adjusting the g-C3N4/TiO2 ratio. Catal. Today 2021, 361, 37–42. [Google Scholar] [CrossRef]
- Ren, K.; Wang, S.; Luo, Y.; Chou, J.P. High-efficiency photocatalyst for water splitting: A Janus MoSSe/XN (X = Ga, Al) van der Waals heterostructure. J. Phys. D Appl. Phys. 2020, 53, 1855041. [Google Scholar] [CrossRef]
- Ananth, A.; Mok, Y.S. Dielectric barrier discharge plasma-mediated synthesis of several oxide, nanomaterials and its characterization. Powder Technol. 2015, 269, 259–266. [Google Scholar] [CrossRef]
- Ananth, A.; Dharaneedharan, S.; Seo, H.-J.; Heo, M.-S.; Boo, J.-H. Soft jet plasma-assisted synthesis of zinc oxide nanomaterials: Morphology controls and antibacterial activity of ZnO. Chem. Eng. J. 2017, 322, 742–751. [Google Scholar] [CrossRef]
- Mariotti, D.; Svrcekm, V.; Hamilton, J.W.H.; Schmidt, M.; Kondo, M. Silicon nanocrystals in liquid media: Optical properties and surface stabilization by microplasma-induced non-equilibrium liquid chemistry. Adv. Funct. Mater. 2012, 22, 954–964. [Google Scholar] [CrossRef]
- Kim, H.; Sakakita, H.; Ohsaki, H.; Katsurai, M. Microwave-excited atmospheric pressure plasma jet with wide aperture for the synthesis of carbon nanomaterials. Jap. J. Appl. Phys. 2015, 54, 01AA02. [Google Scholar] [CrossRef]
- Saito, G.; Akiyama, T. Nanomaterial synthesis using plasma generation in liquid. J. Nanomater. 2015, 2015, 123696. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, Y. A review of plasma-liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef]
- Fierro, A.; Laity, G.; Neuber, A. Optical emission spectroscopy study in the VUV-VIS regimes of a developing low-temperature plasma in nitrogen gas. J. Phys. D Appl. Phys. 2012, 45, 495202. [Google Scholar] [CrossRef]
- Dowling, D.P.; O’Neill, F.T.; Milosavljevic, V.; Law, V.J. DC pulsed atmospheric pressure plasma jet image formation. IEEE Xplore IEEE Trans. Plasma Sci. 2011, 39, 2326–2327. [Google Scholar] [CrossRef] [Green Version]
- Hieda, J.; Saito, N.; Takai, O. Exotic shapes of gold nanoparticles synthesized using plasma in aqueous solution. J. Vac. Sci. Technol. A 2008, 26, 854–856. [Google Scholar] [CrossRef]
- Richmonds, C.; Sankaran, R.M. Plasma-liquid electrochemistry: Rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl. Phys. Lett. 2008, 93, 131501. [Google Scholar] [CrossRef]
- Baran, E.J.; Botto, I.L. Die Raman-Spektren von ZnTiO3 und CdTiO3. Z. Anorg. Chem. 1979, 448, 188–192. [Google Scholar] [CrossRef]
- Bernert, T.; Ruiz-Fuertes, J.; Bayarjargal, L.; Winkler, B. Synthesis and high (pressure, temperature) stability of ZnTiO3 polymorphs studied by Raman spectroscopy. Solid State Sci. 2015, 43, 53–58. [Google Scholar] [CrossRef]
- Fuertes, J.R.; Bernert, T.; He, M.; Winkler, B.; Vinograd, V.L.; Milman, V. Local structure of CuxZn2-xTiO4 inverse spinel. Appl. Phys. Lett. 2014, 105, 071911. [Google Scholar] [CrossRef]
- Maheu, C.; Cardenas, L.; Puzenat, E.; Afanasiev, P.; Geantet, C. UPS and UV spectroscopies combined to position the energy levels of TiO2 anatase and rutile nanopowders. Phys. Chem. Chem. Phys. 2018, 20, 25629–25637. [Google Scholar] [CrossRef]
- Conejeros, S.; Neil, L.; Allan, N.L.; Claeyssens, F.; Hart, J.N. Graphene and novel graphitic ZnO and ZnS nanofilms: The energy landscape, nonstoichiometry and water dissociation. Nanoscale Adv. 2019, 1, 1924–1935. [Google Scholar] [CrossRef]
- Lei, S.; Fan, H.; Ren, X.; Fang, J.; Ma, L.; Liua, Z. Novel sintering and band gap engineering of ZnTiO3 ceramics with excellent microwave dielectric properties. J. Mater. Chem. C 2017, 5, 4040–4047. [Google Scholar] [CrossRef]
- Seo, H.J.; Lee, J.W.; Na, Y.H.; Boo, J.H. Enhancement of Photocatalytic Activities with Nanosized Polystyrene Spheres Patterned Titanium Dioxide Films for Water Purification. Catalysts 2020, 10, 886. [Google Scholar] [CrossRef]
- Doong, R.-A.; Chang, S.-M.; Hung, Y.-C.; Kao, I.-L. Preparation of highly ordered titanium dioxide porous films: Characterization and photocatalytic activity. Sep. Purif. Technol. 2007, 58, 192–199. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals. Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Lee, J.W.; Nam, S.-H.; Yu, J.-H.; Kim, D.I.; Jeong, R.H.; Boo, J.-H. Morphological modulation of urchin-like Zn2SnO4/SnO2 hollow spheres and their applications as photocatalysts and quartz crystal microbalance measurements. Appl. Surf. Sci. 2019, 474, 78–84. [Google Scholar] [CrossRef]
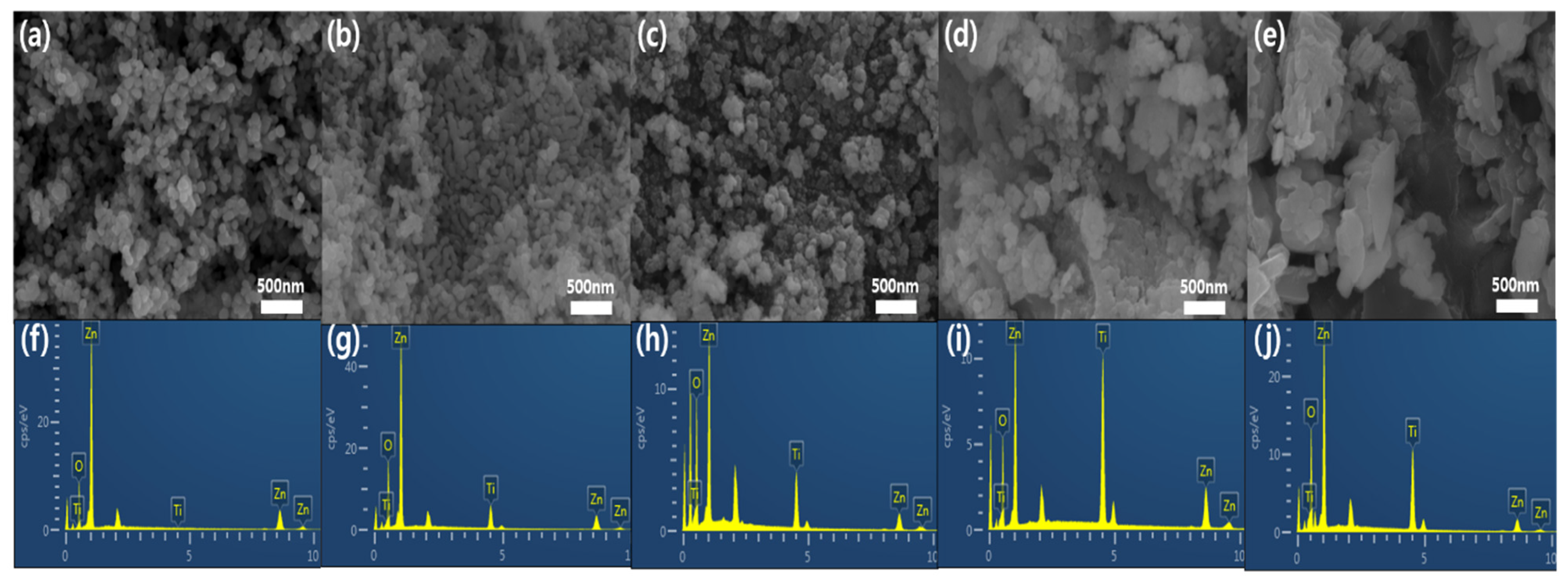


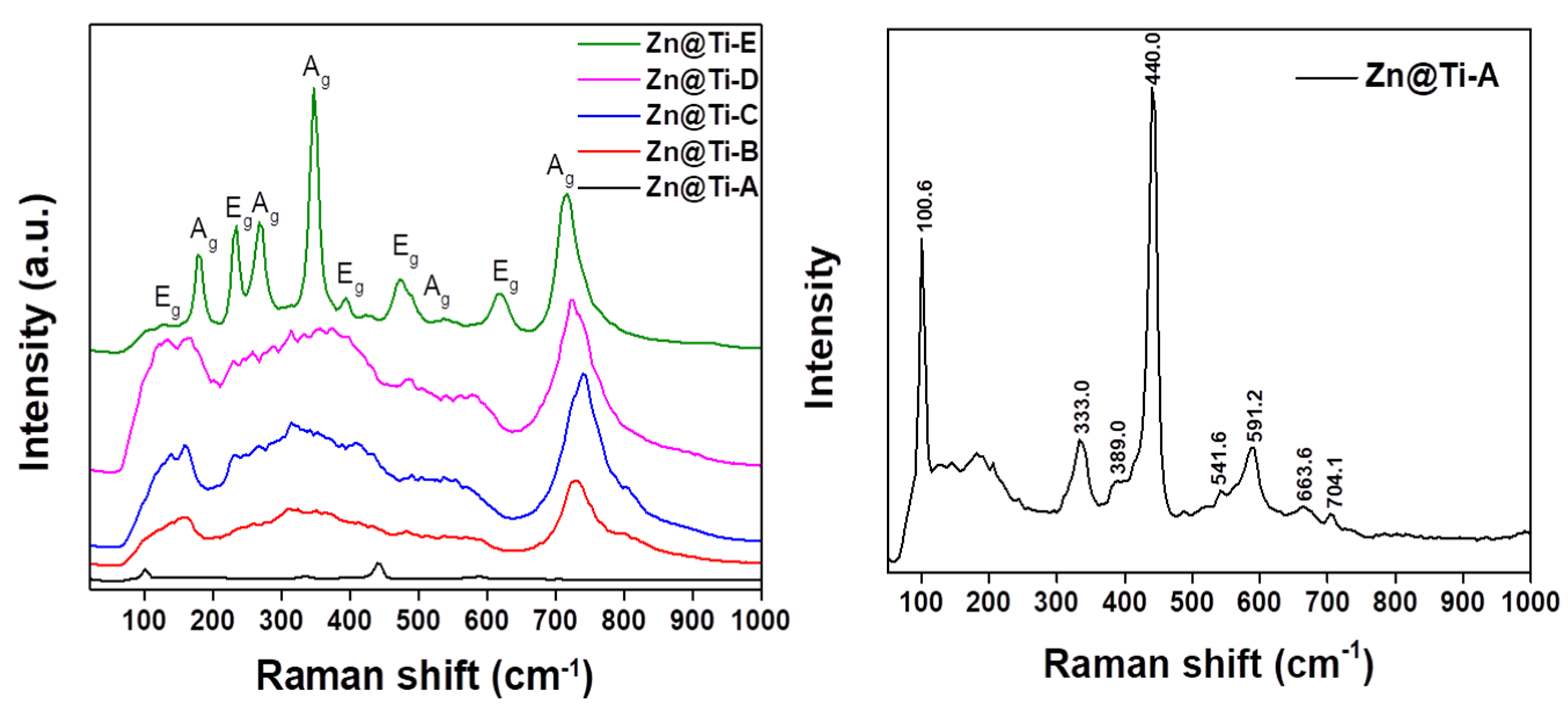
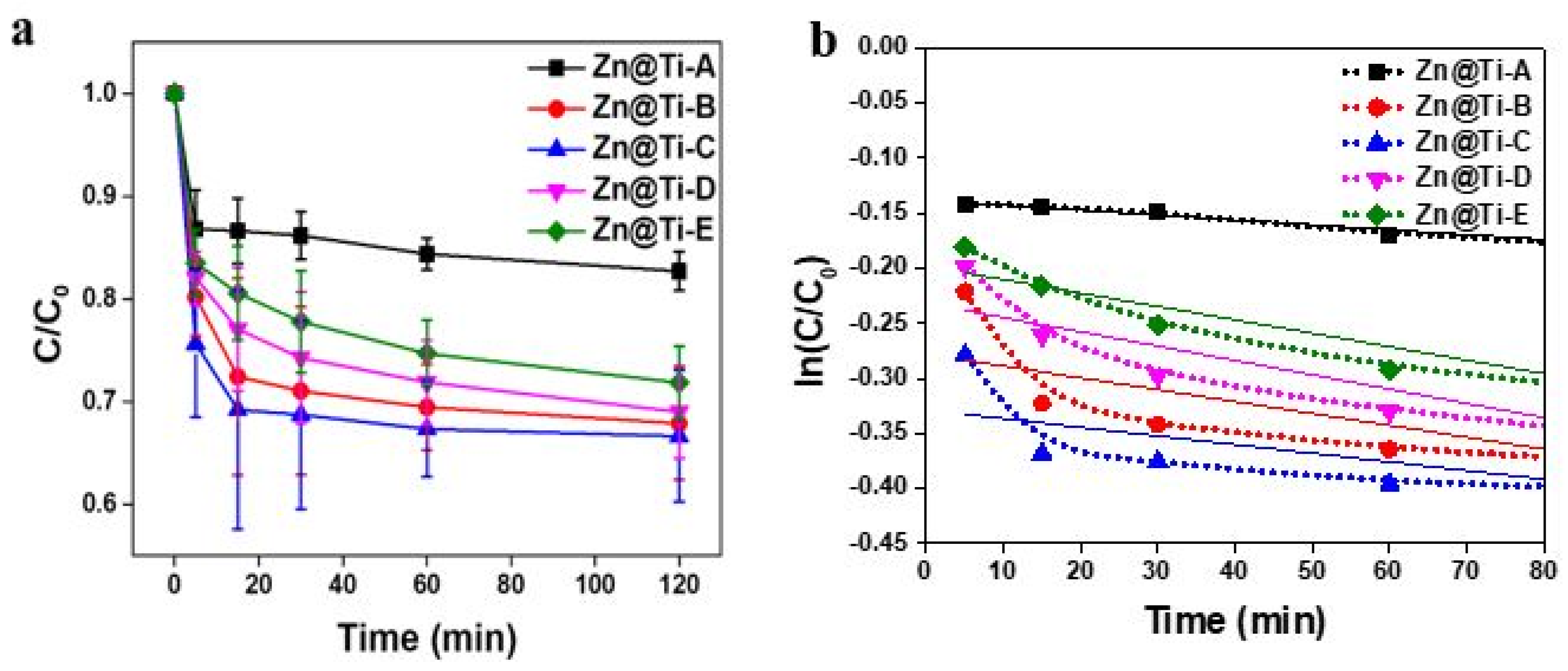
| Element | Zn@Ti-A | Zn@Ti-B | Zn@Ti-C | Zn@Ti-D | Zn@Ti-E |
|---|---|---|---|---|---|
| O | 52.75 | 58.67 | 68.47 | 57.40 | 64.38 |
| Ti | 0.00 | 8.16 | 10.76 | 24.41 | 15.16 |
| Zn | 47.25 | 33.17 | 20.77 | 18.19 | 20.45 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Reaction Time (min.) | 5 | 15 | 30 | 60 | |
|---|---|---|---|---|---|
| C/Co of TiO2 (Degussa P-25) | 0.90 | 0.87 | 0.84 | 0.73 | [30] |
| C/Co of Zn@Ti-A (ZnTiO3) | 0.89 | 0.88 | 0.87 | 0.85 | This Work |
| C/Co of Zn@Ti-C (Zn2.0Ti1.0O3+X) | 0.75 | 0.70 | 0.69 | 0.68 | This Work |
| Name | Zinc Nitrate | Titanium Butoxide | Sodium Hydroxide |
|---|---|---|---|
| Zn@Ti-A | 0.1 mol | 0 mol | 0.1 mol |
| Zn@Ti-B | 0.0875 mol | 0.0125 mol | 0.1 mol |
| Zn@Ti-C | 0.075 mol | 0.025 mol | 0.1 mol |
| Zn@Ti-D | 0.0625 mol | 0.0375 mol | 0.1 mol |
| Zn@Ti-E | 0.05 mol | 0.05 mol | 0.1 mol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.-J.; Yu, J.-H.; Ananth, A.; Jeong, R.-H.; Boo, J.-H. Synthesis of Zinc-Titanium Oxide Nanocomposites by Plasma Jet and Its Application to Photocatalyst. Catalysts 2022, 12, 1020. https://doi.org/10.3390/catal12091020
Seo H-J, Yu J-H, Ananth A, Jeong R-H, Boo J-H. Synthesis of Zinc-Titanium Oxide Nanocomposites by Plasma Jet and Its Application to Photocatalyst. Catalysts. 2022; 12(9):1020. https://doi.org/10.3390/catal12091020
Chicago/Turabian StyleSeo, Hyeon-Jin, Jung-Hoon Yu, Antony Ananth, Rak-Hyun Jeong, and Jin-Hyo Boo. 2022. "Synthesis of Zinc-Titanium Oxide Nanocomposites by Plasma Jet and Its Application to Photocatalyst" Catalysts 12, no. 9: 1020. https://doi.org/10.3390/catal12091020
APA StyleSeo, H.-J., Yu, J.-H., Ananth, A., Jeong, R.-H., & Boo, J.-H. (2022). Synthesis of Zinc-Titanium Oxide Nanocomposites by Plasma Jet and Its Application to Photocatalyst. Catalysts, 12(9), 1020. https://doi.org/10.3390/catal12091020







