Efficient and Eco-Friendly Perspectives for C-H Arylation of Benzothiazole Utilizing Pd Nanoparticle-Decorated Chitosan
Abstract
:1. Introduction
2. Results and Discussion
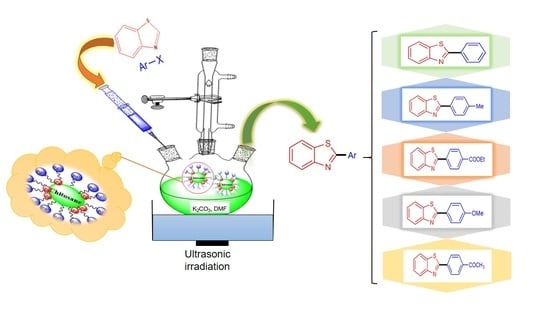
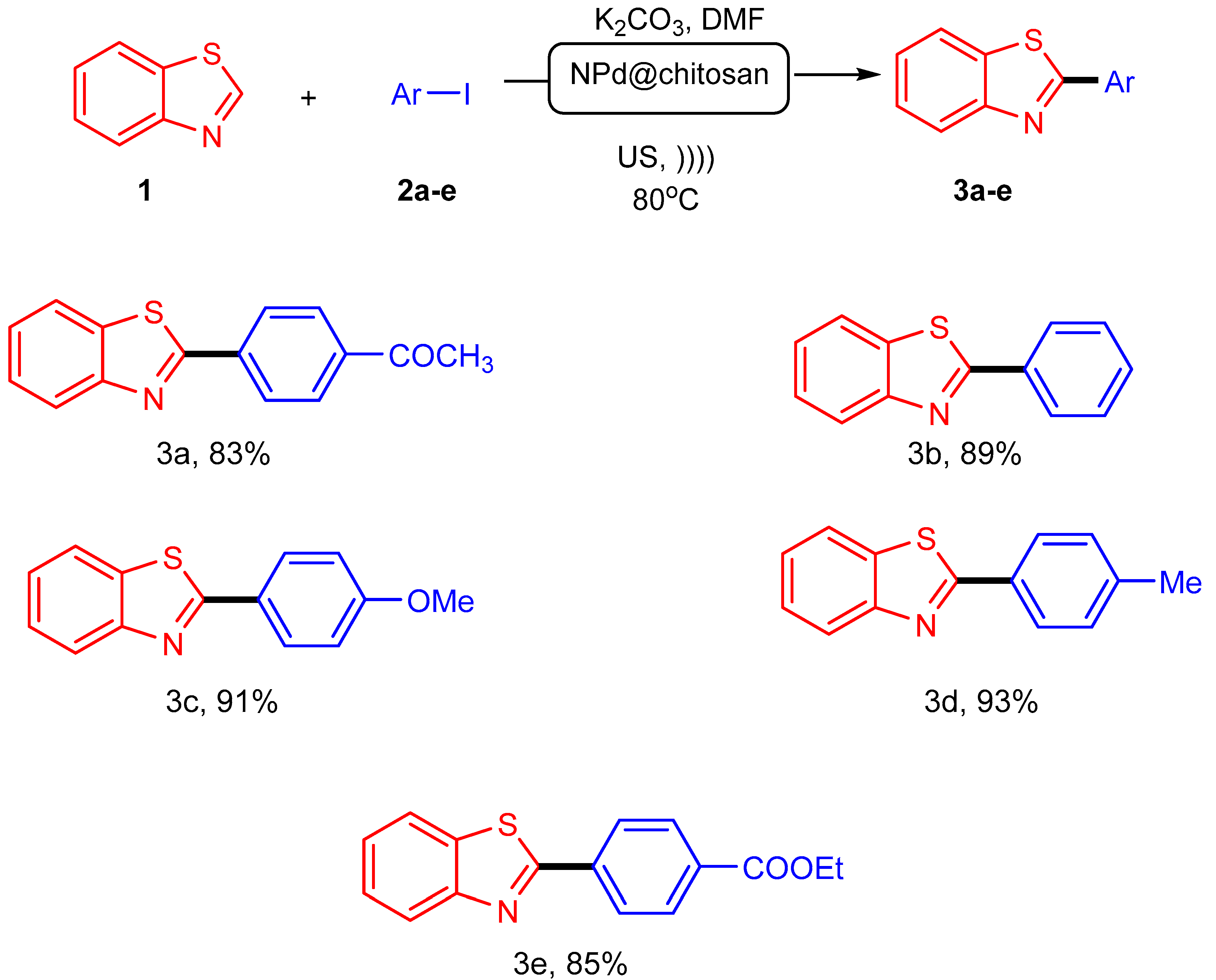
2.1. C-H Arylation of Benzothiazole
2.2. Catalyst Characterization
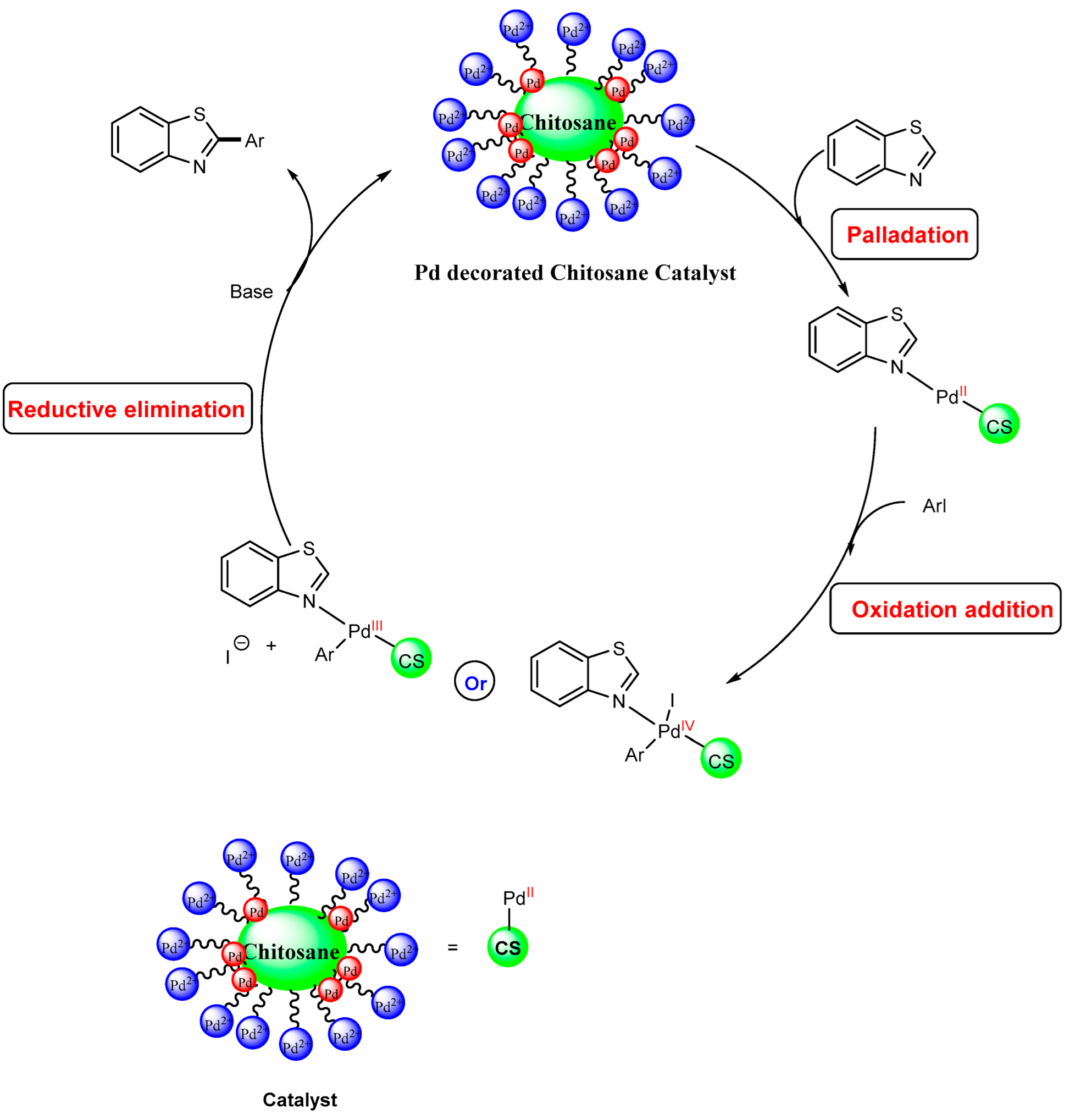
2.3. Tentative Mechanism
3. Experiments
3.1. Reagents
3.2. Synthesis of Pd Nanoparticle-decorated Chitosan Sample (5% Pd@chitosan)
3.3. C-H arylation of Benzothiazole
3.3.1. Silent Reactions
3.3.2. Sonicated Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular Chemistry to enable a Circular Economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar]
- Zuin, V.G.; Kümmerer, K. Chemistry and Materials Science for a Sustainable Circular Polymeric Economy. Nat. Rev. Mater. 2022, 7, 76–78. [Google Scholar]
- Hemmati, S.; Heravi, M.M.; Karmakar, B.; Veisi, H. Green Fabrication of Reduced Graphene Oxide Decorated with Ag Nanoparticles (RGO/Ag NPs) Nanocomposite: A Reusable Catalyst for the Degradation of Environmental Pollutants in Aqueous Medium. J. Mol. Liq. 2020, 319. [Google Scholar] [CrossRef]
- García-Suárez, E.J.; Lara, P.; García, A.B.; Ojeda, M.; Luque, R.; Philippot, K. Efficient and Recyclable Carbon-Supported Pd Nanocatalysts for the Suzuki-Miyaura Reaction in Aqueous-Based Media: Microwave vs Conventional Heating. Appl. Catal. A Gen. 2013, 468, 59–67. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, P.; Huang, D.; Zeng, G.; Lai, C.; Qin, L.; Li, B.; He, J.; Yi, H.; Cheng, M.; et al. Au Nanoparticles Decorated on Activated Coke via a Facile Preparation for Efficient Catalytic Reduction of Nitrophenols and Azo Dyes. Appl. Surf. Sci. 2019, 473, 578–588. [Google Scholar] [CrossRef]
- Nasri, A.; Jaleh, B.; Nezafat, Z.; Nasrollahzadeh, M.; Azizian, S.; Jang, H.W.; Shokouhimehr, M. Fabrication of G-C3N4/Au Nanocomposite Using Laser Ablation and Its Application as an Effective Catalyst in the Reduction of Organic Pollutants in Water. Ceram. Int. 2021, 47, 3565–3572. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Rahmani, S.; Tizhoush, H. The Immobilized Cu Nanoparticles on Magnetic Montmorillonite (MMT@Fe3O4@Cu): As an Efficient and Reusable Nanocatalyst for Reduction and Reductive-Acetylation of Nitroarenes with NaBH4. Polyhedron 2020, 175, 114201. [Google Scholar] [CrossRef]
- Veisi, H.; Najafi, S.; Hemmati, S. Pd(II)/Pd(0) Anchored to Magnetic Nanoparticles (Fe3O4) Modified with Biguanidine-Chitosan Polymer as a Novel Nanocatalyst for Suzuki-Miyaura Coupling Reactions. Int. J. Biol. Macromol. 2018, 113, 186–194. [Google Scholar] [CrossRef]
- Ahmad, P.T.; Jaleh, B.; Nasrollahzadeh, M.; Issaabadi, Z. Efficient Reduction of Waste Water Pollution Using GO/ΓMnO2/Pd Nanocomposite as a Highly Stable and Recoverable Catalyst. Sep. Purif. Technol. 2019, 225, 33–40. [Google Scholar] [CrossRef]
- Ei-Shobaky, G.A.; Abroad, A.S.; Mokhtar, M. Effect of Gamma-Irradiation on Surface and Catalytic Properties of CuO-ZnO/Al2O3 System. J. Radioanal. Nucl. Chem. 1997, 219, 89–94. [Google Scholar]
- Mokhtar, M.; Ohlinger, C.; Schlander, J.H.; Turek, T. Hydrogenolysis of Dimethyl Maleate on Cu/ZnO/Al2O3 Catalysts. Chem. Eng. Technol. 2001, 24, 423–426. [Google Scholar]
- Veisi, H.; Hamelian, M.; Hemmati, S. Palladium Anchored to SBA-15 Functionalized with Melamine-Pyridine Groups as a Novel and Efficient Heterogeneous Nanocatalyst for Suzuki-Miyaura Coupling Reactions. J. Mol. Catal. A Chem. 2014, 395, 25–33. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar]
- Jamwal, N.; Sodhi, R.K.; Gupta, P.; Paul, S. Nano Pd(0) Supported on Cellulose: A Highly Efficient and Recyclable Heterogeneous Catalyst for the Suzuki Coupling and Aerobic Oxidation of Benzyl Alcohols under Liquid Phase Catalysis. Int. J. Biol. Macromol. 2011, 49, 930–935. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar]
- Ma, J.; Sahai, Y. Chitosan Biopolymer for Fuel Cell Applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Ruthenium on Chitosan: A Recyclable Heterogeneous Catalyst for Aqueous Hydration of Nitriles to Amides. Green Chem. 2014, 16, 2122–2127. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous Catalysis on Chitosan-Based Materials: A Review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar]
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Cheng, W.; Zhang, J.; Li, X.; Zhang, S.; She, Y. Chitosan Functionalized Ionic Liquid as a Recyclable Biopolymer-Supported Catalyst for Cycloaddition of CO2. Green Chem. 2012, 14, 654–660. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized Chitosan as a Green, Recyclable, Biopolymer-Supported Catalyst for the [3+2] Huisgen Cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, N.; Singh, R.K.; Gupta, P.; Singh, R.; Jain, S.L. Dual Catalysis with Magnetic Chitosan: Direct Synthesis of Cyclic Carbonates from Olefins with Carbon Dioxide Using Isobutyraldehyde as the Sacrificial Reductant. Dalton Trans. 2015, 44, 11860–11866. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl-Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar]
- Lewis, J.C.; Bergman, R.G.; Ellman, J.A. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc. Chem. Res. 2008, 41, 1013–1025. [Google Scholar] [CrossRef]
- Amii, H.; Uneyama, K. C-F Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183. [Google Scholar] [CrossRef]
- Chen, X.; Engle, K.M.; Wang, D.H.; Yu, J.Q. Palladium(II)-CataIyzed C-H Aetivation/C-C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar]
- Das, D.; Bhutia, Z.T.; Chatterjee, A.; Banerjee, M. Mechanochemical Pd(II)-Catalyzed Direct and C-2-Selective Arylation of Indoles. J. Org. Chem. 2019, 84, 10764–10774. [Google Scholar]
- Albano, G.; Decandia, G.; Capozzi, M.A.M.; Zappimbulso, N.; Punzi, A.; Farinola, G.M. Infrared Irradiation-Assisted Solvent-Free Pd-Catalyzed (Hetero)aryl-aryl Coupling via C−H Bond Activation. ChemSusChem 2021, 14, 3391–3401. [Google Scholar]
- Sharma, A.; Vacchani, D.; Van der Eycken, E. Developments in direct C-H arylation of (hetero)arenes under microwave irradiation. Chemistry 2013, 19, 1158–1168. [Google Scholar] [CrossRef]
- Liu, X.W.; Shi, J.L.; Yan, J.X.; Wei, J.B.; Peng, K.; Dai, L.; Li, C.G.; Wang, B.Q.; Shi, Z.J. Reigoselective Arylation of Thiazole Derivatives at 5-Position via Pd Catalysis under Ligand-Free Conditions. Org. Lett. 2013, 15, 5774–5777. [Google Scholar] [CrossRef]
- Gong, B.; Hong, F.; Kohm, C.; Bonham, L.; Klein, P. Synthesis and SAR of 2-Arylbenzoxazoles, Benzothiazoles and Benzimidazoles as Inhibitors of Lysophosphatidic Acid Acyltransferase-β. Bioorganic Med. Chem. Lett. 2004, 14, 1455–1459. [Google Scholar] [CrossRef]
- Mortimer, C.G.; Wells, G.; Crochard, J.P.; Stone, E.L.; Bradshaw, T.D.; Stevens, M.F.G.; Westwell, A.D. Antitumor Benzothiazoles. 26.1 2-(3,4-Dimethoxyphenyl)-5- Fluorobenzothiazole (GW 610, NSC 721648), a Simple Fluorinated 2-Arylbenzothiazole, Shows Potent and Selective Inhibitory Activity against Lung, Colon, and Breast Cancer Cell Lines. J. Med. Chem. 2006, 49, 179–185. [Google Scholar] [CrossRef]
- Wee Phoon, C.; Yong Ng, P.; Ting, A.E.; Ling Yeo, S.; Mui Sim, M. Biological Evaluation of Hepatitis C Virus Helicase Inhibitors. Bioorganic Med. Chem. Lett. 2001, 11, 1647–1650. [Google Scholar]
- Benazzouz, A.; Boraud, T.; Dube, P. dat, A. Boireau, J.-M. Stutzmann, C. Gross. Eur. J. Pharmacol. 1995, 284, 10–16. [Google Scholar]
- Mokhtar, M.; Alhashedi, B.F.A.; Kashmery, H.A.; Ahmed, N.S.; Saleh, T.S.; Narasimharao, K. Highly Efficient Nanosized Mesoporous CuMgAl Ternary Oxide Catalyst for Nitro-Alcohol Synthesis: Ultrasound-Assisted Sustainable Green Perspective for the Henry Reaction. ACS Omega 2020, 5, 6532–6544. [Google Scholar] [CrossRef]
- Saleh, T.S.; Al-Bogami, A.S.; Mekky, A.E.M.; Alkhathlan, H.Z. Sonochemical Synthesis of Novel Pyrano [3,4-e] [1,3] Oxazines: A Green Protocol. Ultrason. Sonochemistry 2017, 36, 474–480. [Google Scholar] [CrossRef]
- Saleh, T.S.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.N.; Al-Thabaiti, S.A.; Mokhtar, M. Mg-Al Hydrotalcite as an Efficient Catalyst for Microwave Assisted Regioselective 1,3-Dipolar Cycloaddition of Nitrilimines with the Enaminone Derivatives: A Green Protocol. J. Mol. Catal. A Chem. 2013, 367, 12–22. [Google Scholar] [CrossRef]
- Narasimharao, K.; Al-Sabban, E.; Saleh, T.S.; Gallastegui, A.G.; Sanfiz, A.C.; Basahel, S.; Al-Thabaiti, S.; Alyoubi, A.; Obaid, A.; Mokhtar, M. Microwave Assisted Efficient Protocol for the Classic Ullmann Homocoupling Reaction Using Cu-Mg-Al Hydrotalcite Catalysts. J. Mol. Catal. A Chem. 2013, 379, 152–162. [Google Scholar] [CrossRef]
- Bassyouni, F.A.; Saleh, T.S.; Elhefnawi, M.M.; El-Moez, S.I.A.; El-Senousy, W.M.; Abdel-Rehim, M.E. Synthesis, Pharmacological Activity Evaluation and Molecular Modeling of New Polynuclear Heterocyclic Compounds Containing Benzimidazole Derivatives. Arch. Pharmacal Res. 2012, 35, 2063–2075. [Google Scholar] [CrossRef]
- Shahid, A.; Ahmed, N.S.; Saleh, T.S.; Al-Thabaiti, S.A.; Basahel, S.N.; Schwieger, W.; Mokhtar, M. Solvent-Free Biginelli Reactions Catalyzed by Hierarchical Zeolite Utilizing a Ball Mill Technique: A Green Sustainable Process. Catalysts 2017, 7, 84. [Google Scholar] [CrossRef]
- Mady, M.F.; Saleh, T.S.; El-Kateb, A.A.; Abd El-Rahman, N.M.; Abd El-Moez, S.I. Microwave-Assisted Synthesis of Novel Pyrazole and Pyrazolo [3,4-d] Pyridazine Derivatives Incorporating Diaryl Sulfone Moiety as Potential Antimicrobial Agents. Res. Chem. Intermed. 2016, 42, 753–769. [Google Scholar] [CrossRef]
- Al-Bogami, A.S.; Saleh, T.S.; Mekky, A.E.M.; Shaaban, M.R. Microwave Assisted Regioselective Synthesis and 2D-NMR Studies of Novel Azoles and Azoloazines Utilizing Fluorine-Containing Building Blocks. J. Mol. Struct. 2016, 1121, 167–179. [Google Scholar] [CrossRef]
- El Maksod, I.H.A.; Saleh, T.S. The Use of Nano Supported Nickel Catalyst in Reduction of P-Nitrophenol Using Hydrazine as Hydrogen Donor. Green Chem. Lett. Rev. 2010, 3, 127–134. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Menzel, R.; Wang, Y.; Garcia-Gallastegui, A.; Bawaked, S.M.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M. Graphene-Oxide-Supported CuAl and CoAl Layered Double Hydroxides as Enhanced Catalysts for Carbon-Carbon Coupling via Ullmann Reaction. J. Solid State Chem. 2017, 246, 130–137. [Google Scholar] [CrossRef]
- Mason, T.J. The design of ultrasonic reactors for environmental remediation. In Ultrasound in Environmental Protection; Elsevier: Amsterdam, The Netherlands, 2001; pp. 247–268. [Google Scholar]
- Shah, Y.T.; Pandit, A.B.; Moholkar, V.S. Cavitation Reaction Engineering; Springer Science & Business Media: Berlin, Germany, 1999. [Google Scholar]
- Gmehling, J. N, N-Dimethylformamide (DMF) Dynamic Viscosity: Datasheet from “Dortmund Data Bank (DDB)—Thermophysical Properties Edition 2014” in SpringerMaterials; Springer-Verlag Berlin Heidelberg & DDBST GmbH: Oldenburg, Germany. Available online: https://materials.springer.com/thermophysical/docs/vis_c72 (accessed on 4 August 2022).
- Shaikh, N.; Pamidimukkala, P. Magnetic Chitosan Stabilized Palladium Nanostructures: Potential Catalysts for Aqueous Suzuki Coupling Reactions. Int. J. Biol. Macromol. 2021, 183, 1560–1573. [Google Scholar] [CrossRef]
- Tsyrul’nikov, P.G.; Afonasenko, T.N.; Koshcheev, S.V.; Boronin, A.I. State of Palladium in Palladium-Aluminosilicate Catalysts as Studied by XPS and the Catalytic Activity of the Catalysts in the Deep Oxidation of Methane. Kinet. Catal. 2007, 48, 728–734. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical Modification of Chitosan by Phosphorylation: An XPS, FT-IR and SEM Study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/Chitosan Beads for Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef]
- Dong, Y.; Bi, J.; Ming, S.; Zhang, S.; Zhu, D.; Meng, D.; Li, T. Functionalized Chitosan as a Novel Support for Stabilizing Palladium in Suzuki Reactions. Carbohydr. Polym. 2021, 260, 117815. [Google Scholar] [CrossRef]
- Luo, M.; Wang, X.; Meng, T.; Yang, P.; Zhu, Z.; Min, H.; Chen, M.; Chen, W.; Zhou, X. Rapid One-Step Preparation of Hierarchical Porous Carbon from Chitosan-Based Hydrogel for High-Rate Supercapacitors: The Effect of Gelling Agent Concentration. Int. J. Biol. Macromol. 2020, 146, 453–461. [Google Scholar] [CrossRef]
- Kumari, S.; Layek, S.; Pathak, D.D. Palladium Nanoparticles Immobilized on a Magnetic Chitosan-Anchored Schiff Base: Applications in Suzuki-Miyaura and Heck-Mizoroki Coupling Reactions. New J. Chem. 2017, 41, 5595–5604. [Google Scholar] [CrossRef]
- Powers, D.C.; Geibel, M.A.L.; Klein, J.E.M.N.; Ritter, T. Bimetallic Palladium Catalysis: Direct Observation of Pd(III)-Pd(III) Intermediates. J. Am. Chem. Soc. 2009, 131, 17050–17051. [Google Scholar] [CrossRef]
- Mc Glacken, G.P.; Bateman, L.M. Recent Advances in Aryl–Aryl Bond Formation by Direct Arylation. Chem. Soc. Rev. 2009, 38, 2447–2464. [Google Scholar] [CrossRef]
- Ding, Q.; Ji, H.; Wang, D.; Lin, Y.; Yu, W.; Peng, Y. Pd(II)-Catalyzed Ortho Arylation of 2-Arylbenzothiazoles with Aryl Iodides via Benzothiazole-Directed C-H Activation. J. Organomet. Chem. 2012, 711, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Muthukrishnan, A.M. Green Synthesis of Copper-Chitosan Nanoparticles and Study of Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2015, 6, 1000251. [Google Scholar] [CrossRef]


| Entry | Base (Equiv.) | Catalyst | Solvent | Ultrasound Irradiation a | Silent Condition a | ||
|---|---|---|---|---|---|---|---|
| Yield b (%) | Time (h) | Yield b (%) | Time (h) | ||||
| 1 | K2CO3 (1) | None | DMF | - | - | - | - |
| 2 | K2CO3 (1) | Chitosan | DMF | - | - | - | - |
| 3 | K2CO3 (1) | 5%Pd@ Chitosan (0.05 g) | DMF | 81 | 3.0 | 48 | 16 |
| 4 | K2CO3 (1) | 5%Pd@ Chitosan (0.1 g) | DMF | 81 | 3.0 | 48 | 16 |
| 5 | K2CO3 (2) | 5%Pd@ Chitosan (0.05 g) | DMF | 83 | 2.5 | 51 | 16 |
| 6 | K2CO3 (3) | 5%Pd@ Chitosan (0.05 g) | DMF | 83 | 2.5 | 51 | 16 |
| 7 | K2CO3 (2) | 5%Pd@ Chitosan (0.05 g) | ACN | 59 | 4.0 | 38 | 24 |
| 8 | K2CO3 (2) | 5%Pd@ Chitosan (0.05 g) | DMSO | 43 | 5.5 | 33 | 24 |
| 9 | K2CO3 (2) | 5%Pd@ Chitosan (0.05 g) | DCM | 26 | 6.0 | 17 | 36 |
| 10 | K2CO3 (2) | 5%Pd@ Chitosan (0.05 g) | THF | 41 | 5.5 | 33 | 24 |
| 11 | CH3COOK (2) | 5%Pd@ Chitosan (0.05 g) | DMF | 81 | 3.0 | 50 | 16 |
| 12 | Na2CO3 (2) | 5%Pd@ Chitosan (0.05 g) | DMF | 80 | 3.0 | 50 | 16 |
| 13 | K3PO4 (2) | 5%Pd@ Chitosan (0.05 g) | DMF | 79 | 3.0 | 50 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, M.M.M.; Saleh, T.S.; Bawaked, S.M.; Alghamdi, K.S.; Narasimharao, K. Efficient and Eco-Friendly Perspectives for C-H Arylation of Benzothiazole Utilizing Pd Nanoparticle-Decorated Chitosan. Catalysts 2022, 12, 1000. https://doi.org/10.3390/catal12091000
Mostafa MMM, Saleh TS, Bawaked SM, Alghamdi KS, Narasimharao K. Efficient and Eco-Friendly Perspectives for C-H Arylation of Benzothiazole Utilizing Pd Nanoparticle-Decorated Chitosan. Catalysts. 2022; 12(9):1000. https://doi.org/10.3390/catal12091000
Chicago/Turabian StyleMostafa, Mohamed Mokhtar M., Tamer S. Saleh, Salem M. Bawaked, Khadijah S. Alghamdi, and Katabathini Narasimharao. 2022. "Efficient and Eco-Friendly Perspectives for C-H Arylation of Benzothiazole Utilizing Pd Nanoparticle-Decorated Chitosan" Catalysts 12, no. 9: 1000. https://doi.org/10.3390/catal12091000
APA StyleMostafa, M. M. M., Saleh, T. S., Bawaked, S. M., Alghamdi, K. S., & Narasimharao, K. (2022). Efficient and Eco-Friendly Perspectives for C-H Arylation of Benzothiazole Utilizing Pd Nanoparticle-Decorated Chitosan. Catalysts, 12(9), 1000. https://doi.org/10.3390/catal12091000