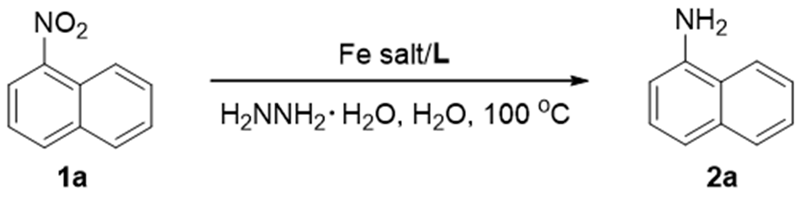
A Reusable FeCl3∙6H2O/Cationic 2,2′-Bipyridyl Catalytic System for Reduction of Nitroarenes in Water
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Experimental Method
3.2.1. General Procedure for Reduction of Nitroarenes
3.2.2. General Procedure for Catalyst Reuse Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Downing, R.S.; Kunkeler, P.J.; van Bekkum, H. Catalytic Syntheses of Aromatic Amines. Catal. Today 1997, 37, 121–136. [Google Scholar] [CrossRef]
- Vogt, P.F.; Gerulis, J.J. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Ono, N. The Nitro Group in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Hu, Z.-N.; Liang, J.; Ding, K.; Ai, Y.; Liang, Q.; Sun, H.-B. Insight into the Selectivity of Nano-Catalytic Nitroarenes Reduction over Other Active Groups by Exploring Hydrogen Sources and Metal Components. Appl. Catal. A Gen. 2021, 626, 118339. [Google Scholar] [CrossRef]
- Grieco, G.; Blacque, O. Microwave-Assisted Reduction of Aromatic Nitro Compounds with Novel Oxo-Rhenium Complexes. Appl. Organometal. Chem. 2022, 36, e6452. [Google Scholar] [CrossRef]
- Knifton, J.F. Homogeneous Catalyzed Reduction of Nitro Compounds. IV. Selective and Sequential Hydrogenation of Nitroaromatics. J. Org. Chem. 1976, 41, 1200–1206. [Google Scholar] [CrossRef]
- Pachisia, S.; Kishan, R.; Yadav, S.; Gupta, R. Half-Sandwich Ruthenium Complexes of Amide-Phosphine Based Ligands: H-Bonding Cavity Assisted Binding and Reduction of Nitro-substrates. Inorg. Chem. 2021, 60, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.N.; Prabha, P.S.; Bhuvanesh, N.S.P.; Karvembu, R. Tuning Acylthiourea Ligands in Ru(II) Catalysts for Altering the Reactivity and Chemoselectivity of Transfer Hydrogenation Reactions, and Synthesis of 3-Isopropoxy-1H-indole through a New Synthetic Approach. J. Organomet. Chem. 2020, 908, 121087. [Google Scholar] [CrossRef]
- Jia, W.-G.; Du, T.-T.; Gao, L.-L.; Du, J. Synthesis, Characterization, and Catalytic Activity of Half-Sandwich Ruthenium Complexes with Pyridine/Phenylene Bridged NHC=E (NHC = N-heterocyclic carbene, E. = S., Se) Ligands. Appl. Organometal. Chem. 2020, 34, e5651. [Google Scholar] [CrossRef]
- Toti, A.; Frediani, P.; Salvini, A.; Rosi, L.; Giolli, C. Hydrogenation of Single and Multiple N–N or N–O Bonds by Ru(II) Catalysts in Homogeneous Phase. J. Organomet. Chem. 2005, 690, 3641–3651. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.A.; Prashar, A.K.; Kinage, A.K.; Kumar, R.; Meijboom, R. Ru(II) Phenanthroline Complex as Catalyst for Chemoselective Hydrogenation of Nitro-Aryls in a Green Process. Ind. Eng. Chem. Res. 2010, 49, 12180–12184. [Google Scholar] [CrossRef]
- Schabel, T.; Belger, C.; Plietker, B. A Mild Chemoselective Ru-Catalyzed Reduction of Alkynes, Ketones, and Nitro Compounds. Org. Lett. 2013, 15, 2858–2861. [Google Scholar] [CrossRef]
- Paul, B.; Chakrabarti, K.; Shee, S.; Maji, M.; Mishra, A.; Kundu, S. A Simple and Efficient in situ Generated Ruthenium Catalyst for Chemoselective Transfer Hydrogenation of Nitroarenes: Kinetic and Mechanistic Studies and Comparison with Iridium Systems. RSC Adv. 2016, 6, 100532–100545. [Google Scholar] [CrossRef]
- Jia, W.-G.; Wang, Z.-B.; Zhi, X.-T. Half-sandwich Ruthenium Complexes with Schiff Base Ligands Bearing a Hydroxyl Group: Preparation, Characterization and Catalytic Activities. Appl. Organometal. Chem. 2020, 34, e5289. [Google Scholar] [CrossRef]
- Sarki, N.; Goyal, V.; Tyagi, N.K.; Puttaswamy; Narani, A.; Ray, A.; Natte, K. Simple RuCl3-catalyzed N-Methylation of Amines and Transfer Hydrogenation of Nitroarenes using Methanol. ChemCatChem 2021, 13, 1722–1729. [Google Scholar] [CrossRef]
- Namdeo, P.K.; Sheokand, S.; Kote, B.S.; Radhakrishna, L.; Kunchur, H.S.; Saini, P.; Ramakrishnan, S.; Balakrishna, M.S. RuII Complexes of 1,2,3-Triazole Appended Tertiary Phosphines, [P(Ph){(o-C6H4)(1,2,3-N3C(Ph)CH}2] and [P(Ph){o-C6H4(CCH)-(1,2,3-N3-Ph)}2]: Highly Active Catalysts for Transfer Hydrogenation of Carbonyl/Nitro Compounds and for α-Alkylation of Ketones. Dalton Trans. 2022, 51, 6795–6808. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.-G.; Zhang, H.; Zhang, T.; Xie, D.; Ling, S.; Sheng, E.-H. Half-Sandwich Ruthenium Complexes with Schiff Base Ligands: Syntheses, Characterization, and Catalytic Activities for the Reduction of Nitroarenes. Organometallics 2016, 35, 503–512. [Google Scholar] [CrossRef]
- Jia, W.-G.; Ling, S.; Zhang, H.-N.; Sheng, E.-H.; Lee, R. Half-Sandwich Ruthenium Phenolate−Oxazoline Complexes: Experimental and Theoretical Studies in Catalytic Transfer Hydrogenation of Nitroarene. Organometallics 2018, 37, 40–47. [Google Scholar] [CrossRef]
- Lin, S.-C.A.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Diruthenium Complex Catalyzed Reduction of Nitroarenes-Investigation of Reaction Pathway. Mol. Catal. 2019, 466, 46–51. [Google Scholar] [CrossRef]
- Harsy, S.G. Homogeneous Hydrogenation of Nitroaliphatic Compounds Catalyzed by Group VIII Transition Metal Phosphine Complexes. Tetrahedron 1990, 46, 7403–7412. [Google Scholar] [CrossRef]
- Chepaikin, E.G.; Khidekel’, M.L.; Ivanova, V.V.; Zakhariev, A.I.; Shopov, D.M. Homogeneous Catalytic Hydrogenation of Aromatic Nitrocompounds by Complexes of the Platinum Group Metal with Dyes. The reaction of Nitrobenzene with a Complex of Rhodium with the Anion-radical of Potassium Endigodisulfonate. J. Mol. Catal. 1981, 10, 115–119. [Google Scholar] [CrossRef]
- Verma, P.K.; Bala, M.; Thakur, K.; Sharma, U.; Kumar, N.; Singh, B. Iron and Palladium(II) Phthalocyanines as Recyclable Catalysts for Reduction of Nitroarenes. Catal. Lett. 2014, 144, 1258–1267. [Google Scholar] [CrossRef]
- Jia, W.-G.; Gao, L.-L.; Wang, Z.-B.; Sun, L.-Y.; Han, Y.-F. Synthesis, Characterization, and Catalytic Activities of Palladium Complexes with Phenylene-Bridged Bis(thione) Ligands. Organometallics 2019, 38, 1946–1954. [Google Scholar] [CrossRef]
- Yang, S.-T.; Shen, P.; Liao, B.-S.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Catalytic Reduction of Nitroarenes by Dipalladium Complexes: Synergistic Effect. Organometallic 2017, 36, 3110–3116. [Google Scholar] [CrossRef]
- Karami, K.; Mousavi, N.S. A Palladium Complex Immobilized onto a Magnetic GO-MnFe2O4 Surface as an Effective and Recyclable Catalyst for the Reduction of p-Nitrophenol. Dalton Trans. 2018, 47, 4175–4182. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liao, S.; Xu, Y.; Yang, B.; Yu, D. Hydrogenation of Nitroaromatics by Polymer-Anchored Bimetallic Palladium-Ruthenium and Palladium-Platinum Catalysts under Mild Conditions. J. Mol. Catal. A Chem. 1997, 120, 247–255. [Google Scholar] [CrossRef]
- Zakhariev, A.; Ivanova, V.; Khidekel, M.L.; Chepaikin, E.G.; Shopov, D. Hydrogenation of Aromatic Nitro Compounds in the Presence of the Platinum(II) Complex of 1-Phenylazo-2-naphthol in DMF. React. Kinet. Catal. Lett. 1978, 8, 195–201. [Google Scholar]
- Corma, A.; González-Arellano, C.; Iglesias, M.; Sánchez, F. Gold Complexes as Catalysts: Chemoselective Hydrogenation of Nitroarenes. Appl. Catal. A 2009, 356, 99–102. [Google Scholar] [CrossRef]
- Jia, G.-W.; Dai, Y.-C.; Zhang, H.-N.; Lu, X.; Sheng, E.-H. Synthesis and Characterization of Gold Complexes with Pyridine-Based SNS Ligands and as Homogeneous Catalysts for Reduction of 4-Nitrophenol. RSC Adv. 2015, 5, 29491–29496. [Google Scholar] [CrossRef]
- Sabater, S.; Mata, J.A.; Peris, E. Dual Catalysis with an IrIII–AuI Heterodimetallic Complex: Reduction of Nitroarenes by Transfer Hydrogenation using Primary Alcohols. Chem. Eur. J. 2012, 18, 6380–6385. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, C.; Cong, X.; Deng, G.; Liu, L.L.; Luo, M.; Zeng, X. Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2021, 143, 1618–1629. [Google Scholar] [CrossRef]
- Zubar, V.; Dewanji, A.; Rueping, M. Chemoselective Hydrogenation of Nitroarenes Using an Air-Stable Base-Metal Catalyst. Org. Lett. 2021, 23, 2742–2747. [Google Scholar] [CrossRef]
- Ioannou, D.I.; Gioftsidou, D.K.; Tsina, V.E.; Kallitsakis, M.G.; Hatzidimitrious, A.G.; Terzidis, M.A.; Angaridis, P.A.; Lykakis, I.N. Selective Reduction of Nitroarenes to Arylamines by the Cooperative Action of Methylhydrazine and a Tris(N-heterocyclic thioamidate) Cobalt(III) Complex. J. Org. Chem. 2021, 86, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Dagur, M.S.; Ali, A.; Gupta, R. Trinuclear {Co2+–M3+–Co2+} Complexes Catalyze Reduction of Nitro Compounds. Dalton Trans. 2015, 44, 17453–17461. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Kumar, P.; Kumar, N.; Kumar, V.; Singh, B. Highly Chemo- and Regioselective Reduction of Aromatic Nitro Compounds Catalyzed by Recyclable Copper(II) as well as Cobalt(II) Phthalocyanines. Adv. Synth. Catal. 2010, 352, 1834–1840. [Google Scholar] [CrossRef]
- Murugesan, K.; Wei, Z.; Chandrashekhar, V.G.; Jiao, H.; Beller, M.; Jagadeesh, R.V. General and Selective Synthesis of Primary Amines Using Ni-Based Homogeneous Catalysts. Chem. Sci. 2020, 11, 4332–4339. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wang, Y.; Zhou, Y.; Yao, Z.-J. N,N-Chelate Nickel(II) Complexes Bearing Schiff Base Ligands as Efficient Hydrogenation Catalysts for Amine Synthesis. J. Organomet. Chem. 2020, 959, 122187. [Google Scholar] [CrossRef]
- Jia, W.-G.; Li, M.; Zhi, X.-T.; Gao, L.-L.; Du, J. Copper(II) Complex with Oxazoline Ligand: Synthesis, Structures and Catalytic Activity for Nitro Compounds Reduction. J. Mol. Struct. 2020, 1217, 128349. [Google Scholar] [CrossRef]
- Sharma, U.; Kumar, N.; Verma, P.K.; Kumar, V.; Singh, B. Zinc Phthalocyanine with PEG-400 as a Recyclable Catalytic System for Selective Reduction of Aromatic Nitro Compounds. Green Chem. 2012, 14, 2289–2293. [Google Scholar] [CrossRef]
- Begum, R.; Rehan, R.; Farooqi, Z.H.; Butt, Z.; Ashraf, S. Physical Chemistry of Catalytic Reduction of Nitroarenes Using Various Nanocatalytic Systems: Past, Present, and Future. J. Nanopart. Res. 2016, 18, 231. [Google Scholar] [CrossRef]
- Romero, A.H. Reduction of Nitroarenes via Catalytic Transfer Hydrogenation Using Formic Acid as Hydrogen Source: A Comprehensive Review. ChemistrySelect 2020, 5, 13054–13075. [Google Scholar] [CrossRef]
- Hoseini, C.; Seysde, M.; Asadi, S.; Heravi, M.M. Application of Bimetallic and Trimetallic Nanoparticles Supported on Graphene as novel Heterogeneous Catalysts in the Reduction of Nitroarenes, Homo-coupling, Suzuki-Miyaura and Sonogashira Reactions. Curr. Org. Chem. 2020, 24, 2216–2234. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene Reduction: A Trusted Model Reaction to Test Nanoparticle Catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- Kadam, H.K.; Tilve, S.G. Advancement in Methodologies for Reduction of Nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on Selective Hydrogenation of Nitroarene by Catalytic, Photocatalytic and Electrocatalytic Reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Najeeb, J.; Sahrif, A.; Intisar, A.; Ahmed, E. Critical Review on the Chemical Reduction of Nitroaniline. RSC Adv. 2020, 10, 19041–19058. [Google Scholar] [CrossRef]
- Sedghi, R.; Heravi, M.M.; Asadi, S.; Nazari, N.; Nabid, M.R. Recently Used Nanocatalysts in Reduction of Nitroarenes. Curr. Org. Chem. 2016, 20, 696–734. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal−Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Amirmahani, N.; Rashidi, M.; Mahmoodi, N.O. Synthetic Application of Gold Complexes on Magnetic Supports. Appl. Organometal. Chem. 2020, 34, e5626. [Google Scholar] [CrossRef]
- Kottappara, R.; Pillai, S.C.; Vijayan, B.K. Copper-Based Nanocatalysts for Nitroarene Reduction-A Review of Recent Advances. Inorg. Chem. Commun. 2020, 121, 108181. [Google Scholar] [CrossRef]
- Lu, G.; Sun, K.; Lin, Y.; Du, Q.; Zhang, J.; Wang, K.; Wang, P. Single-Atomic-Site Iron on N-Doped Carbon for Chemoselective Reduction of Nitroarenes. Nano Res. 2022, 15, 603–611. [Google Scholar] [CrossRef]
- Tian, S.; Hu, M.; Xu, Q.; Gong, W.; Chen, W.; Yang, J.; Zhu, Y.; Chen, C.; He, J.; Liu, Q.; et al. Single-Atom Fe with Fe1N3 Structure Showing Superior Performances for both Hydrogenation and Transfer Hydrogenation of Nitrobenzene. Sci. China Mater. 2021, 64, 642–650. [Google Scholar] [CrossRef]
- Yun, R.; Zhan, F.; Li, N.; Zhang, B.; Ma, W.; Hong, L.; Sheng, T.; Du, L.; Zheng, B.; Liu, S. Fe Single Atoms and Fe2O3 Clusters Liberated from N-Doped Polyhedral Carbon for Chemoselective Hydrogenation under Mild Conditions. ACS Appl. Mater. Interfaces 2020, 12, 34122–34129. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Li, Z.; Gao, G.; Sun, P.; Wang, J.; Zhang, B.; Zhong, J.; Jiang, Z.; Li, F. Graphitic Phosphorus Coordinated Single Fe Atoms for Hydrogenative Transformations. Nat. Commun. 2020, 11, 4074. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.-C.; Yang, W.; Zhang, J.; Li, Y.; Zhao, D.; Liu, S.; Wu, K.; Liu, Q.; Zhang, C.; Wang, D.; et al. Isolated Iron Single-Atomic Site-Catalyzed Chemoselective Transfer Hydrogenation of Nitroarenes to Arylamines. ACS Appl. Mater. Interfaces 2019, 11, 33819–33824. [Google Scholar] [CrossRef] [PubMed]
- Ramadas, K.; Srinivasan, N. Iron-Ammonium Chloride—A Convenient and Inexpensive Reductant. Synth. Commun. 1992, 22, 3189–3195. [Google Scholar] [CrossRef]
- Chandrappa, S.; Vinaya, K.; Ramakrishnappa, T.; Rangappa, K.S. An Efficient Method for Aryl Nitro Reduction and Cleavage of Azo Compounds Using Iron Powder/Calcium Chloride. Synlett 2010, 3019–3022. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Hua, W. Synthesis and Characterization of Fully Conjugated Schiff Base Macrocycles Containing 1,3,4-Oxadiazole Moiety. Synth. Commun. 2002, 32, 3339–3345. [Google Scholar] [CrossRef]
- Xiao, Z.-P.; Wang, Y.-C.; Du, G.-Y.; Wu, J.; Luo, T.; Yi, S.-F. Efficient Reducing System Based on Iron for Conversion of Nitroarenes to Anilines. Synth. Commun. 2010, 40, 661–665. [Google Scholar] [CrossRef]
- Gamble, A.B.; Garner, J.; Gordon, C.P.; O’Conner, S.M.J.; Keller, P.A. Aryl Nitro Reduction with Iron Powder or Stannous Chloride under Ultrasonic Irradiation. Synth. Commun. 2007, 37, 2777–2786. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Wu, Z.; Yan, J.; Wang, M.; Ding, Y. Reduction of Nitroarenes to Aromatic Amines with Nanosized Activated Metallic Iron Powder in Water. Synthesis 2003, 2001–2004. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Prashad, M.; Repič, O.; Blacklock, T.J. A Practical and Chemoselective Reduction of Nitroarenes to Anilines Using Activated Iron. Adv. Synth. Catal. 2005, 347, 217–219. [Google Scholar] [CrossRef]
- Boothroyd, S.R.; Kerr, M.A. A Mild and Efficient Method for the Preparation of Anilines from Nitroarenes. Tetrahedron Lett. 1995, 36, 2411–2414. [Google Scholar] [CrossRef]
- Desai, D.G.; Swami, S.S.; Hapase, S.B. Rapid and Inexpensive Method for Reduction of Nitroarenes to Anilines. Synth. Commun. 1999, 29, 1033–1036. [Google Scholar] [CrossRef]
- Pehlivan, L.; Métay, E.; Laval, S.; Dayoub, W.; Demonchaux, P.; Mignani, G.; Lemaire, M. Iron-Catalyzed Selective Reduction of Nitro Compounds to Amines. Tetrahedron Lett. 2010, 51, 1939–1941. [Google Scholar] [CrossRef]
- Morse, J.R.; Callejas, J.F.; Darling, A.J.; Schaak, R.E. Bulk Iron Pyrite as a Catalyst for the Selective Hydrogenation of Nitroarenes. Chem. Commun. 2017, 53, 4807–4810. [Google Scholar] [CrossRef]
- MacNair, A.J.; Tran, M.-M.; Nelson, J.E.; Sloan, G.U.; Ironmonger, A.; Thomas, S.P. Iron-Catalysed, General and Operationally Simple Formal Hydrogenation Using Fe(OTf)3 and NaBH4. Org. Biomol. Chem. 2014, 12, 5082–5088. [Google Scholar] [CrossRef] [Green Version]
- Desai, D.G.; Swami, S.S.; Dabhade, S.K.; Ghagare, M.G. FeS-NH4Cl-CH3OH-H2O: An Efficient and Inexpensive System for Reduction of Nitroarenes to Anilines. Synth. Commun. 2001, 31, 1249–1251. [Google Scholar] [CrossRef]
- Yoo, B.W.; Choi, J.W.; Hwang, S.K.; Kim, D.Y.; Baek, H.S.; Choi, K.I.; Kim, J.H. A Facile Reduction of Nitroarenes to Anilines Using FeCl3·6H2O/Indium. Synth. Commun. 2003, 33, 2985–2988. [Google Scholar] [CrossRef]
- Deshpande, R.M.; Mahajan, A.N.; Diwakar, M.M.; Ozarde, P.S.; Chaudhari, R.V. Chemoselective Hydrogenation of Substituted Nitroaromatics Using Novel Water-Soluble Iron Complex Catalysts. J. Org. Chem. 2004, 69, 4835–4838. [Google Scholar] [CrossRef]
- Junge, K.; Wendt, B.; Shaikh, N.; Beller, M. Iron-Catalyzed Selective Reduction of Nitroarenes to Anilines Using Organosilanes. Chem. Commun. 2010, 46, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Verma, P.K.; Kumar, N.; Kumar, V.; Bala, M.; Singh, B. Phosphane-Free Green Protocol for Selective Nitro Reduction with an Iron-Based Catalyst. Chem. Eur. J. 2011, 17, 5903–5907. [Google Scholar] [CrossRef]
- Wienhöfer, G.; Sorribes, I.; Boddien, A.; Westerhaus, F.; Junge, K.; Junge, H.; Llusar, R.; Beller, M. General and Selective Iron-Catalyzed Transfer Hydrogenation of Nitroarenes without Base. J. Am. Chem. Soc. 2011, 133, 12875–12879. [Google Scholar] [CrossRef] [PubMed]
- Wienhöfer, G.; Baseda-Krüger, M.; Ziebart, C.; Westerhaus, F.A.; Baumann, W.; Jackstell, R.; Junge, K.; Beller, M. Hydrogenation of Nitroarenes Using Defined Iron–Phosphine Catalysts. Chem. Commun. 2013, 49, 9089–9091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Shaver, M.P.; Thomas, S.P. Chemoselective Nitro Reduction and Hydroamination Using a Single Iron Catalyst. Chem. Sci. 2016, 7, 3031–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Tongdee, S.; Ammaiyappan, Y.; Darcel, C. A Concise Route to Cyclic Amines from Nitroarenes and Ketoacids under Iron-Catalyzed Hydrosilylation Conditions. Adv. Synth. Catal. 2021, 363, 3859–3865. [Google Scholar] [CrossRef]
- Patil, N.M.; Sasaki, T.; Bhanage, B.M. Immobilized Iron Metal-Containing Ionic Liquid-Catalyzed Chemoselective Transfer Hydrogenation of Nitroarenes into Anilines. ACS Sustain. Chem. Eng. 2016, 4, 429–436. [Google Scholar] [CrossRef]
- Sakaki, S.; Mitarai, S.; Ohkubo, K. New Reduction Catalysis of Metalloporphyrins. Catalytic Reduction of Nitrobenzene to Aniline with Tetraphenylporphyrinato-iron(III) Chloride−NaBH4 System. Chem. Lett. 1991, 20, 195–198. [Google Scholar] [CrossRef]
- Wilkinson, H.S.; Tanoury, G.J.; Wald, S.A.; Senanayake, C.H. Chemoselective Reductions of Nitroarenes: Bromoethanol Assisted Phthalocyanatoiron/NaBH4 Reductions. Tetrahedron Lett. 2001, 42, 167–170. [Google Scholar] [CrossRef]
- Dey, R.; Mukherjee, N.; Ahammed, S.; Ranu, B.C. Highly Selective Reduction of Nitroarenes by Iron(0) Nanoparticles in Water. Chem. Commun. 2012, 48, 7982–7984. [Google Scholar] [CrossRef]
- Lee, N.R.; Bikovtseva, A.A.; Cortes-Clerget, M.; Gallou, F.; Lipshutz, B.H. Carbonyl Iron Powder: A Reagent for Nitro Group Reductions under Aqueous Micellar Catalysis Conditions. Org. Lett. 2017, 19, 6518–6521. [Google Scholar] [CrossRef]
- He, S.; Niu, H.; Zeng, T.; Wang, S.; Cai, Y. A Facile and Efficient Method for Continuous Reduction of Nitroaromatic Compounds Through the Cyclic Transformation Between Fe(II)-complexes and Nano Zero–valent Iron. ChemistrySelect 2016, 1, 2821–2825. [Google Scholar] [CrossRef]
- Sharma, C.; Srivastava, A.K.; Soni, A.; Kumari, S.; Joshi, R.K. CO-Free, Aqueous Mediated, Instant and Selective Reduction of Nitrobenzene via Robustly Stable Chalcogen Stabilised Iron Carbonyl Clusters (Fe3E2(CO)9, E. = S, Se, Te). RSC Adv. 2020, 10, 32516–32521. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Wang, J.-C.; Tsai, F.-Y. A Reusable FeCl3·6H2O/Cationic 2,2′-Bipyridyl Catalytic System for the Coupling of Aryl Iodides with Thiols in Water under Aerobic Conditions. Green Chem. 2009, 11, 326–329. [Google Scholar] [CrossRef]
- Hung, T.-T.; Huang, C.-M.; Tsai, F.-Y. Sonogashira–Hagihara Coupling towards Diaryl Alkynes Catalyzed by FeCl3·6H2O/Cationic 2,2’-Bipyridyl. ChemCatChem 2012, 4, 540–545. [Google Scholar] [CrossRef]
- Huang, C.-M.; Peng, W.-S.; Liu, L.-J.; Wu, C.-C.; Tsai, F.-Y. Iron-Catalyzed Conjugate Addition of Aryl Iodides onto Activated Alkenes under Air in Water. Catalysts 2020, 10, 1320. [Google Scholar] [CrossRef]
- Furst, A.; Berlo, R.C.; Hooton, S. Hydrazine as a Reducing Agent for Organic Compounds (Catalytic Hydrazine Reductions). Chem. Rev. 1965, 65, 51–68. [Google Scholar] [CrossRef]
- Handa, S.; Wang, Y.; Gallou, F.; Lipshutz, B.H. Sustainable Fe–ppm Pd Nanoparticle Catalysis of Suzuki-Miyaura Cross-Couplings in Water. Science 2015, 349, 1087–1091. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Handa, S.; Gallou, F.; Lipshutz, B.H. Safe and Selective Nitro Group Reductions Catalyzed by Sustainable and Recyclable Fe/ppm Pd Nanoparticles in Water at Room Temperature. Angew. Chem. Int. Ed. 2016, 55, 8979–8983. [Google Scholar] [CrossRef]
- Gabriel, C.M.; Parmentier, M.; Riegert, C.; Lanz, M.; Handa, S.; Lipshutz, B.H.; Gallou, F. Sustainable and Scalable Fe/ppm Pd Nanoparticle Nitro Group Reductions in Water at Room Temperature. Org. Process. Res. Dev. 2017, 21, 247–252. [Google Scholar] [CrossRef]
- Kantam, M.L.; Bandyopadhyay, T.; Rahman, A.; Reddy, N.M.; Choudary, B.M. Reduction of Nitroaromatics with a New Heterogenised MCM–Silylamine Palladium (II) Catalyst. J. Mol. Catal. A Chem. 1998, 133, 293–295. [Google Scholar] [CrossRef]
- Rahaim, R.J.; Maleczka, R.E. Pd-Catalyzed Silicon Hydride Reductions of Aromatic and Aliphatic Nitro Groups. Org. Lett. 2005, 7, 5087–5090. [Google Scholar] [CrossRef]
- Rahaim, R.J.; Maleczka, R.E. Palladium-Catalyzed Silane/Siloxane Reductions in the One-Pot Conversion of Nitro Compounds into Their Amines, Hydroxylamines, Amides, Sulfon amides, and Carbamates. Synthesis 2006, 3316–3340. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Motoshima, K.; Ishikawa, M.; Hashimoto, Y.; Sugita, K. Peroxisome Proliferator-Activated Receptor Agonists with Phenethylphenylph Thalimide Skeleton Derived from Thalidomide-Related Liver X Receptor Antagonists: Relationship Between Absolute Configuration and Subtype Selectivity. Bioorg. Med. Chem. 2011, 19, 3156–3172. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Shendage, S.S.; Nagarkar, J.M. Choline Chloride Based Deep Eutectic Solvent as an Efficient Solvent for the Benzylation of Phenols. Tetrahedron Lett. 2014, 55, 7243–7246. [Google Scholar] [CrossRef]
- Xia, J.-H.; Jiang, Y.; Gong, S.-M.; Sun, Z.; Wang, Y.-H. Effects of Side Chains with Similar Lengths and Different Structures of Polyimides on Liquid Crystal Alignment Behavior. Chin. J. Polym. Sci. 2014, 32, 1610–1619. [Google Scholar] [CrossRef]
- Ramesh, P.; Fadnavis, N.W. Ammonium Nitrate: A Biodegradable and Efficient Catalyst for the Direct Amidation of Esters under Solvent-free Conditions. Chem. Lett. 2015, 44, 138–140. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, J.-R.; Tsai, F.-Y. Palladium(II)/Cationic 2,2’-Bipyridyl System as a Highly Efficient and Reusable Catalyst for the Mizoroki-Heck Reaction in Water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Hiyama Reaction of Aryl Bromides with Arylsiloxanes Catalyzed by a Reusable Palladium(II)/Cationic Bipyridyl System in Water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]

| Type of Iron Catalyst | H2 Source | Solvent | Temp. (°C) | Ref. |
|---|---|---|---|---|
| Iron single atom site | ||||
| FeSA@NC-20A (0.42 mol%) | N2H4·H2O (3 equiv) | EtOH | rt | [53] |
| Fe1/N-C | H2 (5 bar) | iPrOH | 160 | [54] |
| FeSAs/Fe2O3ACs/NPC | N2H4·H2O (40 equiv) | EtOH | rt | [55] |
| Fe-P900-PCC | H2 (4 Mpa) | Heptane | 150 | [56] |
| Fe1/N−C | N2H4·H2O (5 equiv) | EtOH | 60 | [57] |
| Iron powder (stoichiometric or excess) | ||||
| Fe/NH4Cl | MeOH/H2O | Reflux | [58] | |
| Fe/CaCl2 | EtOH/H2O | 60 | [59] | |
| Fe/HCl | EtOH | 70 | [60] | |
| Fe/NH4Cl | H2O/Acetone | Reflux | [61] | |
| Fe/AcOH | EtOH/H2O | Sonication | [62] | |
| Activated Fe | H2O | 210 | [63] | |
| Fe/HCl | EtOH/H2O | 65 | [64] | |
| Iron salts | ||||
| FeCl3∙6H2O (1.33 mol%) | H2NNMe2 (10.5 equiv) | MeOH | Reflux | [65] |
| FeCl3∙6H2O (3 equiv)/Zn | DMF/H2O | 100 | [66] | |
| Fe(acac)3 (10 mol%) | TMDS (4 equiv) | THF | 60 | [67] |
| FeS2 (0.83 equiv) | H2 (50 bar) | THF/H2O | 120 | [68] |
| Fe(OTf)3 (10 mol%) | NaBH4 (20 equiv) | EtOH | rt | [69] |
| FeS (5 equiv)/NH4Cl | MeOH/H2O | Reflux | [70] | |
| FeCl3∙6H2O (1 equiv)/In | MeOH/H2O | Sonication | [71] | |
| Iron complex | ||||
| Fe(CO)3(PPh3)2 (0.5 mol%) or Fe(CO)3(AsPh3)2 (0.5 mol%) | H2 (80 atm) | C6H6/EtOH | 125 | [6] |
| FeSO4∙7H2O/Na2EDTA (0.075 mol%) | H2 (400 psi) | CH3C6H5/H2O | 150 | [72] |
| FeBr2/PPh3 (10 mol%) | PhSiH3 (2.5 equiv) | CH3C6H5 | 110 | [73] |
| FePc/FeSO4·7H2O (0.5 mol%) | N2H4·H2O (2 equiv) | H2O/EtOH | 120 | [74] |
| Fe(BF4)2 6H2O/PP3 (4 mol%) | HCO2H (4.5 equiv) | EtOH | 40 | [75] |
| [FeF(PP3)][BF4] (2 mol%) | H2 (20 bar) | t-AmOH | 120 | [76] |
| Fe(III)(Furf) (2 mol%) | HSi(OEt)3 (4 equiv) | CH3CN | 80 | [77] |
| Fe(CO)4(IMes) (5 mol%) | PhSiH3 (3 equiv) | CH3C6H5 | 90, hν | [78] |
| ImmFe-IL (3 mol%) | N2H4·H2O (3 equiv) | Ethylene glycol | 110 | [79] |
| (TPP)Fe(III)Cl (0.06 mol%) | NaBH4 (1.6 equiv) | Diglyme | 30 | [80] |
| PcFe(II) (2 mol%) | NaBH4 (2 equiv) | Diglyme | rt | [81] |
| Carbonyl iron powder (5 equiv) | NH4Cl (3 equiv) | H2O | 45 | [83] |
| FeSO4/Citrate (1 mol%) | NaBH4 (400 equiv) | H2O | rt | [84] |
| Fe3Se2(CO)9 (3 mol%) | N2H4·H2O (2 equiv) | H2O | 110 | [85] |
| This work | ||||
| FeCl3∙6H2O/Cationic 2,2′- bipyridyl (1–2 mol%) | N2H4·H2O (4 equiv) | H2O | 100 | |
 | |||
|---|---|---|---|
| Entry | Iron Salt | Duration (h) | Yield (%) b |
| 1 | FeCl2∙4H2O | 6 | 34 |
| 2 | FeBr2 | 6 | 35 |
| 3 | FeC2O4∙2H2O | 6 | 33 |
| 4 | FeSO4∙7H2O | 6 | 11 |
| 5 | FeBr3 | 6 | 32 |
| 6 | Fe2O3∙2H2O | 6 | 0 |
| 7 | FeCl3∙6H2O | 6 | 69 |
| 8 | none | 6 | 0 |
| 9 | FeCl3∙6H2O | 12 | 98 |
| 10 | FeCl3∙6H2O | 24 | 98 |
| 11 c | FeCl3∙6H2O | 24 | 73 |
| 12 d | FeCl3∙6H2O | 12 | 41 |
| 13 e | FeCl3∙6H2O | 12 | 18 |
| 14 f | FeCl3∙6H2O | 12 | 43 |
| 15 g | FeCl3 | 12 | 98 |
| 16 h | FeCl3∙6H2O | 12 | 0 |
| 17 i | FeCl3∙6H2O | 12 | 0 |
| 18 j | FeCl3∙6H2O | 12 | 97 |
| Entry | Nitroarene | Product | Yield (%) b |
|---|---|---|---|
| 1 | 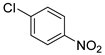 1b 1b | 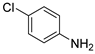 2b 2b | 99 |
| 2 | 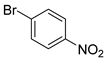 1c 1c | 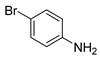 2c 2c | 97 |
| 3 | 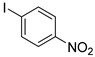 1d 1d | 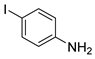 2d 2d | 97 |
| 4 | 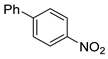 1e 1e | 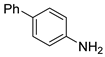 2e 2e | 83 |
| 5 |  1f 1f | 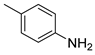 2f 2f | 92 |
| 6 c | 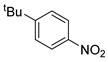 1g 1g |  2g 2g | 99 |
| 7 c | 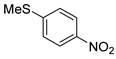 1h 1h | 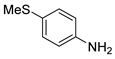 2h 2h | 88 |
| 8 c |  1i 1i | 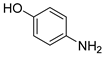 2i 2i | 72 |
| 9 c |  1j 1j | 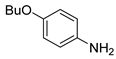 2j 2j | 78 |
| 10 c | 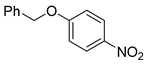 1k 1k | 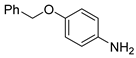 2k 2k | 70 |
| 11 c | 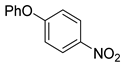 1l 1l | 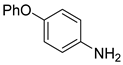 2l 2l | 89 |
| 12 | 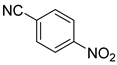 1m 1m | 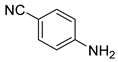 2m 2m | 90 |
| 13 | 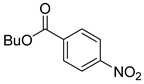 1n 1n |  2n 2n | 86 |
| 14 | 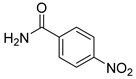 1o 1o | 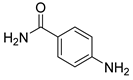 2o 2o | 92 |
| 15 | 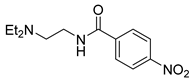 1p 1p | 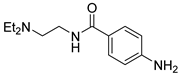 2p 2p | 98 |
| 16 c | 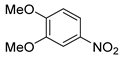 1q 1q | 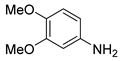 2q 2q | 95 |
| 17 | 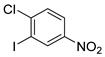 1r 1r | 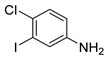 2r 2r | 95 |
| 18 |  1s 1s | 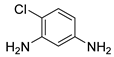 2s 2s | 95 |
| 19 | 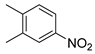 1t 1t | 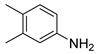 2t 2t | 86 |
| 20 |  1u 1u | 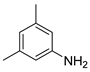 2u 2u | 96 |
| 21 c | 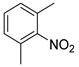 1v 1v | 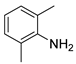 2v 2v | 49 |
| 22 | 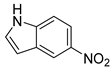 1w 1w | 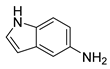 2w 2w | 82 |
| 23 | 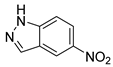 1x 1x |  2x 2x | 93 |
| 24 | 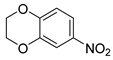 1y 1y | 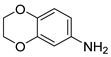 2y 2y | 95 |
| 25 |  1z 1z | 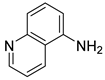 2z 2z | 92 |
| 26 | 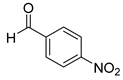 3a 3a |  4a 4a | 83 |
| 27 | 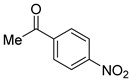 3b 3b | 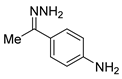 4b 4b | 90 |
| 28 | 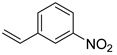 3c 3c | 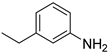 4cʹ 4cʹ | 79 |
| 29 | 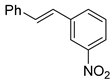 3d 3d | 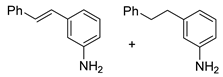 4d + 4d′ 4d + 4d′ | 77 d |
| 30 e |  5a 5a |  6a 6a | 80 |
| 31 e | 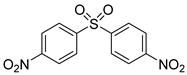 5b 5b | 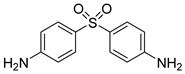 6b 6b | 87 |
 | |||||
|---|---|---|---|---|---|
| Entry | Product | Isolated Yield (%) | |||
| Initial Run | 1st Reuse Run | 2nd Reuse Run | 3rd Reuse Run | ||
| 1 a | 2a | 98 | 94 | 87 | 80 |
| 2 b | 2h | 99 | 93 | 86 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, T.-Y.; Peng, W.-S.; Tang, J.-W.; Tsai, F.-Y. A Reusable FeCl3∙6H2O/Cationic 2,2′-Bipyridyl Catalytic System for Reduction of Nitroarenes in Water. Catalysts 2022, 12, 924. https://doi.org/10.3390/catal12080924
Hung T-Y, Peng W-S, Tang J-W, Tsai F-Y. A Reusable FeCl3∙6H2O/Cationic 2,2′-Bipyridyl Catalytic System for Reduction of Nitroarenes in Water. Catalysts. 2022; 12(8):924. https://doi.org/10.3390/catal12080924
Chicago/Turabian StyleHung, Tsai-Yu, Wen-Sheng Peng, Jing-Wen Tang, and Fu-Yu Tsai. 2022. "A Reusable FeCl3∙6H2O/Cationic 2,2′-Bipyridyl Catalytic System for Reduction of Nitroarenes in Water" Catalysts 12, no. 8: 924. https://doi.org/10.3390/catal12080924
APA StyleHung, T.-Y., Peng, W.-S., Tang, J.-W., & Tsai, F.-Y. (2022). A Reusable FeCl3∙6H2O/Cationic 2,2′-Bipyridyl Catalytic System for Reduction of Nitroarenes in Water. Catalysts, 12(8), 924. https://doi.org/10.3390/catal12080924







