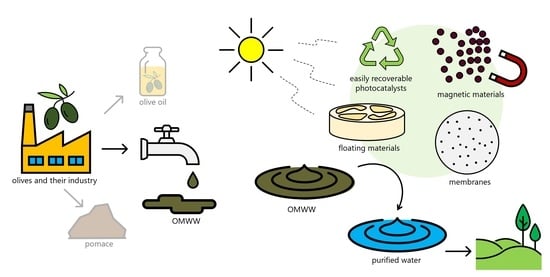
Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials
Abstract
:1. Introduction
2. An Insight into the EU Legislation
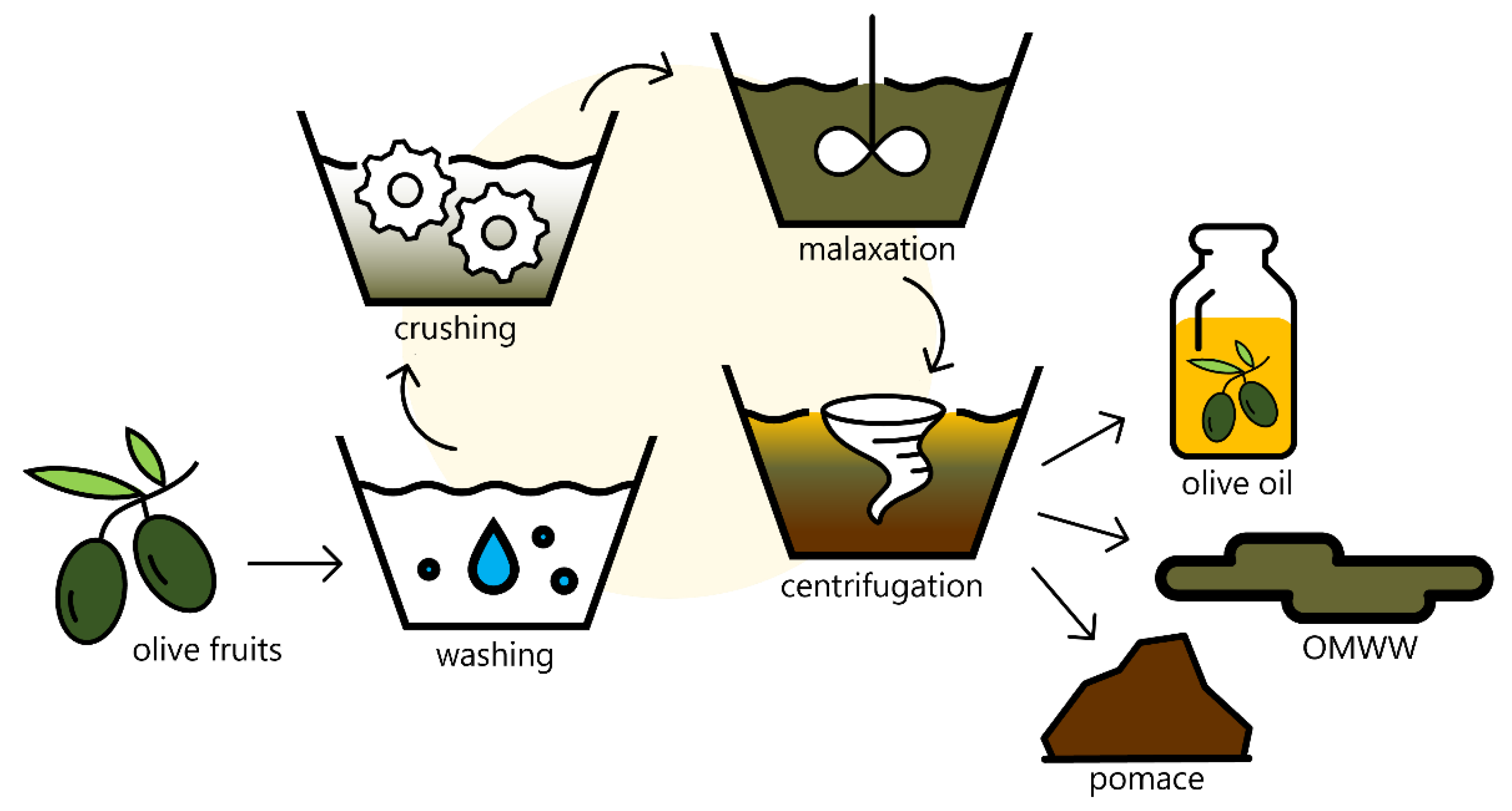
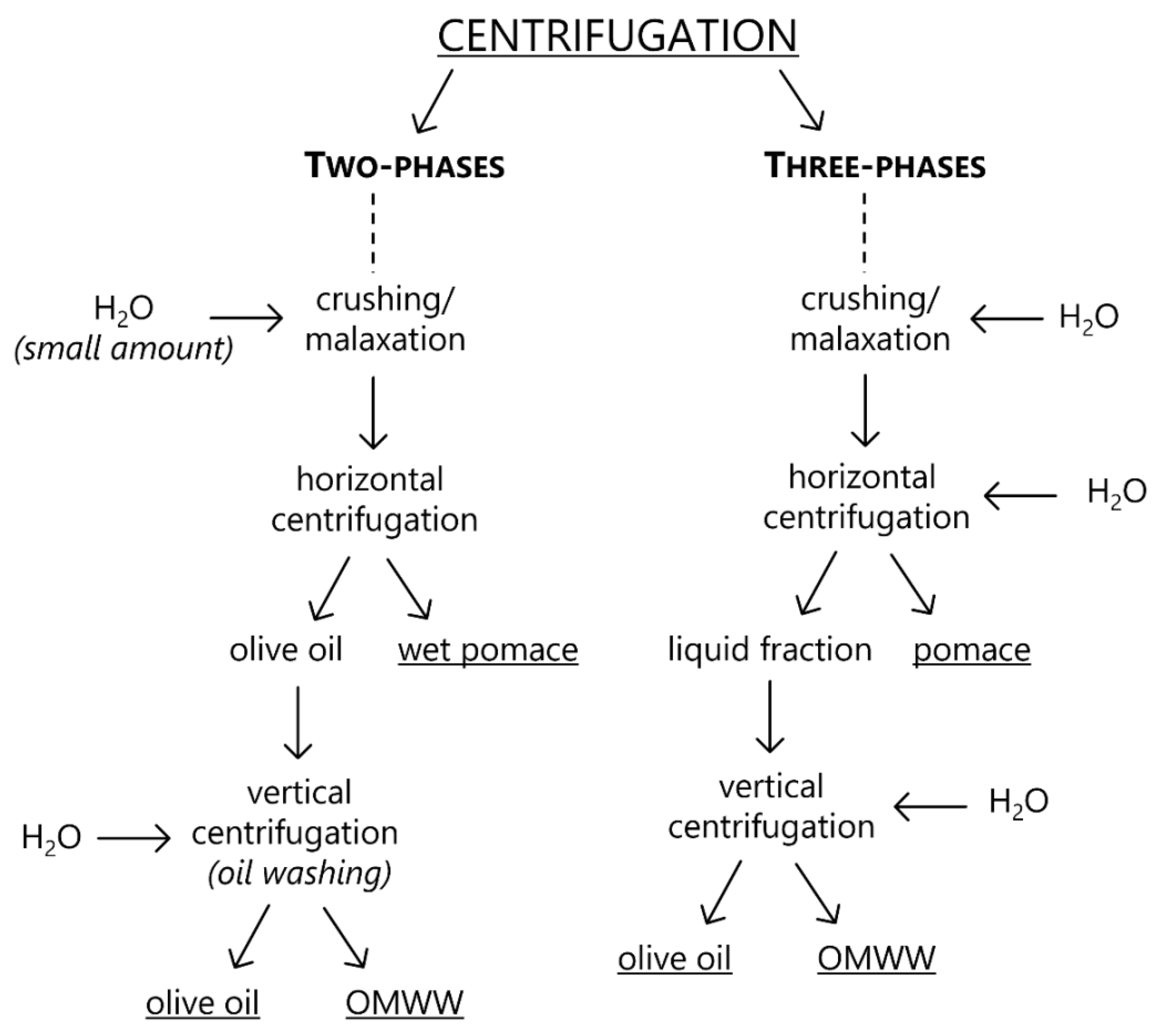
3. Emerging Innovative Approaches for Olive Oil Production
4. Olive Mill Wastewater Treatment
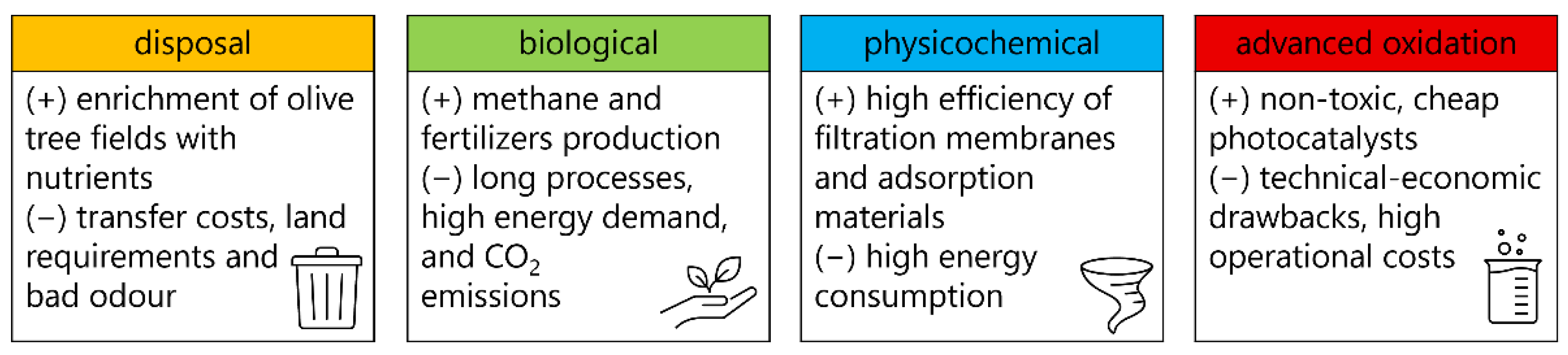
4.1. Disposal Methods
4.2. Biological Methods
4.3. Physicochemical Treatments
4.4. Advanced Oxidation Methods
4.4.1. Photocatalytic Treatments
UV Photocatalysis
Visible/Solar Photocatalysis
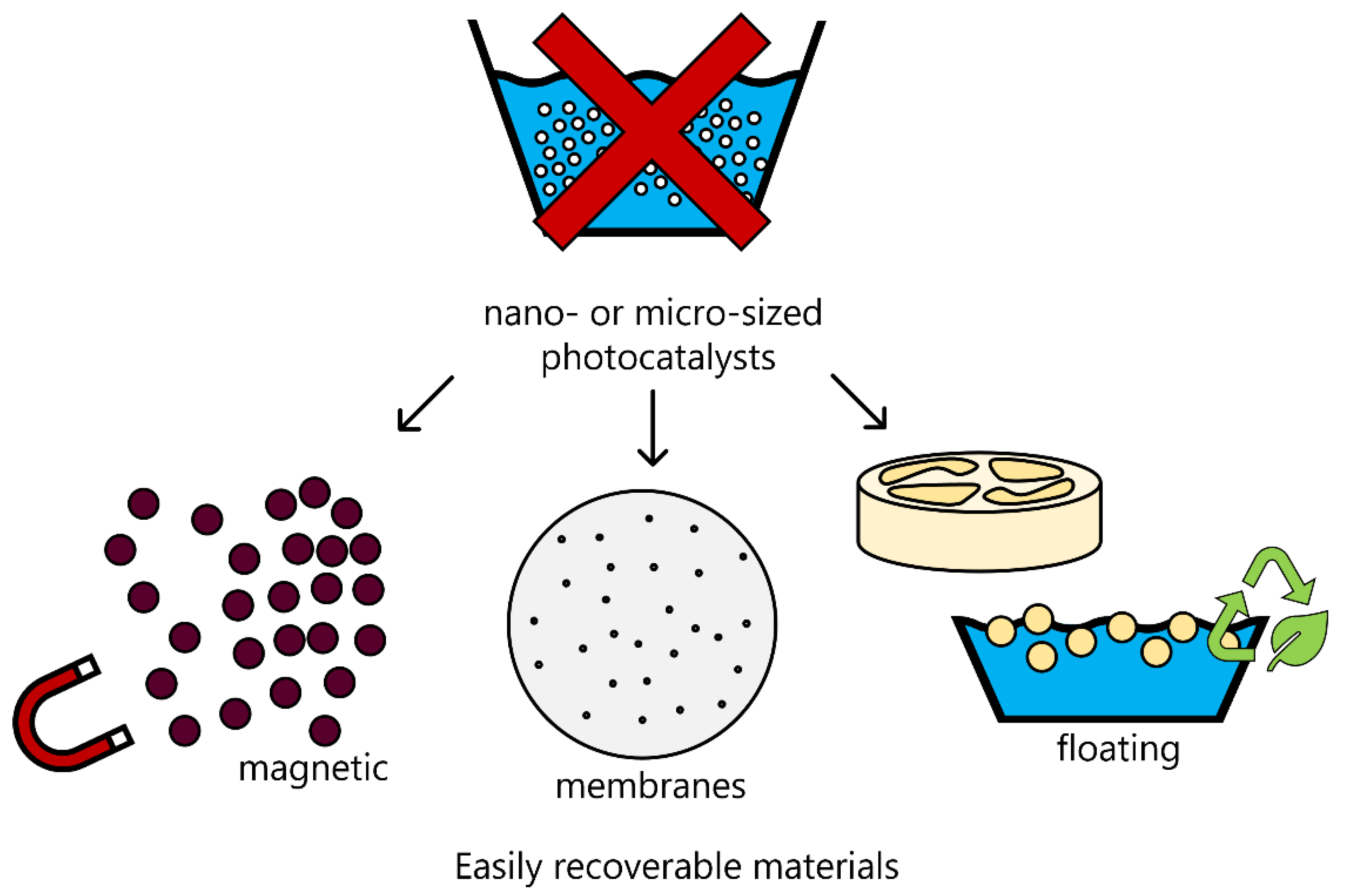
5. From Conventional to Easily Recoverable Magnetic Photocatalysts
6. Perspectives
6.1. Eco-Friendly Materials Used to Treat “Model Pollutants”
6.2. Other Emerging Eco-Friendly Materials (Floating Devices, Membranes)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ducom, G.; Gautier, M.; Pietraccini, M.; Tagutchou, J.P.; Lebouil, D.; Gourdon, M. Comparative analyses of three olive mill solid residues from different countries and processes for energy recovery by gasification. Renew. Energy 2020, 145, 180–189. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Moreno, J.L.; Doula, M. Olive oil waste management EU Legislation: Current situation and policy recommendations. Int. J. Chem. Environ. Eng. Syst. 2012, 3, 65–77. [Google Scholar]
- Mercé Sole, M.; Pons, L.; Conde, M.; Gaidau, C.; Baccardit, A. Characterization of Wet Olive Pomace Waste as Bio Based Resource for Leather Tanning. Materials 2021, 14, 5790. [Google Scholar] [CrossRef] [PubMed]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Bio/Technol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Azaizeh, H.; Abu Tayeh, H.N.; Gerchman, Y. Chapter 2: Valorisation of olive oil indus-try solid waste and production of ethanol and high value-added biomolecules. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–40. ISBN 9780128179512. [Google Scholar]
- Paredes, C.; Cegarra, J.; Roig, A.; Sanchez-Monedero, M.A.; Bernal, M.P. Characterisation of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Potoglou, D.; Kouzeli-Katsiri, A.; Haralambopoulos, D. Solar distillation of olive mill wastewater. Renew. Energy 2004, 29, 569–579. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive-Mill Waste Management—Literature Review and Patent Survey; Typothito-George Dardanos: Athens, Greece, 2004. [Google Scholar]
- Caputo, A.C.; Scacchia, F.; Pelagagge, P.M. Disposal of byproducts in olive oil industry: Waste-to-energy solutions. Appl. Therm. Eng. 2003, 23, 197–214. [Google Scholar] [CrossRef]
- Al-Malah, K.; Azzam, M.O.J.; Abulail, N.I. Olive mills effluent (OME) wastewater post-treatment using activated clay. Sep. Purif. Technol. 2000, 20, 225–234. [Google Scholar] [CrossRef]
- Azzam, M.O.J.; Al-Malah, K.I.; Abu-Lail, N.I. Dynamic posttreatment response of olive mill effluent wastewater using activated carbon. J. Environ. Sci. Health A 2004, 39, 269–280. [Google Scholar] [CrossRef]
- Arvaniti, E.C.; Zagklis, D.P.; Papadakis, V.G.; Paraskeva, C.A. High-added value materials production from OMW: A technical and economical optimization. Int. J. Chem. Eng. 2012, 2012, 607219. [Google Scholar] [CrossRef]
- Djellabi, R.; Giannantonio, R.; Falletta, E.; Bianchi, C.L. SWOT analysis of photocatalytic materials towards large scale environmental remediation. Curr. Opin. Chem. Eng. 2021, 33, 100696. [Google Scholar] [CrossRef]
- Galloni, M.G.; Cerrato, G.; Giordana, A.; Falletta, E.; Bianchi, C.L. Sustainable Solar Light Photodegradation of Diclofenac by Nano- and Micro-Sized SrTiO3. Catalysts 2022, 12, 804. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Removal of COD from olive mill wastewater by Fenton’s reagent: Kinetic study. J. Hazard. Mater. 2009, 168, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.; Bravi, M. Critical flux analyses on differently pretreated olive vegetation wastewater streams: Some case studies. Desalination 2010, 250, 578–582. [Google Scholar] [CrossRef]
- Papaphilippou, P.C.; Yiannapas, C.; Politi, M.; Daskalaki, V.M.; Michael, C.; Kalogerakis, N.; Mantzavinos, D.; Fatta-Kassinos, D. Sequential coagulation–flocculation, solvent extraction and photo-Fenton oxidation for the valorization and treatment of olive mill effluent. Chem. Eng. J. 2013, 224, 82–88. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Applica-tion of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Aziz, K.H.H. Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere 2019, 228, 377–383. [Google Scholar] [CrossRef]
- Podgornik, M.; Bucar-Miklavcic, M.; Levart, A.; Salobir, J.; Rezar, V.; Butinar, B. Chemical Characteristics of Two-Phase Olive-Mill Waste and Evaluation of Their Direct Soil Application in Humid Mediterranean Regions. Agronomy 2022, 12, 1621. [Google Scholar] [CrossRef]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 15 July 2022).
- Council Directive 1999/31/EC on the Landfill of Waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01999L0031-20180704&from=FI (accessed on 10 July 2022).
- Taccogna, G. The Legal Regime of Olive Pomace Deriving from Olive Oil Extraction at Olive Mills, Waste, By-Products and Biomass; On behalf of ARE S.p.A. Agenzia regionale per l’energia della Liguria, member of the community project: “MORE: Market of Olive Residues for Energy”; Department of Public and Procedural Law, University of Genoa: Genoa, Italy, 2010. [Google Scholar]
- EC-DG. Survey of Wastes Spread on Land, Final Report, European Commission, Directorate-General for Environment. 2001. Available online: https://ec.europa.eu/environment/pdf/waste/compost/econanalysis_finalreport.pdf (accessed on 30 June 2022).
- Aydar, A.Y.; Bagdatlioglu, N.; Köseoglu, O. Effect of ultrasound on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM). Grasas Aceites 2017, 68, e189. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A.; Stefanatos, S. Olive Oil Waste Treatment: A Comparative and Critical Presentation of Methods, Advantages & Disadvantages. Crit. Rev. Food Sci. Nutr. 2007, 47, 187–229. [Google Scholar] [PubMed]
- Facts and Definitions: The Olive Oil Source. Available online: http://www.oliveoilsource.com/millandpressfacts.htm (accessed on 25 June 2022).
- Olive Oil Waste Treatment. Available online: http://www.ucm.es/info/improliv/allgem.htm (accessed on 25 June 2022).
- Dakhli, R.D. Agronomic Application of Olive Mill Waste Water: Short-Term Effect on Soil Chemical Properties and Barley Performance Under Semiarid Mediterranean Conditions. EQA-Int. J. Environ. Qual. 2018, 27, 1–17. [Google Scholar]
- Fedeli, E.; Camurati, F. Valorisation des margines et des grignons´epuises par recuperation de quelques composants. In Proceedings of the Seminaire International sur la Valorisation des Sous-Produits de L’olivier PNUD/FAO/COI, Monastir, Tunisia, 15–17 December 1981. [Google Scholar]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Olive Mill Wastewater Valorization through Steam Reforming Using Multifunctional Reactors: Challenges of the Process Intensification. Energies 2022, 15, 920. [Google Scholar] [CrossRef]
- Production of Olive Oil. Available online: http://www.oliveoilsource.com/millandpressfacts3.htm (accessed on 26 June 2022).
- Abenoza, M.; Benito, M.; Saldaña, G.; Alvarez, I.; Raso, J.; Sanchez-Gimeno, A.C. Effects of pulsed electric field on yield extrac-tion and quality of olive oil. Food Bioprocess Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Puértolas, E.; Martínez de Marañón, I. Olive oil pilot-production assisted by pulsed electric field: Impact on extraction yield, chemical parameters and sensory properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Durante, V.; La Notte, D. Working towards the development of innovative ultrasound equipment for the extraction of virgin olive oil. Ultrason. Sonochem. 2013, 20, 1261–1270. [Google Scholar] [CrossRef]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials; Intech Open: London, UK, 2018; pp. 157–169. [Google Scholar]
- Meroni, D.; Djellabi, R.; Ashokkumar, M.; Bianchi, C.L.; Boffito, D.C. Sonoprocessing: From Concepts to Large-Scale Reactors. Chem. Rev. 2022, 122, 3219–3258. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Aguirre, D.; Mobbs, T.; Barbosa-cánovas, G.V. Ultrasound Technologies for Food and Bioprocessing; Epub ahead of print; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-E-Huma, R.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Sordini, B.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Servili, M. Improvement of Olive Oil Mechanical Extraction: New Technologies, Process Efficiency, and Extra Virgin Olive Oil Quality. In Products from Olive Tree Dimitrios Boskou and Maria Lisa Clodoveo; Intech Open: London, UK, 2016; Available online: https://www.intechopen.com/books/products-from-olive-tree/improvement-of-olive-oil-mechanicalextraction-new-technologies-processefficiency-and-extra-virgin (accessed on 20 June 2022). [CrossRef] [Green Version]
- Clodoveo, M.L. An overview of emerging techniques in virgin olive oil extraction process: Strategies in the development of innovative plants. J. Agric. Eng. 2013, 44, 49–59. [Google Scholar] [CrossRef]
- Clodoveo, M.L. New advances in the development of innovative virgin olive oil extraction plants: Looking back to see the future. Food Res. Int. 2013, 54, 726–729. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hachicha Hbaieb, R. Beyond the traditional virgin olive oil extraction systems: Searching innovative and sustainable plant engineering solutions. Food Res. Int. 2013, 54, 1926–1933. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Research and Innovative Ap-proaches to Obtain Virgin Olive Oils with a Higher Level of Bioactive Constituents. Olive Olive Oil Bioact. Const. 2015, 7, 179–215. [Google Scholar] [CrossRef]
- Sun, D.W. Emerging Technologies for Food Processing, 2nd ed.; Elsevier Inc.: Dublin, Ireland, 2014. [Google Scholar]
- Çavdar, H.K.; Yanık, D.K.; Gök, U.; Gogus, F. Optimisation of microwave-assisted extraction of pomegranate (Punica granatum L.) seed oil and evaluation of Its physicochemical and bioactive properties. Food Technol. Biotechnol. 2017, 55, 86–94. [Google Scholar] [PubMed]
- Aydar, A.Y. Chapter: Emerging extraction technologies in olive oil production. In Technological Innovation in the Olive Oil Production Chain; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.; Beltrán, G.; Uceda, M. High-power ultra-sound in olive paste pretreatment. Effect on process yield and virgin olive oil characteristics. Ultrason. Sonochem. 2007, 14, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.A.; Beltrán, G.; Sánchez-Ortiz, A.; Sanchez, S.; Jimenez, A. Continuous high power ultrasound treatment before malaxation, a laboratory scale approach: Effect on virgin olive oil quality criteria and yield. Eur. J. Lipid Sci. Technol. 2016, 118, 332–336. [Google Scholar] [CrossRef]
- Aydar, A.Y. Physicochemical characteristics of extra virgin olive oils obtained by ultrasound assisted extraction from different olive cultivars. Int. J. Sci. Technol. Res. 2018, 4, 1–10. [Google Scholar]
- Bejaoui, M.A.; Beltran, G.; Aguilera, M.P.; Jimenez, A. Continuous conditioning of olive paste by high power ultrasounds: Response surface methodology to predict temperature and its effect on oil yield and virgin olive oil characteristics. LWT-Food Sci. Technol. 2016, 69, 175–184. [Google Scholar] [CrossRef]
- Kadi, H.; Moussaoui, R.; Djadoun, S.; Sharrock, P. Microwave assisted extraction of olive oil pomace by acidic hexane. Iran. J. Chem. Chem. Eng. 2016, 35, 73–79. [Google Scholar]
- Leone, A.; Tamborrino, A.; Zagaria, R.; Sabella, E.; Romaniello, R. Plant innovation in the olive oil extraction process: A comparison of efficiency and energy consumption between microwave treatment and traditional malaxation of olive pastes. J. Food Eng. 2015, 146, 44–52. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Paduano, A.; Di Palmo, T.; Crupi, P.; Corbo, F.; Pesce, V.; Distaso, E.; Tamburrano, P.; Amirante, R. Engineering design and prototype development of a full scale ultrasound system for virgin olive oil by means of numerical and experimental analysis. Ultrason. Sonochem. 2017, 37, 169–181. [Google Scholar] [CrossRef]
- Leal, A.L.; Soria, M.A.; Madeira, L.M. Autothermal reforming of impure glycerol for H2 production: Thermodynamic study including in situ CO2 and/or H2 separation. Int. J. Hydrogen Energy 2016, 41, 2607–2620. [Google Scholar] [CrossRef]
- Agency, I.E. Hydrogen. 2021. Available online: https://www.iea.org/reports/hydrogen (accessed on 12 August 2022).
- Agency, I.E. Global Hydrogen Demand by Sector in the Net Zero Scenario, 2020–2030. 2021. Available online: https://www.iea.org/data-and-statistics/charts/global-hydrogen-demand-by-sector-in-the-net-zero-scenario-2020-2030 (accessed on 12 August 2022).
- Kapellakis, I.E.; Tsagarakis, K.P.; Avramaki, C.; Angelakis, A.N. Olive mill wastewater management in river basins: A case study in Greece. Agric. Water Manag. 2006, 82, 354–370. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G.; Moutsopoulou, M.; Stefanoudaki, E. Application of olive mill wastewater to a Cretan olive orchard: Effects on soil properties, plant performance and the environment. Agric. Ecosyst. Environ. 2010, 138, 293–298. [Google Scholar] [CrossRef]
- Watanabe, K. Microorganisms relevant to bioremediation. Curr. Opin. Biotechnol. 2001, 12, 237–241. [Google Scholar] [CrossRef]
- Enhanced Bioremediation. Available online: http://www.cpeo.org/techtree/ttdescript/ensolmx.htm (accessed on 15 June 2022).
- Ehaliotis, C.; Papadopoulou, K.; Kotsou, M.; Mari, I.; Balis, C. Adaptation and population dynamics of Azotobacter vinelandii during aerobic biological treatment of olive-mill wastewater. FEMS Microbiol. Ecol. 1999, 30, 301–311. [Google Scholar] [CrossRef]
- Di Gioia, D.; Barberio, C.; Spagnesi, S.; Marchetti, L.; Fava, F. Characterization of four olive-mill-wastewater indigenous bacterial strains capable of aerobically degrading hydroxylated and methoxylated monocyclic aromatic compounds. Arch. Microbiol. 2002, 178, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, D.; Bertin, L.; Fava, F.; Marchetti, L. Biodegradation of hydroxylated and methoxylated benzoic, phenylacetic and phenylpropenoic acids present in olive mill wastewaters by two bacterial strains. Res. Microbiol. 2001, 152, 83–93. [Google Scholar] [CrossRef]
- Piperidou, C.I.; Chaidou, C.I.; Stalikas, C.D.; Soulti, K.; Pilidis, G.A.; Balis, C. Bioremediation of olive oil mill wastewater: Chemical alternations induced by Azotobacter vinelandii. J. Agric. Food Chem. 2000, 48, 1941–1948. [Google Scholar] [CrossRef]
- Yesilada, O.; Sik, S.; Sam, M. Biodegradation of olive oil mill wastewater by Coriolus versicolor and Funalia trogii: Effects of agitation, initial COD concentration, inoculum size and immobilization. World J. Microbiol. Biotechnol. 1997, 14, 37–42. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Arvaniti, E.C.; Papadakis, V.G.; Paraskeva, C.A. Sustainability analysis and benchmarking of olive mill wastewater treatment methods. J. Chem. Technol. Biotechnol. 2013, 88, 742–750. [Google Scholar] [CrossRef]
- Tomati, U.; Galli, E.; Fiorelli, F.; Pasetti, L. Fertilizers from composting of olive-mill wastewaters. Int. Biodeterior. Biodegrad. 1996, 38, 155–162. [Google Scholar] [CrossRef]
- Hachicha, S.; Cegarra, J.; Sellami, F.; Hachicha, R.; Drira, N.; Medhioub, K.; Ammar, E. Elimination of polyphenols toxicity from olive mill wastewater sludge by its co-composting with sesame bark. J. Hazard. Mater. 2009, 161, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- New Technologies for Husks and Waste Waters Recycling. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/details/1782 (accessed on 5 June 2022).
- Gavala, H.N.; Skiadas, I.V.; Bozinis, N.A.; Lyberatos, G. Anaerobic codigestion of agricultural industries’ wastewaters. Water Sci. Technol. 1996, 34, 67–75. [Google Scholar] [CrossRef]
- Zouari, N.; Ellouz, R. Toxic effect of coloured olive compounds on the anaerobic digestion of olive oil mill effluent in UASB-like reactors. J. Chem. Technol. Biotechnol. 1996, 66, 414–420. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Kopsahelis, A.; Blika, P.S.; Paraskeva, C.A.; Lyberatos, G. Anaerobic digestion of olive mill wastewater in a periodic anaerobic baffled reactor (PABR) followed by further effluent purification via membrane separation technologies. J. Chem. Technol. Biotechnol. 2009, 84, 909–917. [Google Scholar] [CrossRef]
- Boari, G.; Brunetti, A.; Passino, R.; Rozzi, A. Anaerobic digestion of olive oil mill wastewaters. Agric. Wastes 1984, 10, 161–175. [Google Scholar] [CrossRef]
- Aktas, E.S.; Imre, S.; Ersoy, L. Characterization and lime treatment of olive mill wastewater. Water Res. 2001, 35, 2336–2340. [Google Scholar] [CrossRef]
- Boukhoubza, F.; Jail, A.; Korchi, F.; Idrissi, L.L.; Hannache, H.; Duarte, J.C.; Hassani, L.; Nejmeddine, A. Application of lime and calcium hypochlorite in the dephenolisation and discolouration of olive mill wastewater. J. Environ. Manag. 2009, 91, 124–132. [Google Scholar] [CrossRef]
- Saglık, S.; Ersoy, L.; Imre, S. Oil recovery from lime-treated wastewater of olive mills. Eur. J. Lipid Sci. Technol. 2002, 104, 212–215. [Google Scholar] [CrossRef]
- Ugurlu, M.; Kula, I. Decolourization and removal of some organic compounds from olive mill wastewater by advanced oxidation processes and lime treatment. Environ. Sci. Pollut. Res. 2007, 14, 319–325. [Google Scholar] [CrossRef]
- Inan, H.; Dimoglo, A.; Simsek, H.; Karpuzcu, M. Olive oil mill wastewater treatment by means of electro-coagulation. Sep. Purif. Technol. 2004, 36, 23–31. [Google Scholar] [CrossRef]
- Israilides, C.J.; Vlyssides, A.G.; Mourafeti, V.N.; Karvouni, G. Olive oil wastewater treatment with the use of an electrolysis system. Bioresour. Technol. 1997, 61, 163–170. [Google Scholar] [CrossRef]
- Giannis, A.; Kalaitzakis, M.; Diamadopoulos, E. Electrochemical treatment of olive mill wastewater. J. Chem. Technol. Biotechnol. 2007, 82, 663–671. [Google Scholar] [CrossRef]
- Papastefanakis, N.; Mantzavinos, D.; Katsaounis, A. DSA electrochemical treatment of olive mill wastewater on Ti/RuO2 anode. J. Appl. Electrochem. 2010, 40, 729–737. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Dimou, A.; Mantzavinos, D.; Katsaounis, A. Electrochemical oxidation of model compounds and olive mill wastewater over DSA electrodes: 1. The case of Ti/IrO2 anode. J. Hazard. Mater. 2009, 167, 268–274. [Google Scholar] [CrossRef]
- Ahmed, B.; Limem, E.; Abdel-Wahab, A.; Nasr, B. Photo-Fenton treatment of actual agro-industrial wastewaters. Ind. Eng. Chem. Res. 2011, 50, 6673–6680. [Google Scholar] [CrossRef]
- Aki, S.N.V.K.; Abraham, M.A. An economic evaluation of catalytic supercritical water oxidation: Comparison with alternative waste treatment technologies. Environ. Prog. 1998, 17, 246–255. [Google Scholar] [CrossRef]
- Rivas, F.J.; Gimeno, O.; Portela, J.R.; de la Ossa, E.M.; Beltran, F.J. Supercritical water oxidation of olive oil mill wastewater. Ind. Eng. Chem. Res. 2001, 17, 246–255. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Diamadopoulos, E.; Mantzavinos, D. Effect of key operating parameters on the non-catalytic wet oxidation of olive mill wastewaters. Water Sci. Technol. 2009, 59, 2509–2518. [Google Scholar] [CrossRef] [Green Version]
- Weichgrebe, D.; Vogelpohl, A. A comparative study of wastewater treatment by chemical wet oxidation. Chem. Eng. Process. Process. Intensif. 1994, 33, 199–203. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, P.; Coz, A.; Charalabaki, M.; Kalogerakis, N.; Xekoukoulotakis, N.P.; Mantzavinos, D. Ozonation of weathered olive mill wastewaters. J. Chem. Technol. Biotechnol. 2006, 81, 1570–1576. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on magnetically separable graphitic carbon nitride-based nanocomposites as promising visible-light-driven photocatalysts. J. Mater. Sci. Mater. Electron. 2018, 29, 1719–1747. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A.; Abitorabi, M.; Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. [Google Scholar] [CrossRef]
- Djellabi, R.; Noureen, L.; Dao, V.D.; Meroni, D.; Falletta, E.; Dionysiou, D.D.; Bianchi, C.L. Recent advances and challenges of emerging solar-driven steam and the contribution of photocatalytic effect. Chem. Eng. J. 2022, 431, 134024. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photo-catalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Ovejero, D.; Barco, B.; Bahamonde, A.; Díaz, E.; Faraldos, M. An approach on the comparative behavior of chloro/nitro substituted phenols photocatalytic degradation in water. J. Environ. Chem. Eng. 2019, 7, 103051. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Xekoukoulotakis, N.P.; Mantzavinos, D. Determination of key operating conditions for the photocatalytic treatment of olive mill wastewaters. Catal. Today 2009, 144, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Justino, C.I.; Duarte, K.; Loureiro, F.; Pereira, R.; Antunes, S.C.; Marques, S.M.; Gonçalves, F.; Rocha-Santos, T.A.P.; Freitas, A.C. Toxicity and organic content characterization of olive oil mill wastewater undergoing a sequential treatment with fungi and photo-Fenton oxidation. J. Hazard. Mater. 2009, 172, 1560–1572. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Hodaifa, G.; Víctor-Ortega, M.D.; Martínez-Férez, A. A novel photocatalyst with ferromagnetic core used for the treatment of olive oil mill effluents from two-phase production process. Sci. World J. 2013, 2013, 196470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafi, W.K.; Shannak, B.; Al-Shannag, M.; Al-Anber, Z.; Al-Hasan, M. Treatment of olive mill wastewater by combined advanced oxidation and biodegradation. Sep. Purif. Technol. 2009, 70, 141–146. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Stoller, M. Kinetics and boundary flux optimization of integrated photocatalysis and ultrafiltration process for two-phase vegetation and olive washing wastewaters treatment. Chem. Eng. J. 2015, 279, 387–395. [Google Scholar] [CrossRef]
- Costa, J.C.; Alves, M.M. Posttreatment of olive mill wastewater by immobilized TiO2 photocatalysis. Photochem. Photobiol. 2013, 89, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Ruzmanova, Y.; Stoller, M.; Chianese, A. Photocatalytic treatment of olive mill wastewater by magnetic core titanium dioxide nanoparticles. Chem. Eng. Trans. 2013, 32, 2269–2274. [Google Scholar]
- Gernjak, W.; Maldonado, M.I.; Malato, S.; Cáceres, J.; Krutzler, T.; Glaser, A.; Bauer, R. Pilot-plant treatment of olive mill wastewater (OMW) by solar TiO2 photocatalysis and solar photo-Fenton. Sol. Energy 2004, 77, 567–572. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Xiao, G.; Su, H. Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants. Chin. J. Chem. Eng. 2019, 27, 1416–1428. [Google Scholar] [CrossRef]
- Tomar, L.J.; Chakrabarty, B.S. Synthesis, structural and optical properties of TiO2-ZrO2 nanocomposite by hydrothermal method. Adv. Mater. Lett. 2013, 4, 64–67. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Feizpoor, S.; Seifzadeh, D.; Ghosh, S. Improving visible-light-induced photocatalytic ability of TiO2 through coupling with Bi3O4Cl and carbon dot nanoparticles. Sep. Purif. Technol. 2020, 238, 116404. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Yubuta, K. Integration of carbon dots and polyaniline with TiO2 nanoparticles: Substantially enhanced photocatalytic activity to removal various pollutants under visible light. J. Photochem. Photobiol. A Chem. 2018, 367, 94–104. [Google Scholar] [CrossRef]
- Divya, K.S.; Madhu, A.K.; Umadevi, T.U.; Suprabha, T.; Nair, P.R.; Suresh, M. Improving the photocatalytic performance of TiO2 via hybridizing with graphene. J. Semicond. 2017, 38, 063002. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwinska-Ciesielczyk, K.; Jesionowski, T. Titania-based hybrid materials with ZnO, ZrO2 and MoS2: A review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef] [Green Version]
- Shao, G.N.; Imran, S.M.; Jeon, S.J.; Engole, M.; Abbas, N.; Salman Haider, M.; Kang, S.J.; Kim, H.T. Sol–gel synthesis of photoactive zirconia–titania from metal salts and investigation of their photocatalytic properties in the photodegradation of methylene blue. Powder Technol. 2014, 258, 99–109. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, W.; Tang, J.; Lin, J.; Li, S.; Zhu, M. Dopamine-assisted preparation of Fe3O4@MnO2 yolk@shell microspheres for improved pseudocapacitive performance. Electrochim. Acta 2019, 317, 628–637. [Google Scholar] [CrossRef]
- Karafas, E.S.; Romanias, M.N.; Stefanopoulos, V.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Papagiannakopoulos, P. Effect of metal doped and co-doped TiO2 photocatalysts oriented to degrade indoor/outdoor pollutants for air quality improvement. A kinetic and product study using acetaldehyde as probe molecule. J. Photochem. Photobiol. A Chem. 2019, 371, 255–263. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Tshabalala, Z.P.; Hillie, K.T.; Swart, H.C.; Motaung, D.E. The blue luminescence of p-type NiO nanostructured material induced by defects: H2S gas sensing characteristics at a relatively low operating temperature. Appl. Surf. Sci. 2020, 525, 146002. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Wu, Q.; Wu, J.; Xu, Y. Synthesis of sea-urchin-like Fe3O4/SnO2 heterostructures and its application for environmental remediation by removal of p-chlorophenol. J. Mater. Sci. 2018, 54, 1341–1350. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Satpati, B.; Mohapatra, S. Plasmon-enhanced photoluminescence from SnO2 nanostructures decorated with Au nanoparticles. Appl. Surf. Sci. 2020, 504, 144381. [Google Scholar] [CrossRef]
- Imran, M.; Riaz, S.; Sanaullah, I.; Khan, U.; Sabri, A.N.; Naseem, S. Microwave assisted synthesis and antimicrobial activity of Fe3O4-doped ZrO2 nanoparticles. Ceram. Int. 2019, 45, 10106–10113. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2–ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, C.; Zhai, H.; Liu, B.; Zhao, X.; Fang, D.; Wei, Y. Facile preparation of flexible and stable superhydrophobic non-woven fabric for efficient oily wastewater treatment. Surf. Coat. Technol. 2019, 357, 526–534. [Google Scholar] [CrossRef]
- Elbasuney, S.; Elsayed, M.A.; Mostafa, S.F.; Khalil, W.F. MnO2 nanoparticles supported on porous Al2O3 substrate for wastewater treatment: Synergy of adsorption, oxidation, and photocatalysis. J. Inorg. Organomet. Polym. Mater. 2019, 29, 827–840. [Google Scholar] [CrossRef]
- Yaacob, N.; Sean, G.P.; Nazri, N.A.M.; Ismail, A.F.; Abidin, M.N.Z.; Subramaniam, M.N. Simultaneous oily wastewater adsorption and photodegradation by ZrO2–TiO2 heterojunction photocatalysts. J. Water Process Eng. 2021, 39, 101644. [Google Scholar] [CrossRef]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, A.; Murugesan, V. Solar photocatalytic degradation of azodye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Kobayakawa, K.; Sato, C.; Sato, Y.; Fujishima, A. Continuous-flow photoreactor packed with titanium dioxide immobilized on large silica gel beads to decompose oxalic acid in excess water. J. Photochem. Photobiol. A Chem. 1998, 118, 65–69. [Google Scholar] [CrossRef]
- Kamat, P.V. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem. Rev. 1993, 93, 267–300. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Agostiano, A. UV-induced photocatalytic degradation of azo dyes by organic capped ZnO nanocrystals immobilized onto substrates. Appl. Catal. B Environ. 2005, 62, 1–11. [Google Scholar] [CrossRef]
- Fouad, O.A.; Ismail, A.A.; Zaki, Z.I.; Mohamed, R.M. Zinc oxide thin films prepared by thermal evaporation deposition and its photocatalytic activity. Appl. Catal. B Environ. 2006, 62, 144–149. [Google Scholar] [CrossRef]
- Yeber, M.C.; Rodriguez, J.; Freer, J.; Baeza, J.; Duran, N.H.; Mansilla, D. Advanced oxidation of a pulp mill bleaching wastewater. Chemosphere 1999, 39, 1679–1688. [Google Scholar] [CrossRef]
- Khodja, A.A.; Sheili, T.; Pihichowski, J.F.; Boule, P. Photocatalytic degradation of 2-phenylphenol on TiO2 and ZnO in aqueous suspensions. J. Photochem. Photobiol. A 2001, 141, 231–239. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalyzed oxidation of phenol, 2-chlorophenol and pentachlorophenol chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A Chem. 2001, 85, 247–253. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Photocatalytic decomposition of leather dye: Comparative study of TiO2 supported on alumina and glass beads. J. Photochem. Photobiol. A Chem. 2002, 148, 153–159. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A focus on advanced physico-chemical processes for olive mill wastewater treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Di Somma, I.; Lo Giudice, R.; Marotta, R.; Pinto, G.; Pollio, A. Effect of combined physico-chemical processes on the phytotoxicity of olive mill wastewaters. Water Res. 2008, 42, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Lofrano, G.; Grassi, M.; Belgiorno, V. Pretreatment of olive mill wastewater by chitosan coagulation and advanced oxidation processes. Sep. Purif. Technol. 2008, 63, 648–653. [Google Scholar] [CrossRef]
- Aytar, P.; Gedikli, S.; Sam, M.; Farizoglu, B.; Çabuk, A. Sequential treatment of olive oil mill wastewater with adsorption and biological and photo-Fenton oxidation. Environ. Sci. Pollut. Res. 2013, 20, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Hodaifa, G.; Agabo, C.; Moya, A.J.; Pacheco, R.; Mateo, S. Treatment of olive oil mill wastewater by UV-light and UV/H2O2 system. Int. J. Green Technol. 2015, 1, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Ruzmanova, Y.; Ustundas, M.; Stoller, M.; Chianese, A. Photocatalytic treatment of olive mill wastewater by N-doped titanium dioxide nanoparticles under visible light. Chem. Eng. Trans. 2013, 32, 2233–2238. [Google Scholar]
- Cai, Q.; Zhu, Z.; Chen, B.; Zhang, B. Oil-in-water emulsion breaking marine bacteria for demulsifying oily wastewater. Water Res. 2019, 149, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Guria, C.; Mandal, A. Synthesis, characterization and performance studies of mixed-matrix poly(vinyl chloride)-bentonite ultrafiltration membrane for the treatment of saline oily wastewater. Process. Saf. Environ. Prot. 2018, 116, 703–717. [Google Scholar] [CrossRef]
- Perez-Calderon, J.; Santos, M.V.; Zaritzky, N. Optimal clarification of emulsified oily wastewater using a surfactant/chitosan biopolymer. J. Environ. Chem. Eng. 2018, 6, 3808–3818. [Google Scholar] [CrossRef]
- Cebeci, M.S.; Gokçek, O.B. Investigation of the treatability of molasses and industrial oily wastewater mixture by an anaerobic membrane hybrid system. J. Environ. Manag. 2018, 224, 298–309. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.S.; Meenakshi, S. Encapsulation of metal ions between the biopolymeric layer beads for tunable action on oil particles adsorption from oily wastewater. J. Mol. Liq. 2018, 255, 429–438. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B. Oily wastewater treatment by nano-TiO2-induced photocatalysis. IEEE Nanotechnol. 2017, 11, 2–13. [Google Scholar]
- Yao, T.; Jia, W.; Feng, Y.; Zhang, J.; Lian, Y.; Wu, J.; Zhang, X. Preparation of reduced graphene oxide nanosheet/FexOy/nitrogen-doped carbon layer aerogel as pho-to-Fenton catalyst with enhanced degradation activity and reusability. J. Hazard. Mater. 2019, 362, 62–71. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Reaction mechanism and degradation pathway of rhodamine 6G by photocatalytic treatment. Water Air Soil Pollut. 2017, 228, 291. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Toxicological assessment and UV/TiO2-based induced degradation profile of reactive black 5 dye. Environ. Manag. 2018, 61, 171–180. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Chen, Y.; Jiang, J.; Ma, Y.; Wang, Z.; Huang, W.; Wang, S.; Liu, M.; Ma, D.; et al. Fabrication of hollow flower-like magnetic Fe3O4/C/MnO2/C3N4 composite with enhanced photocatalytic activity. Sci. Rep. 2021, 11, 2597. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Yang, Y.; Chen, Y.; Ma, Y.; Lyu, P.; Cui, A.; Huang, W.; Zhang, Z.; Li, Y.; Si, F. Photocatalytic degradation of MB dye by the magnetically separable 3D flower- like Fe3O4/SiO2/MnO2/BiOBr-Bi photocatalyst. J. Alloys Compd. 2021, 861, 158256. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Baltanás, M.A.; Cassano, A.E. Supported titanium oxide as photocatalyst in water decontamination: State of the art. Catal. Today 1997, 39, 219–231. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Wang, D.; Tang, X.; Yan, Y.; Shi, W.; Wang, Y.; Gao, N.; Yao, X.; Dong, H. Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity. Appl. Catal. B Environ. 2016, 182, 115–122. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, R.; Lu, Z.; Wang, W.; Yan, Y. Dual sensitization effect and conductive structure of Fe3O4@mTiO2/C photocatalyst towards superior photodegradation activity for bisphenol A under visible light. J. Photochem. Photobiol. A Chem. 2019, 382, 111902. [Google Scholar] [CrossRef]
- Tan, J.; Wang, X.; Hou, W.; Zhang, X.; Liu, L.; Ye, J.; Wang, D. Fabrication of Fe3O4@graphene/TiO2 nanohybrid with enhanced photocatalytic activity for isopropanol degradation. J. Alloys Compd. 2019, 792, 918–927. [Google Scholar] [CrossRef]
- Ji, H.Y.; Jing, X.C.; Xu, Y.G.; Yan, J.; Li, H.P.; Li, Y.P.; Huang, L.Y.; Zhang, Q.; Xu, H.; Li, H.M. Magnetic g-C3N4/NiFe2O4 hybrids with enhanced photocatalytic activity. RSC Adv. 2015, 5, 57960–57967. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Huang, J.; Zhu, Y.; Zhu, J.; Yang, X.; Chen, W.; Yao, Y.; Qian, S.; Jiang, H.; et al. In-situ SERS monitoring of reaction catalyzed by multifunctional Fe3O4@TiO2@Ag-Au microspheres. Appl. Catal. B Environ. 2017, 205, 11–18. [Google Scholar] [CrossRef]
- Singh, P.; Sudhaik, A.; Raizada, P.; Shandilya, P.; Sharma, R.; Hosseini-Bandegharaei, A. Photocatalytic performance and quick recovery of BiOI/Fe3O4@graphene oxide ternary photocatalyst for photodegradation of 2,4-dintirophenol under visible light. Mater. Today Chem. 2019, 12, 85–95. [Google Scholar] [CrossRef]
- Paul, A.; Dhar, S.S. Designing Cu2V2O7/CoFe2O4/g-C3N4 ternary nanocomposite: A high performance magnetically recyclable photocatalyst in the reduction of 4-nitrophenol to 4-aminophenol. J. Solid State Chem. 2020, 290, 121563. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Hu, J.; Yan, L. A spray pyrolysis synthesis of MnFe2O4/SnO2 yolk/shell composites for magnetically recyclable photocatalyst. Mater. Lett. 2017, 199, 135–138. [Google Scholar] [CrossRef]
- Tariq, N.; Fatima, R.; Zulfiqar, S.; Rahman, A.; Warsi, M.F.; Shakir, I. Synthesis and characterization of MoO3/CoFe2O4 nanocomposite for photocatalytic applications. Ceram. Int. 2020, 46, 21596–21603. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; Tong, Z.; Liu, Y.; Ma, Y.; Wang, R.; Bi, Y.; Liao, Z. Research progress of magnetic bismuth-based materials in photocatalysis: A review. J. Alloys Compd. 2021, 886, 161096. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Stoller, M.; Ciambelli, P.; Chianese, A. Photo-catalytic Removal of Phenol by Ferromagnetic N-TiO2/SiO2/Fe3O4 Nanoparticles in presence of Visible Light Irradiation. Chem. Eng. Trans. 2016, 47, 235–240. [Google Scholar]
- Hesas, R.H.; Baei, M.S.; Rostami, H.; Gardy, J.; Hassanpour, A. An investigation on the capability of magnetically separable Fe3O4/mordenite zeolite for refinery oily wastewater purification. J. Environ. Manag. 2019, 241, 525–534. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z. Flower-like Ag2MoO4/Bi2MoO6 heterojunctions with enhanced photocatalytic activity under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2017, 71, 156–164. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Betancourt-Cantera, L.G.; Fuentes, K.M.; Bolarín-Miró, A.M.; Aldabe-Bilmes, S.; Cortés-Escobedo, C.A.; Sánchez-De Jesús, F. Enhanced photocatalytic activity of BiFeO3 for water remediation via the addition of Ni2+. Mater. Res. Bull. 2020, 132, 111012. [Google Scholar] [CrossRef]
- Tao, R.; Li, X.; Li, X.; Liu, S.; Shao, C.; Liu, Y. Discrete heterojunction nanofibers of BiFeO3/Bi2WO6: Novel architecture for effective charge separation and enhanced photocatalytic performance. J. Colloid Interface Sci. 2020, 572, 257–268. [Google Scholar] [CrossRef]
- Cirkovic, J.; Radojkovic, A.; Golic, D.L.; Tasic, N.; Cizmic, M.; Brankovic, G.; Brankovic, Z. Visible-light photocatalytic degradation of mordant blue 9 by single-phase BiFeO3 nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104587. [Google Scholar] [CrossRef]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic activity and doping effects of BiFeO3 nanoparticles in model organic dyes. Heliyon 2020, 6, 03237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadenbach, T.; Benitez, M.J.; Morales, A.L.; Costa Vera, C.; Lascano, L.; Quiroz, F.; Debut, A.; Vizuete, K. Nanocasting synthesis of BiFeO3 nanoparticles with enhanced visible-light photocatalytic activity. Beilstein J. Nanotechnol. 2020, 11, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, Z.; Zhong, C.; Lu, Z. Facile synthesis of BiFeO3 nanosheets with enhanced visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2017, 29, 4817–4829. [Google Scholar] [CrossRef]
- Li, Y.A.; Zhang, X.; Chen, L.; Sun, H.; Zhang, H.; Si, W.; Wang, W.; Wang, L.; Li, J. Enhanced magnetic and photocatalytic properties of BiFeO3 nanotubes with ultrathin wall thickness. Vacuum 2021, 184, 109867. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Vladimirova, E.V.; Kandaurov, M.V.; Kellerman, D.G.; Kuznetsov, M.V.; Buldakova, L.U.; Samigullina, R.F. Synthesis of hollow spheres of BiFeO3 from nitrate solutions with tartaric acid: Morphology and magnetic properties. J. Alloys Compd. 2019, 777, 586–592. [Google Scholar] [CrossRef]
- Li, X.; Tang, Z.; Ma, H.; Wu, F.; Jian, R. PVP-assisted hydrothermal synthesis and photocatalytic activity of single-crystalline BiFeO3 nanorods. Appl. Phys. A 2019, 125, 598. [Google Scholar] [CrossRef]
- Bharathkumar, S.; Sakar, M.; Vinod, R.; Balakumar, S. Versatility of electrospinning in the fabrication of fibrous mat and mesh nanostructures of bismuth ferrite (BiFeO3) and their magnetic and photocatalytic activities. Phys. Chem. Chem. Phys. 2015, 17, 17745–17754. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Sun, H.; Li, M.; Shi, W. High-efficiency photocatalytic water splitting by a N-doped porous g-C3N4 nanosheet polymer photocatalyst derived from urea and N, N-dimethylformamide. Inorg. Chem. Front. 2020, 7, 1770–1779. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Lu, X.; Zuo, S.; Li, Z.; Yao, C.; Ni, C. Microwave hydrothermal synthesis of BiP1 − xVxO4/attapulgite nanocomposite with efficient photocatalytic performance for deep desulfurization. Powder Technol. 2018, 327, 467–475. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Guo, F.; Tang, Y.; Lu, C. Construction of CuBi2O4/ Bi2MoO6 p-n heterojunction with nanosheets-on-microrods structure for improved photocatalytic activity towards broad spectrum antibiotics degradation. Chem. Eng. J. 2020, 394, 125009. [Google Scholar] [CrossRef]
- Dieu Cam, N.T.; Pham, H.D.; Pham, T.D.; Thu Phuong, T.T.; Van Hoang, C.; Thanh Tung, M.H.; Trung, N.T.; Huong, N.T.; Thu Hien, T.T. Novel photocatalytic performance of magnetically recoverable MnFe2O4/BiVO4 for polluted antibiotics degradation. Ceram. Int. 2001, 47, 1686–1692. [Google Scholar] [CrossRef]
- Sakhare, P.A.; Pawar, S.S.; Bhat, T.S.; Yadav, S.D.; Patil, G.R.; Patil, P.S.; Sheikh, A.D. Magnetically recoverable BiVO4/NiFe2O4 nanocomposite photocatalyst for efficient detoxification of polluted water under collected sunlight. Mater. Res. Bull. 2020, 129, 110908. [Google Scholar] [CrossRef]
- Rohani Bastami, T.; Ahmadpour, A.; Ahmadi Hekmatikar, F. Synthesis of Fe3O4/Bi2WO6 nanohybrid for the photocatalytic degradation of pharmaceutical ibuprofen under solar light. J. Ind. Eng. Chem. 2017, 51, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Xiu, Z.; Cao, Y.; Xing, Z.; Zhao, T.; Li, Z.; Zhou, W. Wide spectral response photothermal catalysis-fenton coupling systems with 3D hierarchical Fe3O4/Ag/Bi2MoO6 ternary hetero-superstructural magnetic microspheres for efficient high-toxic organic pollutants removal. J. Colloid Interface Sci. 2019, 533, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl. Catal. B Environ. 2020, 274, 119063. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, Z.; Dong, C.; Wang, Z.; Yao, S.; Gao, H.; Liu, Z.; Liu, Y.; Yang, B.; Zhang, H. Ultrathin BiOX (X = Cl, Br, I) nanosheets with exposed {001} facets for photocatalysis. ACS Appl. Nano Mater. 2020, 3, 1981–1991. [Google Scholar] [CrossRef]
- Li, Z.; Huang, G.; Liu, K.; Tang, X.; Peng, Q.; Huang, J.; Ao, M.; Zhang, G. Hierarchical BiOX (X = Cl, Br, I) microrods derived from bismuth-MOFs: In situ synthesis, photocatalytic activity and mechanism. J. Clean. Prod. 2020, 272, 122892. [Google Scholar] [CrossRef]
- Wang, L.; Liu, G.P.; Wang, B.; Chen, X.; Wang, C.T.; Lin, Z.X.; Xia, J.X.; Li, H.M. Oxygen vacancies engineering-mediated BiOBr atomic layers for boosting visible light-driven photocatalytic CO2 reduction. Sol. RRL 2020, 5, 2000480. [Google Scholar] [CrossRef]
- Cao, L.; Ma, D.; Zhou, Z.; Xu, C.; Cao, C.; Zhao, P.; Huang, Q. Efficient photocatalytic degradation of herbicide glyphosate in water by magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation. Chem. Eng. J. 2019, 368, 212–222. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhang, L.; Zhuo, S. A facile route to the synthesis of magnetically separable BiOBr/NiFe2O4 composites with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 419, 586–594. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wang, L.; Zhang, L.; Cao, X.; Guo, Y. Spinel NiFe2O4 nanoparticles decorated BiOBr nanosheets for improving the photocatalytic degradation of organic dye pollutants. J. Taiwan Inst. Chem. Eng. 2018, 85, 257–264. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Zeng, H.; Lin, H.; Li, H.; Tham, K.O.; Mohamed, A.R.; Lim, J.W.; Qin, Z. Magnetic NiFe2O4 nanoparticles decorated on N-doped BiOBr nanosheets for expeditious visible light photocatalytic phenol degradation and hexavalent chromium reduction via a Z-scheme heterojunction mechanism. Appl. Surf. Sci. 2021, 559, 149966. [Google Scholar] [CrossRef]
- Ma, W.; Chen, L.; Zhu, Y.; Dai, J.; Yan, Y.; Li, C. Facile synthesis of the magnetic BiOCl/ ZnFe2O4 heterostructures with enhanced photocatalytic activity under visible- light irradiation. Colloids Surf. A 2016, 508, 135–141. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, A.; Zhang, D.; Feng, C.; Su, S.; Zhang, X.; Xiang, J.; Chen, G.; Wang, Y. Efficient removal of Hg0 from simulated flue gas by novel magnetic Ag2WO4/BiOI/CoFe2O4 photocatalysts. Chem. Eng. J. 2019, 373, 780–791. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, P.; Zhang, X.; Li, H.; Wang, Y.; Sun, Z.; Xiang, J.; Su, S.; Liu, Z. Insights into efficient removal of gaseous Hg0 using AgIO3-Modified BiOI/CoFe2O4 composites through photocatalytic oxidation. Energ. Fuel 2019, 33, 12538–12548. [Google Scholar] [CrossRef]
- Shan, B.; Zhao, Y.; Li, Y.; Wang, H.; Chen, R.; Li, M. High-quality dual-plasmonic Au@Cu2 – xSe nanocrescents with precise Cu2–xSe domain size control and tunable optical properties in the second near-infrared biowindow. Chem. Mater. 2019, 31, 9875–9886. [Google Scholar] [CrossRef]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Liang, Q.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Zhang, X.; Zhao, J.; Hu, Z.; Wang, Z.; Xie, X. Preparation of magnetic nanosphere/nanorod/nanosheet-like Fe3O4/Bi2S3/BiOBr with enhanced (001) and (110) facets to photodegrade diclofenac and ibuprofen under visible LED light irradiation. Chem. Eng. J. 2019, 378, 122169. [Google Scholar] [CrossRef]
- Cong, Y.Q.; Ji, Y.; Ge, Y.H.; Jin, H.; Zhang, Y.; Wang, Q. Fabrication of 3D Bi2O3-BiOI heterojunction by a simple dipping method: Highly enhanced visible-light photoelectrocatalytic activity. Chem. Eng. J. 2017, 307, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, Z.; Farrokhnia, A.; Garcia-Lopez, E.I.; Shoushtari, M.Z. Superparamagnetic recoverable flowerlike Fe3O4@Bi2O3 core-shell with g-C3N4 sheet nanocomposite: Synthesis, characterization, mechanism and kinetic study of photo-catalytic activity. J. Mater. Sci. Mater. Electron. 2019, 31, 1022–1033. [Google Scholar] [CrossRef]
- Gao, N.; Lu, Z.; Zhao, X.; Zhu, Z.; Wang, Y.; Wang, D.; Hua, Z.; Li, C.; Huo, P.; Song, M. Enhanced photocatalytic activity of a double conductive C/Fe3O4/Bi2O3 composite photocatalyst based on biomass. Chem. Eng. J. 2016, 304, 351–361. [Google Scholar] [CrossRef]
- Falletta, E.; Longhi, M.; Di Michele, A.; Boffito, D.C.; Bianchi, C.L. Floatable graphitic carbon nitride/alginate beads for the photodegradation of organic pollutants under solar light irradiation. J. Clean. Prod. 2022, 133641. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhao, J.; Song, J.; Zhou, L.; Wang, J.; Tong, X.; Chen, Y. An alternative to in situ photocatalytic degradation of microcystin-LR by worm-like N, P co-doped TiO2/expanded graphite by carbon layer (NPT-EGC) floating composites. Appl. Catal. B Environ. 2017, 206, 479–489. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photo-catalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef] [PubMed]
- Djellabi, R.; Zhao, X.; Frias Ordonez, M.; Falletta, E.; Bianchi, C.L. Comparison of the photoactivity of several semiconductor oxides in floating aerogel and suspension systems towards the reduction of Cr(VI) under visible light. Chemosphere 2001, 281, 130839. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Zhang, T.; Dong, F.; Huang, H. Readily attainable spongy foam photocatalyst for promising practical photocatalysis. Appl. Catal. B Environ. 2017, 208, 75–81. [Google Scholar] [CrossRef]
- Chawla, H.; Saha, M.; Upadhyay, S.; Rohilla, J.; Ingole, P.P.; Sapi, A.; Szenti, I.; Yadav, M.; Lebedev, V.T.; Chandra, A.; et al. Enhanced photocatalytic activity and easy recovery of visible light active MoSe2/BiVO4 heterojunction immobilized on Luffa cylindrica—Experimental and DFT study. Environ. Sci. Nano 2021, 8, 3028. [Google Scholar] [CrossRef]
- Huang, X.-H.; Hu, T.; Bu, H.; Li, W.X.; Li, Z.L.; Hu, H.J.; Chen, W.Z.; Lin, Y.; Jiang, G.B. Transparent floatable magnetic alginate sphere used as photocatalysts carrier for im-proving photocatalytic efficiency and recycling convenience. Carbohydr. Polym. 2021, 254, 117281. [Google Scholar] [CrossRef]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of tech-nologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Rawindran, H.; Lim, J.W.; Goh, P.S.; Subramaniam, M.N.; Ismail, A.F.; Arzhandi, M.R.D. Simultaneous separation and degradation of surfactants laden in produced water using PVDF/TiO2 photocatalytic membrane. J. Clean. Prod. 2019, 221, 490–501. [Google Scholar] [CrossRef]
- Algamdi, M.S.; Alsohaimi, I.H.; Lawler, J.; Ali, H.M.; Aldawsari, A.M.; Hassan, H.M.A. Fabrication of graphene oxide incorporated polyethersulfone hybrid ultra-filtration membranes for humic acid removal. Sep. Purif. Technol. 2019, 223, 17–23. [Google Scholar] [CrossRef]
- Zhou, K.-G.; McManus, D.; Prestat, E.; Zhong, X.; Shin, Y.; Zhang, H.-L.; Haigh, S.; Casiraghi, C. Self-catalytic membrane photo-reactor made of carbon nitride nanosheets. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 11666–11671. [Google Scholar] [CrossRef] [Green Version]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol using coextruded dual-layer hollowfibre membranes incorporated with a different ratio of TiO2/PVDF. React. Funct. Polym. 2016, 99, 80–87. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J.; Adrus, N.; Hashim, N.A. Antifouling behavior and separation performance of immobilized TiO2 in dual layer hollow fiber membranes. Polym. Eng. Sci. 2017, 58, 1636–1643. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F. Photocatalytic performance ofTiO2/Clinoptilolite: Comparison study in suspension and hybrid photocatalytic membrane reactor. Chemosphere 2019, 228, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Philip, L. Combined biological and photocatalytic treatment of real coke oven wastewater. Chem. Eng. J. 2016, 295, 20–28. [Google Scholar] [CrossRef]
- Salim, N.E.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Qtaishat, M.; Matsuura, T.; Aziz, F.; Salleh, W.N.W. Preparation and characterization of hydrophilic surface modifier macro-molecule modified poly (ether sulfone) photocatalytic membrane for phenol removal. Chem. Eng. J. 2018, 335, 236–247. [Google Scholar] [CrossRef]
- Salim, N.E.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Qtaishat, M.R.; Matsuura, T.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Effects of hydrophilic surface macromolecule modifier loading on PES/O-g-C3N4 hybrid photocatalytic membrane for phenol removal. Appl. Surf. Sci. 2019, 465, 180–191. [Google Scholar] [CrossRef]

| Olives’ Variety | Used Technology a | Investigated Parameters | Dependent Variables | Maximum Extraction Yield (%) b | Ref. |
|---|---|---|---|---|---|
| Edremit | HPU | Ultrasound time, ultrasound temperature, malaxation time | Oil yield, acidity, peroxide value, and antioxidant properties | 9 | [26] |
| Coratina | HPU | Ultrasound application step (After crushing/ before crushing) | Olive paste temperature, energy balance, oil yield, quality indices of oil, minor compounds | 16 | [36] |
| Picual | HPU | Direct/indirect application of ultrasound | Olive paste temperature | 16 | [49] |
| Picual | HPU | Continuous ultrasound application before centrifugation | Oil yield, quality indices, volatile and minor compounds, fatty acid composition | 53 | [50] |
| Edremit, Gemlik, Uslu | HPU | Ultrasound and malaxation time | Oil yield, UV absorbance values acidity, peroxide value, total phenolic content | 68 | [51] |
| Picual | HPU | Olive paste flow, HPU intensity, fruit temperature, olive moisture, and fat content | Olive paste temperature | 17 | [52] |
| Ogliarola Barese | HPU, MW | Thermal effect of US and MW | Malaxation time, oil yield, quality characteristics, and energy efficiency | 17 | [45] |
| Arbequina | PEF | PEF application | Oil yield, acidity, quality characteristics, total phenols, sensory properties | n.d. c | [35] |
| Chemlal | MW | Extraction time, acetic acid content in hexane, irradiation power | Oil yield, total phenols, quality parameters | 6 | [53] |
| Peranzana | MW | Malaxation time and MW | Energy consumption, oil yield, structure modifications of olive pastes | n.d. c | [54] |
| Coratina | HPU | Sonication time | Oil yield, oil quality indices, phenolic composition | n.d. c | [55] |
| OMWW Origin | Type of Process Treatment and Scale | Obtained Results | Ref. |
|---|---|---|---|
| Jordan | (i) O3/UV or (ii) UV/O3, followed by (iii) biodegradation—laboratory scale | COD removal efficiencies up to (i) 91% by UV/O3 followed by biodegradation | [101] |
| Greece | Photocatalytic treatment with TiO2 (Degussa P25)—laboratory scale | 200 mg·L−1 COD residual and complete total phenol removal | [98] |
| Spain | pH-temperature flocculation + ferromagnetic core TiO2 + UV photocatalysis— laboratory and pilot scale | 58.3% COD and 27.5% total phenols removal efficiencies; overall COD removal efficiency up to 91% | [100,102] |
| Portugal | nano-TiO2 immobilized in nonwoven paper— laboratory scale | 90.8 ± 2.7% removal of the phenolic content | [103] |
| Italy | UV/TiO2— laboratory and pilot scale | COD reduction around 50% upon 1.5 g·L−1 nanocatalyst dosage | [104,105] |
| OMWW Origin | Type of Process Treatment and Scale | Obtained Results | Ref. |
|---|---|---|---|
| Spain and Portugal | (i) Solar photocatalysis with TiO2 or added peroxydisulphate, or (ii) solar photo-Fenton—pilot plant | (i) Solar photocatalytic systems did not present sufficientefficacy (ii) 85% COD and up to 100% phenols concentration removal | [105] |
| Italy | (i) Centrifugation + solar photolysis, or (ii) centrifugation + solar modified photo Fenton—laboratory scale | (ii) COD and phenolics removal efficiencies up to 29.3% and 63.6% | [136] |
| Italy | Fenton preceded by coagulation—laboratory scale | 85% COD removal (2 h) | [137] |
| Portugal | Biological (fungi Pleurotus sajor caju) and photo-Fenton oxidation—laboratory scale | COD removal efficiency up to 76% and total phenols up to 92% | [99] |
| Cyprus | Coagulation–flocculation, extraction of phenolics and post-oxidation by photo Fenton—laboratory scale | COD removal about 73 ± 2.3% and total phenols of 87 ± 3.1% | [18] |
| Turkey | Sequential adsorption, biological and photo-Fenton treatment—laboratory scale | 99% phenols reduction and 90% total organic content | [138] |
| Spain | UV/H2O2—laboratory scale | COD removal of 40–48% (30 min) | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloni, M.G.; Ferrara, E.; Falletta, E.; Bianchi, C.L. Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials. Catalysts 2022, 12, 923. https://doi.org/10.3390/catal12080923
Galloni MG, Ferrara E, Falletta E, Bianchi CL. Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials. Catalysts. 2022; 12(8):923. https://doi.org/10.3390/catal12080923
Chicago/Turabian StyleGalloni, Melissa G., Elena Ferrara, Ermelinda Falletta, and Claudia L. Bianchi. 2022. "Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials" Catalysts 12, no. 8: 923. https://doi.org/10.3390/catal12080923
APA StyleGalloni, M. G., Ferrara, E., Falletta, E., & Bianchi, C. L. (2022). Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials. Catalysts, 12(8), 923. https://doi.org/10.3390/catal12080923