Copper-Catalyzed Reactions of Aryl Halides with N-Nucleophiles and Their Possible Application for Degradation of Halogenated Aromatic Contaminants
Abstract
:1. Introduction
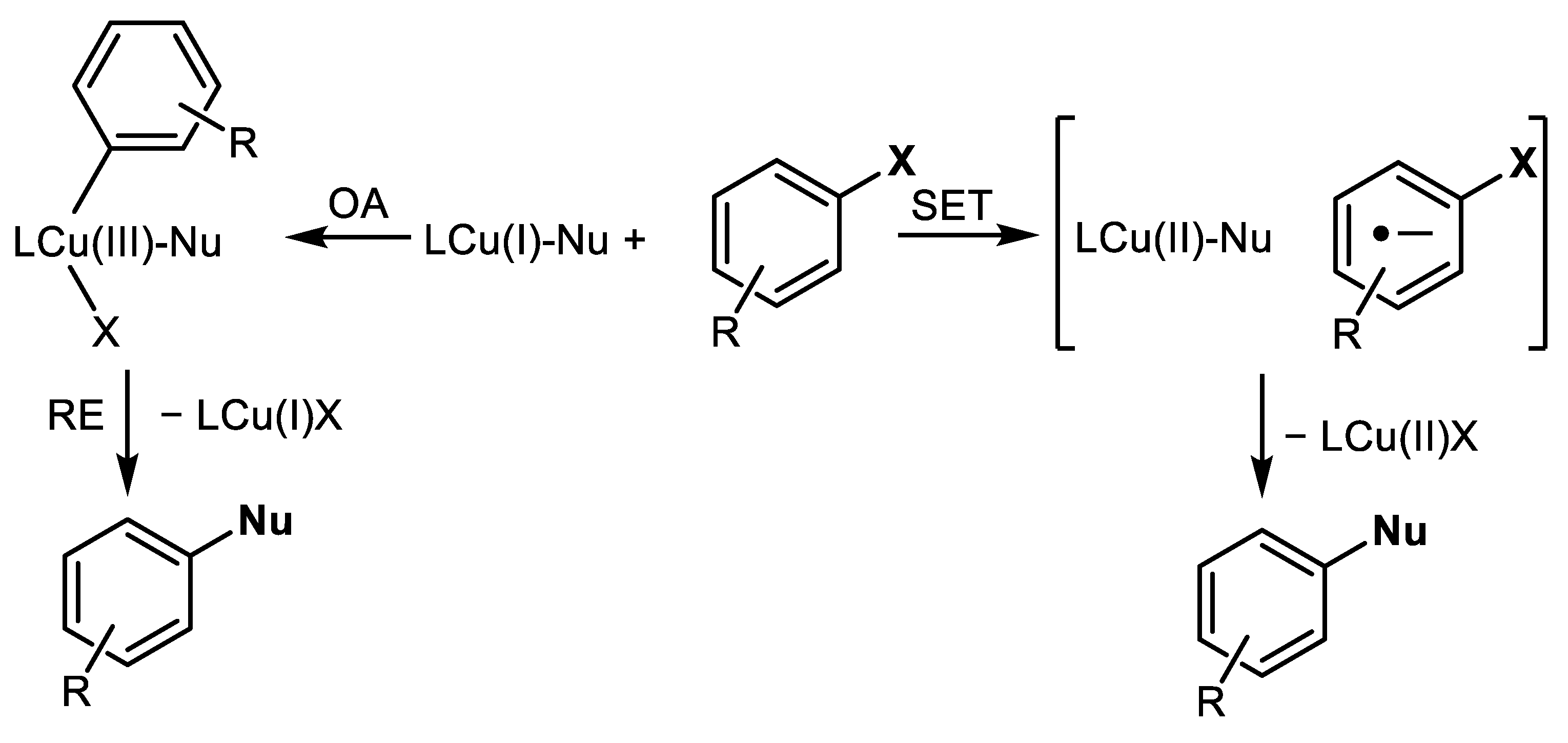
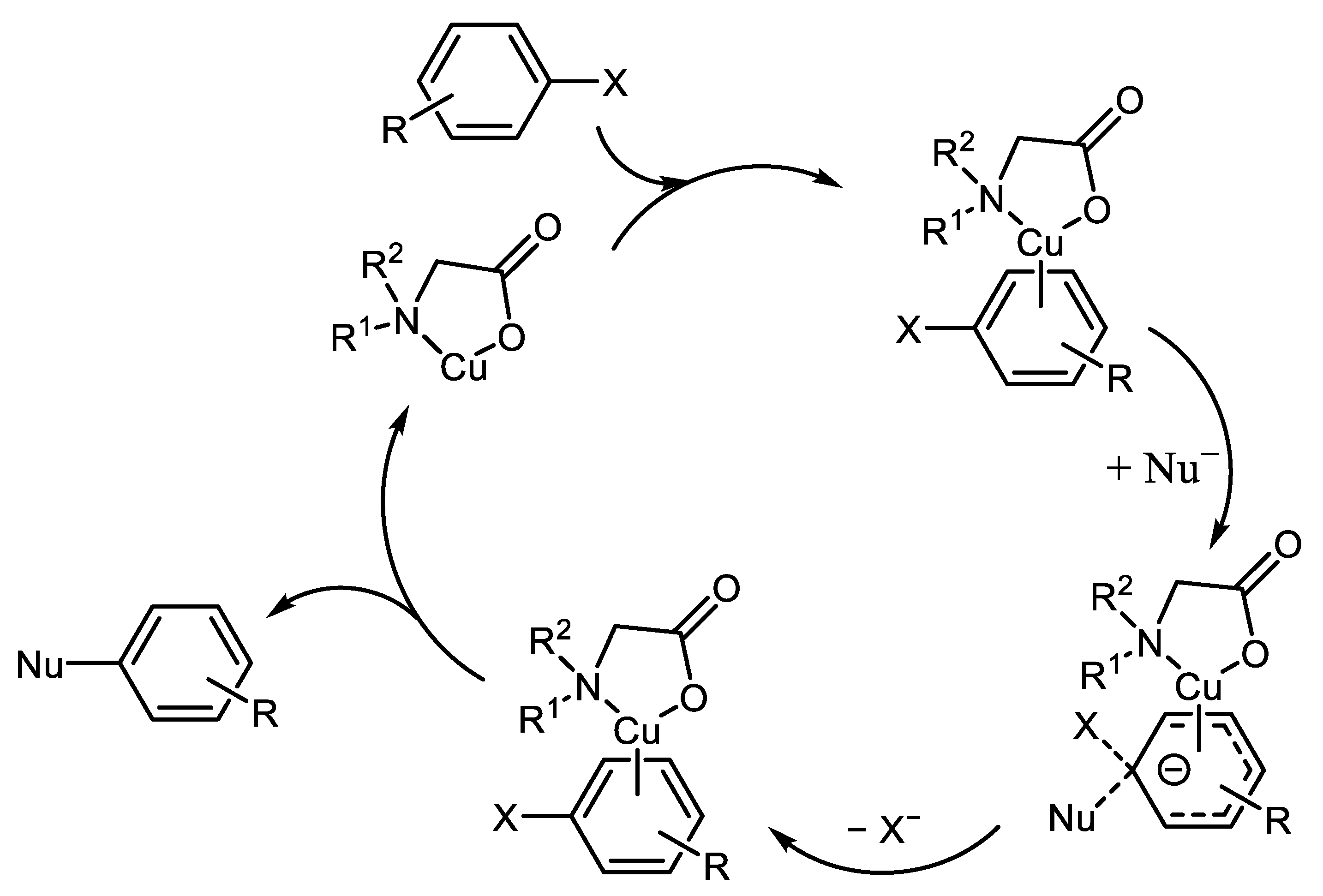
2. History and Modern Trends in Cu-Catalyzed Ar-Xs Transformations with Amines
2.1. Cu-Catalyzed Substitution of Halogen in Ar-Xs with NH3
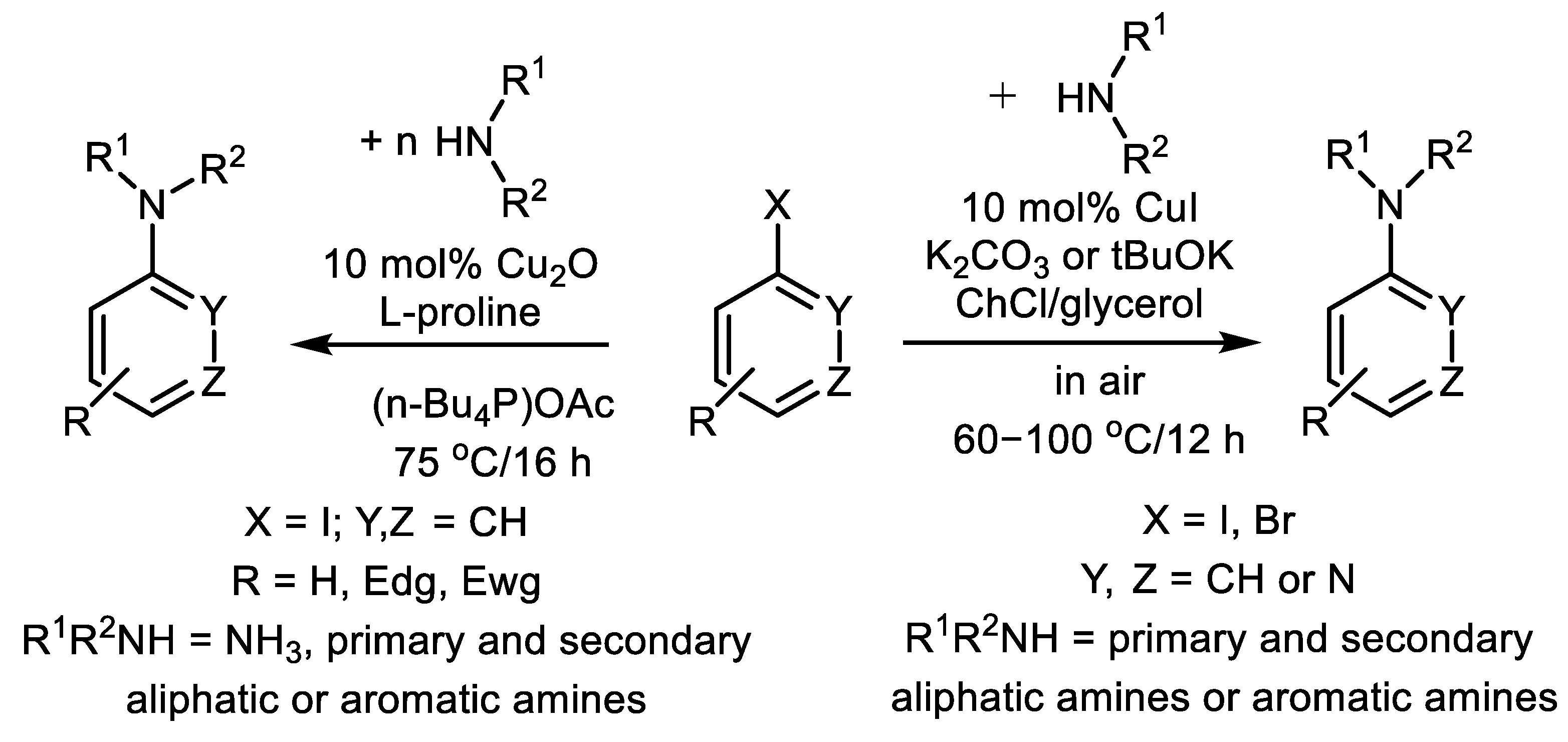
2.2. Cu-Catalyzed Substitution of Halogen in Ar-Xs with Primary and Secondary Amines

2.3. Cu-Catalyzed Functionalization of Ar-Xs in Green Solvents
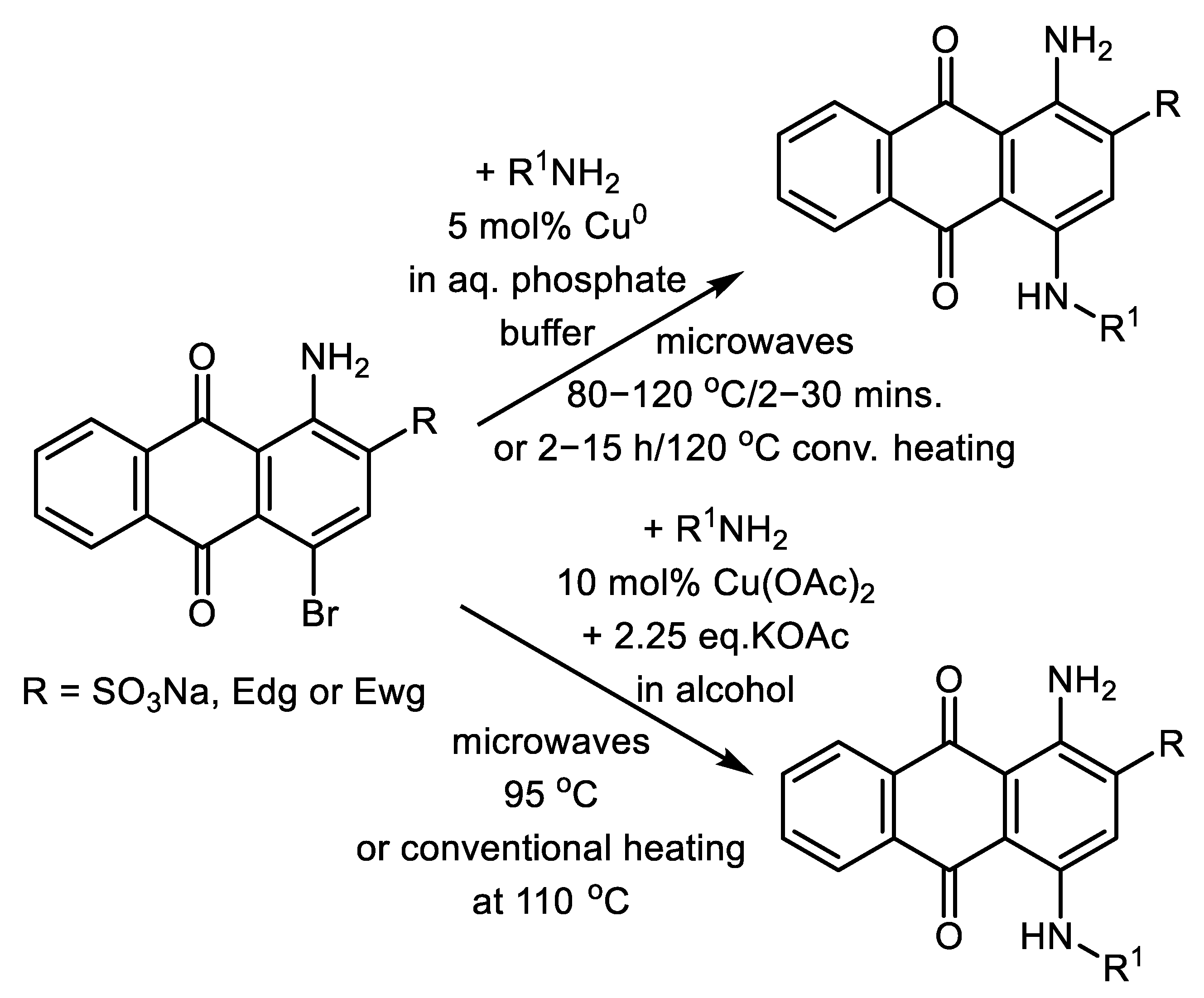
2.4. Microwave Assisted C-N Cross-Couplings
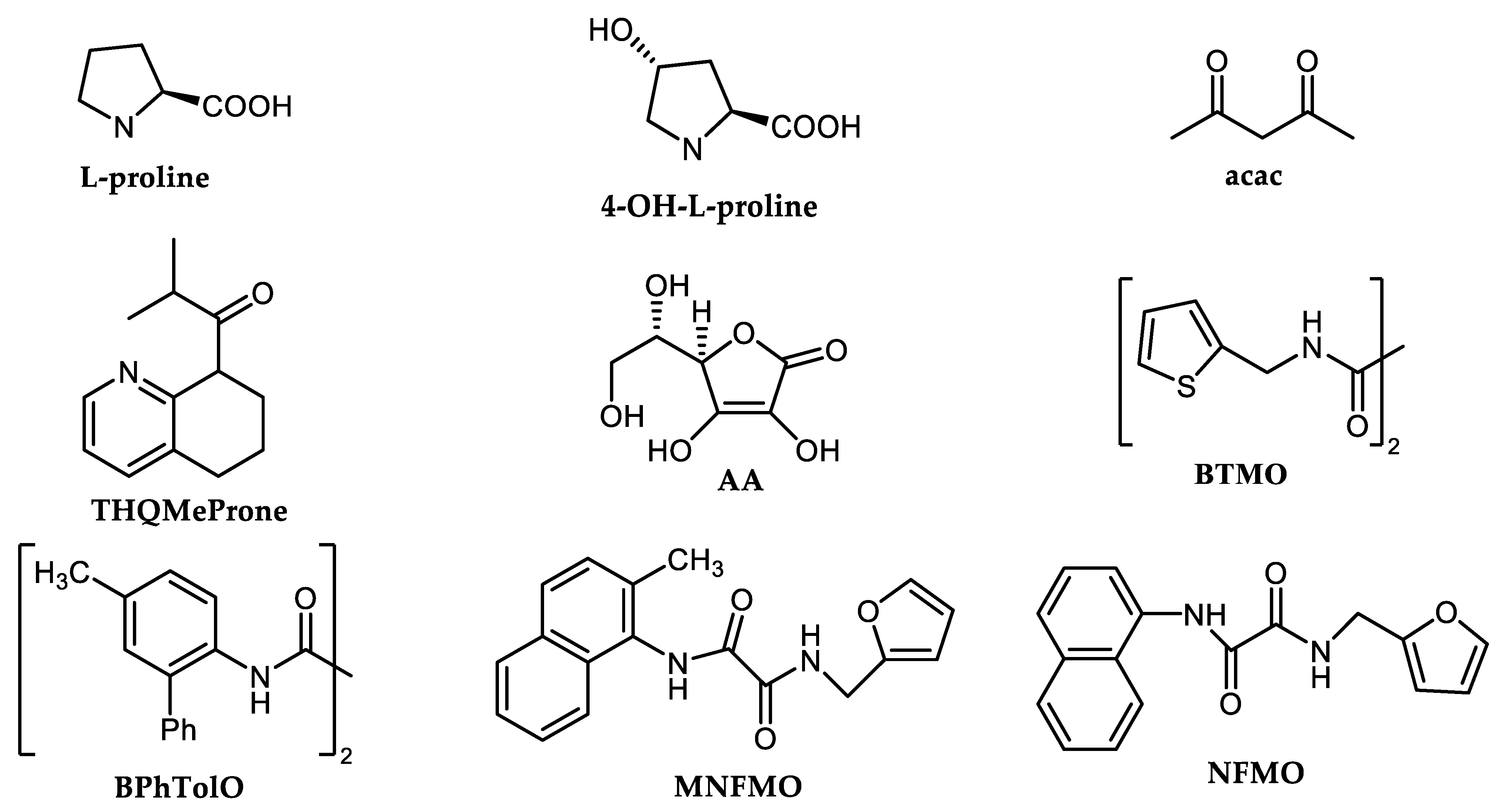
2.5. DH Catalyzed by Reusable Heterogeneous Copper Catalysts
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halden, R.U. On the Need and Speed of Regulating Triclosan and Triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.A. Supported Transition Metal Catalysts for Hydrodechlorination Reactions. ChemCatChem 2011, 3, 800–821. [Google Scholar] [CrossRef]
- Morarji, D.V.; Gurjar, K.K. Theoretical and Experimental Studies: Cu(I)/Cu(II) Catalytic Cycle in CuI/Oxalamide-Promoted C–N Bond Formation. Organometallics 2019, 38, 2502–2511. [Google Scholar] [CrossRef]
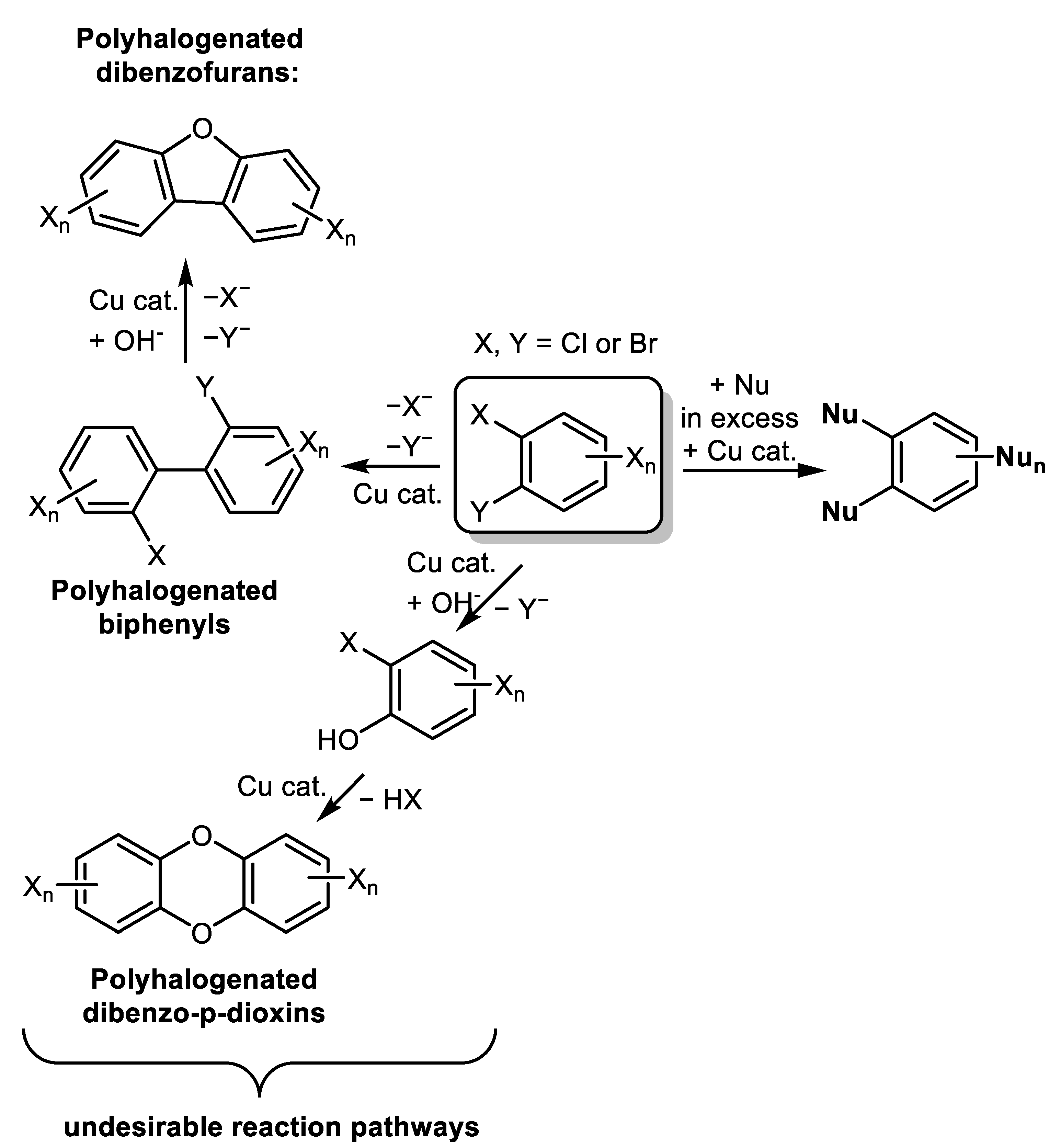
- Weidlich, T. The Influence of Copper on Halogenation/Dehalogenation Reactions of Aromatic Compounds and Its Role in the Destruction of Polyhalogenated Aromatic Contaminants. Catalysts 2021, 11, 378. [Google Scholar] [CrossRef]
- Egan, J.R.; Amlôt, R. Modelling Mass Casualty Decontamination Systems Informed by Field Exercise Data. Int. J. Environ. Res. Public Health 2012, 9, 3685–3710. [Google Scholar] [CrossRef]
- Shekhar, S.; Ahmed, T.S.; Ickes, A.R.; Haibach, M.C. Recent Advances in Nonprecious Metal Catalysis. Org. Process Res. Dev. 2022, 26, 14–42. [Google Scholar] [CrossRef]
- Buono, F.; Nguyen, T.; Qu, B.; Wu, H.; Haddad, N. Recent Advances in Nonprecious Metal Catalysis. Org. Process Res. Dev. 2021, 25, 1471–1495. [Google Scholar] [CrossRef]
- Singer, R.A.; Monfette, S.; Bernhardson, D.J.; Tcyrulnikov, S.; Hansen, E.C. Recent Advances in Nonprecious Metal Catalysis. Org. Process Res. Dev. 2020, 24, 909–915. [Google Scholar] [CrossRef]
- Yashwantrao, G.; Saha, S. Sustainable strategies of C–N bond formation via Ullmann coupling employing earth abundant copper catalyst. Tetrahedron 2021, 97, 132406. [Google Scholar] [CrossRef]
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692. [Google Scholar] [CrossRef]
- Dobrounig, P.; Trobe, M.; Breinbauer, R. Sequential and iterative Pd-catalyzed cross-coupling reactions in organic synthesis. Monatsh. Chem. 2017, 148, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Monnier, F.; Taillefer, M. Catalytic C–C, C–N, and C–O Ullmann-Type Coupling Reactions: Copper Makes a Difference. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, S.; Ebrahimiasl, S.; Hosseinian, A.; Monfared, A.; Vessally, E. Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N–H compounds. RSC Adv. 2019, 9, 8964–8976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-J.; Mankad, N.P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 2020, 49, 8036–8064. [Google Scholar] [CrossRef]
- Thapa, S.; Shrestha, B.; Gurung, S.K.; Giri, R. Copper-catalysed cross-coupling: An untapped potential. Org. Biomol. Chem. 2015, 13, 4816–4827. [Google Scholar] [CrossRef]
- Chemler, S.R. Copper catalysis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 2252–2253. [Google Scholar] [CrossRef]
- Aneeja, T.; Neetha, M.; Afsina, C.M.A.; Anilkumar, G. Progress and prospects in copper-catalyzed C–H functionalization. RSC Adv. 2020, 10, 34429–34458. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef]
- Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W. Copper-Catalyzed C–H Functionalization Reactions: Efficient Synthesis of Heterocycles. Chem. Rev. 2015, 115, 1622–1651. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, Q.; Ma, D. Amino Acid Promoted CuI-Catalyzed C−N Bond Formation between Aryl Halides and Amines or N-Containing Heterocycles. J. Org. Chem. 2005, 70, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-W.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Dicopper Complexes with Anthyridine-Based Ligands: Coordination and Catalytic Activity. Organometallics 2016, 35, 151–158. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, L.; Zhou, C.; Ma, D. ChemInform Abstract: Cu-Catalyzed Ullmann-Type C-Heteroatom Bond Formation: The Key Role of Dinucleating Ancillary Ligands. ChemInform 2014, 45. [Google Scholar] [CrossRef]
- D Senra, J.; CS Aguiar, L.; BC Simas, A. Recent progress in transition-metal-catalyzed cn cross-couplings: Emerging approaches towards sustainability. Curr. Org. Synth. 2011, 8, 53–78. [Google Scholar] [CrossRef]
- Zhu, R.; Xing, L.; Wang, X.; Cheng, C.; Su, D.; Hu, Y. Highly Practical “Ligand-Free-Like” Copper-Catalyzed N-Arylation of Azoles in Lower Nitrile Solvents. Adv. Synth. Catal. 2008, 350, 1253–1257. [Google Scholar] [CrossRef]
- Choudary, B.M.; Sridhar, C.; Kantam, M.L.; Venkanna, G.T.; Sreedhar, B. Design and Evolution of Copper Apatite Catalysts for N-Arylation of Heterocycles with Chloro- and Fluoroarenes. J. Am. Chem. Soc. 2005, 127, 9948–9949. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents. Catalysts 2020, 10, 429. [Google Scholar] [CrossRef]
- Cicco, L.; Hernández-Fernández, J.A.; Salomone, A.; Vitale, P.; Ramos-Martín, M.; González-Sabín, J.; Presa Soto, A.; Perna, F.M.; Capriati, V.; García-Álvarez, J. Copper-catalyzed Goldberg-type C–N coupling in deep eutectic solvents (DESs) and water under aerobic conditions. Org. Biomol. Chem. 2021, 19, 1773–1779. [Google Scholar] [CrossRef]
- Hoffmann, M.M. Polyethylene glycol as a green chemical solvent. Curr. Opin. Coll. Interface Sci. 2022, 57, 101537. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Matveev, D.; Vasilevsky, V.; Volkov, V.; Plisko, T.; Shustikov, A.; Volkov, A.; Bildyukevich, A. Fabrication of ultrafiltration membranes from non-toxic solvent dimethylsulfoxide: Benchmarking of commercially available acrylonitrile co-polymers. J. Environ. Chem. Eng. 2022, 10, 107061. [Google Scholar] [CrossRef]
- Yang, Q.; Sheng, M.; Li, X.; Tucker, C.; Vásquez Céspedes, S.; Webb, N.J.; Whiteker, G.T.; Yu, J. Potential Explosion Hazards Associated with the Autocatalytic Thermal Decomposition of Dimethyl Sulfoxide and Its Mixtures. Org. Process Res. Dev. 2020, 24, 916–939. [Google Scholar] [CrossRef]
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C−N, C−O, C−S, and C−C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef]
- Enthaler, S. Ammonia: An Environmentally Friendly Nitrogen Source for Primary Aniline Synthesis. ChemSusChem 2010, 3, 1024–1029. [Google Scholar] [CrossRef]
- Klinkenberg, J.L.; Hartwig, J.F. Catalytic Organometallic Reactions of Ammonia. Angew. Chem. Int. Ed. 2011, 50, 86–95. [Google Scholar] [CrossRef]
- Lang, F.; Zewge, D.; Houpis, I.N.; Volante, R.P. Amination of aryl halides using copper catalysis. Tetrahedron Lett. 2001, 42, 3251–3254. [Google Scholar] [CrossRef]
- Gaillard, S.; Elmkaddem, M.K.; Fischmeister, C.; Thomas, C.M.; Renaud, J.-L. Highly efficient and economic synthesis of new substituted amino-bispyridyl derivatives via copper and palladium catalysis. Tetrahedron Lett. 2008, 49, 3471–3474. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, J.; Song, Y.; Wang, L.; Zou, G. Hemilabile-coordinated copper promoted amination of aryl halides with ammonia in aqueous ethylene glycol under atmosphere pressure. Appl. Organomet. Chem. 2009, 23, 150–153. [Google Scholar] [CrossRef]
- Thomas, C.; Wu, M.; Billingsley, K.L. Amination–Oxidation Strategy for the Copper-Catalyzed Synthesis of Monoarylamines. J. Org. Chem. 2016, 81, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Atherton, J.H.; Page, M.I. Copper(I)-Catalyzed Amination of Aryl Halides in Liquid Ammonia. J. Org. Chem. 2012, 77, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Hee Seon, J. Simple and convenient copper-catalyzed amination of aryl halides to primary arylamines using NH4OH. Tetrahedron 2016, 72, 5988–5993. [Google Scholar] [CrossRef]
- Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(Naphthalen-1-yl)-N′-alkyl Oxalamide Ligands Enables Cu-Catalyzed Aryl Amination with High Turnovers. Org. Lett. 2017, 19, 2809–2812. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. Assembly of Primary (Hetero)Arylamines via CuI/Oxalic Diamide-Catalyzed Coupling of Aryl Chlorides and Ammonia. Org. Lett. 2015, 17, 5934–5937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Y.; Ma, D. Cu-Mediated Ullmann-Type Cross-Coupling and Industrial Applications in Route Design, Process Development, and Scale-up of Pharmaceutical and Agrochemical Processes. Org. Process Res. Dev. 2022, 26, 1690–1750. [Google Scholar] [CrossRef]
- De, S.; Yin, J.; Ma, D. Copper-Catalyzed Coupling Reaction of (Hetero)Aryl Chlorides and Amides. Org. Lett. 2017, 19, 4864–4867. [Google Scholar] [CrossRef]
- Kim, J.; Chang, S. Ammonium salts as an inexpensive and convenient nitrogen source in the Cu-catalyzed amination of aryl halides at room temperature. ChemComm 2008, 26, 3052–3054. [Google Scholar] [CrossRef]
- Gao, X.; Fu, H.; Qiao, R.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Primary Arylamines via Cascade Reactions of Aryl Halides with Amidine Hydrochlorides. J. Org. Chem. 2008, 73, 6864–6866. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, X.; Zhang, H.; Jiang, Y.; Ma, D. CuI/4-Hydro-l-proline as a More Effective Catalytic System for Coupling of Aryl Bromides with N-Boc Hydrazine and Aqueous Ammonia. J. Org. Chem. 2009, 74, 4542–4546. [Google Scholar] [CrossRef]
- Xia, N.; Taillefer, M. A Very Simple Copper-Catalyzed Synthesis of Anilines by Employing Aqueous Ammonia. Angew. Chem. Int. Ed. 2009, 48, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, Q.; Ding, K. An Efficient Copper-Catalyzed Amination of Aryl Halides by Aqueous Ammonia. Adv. Synth. Catal. 2009, 351, 1722–1726. [Google Scholar] [CrossRef]
- Fantasia, S.; Windisch, J.; Scalone, M. Ligandless Copper-Catalyzed Coupling of Heteroaryl Bromides with Gaseous Ammonia. Adv. Synth. Catal. 2013, 355, 627–631. [Google Scholar] [CrossRef]
- Shaughnessy, K.H.; Ciganek, E.; DeVasher, R.B. Chapter 1: Copper-catalyzed amination of aryl and alkenyl electrophiles. In Organic Reactions; Denmark, S.E., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 85. [Google Scholar]
- Huang, X.; Anderson, K.W.; Zim, D.; Jiang, L.; Klapars, A.; Buchwald, S.L. Expanding Pd-Catalyzed C-N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions. J. Am. Chem. Soc. 2003, 125, 6653–6655. [Google Scholar] [CrossRef]
- Antilla, J.C.; Baskin, J.M.; Barder, T.E.; Buchwald, S.L. Copper−Diamine-Catalyzed N-Arylation of Pyrroles, Pyrazoles, Indazoles, Imidazoles, and Triazoles. J. Org. Chem. 2004, 69, 5578–5587. [Google Scholar] [CrossRef]
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef]
- Altman, R.A.; Buchwald, S.L. 4,7-Dimethoxy-1,10-phenanthroline: An Excellent Ligand for the Cu-Catalyzed N-Arylation of Imidazoles. Org. Lett. 2006, 8, 2779–2782. [Google Scholar] [CrossRef]
- Altman, R.A.; Koval, E.D.; Buchwald, S.L. Copper-Catalyzed N-Arylation of Imidazoles and Benzimidazoles. J. Org. Chem. 2007, 72, 6190–6199. [Google Scholar] [CrossRef]
- Klapars, A.; Buchwald, S.L. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845. [Google Scholar] [CrossRef]
- Sheppard, T.D. Metal-catalysed halogen exchange reactions of aryl halides. Org. Biomol. Chem. 2009, 7, 1043–1052. [Google Scholar] [CrossRef]
- Chen, M.; Ichikawa, S.; Buchwald, S.L. Rapid and efficient copper-catalyzed finkelstein reaction of (Hetero) aromatics under continuous-flow conditions. Angew. Chem. Int. Ed. 2015, 54, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Cristau, H.-J.; Cellier, P.P.; Spindler, J.-F.; Taillefer, M. Highly Efficient and Mild Copper-Catalyzed N- and C-Arylations with Aryl Bromides and Iodides. Chem. Eur. J. 2004, 10, 5607–5622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.J.D.; Jiang, Y.; Fu, H.; Zhao, Y. A Mild and Efficient Method for Copper-Catalyzed Ullmann-Type N-Arylation of Aliphatic Amines and Amino Acids. Synlett 2007, 12, 1836–1842. [Google Scholar] [CrossRef]
- Jiang, D.; Fu, H.; Jiang, Y.; Zhao, Y. CuBr/rac-BINOL-Catalyzed N-Arylations of Aliphatic Amines at Room Temperature. J. Org. Chem. 2007, 72, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Cai, Q. L-Proline promoted Ullmann-type coupling reactions of aryl iodides with indoles, pyrroles, imidazoles or pyrazoles. Synlett 2004, 1, 128–130. [Google Scholar] [CrossRef]
- Cai, Q.Z.W.; Zhang, H.; Zhang, Y.; Ma, D. Preparation of N-Aryl Compounds by Amino Acid-Promoted Ullmann-Type Coupling Reactions. Synthesis 2005, 3, 496–499. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Y.; Yao, J.; Wu, S.; Tao, F. Accelerating Effect Induced by the Structure of α-Amino Acid in the Copper-Catalyzed Coupling Reaction of Aryl Halides with α-Amino Acids. Synthesis of Benzolactam-V8. J. Am. Chem. Soc. 1998, 120, 12459–12467. [Google Scholar] [CrossRef]
- Shafir, A.; Buchwald, S.L. Highly Selective Room-Temperature Copper-Catalyzed C−N Coupling Reactions. J. Am. Chem. Soc. 2006, 128, 8742–8743. [Google Scholar] [CrossRef]
- Xi, Z.; Liu, F.; Zhou, Y.; Chen, W. CuI/L (L=pyridine-functionalized 1,3-diketones) catalyzed C–N coupling reactions of aryl halides with NH-containing heterocycles. Tetrahedron 2008, 64, 4254–4259. [Google Scholar] [CrossRef]
- Lv, X.; Bao, W. A β-Keto Ester as a Novel, Efficient, and Versatile Ligand for Copper(I)-Catalyzed C−N, C−O, and C−S Coupling Reactions. J. Org. Chem. 2007, 72, 3863–3867. [Google Scholar] [CrossRef]
- Modak, A.; Nett, A.J.; Swift, E.C.; Haibach, M.C.; Chan, V.S.; Franczyk, T.S.; Shekhar, S.; Cook, S.P. Cu-Catalyzed C–N Coupling with Sterically Hindered Partners. ACS Catal. 2020, 10, 10495–10499. [Google Scholar] [CrossRef]
- Terrier, F. Nucleophilic Aromatic Displacement: The Influence of the Nitro Group; VCH Publishers: New York, NY, USA, 1991; ISBN 0-89573-312-9. [Google Scholar]
- Mei, X.; August, A.T.; Wolf, C. Regioselective Copper-Catalyzed Amination of Chlorobenzoic Acids: Synthesis and Solid-State Structures of N-Aryl Anthranilic Acid Derivatives. J. Org. Chem. 2006, 71, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Maradolla, M.B.; Amaravathi, M.; Kumar, V.N.; Mouli, G.V.P.C. Regioselective copper-catalyzed amination of halobenzoic acids using aromatic amines. J. Mol. Catal. A Chem. 2007, 266, 47–49. [Google Scholar] [CrossRef]
- Docampo Palacios, M.L.; Pellon Comdom, R.F. Synthesis of 11H-Pyrido [2,1-b]quinazolin-11-one and Derivatives Using Ultrasound Irradiation. Synth. Commun. 2003, 33, 1777. [Google Scholar] [CrossRef]
- Allen, C.F.H.; McKee, G.H.W.; Hartman, W.W.; Weissberger, A. Acridone. Org. Synth. 1939, 19, 6. [Google Scholar] [CrossRef]
- Guo, X.; Rao, H.; Fu, H.; Jiang, Y.; Zhao, Y. An Inexpensive and Efficient Copper Catalyst for N-Arylation of Amines, Amides and Nitrogen-Containing Heterocycles. Adv. Synth. Catal. 2006, 348, 2197–2202. [Google Scholar] [CrossRef]
- Klapars, A.; Antilla, J.C.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycles. J. Am. Chem. Soc. 2001, 123, 7727–7729. [Google Scholar] [CrossRef]
- Klapars, A.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Ha lides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar] [CrossRef]
- Yang, K.; Qiu, Y.; Li, Z.; Wang, Z.; Jiang, S. Ligands for Copper-Catalyzed C-N Bond Forming Reactions with 1 Mol% CuBr as Catalyst. J. Org. Chem. 2011, 76, 3151–3159. [Google Scholar] [CrossRef]
- Liu, W.; Xu, J.; Chen, X.; Zhang, F.; Xu, Z.; Wang, D.; He, Y.; Xia, X.; Zhang, X.; Liang, Y. CuI/2-Aminopyridine 1-Oxide Catalyzed Amination of Aryl Chlorides with Aliphatic Amines. Org. Lett. 2020, 22, 7486–7490. [Google Scholar] [CrossRef]
- Bhunia, S.; Kumar, S.V.; Ma, D. N,N′-Bisoxalamides Enhance the Catalytic Activity in Cu-Catalyzed Coupling of (Hetero)Aryl Bromides with Anilines and Secondary Amines. J. Org. Chem. 2017, 82, 12603–12612. [Google Scholar] [CrossRef] [PubMed]
- Pawar, G.G.; Wu, H.; De, S.; Ma, D. Copper(I) Oxide/N,N′-Bis[(2-furyl)methyl]oxalamide-Catalyzed Coupling of (Hetero)aryl Halides and Nitrogen Heterocycles at Low Catalytic Loading. Adv. Synth. Catal. 2017, 359, 1631–1636. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Qi, L.; Sun, J.-P.; Long, J.-J. Synthesis of an anthraquinonoid disperse reactive dye based on a ligand-free Ullmann reaction. Color. Technol. 2017, 133, 283–292. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, L.; Zhao, Y. Cu(II)-Catalyzed C-N Coupling of (Hetero)aryl Halides and N-Nucleophiles Promoted by α-Benzoin Oxime. Molecules 2019, 24, 4177. [Google Scholar] [CrossRef]
- Yadav, D.K.T.; Rajak, S.S.; Bhanage, B.M. N-arylation of indoles with aryl halides using copper/glycerol as a mild and highly efficient recyclable catalytic system. Tetrahedron Lett. 2014, 55, 931–935. [Google Scholar] [CrossRef]
- Khatri, P.K.; Jain, S.L. Glycerol ingrained copper: An efficient recyclable catalyst for the N-arylation of amines with aryl halides. Tetrahedron Lett. 2013, 54, 2740–2743. [Google Scholar] [CrossRef]
- Bollenbach, M.; Wagner, P.; Aquino, P.G.V.; Bourguignon, J.-J.; Bihel, F.; Salomé, C.; Schmitt, M. d-Glucose: An Efficient Reducing Agent for a Copper(II)-Mediated Arylation of Primary Amines in Water. ChemSusChem 2016, 9, 3244–3249. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Yang, M.; Zhang, F.; Mao, F.; Yu, J.; Xia, X. Room-temperature Cu-catalyzed N-arylation of aliphatic amines in neat water. Org. Biomol. Chem. 2017, 15, 8009–8012. [Google Scholar] [CrossRef]
- Ferlin, F.; Trombettoni, V.; Luciani, L.; Fusi, S.; Piermatti, O.; Santoro, S.; Vaccaro, L. A waste-minimized protocol for copper-catalyzed Ullmann-type reaction in a biomass derived furfuryl alcohol/water azeotrope. Green Chem. 2018, 20, 1634–1639. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Baqi, Y. Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions. Catalysts 2021, 11, 46. [Google Scholar] [CrossRef]
- Zhu, X.; Su, L.; Huang, L.; Chen, G.; Wang, J.; Song, H.; Wan, Y. A Facile and Efficient Oxalyldihydrazide/Ketone-Promoted Copper-Catalyzed Amination of Aryl Halides in Water. Eur. J. Org. Chem. 2009, 2009, 635–642. [Google Scholar] [CrossRef]
- Colacino, E.; Villebrun, L.; Martinez, J.; Lamaty, F. PEG3400–Cu2O–Cs2CO3: An efficient and recyclable microwave-enhanced catalytic system for ligand-free Ullmann arylation of indole and benzimidazole. Tetrahedron 2010, 66, 3730–3735. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. Nat. Protoc. 2010, 5, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Baqi, Y.; Müller, C.E. Rapid and Efficient Microwave-Assisted Copper(0)-Catalyzed Ullmann Coupling Reaction: General Access to Anilinoanthraquinone Derivatives. Org. Lett. 2007, 9, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.M.; Rashed, M.; Wingen, L.; Baqi, Y.; Müller, C.E. Ullmann reactions of 1-amino-4-bromoanthraquinones bearing various 2-substituents furnishing novel dyes. Dyes Pigm. 2016, 131, 33–40. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Vitale, P.; Perna, F.M.; Capriati, V. Reshaping Ullmann Amine Synthesis in Deep Eutectic Solvents: A Mild Approach for Cu-Catalyzed C–N Coupling Reactions with No Additional Ligands. Front. Chem. 2019, 7, 723. [Google Scholar] [CrossRef]
- Mandal, B.; Ghosh, S.; Basu, B. Task-Specific Properties and Prospects of Ionic Liquids in Cross-Coupling Reactions. Top. Curr. Chem. 2019, 377, 30. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Z.; Bao, W. CuI catalyzed C–N bond forming reactions between aryl/heteroaryl bromides and imidazoles in [Bmim]BF4. Tetrahedron 2006, 62, 4756–4761. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling—A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
- Shaabani, A.; Afshari, R. Magnetic Ugi-functionalized graphene oxide complexed with copper nanoparticles: Efficient catalyst toward Ullman coupling reaction in deep eutectic solvents. J. Colloid Interface Sci. 2018, 510, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Zhu, L.; Lan, J.; Xie, R.; You, J. A Concept of Supported Amino Acid Ionic Liquids and Their Application in Metal Scavenging and Heterogeneous Catalysis. J. Am. Chem. Soc. 2007, 129, 13879–13886. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Mehrazin, L.; Hekmati, M.; Izadi, M.; Veisi, H. Biosynthesis of CuO nanoparticles using Rosa canina fruit extract as a recyclable and heterogeneous nanocatalyst for C-N Ullmann coupling reactions. Mater. Chem. Phys. 2018, 214, 527–532. [Google Scholar] [CrossRef]
- Rout, L.; Jammi, S.; Punniyamurthy, T. Novel CuO Nanoparticle Catalyzed C−N Cross Coupling of Amines with Iodobenzene. Org. Lett. 2007, 9, 3397–3399. [Google Scholar] [CrossRef] [PubMed]
- Kantam, M.L.; Yadav, J.; Laha, S.; Sreedhar, B.; Jha, S. N-arylation of heterocycles with activated chloro- and fluoroarenes using nanocrystalline copper(II) oxide. Adv. Synth. Catal. 2007, 349, 1938–1942. [Google Scholar] [CrossRef]
- Nasir Baig, R.B.; Varma, R.S. Magnetic silica supported copper: A modular approach to aqueous Ullmann-type amination of aryl halides. RSC Adv. 2014, 4, 6568–6572. [Google Scholar] [CrossRef]
- Kore, N.; Pazdera, P. New Stable Cu(I) Catalyst Supported on Weakly Acidic Polyacrylate Resin for Green C-N Coupling: Synthesis of N-(Pyridin-4-yl)benzene Amines and N,N-Bis(pyridine-4-yl)benzene Amines. Molecules 2017, 22, 2. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, N.S.; Sreedhar, B.; Kantam, M.L. N-Arylation of nitrogen heterocycles with aryl halides and arylboronic acids catalyzed by cellulose supported copper(0). J. Mol. Catal. A Chem. 2006, 252, 136–141. [Google Scholar] [CrossRef]
- Hemmati, S.; Kamangar, S.A.; Yousefi, M.; Salehi, M.H.; Hekmati, M. Cu(I)-anchored polyvinyl alcohol coated-magnetic nanoparticles as heterogeneous nanocatalyst in Ullmann type C–N coupling reactions. Appl. Organometal. Chem. 2020, 34, e5611. [Google Scholar] [CrossRef]
- Gorginpour, F.; Zali-Boeini, H.; Rudbari, H.A. A quinoxaline-based porous organic polymer containing copper nanoparticles CuNPs@Q-POP as a robust nanocatalyst toward C–N coupling reaction. RSC Adv. 2021, 11, 3655–3665. [Google Scholar] [CrossRef]
- Sreedhar, B.; Arundhathi, R.; Reddy, P.L.; Kantam, M.L. CuI Nanoparticles for C−N and C−O Cross Coupling of Heterocyclic Amines and Phenols with Chlorobenzenes. J. Org. Chem. 2009, 74, 7951–7954. [Google Scholar] [CrossRef] [PubMed]
- Veisi, H.; Hamelian, M.; Hemmati, S.; Dalvand, A. CuI catalyst heterogenized on melamine-pyridines immobilized SBA-15: Heterogeneous and recyclable nanocatalyst for Ullmann-type CN coupling reactions. Tetrahedron Lett. 2017, 58, 4440–4446. [Google Scholar] [CrossRef]
- Veisi, H.; Hamelian, M.; Hemmati, S. Palladium anchored to SBA-15 functionalized with melamine-pyridine groups as a novel and efficient heterogeneous nanocatalyst for Suzuki–Miyaura coupling reactions. J. Mol. Catal. A Chem. 2014, 395, 25–33. [Google Scholar] [CrossRef]
- Veerakumar, P.; Velusamy, N.; Thanasekaran, P.; Lin, K.-C.; Rajagopal, S. Copper supported silica-based nanocatalysts for CuAAC and cross-coupling reactions. React. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Xue, F.; Chen, G.; Yuan, G. Nitrogen-rich copolymeric microsheets supporting copper nanoparticles for catalyzing arylation of N-heterocycles. Appl. Catal. A Gen. 2011, 403, 104–111. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, G.; Chen, C.; Liu, Y.; Zhang, X. Gram-Scale Synthesis of Polymeric Carbon Nitride-Supported Copper: A Practical Catalyst for Ullmann-Type C—N Coupling Modifying Secondary Pyrimidin-2-amines without Additional Ligand. Chin. J. Org. Chem. 2021, 41, 1216–1223. [Google Scholar] [CrossRef]
- Mohan, A.; Dutta, S.; Balusamy, S.; Madav, V. Liquid fuel from waste tires: Novel refining, advanced characterization and utilization in engines with ethyl levulinate as an additive. RSC Adv. 2021, 11, 9807–9826. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Surface-Mediated Formation of Polybrominated Dibenzo-p-dioxins and Dibenzofurans from the High-Temperature Pyrolysis of 2-Bromophenol on a CuO/Silica Surface. Environ. Sci. Technol. 2005, 39, 4857–4863. [Google Scholar] [CrossRef]
- Bhari, R.; Kaur, M.; Sarup Singh, R. Chicken Feather Waste Hydrolysate as a Superior Biofertilizer in Agroindustry. Curr. Microbiol. 2021, 78, 2212–2230. [Google Scholar] [CrossRef]
- Amal Joseph, P.J.; Priyadarshini, S. Copper-Mediated C-X Functionalization of Aryl Halides. Org. Proc. Res. Dev. 2017, 21, 1889–1924. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, H.; Zou, B.; Xie, X.; Zhu, W.; He, G.; Wang, J.; Pan, X.; Chen, Y.; Yuan, Q.; et al. Amino acid-promoted Ullmann-type coupling reactions and their applications in organic synthesis. Pure Appl. Chem. 2009, 81, 227–234. [Google Scholar] [CrossRef]
- Bates, C.G.; Gujadhur, R.K.; Venkataraman, D. A General Method for the Formation of Aryl−Sulfur Bonds Using Copper(I) Catalysts. Organic Lett. 2002, 4, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Jing, B.; Liu, S.; Chae, J.; Liu, Y. Copper-Catalyzed Direct Synthesis of Aryl Thiols from Aryl Iodides Using Sodium Sulfide Aided by Catalytic 1,2-Ethanedithiol. Synlett 2017, 28, 2272–2276. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chen, Y.-L.; Reddy, D.M.; Du, K.; Li, C.-E.; Shih, B.-H.; Xue, Y.-J.; Lee, C.-F. CuI/Oxalic Diamide-Catalyzed Cross-Coupling of Thiols with Aryl Bromides and Chlorides. Chem. Eur. J. 2017, 23, 10087–10091. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.; Jiang, Y.; Ma, D. Assembly of α-(Hetero)aryl Nitriles via Copper-Catalyzed Coupling Reactions with (Hetero)aryl Chlorides and Bromides. Angew. Chem. Int. Ed. 2021, 60, 7082–7086. [Google Scholar] [CrossRef]
- Oeser, P.; Koudelka, J.; Petrenko, A.; Tobrman, T. Recent Progress Concerning the N-Arylation of Indoles. Molecules 2021, 26, 5079. [Google Scholar] [CrossRef]
- Lyakhovich, M.S.; Averin, A.D.; Grigorova, O.K.; Roznyatovsky, V.A.; Maloshitskaya, O.A.; Beletskaya, I.P. Cu(I)- and Pd(0)-Catalyzed Arylation of Oxadiamines with Fluorinated Halogenobenzenes: Comparison of Efficiency. Molecules 2020, 25, 1084. [Google Scholar] [CrossRef]
- Fui, C.J.; Sarjadi, M.S.; Sarkar, S.M.; Rahman, M.L. Recent Advancement of Ullmann Condensation Coupling Reaction in the Formation of Aryl-Oxygen (C-O) Bonding by Copper-Mediated Catalyst. Catalysts 2020, 10, 1103. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q. Copper/Amino Acid Catalyzed Cross-Couplings of Aryl and Vinyl Halides with Nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Diyali, N.; Rasaily, S.; Biswas, B. Metal–Organic Framework: An Emergent Catalyst in C–N Cross-Coupling Reactions. Coord. Chem. Rev. 2022, 469, 214667. [Google Scholar] [CrossRef]
- Huang, H.; Yan, X.; Zhu, W.; Liu, H.; Jiang, H.; Chen, K. Efficient Copper-Promoted N-Arylations of Aryl Halides with Amines. J. Comb. Chem. 2008, 10, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Averin, A.D. Metal-catalyzed reactions for the C(sp2)–N bond formation: Achievements of recent years. Russ. Chem. Rev. 2021, 90, 1359–1396. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Adler, P.; Grison, C. Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis. Catalysts 2019, 9, 214. [Google Scholar] [CrossRef]
- Venkateswarlu, K. Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett. 2021, 19, 3887–3950. [Google Scholar] [CrossRef]
- Weidlich, T.; Krejcova, A.; Prokes, L. Hydrodebromination of 2,4,6-tribromophenol in aqueous solution using Devarda’s alloy. Monatsh. Chem. 2013, 144, 155–162. [Google Scholar] [CrossRef]
- Weidlich, T.; Kamenicka, B.; Melanova, K.; Cicmancova, V.; Komersova, A.; Cermak, J. Hydrodechlorination of Different Chloroaromatic Compounds at Room Temperature and Ambient Pressure-Differences in Reactivity of Cu- and Ni-Based Al Alloys in an Alkaline Aqueous Solution. Catalysts 2020, 10, 994. [Google Scholar] [CrossRef]
- Weidlich, T.; Oprsal, J.; Krejcova, A.; Jasurek, B. Effect of glucose on lowering Al-Ni alloy consumption in dehalogenation of halogenoanilines. Monatsh. Chem. 2015, 146, 613–620. [Google Scholar] [CrossRef]
- Weidlich, T. Applicability of Nickel-Based Catalytic Systems for Hydrodehalogenation of Recalcitrant Halogenated Aromatic Compounds. Catalysts 2021, 11, 1465. [Google Scholar] [CrossRef]
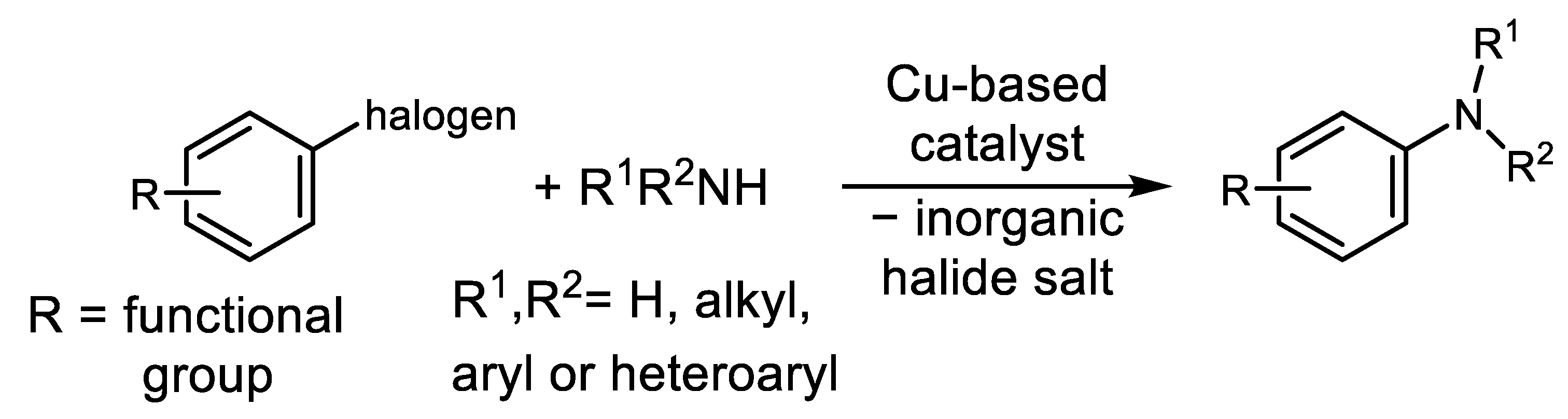

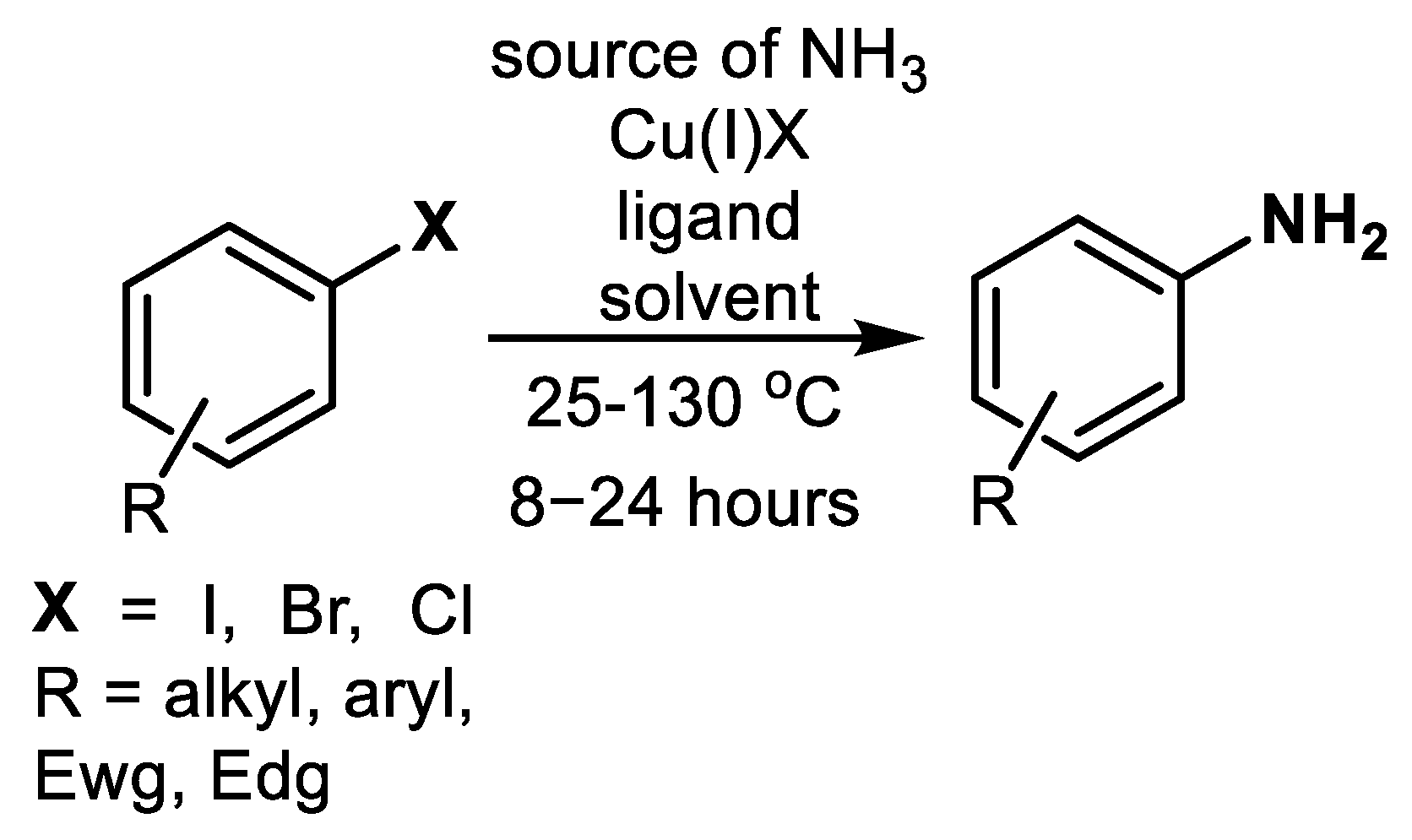







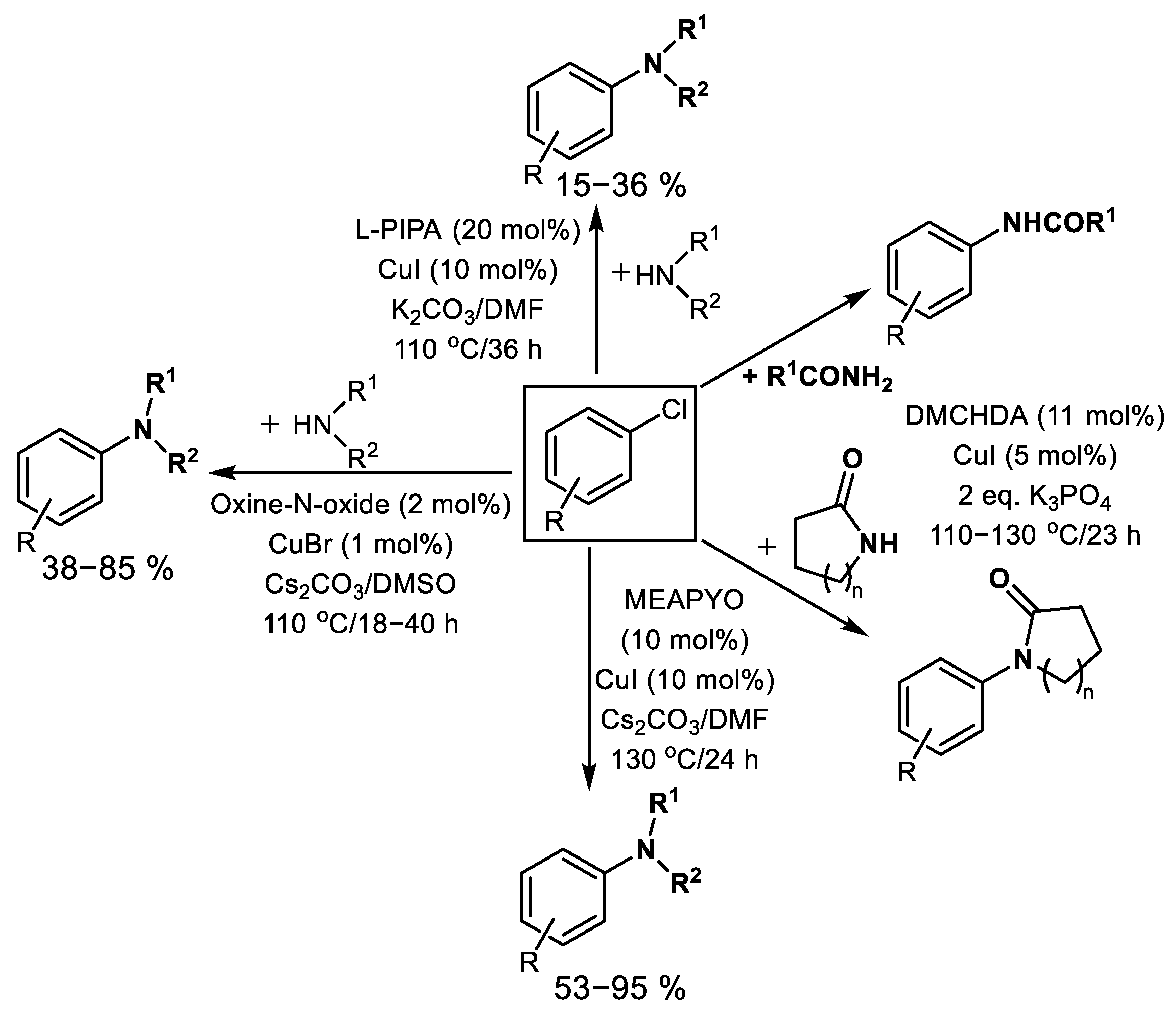
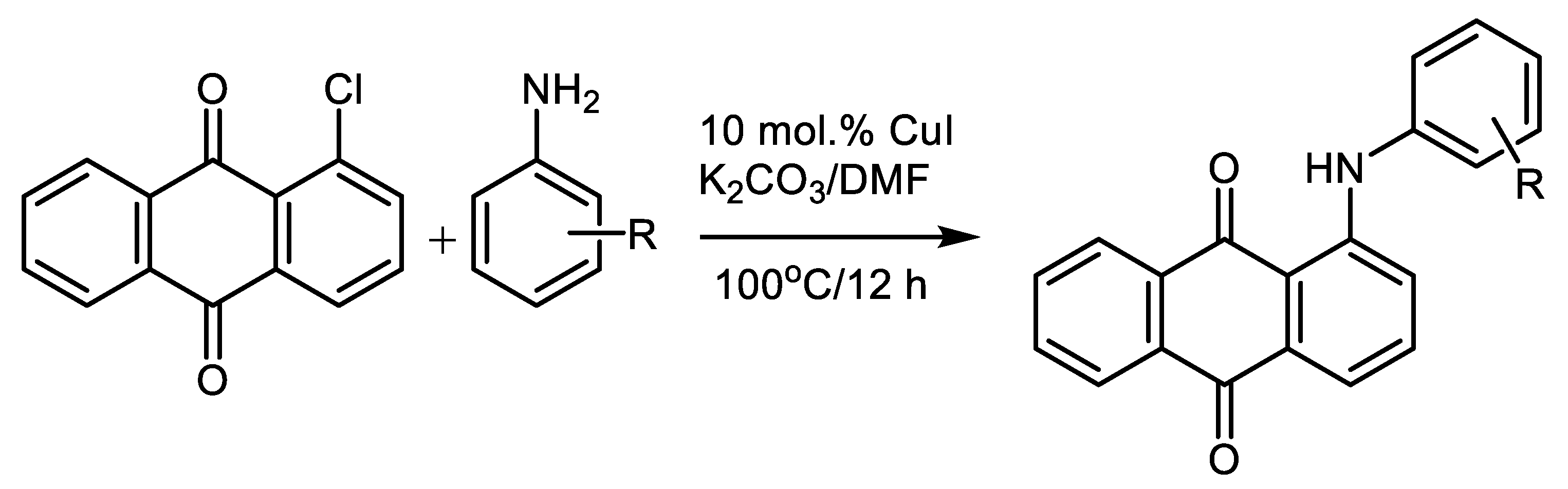
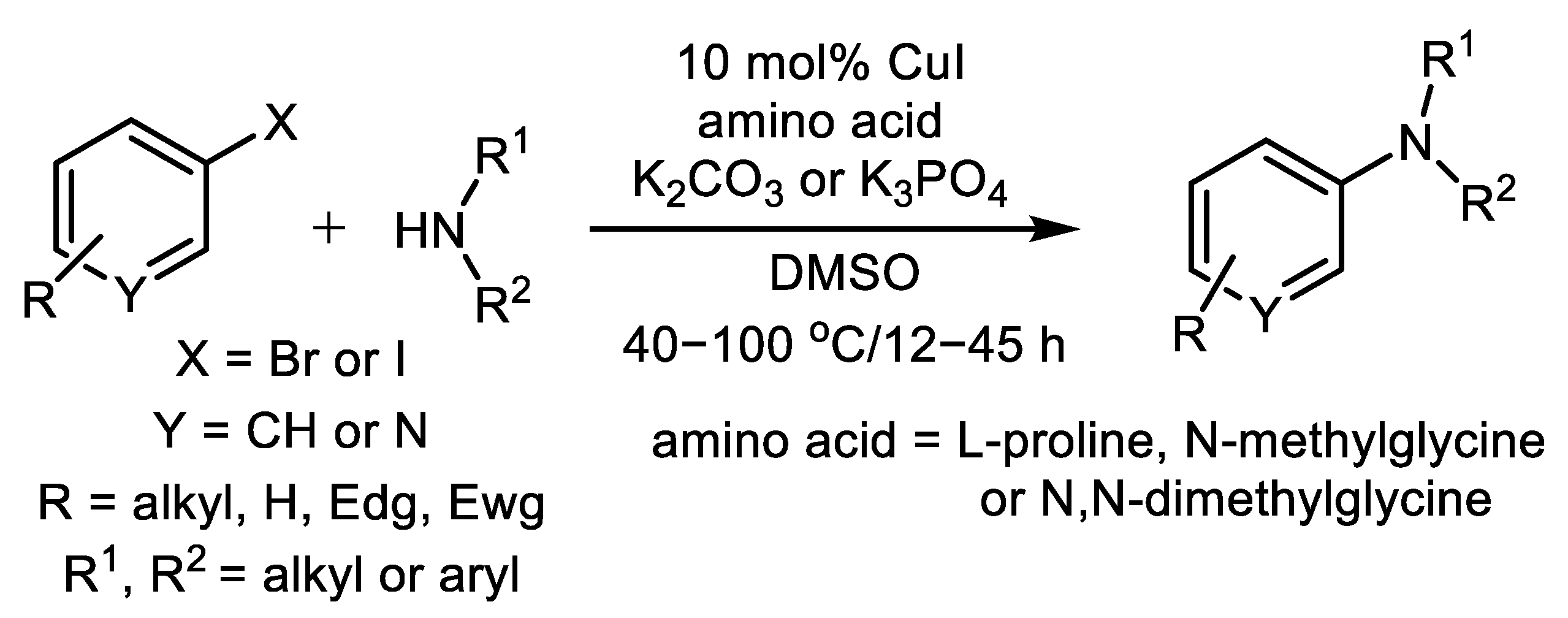
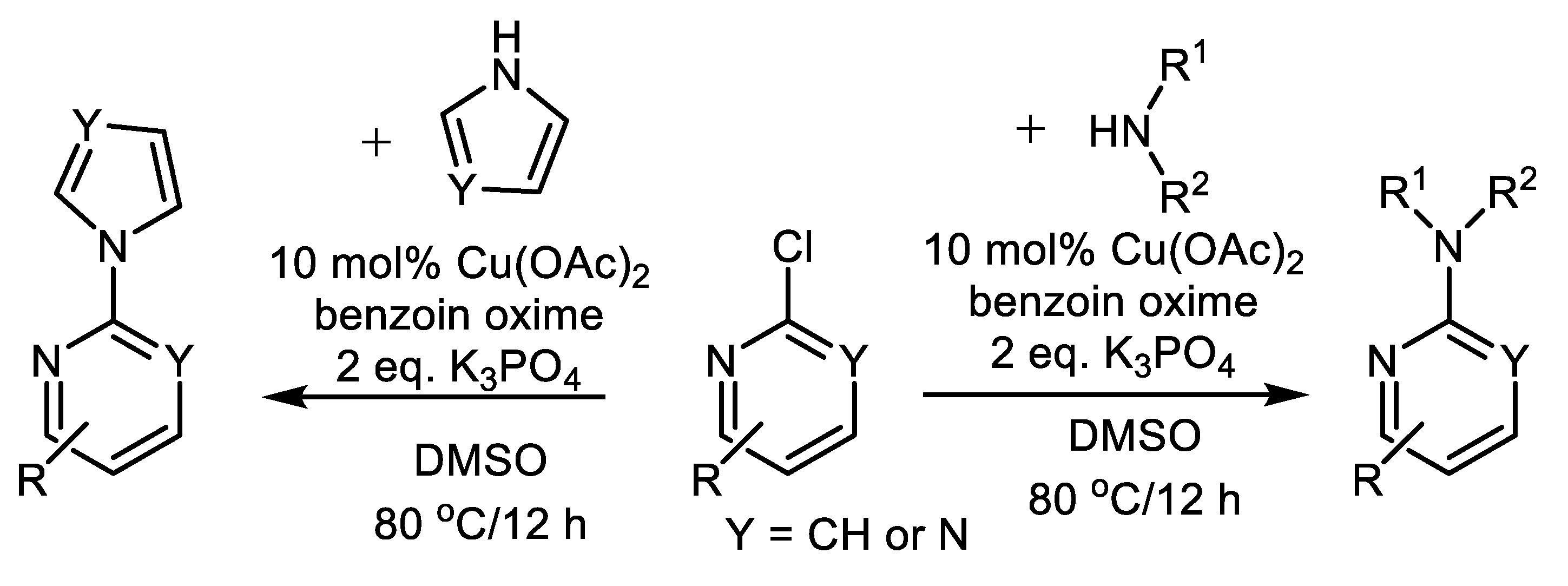
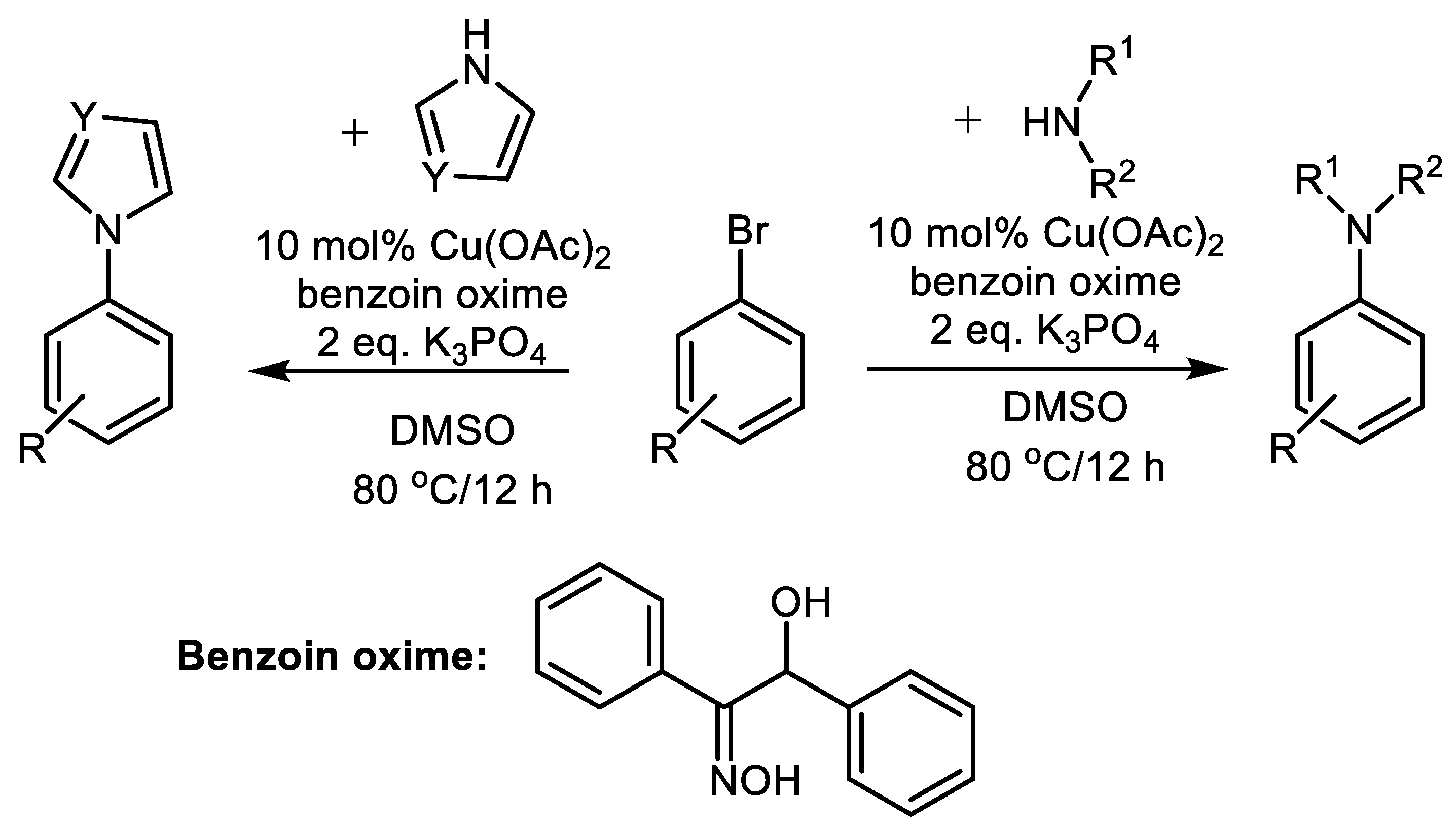
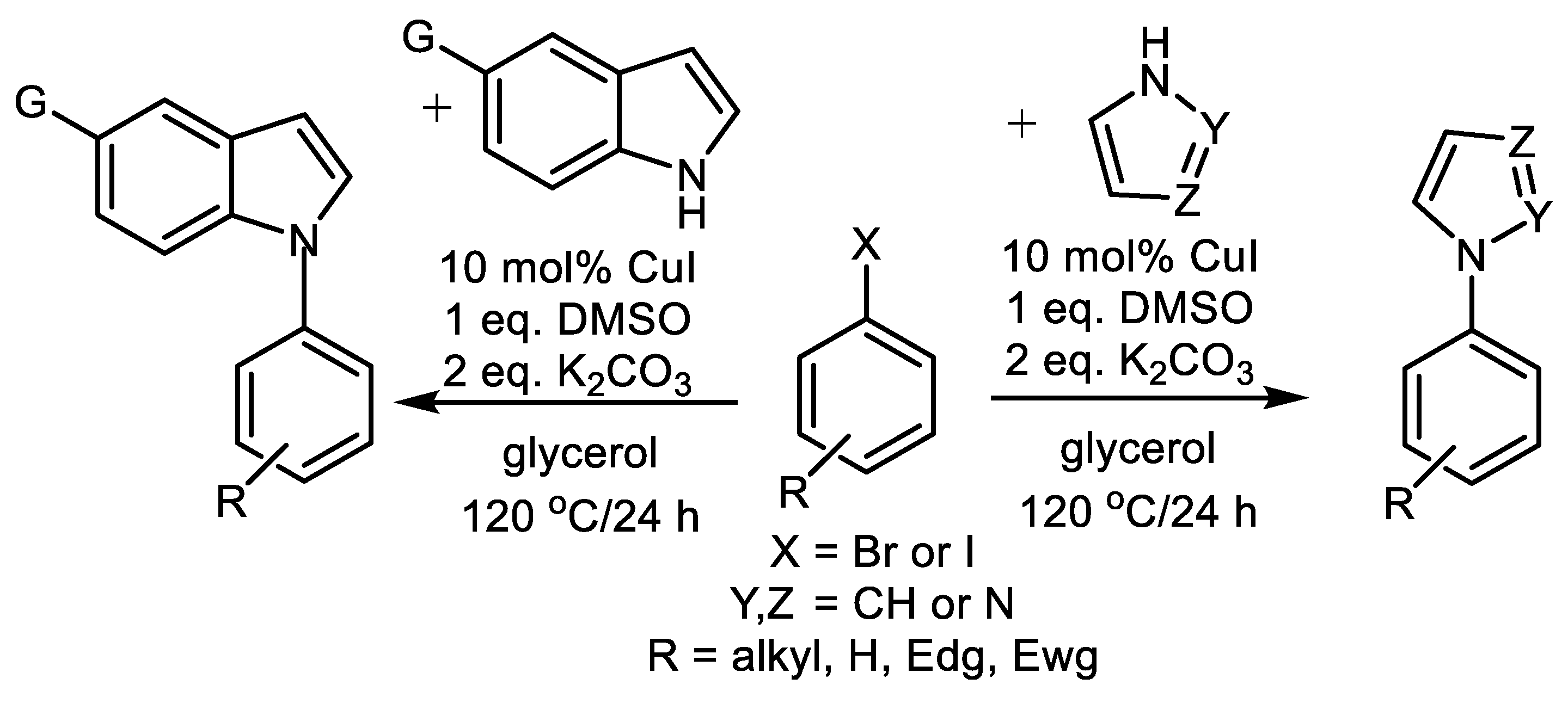
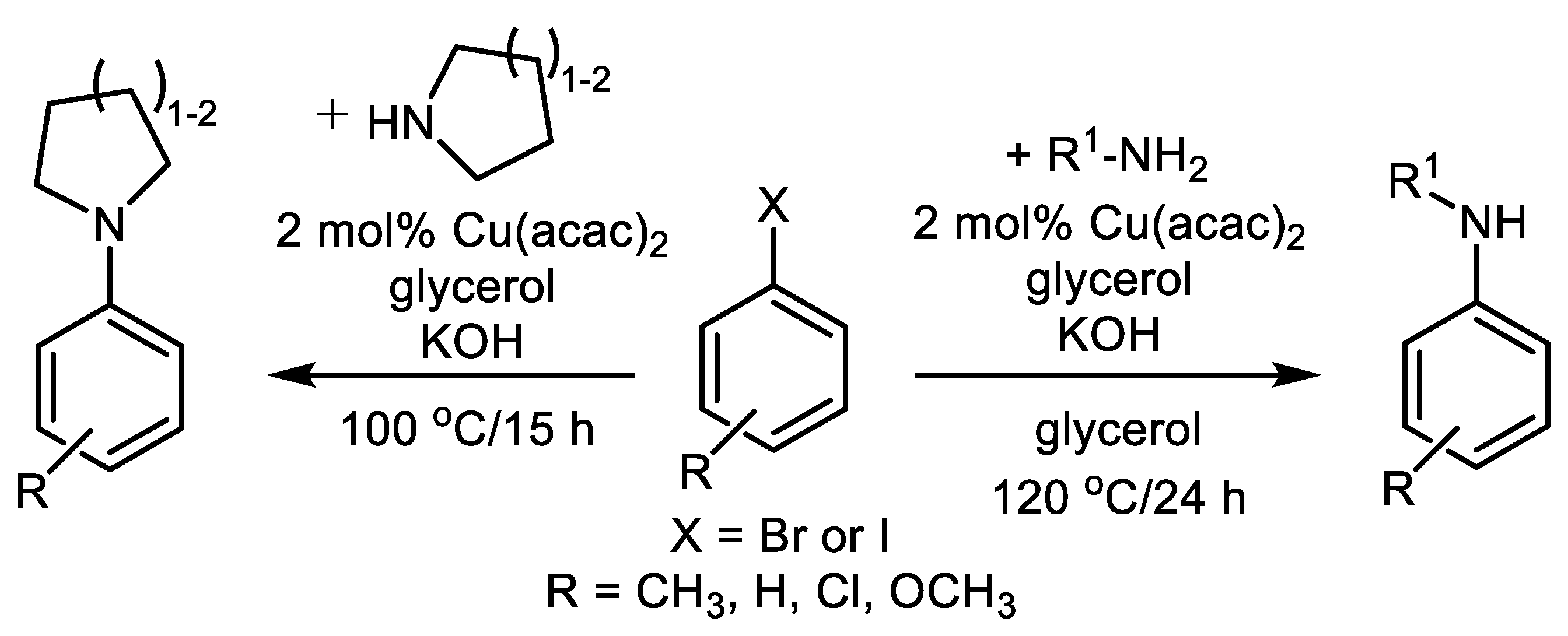

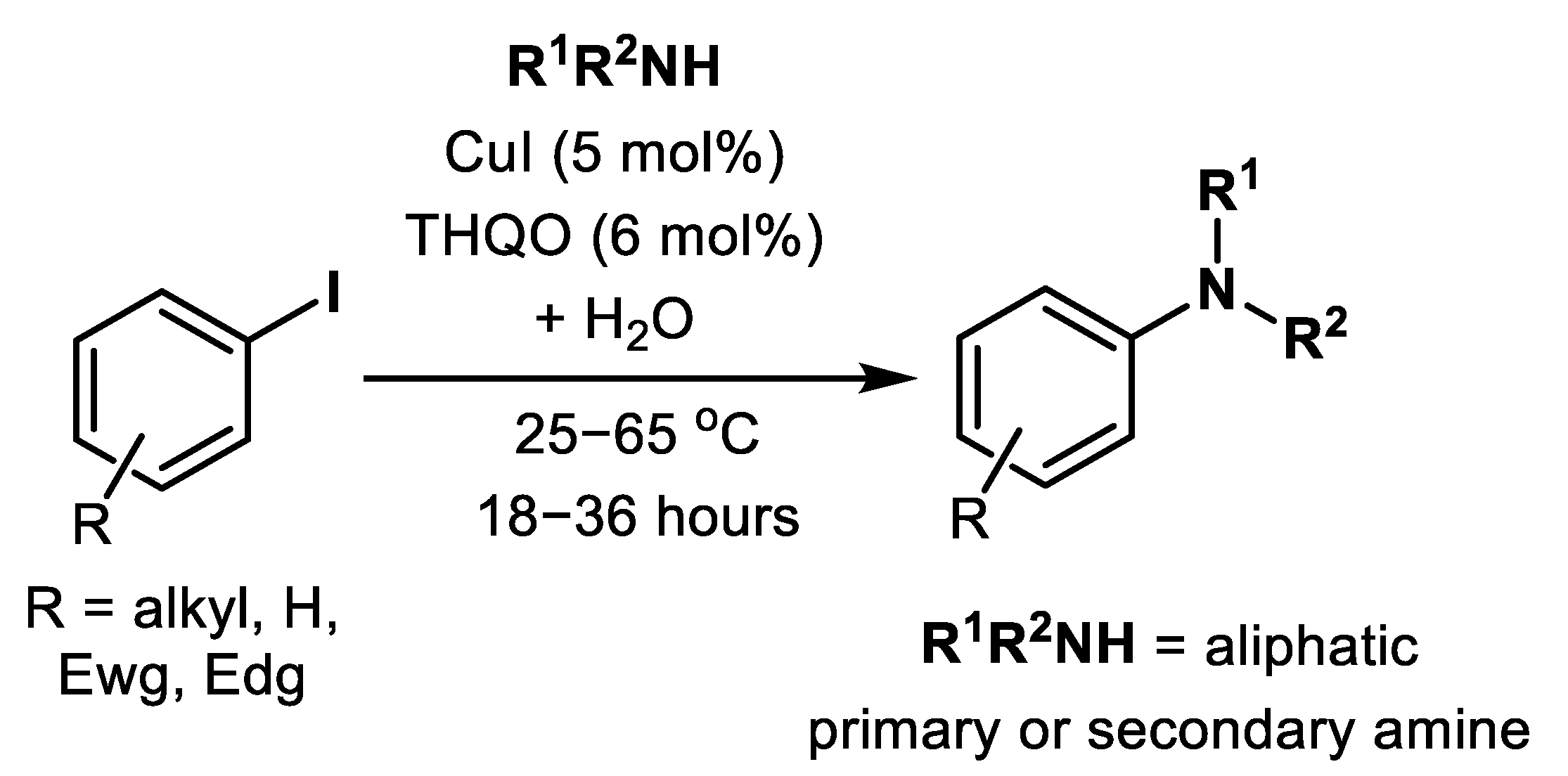
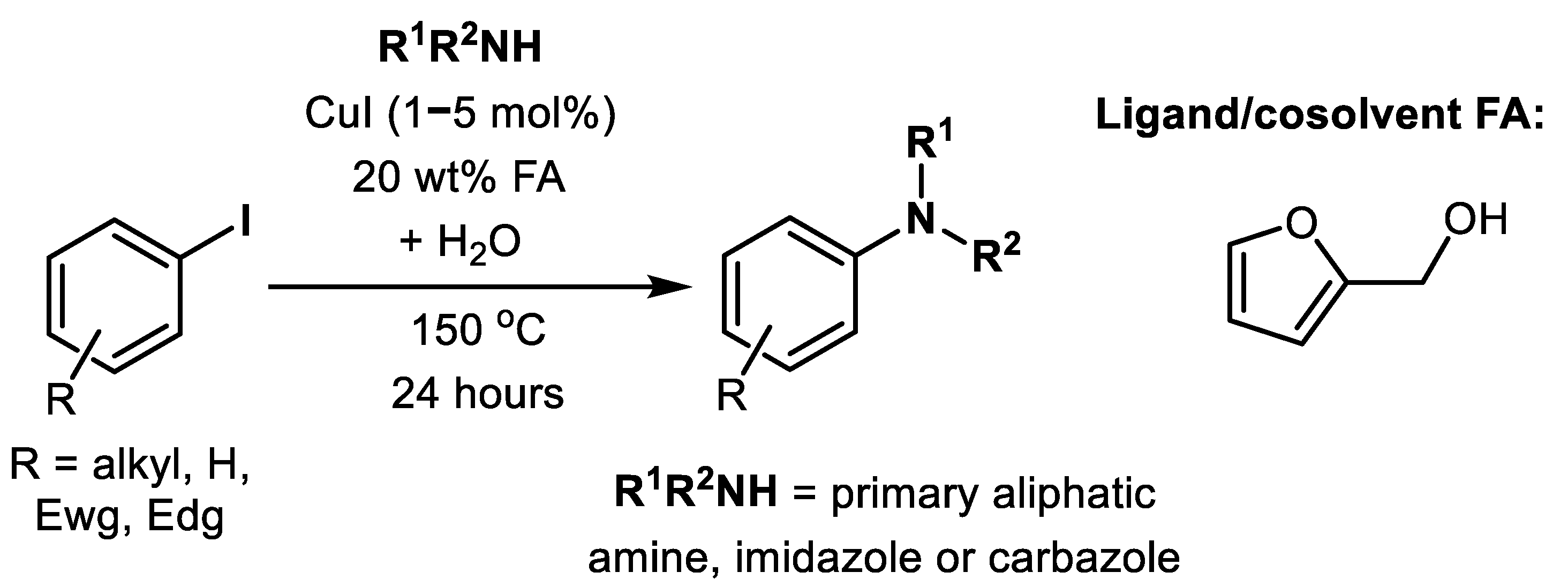






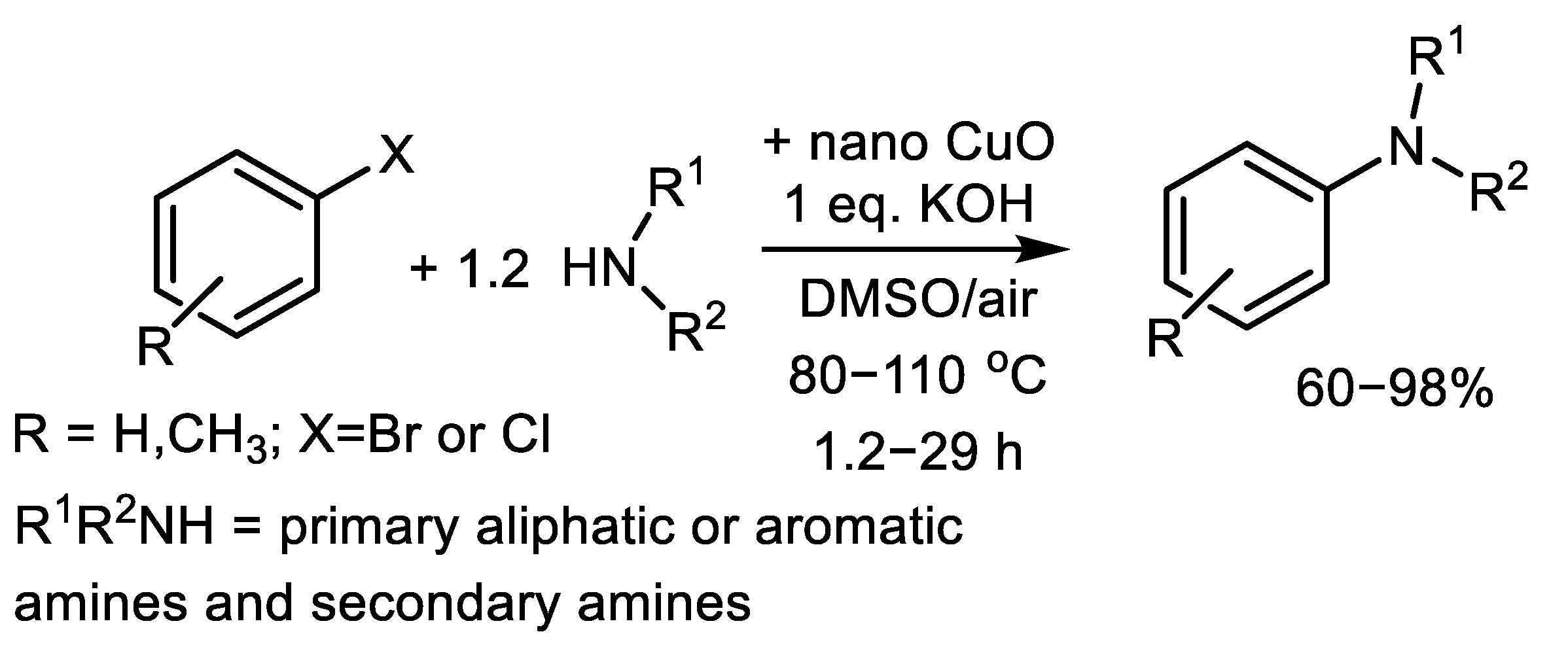


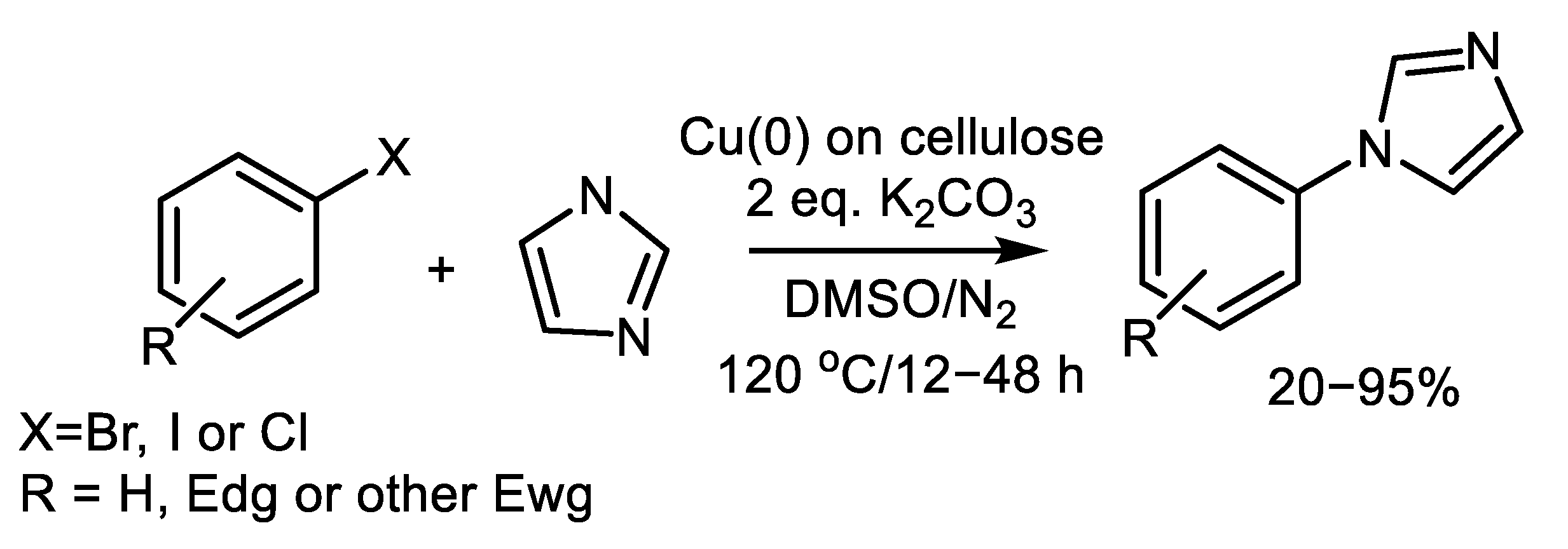
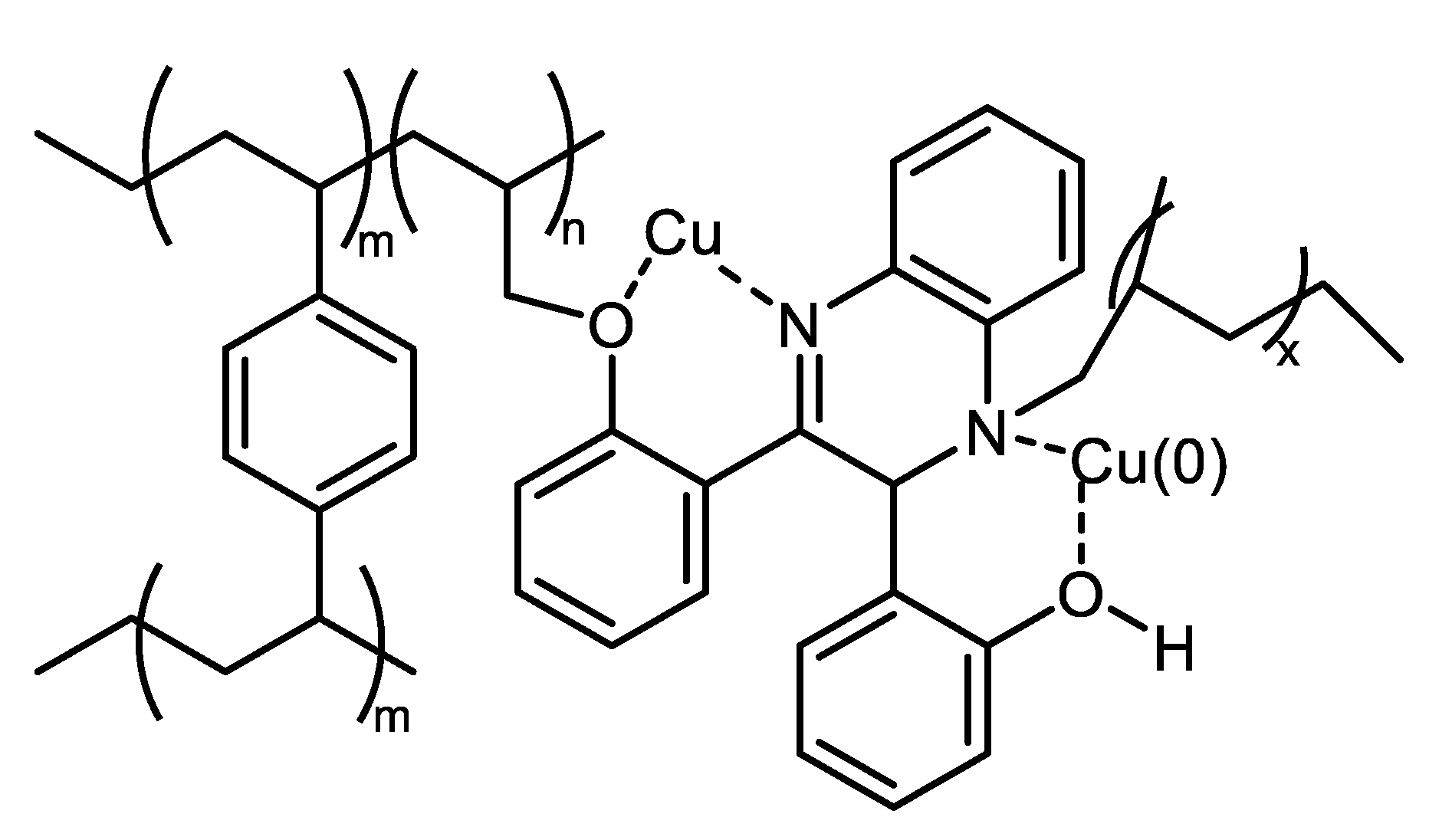
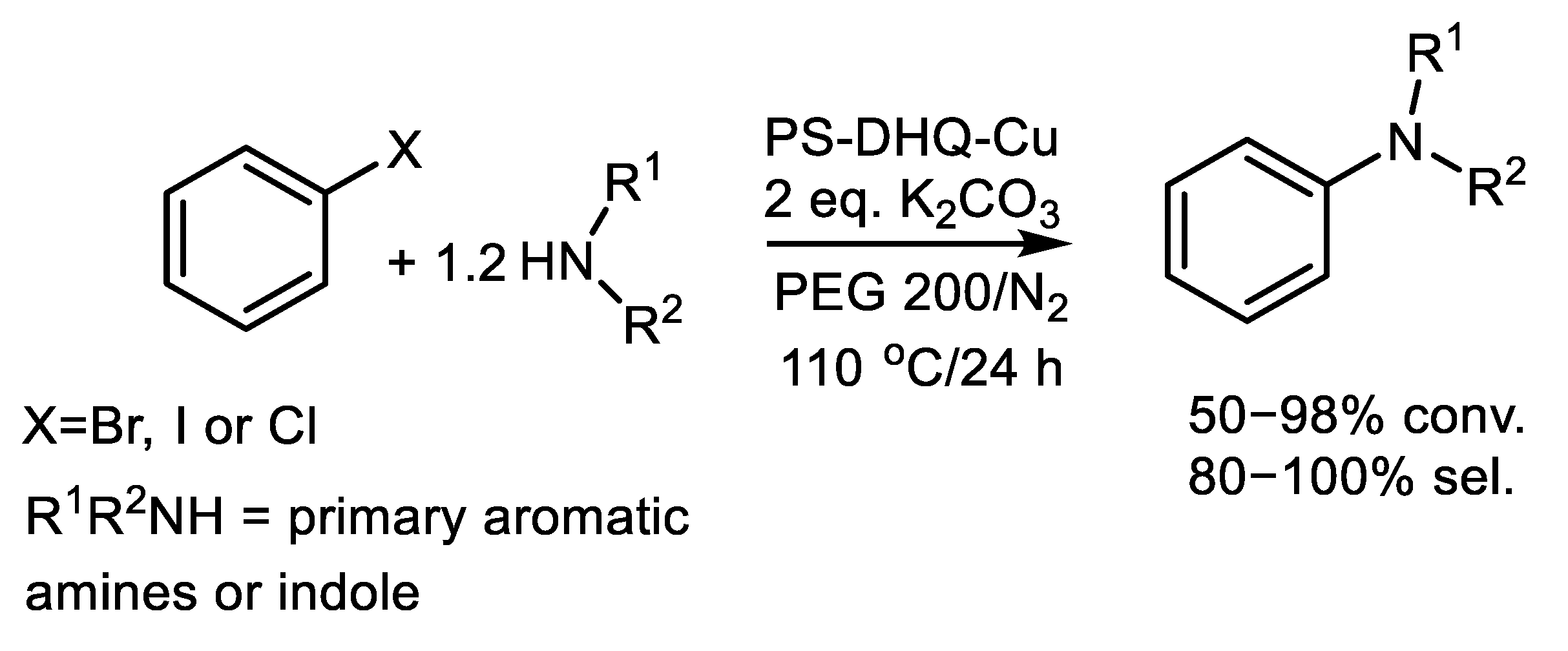
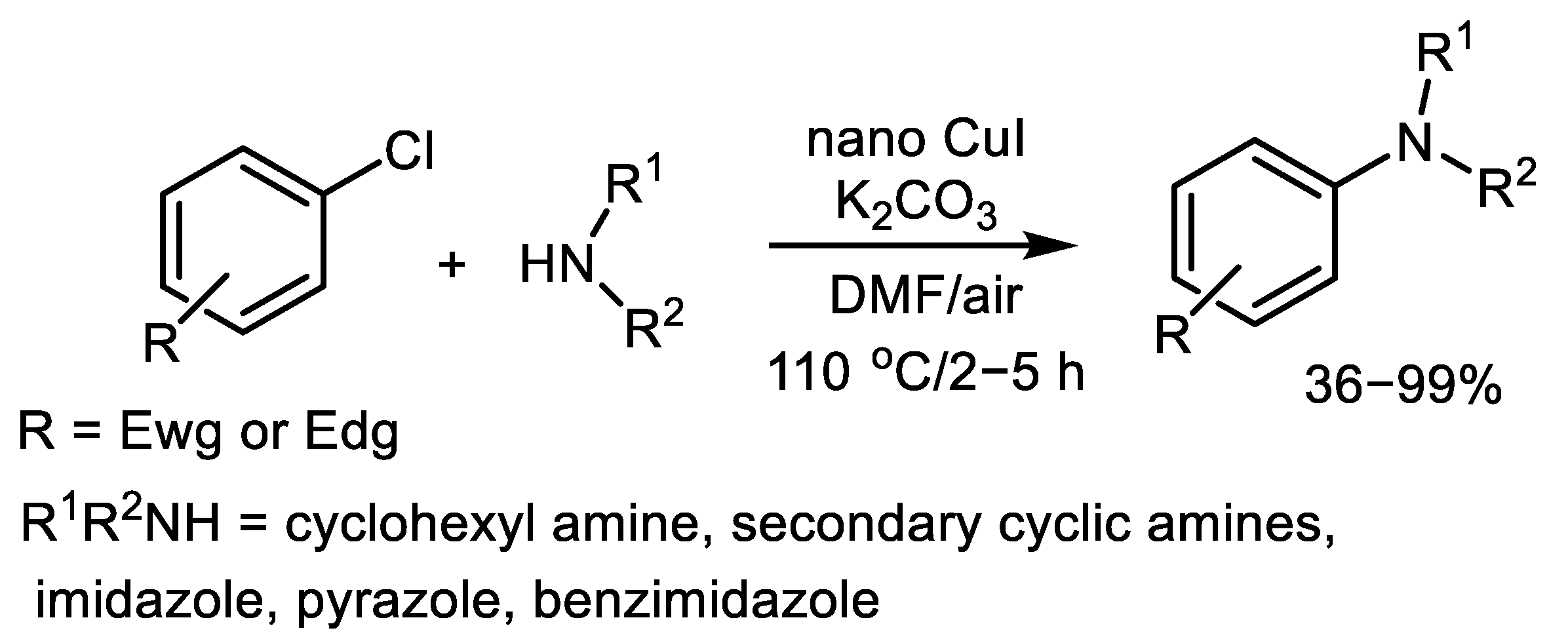

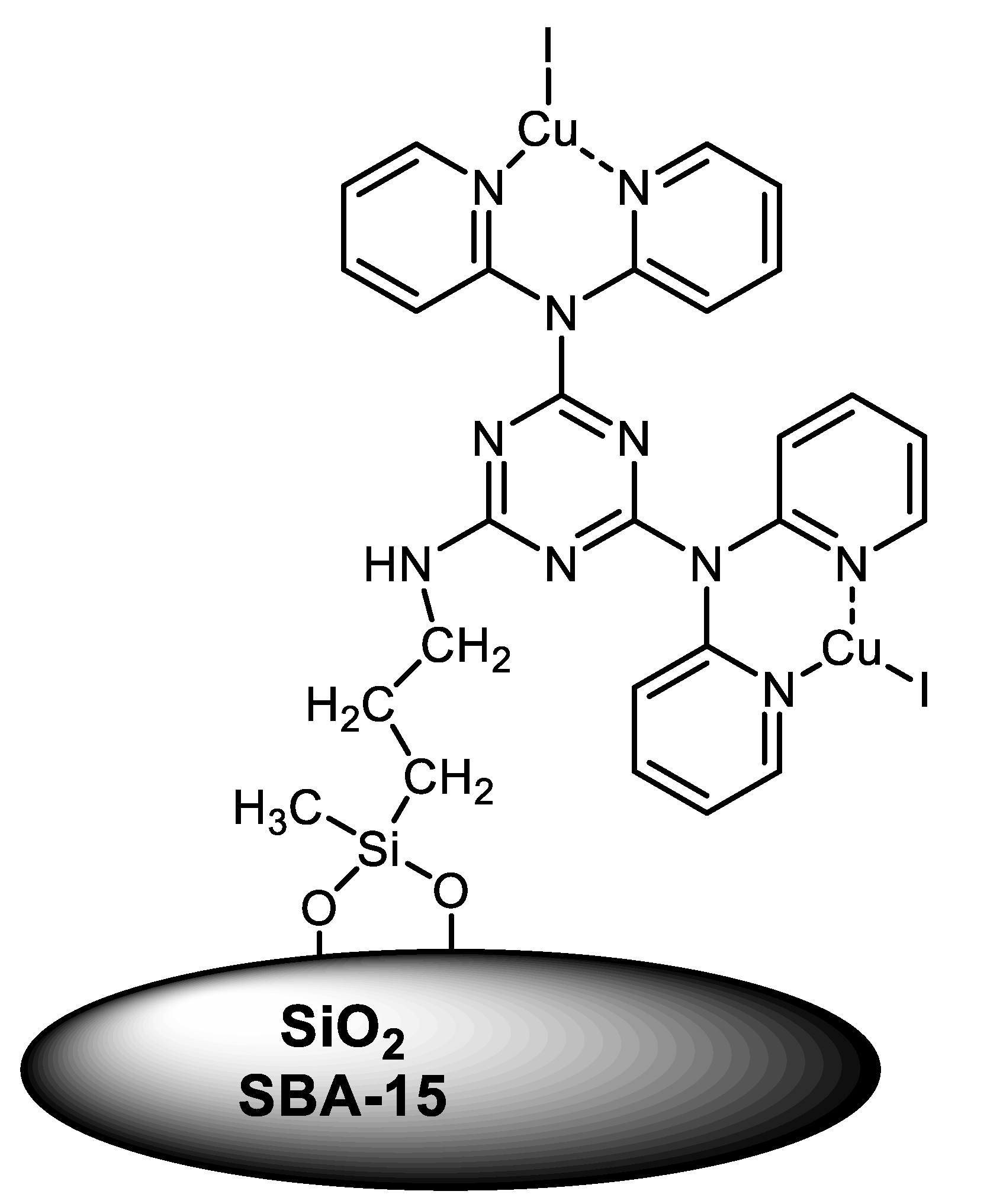
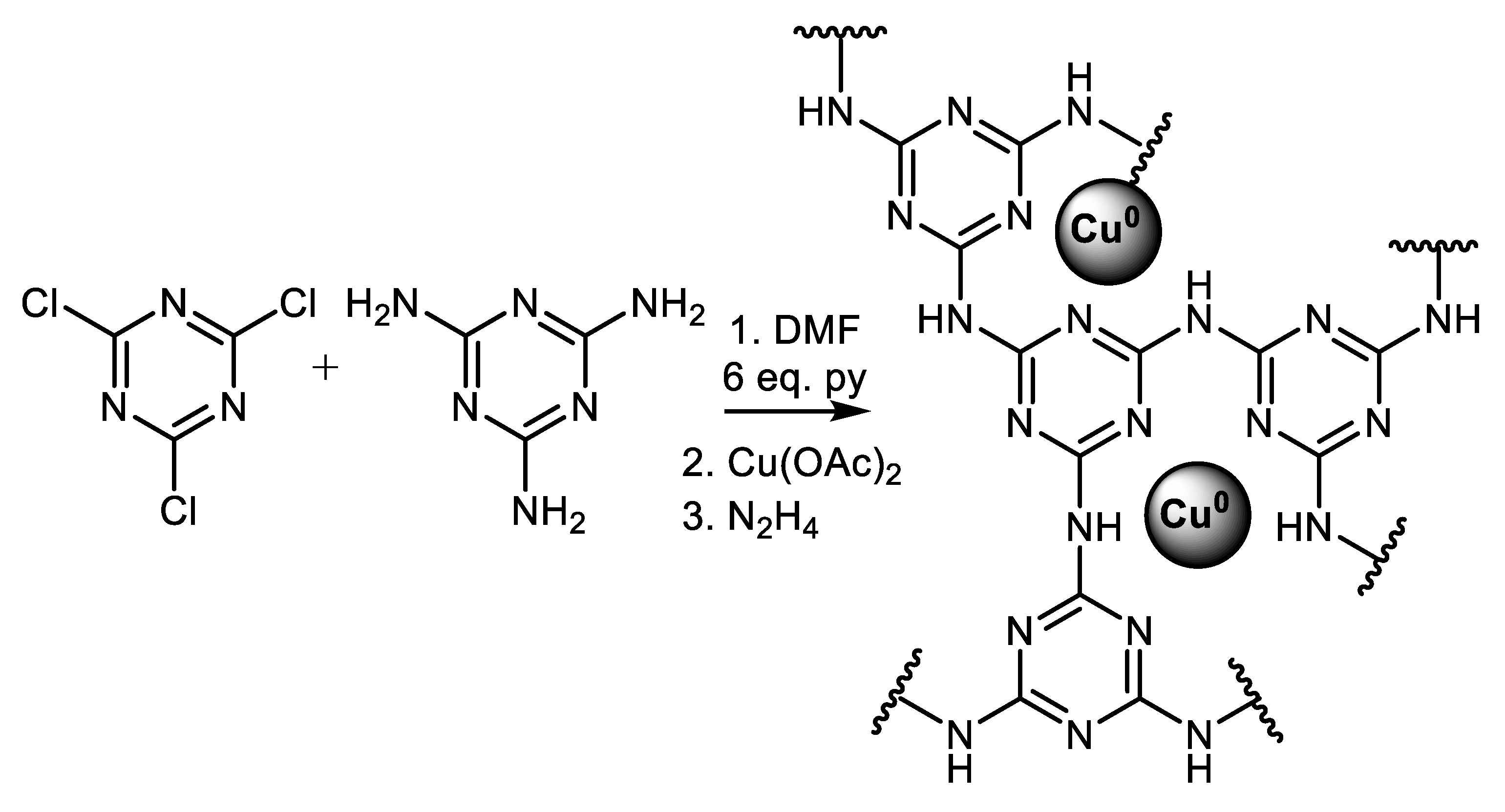
| (Hetero)Aryl Halide (Ar-X) | Added Catalyst | Added Ligand (mol.%) | Base/Solvent | Reaction Conditions | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Bromopyridines or iodobenzene | Cu2O or Cu/CuCl (0.5 wt.%) | no | 8 M NH3 in HOCH2CH2OH | 80 °C/16 h in autoclave flushed Ar | 62–99 | [38] |
| Subst. 2-bromopyridines | Cu2O (2 mol.%) | no | HOCH2CH2OH saturated with NH3 | 100 °C/24 h in autoclave | 54–82 | [39] |
| 4-bromo-acetophenone | CuI (equimolar quantity to substrate) or Cu powder (20 mol.%) | no | 27 wt.% NH3 in H2O + 6 M NH3 in HOCH2CH2OH (3:5) | flushed with N2 85 °C/8–12 h (0.3–1.2 MPa) | 77–86 | [40] |
| Subst. Bromo- and iodobenzenes (chlorobenzenes do not react) | CuI (equimolar quantity to substrate) + Cu powder (20 mol.%) | no | 27 wt.% NH3 in H2O + 6 M NH3 in HOCH2CH2OH (3:5) | flushed with N2 50–85 °C/8–16 h (ambient pressure) | 37–85 | [40] |
| 3- and 4-subst. Iodobenzenes (2-iodo- with low conversion) | CuI (10 mol.%) | L-proline (20 mol.%) | 1 eq. NH4Cl + 3 eq. K2CO3 DMSO + 5 vol.% H2O | Under Ar 25 °C/12 h | 32–98 | [48] |
| 3- and 4-subst. iodobenzenes (2-iodo- with low conversion) | CuI (20 mol.%) | L-proline (40 mol.%) | 1.5 eq. 28% aq. NH4OH + 3 eq. K2CO3 + DMSO | Under Ar 25 °C/24 h | 77–97 | [48] |
| Iodoanilines | CuI (10 mol.%) | L-proline (20 mol.%) | 1.2 eq. of acetamidine.HCl 2–3 eq. Cs2CO3 In DMF | Under N2 110–120 °C/10 h | 64–92 | [49] |
| Subst. bromo- and iodobenzenes (Ar-Cls do not react) | CuI (20 mol.%) | no | 1.2 eq. of valine 1.5 eq. Cs2CO3 in DMSO | (a) 90 °C/24 h under Ar (b) 90 °C/24 h under O2 | 28–90 | [41] |
| (Hetero)Aryl Halide (Ar-X) | Added Catalyst | Added Ligand (mol.%) | Base/Solvent | Reaction Conditions | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Subst. bromobenzenes | CuI (20 mol.%) | 4-OH-L-proline (40 mol.%) | aq. NH4OH + DMSO (1:2) | 50 °C/24 h under N2 | 55–92 | [50] |
| Subst. bromo- and iodobenzenes | Cu(acac)2 (10 mol.%) | Acac (40 mol.%) | 2 eq. Cs2CO3 aq.NH4OH/DMF (0.6:4) | 70–90 °C/24 h under N2 | 23–99 (23% ortho- subst.) | [51] |
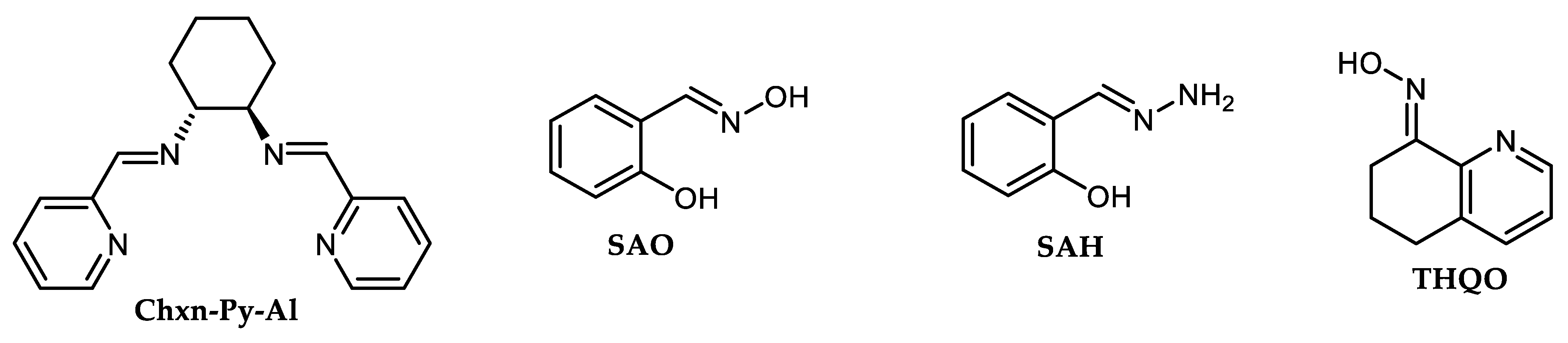
| Subst. Ph-Br (chlorobenzenes do not react) | CuBr (10 mol.%) | THQMeProne (20 mol.%) | 2.5 eq. K3PO4 5 eq. aq.NH4OH in DMSO | 110 °C/24 h under Ar | 52–95 (52% ortho) | [52] |
| Subst. iodobenzenes | CuBr (5 mol.%) | THQMeProne (10 mol.%) | 2.5 eq. K3PO4 5 eq. aq.NH4OH in DMSO | 25 °C/24 h under Ar | 27–95 (27% ortho) | [52] |
| Brominated N-heterocycles | Cu(acac)2 (5 mol.%) | no | 1 eq. K3PO4 20 eq. NH3/DMF | 90 °C/24 h under N2 | 48–88 | [42] |
| Subst. bromo- and iodobenzenes, NO2-chloro-benzenes | CuI (1 mol.%) | AA (1 mol.%) | NH3 (l) | 100 °C/18 h in autoclave | 63–99 | [53] |
| Bromobenzenes | CuI (10 mol.%) | DMEDA (15 mol.%) | 27 wt.% NH3 in H2O/DMSO (3:1) | 130 °C/6–18 h in autoclave flushed Ar | 84–96 | [43] |
| Bromobenzenes | CuI (10 mol.%) | no | 27 wt.% NH3 in H2O/PEG300 (3:1) | 130 °C/12–24 h in autoclave flushed Ar | 85–99 | [43] |
| Subst. bromo- and iodobenzenes | Cu2O (0.1 mol.%) | Ar-Br: MNFMO (0.1 mol.%) Ar-I: NFMO (0.1 mol.%) | 1.3 eq. KOH + 27 wt.% NH3 in H2O + EtOH (2:1) | Ar-Br: 80 °C/24 h Ar-I: 60 °C/24 h Under Ar | 64–98 | [44] |
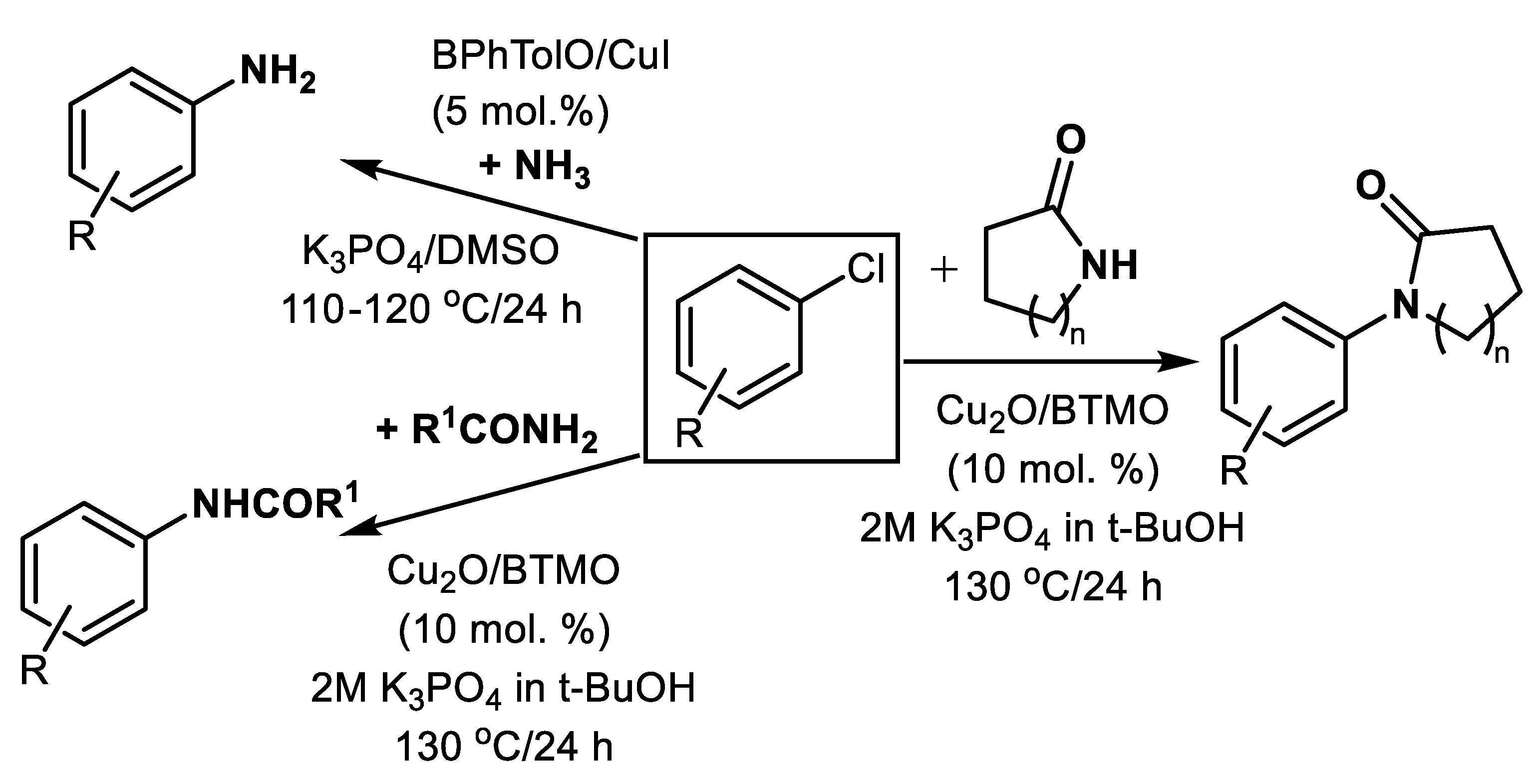
| Subst. Chlorobenzenes and chlorinated heterocycles | CuI (5 mol.%) | BPhTolO (5 mol.%) | 1.1 eq. K3PO4 2 eq. aq.NH4OH in DMS | 110–120 °C 24 h Under Ar | 60–95 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidlich, T.; Špryncová, M.; Čegan, A. Copper-Catalyzed Reactions of Aryl Halides with N-Nucleophiles and Their Possible Application for Degradation of Halogenated Aromatic Contaminants. Catalysts 2022, 12, 911. https://doi.org/10.3390/catal12080911
Weidlich T, Špryncová M, Čegan A. Copper-Catalyzed Reactions of Aryl Halides with N-Nucleophiles and Their Possible Application for Degradation of Halogenated Aromatic Contaminants. Catalysts. 2022; 12(8):911. https://doi.org/10.3390/catal12080911
Chicago/Turabian StyleWeidlich, Tomáš, Martina Špryncová, and Alexander Čegan. 2022. "Copper-Catalyzed Reactions of Aryl Halides with N-Nucleophiles and Their Possible Application for Degradation of Halogenated Aromatic Contaminants" Catalysts 12, no. 8: 911. https://doi.org/10.3390/catal12080911
APA StyleWeidlich, T., Špryncová, M., & Čegan, A. (2022). Copper-Catalyzed Reactions of Aryl Halides with N-Nucleophiles and Their Possible Application for Degradation of Halogenated Aromatic Contaminants. Catalysts, 12(8), 911. https://doi.org/10.3390/catal12080911