Abstract
Enantioselective organocatalysis has quickly established itself as the third pillar of asymmetric catalysis. It is a powerful technology platform, and it has a tremendous impact in both academic and industrial settings. By focusing on pregabalin, as a case study, this Perspective aims to show how a process amenable to industry of a simple chiral molecule can be tackled in several different ways using organocatalysis.
Keywords:
industry; process development; organocatalysis; pregabalin; API; asymmetric catalysis; catalysis 1. Introduction
Sustainability is a critical goal of modern society. In the frame of the pivotal role played by chemistry and catalysis toward the sustainability goals, asymmetric organocatalysis is an important technological platform. Despite being a relatively young field, its high impact has been recognized recently with the award of a Nobel prize to List and MacMillan [1]. Organic molecules were previously sporadically used as catalysts for asymmetric reactions; however, it was the two seminal papers by List [2] and MacMillan [3] that conceptualized the field at its beginning, in 2000. In addition to the breakthrough chemistry shown, the introduction of the term “organocatalysis” [3] was prophetic of the value of the disclosure. Several factors may help to understand why asymmetric organocatalysis is relevant to a sustainable industry; catalysts do not contain endangered elements and can be largely derived from non-depleting resources, general modes of activation, biomimetic aspects, low cost and stability of the catalysts, ease of operation, and relative ease of scale-up.
Asymmetric organocatalysis is a prime example of a young and disruptive field [4,5] that has entered and affected industry just after few years it was discovered; it is routinely screened in industry when developing a commercial process although, for several reasons, few of the processes are reported in the primary literature [6,7,8].
It is interesting to note that some prominent examples of the use of organic molecules as asymmetric catalysts had been reported years before the advent of organocatalysis. In the 1970s, Eder, Sauer, Wiechert (Schering AG) [9] and Hajos and Parrish (Hoffmann-La Roche) [10] developed a Robinson annulation catalyzed by L-proline for the synthesis of key building blocks for steroids, although the mechanism they proposed was not fully convincing. Subsequently, in the 1980s, Dolling and Grabowski (Merck Sharp & Dome) [11] published the first example of a highly enantioselective phase-transfer alkylation reaction, as a key step to (+)-indacrinone, using a Cinchona alkaloid derivative as a catalyst.
Despite all the intrinsic advantages of organocatalysis for an industrial process, its typically high catalyst loadings, due to low TONs and TOFs, have sometimes hampered its adoption in industry, or at least this has been the perception for most of the scientific community. Therefore, considerable efforts have been put forward, especially in academic settings, to address the low productivity exhibited by organocatalysts. Means to recycle and reuse organocatalysts [12,13], the development of highly active catalysts [14,15,16,17], immobilization of organocatalysts over solid supports [18,19,20,21,22,23,24,25], and the combination of different catalytic cycles [26,27,28,29,30,31,32] are some of the strategies that have been reported.
Catalysis is a key technology for the future, and discoveries and developments in academia bring a high added value to industry. Fortunately, the influence of academia on industry can also be the reverse. For example, academia has adopted the Design of Experiments (DoE), a tool used in industry to optimize reactions or verify the robustness of prospective commercial processes and used it in the realm of organocatalysis as well [33,34,35,36].
Driven by our own experience, this Perspective will focus on pregabalin (Lyrica™) as a representative case study, to showcase how a process for a blockbuster drug may be tackled from different angles. Selected reports focusing on asymmetric organocatalysis as the key technology will be discussed, to show how different approaches and solutions may bring about interesting and successful advancements; the reader may refer to previous reviews for more comprehensive related literature overviews [6,7,37].
2. Pregabalin
Pregabalin is the active pharmaceutical ingredient (API) of blockbuster drugs [38] useful for the treatment of many conditions related to the central nervous system, such as seizure and anxiety disorders, neuropathic pain, fibromyalgia, and epilepsy. The API is a relatively simple γ-amino acid, (S)-3-aminomethyl-5-methyl-hexanoic acid [6,7,37,39].
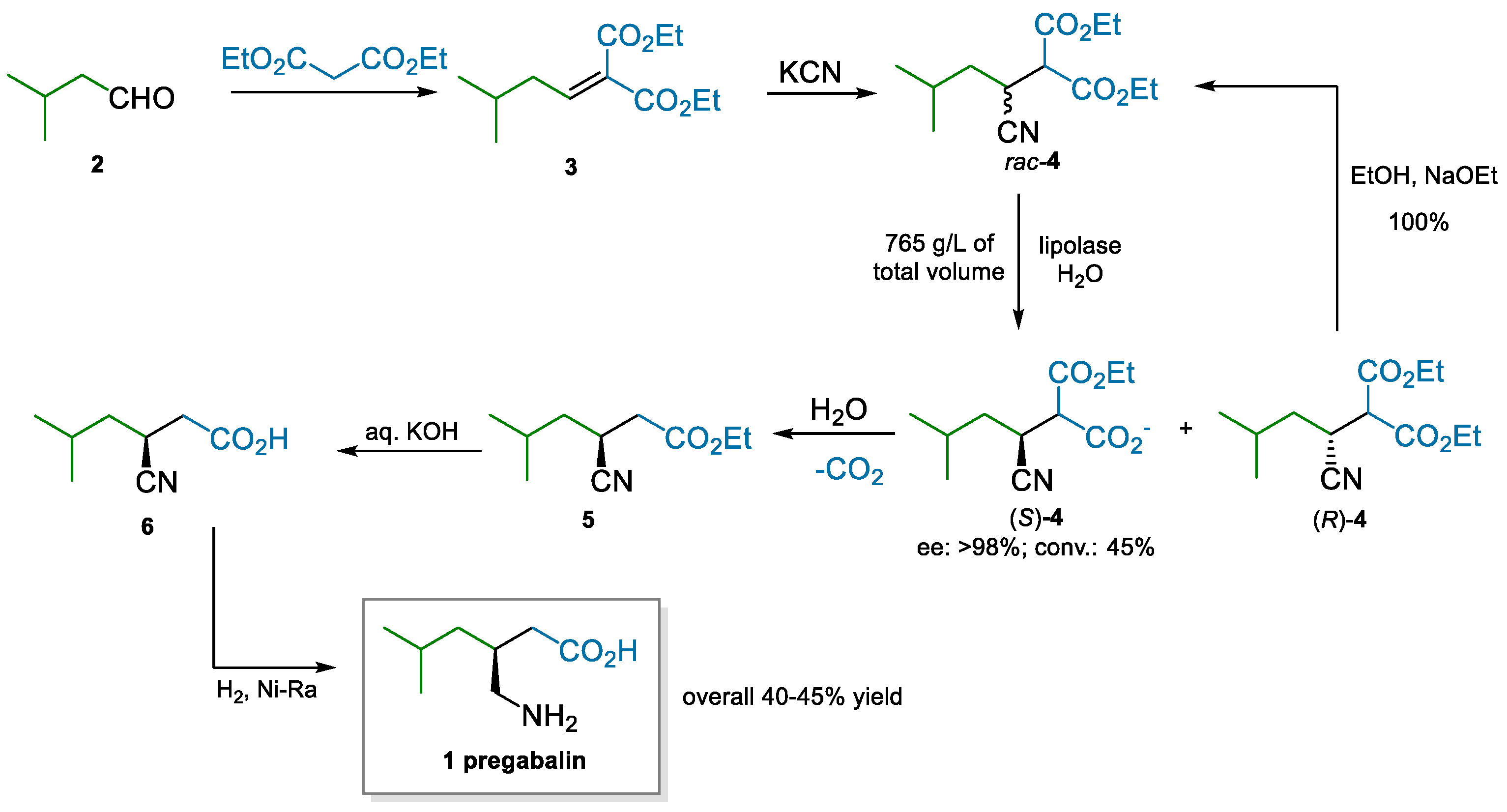
Pfizer, pregabalin’s originator, developed an initial manufacturing process that was based on a classical resolution via mandelic acid in the last step. However, the demand for large volumes of the API required finding more sustainable alternatives to this approach. Enantioselective syntheses were evaluated [40,41,42,43,44]; eventually, an enzymatic kinetic resolution was pivotal to developing an extremely efficient process (Scheme 1) [45]. The route makes use of low-cost materials, simple chemistry, and high throughput due to the highly concentrated enzymatic reaction. A Knoevenagel reaction between diethyl malonate and isovaleraldehyde yields alkylidene malonate 3, which is subjected to cyanide conjugate addition to generate rac-4. An efficient lipolase kinetic resolution, accompanied by simple recycling of the off-enantiomer, produces (S)-4 in high ee and conversion. The subsequent steps are also carried out in water as the reaction medium; decarboxylation, saponification, and reduction of the nitrile yield pregabalin with a high overall yield and in basically enantiopure form.
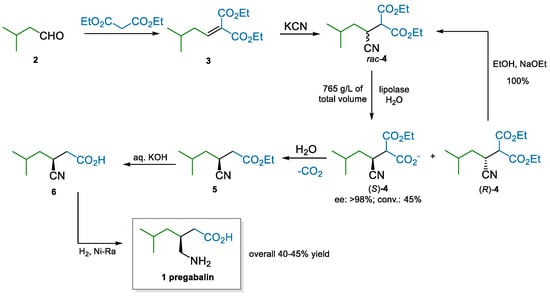
Scheme 1.
Pfizer’s process to pregabalin implementing a lipolase kinetic resolution (2008).
In the following years, many alternative routes to pregabalin have been disclosed. On one hand, the simple structure of pregabalin—a γ-amino acid—made it an ideal benchmark to rapidly prove the utility of new catalytic asymmetric methodologies; on the other hand, more importantly, the huge commercial success of pregabalin stimulated dramatic efforts towards non-infringing routes for generic market production. Overall, the literature reports several classical and kinetic resolution approaches, mostly enzymatic [37], accompanied by several catalytic enantioselective routes [46,47,48].
The γ-amino acid structure of pregabalin lends itself to several combinations of functional group interconversions and disconnections, which would enable use of simple starting materials. However, from a commercial perspective, there are several key factors to consider in addition to the feasibility of the chemistry and the apparent structural simplicity of the starting materials. These key factors include the actual economic viability of all materials including the catalysts, along with the amenability of the reaction conditions to industrialization. In the following paragraphs, we focus on selected relevant organocatalytic approaches that introduced interesting and inventive solutions.
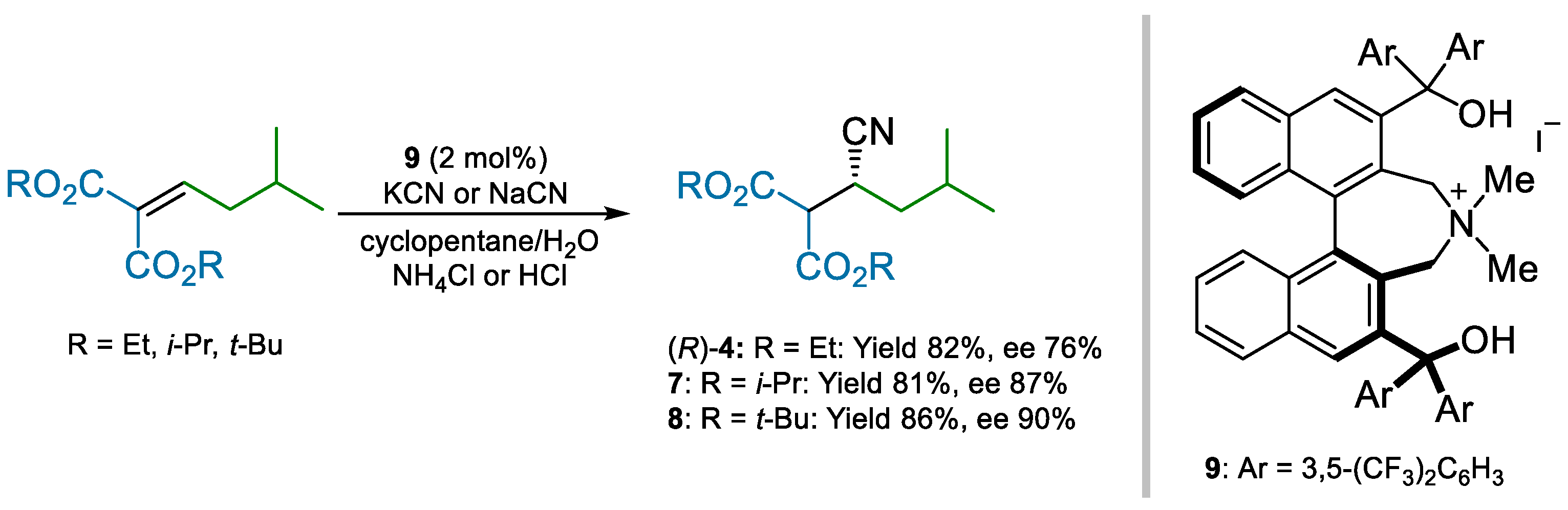
Maruoka and co-workers employed chiral phase-transfer catalyst 9 to impart enantioselectivity to the same conjugate addition used by Pfizer (Scheme 2) [49]. By using a biphasic cyclopentane/water mixture, along with sodium or potassium cyanide as cyanide sources, high ees were obtained. Interestingly, supposedly to quench the enolate resulting from cyanide addition, the use of protic additives proved to be critical to achieving the catalyst turnover.
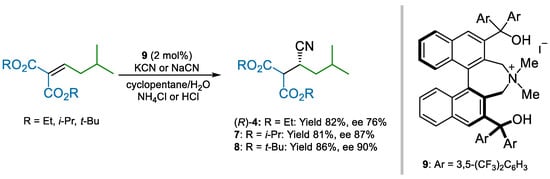
Scheme 2.
Maruoka’s enantioselective conjugate addition of cyanide to alkylidene malonates (2013).
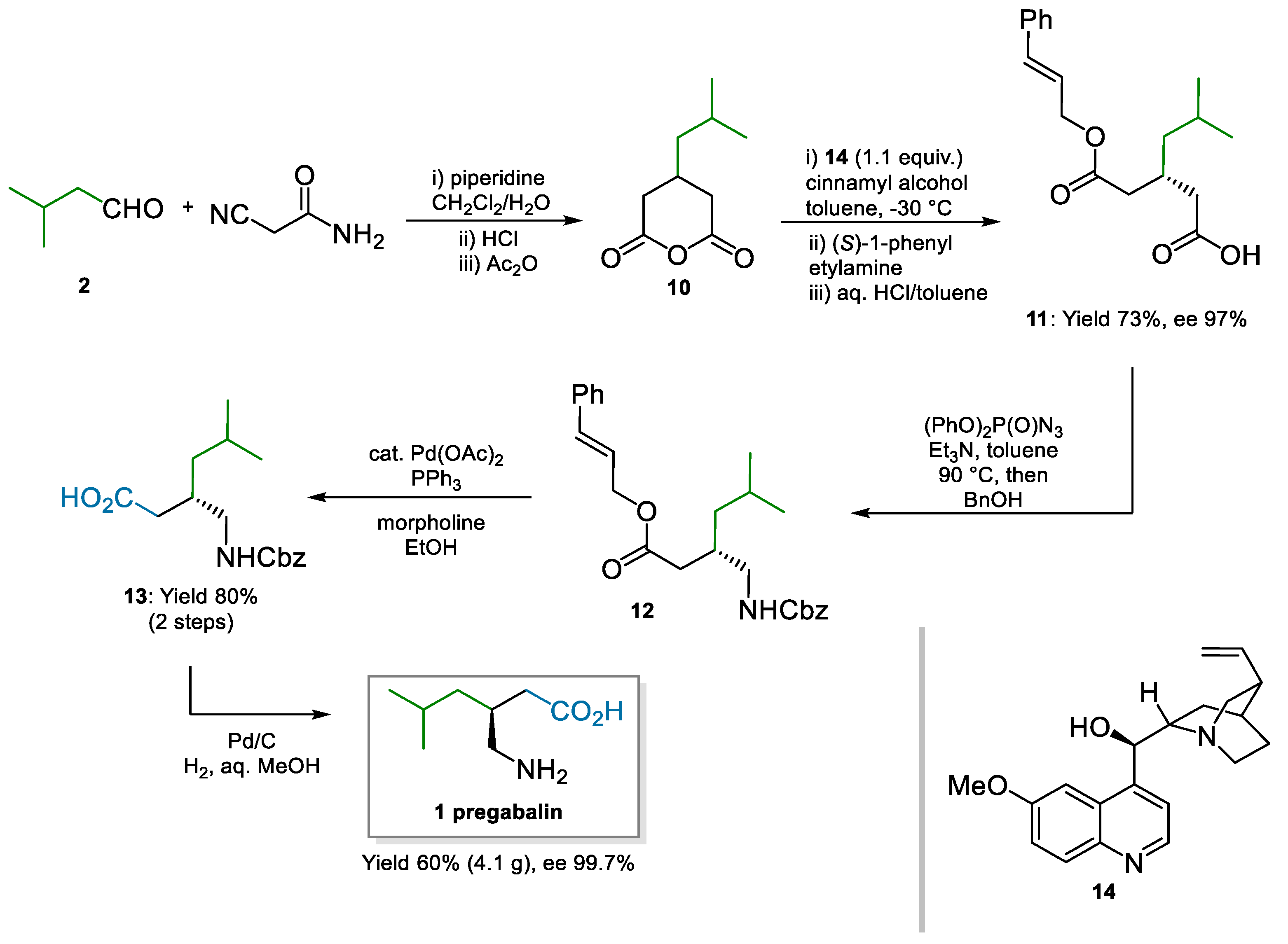
Different but intensely pursued approaches to pregabalin have been based on the desymmetrization of glutaric anhydride 10, which resulted in a chiral monocarboxylic acid intermediate such as 11. The downstream chemistry from this intermediate to pregabalin was rather simplistic and was based on nitrene rearrangements such as Curtius’s or Hofmann’s (Scheme 3). Hameršak and co-workers [50] applied a previous protocol from Bolm’s group [51] to the synthesis of pregabalin. Stoichiometric amounts of quinine 14 were used as a chiral promoter in the desymmetrization step; the moderate enantioenriched product 11 (ca. 70% ee) was crystallized with (S)-1-phenylethylamine to improve the enantiomeric excess to 97%. Curtius rearrangement, via acyl azide, and trapping of the isocyanate with benzyl alcohol afforded 12. Two Pd-catalyzed deprotections in sequence yielded pregabalin in essentially enantiopure form. The overall sequence was demonstrated on a multi-gram scale and did not require chromatographic purifications.
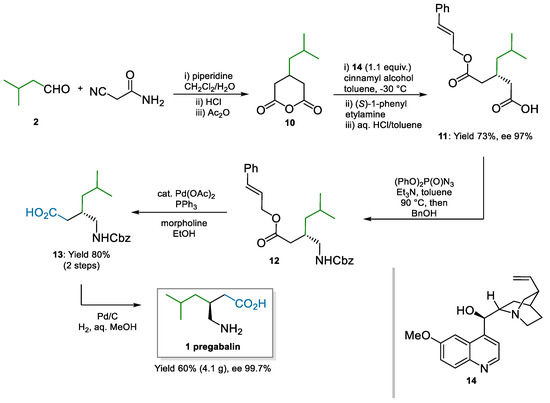
Scheme 3.
Organocatalytic desymmetrization of glutaric anhydride for the synthesis of pregabalin catalyzed by quinine 14 (2007).
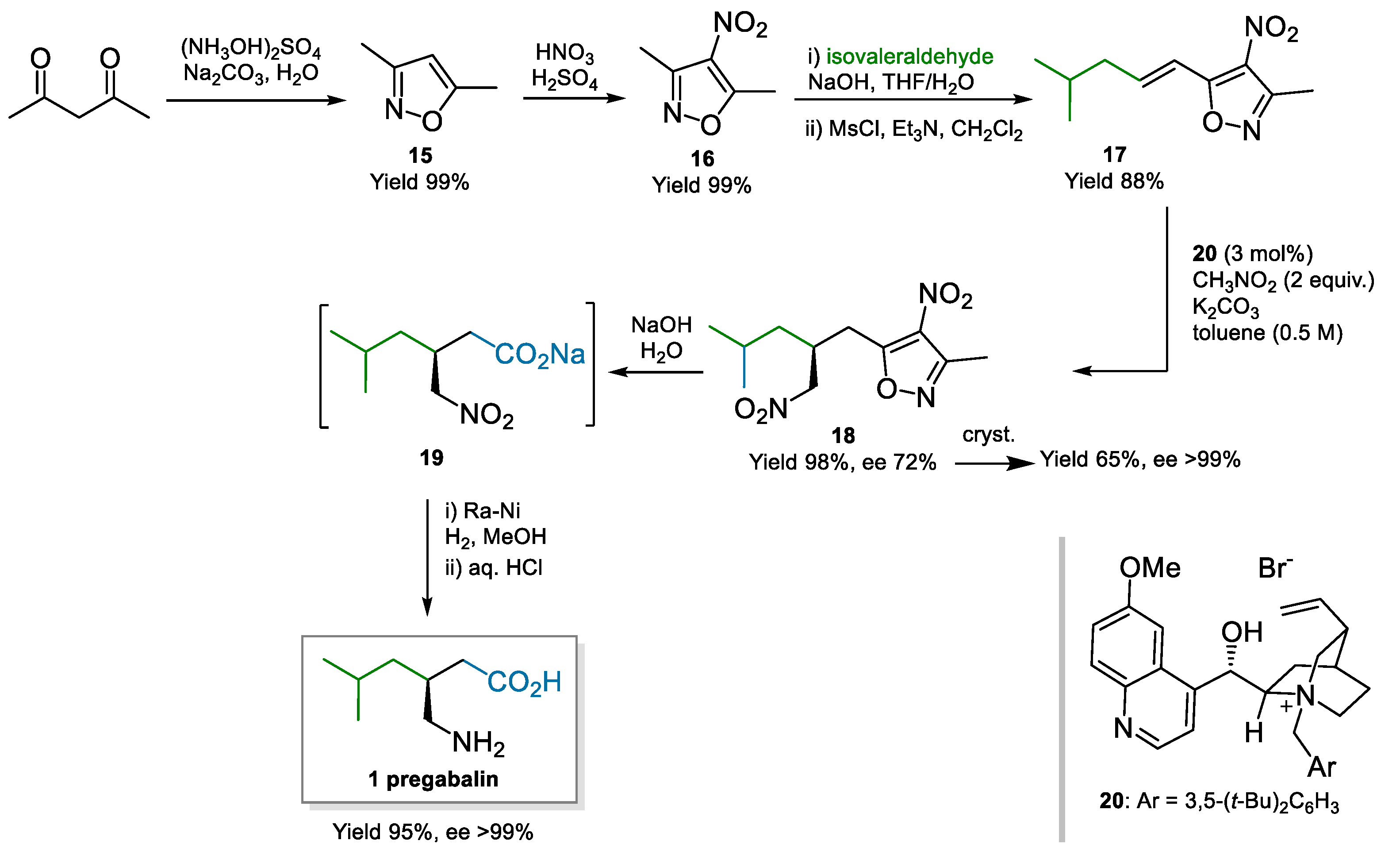
A Cinchona derivative, quaternized at the quinuclidine nitrogen thus inducing a different type of activation, was also employed by Moccia, Adamo, and co-workers who demonstrated a process to pregabalin on kg-scale (Scheme 4) [52,53]. They employed the Cinchona-derived phase-transfer catalyst 20 for the conjugate addition of nitromethane to 17. The key material 17 was based on their brand α,β-unsaturated acid acceptor surrogates derived from 3,5-dimethyl-4-nitroisoxazole 16 [54]. Phase-transfer catalysis imparted good enantioselectivity in the Michael addition (72% ee); further recrystallisation was able to yield enantiopure 18 (>99% ee), with an impact on the yield. Basic hydrolysis unmasked the carboxylate, with subsequent telescoped reduction and pH adjustment providing pregabalin. The process afforded pregabalin in 54% overall yield and >99% ee over six steps, using cost-effective raw materials and catalyst. The possibility to recover and reuse the phase-transfer catalyst through immobilization on a resin-exploiting electrostatic interactions was demonstrated too.
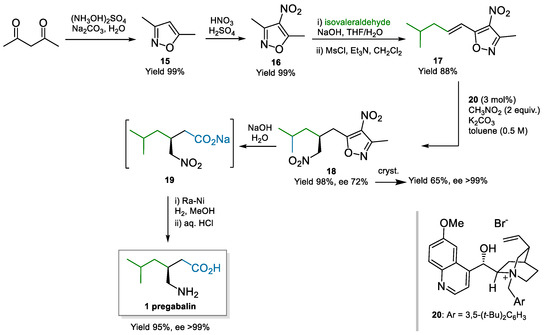
Scheme 4.
Process to pregabalin via enantioselective conjugate addition of nitromethane to Michael acceptor 17 (2013).
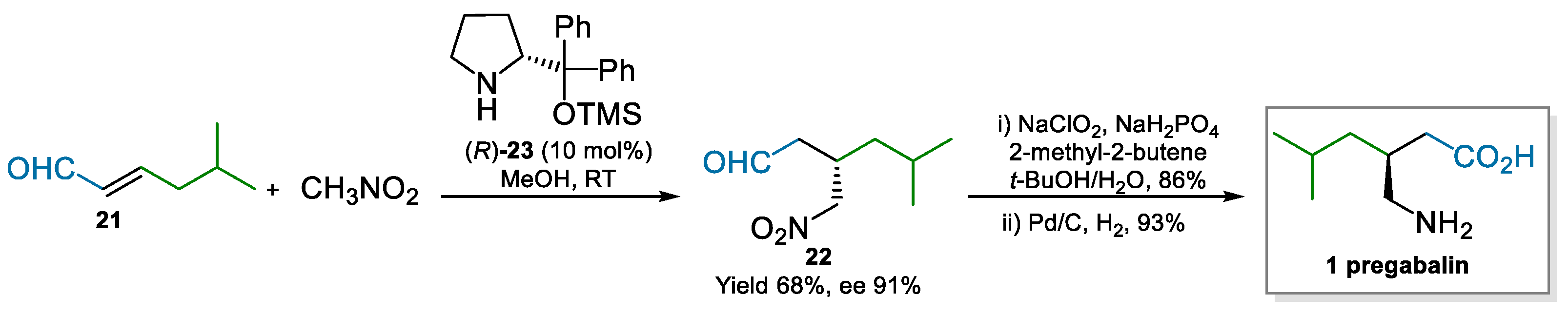
Nitromethane was also used in the enantioselective conjugate addition to 5-methyl-2-hexenal 21. The organocatalytic approach developed by Hayashi and co-workers [55] exploited the Hayashi/Jørgensen catalyst (R)-23, derived by D-Proline, to deliver intermediate 22 in moderate yield and good optical purity, which was easily converted to pregabalin in two steps (Scheme 5). The approach to pregabalin by Hayashi was supposedly used to demonstrate the usefulness of the developed reaction; in fact, as may be expected by an academic paper, they did not tackle process related issues, such as enantiopurity, catalyst loading, or the safety of using nitromethane (vide infra). However, the use of the Hayashi/Jørgensen catalyst 23 is appealing from a commercial point of view; it has been previously used in an industrial setting [56]. Therefore, issues for its production have been already assessed, demonstrating the affordability of its production at scale.
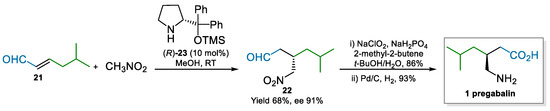
Scheme 5.
Enantioselective conjugated addition of nitromethane to 21 catalyzed by (R)-23 (2007).
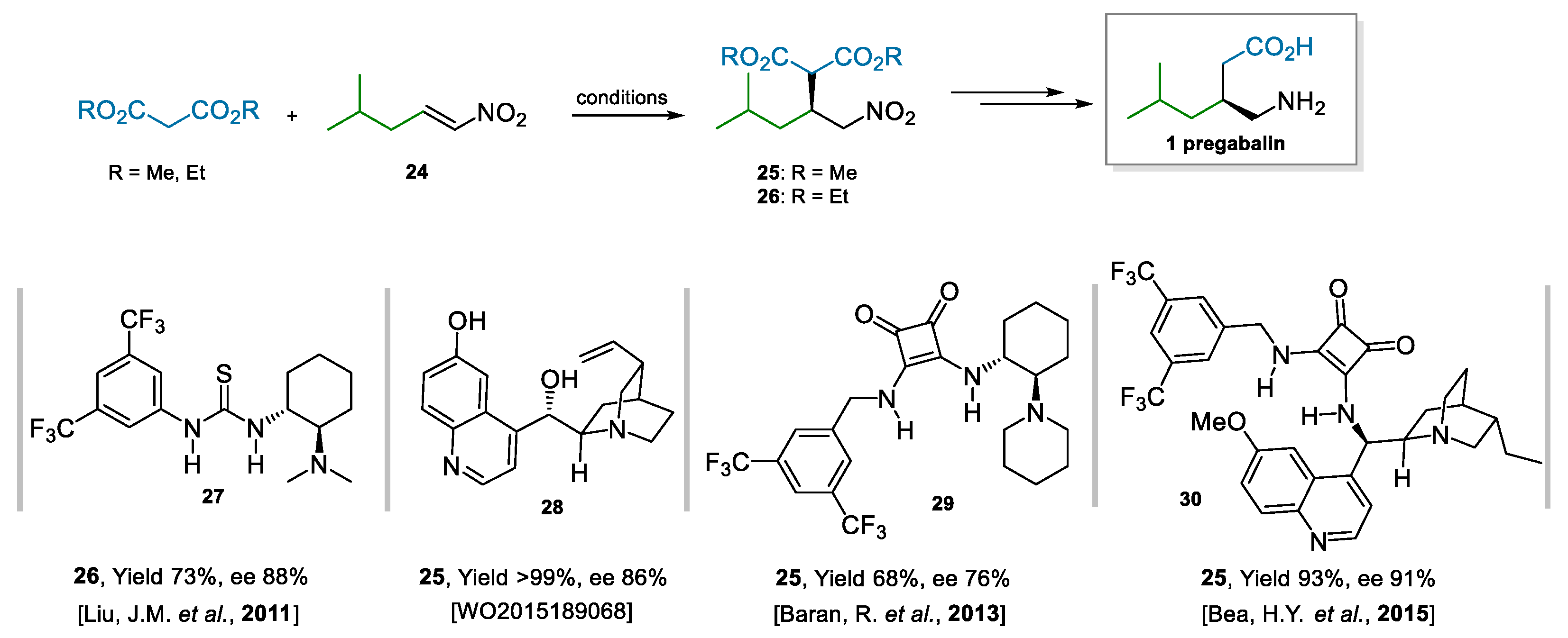
The enantioselective addition of malonates to nitroalkene 24 (Scheme 6) is another successful approach to access pregabalin [57]. Many examples are present in the literature, and they mostly involve the use of bifunctional catalysts, i.e., bearing a tertiary amine adorned with hydrogen-bond donors. Representative examples are Takemoto’s catalyst [58], des-methyl quinine and quinidine derivatives [59], (thio)ureas [60,61], and squaramides [62] derived from Cinchona alkaloids. A selection of representative catalysts and the results they afforded [63,64,65,66] are reported in Scheme 6. Bifunctional catalysts are efficient in imparting enantioselectivity because they coordinate, activate, and bring together both reaction partners. An important challenge of this reaction, as opposed to similar ones using nitrostyrene derivatives, is the instability and the difficulty to prepare and purify (vide infra) aliphatic nitroalkene 24; furthermore, it is less electrophilic than its aromatic counterparts, and it can also give rise to secondary reactions via isomerization of the double bond. This, in turn, is also reflected in higher catalyst loadings needed that will, eventually, also increase the cost of the process.
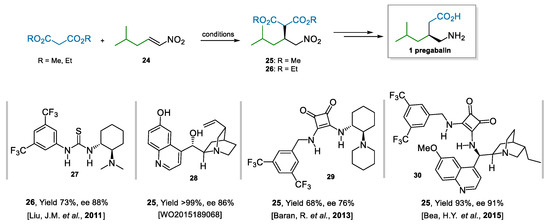
Scheme 6.
Addition of malonates to nitroalkene 24; selected bifunctional catalysts.
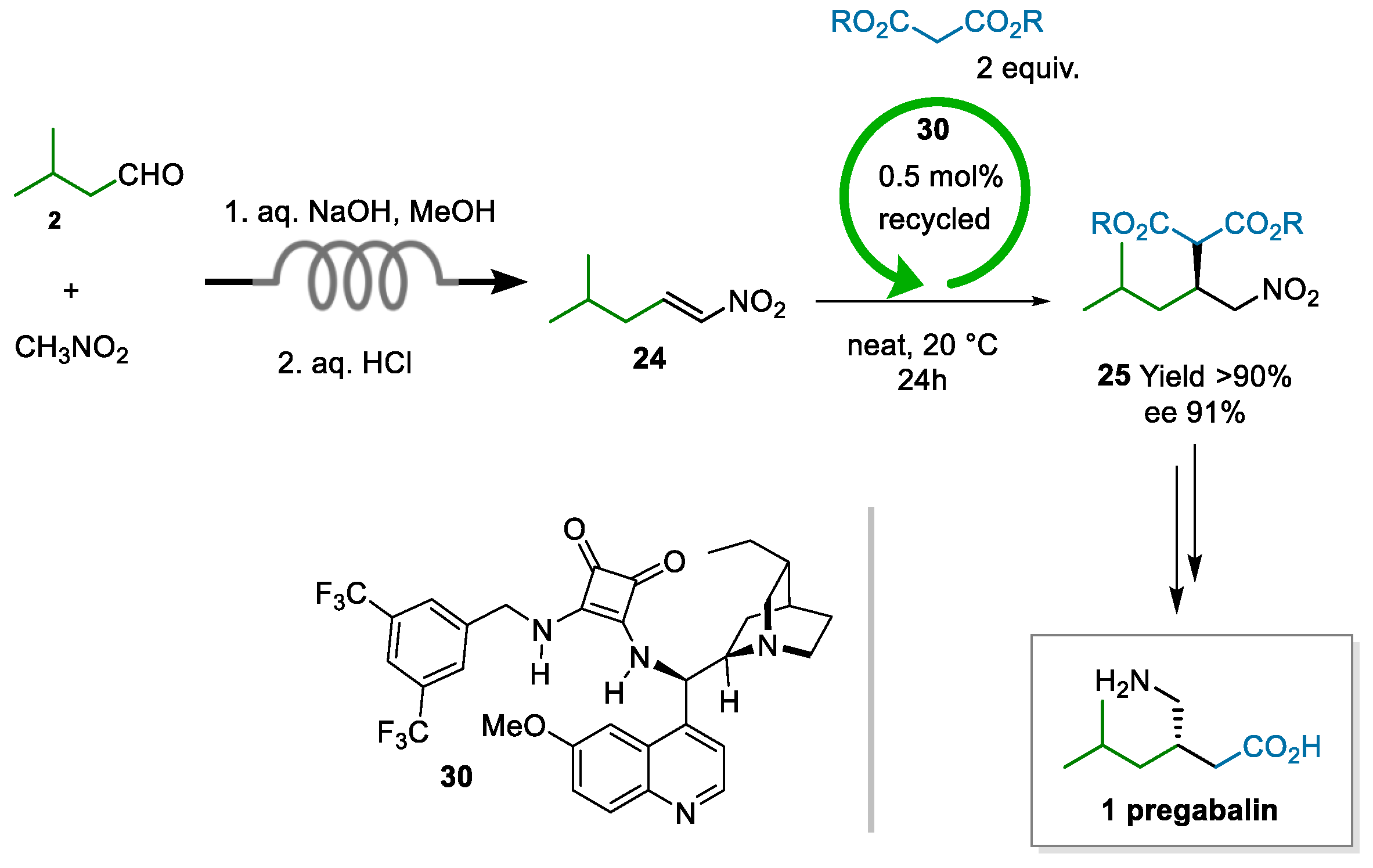
The process developed by Dr. Reddy’s laboratories, indeed, was centered on the enantioselective addition of dimethyl malonate to nitroalkene 24 as the asymmetric step. The innovation lay in the nontrivial application of two enabling technologies, i.e., continuous chemistry for safe access to nitroalkene 24 and asymmetric organocatalysis to impart enantioselectivity in the Michael addition (Scheme 7) [67]. In most contributions reviewed, the production of nitroalkene 24 is a neglected aspect; at Dr. Reddy’s, they showed that an economically viable route needs to circumvent the costly dehydration of the Henry adduct, along with chromatographic purification. Furthermore, purification by distillation should be avoided due to poor thermal stability. Therefore, a flow process was developed to yield 25 of quality high enough for the subsequent step, without requiring purification. The catalytic step, performed under neat conditions, was demonstrated on a >2 Kg scale at 0.5 mol% loading of the squaramide catalyst 30. Moreover, the catalyst was recovered in 59% yield and could be reused without any appreciable loss of activity/selectivity.
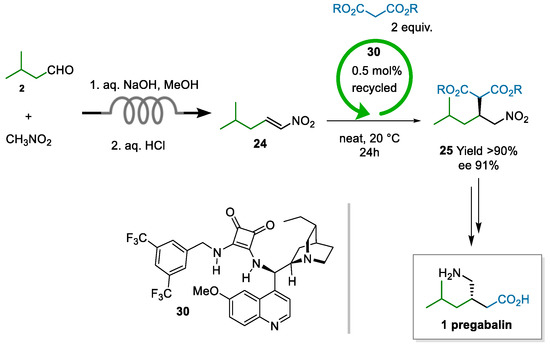
Scheme 7.
Process to pregabalin developed at Dr. Reddy’s (2021; patent application 2013).
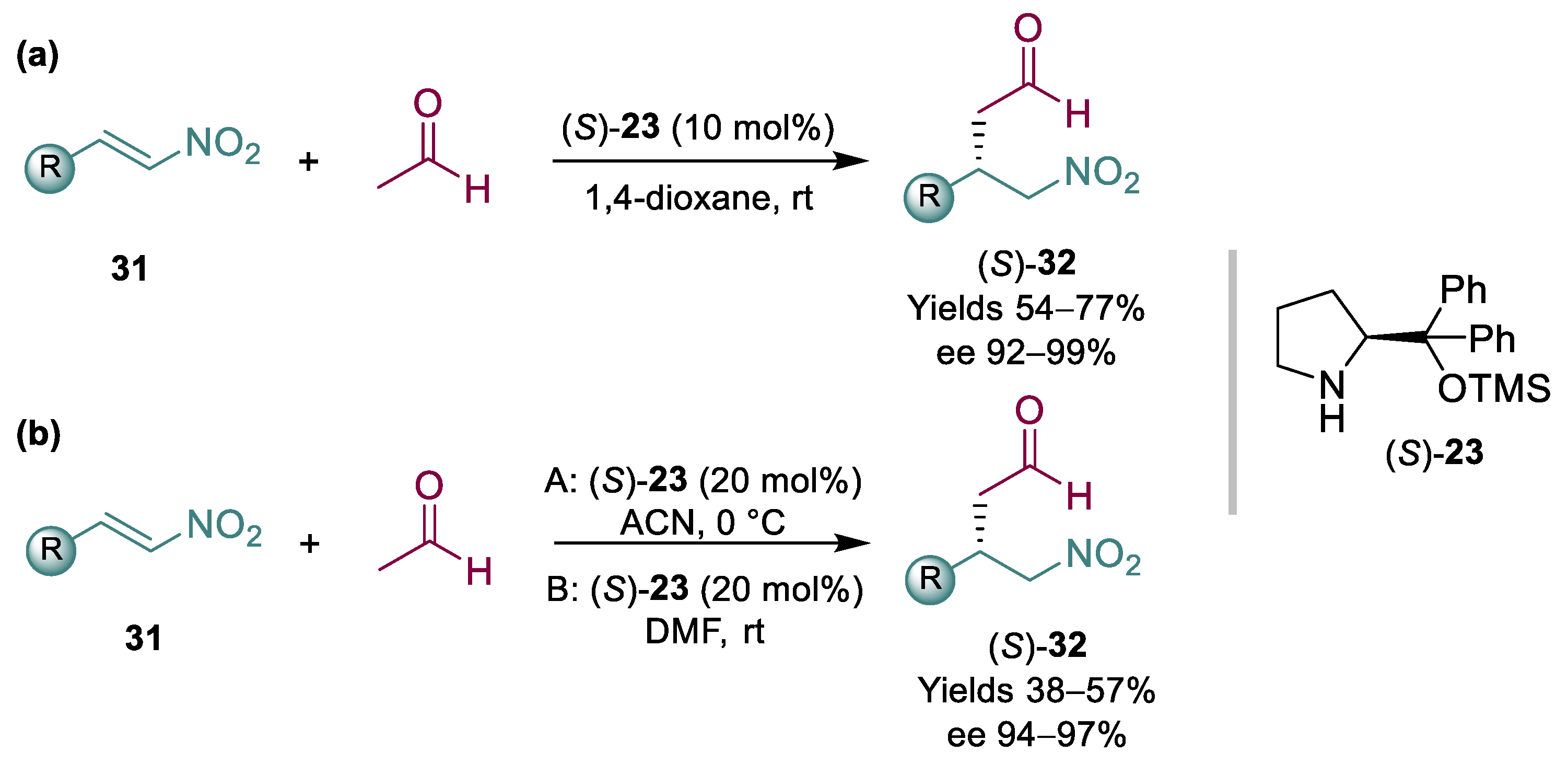
In lieu of malonates, the addition of acetaldehyde to nitroalkene 24 might also be an interesting route to pregabalin; in fact, it would avoid some of the downstream associated with the use of malonates, and it may be catalyzed by Hayashi/Jørgensen catalyst that, as discussed previously, has already been used on scale and is relatively affordable. However, the use of acetaldehyde brings additional challenges; both acetaldehyde and the resulting products are very reactive and can readily give further condensations. Additionally, it is toxic, flammable, and very difficult to handle at scale. In some cases, careful tuning of reaction conditions helped to overcome some of the challenges associated with acetaldehyde [68,69]. Some successful reports where acetaldehyde was used in combination with a range of nitroalkenes 31 have been published (Scheme 8). The first two groundbreaking and successful publications were by List and Hayashi; in one instance, 10 equiv. of acetaldehyde were needed [70] (Scheme 8a), while in another instance, 5 equiv. were added via syringe pump [71] (Scheme 8b). In both cases, relatively high loadings of organocatalyst (S)-23 were required, affording products 32 in moderate to good yields and high ees.
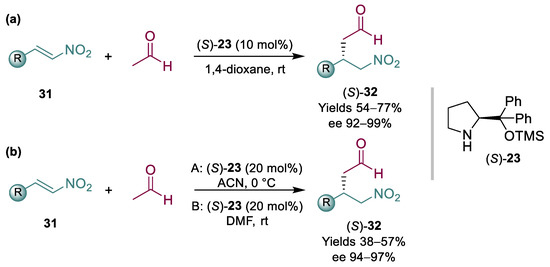
Scheme 8.
Organocatalyzed acetaldehyde addition to nitroalkenes 31 (2008) (a,b).
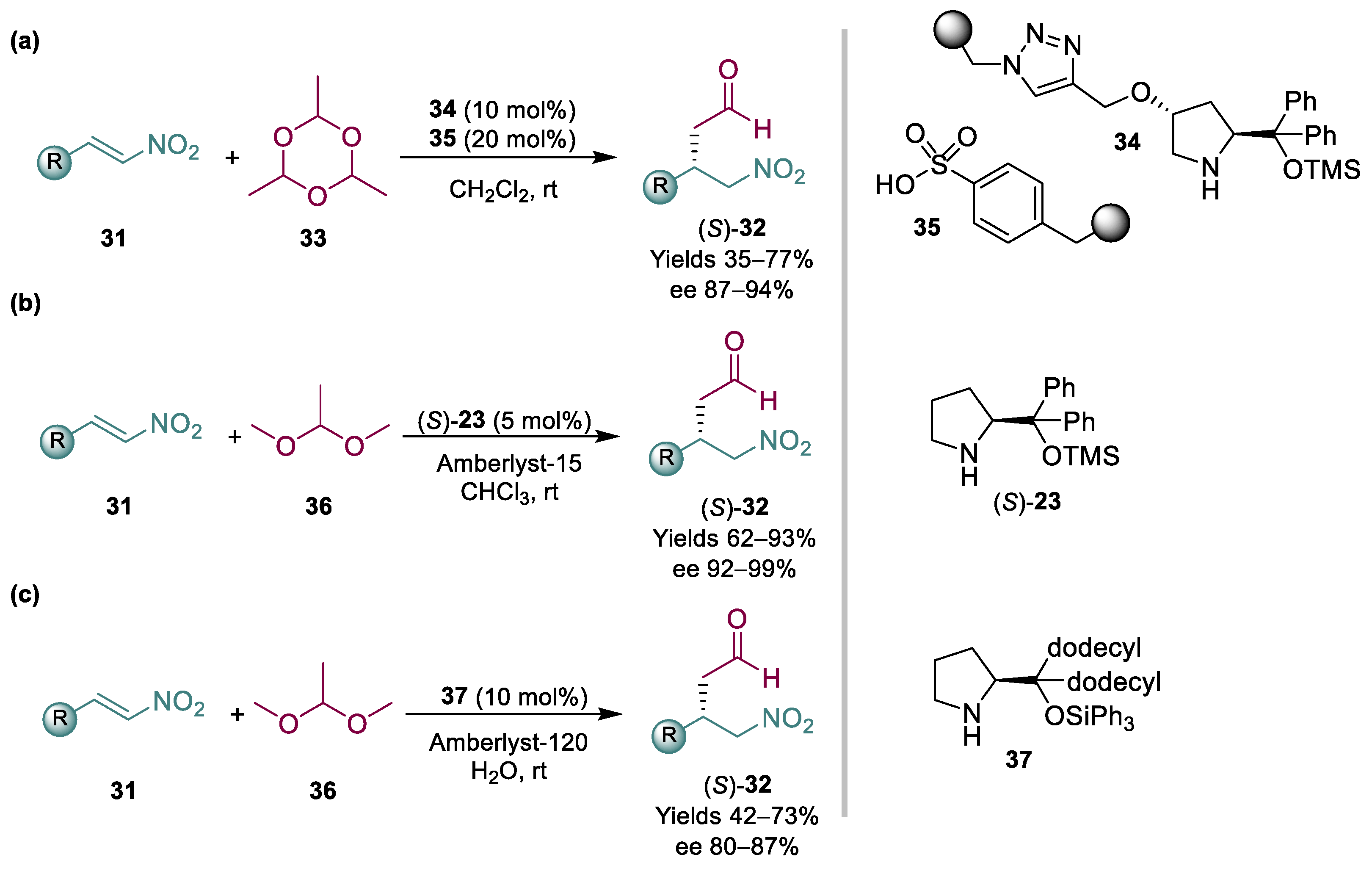
Pericàs and coworkers devised the use of paraldehyde 33 as an acetaldehyde surrogate; by using immobilized organocatalysts and segregating them in teabags, they were able to afford the desired products in good yields and ees (Scheme 9a) [72]. In more detail, a combination of polystyrene-supported aminocatalyst 34, capable of activating the acetaldehyde substrate for the enantioselective addition, was employed in combination with sulfonic acid resins, essential for the generation of acetaldehyde in situ from the paraldehyde precursor. While the equivalents of acetaldehyde were high (10 equiv.), they ingeniously tackled the issue of using a toxic and difficult to handle chemical, providing a good perspective for its application on scale. Addressing these challenges, our group recently reported the use of another, possibly safer to handle and more appealing, acetaldehyde precursor for these reactions: dimethyl acetal 36 (Scheme 9b) [73]. By using a homogeneous Hayashi/Jørgensen catalyst (S)-23, in combination with Amberlyst-15, we were able to afford the desired products in high yields and ees, not only with a range of nitroalkenes 31 but also with the less reactive nitroalkene 24 required for pregabalin, with relatively low catalyst loadings.
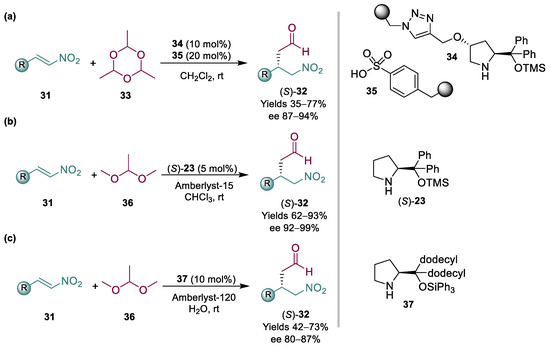
Scheme 9.
Organocatalyzed Michael addition to nitroalkenes 31 employing surrogates of acetaldehyde (2013, 2020, 2022) (a–c).
Although the developed reaction with acetaldehyde dimethyl acetal 36 proved interesting, the use of chloroform as a solvent is desirable to avoid, especially if the process is aimed for an industrial protocol. Therefore, our group decided to explore the possibility of using water as a benign solvent. In order to optimize a reaction involving many variables, many experiments were needed using the one variable at a time (OVAT) approach. With this in mind, we set out to screen the multidimensional space using Design of Experiments, a tool that is routinely used in industry to optimize catalytic reactions but rather limited in academia [34]. Indeed, via a rational exploration of the chemical space, an industrially appealing protocol for the Michael addition of acetaldehyde to nitroalkenes in water was developed using DoE (Scheme 9c) [33]. Unfortunately, a limitation of the reaction in water was its application to aliphatic nitroalkenes; therefore, presently, it cannot be applied to access pregabalin.
3. Conclusions
Asymmetric organocatalysis is, undoubtedly, a key technology platform both in academia and in industry. In recognition of its impact in basic as well as in applied research, a Nobel prize was very recently awarded to leading chemists in the field. By focusing on selected developments for the preparation of pregabalin as a case study, this perspective showed how discoveries in academia help to advance industry and vice-versa. Indeed, organocatalysis proved to be a very powerful multifaceted type of catalysis; in fact, due to several different organocatalytic activation modes, the preparation of a very simple chiral molecule, such as pregabalin, can be tackled with a variety of approaches. Supported by the examples provided as a case study, we believe that collaboration between academia and industry is crucial for the advancement of the field, and the discoveries and developments made in the public and private sector can benefit greatly by cooperation.
Author Contributions
Conceptualization, L.B., F.F., A.C., F.P. and F.S.; writing—original draft preparation, G.G. and A.C.; writing—review and editing, all authors; supervision, A.C.; project administration, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
G.G. is grateful to PON-DOT13OV2OC for an industrial Ph.D. fellowship. F.P. thanks PON-AIM grant number 1842894 for funding. F.F. thanks UNIMORE for funding (FAR-DSV_2021 and FAR-DSV_2019).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- The Nobel Prize in Chemistry 2021—NobelPrize.Org. Available online: https://www.nobelprize.org/prizes/chemistry/2021/summary/ (accessed on 20 July 2022).
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels—Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Asymmetric Aminocatalysis—Gold Rush in Organic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 6138–6171. [Google Scholar] [CrossRef] [PubMed]
- Aukland, M.H.; List, B. Organocatalysis Emerging as a Technology. Pure Appl. Chem. 2021, 93, 1371–1381. [Google Scholar] [CrossRef]
- Carlone, A.; Bernardi, L. Enantioselective Organocatalytic Approaches to Active Pharmaceutical Ingredients—Selected Industrial Examples. Phys. Sci. Rev. 2019, 4, 20180097. [Google Scholar] [CrossRef]
- Bernardi, L.; Carlone, A.; Fini, F. Industrial Relevance of Asymmetric Organocatalysis in the Preparation of Chiral Amine Derivatives. In Methodologies in Amine Synthesis: Challenges and Applications; Ricci, A., Bernardi, L., Eds.; WILEY-VCH GmbH: Boschstr, Germany, 2020; pp. 187–241. [Google Scholar]
- Hughes, D.L. Highlights of the Recent Patent Literature: Focus on Asymmetric Organocatalysis. Org. Process Res. Dev. 2022, in press. [Google Scholar] [CrossRef]
- Eder, U.; Sauer, G.; Wiechert, R. New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures. Angew. Chem. Int. Ed. 1971, 10, 496–497. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. Synthesis and Conversion of 2-Methyl-2-(3-Oxobutyl)-1,3-Cyclopentanedione to the Isomeric Racemic Ketols of the [3.2.1] Bicyclooctane and of the Perhydroindane Series. J. Org. Chem. 1974, 39, 1612–1615. [Google Scholar] [CrossRef]
- Dolling, U.H.; Davis, P.; Grabowski, E.J.J. Efficient Catalytic Asymmetric Alkylations. 1. Enantioselective Synthesis of (+)-Indacrinone via Chiral Phase-Transfer Catalysis. J. Am. Chem. Soc. 1984, 106, 446–447. [Google Scholar] [CrossRef]
- Fulgheri, T.; Della Penna, F.; Baschieri, A.; Carlone, A. Advancements in the Recycling of Organocatalysts: From Classical to Alternative Approaches. Curr. Opin. Green Sustain. Chem. 2020, 25, 100387–100396. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Supported Proline and Proline-Derivatives as Recyclable Organocatalysts. Chem. Soc. Rev. 2008, 37, 1666–1688. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, F.; Gruttadauria, M.; Agrigento, P.; Noto, R. Low-Loading Asymmetric Organocatalysis. Chem. Soc. Rev. 2012, 41, 2406–2447. [Google Scholar] [CrossRef] [PubMed]
- Kaib, P.S.J.; Schreyer, L.; Lee, S.; Properzi, R.; List, B. Extremely Active Organocatalysts Enable a Highly Enantioselective Addition of Allyltrimethylsilane to Aldehydes. Angew. Chem. Int. Ed. 2016, 55, 13200–13203. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rapún, R.; Fan, X.; Sayalero, S.; Bahramnejad, M.; Cuevas, F.; Pericàs, M.A. Highly Active Organocatalysts for Asymmetric Anti-Mannich Reactions. Chem. Eur. J. 2011, 17, 8780–8783. [Google Scholar] [CrossRef]
- Bae, H.Y.; Höfler, D.; Kaib, P.S.J.; Kasaplar, P.; De, C.K.; Döhring, A.; Lee, S.; Kaupmees, K.; Leito, I.; List, B. Approaching Sub-Ppm-Level Asymmetric Organocatalysis of a Highly Challenging and Scalable Carbon–Carbon Bond Forming Reaction. Nat. Chem. 2018, 10, 888–894. [Google Scholar] [CrossRef]
- Benaglia, M.; Alessandra, M. Catalyst Immobilization: Methods and Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–477. [Google Scholar]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-Supported Organic Catalysts. Chem. Rev. 2003, 103, 3401–3429. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, S.; Cheng, J.P. Non-Covalent Immobilization of Asymmetric Organocatalysts. Catal. Sci. Tech. 2011, 1, 507–516. [Google Scholar] [CrossRef]
- Lai, J.; Fianchini, M.; Pericas, M.A. Development of Immobilized Spinol-Derived Chiral Phosphoric Acids for Catalytic Continuous Flow Processes. Use in the Catalytic Desymmetrization of 3,3-Disubstituted Oxetanes. Acs Catal. 2020, 10, 14971–14983. [Google Scholar] [CrossRef]
- Ragno, D.; Di Carmine, G.; Brandolese, A.; Bortolini, O.; Giovannini, P.P.; Massi, A. Immobilization of Privileged Triazolium Carbene Catalyst for Batch and Flow Stereoselective Umpolung Processes. ACS Catal. 2017, 7, 6365–6375. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis. Adv. Synth. Cat. 2006, 348, 1391–1412. [Google Scholar] [CrossRef]
- Di Carmine, G.; Forster, L.; Wang, S.; Parlett, C.; Carlone, A.; D’Agostino, C. NMR Relaxation Time Measurements of Solvent Effects in an Organocatalysed Asymmetric Aldol Reaction over Silica SBA-15 Supported Proline. React. Chem. Eng. 2022, 7, 269–274. [Google Scholar] [CrossRef]
- Di Carmine, G.; Pesciaioli, F.; Wang, S.; Sinibaldi, A.; Giorgianni, G.; Parlett, C.M.A.; Carlone, A.; D’Agostino, C. Insights into Substituent Effects of Benzaldehyde Derivatives in a Heterogenous Organocatalyzed Aldol Reaction. ChemCatChem 2022, 14, e202200405. [Google Scholar] [CrossRef]
- Sinibaldi, A.; Nori, V.; Baschieri, A.; Fini, F.; Arcadi, A.; Carlone, A. Organocatalysis and beyond: Activating Reactions with Two Catalytic Species. Catalysts 2019, 9, 928. [Google Scholar] [CrossRef]
- Allen, A.E.; MacMillan, D.W.C. Synergistic Catalysis: A Powerful Synthetic Strategy for New Reaction Development. Chem. Sci. 2012, 3, 633–658. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Sinibaldi, A.; Nori, V.; Giorgianni, G.; di Carmine, G.; Pesciaioli, F. Synergistic Strategies in Aminocatalysis. Chem. Eur. J. 2022, e202200818. [Google Scholar] [CrossRef]
- Inamdar, S.M.; Shinde, V.S.; Patil, N.T. Enantioselective Cooperative Catalysis. Organ. Biomol. Chem. 2015, 13, 8116–8162. [Google Scholar] [CrossRef]
- Afewerki, S.; Córdova, A. Combinations of Aminocatalysts and Metal Catalysts: A Powerful Cooperative Approach in Selective Organic Synthesis. Chem. Rev. 2016, 116, 13512–13570. [Google Scholar] [CrossRef]
- Wende, R.C.; Schreiner, P.R. Evolution of Asymmetric Organocatalysis: Multi- and Retrocatalysis. Green Chem. 2012, 14, 1821–1849. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.-Q.; Du, W.; Chen, Y.-C. Asymmetric Organocatalysis Involving Double Activation. Tetrahedron Chem. 2022, 2, 100017. [Google Scholar] [CrossRef]
- Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. DoE-Driven Development of an Organocatalytic Enantioselective Addition of Acetaldehyde to Nitrostyrenes in Water. Chem. Eur. J. 2022, 28, e202104524. [Google Scholar] [CrossRef]
- Nori, V.; Sinibaldi, A.; Pesciaioli, F.; Carlone, A. Impact of Design of Experiments in the Optimisation of Catalytic Reactions in Academia. Synthesis 2022, 54. [Google Scholar] [CrossRef]
- Renzi, P.; Kronig, C.; Carlone, A.; Eröksüz, S.; Berkessel, A.; Bella, M. Kinetic Resolution of Oxazinones: Rational Exploration of Chemical Space through the Design of Experiments. Chem. Eur. J. 2014, 20, 11768–11775. [Google Scholar] [CrossRef] [PubMed]
- Renzi, P.; Bella, M. Design of Experiments: A Rational Approach toward Non-Covalent Asymmetric Organocatalysis. Synlett 2017, 28, 306–315. [Google Scholar] [CrossRef]
- Adamo, M.F.A.; Kelly, B.G.; Moccia, M. Recent Advances in the Preparation of Active Pharmaceutical Ingredient (S)-Pregabalin. Chim. Oggi Chem. Today 2016, 34, 54–57. [Google Scholar]
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Edu. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Silverman, R.B. From Basic Science to Blockbuster Drug: The Discovery of Lyrica. Angew. Chem. Int. Ed. 2008, 47, 3500–3504. [Google Scholar] [CrossRef]
- Burk, M.J.; de Koning, P.D.; Grote, T.M.; Hoekstra, M.S.; Hoge, G.; Jennings, R.A.; Kissel, W.S.; Le, T.v.; Lennon, I.C.; Mulhern, T.A.; et al. An Enantioselective Synthesis of (S)-(+)-3-Aminomethyl-5-Methylhexanoic Acid via Asymmetric Hydrogenation. J. Org. Chem. 2003, 68, 5731–5734. [Google Scholar] [CrossRef]
- Hoge, G.; Wu, H.P.; Kissel, W.S.; Pflum, D.A.; Greene, D.J.; Bao, J. Highly Selective Asymmetric Hydrogenation Using a Three Hindered Quadrant Bisphosphine Rhodium Catalyst. J. Am. Chem. Soc. 2004, 126, 5966–5967. [Google Scholar] [CrossRef]
- Debarge, S.; McDaid, P.; O’Neill, P.; Frahill, J.; Wong, J.W.; Carr, D.; Burrell, A.; Davies, S.; Karmilowicz, M.; Steflik, J. Evaluation of Several Routes to Advanced Pregabalin Intermediates: Synthesis and Enantioselective Enzymatic Reduction Using Ene-Reductases. Org. Process Res. Dev. 2014, 18, 109–121. [Google Scholar] [CrossRef]
- Process for the Preparation of (S)—3—CYANO—5—METHYLHEXANOIC Acid Derivatives and of Pregabalin, WO2012025861. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012025861 (accessed on 6 July 2022).
- Winkler, C.K.; Clay, D.; Davies, S.; O’Neill, P.; McDaid, P.; Debarge, S.; Steflik, J.; Karmilowicz, M.; Wong, J.W.; Faber, K. Chemoenzymatic Asymmetric Synthesis of Pregabalin Precursors via Asymmetric Bioreduction of β-Cyanoacrylate Esters Using Ene-Reductases. J. Org. Chem. 2013, 78, 1525–1533. [Google Scholar] [CrossRef]
- Martinez, C.A.; Hu, S.; Dumond, Y.; Tao, J.; Kelleher, P.; Tully, L. Development of a Chemoenzymatic Manufacturing Process for Pregabalin. Org. Process Res. Dev. 2008, 12, 392–398. [Google Scholar] [CrossRef]
- Ordóñez, M.; Cativiela, C. Stereoselective Synthesis of γ-Amino Acids. Tetrahedron: Asymm. 2007, 18, 3–99. [Google Scholar] [CrossRef]
- Ordóñez, M.; Cativiela, C.; Romero-Estudillo, I. An Update on the Stereoselective Synthesis of γ-Amino Acids. Tetrahedron: Asymm. 2016, 27, 999–1055. [Google Scholar] [CrossRef]
- Han, J.; Escorihuela, J.; Fustero, S.; Landa, A.; Soloshonok, V.A.; Sorochinsky, A. Asymmetric Michael Addition in Synthesis of β-Substituted GABA Derivatives. Molecules 2022, 27, 3797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shirakawa, S.; Maruoka, K. Phase-Transfer-Catalyzed Asymmetric Conjugate Cyanation of Alkylidenemalonates with KCN in the Presence of a Brønsted Acid Additive. Org. Lett. 2013, 15, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Hameršak, Z.; Stipetić, I.; Avdagić, A. An Efficient Synthesis of (S)-3-Aminomethyl-5-Methylhexanoic Acid (Pregabalin) via Quinine-Mediated Desymmetrization of Cyclic Anhydride. Tetrahedron Asymm. 2007, 18, 1481–1485. [Google Scholar] [CrossRef]
- Bolm, C.; Schiffers, I.; Dinter, C.L.; Gerlach, A. Practical and Highly Enantioselective Ring Opening of Cyclic Meso-Anhydrides Mediated by Cinchona Alkaloids. J. Org. Chem. 2000, 65, 6984–6991. [Google Scholar] [CrossRef]
- Moccia, M.; Cortigiani, M.; Monasterolo, C.; Torri, F.; del Fiandra, C.; Fuller, G.; Kelly, B.; Adamo, M.F.A. Development and Scale-up of an Organocatalytic Enantioselective Process to Manufacture (S)-Pregabalin. Org. Process Res. Dev. 2015, 19, 1274–1281. [Google Scholar] [CrossRef]
- WO2013076225 Process for the Preparation of Gamma Amino Acids and Intermediates Used in Said Process. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013076225 (accessed on 23 July 2022).
- Nagaraju, S.; Sathish, K.; Kashinath, D. Applications of 3,5-Dialkyl-4-Nitroisoxazoles and Their Derivatives in Organic Synthesis. ChemistrySelect 2021, 6, 7736–7793. [Google Scholar] [CrossRef]
- Gotoh, H.; Ishikawa, H.; Hayashi, Y. Diphenylprolinol Silyl Ether as Catalyst of an Asymmetric, Catalytic, and Direct Michael Reaction of Nitroalkanes with α,β-Unsaturated Aldehydes. Org. Lett. 2007, 9, 5307–5309. [Google Scholar] [CrossRef]
- Xu, F.; Zacuto, M.; Yoshikawa, N.; Desmond, R.; Hoerrner, S.; Itoh, T.; Journet, M.; Humphrey, G.R.; Cowden, C.; Strotman, N.; et al. Asymmetric Synthesis of Telcagepant, a CGRP Receptor Antagonist for the Treatment of Migraine. J. Org. Chem. 2010, 75, 7829–7841. [Google Scholar] [CrossRef] [PubMed]
- Bassas, O.; Huuskonen, J.; Rissanen, K.; Koskinen, A.M.P. A Simple Organocatalytic Enantioselective Synthesis of Pregabalin. Eur. J. Org. Chem. 2009, 2009, 1340–1351. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Tang, L.; Deng, L. Highly Enantioselective Conjugate Addition of Malonate and β-Ketoester to Nitroalkenes: Asymmetric C-C Bond Formation with New Bifunctional Organic Catalysts Based on Cinchona Alkaloids. J. Am. Chem. Soc. 2004, 126, 9906–9907. [Google Scholar] [CrossRef]
- Ye, J.; Dixon, D.J.; Hynes, P.S. Enantioselective Organocatalytic Michael Addition of Malonate Esters to Nitro Olefins Using Bifunctional Cinchonine Derivatives. Chem. Comm. 2005, 35, 4481–4483. [Google Scholar] [CrossRef]
- McCooey, S.H.; Connon, S.J. Urea- and Thiourea-Substituted Cinchona Alkaloid Derivatives as Highly Efficient Bifunctional Organocatalysts for the Asymmetric Addition of Malonate to Nitroalkenes: Inversion of Configuration at C9 Dramatically Improves Catalyst Performance. Angew. Chem. Int. Ed. 2005, 44, 6367–6370. [Google Scholar] [CrossRef]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives Are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, X.; Ge, Z.M.; Sun, Q.; Cheng, T.M.; Li, R.T. Solvent-Free Organocatalytic Michael Addition of Diethyl Malonate to Nitroalkenes: The Practical Synthesis of Pregabalin and γ-Nitrobutyric Acid Derivatives. Tetrahedron 2011, 67, 636–640. [Google Scholar] [CrossRef]
- WO2015189068 Method for the Preparation of Beta-Substituted Gamma-Amino Carboxylic Acids. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015189068 (accessed on 31 July 2022).
- Baran, R.; Veverková, E.; Škvorcová, A.; Šebesta, R. Enantioselective Michael Addition of 1,3-Dicarbonyl Compounds to a Nitroalkene Catalyzed by Chiral Squaramides—A Key Step in the Synthesis of Pregabalin. Org. Biomol. Chem. 2013, 11, 7705–7711. [Google Scholar] [CrossRef]
- Bae, H.Y.; Song, C.E. Unprecedented Hydrophobic Amplification in Noncovalent Organocatalysis “on Water”: Hydrophobic Chiral Squaramide Catalyzed Michael Addition of Malonates to Nitroalkenes. ACS Catal. 2015, 5, 3613–3619. [Google Scholar] [CrossRef]
- Carlone, A.; Bernardi, L.; McCormack, P.; Warr, T.; Oruganti, S.; Cobley, C.J. Asymmetric Organocatalysis and Continuous Chemistry for an Efficient and Cost-Competitive Process to Pregabalin. Org. Process Res. Dev. 2021, 25, 2795–2805. [Google Scholar] [CrossRef]
- Yang, J.W.; Chandler, C.; Stadler, M.; Kampen, D.; List, B. Proline-Catalysed Mannich Reactions of Acetaldehyde. Nature 2008, 452, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Itoh, T.; Aratake, S.; Ishikawa, H. A Diarylprolinol in an Asymmetric, Catalytic, and Direct Crossed-Aldol Reaction of Acetaldehyde. Angew. Chem. Int. Ed. 2008, 47, 2082–2084. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Itoh, T.; Ohkubo, M.; Ishikawa, H. Asymmetric Michael Reaction of Acetaldehyde Catalyzed by Diphenylprolinol Silyl Ether. Angew. Chem. Int. Ed. 2008, 47, 4722–4724. [Google Scholar] [CrossRef]
- García-García, P.; Ladépêche, A.; Halder, R.; List, B. Catalytic Asymmetric Michael Reactions of Acetaldehyde. Angew. Chem. Int. Ed. 2008, 47, 4719–4721. [Google Scholar] [CrossRef]
- Fan, X.; Rodríguez-Escrich, C.; Sayalero, S.; Pericàs, M.A. Paraldehyde as an Acetaldehyde Precursor in Asymmetric Michael Reactions Promoted by Site-Isolated Incompatible Catalysts. Chem. Eur. J. 2013, 19, 10814–10817. [Google Scholar] [CrossRef]
- Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Organocatalyzed Michael Addition to Nitroalkenes via Masked Acetaldehyde. Catalysts 2020, 10, 1296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).