Hydrogen Production and Degradation of Ciprofloxacin by Ag@TiO2-MoS2 Photocatalysts
Abstract
:1. Introduction
2. Results and Discussion
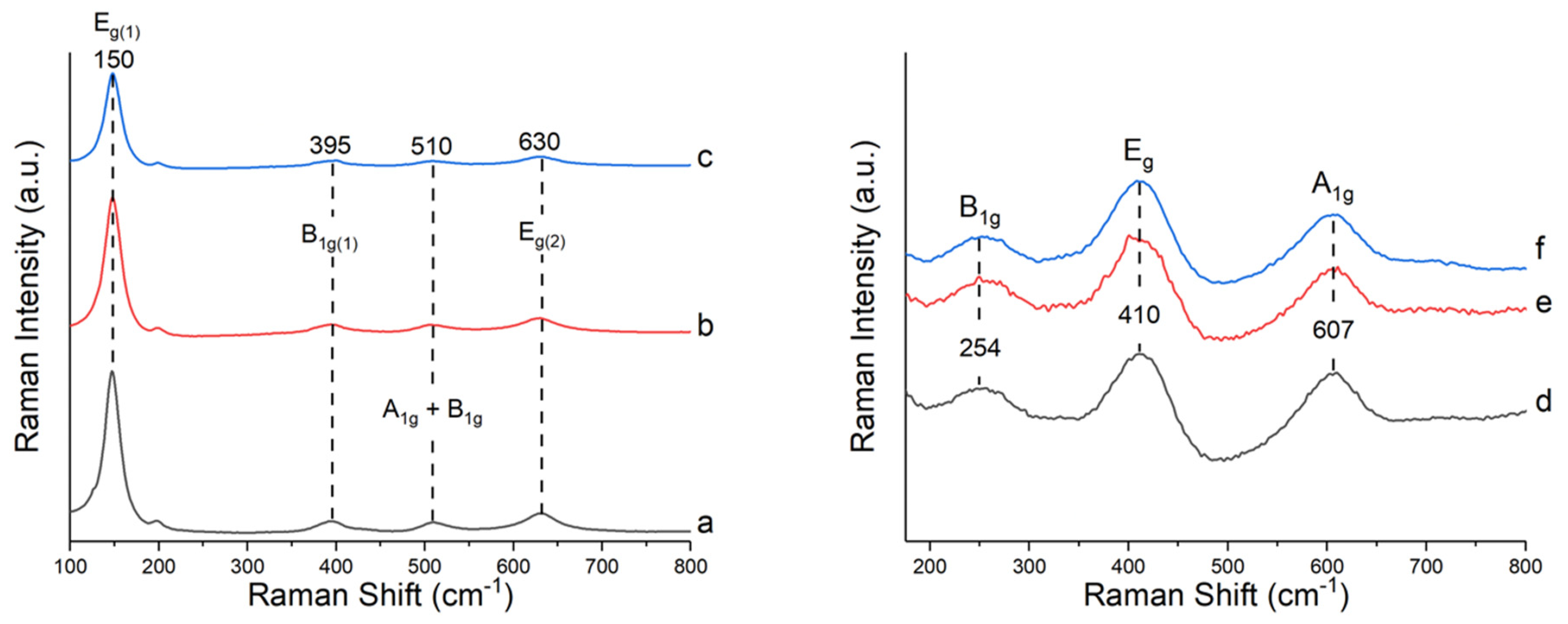
2.1. Characterization of the Catalysts
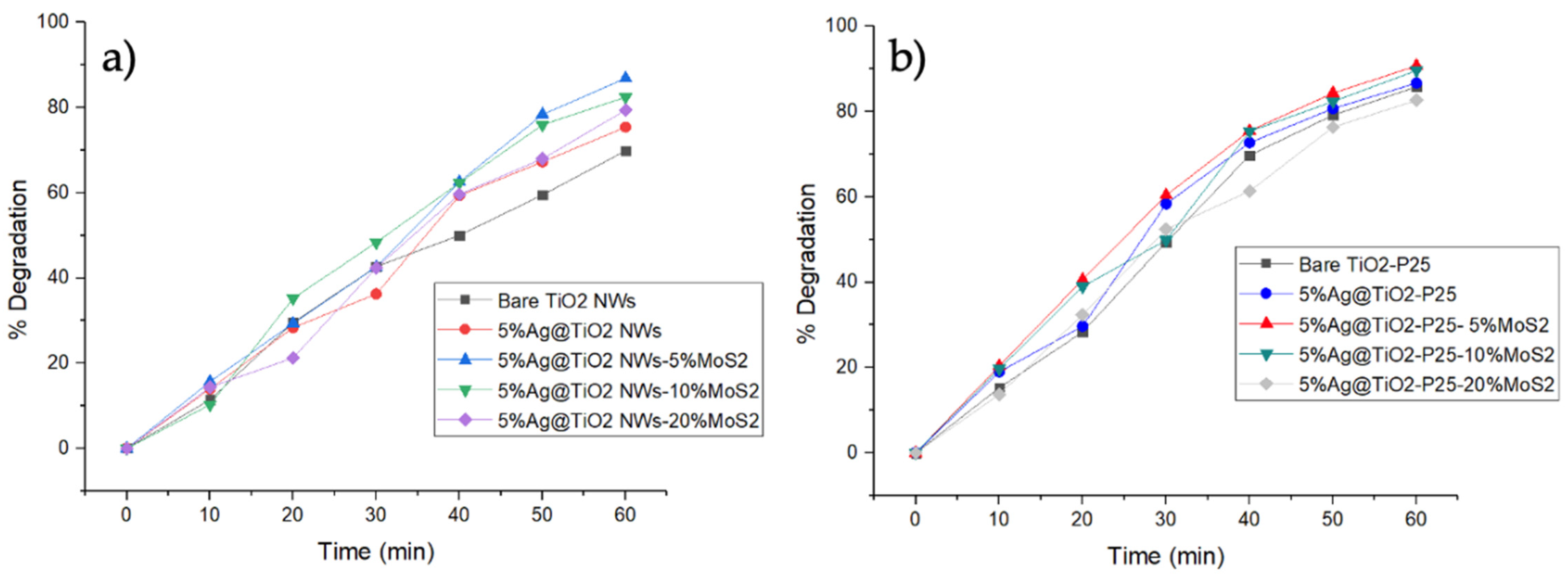
2.2. Photodegradation of Ciprofloxacin
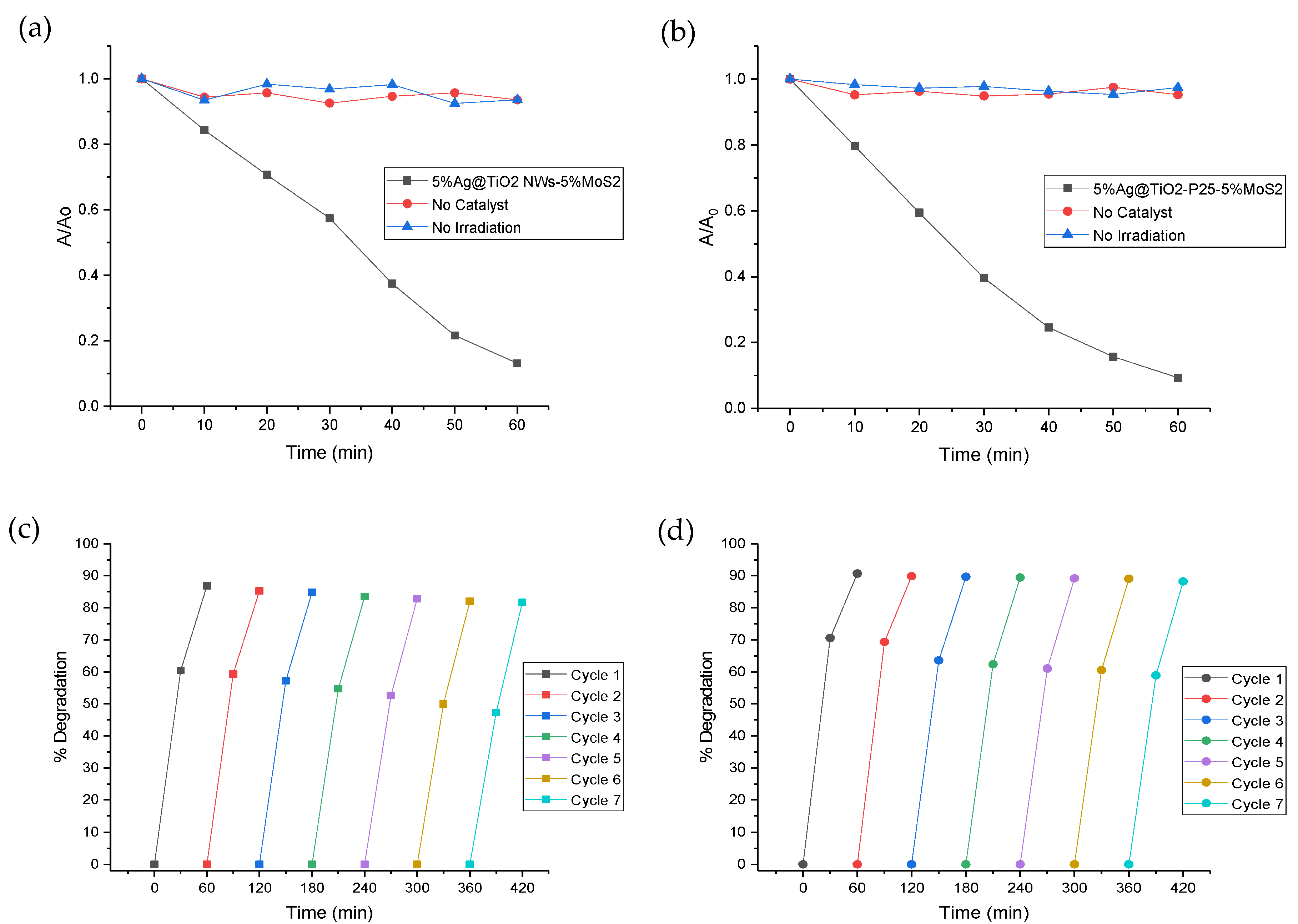
2.3. Stability Tests
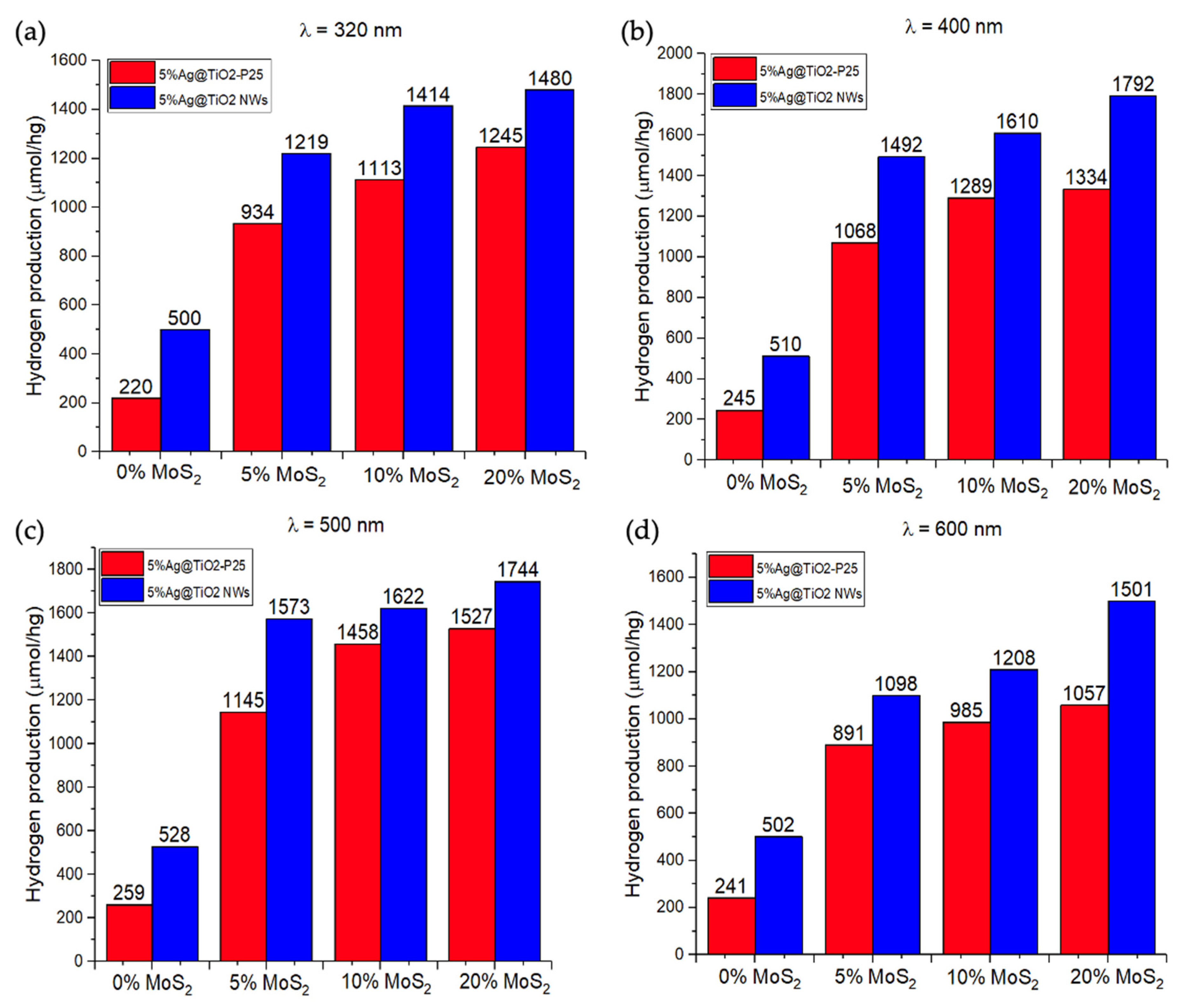
2.4. Hydrogen Production by Water Splitting
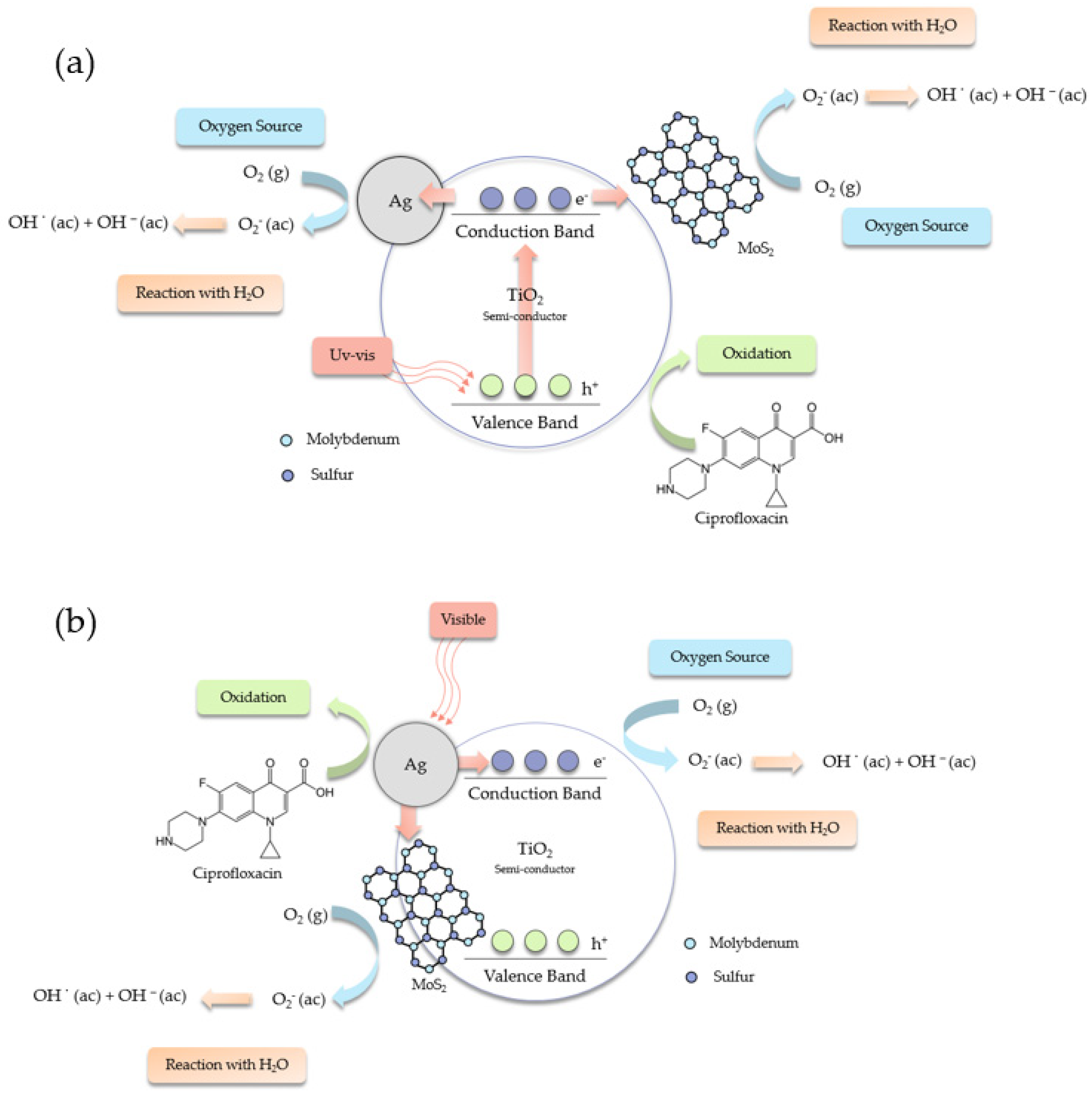
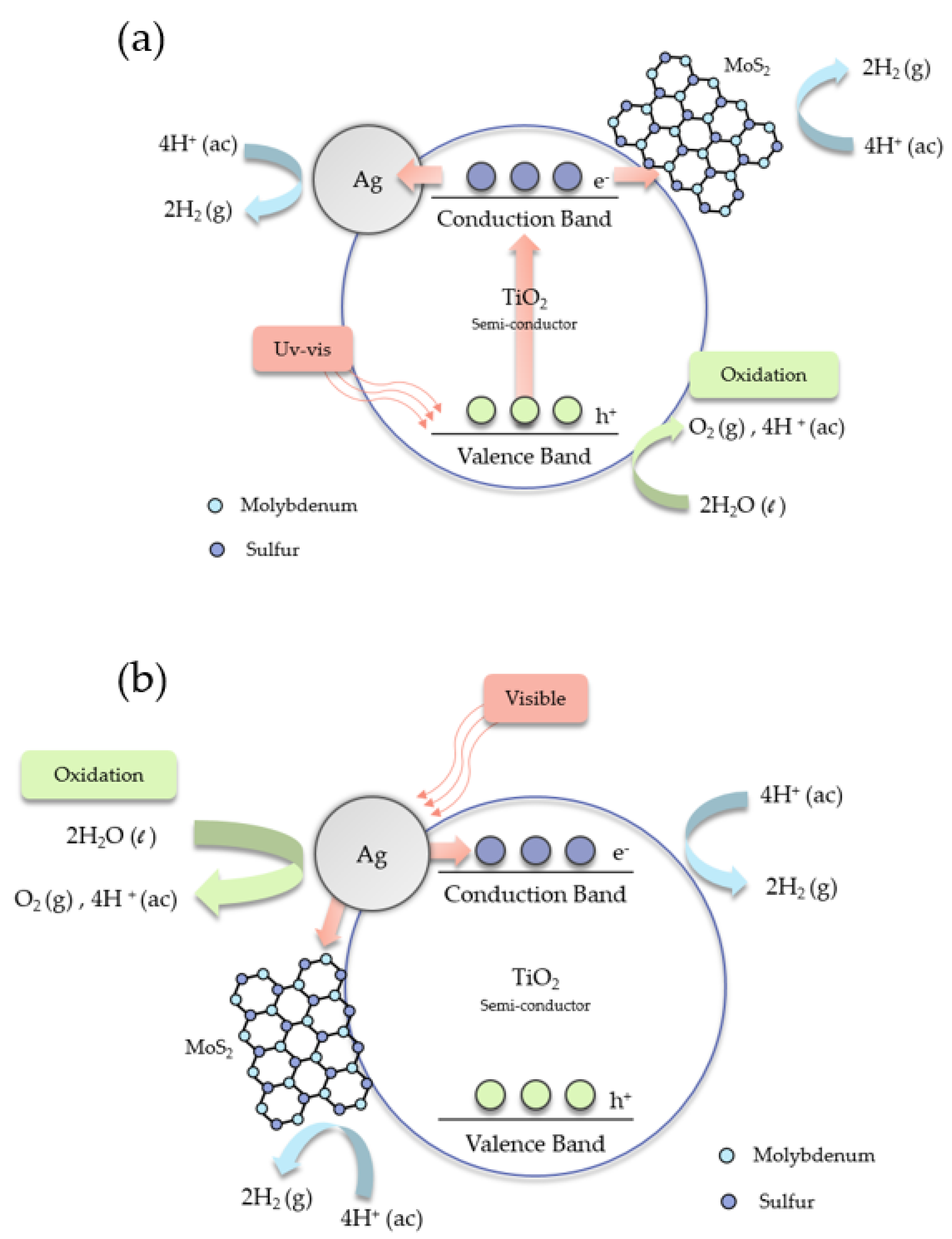
2.5. Mechanisms for the Degradation of Ciprofloxacin and the Production of Hydrogen
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Titanium Oxide Nanowires (TiO2 NWs)
3.3. Incorporation of Ag NPs on TiO2NWs and TiO2-P25
3.4. Incorporation of MoS2 on 5%Ag@TiO2 NWs and 5%Ag@TiO2-P25
3.5. Characterization of the Catalysts
3.6. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, A.; Gautam, A.; Sk, S.; Gavali, D.S.; Thapa, R.; Pal, U. Controlled Loading of MoS2 on Hierarchical Porous TiO2 for Enhanced Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2021, 125, 11950–11962. [Google Scholar] [CrossRef]
- Jin, H.; Song, T.; Paik, U.; Qiao, S.-Z. Metastable Two-Dimensional Materials for Electrocatalytic Energy Conversions. Acc. Mater. Res. 2021, 2, 559–573. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Das, M.R.; Ibrahim, M.M.; Altalhi, T.; El-Sheshtawy, H.S.; Szunerits, S.; Boukherroub, R.; Amin, M.A. CoS2 Nanoparticles Supported on RGO, g-C3N4, BCN, MoS2, and WS2 Two-Dimensional Nanosheets with Excellent Electrocatalytic Performance for Overall Water Splitting: Electrochemical Studies and DFT Calculations. ACS Appl. Energy Mater. 2021, 4, 1269–1285. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Gao, W.; Yang, G.; Qureshi, A.H.; Chen, M.; Yao, X.; Zhou, M.; Zhang, X.; Liu, Y. High Electrocatalytic Activity of Defected MX2/Graphene Heterostructures (M = Mo, W.; X = S, Se) for Hydrogen Evolution Reaction. J. Phys. Chem. C 2021, 125, 15292–15300. [Google Scholar] [CrossRef]
- Brinda, K.N.; Malecki, J.G.; Yhobu, Z.; Nagaraju, D.H.; Budagumpi, S.; Erakulan, E.S.; Thapa, R. Novel Carbene Anchored Molecular Catalysts for Hydrogen Evolution Reactions. J. Phys. Chem. C 2021, 125, 3793–3803. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, S. Influence of Group Iii and Iv Elements on the Hydrogen Evolution Reaction of MoS2 Disulfide. J. Phys. Chem. C 2021, 125, 11848–11856. [Google Scholar]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Hydrogen Production via Pd-TiO2 Photocatalytic Water Reaction Mechanism. Catalysts 2021, 11, 405. [Google Scholar] [CrossRef]
- Tahir, M. Investigating the Influential Effect of Etchant Time in Constructing 2D/2D HCN/MXene Heterojunction with Controlled Growth of TiO2NPs for Stimulating Pho.otocatalytic H2Production. Energy Fuels 2021, 35, 6807–6822. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S. Engineered Graphitic Carbon Nitride-Based Photocatalysts for Visible-Light-Driven Water Splitting: A Review. Energy Fuels 2021, 35, 6504–6526. [Google Scholar] [CrossRef]
- Kwon, H.; Bae, D.; Won, D.; Kim, H.; Kim, G.; Cho, J.; Park, H.J.; Baik, H.; Jeong, A.R.; Lin, C.H.; et al. Nanoporous Silver Telluride for Active Hydrogen Evolution. ACS Nano 2021, 15, 6540–6550. [Google Scholar] [CrossRef]
- Li, Y.; Zuo, S.; Li, Q.H.; Wu, X.; Zhang, J.; Zhang, H.; Zhang, J. Vertically Aligned MoS2 with In-Plane Selectively Cleaved Mo−S Bond for Hydrogen Production. Nano Lett. 2021, 21, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xi, C.; Zhang, R.; Liu, T.; Zou, P.; Wu, D.; Guo, Q.; Mao, J.; Xin, H.; Yang, J. Activating Edge-Mo of 2H-MoS2 via Coordination with Pyridinic N-C for PH-Universal Hydrogen Evolution Electrocatalysis. ACS Catal. 2021, 11, 4486–4497. [Google Scholar] [CrossRef]
- Singh, V.K.; Gupta, U.; Mukherjee, B.; Chattopadhyay, S.; Das, S. MoS2 Nanosheets on MoNi4/MoO2Nanorods for Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 886–896. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Chen, B. Uniformly Dispersed Metal Sulfide Nanodots on G-C3N4 as Bifunctional Catalysts for High-Efficiency Photocatalytic H2 and H2O2 Production under Visible-Light Irradiation. Energy Fuels 2021, 35, 10746–10755. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Zheng, Y.; Liu, Y.; Tian, Z.; Shi, Y.; Wang, Z.; Ma, J.; Zheng, W. Synergistic Effects of Tungsten Doping and Sulfur Vacancies in MoS2 on Enhancement of Hydrogen Evolution. J. Phys. Chem. C 2021, 125, 11369–11379. [Google Scholar] [CrossRef]
- Pinilla, S.; Machín, A.; Park, S.H.; Arango, J.C.; Nicolosi, V.; Márquez-Linares, F.; Morant, C. TiO2-Based Nanomaterials for the Production of Hydrogen and the Development of Lithium-Ion Batteries. J. Phys. Chem. B 2018, 122, 972–983. [Google Scholar] [CrossRef]
- Platero, F.; López-Martín, A.; Caballero, A.; Rojas, T.C.; Nolan, M.; Colón, G. Overcoming Pd-TiO2 Deactivation during H2 Production from Photoreforming Using Cu@Pd Nanoparticles Supported on TiO2. ACS Appl. Nano Mater. 2021, 4, 3204–3219. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Zhou, W.; Chen, M.; Su, M.; Luo, X.; Yu, T.; Yuan, C. MoS2Nanoribbons with a Prolonged Photoresponse Lifetime for Enhanced Visible Light Photoelectrocatalytic Hydrogen Evolution. Inorg. Chem. 2021, 60, 1991–1997. [Google Scholar] [CrossRef]
- Özgür, D.Ö.; Özkan, G.; Atakol, O.; Çelikkan, H. Facile Ion-Exchange Method for Zn Intercalated MoS2 As an Efficient and Stable Catalyst toward Hydrogen Evaluation Reaction. ACS Appl. Energy Mater. 2021, 4, 2398–2407. [Google Scholar] [CrossRef]
- Lin, H.; Ma, X.; Wang, H.; Wang, B.; Li, S.X.; Yi, X.; Li, Y.Y.; Wang, L. Promoted Interfacial Charge Transport and Separation of Size-Uniform Zn, Ni-Doped CdS-1T/2H O-MoS2 Nanoassemblies for Efficient Visible-Light Photocatalytic Water Splitting. Cryst. Growth Des. 2021, 21, 1278–1289. [Google Scholar] [CrossRef]
- Huerta-Aguilar, C.A.; García-Gutiérrez, Y.S.; Thangarasu, P. Crystal plane directed interaction of TiO2 [1 0 1] with AgNPs [1 1 1] silver nanoparticles enhancing solar light induced photo-catalytic oxidation of ciprofloxacin: Experimental and theoretical studies. Chem. Engi. J. 2020, 394, 124286. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Ducongé, J.; Arango, J.C.; Morant, C.; Márquez, F. Synthesis and Characterization of Au@TiO2 NWs and their Catalytic Activity by Water Splitting: A Comparative Study with Degussa P25. AJEAS 2017, 10, 298–311. [Google Scholar]
- Machín, A.; Cotto, M.; Duconge, J.; Arango, J.C.; Morant, C.; Pinilla, S.; Soto-Vázquez, L.; Resto, E.; Márquez, F. Hydrogen production via water splitting using different Au@ZnO catalysts under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 385–394. [Google Scholar] [CrossRef]
- Machín, A.; Arango, J.C.; Fontánez, K.; Cotto, M.; Duconge, J.; Soto-Vázquez, L.; Resto, E.; Petrescu, F.I.T.; Morant, C.; Márquez, F. Biomimetic Catalysts Based on Au@ZnO–Graphene Composites for the Generation of Hydrogen by Water Splitting. Biomimetics 2020, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Machín, A.; Soto-Vázquez, L.; Colón-Cruz, C.; Valentín-Cruz, C.A.; Claudio-Serrano, G.J.; Fontánez, K.; Resto, E.; Petrescu, F.I.; Morant, C.; Márquez, F. Photocatalytic Activity of Silver-Based Biomimetics Composites. Biomimetics 2021, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Piao, C.; Li, S.; Tang, J.; Fang, D. Construction of Fixed Z-Scheme Ag AgBr/Ag/TiO2 Photocatalyst Composite Film for Malachite Green Degradation with Simultaneous Hydrogen Production. J. Power Sources 2020, 469, 228430. [Google Scholar] [CrossRef]
- Lu, L.; Wang, G.; Xiong, Z.; Hu, Z.; Liao, Y.; Wang, J.; Li, J. Enhanced Photocatalytic Activity under Visible Light by the Synergistic Effects of Plasmonics and Ti3+ Doping at the Ag/TiO2−x Heterojunction. Ceram. Int 2020, 46, 1–11. [Google Scholar] [CrossRef]
- Soto-Vázquez, L.; Rolón-Delgado, F.; Rivera, K.; Cotto, M.C.; Ducongé, J.; Morant, C.; Pinilla, S.; Márquez-Linares, F. Catalytic use of TiO2 nanowires in the photodegradation of Benzophenone-4 as an active ingredient in sunscreens. J. Environ. Manag. 2019, 247, 822–828. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Ho, Y.; Ma, C.; Luong, T.; Wei, L.; Yen, T.; Hsu, W.; Chang, W.; Chu, Y.; Tu, Y.; Pande, K.P.; et al. Layered MoS2 Grown on c -Sapphire by Pulsed Laser Deposition. Phys. Status Solidi Rapid Res. Lett. 2015, 191, 187–191. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Bhoyate, B.; Morey, T.; Neria, B.L.; Vasiraju, V.; Gupta, G.; Palchoudhury, S.; Kahol, P.K.; Mishra, S.R.; et al. MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. J. Carbon Res. 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Lorenzo, M.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles After Thermal Treatments and HF. Etching Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Ibukun, O.; Evans, P.E.; Dowben, P.A.; Jeong, H.K. Titanium dioxide-molybdenum disulfide for photocatalytic degradation of methylene blue. Chem. Phys. 2019, 1, 110419. [Google Scholar] [CrossRef]
- Askari, M.B.; Kalourazi, A.F.; Seifi, M.; Shahangian, S.S.; Askari, N.; Manjili, T.J. Hydrothermal Synthesis of Molybdenum Disulfide (MoS2) and Study of Structure, Optical, Electrical and High Antibacterial Properties. Optik 2018, 174, 154–162. [Google Scholar] [CrossRef]
- Li, M.; Cui, Z.; Li, E. Silver-Modified MoS2 Nanosheets as a High-Efficiency Visible-Light Photocatalyst for Water Splitting. Ceram. Int. 2019, 45, 14449–14456. [Google Scholar] [CrossRef]
- Han, E.; Vijayarangamuthu, K.; Youn, J.; Park, Y.K.; Jung, S.C.; Jeon, K.J. Degussa P25 TiO2 modified with H2O2 under microwave treatment to enhance photocatalytic properties. Catal. Today 2018, 303, 305–312. [Google Scholar] [CrossRef]
- Suriani, A.B.; Muqoyyanah; Mohamed, A.; Othman, M.H.D.; Mamat, M.H.; Ahmad, M.K.; Nayan, N.; Khalil Abdul, H.P.S. Reduced graphene oxide-multiwalled carbon nanotubes hybrid film with low Pt loading as counter electrode for improved photovoltaic performance of dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2018, 1, 10723–10743. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C. Optimal Size of Silver Nanoparticles for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. 2011, 115, 1403–1409. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, S.; Liu, X.; Chen, L. Surface-enhanced Raman Scattering Study of Silver Nanoparticles Prepared by Using MC as a Template. J. Nanomat. 2013, 1, 984831. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, Y.; Wang, Z. TiO2, MoS2, And Tio2/MoS2 Heterostructures for Use in Organic Dyes Degradation. Chem. Select. 2018, 3, 1713–1718. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Gharaghani, M.A. Photocatalytic Degradation of Ciprofloxacin Antibiotic by TiO2 Nanoparticles Immobilized on a Glass Plate. Chem. Eng. Comm. 2019, 207, 56–72. [Google Scholar] [CrossRef]
- Costa, L.N.; Nobre, F.X.; de Oliveira Lobo, A.; de Matos, J.M.E. Photodegradation of Ciprofloxacin Using TiO2/SnO2 Nanostructures. Environ. Nanotech. Monit. Manag. 2021, 16, 100466. [Google Scholar]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, O. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Arshad, Z.; Shahid, S.; Arshad, I.; Rizwan, K.; Sher, M.; Fatima, U. Synthesis of TiO2/Graphene Oxide Nanocomposites for Their Enhanced Photocatalytic activity Against Methylene Blue Dye and Ciprofloxacin. J. Com. B. 2019, 175, 107120. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Yang, Y.; Sun, T.; Hu, X.; Zhu, C.; Liu, H.; Wang, Q.; Li, X.; Fan, J. Plasmonic Ag Deposited TiO2 Nano-Sheet Film for Enhanced Photocatalytic Hydrogen Production by Water Splitting. Nanotechnology 2014, 25, 165401. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-Doped TiO2 Photocatalysts with Effective Charge Transfer for Highly Efficient Hydrogen Production through Water Splitting. Int. J. Hydrogen. Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef]
- Ou, W.; Pan, J.; Liu, Y.; Li, S.; Li, H.; Zhao, W.; Wang, J.; Song, C.; Zheng, Y.; Li, C. Two-Dimensional Ultrathin MoS2-Modified Black Ti3+–TiO2 Nanotubes for Enhanced Photocatalytic Water Splitting Hydrogen Production. J. Energy Chem. 2020, 43, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.S.; Rocha, R.D.C.; Pereira, E.C. Photodegradation of Ciprofloxacin antibiotic over TiO2 grown by PEO: Ecotoxicity response in Lactuca sativa L. and Lemna minor. Int. J. Environ. Sci. Technol 2021, 1–10. [Google Scholar] [CrossRef]
- Saravanan, R.; Manoj, D.; Qin, J.; Naushad, M.; Gracia, F.; Lee, A.F.; Khan, M.M.; Gracia-Pinilla, M.A. Mechanothermal synthesis of Ag/TiO2 for photocatalytic methyl orange degradation and hydrogen production. Process Saf. Environ. Prot. 2018, 120, 339–347. [Google Scholar] [CrossRef]
- Lin, Y.; Renb, P.; Wei, C. Fabrication of MoS2/TiO2 heterostructures with enhanced photocatalytic activity. CrystEngComm 2019, 21, 3439–3450. [Google Scholar] [CrossRef]
- Cotto, M.T.; Campo, E.; Gómez, A.; Morant, C.; Márquez, F. Photocatalytic degradation of rhodamine-B under UV-visible light irradiation using different nanostructured catalysts. Am. Chem. Sci. J. 2013, 3, 178–202. [Google Scholar] [CrossRef] [Green Version]
- Naldoni, A.; D’Arienzo, M.; Altomare, M.; Marelli, M.; Scotti, R.; Morazzoni, F.; Selli, E.; Dal Santo, V. Pt and Au/TiO2 photocatalysts for methanol reforming: Role of metal nanoparticles in tuning charge trapping properties and photoefficiency. Appl. Catal. B 2013, 130, 239–248. [Google Scholar] [CrossRef]
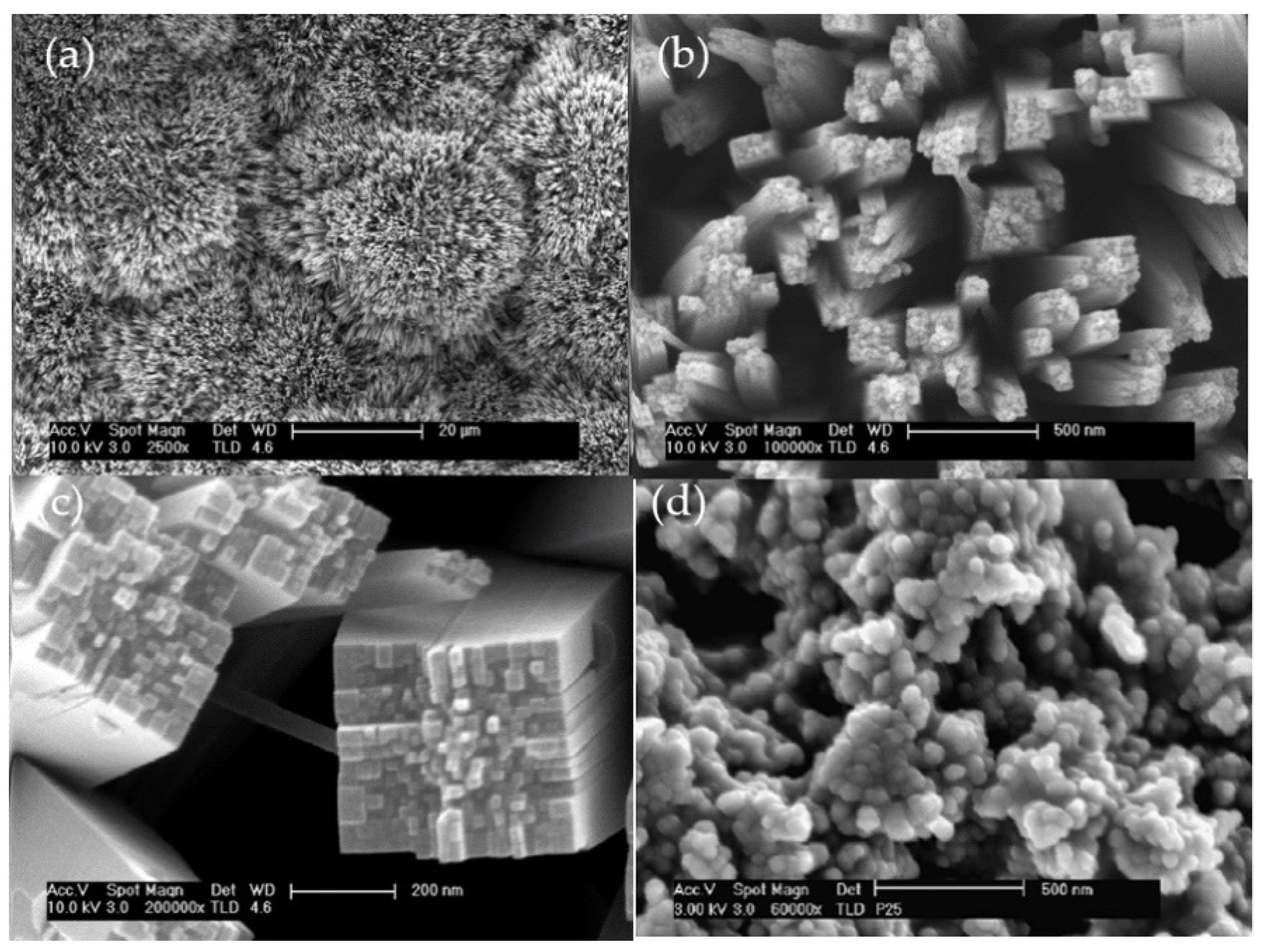

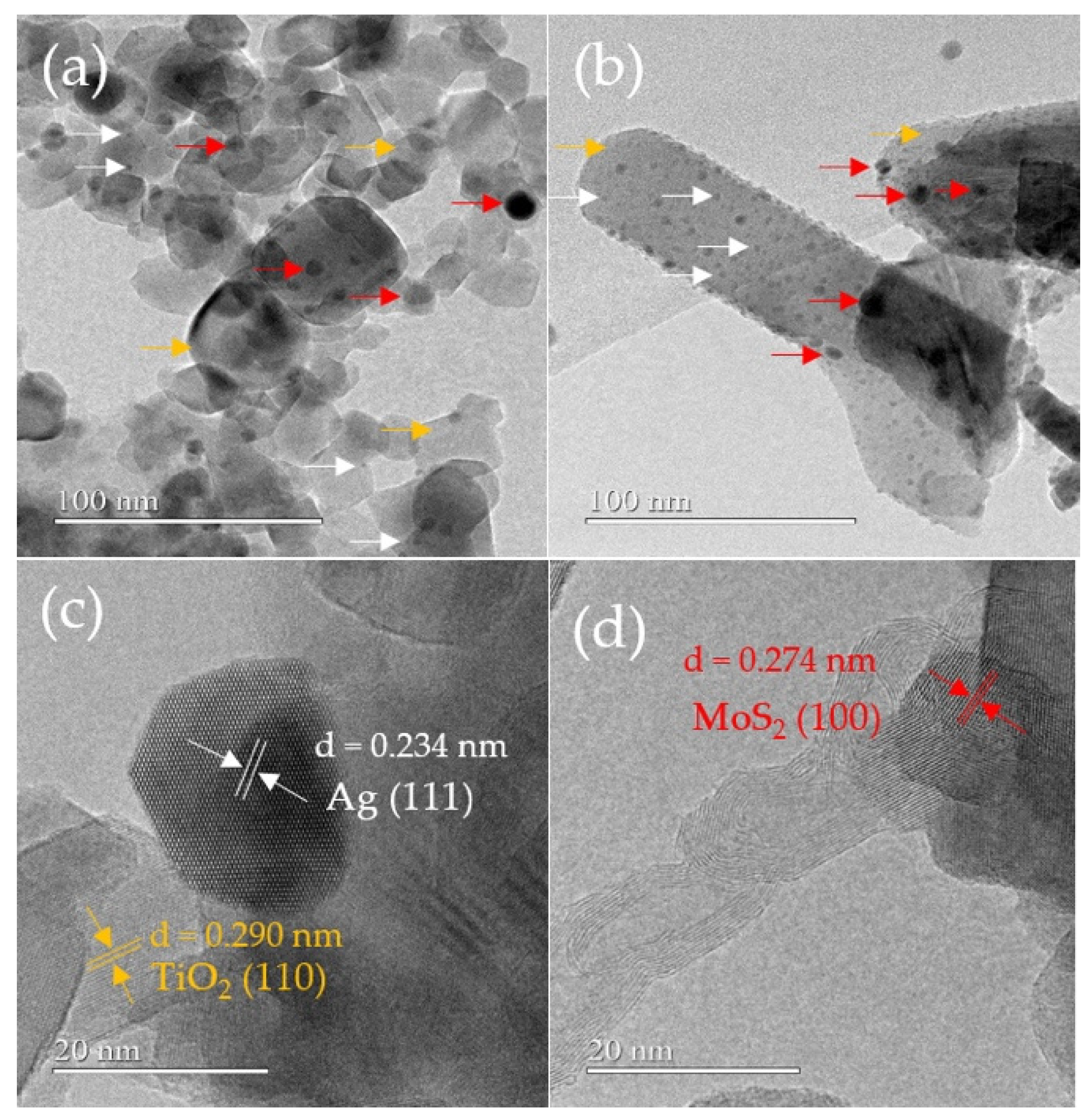
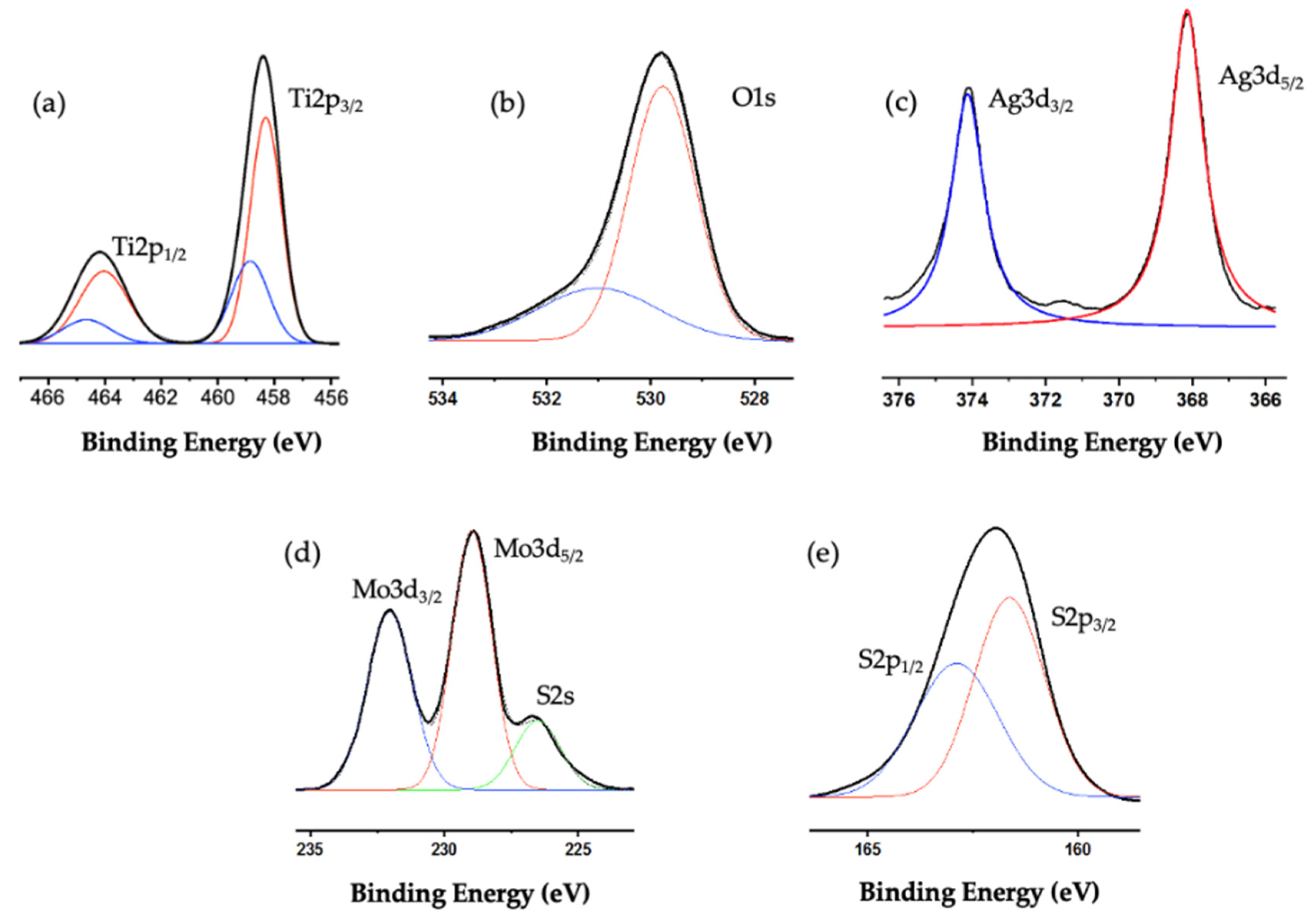
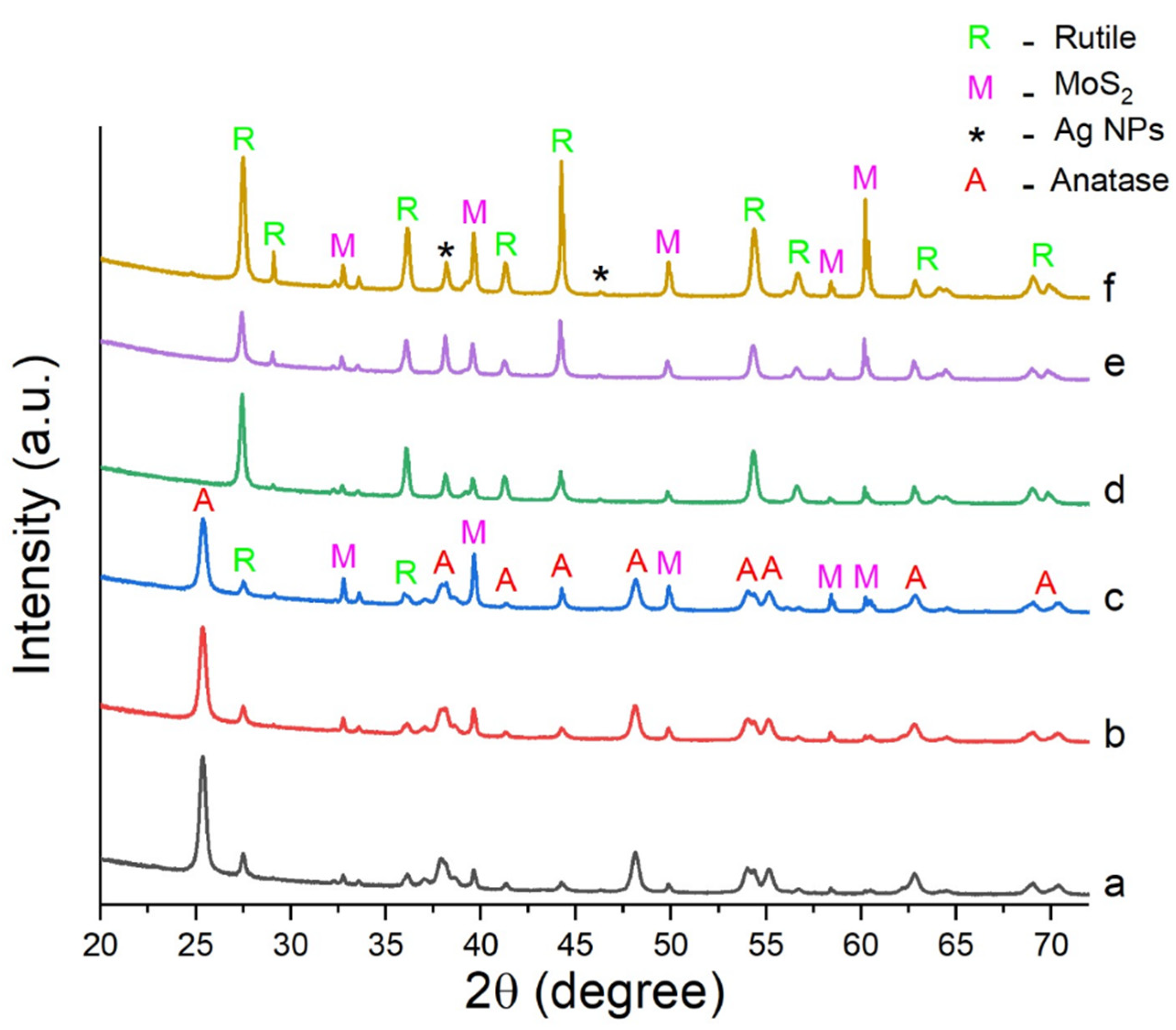
| Catalyst | TiO2-P25 (m2g−1) | TiO2NWs (m2g−1) |
|---|---|---|
| 0% (Unmodified) | 53 | 403 |
| 5% MoS2 | 98 | 429 |
| 10% MoS2 | 129 | 443 |
| 20% MoS2 | 172 | 491 |
| Catalyst | Ag Loading (wt.%) | MoS2 Loading (wt.%) | Degradation (%) * |
|---|---|---|---|
| TiO2-P25 | 5 | 0 | 87 |
| 5 | 91 | ||
| 10 | 90 | ||
| 20 | 83 | ||
| TiO2NWs | 5 | 0 | 75 |
| 5 | 87 | ||
| 10 | 82 | ||
| 20 | 79 | ||
| TiO2-P25 (pristine) | - | - | 86 |
| TiO2NWs (pristine) | - | - | 70 |
| Catalyst | Apparent Rate 1 | R2 |
|---|---|---|
| TiO2NWs | 0.019 | 0.99 |
| 5%Ag@TiO2NWs | 0.022 | 0.98 |
| 5%Ag@TiO2NWs-5%MoS2 | 0.029 | 0.96 |
| 5%Ag@TiO2NWs-10%MoS2 | 0.027 | 0.99 |
| 5%Ag@TiO2NWs-20%MoS2 | 0.023 | 0.98 |
| TiO2-P25 | 0.030 | 0.98 |
| 5%Ag@TiO2-P25 | 0.032 | 0.99 |
| 5%Ag@TiO2-P25-5%MoS2 | 0.036 | 0.99 |
| 5%Ag@TiO2-P25-10%MoS2 | 0.034 | 0.98 |
| 5%Ag@TiO2-P25-20%MoS2 | 0.027 | 0.99 |
| Catalyst. | Cycle | Degradation (%) | Difference (%) |
|---|---|---|---|
| 5%Ag@TiO2 NWs-5%MoS2 | 1 | 86.87 | 6.34 |
| 7 | 81.69 | ||
| 5%Ag@TiO2-P25-5%MoS2 | 1 | 90.72 | 2.83 |
| 7 | 88.22 |
| Catalyst | Maximum H2 Production (Unmodified Catalyst, µmol/hg) ** | Maximum H2 Production by the Modified Catalysts at Different Wavelengths (µmol/hg) | |||
|---|---|---|---|---|---|
| 320 nm | 400 nm | 500 nm | 600 nm | ||
| 5%Ag@TiO2NWs-20%MoS2 | 56; TiO2NWs | 1480 | 1792 | 1744 | 1501 |
| 5%Ag@TiO2-P25-20%MoS2 | 103; TiO2-P25 | 1245 | 1344 | 1527 | 1057 |
| Reference | H2 Production (μmol/gh) | Source (nm) | Irradiation Time (h) | Crystal Structure of TiO2 * | Reaction Mixture | Ag or MoS2 (% wt.) |
|---|---|---|---|---|---|---|
| [45] | 810 | λ > 400 | 3 | A:R | Water: Ethanol | 1.5 (Ag) |
| [46] | 23496 | λ = 254 | 6 | A:R | Water: 0.1N Na2S + 0.1Na2SO3 | 1.5 (Ag) |
| [25] | 1119 | λ = 500 | 2 | R | S + | 10 (Ag) |
| [47] | 1600 | λ= 280–700 | 4 | A | Water: 0.35 M Na2S and 0.25 M Na2SO3 | 50 (MoS2) |
| [48] | 713.15 | λ = 250 | 4 | A/R | Water: TEOA | 16 (MoS2) |
| This work | 1744 | λ = 400 | 2 | R | S and | 5 (Ag) 20 (MoS2) |
| This work | 1527 | λ = 500 | 2 | A:R | S and | 5 (Ag) 20 (MoS2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Fontánez, K.; García, D.; Sampayo, P.; Colón-Cruz, C.; Claudio-Serrano, G.J.; Soto-Vázquez, L.; Resto, E.; Petrescu, F.I.; Morant, C.; et al. Hydrogen Production and Degradation of Ciprofloxacin by Ag@TiO2-MoS2 Photocatalysts. Catalysts 2022, 12, 267. https://doi.org/10.3390/catal12030267
Machín A, Fontánez K, García D, Sampayo P, Colón-Cruz C, Claudio-Serrano GJ, Soto-Vázquez L, Resto E, Petrescu FI, Morant C, et al. Hydrogen Production and Degradation of Ciprofloxacin by Ag@TiO2-MoS2 Photocatalysts. Catalysts. 2022; 12(3):267. https://doi.org/10.3390/catal12030267
Chicago/Turabian StyleMachín, Abniel, Kenneth Fontánez, Diego García, Paola Sampayo, Carla Colón-Cruz, Gerardo J. Claudio-Serrano, Loraine Soto-Vázquez, Edgard Resto, Florian I. Petrescu, Carmen Morant, and et al. 2022. "Hydrogen Production and Degradation of Ciprofloxacin by Ag@TiO2-MoS2 Photocatalysts" Catalysts 12, no. 3: 267. https://doi.org/10.3390/catal12030267
APA StyleMachín, A., Fontánez, K., García, D., Sampayo, P., Colón-Cruz, C., Claudio-Serrano, G. J., Soto-Vázquez, L., Resto, E., Petrescu, F. I., Morant, C., & Márquez, F. (2022). Hydrogen Production and Degradation of Ciprofloxacin by Ag@TiO2-MoS2 Photocatalysts. Catalysts, 12(3), 267. https://doi.org/10.3390/catal12030267











