Crystalline Violet Wastewater Treatment by Low-Temperature Plasma Combined with Industrial Solid Waste Red Mud
Abstract
1. Introduction
2. Results and Discussion
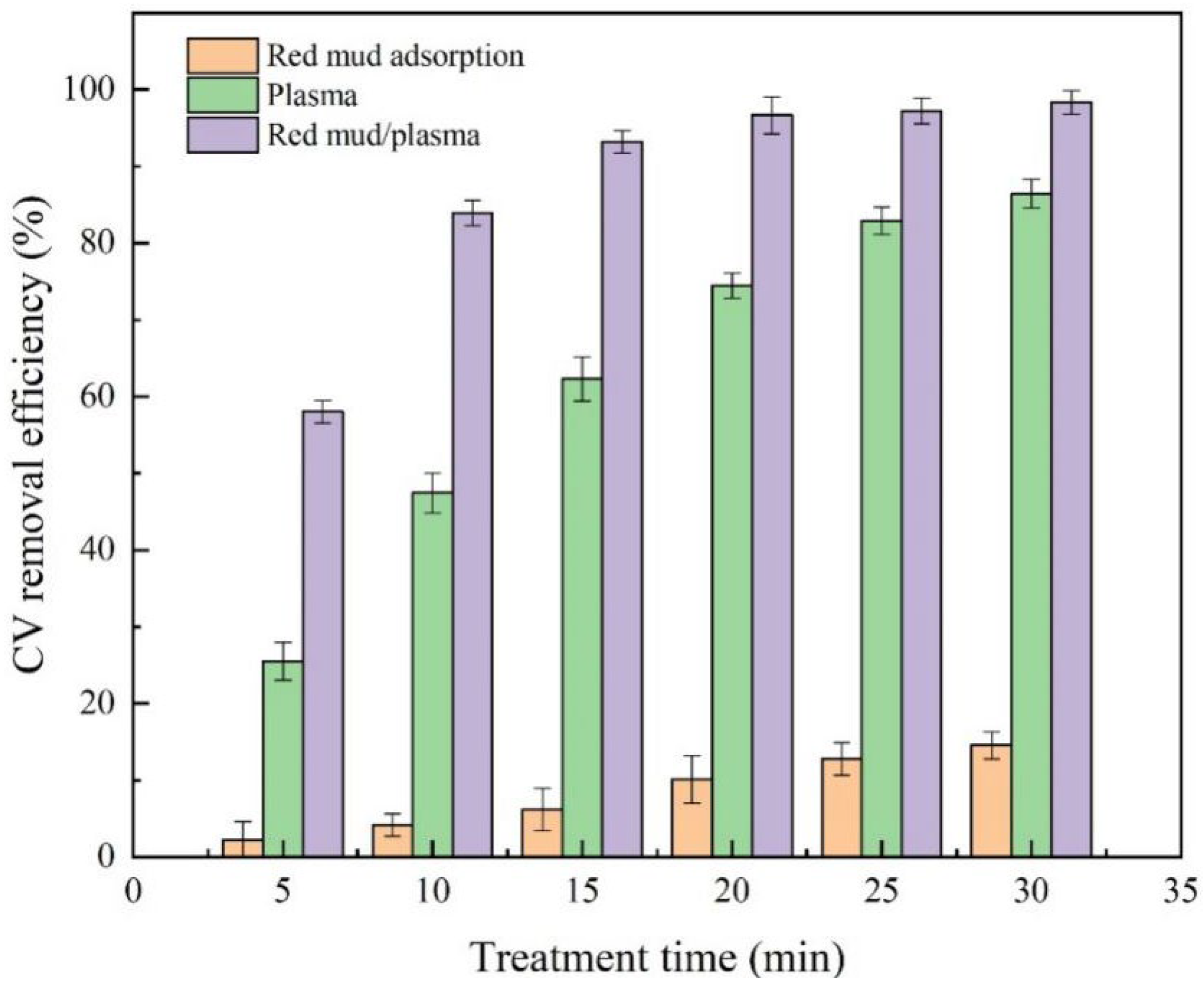
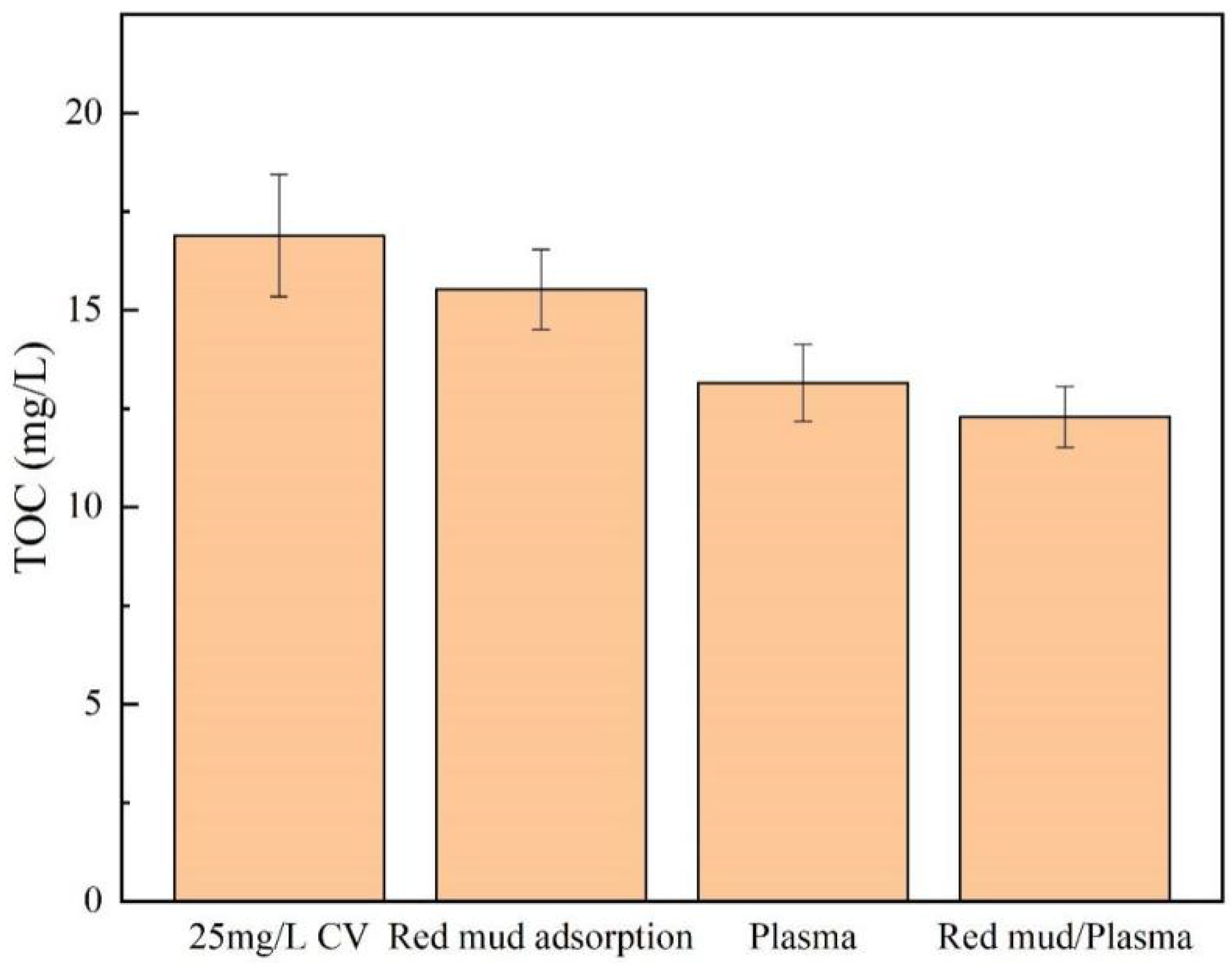
2.1. Synergistic Effect of Red Mud/Plasma Treatment System
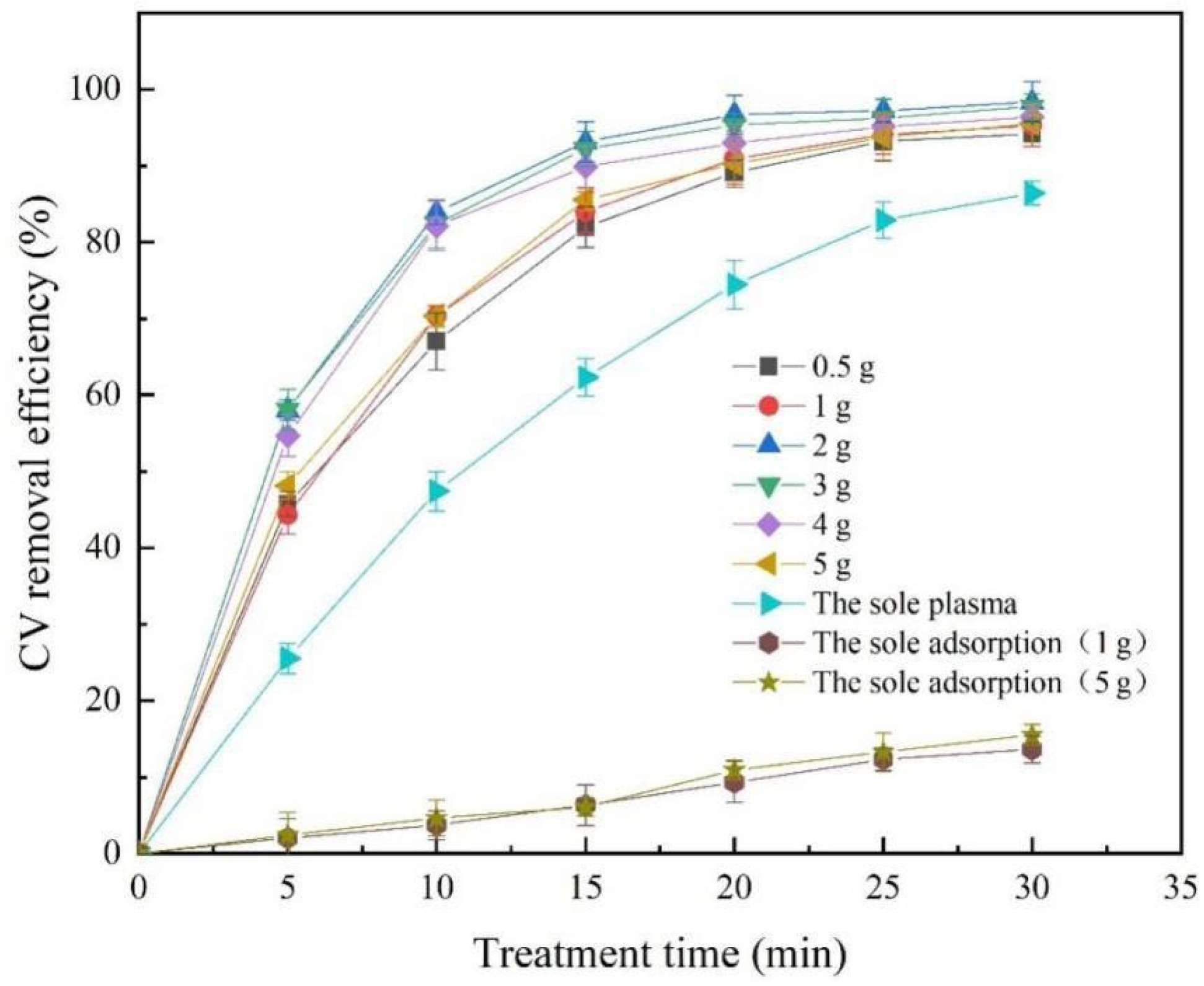
2.2. Effect of Red Mud Addition on the Removal Efficiency of CV
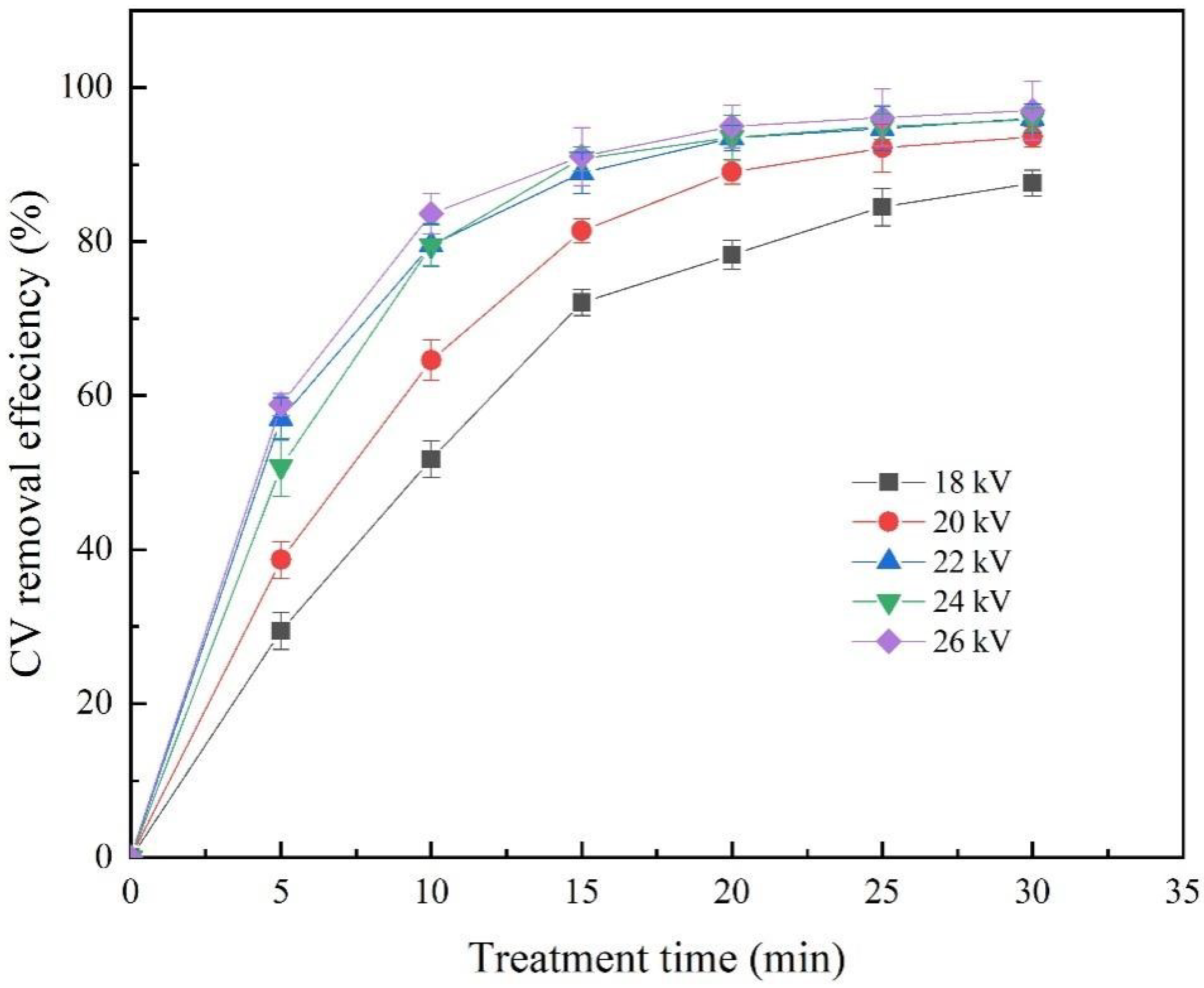
2.3. Effect of Discharge Voltage on the Removal Efficiency of CV
2.4. Effect of Initial pH on the Removal Efficiency of CV
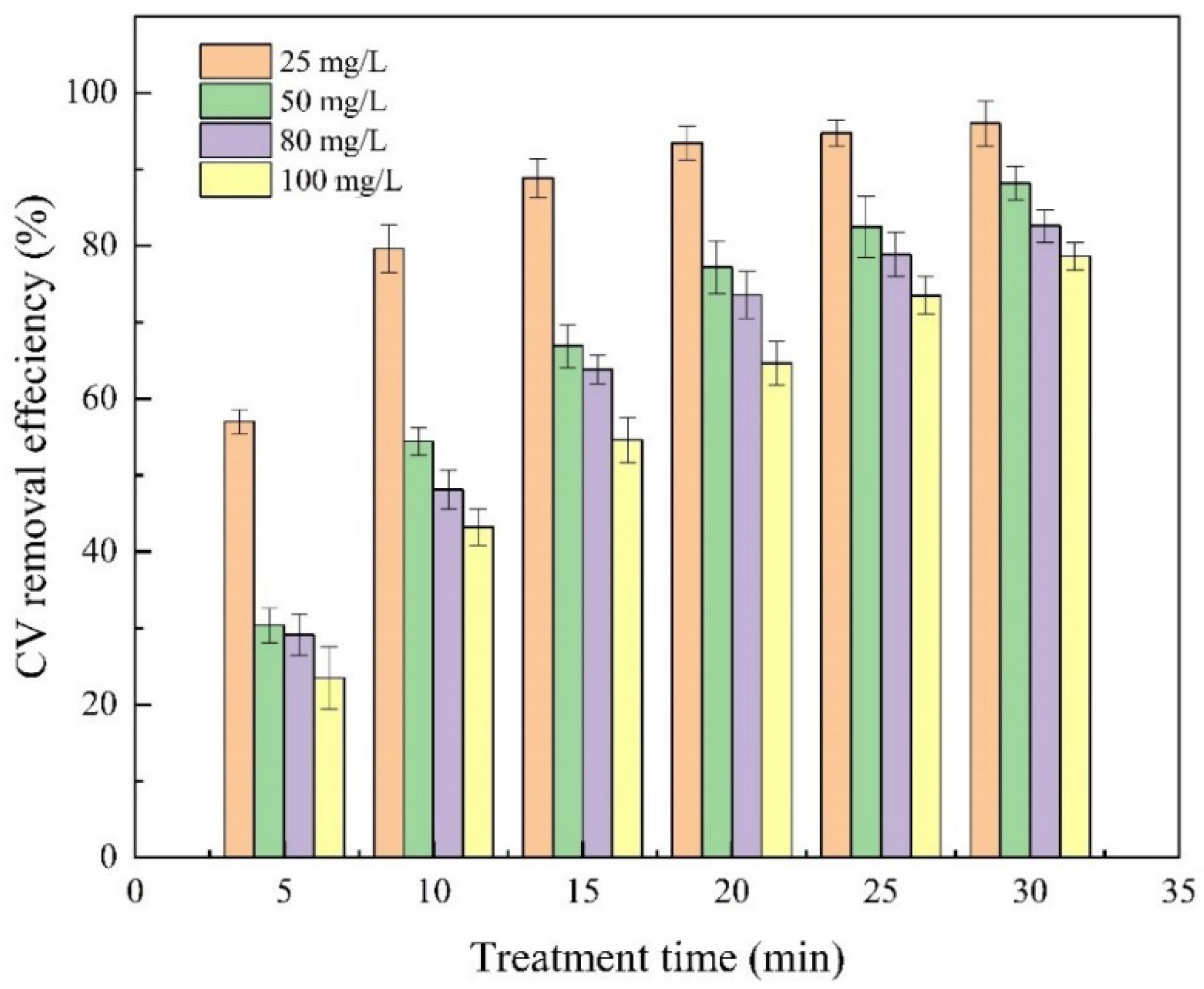
2.5. Effect of Initial Solution Concentration on the Removal Efficiency of CV
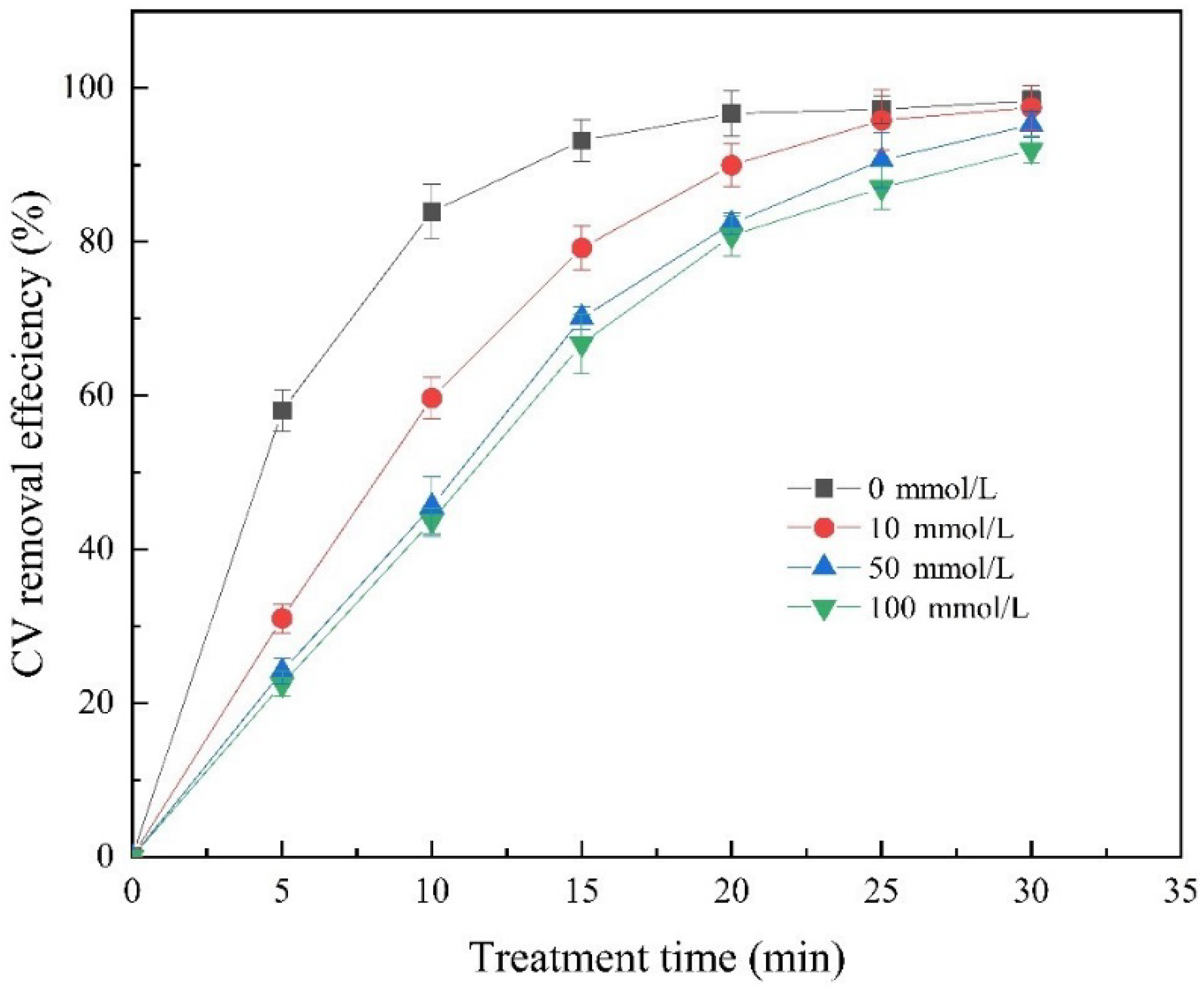
2.6. Radical Quenching Experiments
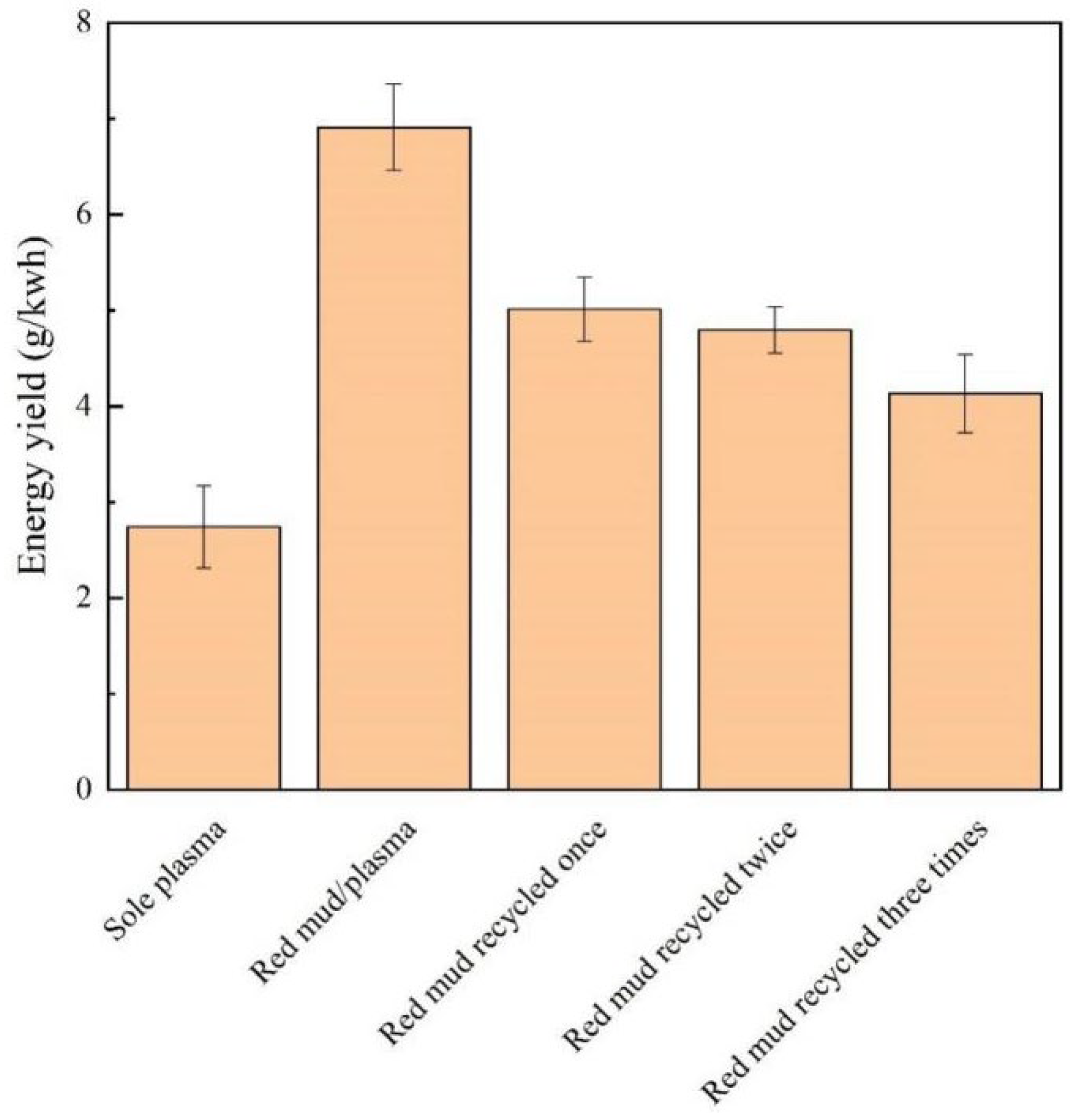
2.7. Red Mud Recycling Experiments
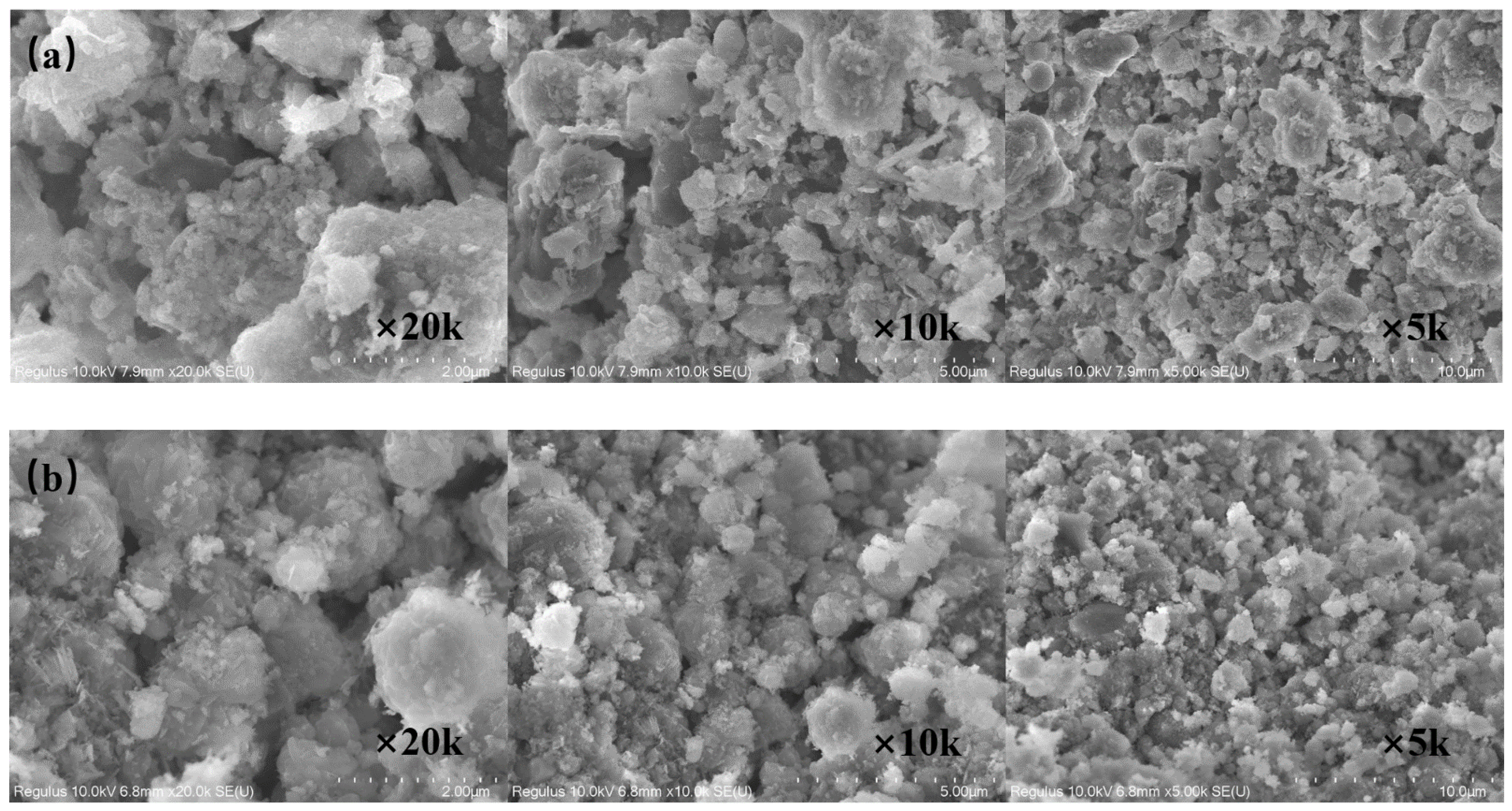
2.8. Characterisation of the Red Mud
3. Materials and Methods Experimental
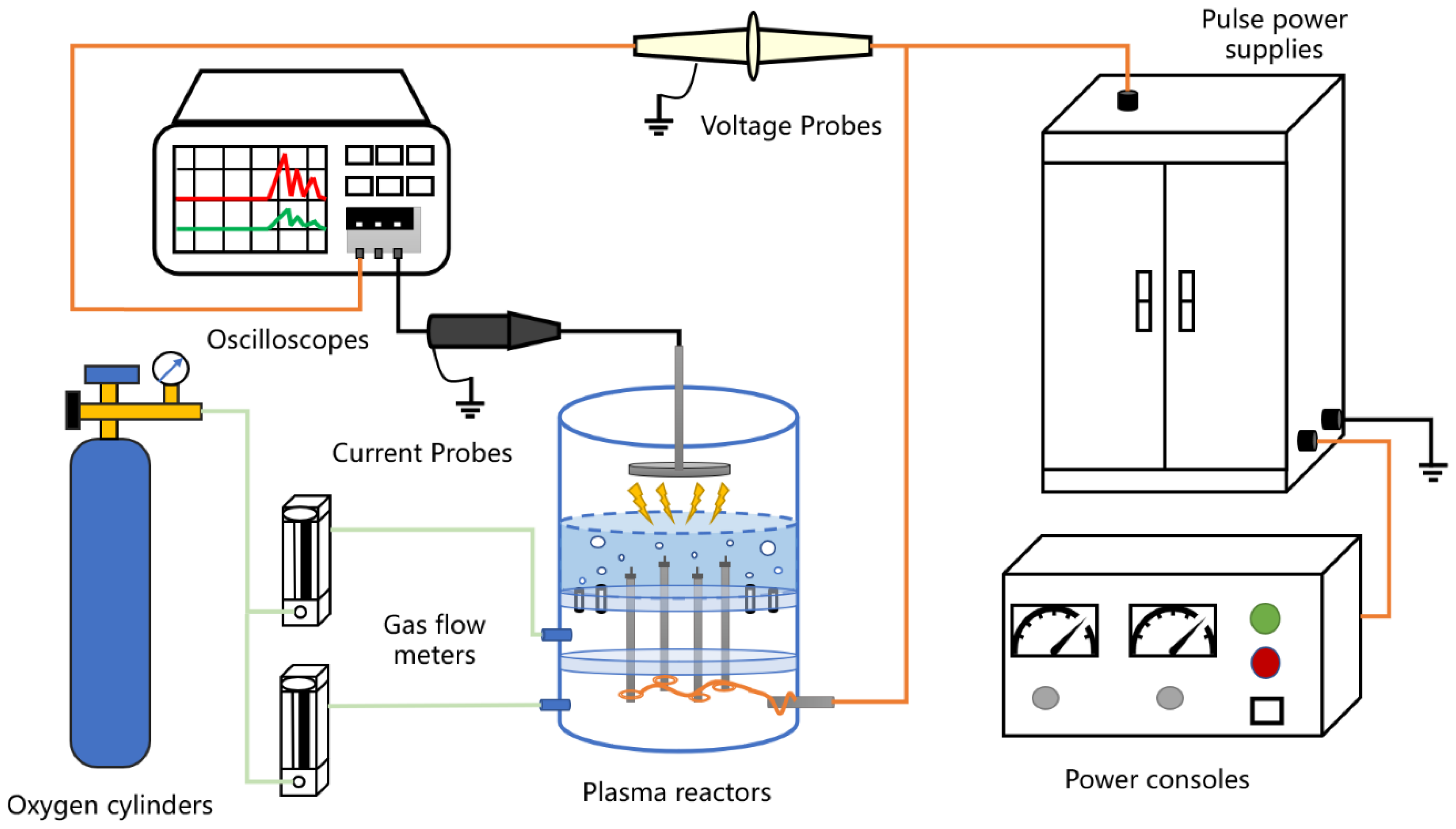
3.1. Experimental Set-Up
3.2. Red Mud Preparation
3.3. Experimental Procedure
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alzahrani, E. Zinc Oxide Nanopowders Prepared by the Sol-Gel Process for the Efficient Photodegradation of Methyl Orange. Curr. Anal. Chem. 2016, 12, 465–475. [Google Scholar] [CrossRef]
- Cai, H.; Mei, Y.D.; Chen, J.H.; Wu, Z.H.; Lan, L.; Zhu, D. An analysis of the relation between water pollution and economic growth in China by considering the contemporaneous correlation of water pollutants. J. Clean. Prod. 2020, 276, 122783. [Google Scholar] [CrossRef]
- Sarma, G.K.; Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag. 2016, 171, 1–10. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ulaganathan, P.; Sivasubramanian, S.; Esakkimuthu, S.; Krishnaswamy, S.; Subramaniam, S. Trichoderma asperellum laccase mediated crystal violet degradation-Optimization of experimental conditions and characterization. J. Environ. Chem. Eng. 2017, 5, 222–231. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.S.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ye, Y.H.; Chen, X.F.; Da, X.W.; Qiu, M.H.; Fan, Y.Q. Charged modified tight ceramic ultrafiltration membranes for treatment of cationic dye wastewater. Chin. J. Chem. Eng. 2022, 41, 267–277. [Google Scholar] [CrossRef]
- Gao, J.; Gu, P.D.; Yuan, L.; Zhong, F.C. Degradation of Dye Wastewater by ns-Pulse DBD Plasma. Plasma Sci. Technol. 2013, 15, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D.Z.; Cheng, L.; Li, Y.P. Preparation and characterization of Fe2O3-CeO2-TiO2/gamma-Al2O3 catalyst for degradation dye wastewater. J. Environ. Sci.-China 2006, 18, 1189–1192. [Google Scholar] [CrossRef]
- Jiao, S.; Zhao, Y.M.; Li, C.S.; Wang, B.S.; Qu, Y. Recyclable adsorbent of BiFeO3/Carbon for purifying industrial dye wastewater via photocatalytic reproducible. Green Energy Environ. 2019, 4, 66–74. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Iqbal, J.; Yang, Z.R.; Du, Y.P. Preparation of magnetic chitosan corn straw biochar and its application in adsorption of amaranth dye in aqueous solution. Int. J. Biol. Macromol. 2022, 199, 234–242. [Google Scholar] [CrossRef]
- Carvalho, J.R.S.; Amaral, F.M.; Florencio, L.; Kato, M.T.; Delforno, T.P.; Gavazza, S. Microaerated UASB reactor treating textile wastewater: The core microbiome and removal of azo dye Direct Black 22. Chemosphere 2020, 242, 125157. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, A.; Haghighat, G.; Shaibani, P.M.; Van Neste, C.W.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Degradation of pharmaceutical contaminants in water by an advanced plasma treatment. Desalin. Water Treat. 2019, 139, 202–221. [Google Scholar] [CrossRef]
- Abd El-Gelil, G.M.; Mansour, M.S.; Ebrahiem, E.E.; EL-Shazly, A.H.; Abou-Gabal, H. Degradation of Eosin Y in Water by Corona Treatment with a Dielectric Barrier Discharge Plasma. Chem. Eng. Technol. 2020, 43, 2015–2022. [Google Scholar] [CrossRef]
- Hu, X.; Wang, B. Removal of pefloxacin from wastewater by dielectric barrier discharge plasma: Mechanism and degradation pathways. J. Environ. Chem. Eng. 2021, 9, 105720. [Google Scholar] [CrossRef]
- Duana, L.J.; Jiang, N.; Lu, N.; Shang, K.F.; Li, J.; Wu, Y. Synergetic effect of TiO2 and Fe3+ as co-catalysts for enhanced phenol degradation in pulsed discharge system. Appl. Catal. B-Environ. 2018, 221, 521–529. [Google Scholar] [CrossRef]
- Huang, J.; Puyang, C.; Wang, Y.; Zhang, J.; Guo, H. Hydroxylamine activated by discharge plasma for synergetic degradation of tetracycline in water: Insight into performance and mechanism. Sep. Purif. Technol. 2022, 300, 121913. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Wu, Q.; Zhou, G.; Yi, C. Kinetic analysis of acid orange 7 degradation by pulsed discharge plasma combined with activated carbon and the synergistic mechanism exploration. Chemosphere 2016, 159, 221–227. [Google Scholar] [CrossRef]
- Chen, W.G.; Wu, H.X.; Fan, J.W.; Fang, Z.; Lin, S.H. Activated persulfate by DBD plasma and activated carbon for the degradation of acid orange II. Plasma Sci. Technol. 2020, 22, 34009. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xiong, X.Y.; Han, Y.; Yuan, H.; Deng, S.H.; Xiao, H.; Shen, F.; Wu, X.B. Application of titanium dioxide-loaded activated carbon fiber in a pulsed discharge reactor for degradation of methyl orange. Chem. Eng. J. 2010, 162, 1045–1049. [Google Scholar] [CrossRef]
- Fan, J.W.; Wu, H.X.; Liu, R.Y.; Meng, L.Y.; Sun, Y.J. Review on the treatment of organic wastewater by discharge plasma combined with oxidants and catalysts. Environ. Sci. Pollut. R 2021, 28, 2522–2548. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.T.; Qiu, S.; Wu, M.B.; Zhang, Q.H.; Yan, Z.F.; Xue, Q.Z. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Shang, K.F.; Li, J.; Morent, R. Hybrid electric discharge plasma technologies for water decontamination: A short review. Plasma Sci. Technol. 2019, 21, 5–13. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.A.; Li, R.; Li, X.; Chen, H.; Xue, G.; Ognier, S. Reactive Blue Degradation in Aqueous Medium by Fe-Doping TiO2 Catalytic Nonthermal Plasma. IEEE Trans. Plasma Sci. 2015, 43, 3234–3241. [Google Scholar] [CrossRef]
- Zhou, R.S.; Zhou, R.W.; Zhang, X.H.; Tu, S.; Yin, Y.W.; Yang, S.Z.; Ye, L.Y. An efficient bio-adsorbent for the removal of dye: Adsorption studies and cold atmospheric plasma regeneration. J. Taiwan Inst. Chem. Eng. 2016, 68, 372–378. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.H.; Sun, W. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Ye, J.; Cong, X.N.; Zhang, P.Y.; Hoffmann, E.; Zeng, G.M.; Liu, Y.; Fang, W.; Wu, Y.; Zhang, H.B. Interaction between phosphate and acid-activated neutralized red mud during adsorption process. Appl. Surf. Sci. 2015, 356, 128–134. [Google Scholar] [CrossRef]
- Xu, B.B.; Qi, F.; Zhang, J.Z.; Li, H.N.; Sun, D.Z.; Robert, D.; Chen, Z.L. Cobalt modified red mud catalytic ozonation for the degradation of bezafibrate in water: Catalyst surface properties characterization and reaction mechanism. Chem. Eng. J. 2016, 284, 942–952. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Busto, R.V.; Goncalves, M.; Coelho, L.H.G. Assessment of the use of red mud as a catalyst for photodegradation of bisphenol A in wastewater treatment. Water Sci. Technol. 2016, 74, 1283–1295. [Google Scholar] [CrossRef][Green Version]
- de Lima, B.A.; de Castro, P.P.R.; Franca, A.B.; Baston, E.P.; Lofrano, R.C.Z.; Samanamud, G.R.L.; Loures, C.C.A.; Naves, L.L.R.; Naves, F.L. Evaluation of the effectiveness of red mud-supported catalysts in combination with ozone and TiO2 in the treatment of solution containing benzene, toluene, and xylene. Environ. Monit. Assess. 2018, 190, 560. [Google Scholar] [CrossRef]
- Inamuddin; Asiri, A.M. Sustainable Green Chemical Processes and their Allied Applications; Springer: New York, NY, USA, 2020. [Google Scholar]
- Anpilov, A.M.; Barkhudarov, E.M.; Bark, Y.B.; Zadiraka, Y.V.; Christofi, M.; Kozlov, Y.N.; Kossyi, I.A.; Kop’ev, V.A.; Silakov, V.P.; Taktakishvili, M.I.; et al. Electric discharge in water as a source of UV radiation, ozone and hydrogen peroxide. J. Phys. D Appl. Phys. 2001, 34, 993–999. [Google Scholar] [CrossRef]
- Cheng, M.M.; Song, W.J.; Ma, W.H.; Chen, C.C.; Zhao, J.C.; Lin, J.; Zhu, H.Y. Catalytic activity of iron species in layered clays for photodegradation of organic dyes under visible irradiation. Appl. Catal. Environ. 2008, 77, 355–363. [Google Scholar] [CrossRef]
- Sum, O.S.N.; Feng, J.Y.; Hu, X.J.; Yue, P.L. Photo-assisted fenton mineralization of an azo-dye acid black 1 using a modified laponite clay-based Fe nanocomposite as a heterogeneous catalyst. Top. Catal. 2005, 33, 233–242. [Google Scholar] [CrossRef]
- Tarkwa, J.B.; Acayanka, E.; Jiang, B.; Oturan, N.; Kamgang, G.Y.; Laminsi, S.; Oturan, M.A. Highly efficient degradation of azo dye Orange G using laterite soil as catalyst under irradiation of non-thermal plasma. Appl. Catal. Environ. 2019, 246, 211–220. [Google Scholar] [CrossRef]
- Reddy, D.R.; Dinesh, G.K.; Anandan, S.; Sivasankar, T. Sonophotocatalytic treatment of Naphthol Blue Black dye and real textile wastewater using synthesized Fe doped TiO2. Chem. Eng. Process. 2016, 99, 10–18. [Google Scholar] [CrossRef]
- Wang, Z.J.; Jiang, S.; Liu, K.F. Treatment of Wastewater with High Conductivity by Pulsed Discharge Plasma. Plasma Sci. Technol. 2014, 16, 688–694. [Google Scholar] [CrossRef]
- Liu, X.H.; Cheng, C.; Xu, Z.M.; Hu, S.H.; Shen, J.; Lan, Y.; Chu, P.K. Degradation of tetracycline in water by gas-liquid plasma in conjunction with rGO-TiO2 nanocomposite. Plasma Sci. Technol. 2021, 23, 11. [Google Scholar] [CrossRef]
- Li, J.; Sato, M.; Ohshima, T. Degradation of phenol in water using a gas-liquid phase pulsed discharge plasma reactor. Thin Solid Films 2007, 515, 4283–4288. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Yang, G.; Zhang, Y.Z.; Zhang, X.H.; Deng, S.H. Degradation of Dye Wastewater by Pulsed High-Voltage Discharge Combined with Spent Tea Leaves. Plasma Sci. Technol. 2014, 16, 1135–1140. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Li, J.; Wu, Y. Synergistic degradation of bisphenol A by pulsed discharge plasma with granular activated carbon: Effect of operating parameters, synergistic mechanism and possible degradation pathway. Vacuum 2018, 156, 402–410. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. Effic. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Huang, Y.R.; Kong, Y.; Li, H.Z.; Wei, X.M. Removal of crystal violet by ultraviolet/persulfate: Effects, kinetics and degradation pathways. Environ. Technol. Innov. 2020, 18, 100780. [Google Scholar] [CrossRef]
- Saini, B.; Dey, A. Synthesis and characterization of copolymer adsorbent for crystal violet dye removal from water. Mater. Today Proc. 2022, 61, 342–350. [Google Scholar] [CrossRef]
- Oloo, C.M.; Onyari, J.M.; Wanyonyi, W.C.; Wabomba, J.N.; Muinde, V.M. Adsorptive removal of hazardous crystal violet dye form aqueous solution using Rhizophora mucronata stem-barks: Equilibrium and kinetics studies. Environ. Toxicol. Chem. 2020, 2, 64–72. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Sun, Y.B.; Zhang, C.X.; Yu, Z.Q. Decomposition of acetaminophen in water by a gas phase dielectric barrier discharge plasma combined with TiO2-rGO nanocomposite: Mechanism and degradation pathway. J. Hazard. Mater. 2017, 323, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Qu, G.Z.; Ren, J.Y.; Yan, Q.H.; Sun, Q.H.; Liang, D.L.; Hu, S.B. Evaluation of the potentials of humic acid removal in water by gas phase surface discharge plasma. Water Res. 2016, 89, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chen, L.; Wang, H.L.; Yan, Z.C. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Joshi, A.A.; Locke, B.R.; Arce, P.; Finney, W.C. Formation of Hydroxyl Radicals, Hydrogen-Peroxide and Aqueous Electrons by Pulsed Streamer Corona Discharge in Aqueous-Solution. J. Hazard. Mater. 1995, 41, 3–30. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.; Sadjadi, M.S.; Ghaznavi-Ghoushchi, M.B. Enhanced photocatalytic activity of ZrO2-CdZrO3-s nanocomposites for degradation of Crystal Violet dye under sunlight. J. Photochem. Photobiol. A 2022, 426, 113746. [Google Scholar] [CrossRef]
- Fahoul, Y.; Tanji, K.; Zouheir, M.; El Mrabet, I.; Naciri, Y.; Hsini, A.; Nahali, L.; Kherbeche, A. Novel River Sediment@ZnO-Co nanocomposite for photocatalytic degradation and COD reduction of crystal violet under visible light. J. Mol. Struct. 2022, 1253, 132298. [Google Scholar] [CrossRef]
- Berardinelli, A.; Hamrouni, A.; Dirè, S.; Ceccato, R.; Camera-Roda, G.; Ragni, L.; Palmisano, L.; Parrino, F. Features and application of coupled cold plasma and photocatalysis processes for decontamination of water. Chemosphere 2021, 262, 128336. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.H.; Lu, S.D.; Peng, J.H.; Zhang, L.B.; Srinivasakannan, C.; Guo, S.H.; Li, W. Removal of cadmium from aqueous solutions using red mud granulated with cement. T. Nonferr. Metal. Soc. 2012, 22, 3140–3146. [Google Scholar] [CrossRef]
- Wang, H.J.; Guo, H.; Liu, Y.J.; Yi, C.W. Regeneration of Acid Orange 7 Exhausted Granular Activated Carbon Using Pulsed Discharge Plasmas. Plasma Sci. Technol. 2015, 17, 881–886. [Google Scholar] [CrossRef]
- Guo, T.F.; Yang, H.Q.; Liu, Q.Y.; Gu, H.N.; Wang, N.; Yu, W.B.; Dai, Y. Adsorptive removal of phosphate from aqueous solutions using different types of red mud. Water Sci. Technol. 2018, 2017, 570–577. [Google Scholar] [CrossRef]
- Romero-Ibarra, I.C.; Ortiz-Landeros, J.; Pfeiffer, H. Microstructural and CO2 chemisorption analyses of Li4SiO4: Effect of surface modification by the ball milling process. Thermochim. Acta 2013, 567, 118–124. [Google Scholar] [CrossRef]
- Tsamo, C.; Djonga, P.N.D.; Dikdim, J.M.D.; Kamga, R. Kinetic and Equilibrium Studies of Cr(VI), Cu(II) and Pb(II) Removal from Aqueous Solution Using Red Mud, a Low-Cost Adsorbent. Arab. J. Sci. Eng. 2018, 43, 2353–2368. [Google Scholar] [CrossRef]
- Li, X.; Ji, M.; Nghiem, L.D.; Zhao, Y.; Liu, D.; Yang, Y.; Wang, Q.; Trinh, Q.T.; Vo, D.V.N.; Pham, V.Q.; et al. A novel red mud adsorbent for phosphorus and diclofenac removal from wastewater. J. Mol. Liq. 2020, 303, 112286. [Google Scholar] [CrossRef]
- Sahu, R.C.; Patel, R.; Ray, B.C. Utilization of activated CO2-neutralized red mud for removal of arsenate from aqueous solutions. J. Hazard. Mater. 2010, 179, 1007–1013. [Google Scholar] [CrossRef]
- Castaldi, P.; Silvetti, M.; Santona, L.; Enzo, S.; Melis, P. XRD, FTIR, and thermal analysis of bauxite ore-processing waste (red mud) exchanged with heavy metals. Clays Clay Miner. 2008, 56, 461–469. [Google Scholar] [CrossRef]
- Kazak, O.; Eker, Y.R.; Akin, I.; Bingol, H.; Tor, A. Green preparation of a novel red mud@carbon composite and its application for adsorption of 2,4-dichlorophenoxyacetic acid from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 23057–23068. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, H.N.; Xu, B.B.; Sun, D.Z. Heating Activated Red Mud Catalytic Ozonation for Degradation Nitrobenzene from Aqueous Solution: Performance and Influence of Preparation Factors. J. Nanosci. Nanotechnol. 2014, 14, 6984–6990. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.B.; Qi, F.; Sun, D.Z.; Chen, Z.L.; Robert, D. Cerium doped red mud catalytic ozonation for bezafibrate degradation in wastewater: Efficiency, intermediates, and toxicity. Chemosphere 2016, 146, 22–31. [Google Scholar] [CrossRef]
- Fan, J.W.; Wu, H.X.; Liu, R.Y.; Meng, L.Y.; Fang, Z.; Liu, F.; Xu, Y.H. Non-thermal plasma combined with zeolites to remove ammonia nitrogen from wastewater. J. Hazard. Mater. 2020, 401, 123627. [Google Scholar] [CrossRef]
- Wu, H.X.; Fan, J.W.; Sun, Y.J.; Liu, R.Y.; Jin, J.C.; Li, P.C. Removal of ammonia nitrogen and phenol by pulsed discharge plasma combined with modified zeolite catalyst. J. Environ. Manag. 2021, 299, 113590. [Google Scholar] [CrossRef]
- Sarwar, Z.; Tichonovas, M.; Krugly, E.; Masione, G.; Abromaitis, V.; Martuzevicius, D. Graphene oxide loaded fibrous matrixes of polyether block amide (PEBA) elastomer as an adsorbent for removal of cationic dye from wastewater. J. Environ. Manag. 2021, 298, 113466. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Synthesis of Guar gum/bentonite a novel bionanocomposite: Isotherms, kinetics and thermodynamic studies for the removal of Pb (II) and crystal violet dye. J. Mol. Liq. 2018, 249, 805–814. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma. Chem. Eng. J. 2017, 313, 1033–1041. [Google Scholar] [CrossRef]


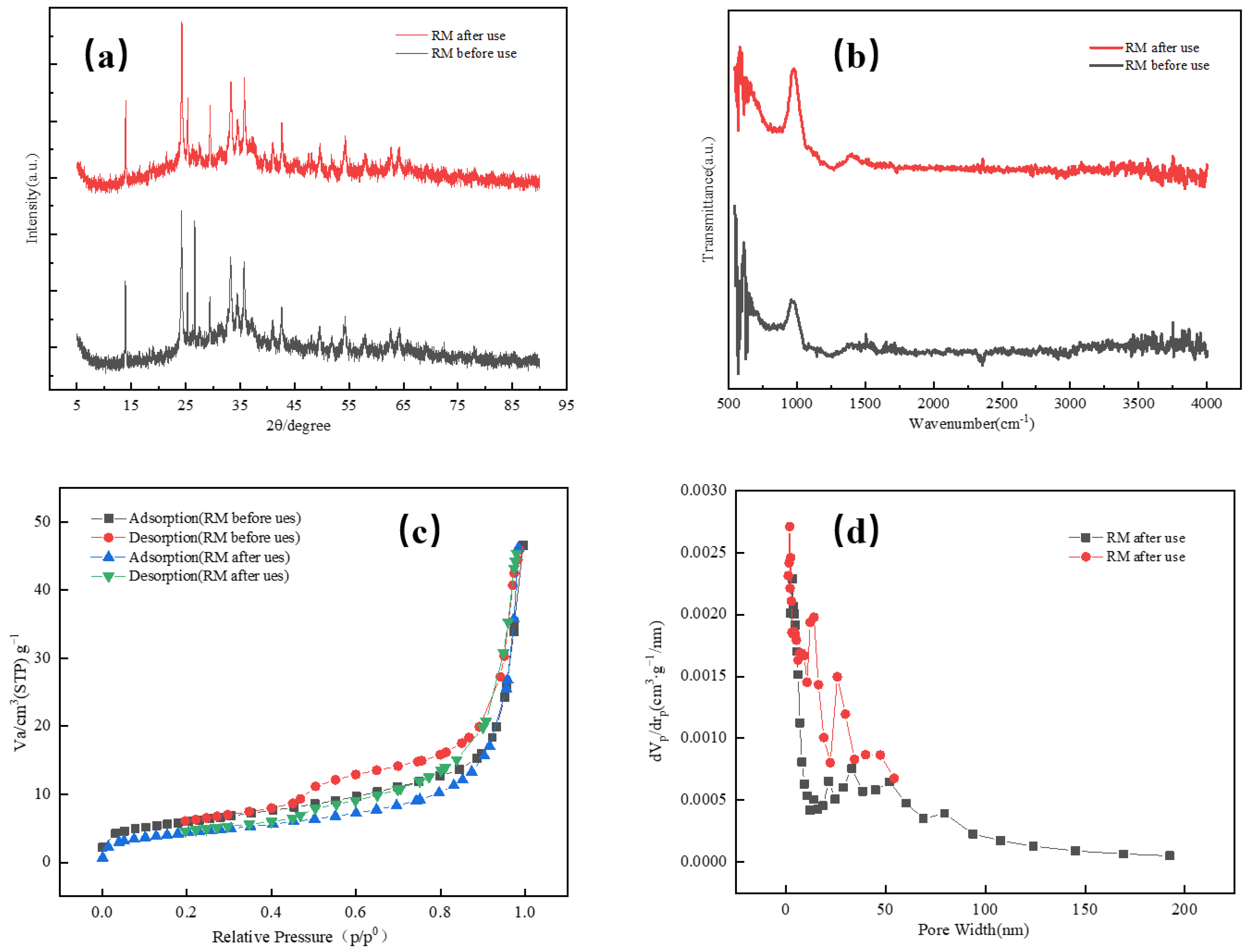
| Red Mud Sample | Fe Concentration in Solution C1 (mg/L) | Al Concentration in Solution C2 (mg/L) |
|---|---|---|
| not recycled | 3.323 | 5.272 |
| first cycle | 3.117 | 3.172 |
| second cycle | 2.740 | 1.886 |
| third cycle | 1.336 | 1.651 |
| Sample | BET Surface Area (m2/g) | Hole Wall Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| Red mud before use | 20.936 | 16.621 | 0.066827 |
| Red mud after use | 15.372 | 14.101 | 0.06397 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wu, H.; Sun, Y.; Wu, Q.; Bi, J.; Jin, J.; Fang, M.; Shi, Z. Crystalline Violet Wastewater Treatment by Low-Temperature Plasma Combined with Industrial Solid Waste Red Mud. Catalysts 2022, 12, 908. https://doi.org/10.3390/catal12080908
Zhang W, Wu H, Sun Y, Wu Q, Bi J, Jin J, Fang M, Shi Z. Crystalline Violet Wastewater Treatment by Low-Temperature Plasma Combined with Industrial Solid Waste Red Mud. Catalysts. 2022; 12(8):908. https://doi.org/10.3390/catal12080908
Chicago/Turabian StyleZhang, Weiwei, Haixia Wu, Yongjun Sun, Qu Wu, Jiliang Bi, Juncheng Jin, Minglong Fang, and Zhiru Shi. 2022. "Crystalline Violet Wastewater Treatment by Low-Temperature Plasma Combined with Industrial Solid Waste Red Mud" Catalysts 12, no. 8: 908. https://doi.org/10.3390/catal12080908
APA StyleZhang, W., Wu, H., Sun, Y., Wu, Q., Bi, J., Jin, J., Fang, M., & Shi, Z. (2022). Crystalline Violet Wastewater Treatment by Low-Temperature Plasma Combined with Industrial Solid Waste Red Mud. Catalysts, 12(8), 908. https://doi.org/10.3390/catal12080908






