Levulinic Acid Is a Key Strategic Chemical from Biomass †
Abstract
:1. Introduction
2. Possible Biomass Feedstock for Levulinic Acid (LA) Production
3. Possible Catalysts for Accelerated Levulinic Acid (LA) Production from Biomass
4. Possible Biochemicals from Levulinic Acid
4.1. Alkyl Levulinates from Levulinic Acid
4.2. Hydrogenation of LA to γ-Valerolactone
4.3. Reductive Amination of LA to N-Substituted Pyrrolidones and N-Substituted Pyrrolidinones
5. Possible Biofuels from Levulinic Acid (LA)
6. Possible Biomaterials from Levulinic Acid (LA)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulidindi, I.N.; Gedanken, A. Can Biofuels Alleviate the Energy and Environmental Crisis? Nova Science Publishers: New York, NY, USA, 2019; pp. 159–203. [Google Scholar]
- Appaturi, J.N.; Andas, J.; Ma, Y.K.; Phoon, B.L.; Batagarawa, S.M.; Koerunnisa, F.; Hussin, M.H.; Ng, E.P. Recent advances in heterogeneous catalyst for the synthesis of alkyl levulinate biofuel additives from renewable levulinic acid: A comprehensive review. Fuel 2022, 323, 124362. [Google Scholar] [CrossRef]
- Al-Lami, M.; Szilagyi, A.; Havasi, D.; Mika, L.T. 1,4-pentanediol: Vapour pressure, density viscosity, refractive index, and its isobaric vapour-liquid equilibrium with 2-methyltetrahydrofuran. J. Chem. Eng. Data 2022, 67, 1450–1459. [Google Scholar] [CrossRef]
- Gautam, P.; Barman, S.; Ali, A. A comprehensive study on the performance of acid catalysts in the synthesis of levulinate ester using biomass derived levulinic acid: A review. Biofuels Bioprod. Biorefin. 2022, 16, 1095–1115. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dou, Y.W.; Gu, B.; Oldani, C.; Tang, Q.H.; Jing, F.L.; Cao, Q.U.; Fang, W.H. Direct conversion of fructose to levulinic acid in water medium catalysed by a reusable perfluoro-sulfonic acid aquivion® resin. Mol. Catal. 2022, 520, 112159. [Google Scholar] [CrossRef]
- Scelfo, S.; Geobaldo, F.; Pirone, R.; Russo, N. Catalytic wet air oxidation of D-glucose by perovskite type oxides (Fe, Co, Mn) for the synthesis of value-added chemicals. Carbohydr. Res. 2022, 514, 108529. [Google Scholar] [CrossRef]
- Lee, B.W.; Seo, J.Y.; Jeong, K.H.; Choi, J.K.; Cho, K.Y.; Cho, S.H.; Back, K.Y. Efficient production of levulinic acid using metal-organic frame work catalyst: Role of Bronsted acid and flexibility. Chem. Eng. J. 2022, 444, 136566. [Google Scholar] [CrossRef]
- Li, X.Y.; Xu, H.C.; Hu, W.X.; Zhou, H.R.; Zhu, Y.M.; Lu, L.F.; Si, C.L. One step synthesis of Mo-doped carbon microspheres for volarization of corn cob to levulinic acid. Ind. Crops Prod. 2022, 184, 115019. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megias-Sayago, C.; Ivanova, S.; Ammari, F.; Centeno, M.A.; Odriozola, J.A. Pursuing efficient systems for glucose transformation to levulinic acid: Homogeneous vs heterogeneous catalysts and the effect of their co-action. Fuel 2022, 318, 123712. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Q.G.; Ma, D.C.; Xing, F.F.; Zeng, X.; Huang, X.X.; Teng, J.Y.; Feng, F. Kinetics for glucose conversion to levulinic acid over solid acid catalyst in γ-valerolactone solution. Biochem. Eng. J. 2020, 180, 108360. [Google Scholar] [CrossRef]
- Morakile, T.; Mandegari, M.; Farzad, S.; Gorgens, J.F. Comparative techno-economic assessment of sugar cane biorefineries producing glutamic acid, levulinic acid and xylitol from sugar cane. Ind. Crops Prod. 2022, 184, 115053. [Google Scholar] [CrossRef]
- Kumar, P.; Zeraati, A.S.; Ro, S.; Miller, K.A.; Wang, A.G.; Alemany, L.B.; Al-Attas, T.A.; Trivedi, D.; Ajayan, P.M.; Hu, J.G.; et al. Metal-free sulfonate/sulphate functionalized carbon nitride for direct conversion of glucose to levulinic acid. ACS Sustain. Chem. Eng. 2022, 10, 6230–6243. [Google Scholar] [CrossRef]
- Han, S.; Lee, S.M.; Kim, J.S. Kinetic study of glucose conversion to 5-hydroxymethylfurfural and levulinic acid catalysed by sulphuric acid. Korean Chem. Eng. Res. 2022, 60, 193–201. [Google Scholar]
- Hes, N.; Mylin, A.; Prudius, S. Catalytic production of levulinic and formic acids from fructose over superacid ZrO2-SiO2-SnO2 catalyst. Colloids Interfaces 2022, 6, 4. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.C.; Harg, B.T.; Ning, X.; Wall, D.; Murphy, J.D. Co-production of hydrochar, levulinic acid and value-added chemicals by microwave assisted hydrothermal carbonization of seaweed. Chem. Eng. J. 2022, 441, 135915. [Google Scholar] [CrossRef]
- Ringani, R.; Azis, M.M.; Rochmadi; Budiman, A. Kinetic study of levulinic acid from Spirulina platensis residue. Appl. Biochem. Biotechnol. 2022, 194, 2684–2699. [Google Scholar] [CrossRef]
- Mongkolpichayarak, I.; Jiraroh, D.; Anutrasakda, W.; Ngamcharussrivichai, C.; Samec, J.S.M.; Tungasmita, D.N. Cr/MCM-22 catalyst for the synthesis of levulinic acid from green hydrothermolysis of renewable biomass resources. J. Catal. 2022, 405, 373–384. [Google Scholar] [CrossRef]
- Taghavi, S.; Ghedini, E.; Menegazzo, F.; Maki-Arvela, P.; Peurla, M.; Zendehdel, M.; Cruciani, G.; Michele, A.D.; Murzin, D.Y.; Signoretto, M. CuZSM-5@HSM composite as an efficient micro-mesoporous catalyst for conversion of sugars into levulinic acid. Catal. Today 2022, 390–391, 146–161. [Google Scholar] [CrossRef]
- Dutta, S.; Zhang, Q.Z.; Cao, Y.; Wu, C.F.; Moustakas, K.; Zhang, S.C.; Wong, K.H.; Tsang, D.C.W. Catalytic valorisation of various paper wastes into levulinic acid, hydroxymethyl furfural, and furfural: Influence of feedstock properties and ferric chloride. Bioresour. Technol. 2022, 357, 127376. [Google Scholar] [CrossRef]
- Hak, C.; Panchai, P.; Nutongkaew, T.; Grisdanurak, N.; Tulaphol, S. One pot levulinic acid production from rice straw by acid hydrolysis in deep eutectic solvent. Chem. Eng. Commun. 2022, 1–14. [Google Scholar] [CrossRef]
- Ukarde, T.M.; Pawar, H.S. PolyE-IL, an efficient and recyclable Bronsted acid catalyst for conversion of rice straw into levulinic and other organic acids. Energy Fuels 2022, 36, 1592–1603. [Google Scholar] [CrossRef]
- Yukesel, D.E.; Ballica, L.; Cengiz, N.; Saglam, M.; Yuksel, M. Aromatic sulfonic acid-catalyzed conversion of safflower stalk into levulinic acid. Biomass Convers. Biorefin. 2022, 2022, 1–2. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhang, S.B.; Yan, B.C.; Cai, Q.J.; Zhang, S.P. Lignin-based solid acid catalyst for cellulose residue conversion into levulinic acid in biphasic system. Ind. Crops Prod. 2022, 178, 114523. [Google Scholar] [CrossRef]
- Antunes, M.M.; Silva, A.F.; Fernandes, A.; Valente, A.A. γ-valerolactone synthesis from α-angelica lactone and levulinic acid over biobased multifunctional nanohybrid catalysts. Catal. Today 2022, 394–396, 268–281. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Selvaraj, M.; Rajabathar, J.R.; Khoerunnisa, F.; Rigolet, S.; Daou, T.J.; Maireles-Torres, P.; El-Bahy, S.M.; El-bahy, Z.M.; Ng, E.P. Highly efficient non-microwave instant heating synthesis of hexyl levulinate fuel additive enhanced by sulphated nanosilica catalyst. Microporous Mesoporous Mater. 2022, 331, 111645. [Google Scholar] [CrossRef]
- Canadell, E.; Badia, J.H.; Ramirez, E.; Fite, C.; Iborra, M.; Tejero, J. Determination of thermodynamic properties for the esterification of levulinic acid with 1-butene. Ind. Eng. Chem. Res. 2022, 61, 8313–8322. [Google Scholar] [CrossRef]
- Singh, G.; Gahtori, J.; Poddar, M.K.; Samanta, C.; Bhattacharya, S.; Birader, A.V.; Bordoloi, A. Studies on synthesis of sub-nanometer size Pt particles stabilized on ZrO2 matrix for formic acid mediated synthesis of γ-valerolactone. ChemistrySelect 2022, 7, e202200029. [Google Scholar] [CrossRef]
- Fuchineco, D.A.B.; Heredia, A.C.; Mendoza, S.M.; Rodriguez-Castellon, E. Esterification of levulnic acid to methyl levulinate over Zr-MOF catalysts. Chem Eng. 2022, 6, 26. [Google Scholar]
- Deng, C.Q.; Liu, J.L.; Luo, J.H.; Cau, L.J.; Deng, J.; Fu, Y. Proton-promoted nickel-catalyzed asymmetric hydrogenation of aliphatic ketoacids. Angew. Chem. Int. Ed. 2022, 61, e202115983. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhao, H.C.; Fang, J.; Li, J.F.; Gao, W.; Ma, K.X.; Liu, C.A.; Yang, H.R.Y.; Ren, X.U.; Dong, Z.P. Ru nanoparticles anchored on porous N-doped carbon nanospheres for efficient catalytic hydrogenation of levulinic acid to γ-valerolactone under solvent-free conditions. J. Colloid Interface Sci. 2022, 623, 905–914. [Google Scholar] [CrossRef]
- Dolui, P.; Tiwari, V.; Saini, P.; Karmaker, T.; Makhal, K.; Goel, H.; Elias, A.J. A catalyst and solvent free route for the synthesis of N-substituted pyrrolidones from levulinic acid. Chem. Eur. J. 2022, 28, e202200829. [Google Scholar] [CrossRef]
- Moreira, A.F.S.; Souza, C.B.; Pinheiro, W.; de Freitus, F.A.; Lachter, E.R. Catalytic conversion of levulinic acid in higher added value products: Sustainable routes for esters production. Rev. Virtual Cuimica 2022, 14, 380–392. [Google Scholar] [CrossRef]
- Bucchianico, D.D.M.D.; Wang, Y.J.; Buvat, J.C.; Pan, Y.; Moreno, V.C.; Leveneur, S. Production of levulinic acid and alkyl levulinates: A process insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Wu, P.D.; Li, H.; Fang, I. Synergistic catalysis of Co-Zr/CNx bimetallic nanoparticles enables reductive amination of biobased levulinic acid. Adv. Sustain. Syst. 2022, 6, 2100321. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, J.G.; Cui, H.Y.; Li, Z.H.; Wang, M.; Yi, W.M. Spontaneous biphasic system with lithium chloride hydrate for efficient esterification of levulinic acid. ChemistrySelect 2022, 7, e202200347. [Google Scholar] [CrossRef]
- Lopez-Aguado, C.; del Monte, D.M.; Paniagna, M.; Morales, G.; Melero, J.A. Techno-economic assessment of conceptual design for gamma-valerolactone production over a bifunctional Zr-Al-beta catalyst. Ind. Eng. Chem. Res. 2022, 61, 5547–5556. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, Y.; Mu, X.Q.; Nie, Y. A sustainable approach for synthesizing (R)-4-aminopentanoic acid from levulinic acid catalysed by structure-guided tailored glutamate dehydrogenase. Front. Bioeng. Biotechnol. 2022, 9, 770302. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, E.; Lilas, P.; Diamantopoulou, P.; Fakas, C.; Kritinakis, I.; Patatsi, E.; Gabrielaton, E.; van Muyden, A.P.; Dyson, P.J.; Papadogianakis, G. Hydrognation of the pivotal biorefinery platform molecule levulinic acid into renewable fuel γ-valerolactone catalysed by unprecenented highly active and stable ruthenium nanoparticles in aqueous medium. Renew. Energy 2022, 192, 35–45. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.Y.; Zhang, J.H.; He, L.; Peng, L.C. Organic acid-assisted design of zirconium-lignocellulose hybrid for highly efficient upgrading of levulinic acid to γ-valerolactone. Fuel 2022, 315, 123150. [Google Scholar] [CrossRef]
- Raguindin, R.Q.; Desalegn, B.Z.; Vishwanath, H.; Gebresillase, M.N.; Seo, J.G. Enhanced hydrogenation of levulinic acid over ordered mesoporous alumina-supported catalyst: Elucidating the effect of fabrication strategy. ChemSusChem 2022, 15, e202102662. [Google Scholar] [CrossRef]
- Raguindin, R.Q.; Desalegn, B.Z.; Gebresillase, M.N.; Seo, J.G. York-shell nickel-cobal phosphides as bifunctional catalysts in the solvent-free hydrogenation of levulinic acid to gamma-valerolactone. Renew. Energy 2022, 191, 763–774. [Google Scholar] [CrossRef]
- Seenivasan, K.; Tran, T.P.; Mohan, P.; Ton, N.N.T.; Thakur, A.; Chammingkwan, P.; Rawat, D.S.; Tanike, T. Graphene oxide frame work-confined Ru (Ru@GOF) as recyclable catalyst for hydrogenation of levulinic acid into γ-valerolactone with formic acid. J. Mater. Sci. 2022, 57, 11714–11724. [Google Scholar] [CrossRef]
- Kumarvel, S.; Thiripuranthagan, S.; Eruappan, E.; Durai, M. Mesoporous Ru/Sn-SBA-15 catalyst: Synthesis, characterization and catalytic activity towards hydrogenation of levulinic acid. J. Porous Mater. 2022, 29, 1083–1095. [Google Scholar] [CrossRef]
- de Abreu, C.R.A.; Simon, P.; Wojcieszak, R.; de Souza, P.M.; Toniolo, F.S. Effect of Rhenium on the catalytic activity of activated carbon-supported nickel applied in the hydrogenation of furfural and levulinic acid. Top. Catal. 2022, 65, 902–914. [Google Scholar] [CrossRef]
- Chhabra, T.; Rohilla, J.; Krishnan, V. Nanoarchitectonics of phosphomolybdic acid supported on activated charcoal for selective conversion of furfuryl alcohol and levulinic acid to alkyl levulinates. Mol. Catal. 2022, 519, 112135. [Google Scholar] [CrossRef]
- Wu, J.C.; Zhu, Y.T.; Liao, P.Z.; Xu, T.Y.; Lu, L.Y.; Zhang, X.H.; Liu, Q.Y.; Ma, L.L.; Wang, C.U. Sustainable metal-lignosulfonate catalyst for efficient catalytic transfer hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. A Gen. 2022, 635, 118556. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Wang, B.W.; Yan, F.Y.; Xu, W.S.; Bai, G.Y.; Li, Y.; Yan, X.L.; Chen, L.G. Boron modified Cu/Al2O3 catalysts for the selective reductive amination of levulinic acid to N-substituted pyrrolidinones. ChemCatChem 2022, 14, e202200311. [Google Scholar] [CrossRef]
- Rapeyko, A.; Rodenas, M.; Xamena, F.X.L. Zr-containing UiO-66 metal-organic frame works as highly selective heterogeneous acid catalysts for the direct ketalization of levulinic acid. Adv. Sustain. Syst. 2022, 6, 2100451. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, J.G.; Cui, H.Y.; Li, Z.H.; Wang, M.; Yi, W.M. Promotion effect of molten salt hydrate on co-esterification of biomass-derived levulinic and formic acids. Fuel 2022, 321, 124077. [Google Scholar] [CrossRef]
- Yu, N.X.; Lu, H.F.; Yang, W.; Zheng, Y.X.; Hu, Q.; Liu, Y.Y.; Wu, K.J.; Liang, B. Transfer hydrogenation of levulinic acid to γ-valerolactone over acid site-modified CuNi alloy. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Adesina, A.; Lokhat, D. Process development for the production of ethylene and propylene from lignocellulosic biomass via the chemical route using an O-cresol based recovery strategy. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Liu, X.F.; Yu, D.Y.; Luo, H.Y.; Li, C. Catalytic upgrading of lignocellulosic biomass sugars toward biofuel 5-ethoxymethyl furfural. Front. Chem. 2022, 9, 831102. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.S.; Chen, L.G.; Liu, Y.; Zhang, X.G.; Liu, J.U.; Zhang, Q.; Wang, C.U.; Ma, L.L. One-pot conversion of biomass derived levulinic acid to furanic biofuel 2-methyl tetrahydrofuran over biometallic NiCo/γ-Al2O3 catalysts. Mol. Catal. 2022, 524, 112317. [Google Scholar] [CrossRef]
- Bunrit, A.; Butburee, T.; Liu, M.J.; Huang, Z.P.; Meeporn, K.; Phawa, C.; Zhang, J.; Kuboon, S.C.; Liu, H.F.; Faungnawaki, K.; et al. Photo-thermo dual catalysis of luvulinic acid and levulinate ester to γ-valerolactone. ACS Catal. 2022, 12, 1677–1685. [Google Scholar] [CrossRef]
- Hijazi, A.; Khalaf, N.; Kwapinski, W.; Leahy, J.J. Catalytic valorisation of biomass levulnic acid into gamma valerolactone using formic acid as a H2 donor: A critical review. RSC Adv. 2022, 12, 13673–13694. [Google Scholar] [CrossRef]
- Juarez, P.; Lopez-Aguado, C.; Paniagua, M.; Melero, J.A.; Mariscal, R.; Morales, G. Self-condensation of levulinic acid into bio-jet fuel precursors over acid zeolites: Elucidating the role of nature, strength, and density of acid sites. Appl. Catal. A Gen. 2022, 631, 118480. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pendem, C.; Goyal, R.; Khatun, R.; Khan, T.S.; Samanta, C.; Chiang, K.; Shah, K.; Haider, M.A.; Bal, R. Study of γ-valerolactone production from hydrogenation of levulinic acid over nanostructured Pt-hydrotalcite catalysts at low temperature. Fuel 2022, 323, 124272. [Google Scholar] [CrossRef]
- Kondawar, S.; Rode, C. Ionic liquids for the sustainable transformation of levulinic acid to γ-valerolactone (GVL). Curr. Opin. Green Sustain. Chem. 2022, 35, 100607. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Mikhailov, S.F.; Kuchkina, N.V.; Bykov, A.V.; Vasiliev, A.L.; Ezernitskaya, M.G.; Golovin, A.L.; Nikoshvili, L.Z.; Sulman, M.G.; Shifrina, Z.B. Ru@hyperbranched polymer for hydrogenation of levulinic acid to gamma-valerolactone: The role of the catalyst support. Int. J. Mol. Sci. 2022, 23, 799. [Google Scholar] [CrossRef]
- Meng, Y.; Jian, Y.M.; Chen, D.D.; Huang, J.S.; Zhang, H.; Li, H. Reductive upgrading of biomass-based levulinic acid to γ-valerolactone over Ru-based single atom catalysts. Front. Chem. 2022, 10, 895198. [Google Scholar] [CrossRef]
- Pothu, R.; Gundeboyina, R.; Boddula, R.; Perugopu, V.; Ma, J. Recent advances in biomass-derived platform chemicals to valeric acid synthesis. New J. Chem. 2022, 46, 5907–5921. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.K. Metabolic engineering of Escherichia Coli for production of polyhydroxyalkanoates with hydroxyvaleric acid derived from levulinic acid. J. Microbiol. Biotechnol. 2022, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
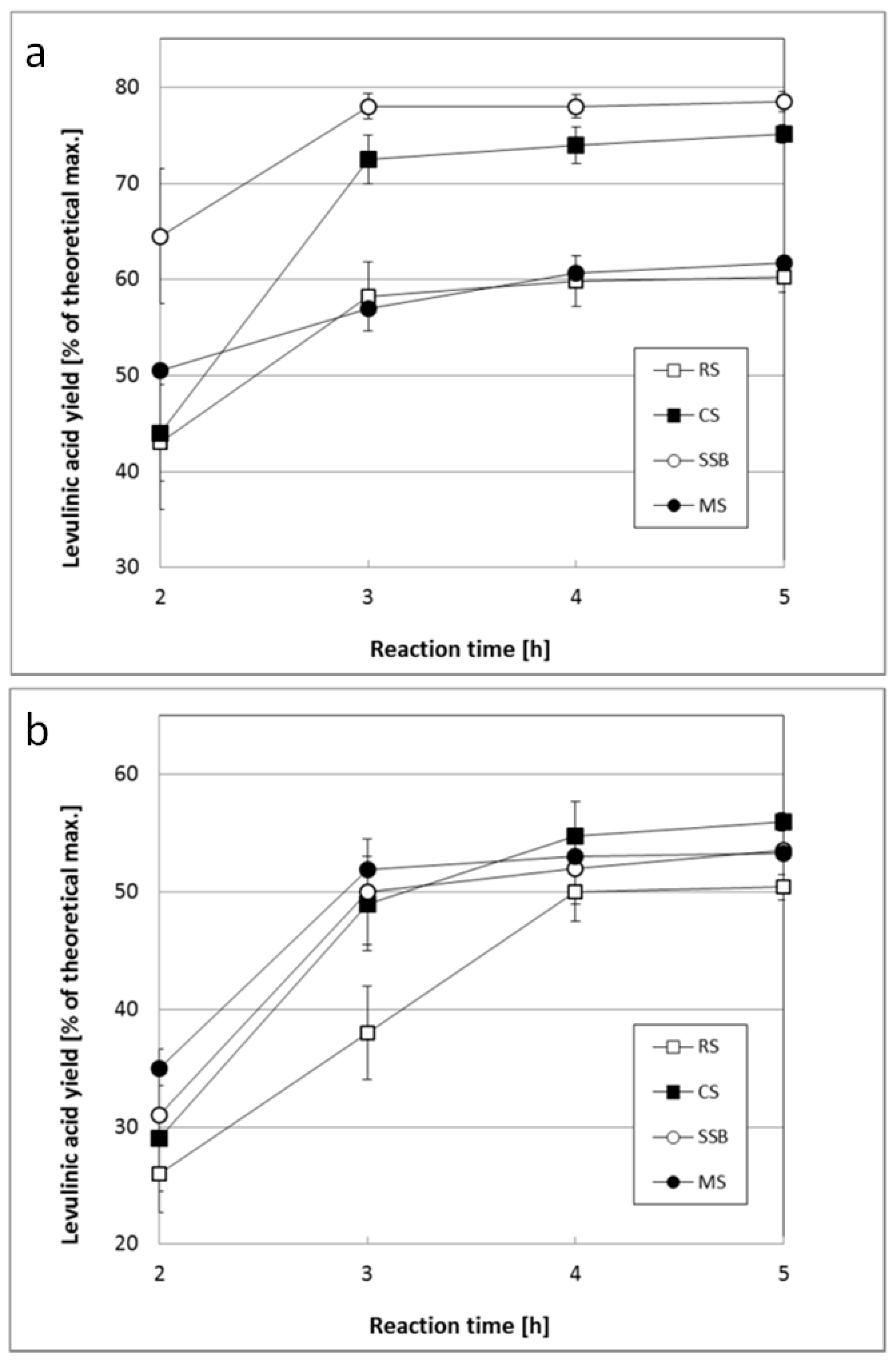
- Pulidindi, I.N.; Kim, T.H. Conversion of levulinic Acid from various herbaceous biomass species using hydrochloric acid and effects of particle size and delignification. Energies 2018, 11, 621. [Google Scholar] [CrossRef]
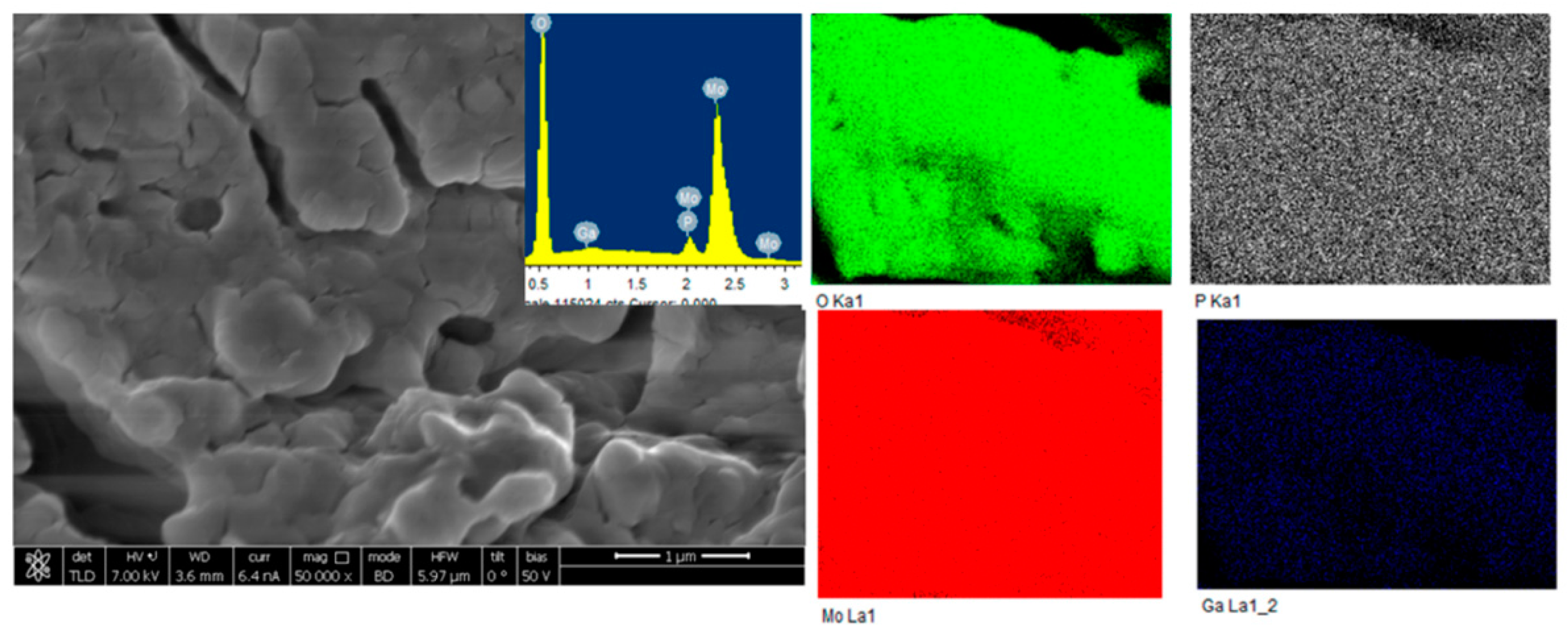
- Kumar, V.B.; Pulidindi, I.N.; Gedanken, A. Development of Ga salt of molybdophosphoric acid for biomass conversion to levulinic Acid. Energy Fuels 2016, 30, 10583–10591. [Google Scholar] [CrossRef]
- Kumar, V.B.; Pulidindi, I.N.; Gedanken, A. Ga modified zeolite based solid acid catalyst for levulinic acid production. ChemistrySelect 2016, 1, 5952–5960. [Google Scholar] [CrossRef]
- Tabah, B.; Pulidindi, I.N.; Gedanken, A. A study on fermentation kinetics for accelerated production of bioethanol from glucose, sucrose, and molasses. J. Bioprocess. Biotech. 2015, 5, 232. [Google Scholar] [CrossRef]
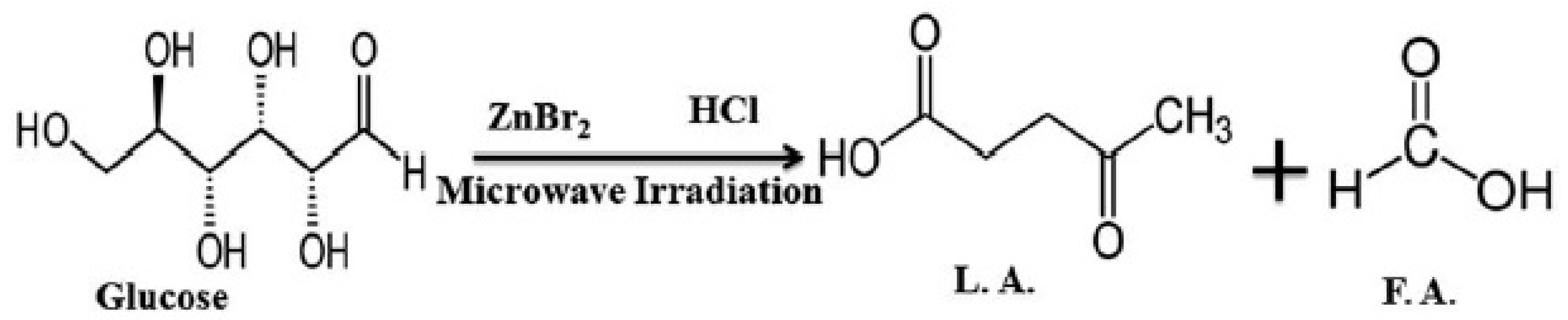
- Kumar, V.B.; Pulidindi, I.N.; Gedanken, A. Synergistic catalytic effect of ZnBr2-HCl system for levulinic acid production. RSC Adv. 2015, 5, 11043–11048. [Google Scholar] [CrossRef]
- Kumar, V.B.; Pulidindi, I.N.; Gedanken, A. Selective conversion of starch to glucose using carbon based solid acid catalyst. Renew. Energy 2015, 78, 141–145. [Google Scholar] [CrossRef]
- Klein, M.; Varvak, A.; Segal, E.; Markovsky, B.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Sonochemical synthesis of HSiW/graphene catalysts for enhanced biomass hydrolysis. Green Chem. 2015, 17, 2418–2425. [Google Scholar] [CrossRef]
- Victor, A.; Pulidindi, I.N.; Gedanken, A. Levulinic acid production from Cicer arietinum, Cotton, Pinus radiata and Sugar cane bagasse. RSC Adv. 2014, 4, 44706–44711. [Google Scholar] [CrossRef]
- Pulidindi, I.N.; Gedanken, A. Biofuels and biochemicals from biomass. Open J. Chem. 2021, 7, 22–24. [Google Scholar]
- Pulidindi, I.N. Development and Exploitation of Carbon Materials from Plant Sources. Ph.D. Thesis, IIT Madras, Chennai, India, 2009. [Google Scholar]
- Misono, M. Characterization of acidic properties of heteropoly compounds in relation to heterogeneous catalysis. In Catalysis by Acids and Bases; Imelic, B., Naccache, C., Coudurier, G., Taarit, Y.B., Vedrine, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 147–156. [Google Scholar]
- Misono, M. Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten. Catal. Rev. Sci. Eng. 1987, 29, 269–321. [Google Scholar] [CrossRef]
- Misono, M. Heterogeneous catalysis by heteropoly compounds. An attempt of molecular design of practical solid acid catalysts. Catal. Lett. 1992, 12, 63–72. [Google Scholar] [CrossRef]
- Misono, M.; Okuhara, T. ; Okuhara, T. Solid superacid catalysts. Chem. Tech. 1993, 23, 23–29. [Google Scholar]
- Misono, M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 13, 1141–1152. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Neel, P.I.; Varadarajan, T.K. Preparation of Activated Carbon from Botanical Source. Indian Patent No. IN200700376-I4, 23 February 2007. Patent Assignee: Indian Institutes Technology. [Google Scholar]
- Viswanathan, B.; Neel, P.I.; Varadarajan, T.K. Development of carbon materials for energy and environmental applications. Catal. Surv. Asia 2009, 13, 164–183. [Google Scholar] [CrossRef]
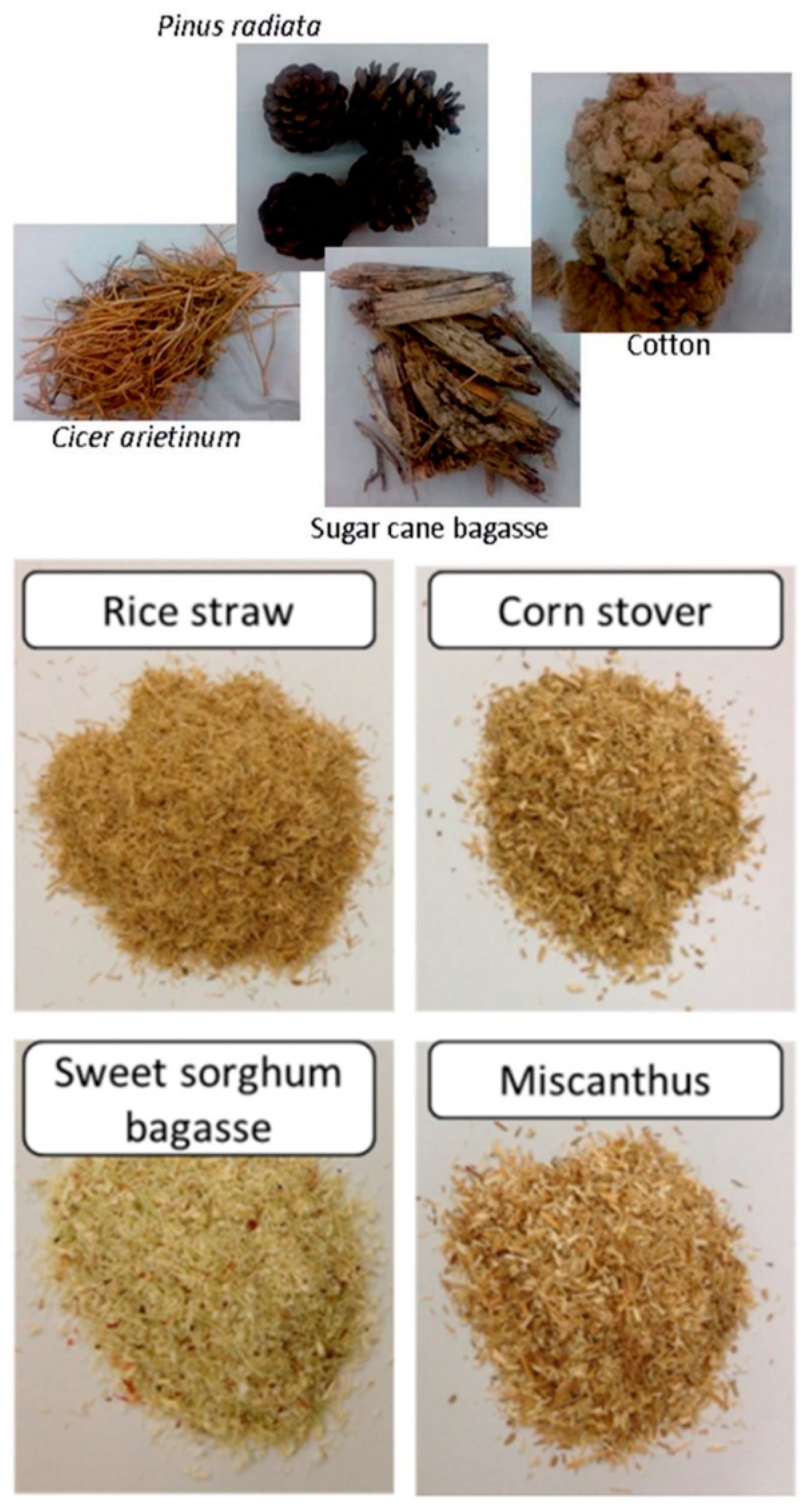




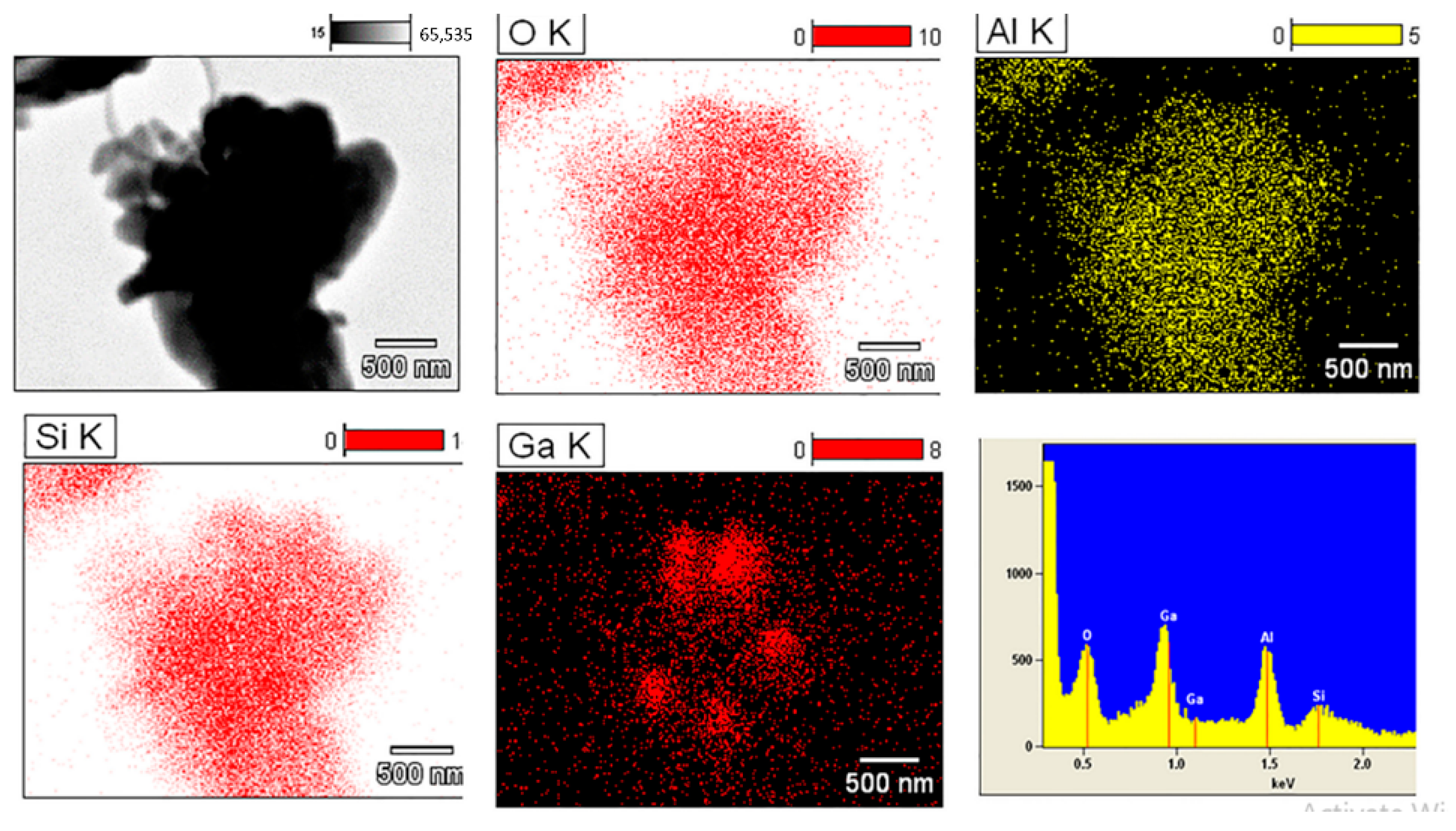

| Feedstock for LA Production | Catalyst | Yield of LA | Reference |
|---|---|---|---|
| Fructose | Aquivion®P98 PFSA, a commercial perfluorosulfonic acid resin in a pellet form | 100 mol% at 120 °C for 30 h; | [5] |
| Glucose | LaMnO3 | 69.5 mol%; | [6] |
| Glucose | MOF (UIO-66-NH-R-SO3H) | 71.6 mol% at 170 °C; | [7] |
| Corn cob | Mo doped carbon microspheres; presence of Lewis acid sites, namely, Mo2C and Mo6+ promoted the isomerization of glucose to fructose | 33.02 (% of theoretical maximum) yield of LA at 195 °C in 90 min; | [8] |
| Glucose | Para-toluene sulfonic acid (PTSA) functionalized activated carbon with CaCl2 | 61 mol% at 175 °C in 120 min with MIBK-H2O solvent system; | [9] |
| Fructose | ZrO2-SiO2-SnO2 Solid super acid catalyst with H0 = −14.52 | 80 mol% at 180 °C in 3.5 h; | [14] |
| Laminaria digitata (wild brown seaweed) | 4 wt.% H2SO4 | 12.5 wt.% at 200 °C in 30 min; | [15] |
| Spirulina platensis residue | 1 M H2SO4 | 16.36 wt.% at 180 °C for 30 min; | [16] |
| Glucose | Cr-MCM-22 | 60 wt.% at 200 °C in 1 h; | [17] |
| Glucose and cellulose | Cu HZSM-5-HMS (hexagonal mesoporous silica) hybrid catalyst | 45 and 30 mol% from glucose and cellulose, respectively. Ideal conditions for glucose conversion are: 200 °C; 10 bar N2; 5 h; Ideal conditions for cellulose conversion are: 230 °C; 5 h; | [18] |
| Newspaper wastes with crystalline cellulose | 0.5 M H2SO4 | 23–27 wt.% obtained from sanitary papers, tracing/parchment papers and paper food box; 180 °C; 5 min, 1:1 GVL/H2O solvent; | [19] |
| Rice straw | 1.5 wt.% HCl | 52 mol% obtained from rice straw pretreated with choline chloride—oxalic acid deep eutectic solvent; Pretreatment: 100 °C for 2 h; Acid hydrolysis: 120 °C for 2 h; | [20] |
| Rice straw | Polyehtyleneimine functionalized acidic ionic liquid (PolyE-IL) with HSO4− counter cation | 65.5 (% of theoretical maximum) from pretreated rice straw; Hydrolysis conditions: 210 °C for 120 min; | [21] |
| Safflower stalk with 45.2 wt.% cellulose content | 0.3 M PTSA | 30 (% of theoretical maximum) at 200 °C for 120 min; | [22] |
| Cellulosic residue from corn stover | Phosphoric acid activated lignin based activated carbon | 67.9 mol% at 190 °C in 150 min in MIBK/H2O-NaCl medium; | [23] |
| Biochemical from LA | Catalyst | Product Yield | Reference |
|---|---|---|---|
| GVL | Hf-FDCA (catalyst prepared from the hybrid of a metal precursor and 2,5 furan dicarboxylic acid | 88% yield of GVL at 180 °C in 24 h; | [24] |
| Hexyl levulinate | Sulfated silica prepared by the sulfonation of amorphous bamboo leaf ash | 95.2% conversion of LA at 90 °C in 7 min with 98.0% selectivity to hexyl levulinate; catalyst reused six times; | [25] |
| GVL | 2 wt.% Pt on ZrO2 | 97% GVL yield; formic acid used as hydrogen source; T = 140 °C; t = 180 min; triethyl amine used to facilitate formic acid decomposition; catalyst reused for four consecutive cycles; | [27] |
| Methyl levulinate | MOF containing Zr metal cluster with amino terephthalic acid (UiO-66-NH2) ligand | 85.89% yield of methyl levulinate in an autoclave in 1 h; T = 130 °C; P = 30 bar N2 pressure; k = 3.57 × 10−3 min−1; activation energy = 48.99 kJ/mol; | [28] |
| Chiral GVL | Nickel phosphine complex; Ni(OTf)2; (S, S) Ph-BPE/(R, R) Ph-BPE; TFE; 50 °C; 12 h; in a gram scale preparation | 78.6% yield of (R)-GVL with 96% ee; 79.05% yield of (S)-GVL with 96% ee; | [29] |
| GVL | Ru nanoparticles anchored on hierarchical porous N-doped carbon nanospheres (3 wt.% Ru/HPNC) | GVL yield > 99% at 100 °C in 2 h under solvent-free conditions; 2.5 MPa H2; catalyst reused for six reaction cycles; | [30] |
| n-butyl levulinate (BL) | LiCl·3H2O + AlCl3 (molten salt hydrates) with microwave irradiation | 95.5% yield of n-butyl levulinate; T = 100 °C; t = 2.5 h; activation energy of n-butyl levulinate formation with LiCl 3H2O and LiCl. 3H2O + AlCl3 were 70.9 kJ/mol and 31.8 kJ/mol respectively; | [35] |
| GVL | Zr-Al beta | 85.5% yield of GVL; isopropyl alcohol used as hydrogen donor; | [36] |
| (R)-4-amino pentanoic acid | Glutamate dehydrogenase coupled with formate dehydrogenase | 97% conversion of LA with a (R)-4-amino pentanoic acid stereoselectivity of >99% in 11 h; | [37] |
| GVL | Ru/PEG (ruthenium nanoparticles stabilized by water soluble polymer PED) | 82 mol% conversion of LA with 99.2 mol% selectivity for GVL; T = 140 °C; t = 15 min; | [38] |
| GVL | Zr@PS-FA (successful coordination observed in the catalyst between Zr4+ and OH and COOH groups of the partially hydrolysed Pinnistum sinese in formic acid) | 95.6 mol% yield of GVL; T = 180 °C; t = 1.5 h; TOF = 9.76 h−1 | [39] |
| 2-methyl tetrahydrofuran | Ni-Cu-OMA (ordered mesoporous alumina) | 73.0% selectivity towards 2-MTHF; two steps are involved in the production of 2-MTHF; Step 1: 190 °C; 30 bar H2; 4 h Step 2: 230 °C; 50 bar H2; 12 h | [41] |
| GVL | Ru@GOF (Ru nanoparticles confined in the gallery space of graphene oxide frameworks pillared with organic linkers) | 93 mol% yield of GVL; T = 90 °C; t = 8 h; TOF = 7240 h−1; catalyst reusable for at least five reaction runs | [42] |
| GVL | 5% Ru/Sn-SBA-15 | LV conversion—99%; GVL selectivity—98%; T = 250 °C; t = 3 h; H2 flow = 25 mL/min; | [43] |
| GVL | 10 wt.% Re supported on activated carbon | LA conversion—100% GVL selectivity—99% T = 120 °C; t = 4 h; | [44] |
| Ethyl levulinate | H3PMo12O40/Activated carbon | Ethyl levulinate yield = 80%; T = 80 °C; t = 15 h; | [45] |
| Nitrogenous Chemical | Catalyst | Product Yield | Reference |
|---|---|---|---|
| N-substituted pyrrolidones | No catalyst; HBpin used as reducing agent | 28–94% yield of N-substituted pyrrolidones; | [31] |
| 5-methyl-2-pyrrolidone | 20 molar percent of Zr in the Co, Zr bimetallic carbon-nitrogen doped catalyst (Co-Zr@chitosan-20) | 92.5% yield of 5-methyl-2-pyrrolidone; ammonia as N source; T = 130 °C; P = 30 bar H2; 24 h; | [34] |
| N-butyl-5-methyl-2-pyrrolidinone | Cu10/AlB3O | N-butyl-5-methyl-2-pyrrolidinone yield = 94%; LA conversion = 99%; stable catalytic performance for 200 h; T = 200 °C; LHSV = 0.3 h−1; 1,4-dioxane solvent; LA:n-butyl amine mole ratio = 1:1; H2 pressure, 3 MPa; | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Victor, A.; Sharma, P.; Pulidindi, I.N.; Gedanken, A. Levulinic Acid Is a Key Strategic Chemical from Biomass. Catalysts 2022, 12, 909. https://doi.org/10.3390/catal12080909
Victor A, Sharma P, Pulidindi IN, Gedanken A. Levulinic Acid Is a Key Strategic Chemical from Biomass. Catalysts. 2022; 12(8):909. https://doi.org/10.3390/catal12080909
Chicago/Turabian StyleVictor, Amudhavalli, Pankaj Sharma, Indra Neel Pulidindi, and Aharon Gedanken. 2022. "Levulinic Acid Is a Key Strategic Chemical from Biomass" Catalysts 12, no. 8: 909. https://doi.org/10.3390/catal12080909
APA StyleVictor, A., Sharma, P., Pulidindi, I. N., & Gedanken, A. (2022). Levulinic Acid Is a Key Strategic Chemical from Biomass. Catalysts, 12(8), 909. https://doi.org/10.3390/catal12080909









