Synthesis of Self-Supported Cu/Cu3P Nanoarrays as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction
Abstract
:1. Introduction
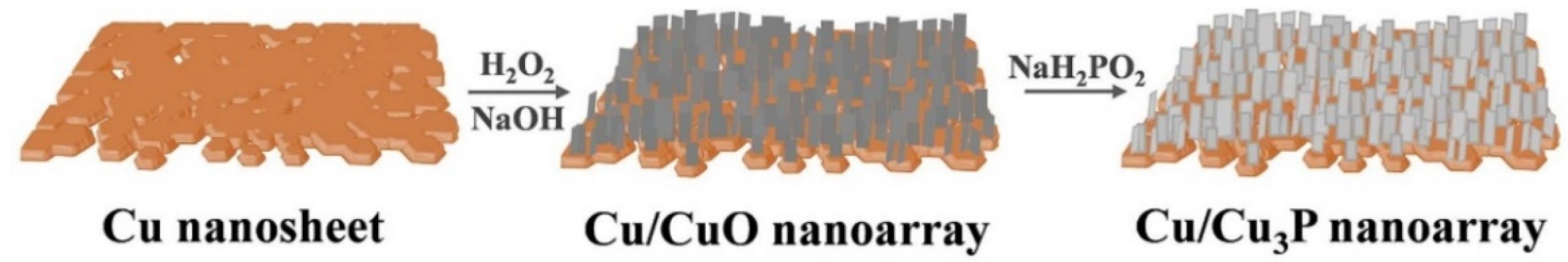
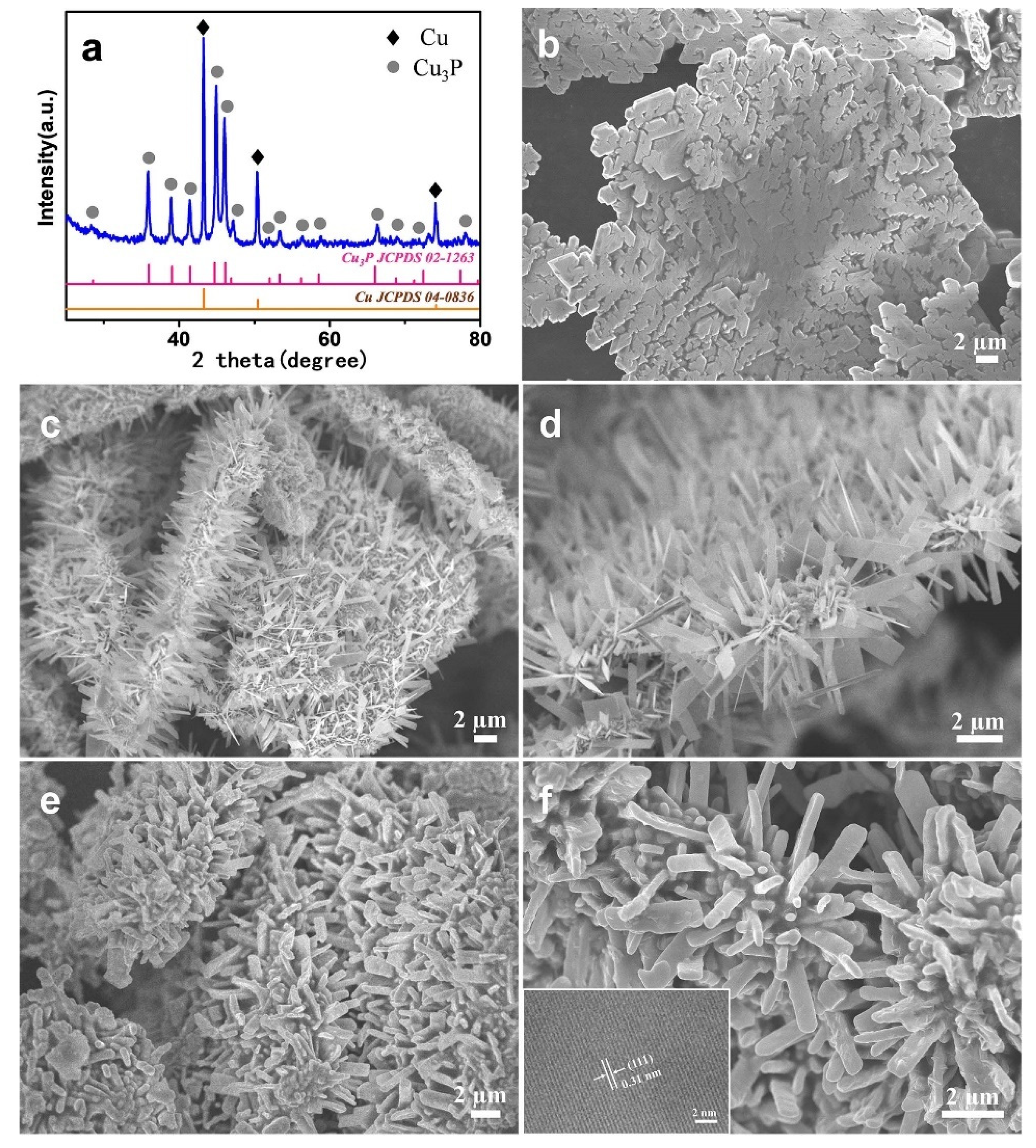
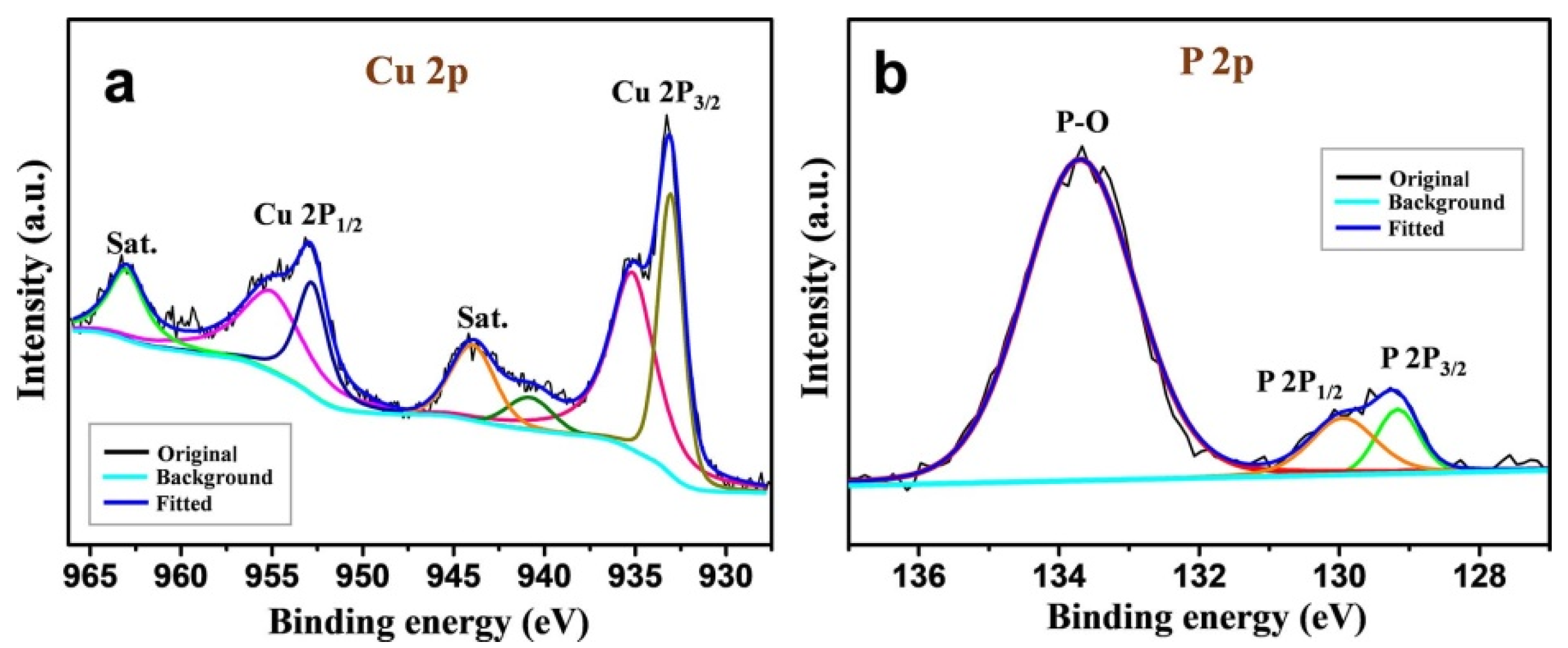
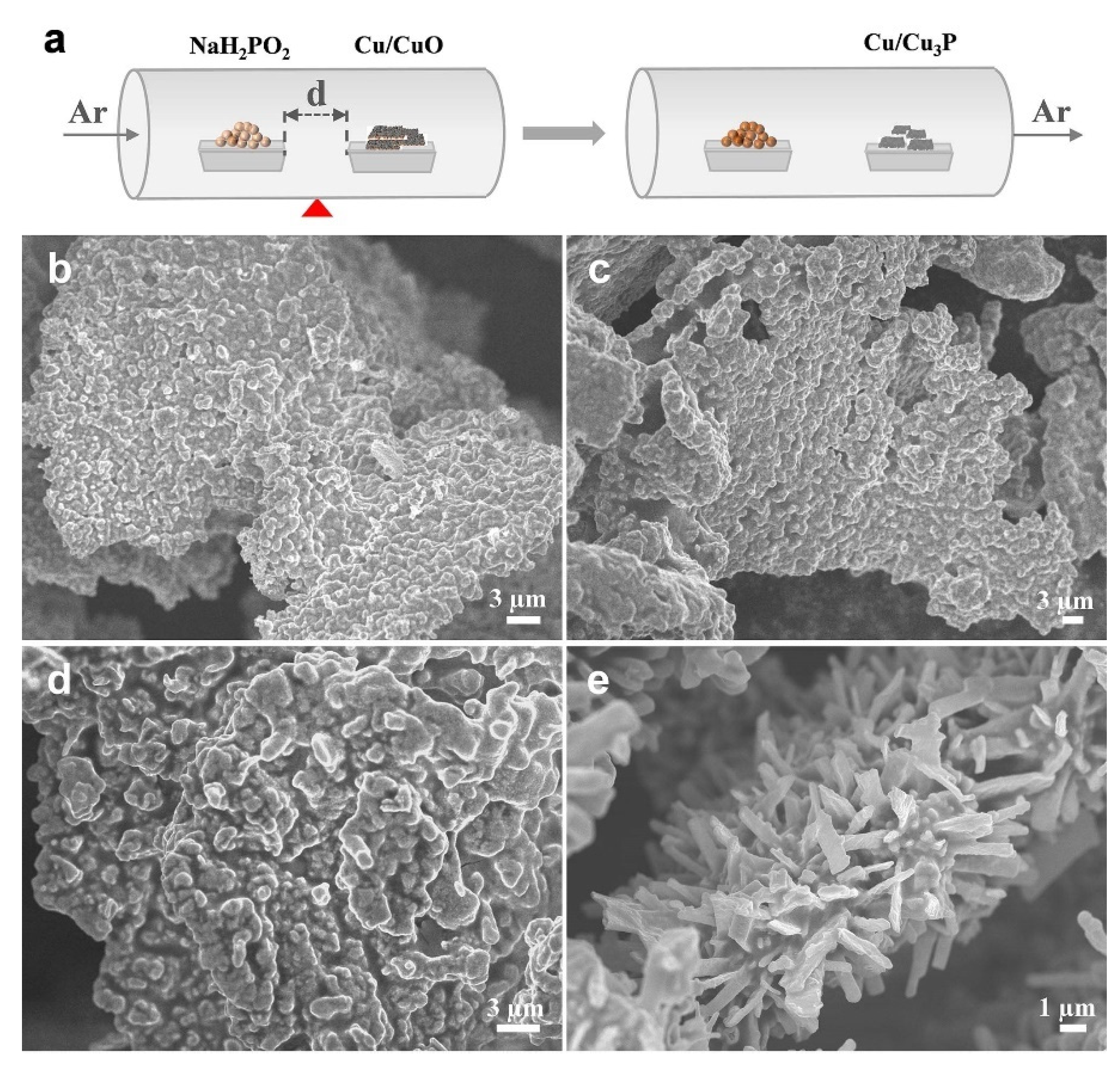
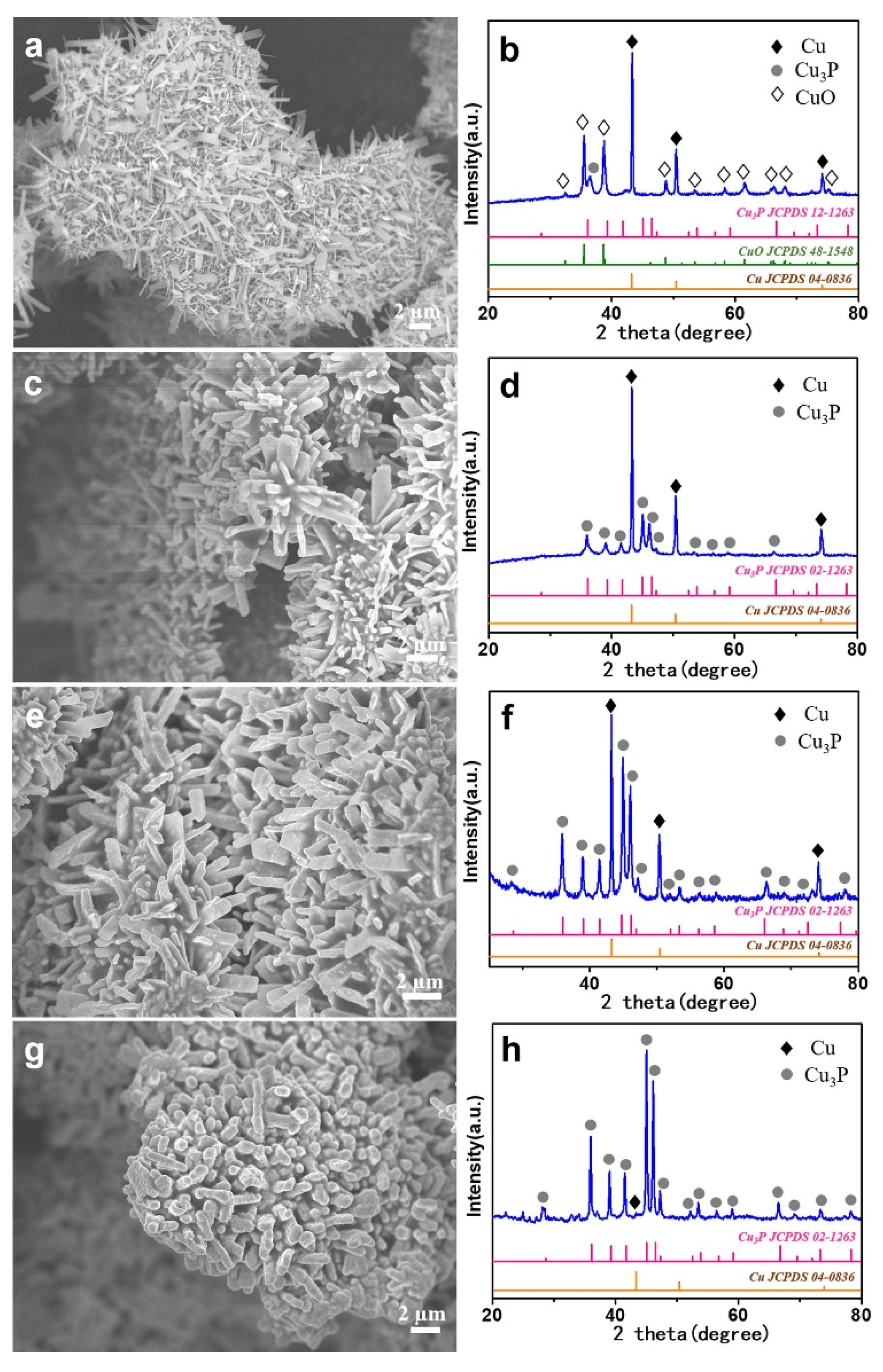
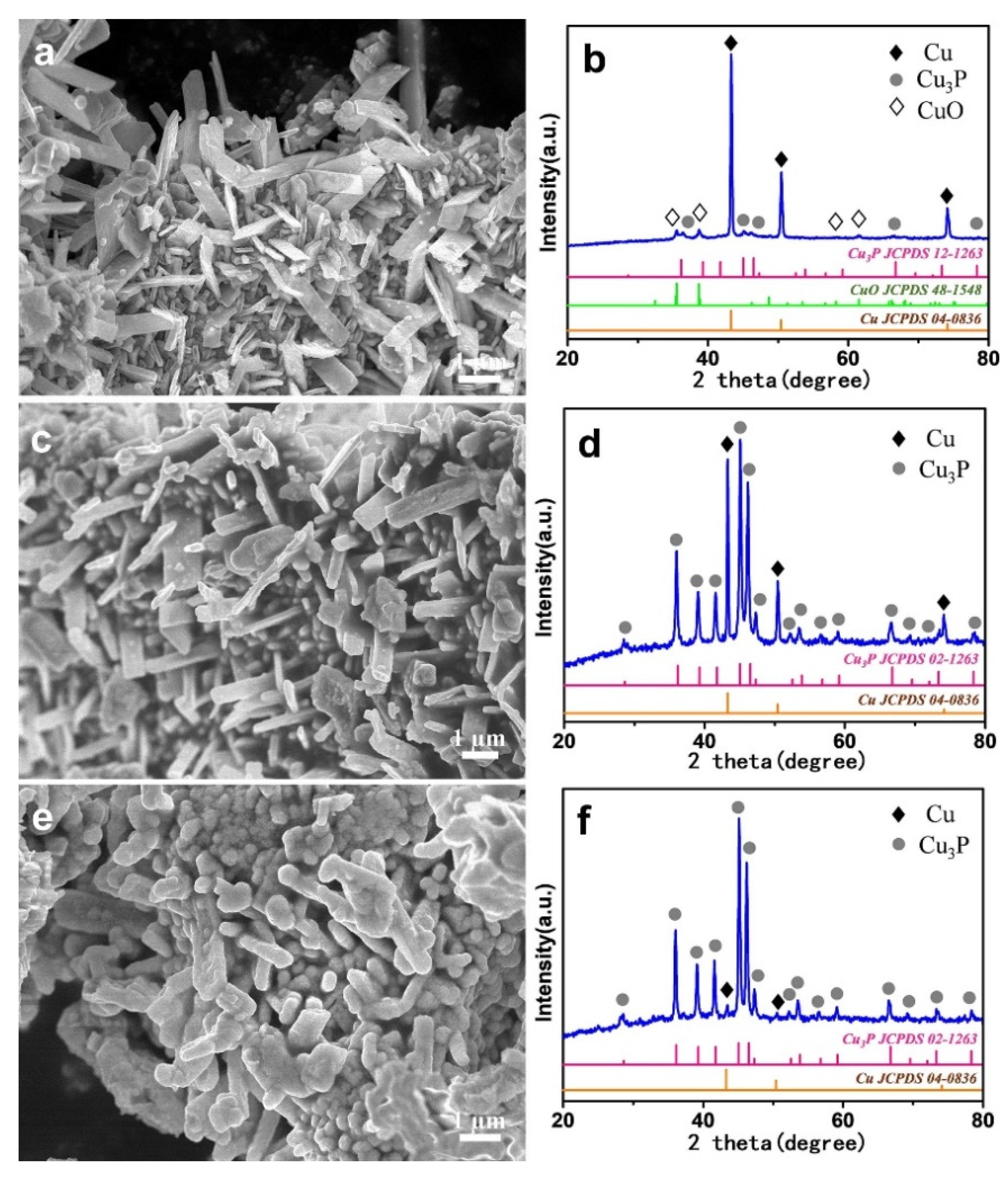
2. Results
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Cu Nanosheets
3.3. Synthesis of Cu/CuO Nanoarrays
3.4. Synthesis of Cu/Cu3P Nanoarrays
3.5. Material Characterization
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Li, W.; Lu, L.; Song, W. Tuning active sites on cobalt/nitrogen doped graphene for electrocatalytic hydrogen and oxygen evolution. Electrochim. Acta 2018, 265, 497–506. [Google Scholar] [CrossRef]
- Jebaslinhepzybai, B.T.; Prabu, N.; Sasidharan, M. Facile galvanic replacement method for porous nanoparticles as an efficient HER electrocatalyst. Int. J. Hydrog. Energy 2020, 45, 11127–11137. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, C.; Zhang, X.; Chen, J. Preparation of NiCoP Hollow Quasi-Polyhedra and Their Electrocatalytic Properties for Hydrogen Evolution in Alkaline Solution. ACS Appl. Mater. Interfaces 2017, 9, 5982–5991. [Google Scholar] [CrossRef]
- Martini, B.K.; Maia, G. Using a combination of Co, Mo, and Pt oxides along with graphene nanoribbon and MoSe2 as efficient catalysts for OER and HER. Electrochim. Acta 2021, 391, 138907. [Google Scholar] [CrossRef]
- Yu, J.; Dai, Y.; He, Q.; Cheng, C.; Ni, M. Robust non-Pt noble metal-based nanomaterials for electrocatalytic hydrogen generation. Appl. Phys. Rev. 2020, 7, 041304. [Google Scholar] [CrossRef]
- El-Hallag, I.; Elsharkawy, S.; Hammad, S. The effect of electrodeposition potential on catalytic properties of Ni nanoparticles for hydrogen evolution reaction (HER) in alkaline media. J. Appl. Electrochem. 2022, 52, 907–918. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [Green Version]
- Popczun, E.J.; Mckone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, H.; Zhang, Q.; Zhang, Z.; Liu, H. Correction to: Heterostructures of MXenes and CoNx-Graphene as highly active electrocatalysts for hydrogen evolution reaction in alkaline media. J. Appl. Electrochem. 2021, 51, 1109. [Google Scholar] [CrossRef]
- Pu, M.; Wang, D.; Zhang, Z.; Guo, Y.; Guo, W. Flexoelectricity enhanced water splitting and hydrogen evolution reaction on grain boundaries of monolayer transition metal dichalcogenides. Nano Res. 2022, 15, 978–984. [Google Scholar] [CrossRef]
- Pak, S.; Lim, J.; Hong, J.; Cha, S.N. Enhanced Hydrogen Evolution Reaction in Surface Functionalized MoS2 Monolayers. Catalysts 2021, 11, 70. [Google Scholar] [CrossRef]
- Yan, X.; Huang, S.; Yang, F.; Sun, S.; Li, Y. Enhanced Catalytic Hydrogen Evolution Reaction Performance of Highly Dispersed Ni2P Nanoparticles Supported by P-Doped Porous Carbon. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126308. [Google Scholar] [CrossRef]
- Kluge, R.M.; Haid, R.W.; Stephens, I.; Callevallejo, F.; Bandarenka, A.S. Monitoring the active sites for the hydrogen evolution reaction at model carbon surfaces. Phys. Chem. Chem. Phys. 2021, 23, 10051–10058. [Google Scholar] [CrossRef]
- Kita, H.; Shen, Y.; Gao, Y. Mass transfer effect in hydrogen evolution reaction on Pt single-crystal electrodes in acid solution. J. Electroanal. Chem. 1992, 334, 351–357. [Google Scholar] [CrossRef]
- Yang, B.; Xu, J.; Duan, B.; Wang, J.; Liu, B. Amorphous phosphatized ruthenium-iron bimetallic nanoclusters with Pt-like activity for hydrogen evolution reaction. Appl. Catal. B Environ. 2021, 283, 119583. [Google Scholar] [CrossRef]
- Lai, Y.; Zhang, Z.; Tan, Y.; Yu, L.; Wu, W.; Wang, Z.; Jiang, T.; Gao, S.; Cheng, N. Electronic modulation of Pt nanoclusters through tuning the interface of Pt-SnO2 clusters for enhanced hydrogen evolution catalysis. Chem. Eng. J. 2022, 435, 135102. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Chen, Y.; Wang, H.; Wang, Y. Improving alkaline hydrogen evolution reaction kinetics on molybdenum carbide: Introducing Ru dopant. J. Catal. 2020, 392, 313–321. [Google Scholar] [CrossRef]
- Cai, J.; Liao, X.; Li, P.; Wang, Q.; Huang, H.; Lyu, Z.; Lin, J.; Xie, S. Penta-Twinned Rh@Pt Core-Shell nanobranches with engineered shell thickness for reversible and active hydrogen redox electrocatalysis. Chem. Eng. J. 2022, 429, 132414. [Google Scholar] [CrossRef]
- Yang, Q.; Li, G.; Manna, K.; Fan, F.; Sun, Y. Topological Engineering of Pt-Group-Metal-Based Chiral Crystals toward High-Efficiency Hydrogen Evolution Catalysts. Adv. Mater. 2020, 32, 1908518. [Google Scholar] [CrossRef]
- Jy, A.; Dan, W.B.; Zhe, Z.B.; Wy, A.; Hs, A.; Yq, A.; Sw, B.; Yukc, D.; Yz, C. Facile synthesis of Nafion-supported Pt nanoparticles with ultra-low loading as a high-performance electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2020, 566, 505–512. [Google Scholar]
- Tiwari, J.N.; Dang, N.K.; Sultan, S.; Thangavel, P.; Jeong, H.Y.; Kim, K.S. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nat. Sustain. 2020, 3, 556–563. [Google Scholar] [CrossRef]
- Bae, S.Y.; Mahmood, J.; Jeon, I.Y.; Baek, J.B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020, 5, 43–56. [Google Scholar] [CrossRef]
- Bonde, J.; Moses, P.G.; Jaramillo, T.F.; Nørskov, J.K.; Chorkendorff, I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 2008, 140, 219–231. [Google Scholar] [CrossRef]
- Reeve, R.W.; Christensen, P.A.; Dickinson, A.J.; Hamnett, A.; Scott, K. Methanol-tolerant oxygen reduction catalysts based on transition metal sulfides and their application to the study of methanol permeation. Electrochim. Acta 2000, 45, 4237–4250. [Google Scholar] [CrossRef]
- Ys, A.; Ch, B.; Js, A.; Yz, A.; Jn, C.; Yong, H.A. One-step construction of a transition-metal surface decorated with metal sulfide nanoparticles: A high-efficiency electrocatalyst for hydrogen generation. J. Colloid Interface Sci. 2020, 558, 1–8. [Google Scholar]
- Dolmatov, V.S.; Kuznetsov, S.A. Synthesis of Refractory Metal Carbides on Carbon Fibers in Molten Salts and Their Electrocatalytic Properties. J. Electrochem. Soc. 2021, 168, 122501. [Google Scholar] [CrossRef]
- Díaz-Coello, S.; Afonso, M.M.; Palenzuela, J.A.; Pastor, E.; García, G. Composite materials from transition metal carbides and ionic liquids as electrocatalyst for hydrogen evolution in alkaline media. J. Electroanal. Chem. 2021, 898, 115620. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Yang, Z.; Yang, P.; Ma, J. Environmental applications of two-dimensional transition metal carbides and nitrides for water purification: A review. Environ. Chem. Lett. 2022, 20, 633–660. [Google Scholar] [CrossRef]
- Yan-Juan, L.I.; Wang, M.; Liu, S.; Gao, J.X.; Yan, X. Preparation and properties of transition metal nitrides caged in N-doped hollow porous carbon sphere for oxygen reduction reaction. Trans. Nonferrous Met. Soc. China 2021, 31, 1427–1438. [Google Scholar]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326.1–1806326.18. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Wang, F.; Li, L.; Chen, H.; Wei, Z. Rational design of porous Ni-Co-Fe ternary metal phosphides nanobricks as bifunctional electrocatalysts for efficient overall water splitting. Appl. Catal. B Environ. 2022, 310, 121353. [Google Scholar] [CrossRef]
- Han, P.; Li, B.; Dang, Y.; Zhou, Y. A Novel Trimetal Phosphide with Amorphous Porous Structure for the Enhanced Electrocatalysis of Oxygen Evolution Reaction. J. Electrochem. Soc. 2021, 168, 116510. [Google Scholar]
- Wy, A.; Yg, A.; Zhi, C.A.; Ying, Z.A.; Zw, A.; Lei, W. Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides. Chin. J. Catal. 2021, 42, 1876–1902. [Google Scholar]
- Liu, L.; Li, N.; Han, J.; Yao, K.; Liang, H. Multicomponent transition metal phosphide for oxygen evolution. Int. J. Miner. Metall. Mater. 2022, 29, 503–512. [Google Scholar] [CrossRef]
- Jian, L.; Gao, X.; Liu, B.; Feng, Q.; Li, X.B.; Huang, M.Y.; Liu, Z.; Zhang, J.; Tung, C.H.; Wu, L.Z. Graphdiyne: A Metal-Free Material as Hole Transfer Layer to Fabricate Quantum Dot-Sensitized Photocathodes for Hydrogen Production. J. Am. Chem. Soc. 2016, 138, 3954. [Google Scholar]
- Cao, R.; Hu, F.; Zhang, T.; Shao, W.; Jian, X. Bottom-up fabrication of triazine-based frameworks as metal-free materials for supercapacitors and oxygen reduction reaction. RSC Adv. 2021, 11, 8384–8393. [Google Scholar] [CrossRef]
- Wei, S.; Qi, K.; Jin, Z.; Cao, J.; Zheng, W.; Chen, H.; Cui, X. One-Step Synthesis of a Self-Supported Copper Phosphide Nanobush for Overall Water Splitting. ACS Omega 2016, 1, 1367–1373. [Google Scholar] [CrossRef]
- Ping, L.; Rodriguez, J.A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P (001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar]
- Zhang, W.; Hong, J.; Zheng, J.; Huang, Z.; Zhou, J.; Xu, R. Nickel-thiolate complex catalyst assembled in one step in water for solar H2 production. J. Am. Chem. Soc. 2011, 133, 20680–20683. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Z.; Asiri, A.M.; Ping, J.; Sun, X. Cobalt phosphide nanoparticles film growth on carbon cloth: A high-performance cathode for electrochemical hydrogen evolution. Int. J. Hydrog. Energy 2014, 39, 16806–16811. [Google Scholar] [CrossRef]
- Cao, S.; Yong, C.; Wang, C.J.; He, P.; Fu, W.F. Highly efficient photocatalytic hydrogen evolution by nickel phosphide nanoparticles from aqueous solution. Chem. Commun. 2014, 50, 10427–10429. [Google Scholar] [CrossRef]
- Mcenaney, J.M.; Crompton, J.C.; Callejas, J.F.; Popczun, E.J.; Biacchi, A.J.; Lewis, N.S.; Schaak, R.E. Amorphous Molybdenum Phosphide Nanoparticles for Electrocatalytic Hydrogen Evolution. Chem. Mater. 2014, 26, 4826–4831. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, Y.; Yuan, M.; Zhe, L.; Wei, B. Direct growth of Ni–Fe phosphides nanohybrids on NiFe foam for highly efficient water oxidation. J. Alloys Compd. 2020, 847, 156363. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sheng, M.; Bhushan, M.W.; Sun, Y. Hierarchically Porous Urchin-Like Ni2P Superstructures Supported on Nickel Foam as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2016, 6, 714–721. [Google Scholar] [CrossRef]
- Gao, X.; Lu, K.; Chen, J.; Min, J.; Tan, M. NiCoP–CoP heterostructural nanowires grown on hierarchical Ni foam as a novel electrocatalyst for efficient hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 23205–23213. [Google Scholar] [CrossRef]
- Yang, Z.; Tuo, Y.; Lu, Q.; Chen, C.; Liu, M.; Liu, B.; Duan, X.; Zhou, Y.; Zhang, J. Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting. Green Energy Environ. 2020, 2, 236–245. [Google Scholar] [CrossRef]
- Hou, C.C.; Chen, Q.Q.; Wang, C.J.; Liang, F.; Lin, Z.; Fu, W.F.; Chen, Y. Self-Supported Cedarlike Semimetallic Cu3P Nanoarrays as a 3D High-Performance Janus Electrode for Both Oxygen and Hydrogen Evolution under Basic Conditions. ACS Appl. Mater. Interfaces 2016, 8, 23037–23048. [Google Scholar] [CrossRef]
- Shiri, P. An overview on the copper-promoted synthesis of five-membered heterocyclic systems. Appl. Organomet. Chem. 2020, 34, 5600–5622. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, X.; Gao, H.; Lu, G.; Dong, W.; Wang, G. Cu@Cu3P core-shell nanowires attached on Ni foam as high-performance electrocatalyst for hydrogen evolution reaction. Chemistry 2018, 25, 1083–1089. [Google Scholar] [PubMed]
- Fedorov, A.; Saraev, A.; Kremneva, A.; Selivanova, A.; Vorokhta, M.; Šmíd, B.; Bulavchenko, O.; Yakovlev, V.; Kaichev, V. Kinetic and mechanistic study of CO oxidation over nanocomposite CuFeAl oxide catalysts. ChemCatChem 2020, 19, 4911–4921. [Google Scholar] [CrossRef]
- Rong, J.; Xu, J.; Qiu, F.; Zhu, Y.; Fang, Y.; Xu, J.; Zhang, T. Sea Urchin-Like MOF-Derived Formation of Porous Cu3P@C as an Efficient and Stable Electrocatalyst for Oxygen Evolution and Hydrogen Evolution Reactions. Adv. Mater. Interfaces 2019, 6, 1900502. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Liu, L.; Chen, H.; Hu, X.; Qian, J.; Huang, D.; Zhang, B.; Tang, J. Self-supported Cu3P nanowire electrode as an efficient electrocatalyst for the oxygen evolution reaction. RSC Adv. 2021, 11, 34137–34143. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Wang, K.; Wumaer, M.; Guo, C.; Tian, B.; Zhang, L.; Akram, N.; Wang, J. Synthesis of hollow Cu@Cu3-xP core-shell nanostructure as dual-functional catalyst with copper vacancy for enhancing chemical reduction and photocatalytic performance. Appl. Surf. Sci. 2022, 589, 153031. [Google Scholar] [CrossRef]
- Ma, L.; Shen, X.; Zhou, H.; Zhu, J.; Xi, C.; Ji, Z.; Kong, L. Synthesis of Cu3P nanocubes and their excellent electrocatalytic efficiency for the hydrogen evolution reaction in acidic solution. RSC Adv. 2016, 6, 9672–9677. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. 2015, 126, 9731–9735. [Google Scholar] [CrossRef]
- Tian, T.; Ai, L.; Jiang, J. Metal–organic framework-derived nickel phosphides as efficient electrocatalysts toward sustainable hydrogen generation from water splitting. RSC Adv. 2015, 5, 10290–10295. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, R.; Zhang, J.; Shi, Y.; Zhang, B. Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 2013, 49, 6656–6658. [Google Scholar] [CrossRef]
- Dang, R.; Song, L.L.; Dong, W.J.; Li, C.R.; Zhang, X.B. Synthesis and self-assembly of large-area Cu nanosheets and their application as an aqueous conductive ink on flexible electronics. ACS Appl. Mater. Interfaces 2014, 6, 622–629. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, R.; Xu, X.; Xie, M.; Liu, J. Synthesis of Self-Supported Cu/Cu3P Nanoarrays as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Catalysts 2022, 12, 762. https://doi.org/10.3390/catal12070762
Dang R, Xu X, Xie M, Liu J. Synthesis of Self-Supported Cu/Cu3P Nanoarrays as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Catalysts. 2022; 12(7):762. https://doi.org/10.3390/catal12070762
Chicago/Turabian StyleDang, Rui, Xiufeng Xu, Mengmeng Xie, and Jian Liu. 2022. "Synthesis of Self-Supported Cu/Cu3P Nanoarrays as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction" Catalysts 12, no. 7: 762. https://doi.org/10.3390/catal12070762
APA StyleDang, R., Xu, X., Xie, M., & Liu, J. (2022). Synthesis of Self-Supported Cu/Cu3P Nanoarrays as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Catalysts, 12(7), 762. https://doi.org/10.3390/catal12070762





