Catalytic Oxidation of Toluene over Fe-Rich Palygorskite Supported Manganese Oxide: Characterization and Performance
Abstract
:1. Introduction
2. Results
2.1. Material Characterization
2.1.1. XRD and Raman Analysis
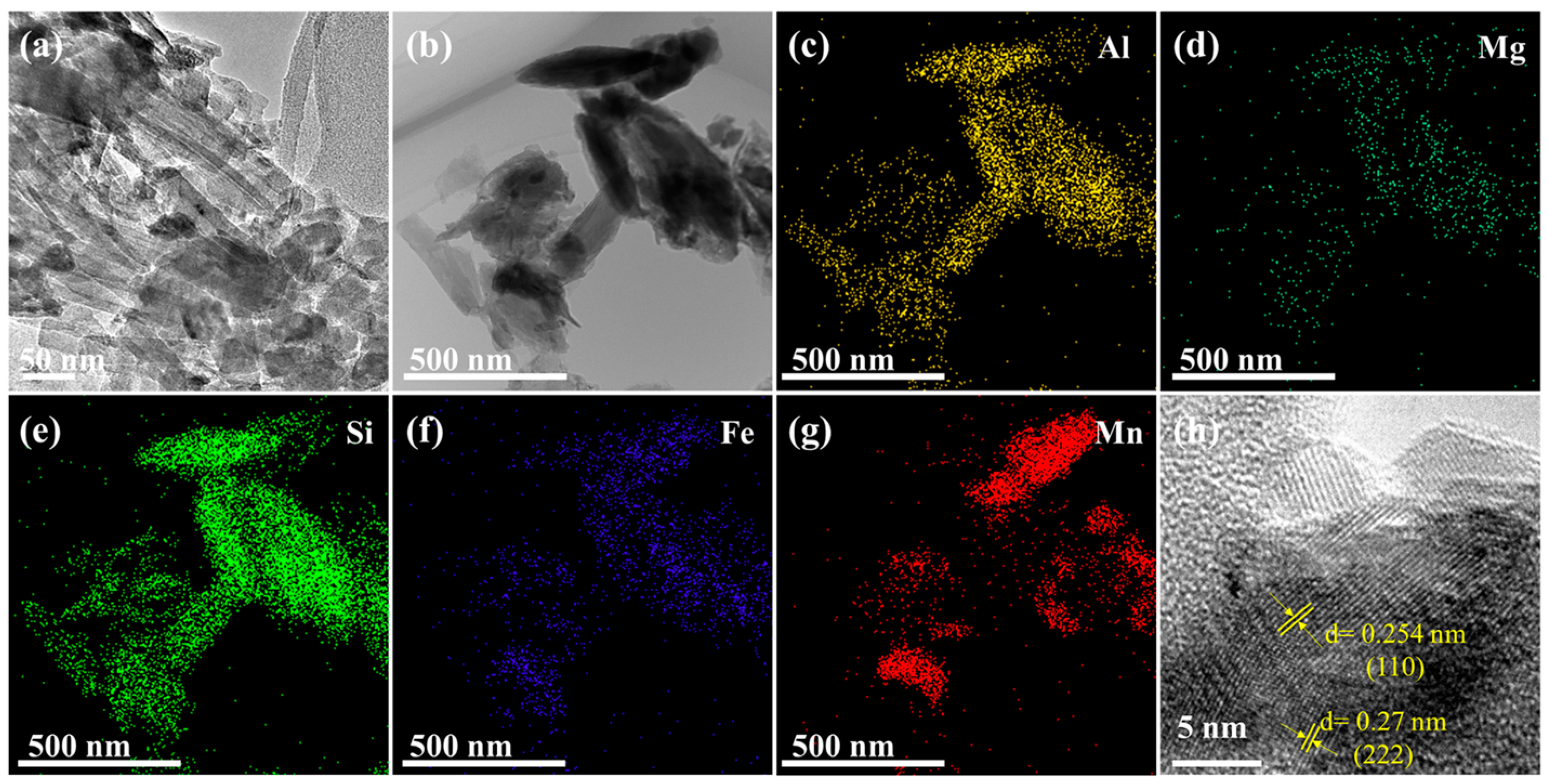
2.1.2. TEM Analysis
2.1.3. XPS Analysis
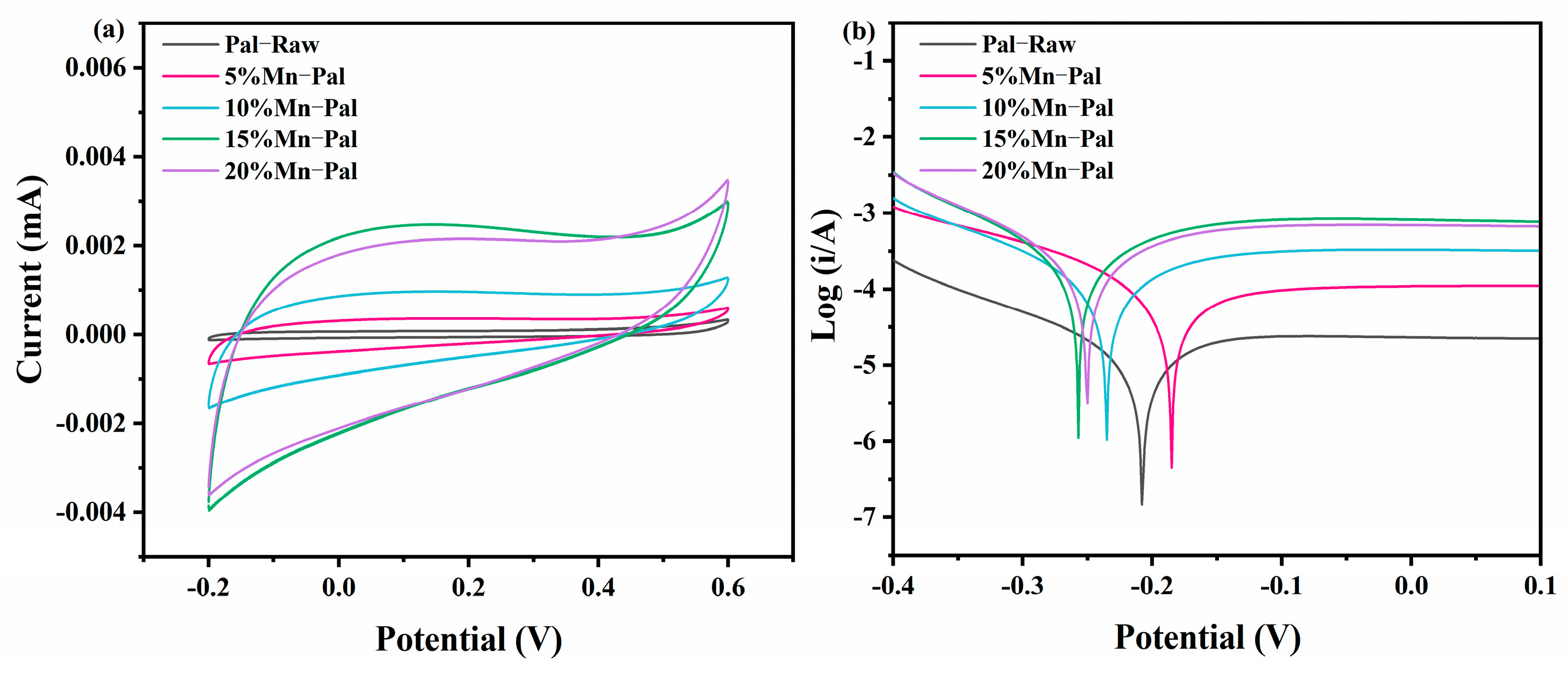
2.1.4. Electrochemical Property
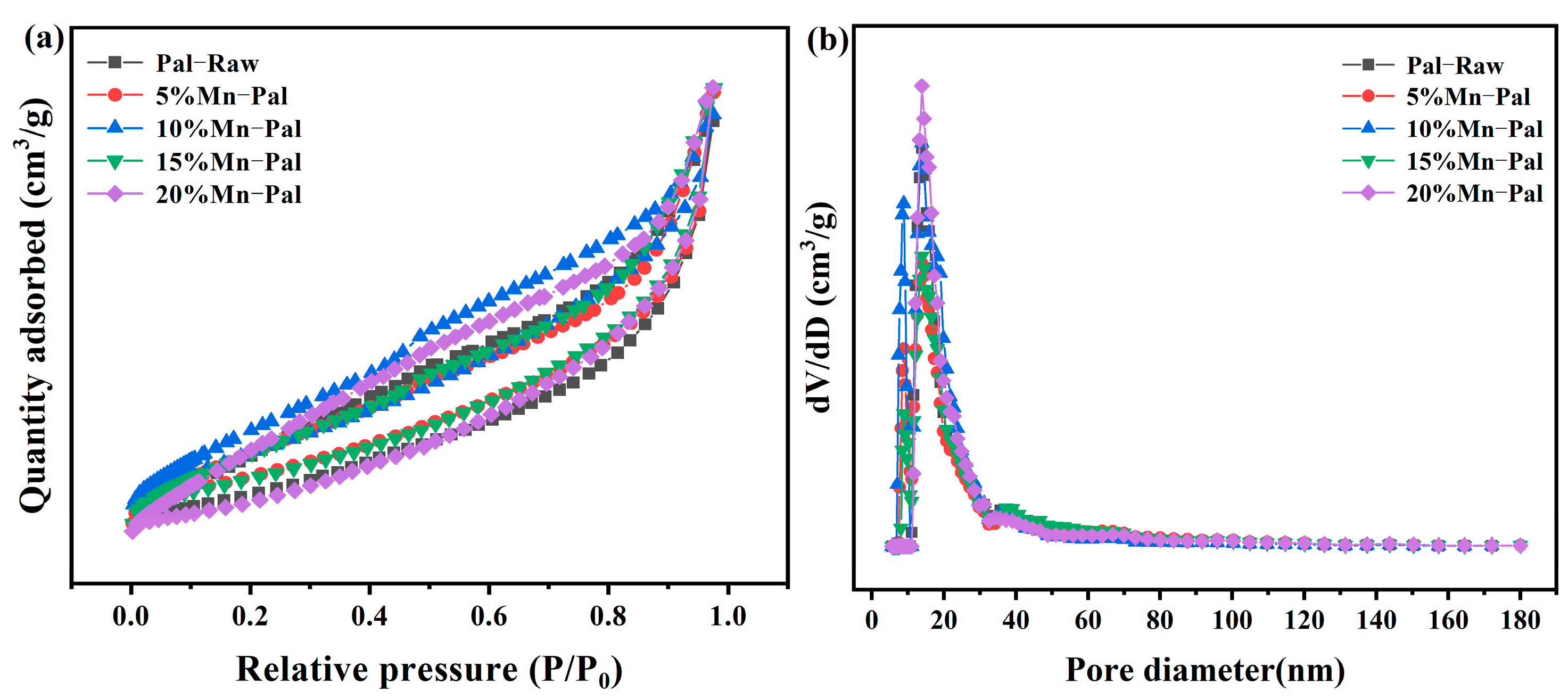
2.1.5. BET Analysis
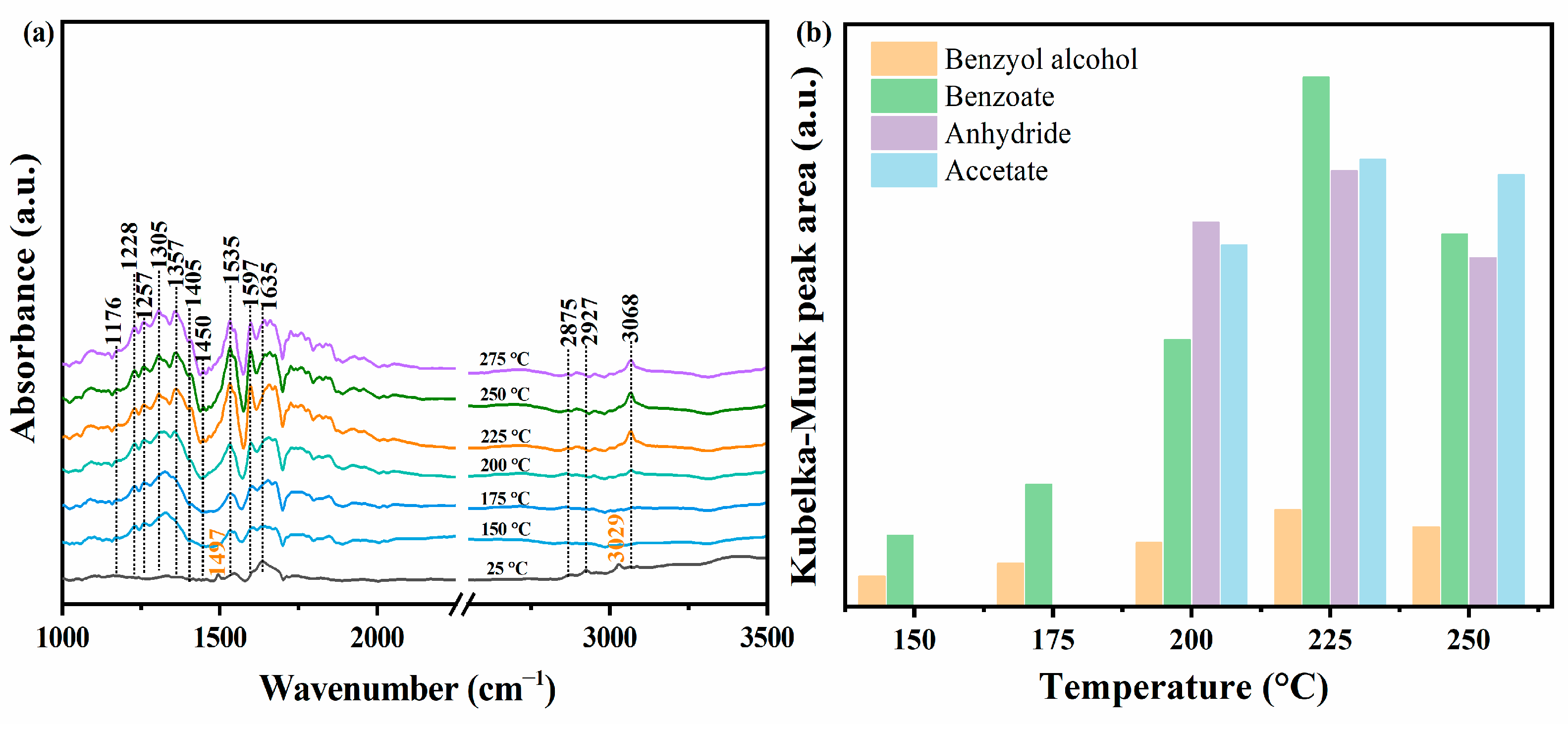
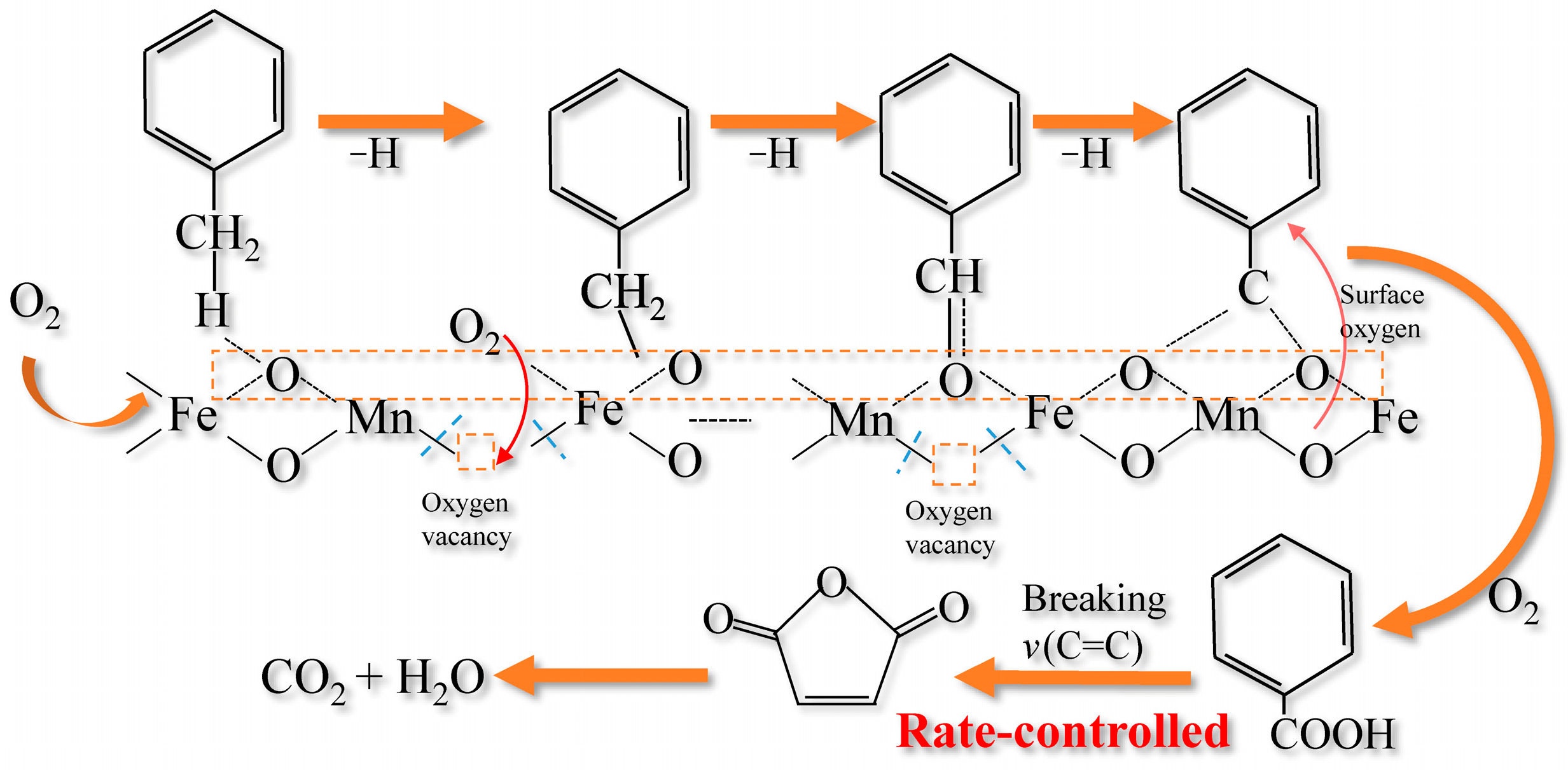
2.1.6. Mechanism Analysis
2.2. Evaluation of Catalytic Activity
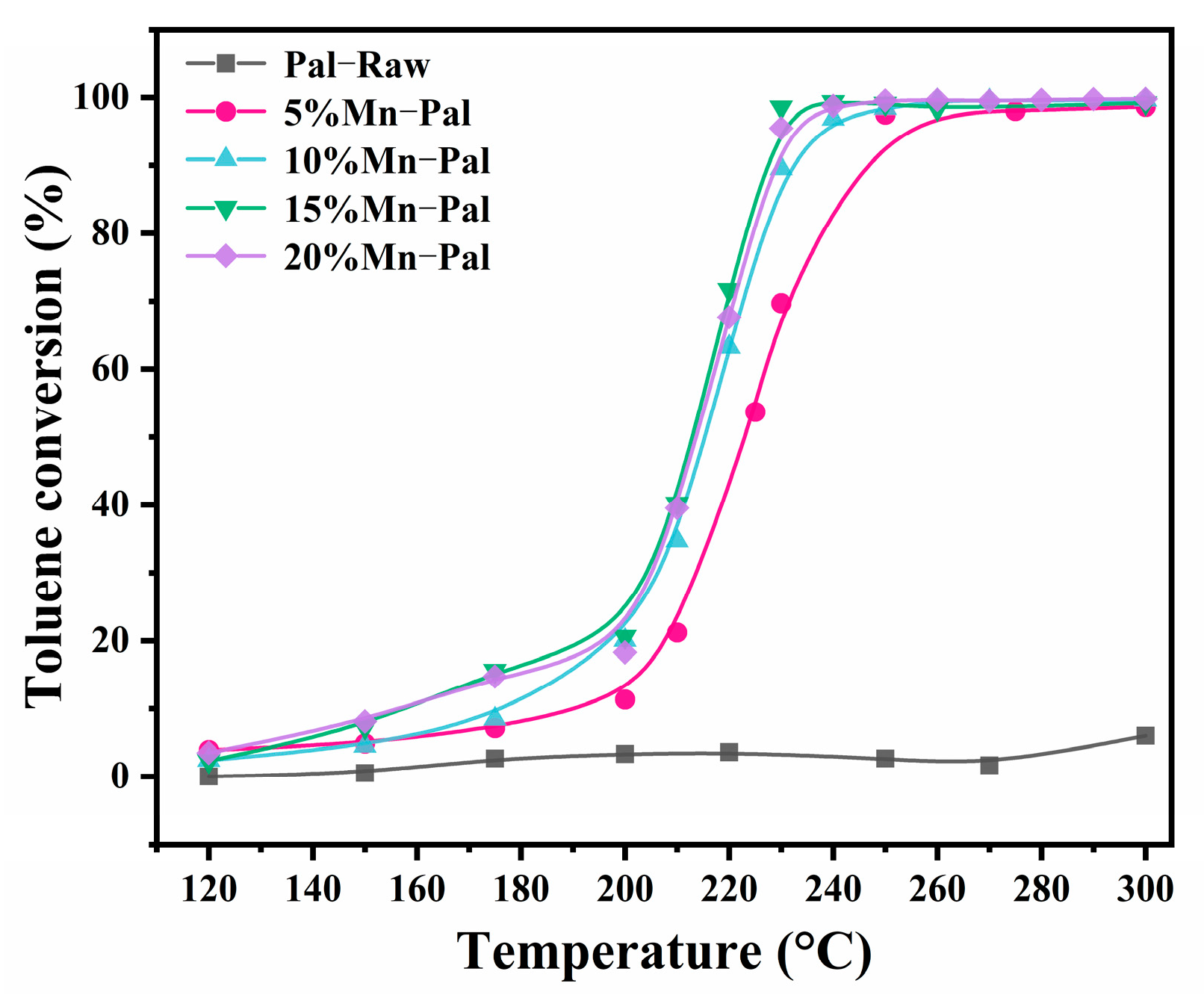
2.2.1. Catalytic Performance
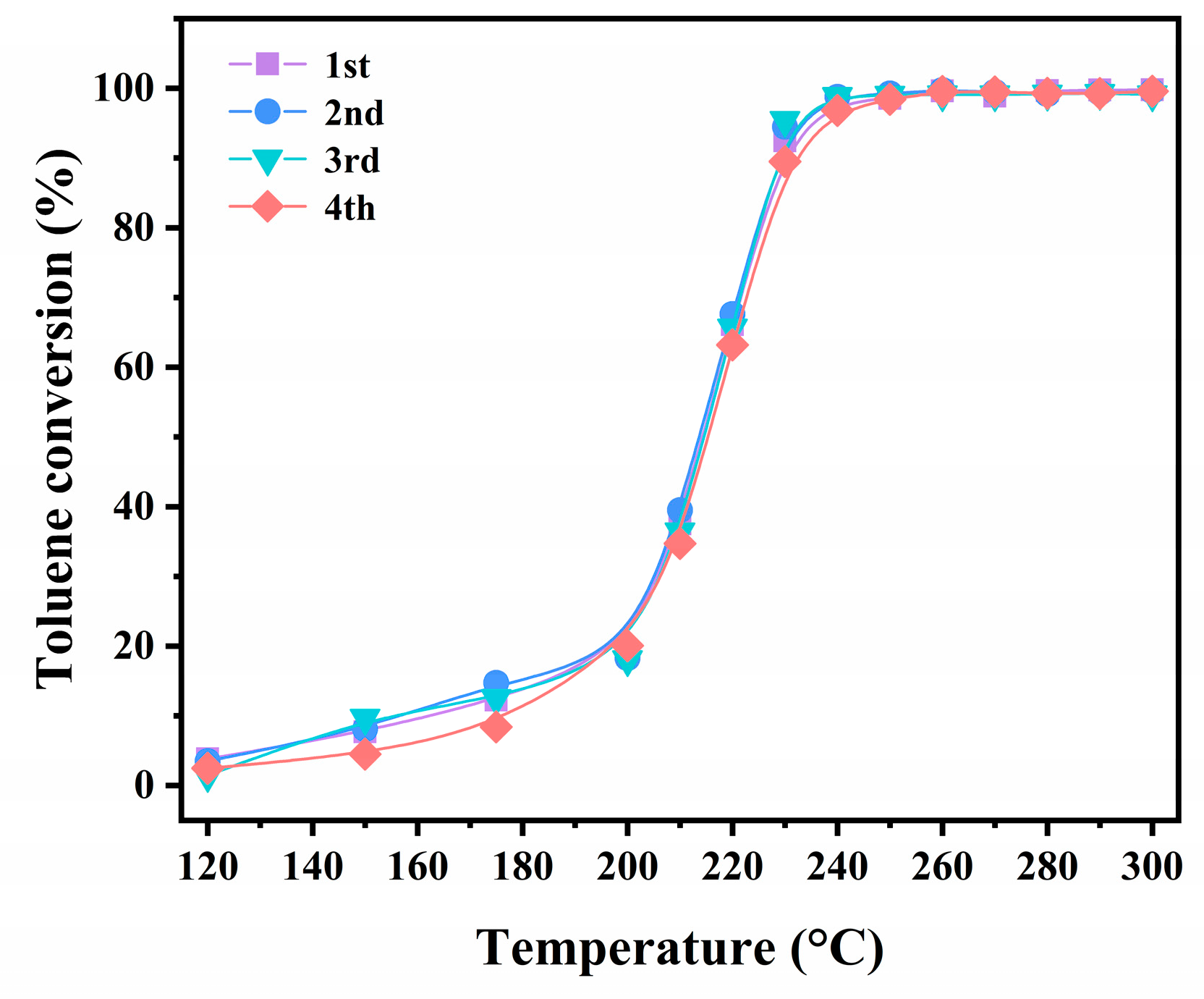
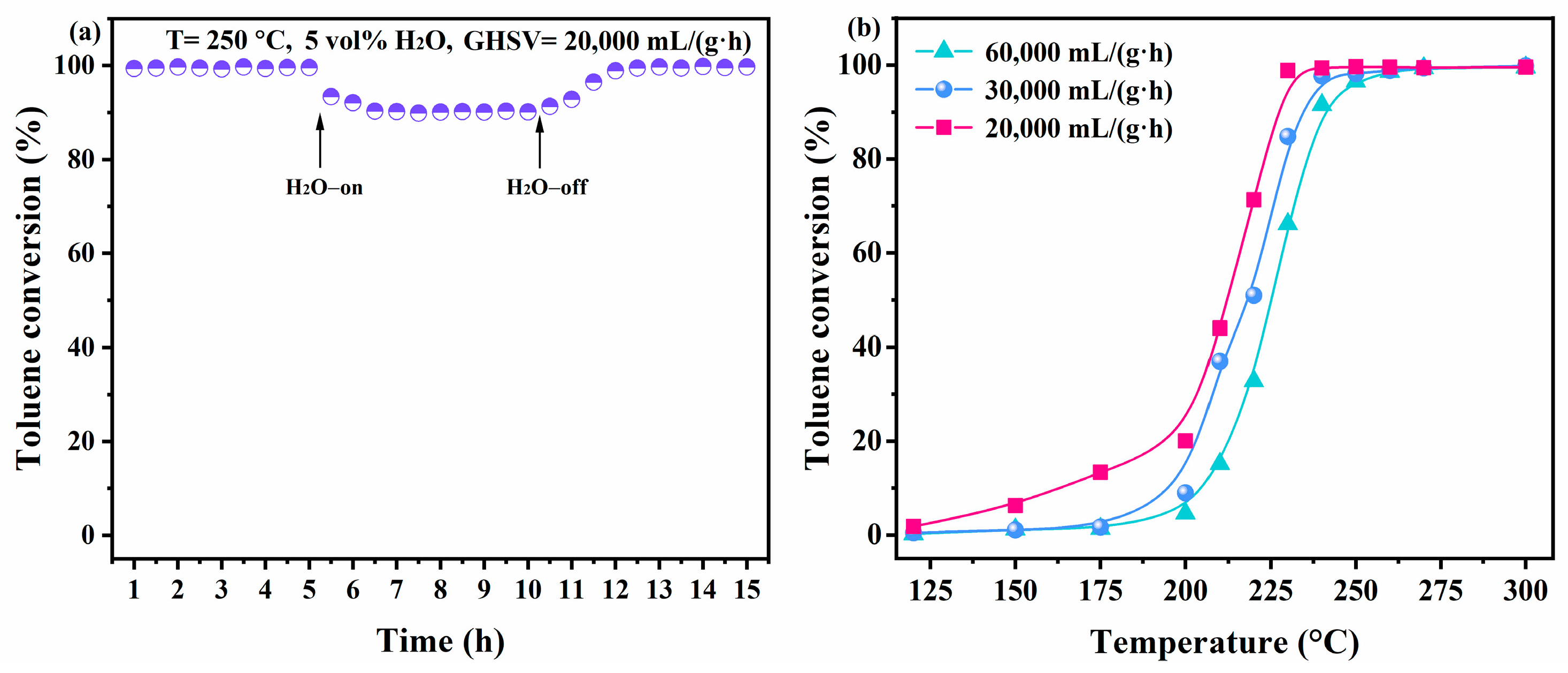
2.2.2. Stability Test
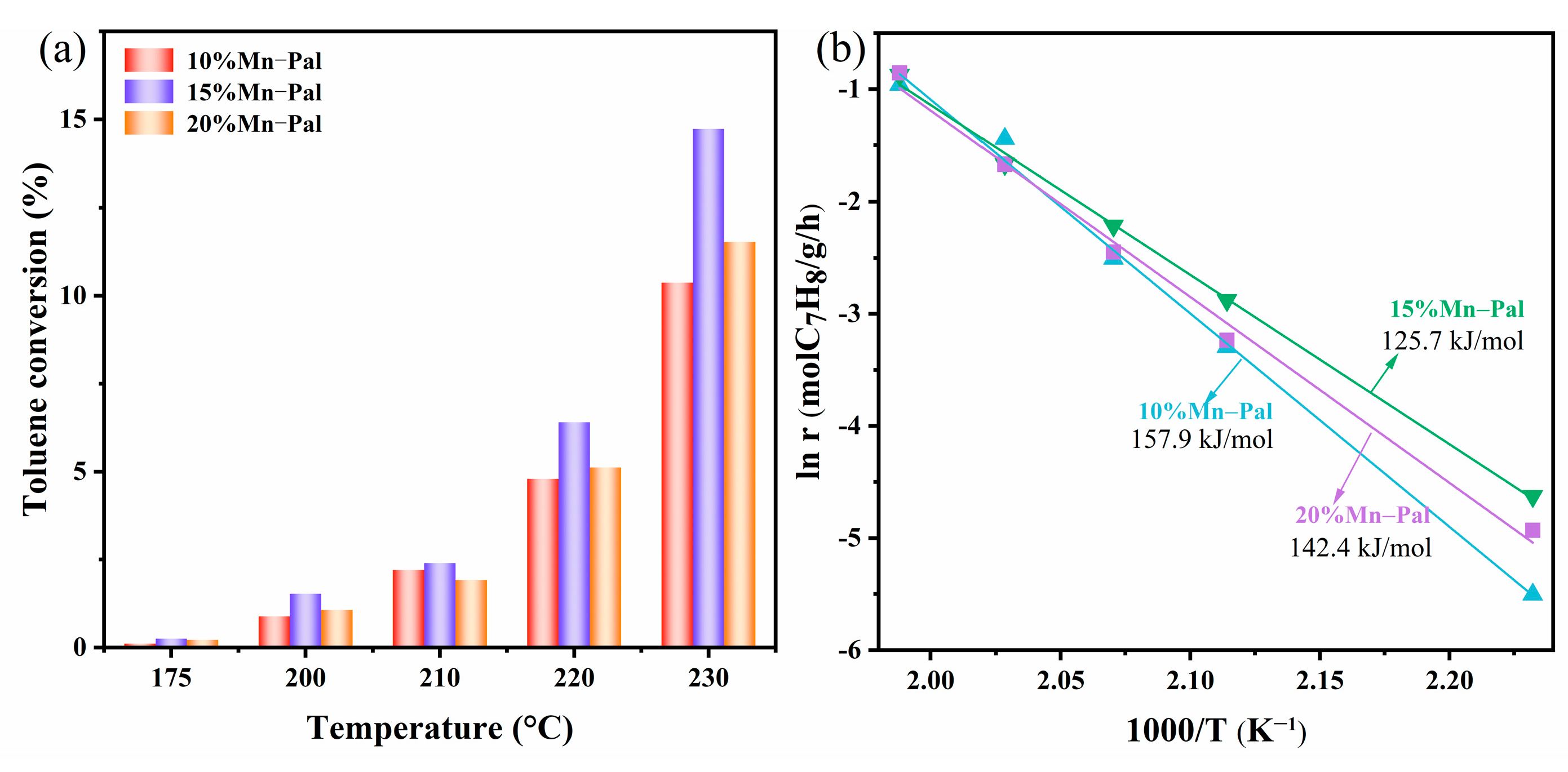
2.2.3. Kinetic Study
3. Discussion
4. Materials and Methods
4.1. Catalyst Synthesis
4.1.1. Materials
4.1.2. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Activity Measurement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Wei, G.; He, H.; Liang, X.; Chen, H.; Xi, Y.; Zhu, J. The catalytic oxidation of formaldehyde over palygorskite-supported copper and manganese oxides: Catalytic deactivation and regeneration. Appl. Surf. Sci. 2019, 464, 287–293. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Zhang, H.; Li, L.; Zhou, P.; Meng, X.; Guo, M.; Jia, J.; Sun, T. In situ fabrication of highly active gamma-MnO2/SmMnO3 catalyst for deep catalytic oxidation of gaseous benzene, ethylbenzene, toluene, and o-xylene. J. Hazard. Mater. 2019, 362, 178–186. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wu, X.; Li, S.; Li, W.; Chen, Y. Porous Mn–Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Lu, A.; Sun, H.; Zhang, N.; Che, L.; Shan, S.; Luo, J.; Zheng, J.; Yang, L.; Peng, D.-L.; Zhong, C.-J.; et al. Surface Partial-Charge-Tuned Enhancement of Catalytic Activity of Platinum Nanocatalysts for Toluene Oxidation. ACS Catal. 2019, 9, 7431–7442. [Google Scholar] [CrossRef]
- Li, L.; Wahab, M.A.; Li, H.; Zhang, H.; Deng, J.; Zhai, X.; Masud, M.K.; Hossain, M.S.A. Pt-Modulated CuMnOx Nanosheets as Catalysts for Toluene Oxidation. ACS Appl. Nano Mater. 2021, 4, 6637–6647. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: Highly effective catalysts for the removal of toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Li, G.; An, T.; Shi, H.; Li, Y. Visible-light-enhanced photothermocatalytic activity of ABO3-type perovskites for the decontamination of gaseous styrene. Appl. Catal. B Environ. 2017, 209, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, E.J.; Park, C.H.; Han, S.W.; Seo, H.O.; Kim, Y.D. Activity of catalysts consisting of Fe2O3 nanoparticles decorating entire internal structure of mesoporous Al2O3 bead for toluene total oxidation. Catal. Today. 2017, 295, 56–64. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464–118479. [Google Scholar] [CrossRef]
- Li, B.; Yang, Q.; Peng, Y.; Chen, J.; Deng, L.; Wang, D.; Hong, X.; Li, J. Enhanced low-temperature activity of LaMnO3 for toluene oxidation: The effect of treatment with an acidic KMnO4. Chem. Eng. J. 2019, 366, 92–99. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, Y.; Peng, X.; Pan, W. Copper modified manganese oxide with tunnel structure as efficient catalyst for low-temperature catalytic combustion of toluene. Chem. Eng. J. 2019, 369, 758–765. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Qin, Y.; Fu, Q.; Sun, H.; Duan, X. Revealing the Highly Catalytic Performance of Spinel CoMn2O4 for Toluene Oxidation: Involvement and Replenishment of Oxygen Species Using In Situ Designed-TP Techniques. ACS Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, G.; Yang, D.; Ishihara, T.; Guo, L. Oxygen vacancy engineering in Fe doped akhtenskite-type MnO2 for low-temperature toluene oxidation. Appl. Catal. B Environ. 2021, 285, 119873–119888. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Guo, L. Enhanced Toluene Combustion over Highly Homogeneous Iron Manganese Oxide Nanocatalysts. ACS Appl. Nano Mater. 2018, 1, 1066–1075. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Deng, W.; Han, J.; Qin, L.; Zhao, B.; Guo, L.; Xing, F. Study on the structure-activity relationship of Fe-Mn oxide catalysts for chlorobenzene catalytic combustion. Chem. Eng. J. 2020, 395, 125172–1125184. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, W.; Xu, Z.; Chen, J.; Jia, H.; Chen, J. Hydrolysis driving redox reaction to synthesize Mn-Fe binary oxides as highly active catalysts for the removal of toluene. Chem. Eng. J. 2017, 330, 281–293. [Google Scholar] [CrossRef]
- Yusuf, S.; Haribal, V.; Jackson, D.; Neal, L.; Li, F. Mixed iron-manganese oxides as redox catalysts for chemical looping–oxidative dehydrogenation of ethane with tailorable heat of reactions. Appl. Catal. B Environ. 2019, 257, 117885–117893. [Google Scholar] [CrossRef]
- Wang, C.; Zou, X.; Liu, H.; Chen, T.; Suib, S.L.; Chen, D.; Xie, J.; Li, M.; Sun, F. A highly efficient catalyst of palygorskite-supported manganese oxide for formaldehyde oxidation at ambient and low temperature: Performance, mechanism and reaction kinetics. Appl. Surf. Sci. 2019, 486, 420–430. [Google Scholar] [CrossRef]
- Han, Z.; Wang, C.; Zou, X.; Chen, T.; Dong, S.; Zhao, Y.; Xie, J.; Liu, H. Diatomite-supported birnessite–type MnO2 catalytic oxidation of formaldehyde: Preparation, performance and mechanism. Appl. Surf. Sci. 2020, 502, 144201–144210. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Applied. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Liu, P.; Wei, G.; Liang, X.; Chen, D.; He, H.; Chen, T.; Xi, Y.; Chen, H.; Han, D.; Zhu, J. Synergetic effect of Cu and Mn oxides supported on palygorskite for the catalytic oxidation of formaldehyde: Dispersion, microstructure, and catalytic performance. Applied. Clay Sci. 2018, 161, 265–273. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Chen, T.; Qing, C.; Zou, X.; Xie, J.; Zhang, X. Synthesis of palygorskite-supported Mn1−xCexO2 clusters and their performance in catalytic oxidation of formaldehyde. Applied. Clay Sci. 2018, 159, 50–59. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, T.; Liu, H.; Zhang, P.; Wang, C.; Dong, S.; Chen, D.; Xie, J.; Zou, X.; Suib, S.L.; et al. High catalytic performance of the Al-promoted Ni/Palygorskite catalysts for dry reforming of methane. Applied. Clay Sci. 2020, 188, 105498–105508. [Google Scholar] [CrossRef]
- Zou, X.; Chen, T.; Zhang, P.; Chen, D.; He, J.; Dang, Y.; Ma, Z.; Chen, Y.; Toloueinia, P.; Zhu, C.; et al. High catalytic performance of Fe-Ni/Palygorskite in the steam reforming of toluene for hydrogen production. Appl. Energ. 2018, 226, 827–837. [Google Scholar] [CrossRef]
- Han, Y.-F.; Chen, F.; Zhong, Z.-Y.; Ramesh, K.; Widjaja, E.; Chen, L.-W. Synthesis and characterization of Mn3O4 and Mn2O3 nanocrystals on SBA-15: Novel combustion catalysts at low reaction temperatures. Catal. Commun. 2006, 7, 739–744. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.; Chen, C.; He, C.; Hao, Z.; Shen, Z.; Liu, H. Sphere-Shaped Mn3O4 Catalyst with Remarkable Low-Temperature Activity for Methyl-Ethyl-Ketone Combustion. Environ. Sci. Technol. 2017, 51, 6288–6297. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Wang, L.; Wen, T.; Liu, H.; Zhang, J.; Liu, Z.; Zhu, C.; Long, C. Mesoporous poorly crystalline alpha-Fe2O3 with abundant oxygen vacancies and acid sites for ozone decomposition. Sci. Total Environ. 2022, 804, 150161. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Ma, J.; Shao, X.; Chen, Y.; He, H. Boosting the Dispersity of Metallic Ag Nanoparticles and Ozone Decomposition Performance of Ag-Mn Catalysts via Manganese Vacancy-Dependent Metal-Support Interactions. Environ. Sci. Technol. 2021, 55, 16143–16152. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Zhou, X.; Sun, P.; Hu, X.; Shimanoe, K.; Lu, G.; Yamazoe, N. Hierarchical alpha-Fe2O3/NiO composites with a hollow structure for a gas sensor. ACS Appl Mater Interfaces 2014, 6, 12031–12037. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, R.; Li, H.; Lan, L.; Zhou, W. Optimized Synthesis Routes of MnOx-ZrO2 Hybrid Catalysts for Improved Toluene Combustion. Catalysts 2021, 11, 1037–1050. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Zhang, J.-Y.; Chen, Y.; Yang, X.; Song, W.; Wei, L.; Li, W. Synergy of Mn and Ni enhanced catalytic performance for toluene combustion over Ni-doped α-MnO2 catalysts. Chem. Eng. J. 2020, 388, 124244–124255. [Google Scholar] [CrossRef]
- Awaya, K.; Koyanagi, Y.; Hatakeyama, K.; Ohyama, J.; Guo, L.; Masui, T.; Ida, S. Catalytic Toluene Combustion over Metastable Layered Manganese Cobalt Oxide Nanosheet Catalysts. Ind. Eng. Chem. Res. 2021, 60, 16930–16938. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Jiang, X.; Ren, Y. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation. J. Hazard. Mater. 2020, 391, 122181. [Google Scholar] [CrossRef]
- Chen, B.; Wu, B.; Yu, L.; Crocker, M.; Shi, C. Investigation into the Catalytic Roles of Various Oxygen Species over Different Crystal Phases of MnO2 for C6H6 and HCHO Oxidation. ACS Catal. 2020, 10, 6176–6187. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.; Xiang, W. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides. Chem. Eng. J. 2020, 387, 124101–124112. [Google Scholar] [CrossRef]
- Song, W.; Poyraz, A.S.; Meng, Y.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Mesoporous Co3O4 with Controlled Porosity: Inverse Micelle Synthesis and High-Performance Catalytic CO Oxidation at −60 °C. Chem. Mater. 2014, 26, 4629–4639. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; An, X.; Shi, J.; Shangguan, W.; Hao, X.; Xu, G.; Tang, B.; Abudula, A.; Guan, G. Generation of abundant defects in Mn-Co mixed oxides by a facile agar-gel method for highly efficient catalysis of total toluene oxidation. Appl. Catal. B Environ. 2021, 282, 119560–119571. [Google Scholar] [CrossRef]
- Liu, F.; Rong, S.; Zhang, P.; Gao, L. One-step synthesis of nanocarbon-decorated MnO2 with superior activity for indoor formaldehyde removal at room temperature. Appl. Catal. B Environ. 2018, 235, 158–167. [Google Scholar] [CrossRef]
- Hao, L.; Ning, J.; Luo, B.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, J.; Thomas, A.; Zhi, L. Structural evolution of 2D microporous covalent triazine-based framework toward the study of high-performance supercapacitors. J. Am. Chem. Soc. 2015, 137, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.Y.; Suib, S.L. Structure-property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, J.; Wang, Y.; Yin, R.; Yang, W.; Wang, G.; Si, W.; Peng, Y.; Li, J. Interaction Mechanism for Simultaneous Elimination of Nitrogen Oxides and Toluene over the Bifunctional CeO2-TiO2 Mixed Oxide Catalyst. Environ. Sci. Technol. 2022, 56, 4467–4476. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Tejedor-Tejedor, I.; Coronado, J.M.; Anderson, M.A. Operando FTIR study of the photocatalytic oxidation of methylcyclohexane and toluene in air over TiO2–ZrO2 thin films: Influence of the aromaticity of the target molecule on deactivation. Appl. Catal. B Environ. 2011, 101, 283–293. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.a.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B Environ. 2020, 260, 118150–118159. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Chen, J.; Wang, C.; Yin, H.; Wang, H.; You, C.; Li, J. Performance and Mechanism of Photocatalytic Toluene Degradation and Catalyst Regeneration by Thermal/UV Treatment. Environ. Sci. Technol. 2020, 54, 14465–14473. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Zhang, Y. Preparation and characterization of manganese oxides supported on functionalized halloysite nanotubes with enhanced catalytic oxidation for toluene. Applied. Clay Sci. 2021, 209, 106147–106155. [Google Scholar] [CrossRef]
- Li, K.; Chen, C.; Zhang, H.; Hu, X.; Sun, T.; Jia, J. Effects of phase structure of MnO2 and morphology of δ-MnO2 on toluene catalytic oxidation. Appl. Surf. Sci. 2019, 496, 143662–143671. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Song, Z.; Liu, W.; Zhao, J.; Ma, Z.a.; Zhao, M.; Xing, Y. Insight into the effect of oxygen species and Mn chemical valence over MnO on the catalytic oxidation of toluene. Appl. Surf. Sci. 2019, 493, 9–17. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over MnOx–CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2008, 84, 303–312. [Google Scholar] [CrossRef]
- Liao, Y.; Fu, M.; Chen, L.; Wu, J.; Huang, B.; Ye, D. Catalytic oxidation of toluene over nanorod-structured Mn–Ce mixed oxides. Catal. Today 2013, 216, 220–228. [Google Scholar] [CrossRef]
- Deng, J.; He, S.; Xie, S.; Yang, H.; Liu, Y.; Guo, G.; Dai, H. Ultralow Loading of Silver Nanoparticles on Mn2O3 Nanowires Derived with Molten Salts: A High-Efficiency Catalyst for the Oxidative Removal of Toluene. Environ. Sci. Technol. 2015, 49, 11089–11095. [Google Scholar] [CrossRef] [PubMed]

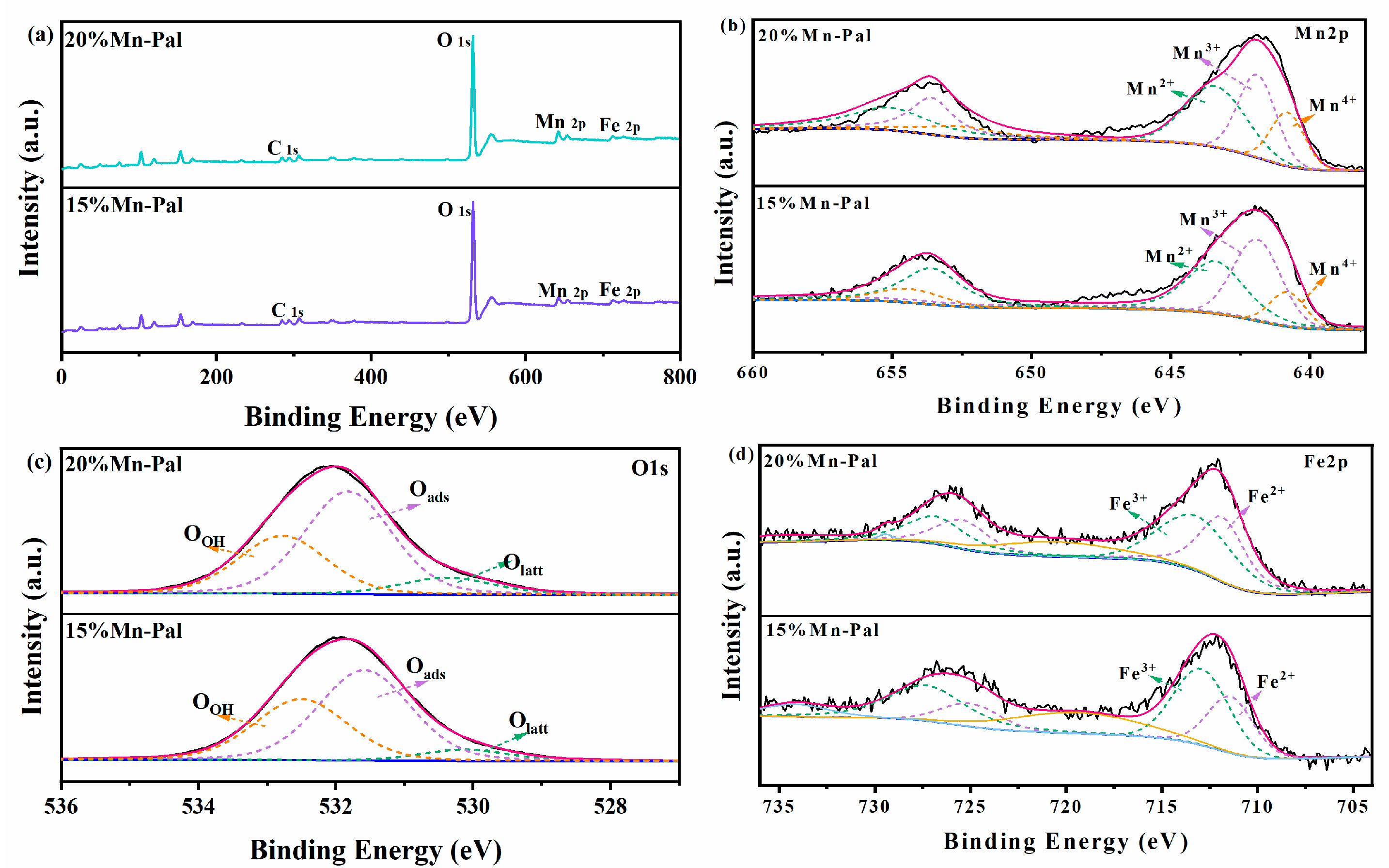
| Catalyst | Mn2+% | Mn3+% | Mn4+% | Fe2+% | Fe3+% | Olatt% | Oabs% | OOH% |
|---|---|---|---|---|---|---|---|---|
| 15%Mn-Pal | 15.8 | 56.1 | 28.1 | 33.3 | 66.7 | 4.9 | 57.4 | 37.7 |
| 20%Mn-Pal | 18.9 | 49.8 | 31.3 | 45.8 | 54.2 | 11.9 | 55.5 | 32.6 |
| Catalysts | Mn Loading (wt%) | SBET (m2/g) | Total Pore Volume (cm3/g) | |
|---|---|---|---|---|
| Theoretical | Actual | |||
| Pal-Raw | – | – | 48.1 | 0.14 |
| 5%Mn-Pal | 5 | 4.2 | 56.2 | 0.16 |
| 10%Mn-Pal | 10 | 9.4 | 59.6 | 0.15 |
| 15%Mn-Pal | 15 | 14.2 | 82.7 | 0.17 |
| 20%Mn-Pal | 20 | 19.0 | 52.4 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Chen, T.; Xu, F.; Liu, H.; Wang, C.; Zhang, Y.; Ji, M.; Xu, C.; Zhu, C.; Li, Z.; et al. Catalytic Oxidation of Toluene over Fe-Rich Palygorskite Supported Manganese Oxide: Characterization and Performance. Catalysts 2022, 12, 763. https://doi.org/10.3390/catal12070763
Dong S, Chen T, Xu F, Liu H, Wang C, Zhang Y, Ji M, Xu C, Zhu C, Li Z, et al. Catalytic Oxidation of Toluene over Fe-Rich Palygorskite Supported Manganese Oxide: Characterization and Performance. Catalysts. 2022; 12(7):763. https://doi.org/10.3390/catal12070763
Chicago/Turabian StyleDong, Shiwei, Tianhu Chen, Fan Xu, Haibo Liu, Can Wang, Yinsheng Zhang, Minghao Ji, Chengrui Xu, Chengzhu Zhu, Zhiguo Li, and et al. 2022. "Catalytic Oxidation of Toluene over Fe-Rich Palygorskite Supported Manganese Oxide: Characterization and Performance" Catalysts 12, no. 7: 763. https://doi.org/10.3390/catal12070763
APA StyleDong, S., Chen, T., Xu, F., Liu, H., Wang, C., Zhang, Y., Ji, M., Xu, C., Zhu, C., Li, Z., & Zou, X. (2022). Catalytic Oxidation of Toluene over Fe-Rich Palygorskite Supported Manganese Oxide: Characterization and Performance. Catalysts, 12(7), 763. https://doi.org/10.3390/catal12070763






