Abstract
The hydrogenation of polyaromatic compounds (PACs) present in mineral oils is of great importance when it comes to the desired product properties and the minimization of health hazards; however, the presence of organonitrogen inhibits the conversion of these compounds. In this study, the inhibition effects of different types of organonitrogen compounds (acridine (ACR) and carbazole (CBZ)-basic and nonbasic organonitrogen) on the hydrodearomatization (HDA) of phenanthrene over a sulfided commercial NiMo/Al2O3 catalyst were investigated in a microflow trickle-bed reactor at a temperature range of 280 to 320 °C and at a total pressure of 120 barg. Analysis of the experimental results shows that the hydrogenation of phenanthrene is significantly decreased in the presence of organonitrogen, with acridine showing stronger inhibiting effects. The extent of hydrodenitrogenation (HDN) is shown to correlate with the inhibition degree with a higher extent of HDN being achieved for carbazole than for acridine. Results from co-feeding different nitrogen types (acridine and carbazole) indicate that basic nitrogen is the dominating type of organonitrogen inhibitor. Recovery of catalyst activity in the absence of organonitrogen indicates fully reversible deactivation suggesting that inhibition relates to competitive adsorption and slower reaction rate of HDN compared to HDA.
1. Introduction
Our everyday lives are surrounded by products containing mineral oils; lubricants, adhesives, transformer oils, printing inks, and tires are a few examples. The raw materials used to produce such mineral oils contain significant amounts of polyaromatic compounds (PACs), which are known carcinogens. Increasing awareness of the health and environmental impacts of these PACs is driving demand for products with lower levels of such compounds.
The reduction in PACs is accomplished via hydrotreating, usually over sulfided catalysts. Hydrogenation reactions, including hydrodearomatization (HDA), are mainly equilibrium reactions that are heavily influenced by the partial pressure of hydrogen; thus, very high hydrogen partial pressures (>100 bar) are often employed.
In addition to PACs, organonitrogen compounds are present in the feedstock and are believed to be some of the most inhibitory compounds in hydrotreatment [1]. Nitrogen is present typically in heterocycles, such as pyrroles and pyridines, and can be categorized either as ‘nonbasic’ or ‘basic’, depending on whether the electron pair of the nitrogen atom is participating in the aromaticity [2]. Pyridine is an example of a basic nitrogen compound, while pyrrole is an example of a nonbasic nitrogen compound.
The effect of the presence of organonitrogen on hydrodesulfurization (HDS) activity has been studied mainly for different fuel fractions. Zeuthen et al. [3], who studied the effect of organic nitrogen on HDS of a diesel feed, concluded that carbazoles (nonbasic nitrogen compounds) were the more refractory compounds during gas oil hydrotreating and that acridine (a basic nitrogen compound) was the most significant inhibitor of HDS, which also hindered the hydrodenitrogenation (HDN) of carbazoles. Model compounds have been used extensively to study important reactions in hydrotreating of mineral oils and petroleum distillates [4,5,6,7,8,9,10,11]. Such studies have provided further evidence of the inhibitory effect of acridine [12,13] and carbazole [13,14,15] on HDS.
While a handful of studies exist on the effects of organonitrogen on HDS activity with concrete conclusions at a high level of consensus, the effects of organonitrogen on the hydrogenation of aromatic structures are still a matter of debate. As organonitrogen compounds are some of the most important inhibitors for the removal of PACs, it is essential to understand how these species effect the hydrogenation reaction pathway.
Beltramone et al. [16], while studying the effect of various nitrogen compounds on the hydrogenation of phenanthrene and substituted dibenzothiophene over a NiMo catalyst system at a pressure of 70 atm, concluded that nonbasic nitrogen is less inhibitory than basic nitrogen. However, in contrast to findings of other studies, the authors concluded that ammonia was found to be the most inhibiting nitrogen compound for the hydrogenation reactions.
The majority of hydrotreating studies using model compounds have focused on diesel hydrotreating and the effect of organonitrogen inhibition on HDS, while for lubricant hydrotreating, higher pressures are typically used, and HDA is of greater interest. This study seeks to build upon the previous findings [14]; here, however, the focus is on HDA, rather than HDS.
In this work, the effect of organonitrogen types on the hydrogenation of PACs is studied over a sulfided commercial NiMo/Al2O3 catalyst in a trickle-flow reactor. The model compounds phenanthrene, acridine, and carbazole are chosen as a model system for lubricant production. As is the case with a real feedstock, where basic and nonbasic nitrogen compounds are simultaneously present in the reactant solution, the influence of the presence of acridine and carbazole on the hydrogenation of a model PAC is investigated at pressure relevant for lubrication base oil production.
2. Results and Discussion
The hydrogenation of phenanthrene (PHE) and the effect of the addition of the organic nitrogen compounds, acridine (ACR), and carbazole (CBZ) were studied in a microflow trickle-bed reactor. The hydrogenation reaction network of phenanthrene is presented in Figure 1 [7,17] and represents a ring-by-ring hydrogenation as described by Korre et al. [17]. The five products are 9,10-dihydrophenanthrene (DIPHE), 1,2,3,4-tetrahydrophenanthrene (TETPHE), 1,2,3,4,4a,9,10,10a-octahydrophenanthrene (asymmetrical-octahydrophenanthrene, ASYM), 1,2,3,4,5,6,7,8-octahydrophenanthrene (symmetrical-octahydrophenanthrene, SYM), and perhydrophenanthrene (PHP) (Figure 2). In the absence of organonitrogen in the reactant feed, all five hydrogenation products were detected and quantified using GC-MS and GC-FID, respectively. When organonitrogen was present in the reactant feed, a significant decrease in phenanthrene conversion was observed with a simultaneous swift in the product distribution, as shown in Figure 3, Figure 4 and Figure 5. The HDN of both carbazole and acridine was investigated, and the results are seen in Figure 6. This figure shows that the extent of HDN was significantly higher for carbazole than for acridine. Finally, experiments of co-feeding acridine and carbazole together with phenanthrene at a reactor temperature of 300 °C showed a small difference in phenanthrene conversion compared to solely feeding acridine with phenanthrene, while a very low extent of HDN was observed; these results are shown in Table 2 and Figure 9. For all the experimental runs, the carbon balance of phenanthrene and its hydrogenation products was typically 92% ± 1.6%. No hydrocracking products were detected, which is in line with the low treatment temperatures employed in the study [5]. Traces of anthracenes were observed but account for <0.01% of the total carbon fed, indicating that isomerization activity of the catalyst under investigated conditions is negligible.
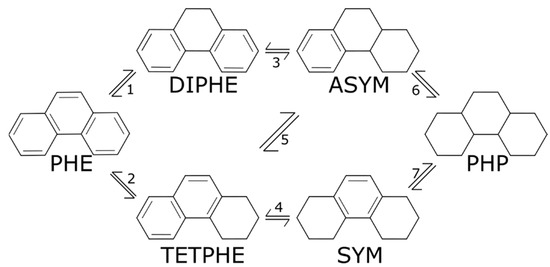
Figure 1.
Reaction network for the hydrogenation of phenanthrene (PHE) and its products: 9,10-dihydrophenanthrene (DIPHE), 1,2,3,4-tetrahydrophenanthrene (TETPHE), 1,2,3,4,4a,9,10,10a-octahydrophenanthrene (ASYM), 1,2,3,4,5,6,7,8-octahydrophenanthrene (SYM), and perhydrophenanthrene (PHP).
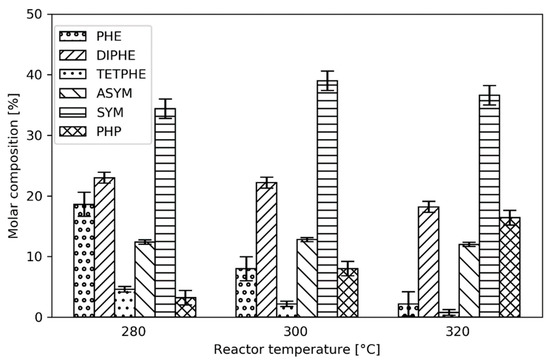
Figure 2.
Product distribution from the hydrogenation of phenanthrene at different reactor temperatures.
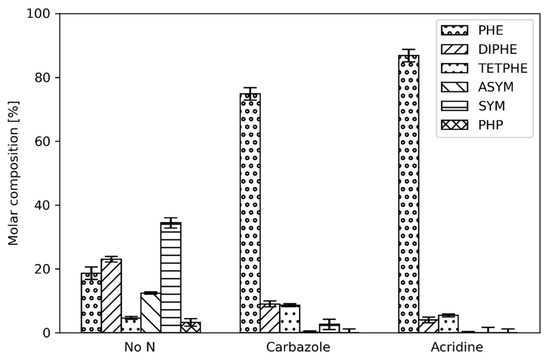
Figure 3.
Phenanthrene product distribution with 1.2 mmol/dm3 organic nitrogen (carbazole or acridine) present in the reactant feed at a reactor temperature of 280 °C.
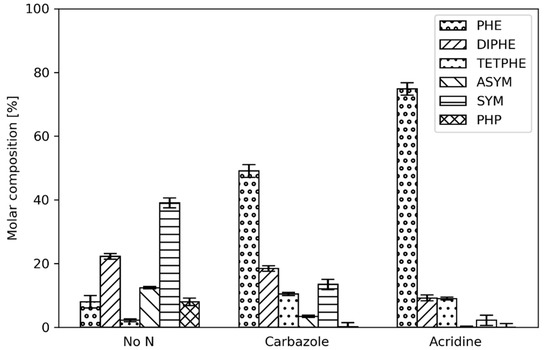
Figure 4.
Phenanthrene product distribution with 1.2 mmol/dm3 organic nitrogen (carbazole or acridine) present in the reactant feed at a reactor temperature of 300 °C.
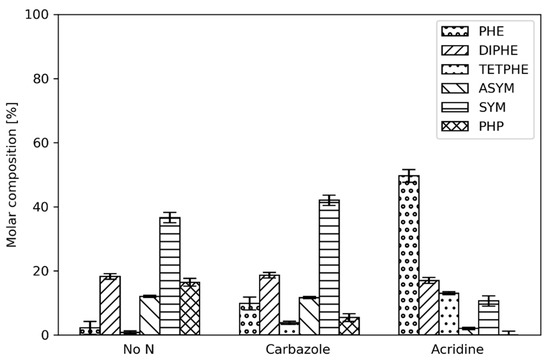
Figure 5.
Phenanthrene product distribution with 1.2 mmol/dm3 organic nitrogen (carbazole or acridine) present in the reactant feed at a reactor temperature of 320 °C.
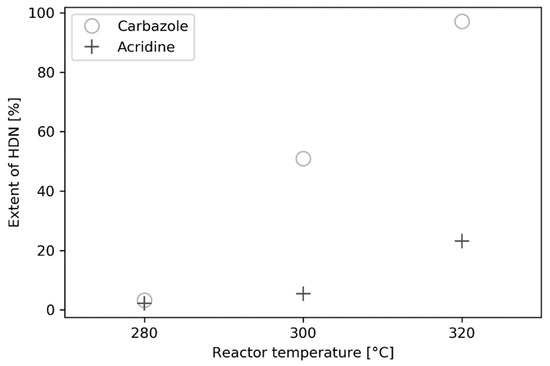
Figure 6.
Extent of nitrogen removal, HDN, for carbazole and acridine at different reactor temperatures at a concentration of 1.2 mmol/dm3. Error bars not shown in the figure; however, they are approx. ±2%.
2.1. Phenanthrene Hydrogenation
DIPHE and TETPHE are the primary products of phenanthrene, whereas SYM and ASYM are secondary products. PHP is a tertiary product formed from both ASYM and SYM, as seen in Figure 1. In Figure 2, the distributions of these phenanthrene hydrogenation products at different reactor temperatures are presented. As expected, when the reactor temperature is increased, the conversion of phenanthrene increases. Moreover, the content of DIPHE does not change significantly, indicating that the hydrogenation of phenanthrene to DIPHE is thermodynamically limited. Comparing the ratio of phenanthrene and DIPHE with the equilibrium data reported by Frye (See Table S1 in Supplementary information), it is clear that the reaction is, indeed, under thermodynamic control [18]. Interestingly, the TETPHE content is very low, indicating that TETPHE is most likely rapidly hydrogenated to the SYM and ASYM products. Moreover, the fully hydrogenated product, PHP, is always formed at the present conditions.
While hydrogenation reactions are typically reversible, for the current system only the phenanthrene to DIPHE could be considered to be under thermodynamic control, while the rest of the reactions can be regarded as irreversible and thus kinetically controlled [18]. Korre et al. [17,19] have reported three types of aromatic hydrogen addition reactions, namely: (1) benzenic (three moles of H2); (2) naphthalenic (two moles of H2); and (3) phenanthrenic (one mole of H2). This suggests that the formation of PHP and ASYM (reactions 3, 6, and 7 according to Figure 1) are most likely the slowest reactions as they are all benzenic hydrogen additions. Thus, the presence of ASYM, and especially PHP, indicates that the catalyst has a high hydrogenation activity. However, the formation of ASYM from TET (reaction 5) is plausibly slow due to the sterically hindered middle ring hydrogenation, whereas the rate of SYM formation is most likely higher, and thus is the preferred pathway for TET (reaction 4) [7]. While the formation of PHP is also from benzenic hydrogen addition, plausible steric hindrances occur during its formation from SYM (reaction 7) compared to its formation from ASYM (reaction 6).
2.2. Addition of Organic Nitrogen
While it is of interest to investigate only the hydrogenation of phenanthrene, a real system would typically also have inhibitory compounds present in the reactant mix. In this section, the results from the addition of two types of organonitrogen compound (namely, carbazole and acridine) to the reactant feed at three different reactor temperatures are presented and discussed. In Figure 3, Figure 4 and Figure 5, the product distribution from phenanthrene hydrogenation in the presence of either carbazole (nonbasic) or acridine (basic) is shown for different reactor temperatures.
As shown in Figure 3, the introduction of carbazole and acridine has a detrimental effect on the phenanthrene hydrogenation at 280 °C. Not only is the conversion of phenanthrene significantly lower (higher molar fraction), but also fewer products of the deep hydrogenation are observed; ASYM and PHP were below the detection limits indicating that these species were most likely not formed. At this low reactor temperature, the effects of carbazole and acridine on the hydrogenation of phenanthrene are similar.
At higher reactor temperatures (Figure 4) the inhibition effect of carbazole is diminished compared to the lower reactor temperature (280 °C, Figure 3). While phenanthrene and DIPHE predominate in the products, SYM has a higher yield than TETPHE. In addition, ASYM and traces of PHP are formed. When acridine is present, slightly higher conversions of phenanthrene and higher yields of DIPHE and TETPHE are observed compared to at the lower temperature. Moreover, the ratio of phenanthrene and DIPHE is lower than when acridine was not fed, indicating that the conversion of phenanthrene to DIPHE reaction (reaction 2) is not at equilibrium and is now under kinetic control instead.
At 320 °C the organonitrogen addition still affects the PACs hydrogenation and the product distribution (Figure 5), however, it is not as severe as it is at lower temperatures. This is especially true for carbazole, where SYM becomes the most abundant product compared to phenanthrene, which predominates at 300 °C. Additionally, the yield of PHP is increased. More importantly, the effect of acridine is not as pronounced compared to the lower reactor temperatures. While phenanthrene is still the most abundant constituent in the liquid outlet, the yield of the hydrogenation products is increased.
Based on the results of the product distribution of phenanthrene at different reactor temperatures, it can be concluded that organonitrogen compounds have a detrimental effect on the hydrogenation of PACs. Especially inhibitory is acridine, representing the basic nitrogen category, as its addition to the reactant feed causes a significant decrease in conversion of phenanthrene and especially toward the deep hydrogenation products across all tested temperatures.
2.3. Hydrodenitrogenation of Carbazole and Acridine
In addition to investigating the product distribution of phenanthrene hydrogenation, the removal of the organic nitrogen also was studied. While the product distribution of phenanthrene is analyzed easily and quantified using GC-FID, the same was not true for carbazole and acridine. The mechanism of nitrogen atom removal involves complete hydrogenation of carbazole and acridine prior to the removal and, similar to phenanthrene, a spectrum of partly or completely hydrogenated products can be formed. However, not all of these could be detected and quantified using GC-FID and/or GC-MS. Thus, total nitrogen analysis was deemed to be the most reliable means to quantify the nitrogen removal, i.e., the remaining content of organic nitrogen in the liquid product. In Figure 6, the extent of HDN is shown for carbazole and acridine at the three different reactor temperatures tested.
Figure 6 shows the HDN rate of carbazole is higher than that of acridine, since the nitrogen concentration observed in the product is lower, at the same space time. This is most likely due the molecular structure and, therefore, reactivity of the C–N bond. For N removal (C–N cleavage) from carbazole and acridine, the nitrogen atom requires sp3-hybridization. Moreover, the C–N cleavage occurs mainly through elimination or substitution reactions [20,21,22]. Therefore, it is relevant to describe the HDN networks of carbazole and acridine, as this reveals that several of the conversion steps are hydrogenation reactions taking place prior to the C–N bond cleavage reactions. In Figure 7 and Figure 8, the HDN networks for carbazole and acridine, respectively, are shown.
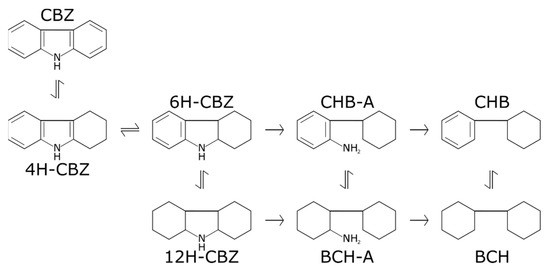
Figure 7.
HDN network for carbazole (CBZ) to its HDN products: tetrahydrocarbazole (4H-CBZ), hexahydrocarbazole (6H-CBZ), dodecahydrocarbazole (12H-CBZ), cyclohexylbenzeneamine (CHB-A), bicyclohexylamine (BCH-A), cyclohexylbenzene (CHB), and bicyclohexane (BCH). Based on [21,23,24,25].
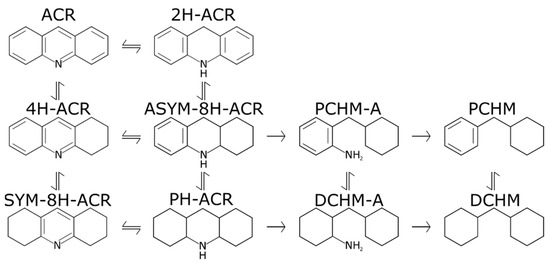
Figure 8.
HDN network of acridine to its HDN products: 9,10-dihydroacridine (2H-ACR), 1,2,3,4-tetrahydroacridine(4H-ACR), 1,2,3,4,4a,9,9a,10-octahydroacridine (ASYM-8H-ACR), 1,2,3,4,5,6,7,8-octahydroacridine (SYM-8A-ACR), perhydroacridine (PH-ACR), phenylcyclohexylmethaneamine (PCHM-A), dicyclohexylmethaneamine (DCHM-A), phenylcyclohexylmethane (PCHM), and dicyclohexylmethane (DCHM).
In the case of carbazole and its hydrogenation (but still nitrogen-containing) products, it was mainly carbazole itself and 4H-CBZ that were detected at measurable concentrations in the liquid product, where carbazole was always the most abundant of the two. Steele and Chirico [25] have concluded that the HDN of carbazole is typically under thermodynamic control, indicating that 4H-CBZ is the thermodynamically dominant product at the tested process conditions. The above suggests that there is a large driving force to hydrogenate carbazole to 4H-CBZ, while the formation of 6H-CBZ is most likely thermodynamically limited. However, in our tests, carbazole is typically more abundant than 4H-CBZ, which could be due to the C–N breaking reactions of 6H-CBZ driving the reactions forward. Neither of the amines (CHB-A and BCH-A) were detected, probably due to them being rapidly undergoing further C–N bond cleavage after being formed [21]. The largest barrier is plausibly the hydrogenation of 4H-CBZ to 6H-CBZ due to thermodynamic constraints (see Table S2 in Supplementary information) or the subsequent ring opening and C–N bond scission. Moreover, both CHB and BCH were detected, with BCH being more abundant at all tested conditions, possibly because of fast subsequent hydrogenation of CHB to BCH. Additionally, the self-inhibition of organic nitrogen during HDN might also hinder the hydrogenation of carbazoles [23] and result in a nonequilibrium conversion. To evaluate this, an additional experiment with higher carbazole content in the feed was performed with a reactor temperature of 300 °C. The results are presented in Table 1. Comparing the extent of HDN for the two cases, carbazole self-inhibition is evident.

Table 1.
The extent of HDN at a reactor temperature of 300 °C with different carbazole content in the reactant feed.
Analyzing the liquid products when acridine was introduced, it can be concluded that while the extent of HDN is very low (Figure 6), the conversion of acridine is always very high, as it was not detected either by GC-FID or GC-MS. However, some of the hydrogenation products of acridine were detected with the most abundant one being the perhydroacridine (PH-ACR); moreover, traces of SYM-8H-ACR were detected. This finding indicates that the abovementioned hydrogenation products act as the main inhibitor. The latter corroborates the previous literature which suggested that SYM-8H-ACR was the “main inhibitor” of acridine HDN [26,27]. The fact that PH-ACR is the main product can be attributed to the higher H2 pressures employed in the present study, which can promote further hydrogenation of SYM-8H-ACR to PH-ACR. The greater inhibitory effect of the hydrogenation products of organic nitrogen compounds can be correlated to the adsorption coefficient of the molecule, which is positively correlated with the hydrogenation degree and the basicity of the molecule [13,26,27,28]. The latter has also been suggested by Lio et al. [29] based on DFT calculations on NiMoS-nanocluster for acridine and carbazole and some of their HDN products. Therefore, while the presence of acridine shows that this largely affects the phenanthrene hydrogenation, it is not acridine itself but rather its hydrogenation products that are the most inhibitory.
Albeit the extent of HDN for acridine was very low, especially at 280 °C and 300 °C; dicyclohexylmethane (DCHM) was detected at all the temperatures (180 m/z in Figures S1 and S2 in Supplementary information), indicating that HDN of acridine does take place. This suggests that the first ring opening (C–N bond split) is likely to be limiting the HDN as neither of the amines are detected.
While DCHM is detected, PH-ACR is found to be the most abundant acridine product, indicating that the position of the nitrogen atom is important. This is supported by findings from Miki and Sugimoto [30], who investigated the HDN of isoquinoline and concluded that it was ten times faster than the HDN of quinoline. This is attributed to the increased rate for the ring opening C–N bond scission reaction for the hydrogenation products of isoquinoline than for quinoline [21]. Similarly, for the HDN of pyridine, the one ring analogue of acridine, protonation can occur and form pyridinium ions. The latter has been shown to be a spectator in the HDN network over a Ni2P and MoS2-based catalyst [31,32,33]. Concluding, when comparing the HDN of carbazole and acridine, Figure 6, it is clear that carbazole undergoes HDN more easily; therefore, it can be concluded that while acridine is very reactive, the C–N bond scission reactions are significantly slower than for carbazole.
2.4. Co-Feeding Organic Nitrogen and Phenanthrene
While the separate addition of carbazole and acridine to the reactant feed is necessary for understanding the effects of the individual components on the hydrogenation of phenanthrene, both of the organic nitrogen types are always present in real mixtures. Therefore, investigating the mixture of carbazole and acridine on the hydrogenation of phenanthrene is also of interest. Figure 9, shows the product distributions from the hydrogenation of phenanthrene at 300 °C in the absence and presence of acridine, as well as in the presence of both acridine and carbazole are shown.
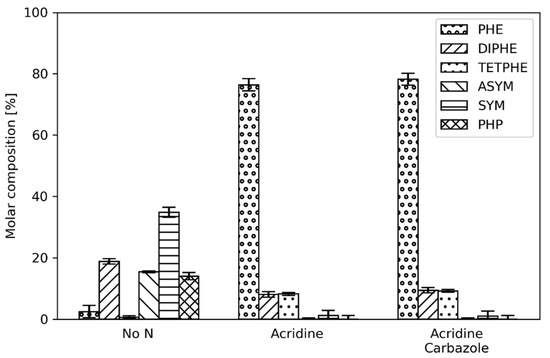
Figure 9.
Phenanthrene product distribution in the presence of 2.4 mmol/dm3 acridine and the combination of acridine and carbazole (2.4 mmol/dm3 of each, i.e., 4.8 mmol/dm3 in total) at a reactor temperature of 300 °C.
As shown, combination of carbazole and acridine in the reactant feed alters the product distribution of phenanthrene, similar to the case of only acridine addition. This indicates that acridine and, more likely, its hydrogenated products are not only inhibiting the hydrogenation of phenanthrene but also the hydrogenation of carbazole. In fact, when comparing the extent of HDN (Table 2) it can be noted that barely any HDN occurred.

Table 2.
The extent of HDN at a reactor temperature of 300 °C with different organic nitrogen compounds present at a concentration of 2.4 mmol/dm3.
This further supports that acridine is the most potent inhibitor and inhibits the conversion of the nonbasic carbazole (HDN) as well as of phenanthrene (HDA) [34,35,36]. This behavior is similar to the observations of Rabarihoela-Rakotovao et al. [27] during HDS of substituted dibenzothiophenes. This supports the plausibility of weak adsorption of nonbasic nitrogen compounds on the active sites as opposed to basic ones, whereas their respective reactivity follows the opposite trend [37]. This is in agreement with the results presented here for HDN of carbazole and acridine; however, carbazole was removed to a large extent when not inhibited by acridine.
Thus, the inhibitory effect of both types of organic nitrogen on the hydrogenation of PACs can be attributed to their higher adsorption strength on the catalyst. This implies competitive adsorption on the catalyst surface and means that the organic nitrogen compounds outcompete phenanthrene for the hydrogenation sites on the catalyst. The case that stands out is when carbazole is added to the reactant mixture at 320 °C; the conversion is indeed lower compared to no nitrogen addition; however, some of PHP is formed, and SYM is the most abundant product once again. This could be related to the HDN conversion of carbazole, where carbazole and its hydrogenated products are almost completely removed. This limits competition for hydrogenation sites between the aromatic hydrocarbons and carbazole (and its hydrogenated products) to only a part of the catalyst bed, resulting in a significantly higher conversion of phenanthrene and formation of deep hydrogenation products, such as PHP. Similar behavior has been observed by Ho [14] while investigating the HDS of 4,6-diethyldibenzothiophene in presence of carbazole. In one of the experiments, the carbazole compound was completely removed but a significant effect on the HDS was still observed; it was suggested that the remaining catalyst bed that is not performing any HDN, accounted for most of the HDS activity. This suggests that ammonia is significantly less inhibitory compared to organic nitrogen compounds contrary to the findings by Beltramone et al. [16]. Furthermore, increasing the temperature does not only increase the activity for the hydrogenation reactions but also lowers the relative coverage of strongly adsorbed compounds [38].
The product distributions of phenanthrene when acridine was present in the reactant stream at 280 °C and 300 °C were quite similar (Figure 4 and Figure 9) with the HDN of acridine essentially negligible at those reactor temperatures (Figure 6). Therefore, the effect of the total nitrogen concentration in the reactant feed was investigated. Figure 10 shows the phenanthrene product distributions at 300 °C with varying total nitrogen concentrations in the reactant feed, where the ratio between carbazole and acridine was kept at 50%.
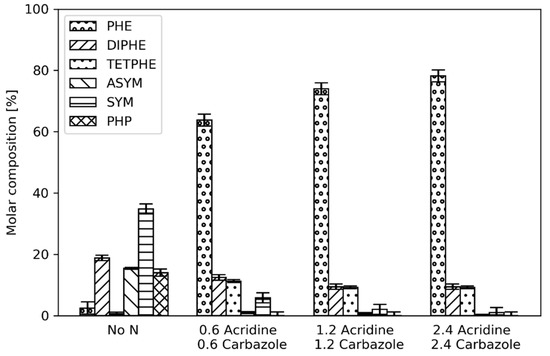
Figure 10.
Phenanthrene product distribution from hydrogenation at 300 °C with varying content of carbazole and acridine in the reactant feed. The number next to the nitrogen compound is the concentration in mmol/dm3.
As shown, the result of varying the total nitrogen concentration did not affect the hydrogenation of phenanthrene to a large extent with the extent of HDN being very low for all cases as mentioned earlier in Table 2. This suggests that when basic nitrogen compounds are present in the reactant feed, they will be the dominating inhibitor not only for the HDA but also HDN of the nonbasic nitrogen compounds. Therefore, in such a case when the HDN rate of basic nitrogen is low, they are most likely the compounds with highest coverage on the surface of the catalyst and significant HDN of nonbasic nitrogen will not occur until the basic nitrogen compounds have been converted.
3. Materials and Methods
3.1. Experimental Setup
The hydrogenation experiments were performed in a high-pressure trickle-bed reactor system, the schematic overview of which is shown in Figure 11. The system consisted of a modified microactivity reactor system (PID Eng&Tech Micromeritics), which included liquid feeding pumps, a trickle-bed microflow reactor, a gas–liquid separator, and an automatic liquid sampling system. The liquid reactant was fed by means of HPLC pumps (Gilson 307 or Gilson 305) at a typical feeding rate of 0.1 cm3/min. High-pressure hydrogen (99.9%, Air Liquide, Paris, France) was used as hydrogenation agent. The liquid and gas reactants were mixed prior to the introduction to the tubular stainless-steel reactor (Sandvik 3R60). The reactor was loaded with silicon carbide (SiC) particles and a sulfided commercial NiMoS hydrotreating catalyst supported on alumina, using a loading scheme that is explained below. The hydrogen feed rate was controlled by a Bronkhorst mass flow controller (EL-FLOW Select F-230M). The reactor was electrically heated with the temperature being monitored by a K-type thermocouple located in the middle of the catalyst bed. Moreover, the reactor tube was encased in an aluminum jacket, which ensured that the catalytic bed was isothermal. The reactor effluent was routed to a stainless-steel gas–liquid separator, where the liquid level was controlled by a valve on the liquid side and determined with a capacitive level sensor. The gaseous effluent passed through a needle-valve, which controlled the system pressure.
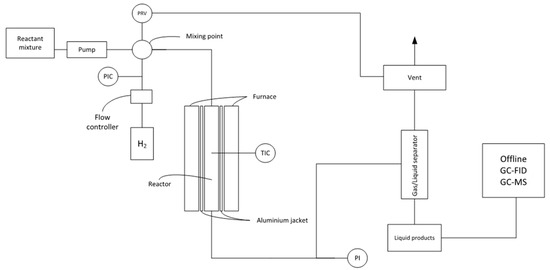
Figure 11.
Schematics of the experimental setup.
A schematic of the reactor loading protocol is shown in Figure 12. The reactor was a 28 cm long stainless-steel tube with an inner diameter of 10 mm. Various sizes of SiC were used to minimize particle slip in the reactor. SiC was used for improving isothermicity of the bed, ensuring complete catalyst irrigation, minimizing wall effects, and reducing axial dispersion [39].
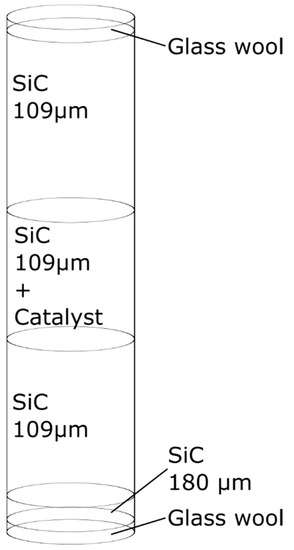
Figure 12.
Schematic of the reactor loading of different SiC sizes (average particle diameter) and catalyst. The bed section that contains catalyst bed is positioned in the middle of the reactor and is approximately 7 cm long.
3.2. Sulfidation Protocol
The catalyst used in the study was a commercial alumina supported NiMo catalyst, received in the metal oxide form with typical metal loadings 8.5–13.5 and 2–2.8 wt% for Mo and Ni, respectively [40,41]. The catalyst was sulfided with a dimethyl disulfide (DMDS, Sigma-Aldrich, St. Louis, MO, USA >99% purity)/decalin (Merck, Readington, NJ, USA, technical grade) solution containing 0.85 mol S/dm3 (approx. 3 wt.% S). The sulfidation was performed at a system pressure of 120 barg, which was also the operating pressure of the hydrogenation experiments. Sulfidation liquid was used for pre-wetting of the catalyst bed for about one hour at a feed rate of 0.3 cm3/min. Thereafter, 90 cm3/min hydrogen was added to the system and the reactor was heated to 150 °C and kept there for two hours followed by a temperature ramp of 2 °C/min to 350 °C and held for 4 h. After sulfidation, the feed to the reactor was changed to typical reactant feed and the temperature lowered to the reaction conditions for stabilization of the catalyst activity.
3.3. Hydrogenation Experiments
To investigate the effect of organic nitrogen on the hydrogenation of phenanthrene, a series of hydrogenation tests were performed. The typical reactant feed consisted of 50 mmol/dm3 phenanthrene (approx. 1 wt.%, Sigma-Aldrich, 98% purity), 85 mmol/dm3 tetradecane (approx. 2 wt.%, Sigma-Aldrich, >99% purity, used as internal standard in offline GC analysis), and 14 mmol/dm3 DMDS (Sigma-Aldrich, >99% purity, equivalent to 940 mg S/dm3) in decahydronaphthalene (or decalin, Merck, technical grade). Moreover, the organic nitrogen compounds used in this study were carbazole (Sigma-Aldrich, 95% purity) and acridine (Sigma-Aldrich, 98% purity). In this study, the catalyst, mass reactor temperature, and organonitrogen concentrations were varied in the range shown in Table 3. The operating pressure of the reactor was 120 barg, and no pressure drop over the catalytic bed was observed. This pressure was chosen to reduce the thermodynamic influence on the hydrogenation reactions of the organonitrogen compounds [42]. In all the experiments, no or negligible deactivation was observed.

Table 3.
Experimental conditions used in this study.
3.4. Analytical Methods
The liquid samples were analyzed offline by an Agilent 7890A gas chromatograph (GC) equipped with a flame ionization detector (FID). The GC-FID was equipped with a 30 m HP-5 column (320 µm internal diameter, 0.25 µm film thickness) and operated in constant flow mode (1.9 cm3/min or 32.7 cm/s) with helium as the carrier gas. The sample inlet was kept at 310 °C. A total of 1 µL samples was injected at a 100:1 split ratio. The GC oven was initially kept at 60 °C for 5 min, followed by a 10 °C/min ramp up to a temperature of 220 °C; thereafter, a 30 °C/min ramp was applied until a final temperature of 310 °C, which it was kept for 5 min. The FID was set to 310 °C, and the gas flows were 35 cm3/min hydrogen, 350 cm3/min of air, and 40 cm3/min of helium as makeup. Standards of phenanthrene were prepared for quantification of phenanthrene and its hydrogenated products. The products had the same response factor as phenanthrene, as described in Korre et al. [17]. Tetradecane was used as the internal standard. Unknown peaks in the GC-FID chromatogram were identified by an Agilent 7890B-5975 MSD GC-MS using NIST libraries. An HP-5ms column and the same temperature programming were used for the GC-MS. The error in the measurement was estimated to be typically less than 2% from a t-test of multiple samples with the same concentration. The total nitrogen content was analyzed using a PAC ANTEK ElemeNtS analyzer, which was calibrated according to ASTM D4629, with readily available standards. This analysis method was based on oxidative combustion and chemiluminescence detection.
4. Conclusions
The hydrogenation of PACs in lubricant feeds in the presence of organonitrogen was investigated in a model system using a microflow trickle-bed reactor loaded with a commercial sulfided alumina supported NiMoS catalyst. The model reaction for PAC conversion was the HDA of phenanthrene, and the basic and nonbasic nitrogen compounds were acridine and carbazole, respectively. At the investigated conditions, phenanthrene was readily hydrogenated to deep hydrogenation products, such as perhydrophenanthrene; however, the addition of organonitrogen in the reactant feed decreased the conversion and significantly altered the product distribution of phenanthrene. Basic nitrogen shows stronger inhibition effects on HDA reactions and undergoes full hydrogenation without significant extent in HDN reactions. Conversely, while also inhibitory, nonbasic nitrogen (carbazole) did not have as significant effect on the phenanthrene hydrogenation and underwent HDN to a larger extent when compared to basic nitrogen (acridine). Co-presence of both types of nitrogen compounds resembles the behavior of the basic nitrogen with minor alternation in phenanthrene hydrogenation products; the HDN rates are diminished and the organonitrogen compounds completely dominate the coverage of the catalyst surface. Even though the total nitrogen concentration has an impact on the overall HDA product distribution, the overall HDA conversion and its relative rates of the individual hydrogenation steps are greatly affected by the relative ratios of organonitrogen type, with the basic organonitrogen compounds strongly affecting the product distribution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12070736/s1, Table S1: Modified equilibrium constants for the phenanthrene hydrogenation reaction 1 and 2. Table S2: Modified equilibrium constants for the two first hydrogenation steps for carbazole HDN. Figure S1: Chromatogram for the 180 m/z in the liquid product when acridine was present in the reactant feed; 180 is the qualifying ion for DCHM. Figure S2: Ion spectrum for the peak containing 180 m/z, from the experiment with a reactor temperature of 320 °C and acridine is in the reactant feed. References [18,25] are cited in the supplementary materials.
Author Contributions
J.V.B. was responsible for conceptualization, methodology, experimentation, investigation, formal analysis, data curation, writing—original draft preparation, writing—review and editing. E.K. and S.L.H. were responsible for original draft preparation, writing—review and editing and supervision. L.J.P. was responsible for original draft preparation, writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Nynas AB.
Data Availability Statement
All the reported data is available in the main text and supporting information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Furimsky, E.; Massoth, F.E. Deactivation of hydroprocessing catalysts. Catal. Today 1999, 52, 381–495. [Google Scholar]
- Sau, M.; Basak, K.; Manna, U.; Santra, M.; Verma, R.P. Effects of organic nitrogen compounds on hydrotreating and hydrocracking reactions. Catal. Today 2005, 109, 112–119. [Google Scholar]
- Zeuthen, P.; Knudsen, K.G.; Whitehurst, D.D. Organic nitrogen compounds in gas oil blends, their hydrotreated products and the importance to hydrotreatment. Catal. Today 2001, 65, 307–314. [Google Scholar]
- Yang, H.; Wang, Y.; Jiang, H.; Weng, H.; Liu, F.; Li, M. Kinetics of phenanthrene hydrogenation system over CoMo/Al2O3 catalyst. Ind. Eng. Chem. Res. 2014, 53, 12264–12269. [Google Scholar]
- Zhang, D.; Zhao, J.; Zhang, Y.; Lu, X. Catalytic hydrogenation of phenanthrene over NiMo/Al2O3 catalysts as hydrogen storage intermediate. Int. J. Hydrog. Energy 2016, 41, 11675–11681. [Google Scholar]
- Wang, K.; Guan, J.; He, D.M.; Zhang, Q.M. The influences of reaction conditions on phenanthrene hydrogenation over NiW/Al2O3 catalyst. Adv. Mater. Res. 2012, 512–515, 2200–2206. [Google Scholar]
- Schachtl, E.; Yoo, J.S.; Gutiérrez, O.Y.; Studt, F.; Lercher, J.A. Impact of ni promotion on the hydrogenation pathways of phenanthrene on MoS2/γ-Al2O3. J. Catal. 2017, 352, 171–181. [Google Scholar]
- Celis-Cornejo, C.M.; Pérez-Martínez, D.J.; Orrego-Ruiz, J.A.; Baldovino-Medrano, V.G. Identification of refractory weakly basic nitrogen compounds in a deeply hydrotreated vacuum gas oil and assessment of the effect of some representative species over the performance of a Ni–MoS2/y-zeolite–alumina catalyst in phenanthrene hydrocracking. Energy Fuels 2018, 32, 8715–8726. [Google Scholar]
- Morales-Valencia, E.M.; Castillo-Araiza, C.O.; Giraldo, S.A.; Baldovino-Medrano, V.G. Kinetic assessment of the simultaneous hydrodesulfurization of dibenzothiophene and the hydrogenation of diverse polyaromatic structures. ACS Catal. 2018, 8, 3926–3942. [Google Scholar]
- Vogelgsang, F.; Shi, H.; Lercher, J.A. Toward quantification of active sites and site-specific activity for polyaromatics hydrogenation on transition metal sulfides. J. Catal. 2021, 403, 98–110. [Google Scholar]
- Schachtl, E.; Zhong, L.; Kondratieva, E.; Hein, J.; Gutiérrez, O.Y.; Jentys, A.; Lercher, J.A. Understanding Ni promotion of MoS2/γ-Al2O3 and its implications for the hydrogenation of phenanthrene. ChemCatChem 2015, 7, 4118–4130. [Google Scholar]
- Ishihara, A.; Lee, J.; Dumeignil, F.; Takashi, M.; Qian, E.W.; Kabe, T. Elucidation of retarding effects of sulfur and nitrogen compounds on aromatic compounds hydrogenation. Energy Fuels 2003, 17, 1338–1345. [Google Scholar]
- Koltai, T.; Macaud, M.; Guevara, A.; Schulz, E.; Lemaire, M.; Bacaud, R.; Vrinat, M. Comparative inhibiting effect of polycondensed aromatics and nitrogen compounds on the hydrodesulfurization of alkyldibenzothiophenes. Appl. Catal. A Gen. 2002, 231, 253–261. [Google Scholar]
- Ho, T. Inhibiting effects in hydrodesulfurization of 4,6-diethyldibenzothiophene. J. Catal. 2003, 219, 442–451. [Google Scholar]
- Ho, T. Poisoning effect of ethylcarbazole on hydrodesulfurization of 4,6-diethyldibenzothiophene. J. Catal. 2004, 222, 450–460. [Google Scholar]
- Beltramone, A.R.; Crossley, S.; Resasco, D.E.; Alvarez, W.E.; Choudhary, T.V. Inhibition of the hydrogenation and hydrodesulfurization reactions by nitrogen compounds over NiMo/Al2O3. Catal. Lett. 2008, 123, 181–185. [Google Scholar]
- Korre, S.C.; Klein, M.T.; Quann, R.J. Polynuclear aromatic hydrocarbons hydrogenation. 1. Experimental reaction pathways and kinetics. Ind. Eng. Chem. Res. 1995, 34, 101–117. [Google Scholar]
- Frye, C.G. Equilibria in the hydrogenation of polycyclic aromatics. J. Chem. Eng. Data 1962, 7, 592–595. [Google Scholar]
- Korre, S.C.; Neurock, M.; Klein, M.T.; Quann, R.J. Hydrogenation of polynuclear aromatic hydrocarbons. 2. Quantitative structure/reactivity correlations. Chem. Eng. Sci. 1994, 49, 4191–4210. [Google Scholar]
- Bachrach, M.; Marks, T.J.; Notestein, J.M. Understanding the hydrodenitrogenation of heteroaromatics on a molecular level. ACS Catal. 2016, 6, 1455–1476. [Google Scholar]
- Prins, R. Catalytic hydrodenitrogenation. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2001; Volume 46, pp. 399–464. [Google Scholar]
- Bello, S.S.; Wang, C.; Zhang, M.; Gao, H.; Han, Z.; Shi, L.; Su, F.; Xu, G. A review on the reaction mechanism of hydrodesulfurization and hydrodenitrogenation in heavy oil upgrading. Energy Fuels 2021, 35, 10998–11016. [Google Scholar]
- Furimsky, E.; Massoth, F.E. Hydrodenitrogenation of petroleum. Catal. Rev. 2005, 47, 297–489. [Google Scholar]
- Bowker, R.H.; Ilic, B.; Carrillo, B.A.; Reynolds, M.A.; Murray, B.D.; Bussell, M.E. Carbazole hydrodenitrogenation over nickel phosphide and Ni-rich bimetallic phosphide catalysts. Appl. Catal. A Gen. 2014, 482, 221–230. [Google Scholar]
- Steele, W.V.; Chirico, R.D. Thermodynamics of the Hydrodenitrogenation of Carbazole; National Institute for Petroleum and Energy Research: Bartlesville, OK, USA; 43p, p. 43.
- Rabarihoela-Rakotovao, V.; Brunet, S.; Berhault, G.; Perot, G.; Diehl, F. Effect of acridine and of octahydroacridine on the HDS of 4,6-dimethyldibenzothiophene catalyzed by sulfided NiMop/Al2O3. Appl. Catal. A Gen. 2004, 267, 17–25. [Google Scholar]
- Rabarihoela-Rakotovao, V.; Diehl, F.; Brunet, S. Deep HDS of diesel fuel: Inhibiting effect of nitrogen compounds on the transformation of the refractory 4,6-dimethyldibenzothiophene over a NiMoP/Al2O3 catalyst. Catal. Lett. 2008, 129, 50–60. [Google Scholar]
- Egorova, M.; Prins, R. Competitive hydrodesulfurization of 4,6-dimethyldibenzothiophene, hydrodenitrogenation of 2-methylpyridine, and hydrogenation of naphthalene over sulfided NiMo/γ-Al2O3. J. Catal. 2004, 224, 278–287. [Google Scholar]
- Liu, X.; Fan, X.; Wang, L.; Sun, J.; Wei, Q.; Zhou, Y.; Huang, W. Competitive adsorption between sulfur- and nitrogen-containing compounds over nimos nanocluster: The correlations of electronegativity, morphology and molecular orbital with adsorption strength. Chem. Eng. Sci. 2021, 231, 116313. [Google Scholar]
- Miki, Y.; Sugimoto, Y. Hydrodenitrogenation of isoquinoline. Appl. Catal. A Gen. 1999, 180, 133–140. [Google Scholar]
- Gott, T.; Oyama, S.T. A general method for determining the role of spectroscopically observed species in reaction mechanisms: Analysis of coverage transients (act). J. Catal. 2009, 263, 359–371. [Google Scholar]
- Temel, B.; Tuxen, A.K.; Kibsgaard, J.; Topsøe, N.-Y.; Hinnemann, B.; Knudsen, K.G.; Topsøe, H.; Lauritsen, J.V.; Besenbacher, F. Atomic-scale insight into the origin of pyridine inhibition of MoS2-based hydrotreating catalysts. J. Catal. 2010, 271, 280–289. [Google Scholar]
- Humbert, S.; Izzet, G.; Raybaud, P. Competitive adsorption of nitrogen and sulphur compounds on a multisite model of NiMoS catalyst: A theoretical study. J. Catal. 2016, 333, 78–93. [Google Scholar]
- Boesen, R.R. Investigation and Modelling of Diesel Hydrotreating Reactions; Technical University of Denmark: Kgs. Lyngby, Denmark, 2011. [Google Scholar]
- Ferdous, D.; Dalai, A.K.; Adjaye, J. Comparison of hydrodenitrogenation of model basic and nonbasic nitrogen species in a trickle bed reactor using commercial NiMo/Al2O3 catalyst. Energy Fuels 2003, 17, 164–171. [Google Scholar]
- Nguyen, M.-T.; Pirngruber, G.D.; Chainet, F.; Tayakout-Fayolle, M.; Geantet, C. Indole hydrodenitrogenation over alumina and silica–alumina-supported sulfide catalysts—Comparison with quinoline. Ind. Eng. Chem. Res. 2017, 56, 11088–11099. [Google Scholar]
- Liu, X.; Ding, S.; Wei, Q.; Zhou, Y.; Zhang, P.; Xu, Z. DFT insights in to the hydrodenitrogenation behavior differences between indole and quinoline. Fuel 2021, 285, 119039. [Google Scholar]
- Sun, M.; Nelson, A.E.; Adjaye, J. Adsorption thermodynamics of sulfur- and nitrogen-containing molecules on NiMoS: A dft study. Catal. Lett. 2006, 109, 133–138. [Google Scholar]
- Sie, S.T.; Krishna, R. Process development and scale up: III. Scale-up and scale-down of trickle bed processes. Rev. Chem. Eng. 1998, 14, 203–252. [Google Scholar]
- Bartholomew, C.H.; Farrauto, R.J. Petroleum refining and processing. In Fundamentals of Industrial Catalytic Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 635–704. [Google Scholar]
- Eijsbouts, S.; van den Oetelaar, L.C.A.; Rayner, M.; Govaers, H.; Boonen, T. Combined HR TEM and STEM-EDX evaluation—The key to better understanding of the Co-Mo sulfide active phase in real-life Co-Mo-P/Al2O3 catalysts. J. Catal. 2021, 403, 56–73. [Google Scholar]
- Machida, M.; Sakao, Y.; Ono, S. Influence of hydrogen partial pressure on hydrodenitrogenation of pyridine, aniline and quinoline. Appl. Catal. A Gen. 1999, 187, L73–L78. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).