Highly Efficient Decarboxylation of L-Lysine to Cadaverine Catalyzed by RuO2 Encapsulated in FAU Zeolite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization for RuO2@FAU Catalysts
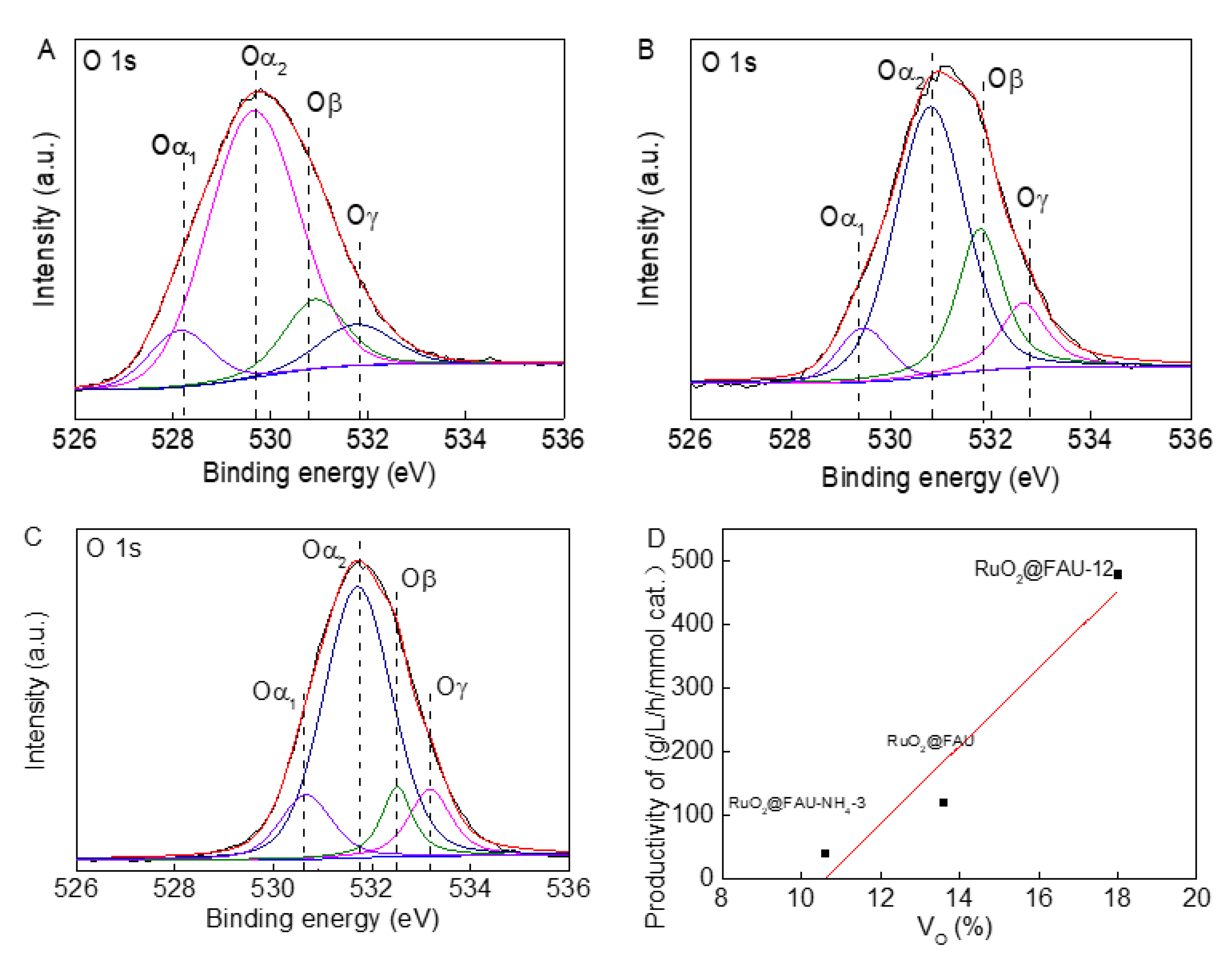
2.2. Synthesis and Characterization of Modified RuO2@FAU Catalysts
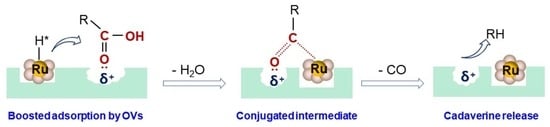
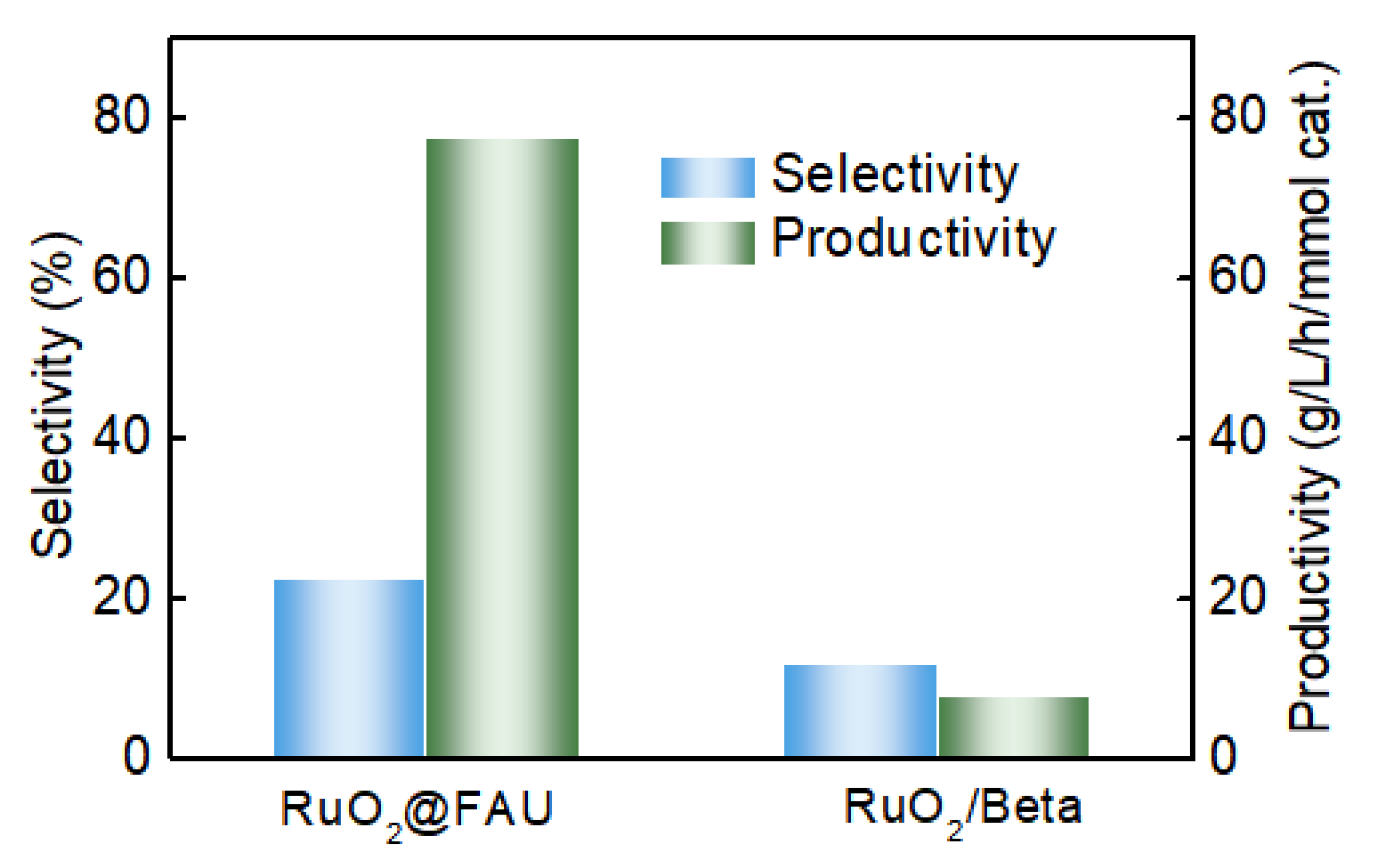
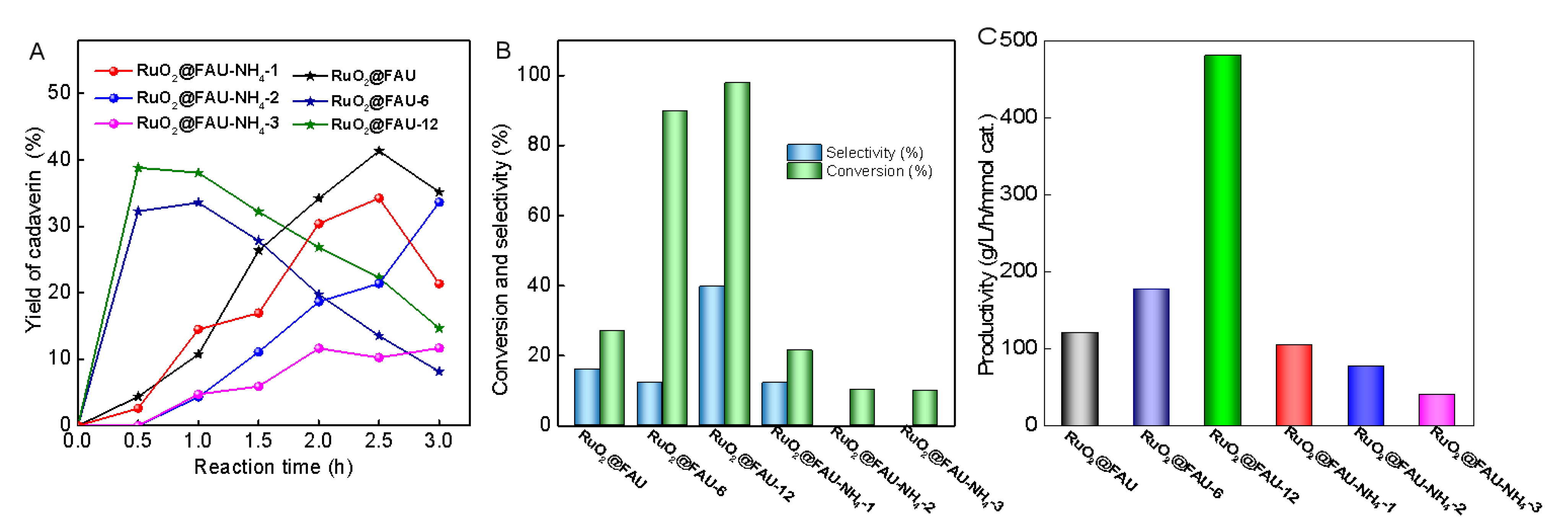
2.3. Enhanced Performance in Decarboxylation of L-Lysine to Cadaverine
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of RuO2@FAU
3.3. Synthesis of RuO2@FAU with Different Si/Al Ratio
3.4. Modification of RuO2@FAU
3.5. Characterizations
3.6. Evaluation in Decarboxylation of L-Lysine to Cadaverine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, P.; Li, X.; Liu, H.; Li, Z.; Liu, J.; Zhuang, W.; Wu, J.; Ying, H. Thermodynamics, crystal structure, and characterization of a bio-based nylon 54 monomer. CrystEngComm 2019, 21, 7069–7077. [Google Scholar] [CrossRef]
- Qian, Z.G.; Xia, X.X.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of cadaverine: A five carbon diamine. Biotechnol. Bioeng. 2011, 108, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Scott, E.L.; Zeeland, T.A.; Sanders, J.P.M. The use of l-lysine decarboxylase as a means to separate amino acids by electrodialysis. Green Chem. 2011, 13, 624–630. [Google Scholar] [CrossRef]
- Kwak, D.H.; Lim, H.G.; Yang, J.; Seo, S.W.; Jung, G.Y. Synthetic redesign of Escherichia coli for cadaverine production from galactose. Biotechnol. Biofuels 2017, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Kind, S.; Wittmann, C. Bio-based production of the platform chemical 1,5-diaminopentane. Appl. Microbial. Biot. 2011, 91, 1287–1296. [Google Scholar] [CrossRef]
- Moon, Y.M.; Yang, S.Y.; Choi, T.R.; Jung, H.R.; Song, H.S.; Han, Y.H.; Park, H.Y.; Bhatia, S.K.; Gurav, R.; Park, K.; et al. Enhanced production of cadaverine by the addition of hexadecyltrimethylammonium bromide to whole cell system with regeneration of pyridoxal-5′-phosphate and ATP. Enzym. Microb. Technol. 2019, 127, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhao, Y.; Ji, X.; Yao, J.; Busk, P.K.; Lange, L.; Huang, Y.; Zhang, S. Advances in bio-nylon 5X: Discovery of new lysine decarboxylases for the high-level production of cadaverine. Green Chem. 2020, 22, 8656–8668. [Google Scholar] [CrossRef]
- Schouwer, F.; Claes, L.; Claes, N.; Bals, S.; Degreve, J.; De Vos, D.E. Pd-catalyzed decarboxylation of glutamic acid and pyroglutamic acid to bio-based 2-pyrrolidone. Green Chem. 2015, 17, 2263–2270. [Google Scholar] [CrossRef]
- Verduyckt, J.; Van Hoof, M.; Schouwer, F.; Wolberg, M.; Kurttepeli, M.; Eloy, P.; Gaigneaux, E.M.; Bals, S.; Kirschhock, C.E.A.; De Vos, D.E. PdPb-catalyzed decarboxylation of proline to pyrrolidine: Highly selective formation of a biobased amine in water. ACS Catal. 2016, 6, 7303–7310. [Google Scholar] [CrossRef] [Green Version]
- Verduyckt, J.; Coeck, R.; De Vos, D.E. Ru-Catalyzed Hydrogenation–Decarbonylation of Amino Acids to Bio-based Primary Amines. ACS Sustain. Chem. Eng. 2017, 5, 3290–3295. [Google Scholar] [CrossRef]
- Xie, S.; Jia, C.; Wang, Z.; Ong, S.S.G.; Zhu, M.J.; Lin, H. Mechanistic Insight into Selective Deoxygenation of l-Lysine to Produce Biobased Amines. ACS Sustain. Chem. Eng. 2020, 8, 11805–11817. [Google Scholar] [CrossRef]
- Lv, X.; Ma, Z.; Li, X.; Zhang, Y.; Huang, Y.; Li, T. Highly efficient decarboxylation of l-lysine to cadaverine catalyzed by supported ruthenium oxide. Catal. Commun. 2021, 158, 106339. [Google Scholar] [CrossRef]
- Ma, Z.; Xin, Z.; Qin, S.; Huang, Y. Mn-Doped Highly Dispersed RuO2 Catalyst with Abundant Oxygen Vacancies for Efficient Decarboxylation of l-Lysine to Cadaverine. ACS Sustain. Chem. Eng. 2021, 9, 13480–13490. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Zhao, S.; Chen, W.; Shi, L.; Wang, Y.; Dong, J.; Li, Z.; Li, F.; Chen, C.; et al. Atomically Dispersed Ruthenium Species Inside Metal-Organic Frameworks: Combining the High Activity of Atomic Sites and the Molecular Sieving Effect of MOFs. Angew. Chem. Int. Ed. 2019, 58, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, R.; Pan, X.; Wang, C.; Fan, M.; Zhu, Y.; Wang, Y.; Peng, J. Catalyst design strategies towards highly shape-selective HZSM-5 for para-xylene through toluene alkylation. Green Energy Environ. 2020, 5, 385–393. [Google Scholar] [CrossRef]
- Zhan, B.Z.; Iglesia, E. RuO2 Clusters within LTA Zeolite Cages: Consequences of Encapsulation on Catalytic Reactivity and Selectivity. Angew. Chem. 2007, 119, 3771–3774. [Google Scholar] [CrossRef]
- Altwasser, S.; Glaser, R.; Lo, A.S.; Liu, P.H.; Chao, K.J. Incorporation of RuO2 nanoparticles into MFI-type zeolites. Micropor. Mesopor. Mater. 2006, 89, 109–122. [Google Scholar] [CrossRef]
- Zhan, B.; White, M.A.; Sham, T.; Pincock, J.A.; Doucet, R.J.; Rao, K.V.R.; Robertson, K.N.; Cameron, T.S. Zeolite-Confined Nano-RuO2: A Green, Selective, and Efficient Catalyst for Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2003, 125, 2195–2199. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; He, J.; Liu, Y.; Geng, H.; Chen, S.; Lin, L.; Liu, M.; Chen, T.; Jiang, Q.; et al. Enhanced catalytic performance through in situ encapsulation of ultrafine Ru clusters within a high-aluminum zeolite. ACS Catal. 2022, 12, 1847–1856. [Google Scholar] [CrossRef]
- Xiong, P.; He, P.; Qu, Y.X.; Wang, L.G.; Cao, Y.; Xu, S.; Chen, J.Q.; Ammar, M.; Li, H.Q. The adsorption properties of NaY zeolite for separation of ethylene glycol and 1,2-butanediol: Experiment and molecular modelling. Green Energy Environ. 2021, 6, 102–113. [Google Scholar] [CrossRef]
- Devadas, A.; Baranton, S.; Coutanceau, C. Green synthesis and modification of RuO2 materials for the oxygen evolution reaction. Front. Energy Res. 2020, 8, 571704. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zhou, W.; Zhang, X.; Zhang, B.; Yu, Y. Structurally disordered RuO2 nanosheets with rich oxygen vacancies for enhanced nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. 2022, 61, e202202604. [Google Scholar]
- Chen, L.; Li, Y.; Zhang, X.; Zhang, Q.; Wang, T.; Ma, L. Mechanistic insights into the effects of support on the reaction pathway for aqueous-phase hydrogenation of carboxylic acid over the supported Ru catalysts. Appl. Catal. A Gen. 2014, 478, 117–128. [Google Scholar] [CrossRef]
- Lv, L.; Wang, S.; Ding, Y.; Zhang, L.; Gao, Y.; Wang, S. Mechanistic insights into the contribution of Lewis acidity to brominated VOCs combustion over titanium oxide supported Ru catalyst. Chemosphere 2021, 263, 128112. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Browne, M.P.; Nolan, H.; Duesberg, G.S.; Colavita, P.E.; Lyons, M.E.G. Low-overpotential high-activity mixed manganese and ruthenium oxide electrocatalysts for oxygen evolution reaction in alkaline media. ACS Catal. 2016, 6, 2408–2415. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Ji, C.; Sun, Y.; Wang, Y.; Zhao, Z.; Lv, Z. Surface basicity induced RuO2 modification on nanosized Li-rich cathode Li1.26Fe0.22Mn0.52O2 with superior electrochemical performance. J. Electrochem. Soc. 2016, 163, A2040. [Google Scholar] [CrossRef]
- Qadir, K.; Joo, S.H.; Mun, B.S.; Butcher, D.R.; Renzas, J.R.; Aksoy, F.; Liu, Z.; Somorjai, G.A.; Park, J.Y. Intrinsic relation between catalytic activity of CO oxidation on Ru nanoparticles and Ru oxides uncovered with ambient pressure XPS. Nano Lett. 2012, 12, 5761–5768. [Google Scholar] [CrossRef]
- Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Huang, K.; Zu, D.; et al. Tuning defects in oxides at room temperature by lithium reduction. Nat. Commun. 2018, 9, 1302. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Sun, X.; Wu, B.; Li, B.; Ning, Z.; Li, J.; Wang, N. Ru/RuO2 nanoparticle composites with N-doped reduced graphene oxide as electrocatalysts for hydrogen and oxygen evolution. ACS Appl. Nano Mater. 2020, 3, 12269–12277. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, P.; Zhang, M.; Zhou, T.; Zhang, L.; Chu, W.; Wu, C.; Xie, Y. Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. ACS Catal. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Weitkamp, J.; Ernst, S.; Hunger, M.; Roser, T.; Huber, S.; Schubert, U.A.; Thomasson, P.; Knozinger, H. Solid state ion exchange of alkali metal cations into zeolite Y: Physicochemical characterization and catalytic tests. Stud. Surf. Sci. Catal. 1996, 101, 731–740. [Google Scholar]
- Marra, G.L.; Fitch, A.N.; Zecchina, A.; Ricchiardi, G.; Salvalaggio, M.; Bordiga, S.; Lamberti, C. Cation location in dehydrated Na–Rb–Y zeolite: An XRD and IR study. J. Phys. Chem. B 1997, 101, 10653–10660. [Google Scholar] [CrossRef]
- Li, J.; Davis, R.J. Raman spectroscopy and dioxygen adsorption on Cs-loaded zeolite catalysts for butene isomerization. J. Phys. Chem. B 2005, 109, 7141–7148. [Google Scholar] [CrossRef]
- Ristic, M.; Marcius, M.; Petrovic, Z.; Ivanda, M.; Music, S. Formation and characterization of ribbon-like RuO2/Ru fibers. Mater. Lett. 2015, 156, 142–145. [Google Scholar] [CrossRef]
- López, N.; Gómez-Segura, J.; Marín, R.P.; Pérez-Ramírez, J. Mechanism of HCl oxidation (Deacon process) over RuO2. J. Catal. 2008, 255, 29–39. [Google Scholar] [CrossRef]
- Anjum, S.M.; Riazunnisa, K. Fine ultra-small ruthenium oxide nanoparticle synthesis by using Catharanthus roseus and Moringa oleifera leaf extracts and their efficacy towards in vitro assays, antimicrobial activity and catalytic: Adsorption kinetic studies using methylene blue dye. J. Clust. Sci. 2022, 33, 1103–1117. [Google Scholar] [CrossRef]
- Bepari, S.; Li, X.; Abrokwah, R.; Mohammad, N.; Arslan, M.; Kuila, D. Co-Ru catalysts with different composite oxide supports for Fischer–Tropsch studies in 3D-printed stainless steel microreactors. Appl. Catal. A Gen. 2020, 608, 117838. [Google Scholar] [CrossRef]
- Prasanth, K.P.; Pillai, R.S.; Peter, S.A.; Bajaj, H.C.; Jasra, R.V.; Chung, H.D.; Kim, T.H.; Song, S.D. Hydrogen uptake in palladium and ruthenium exchanged zeolite X. J. Alloy. Compd. 2008, 466, 439–446. [Google Scholar] [CrossRef]
- Cao, X.; Long, F.; Zhai, Q.; Liu, P.; Xu, J.; Jiang, J. Enhancement of fatty acids hydrodeoxygenation selectivity to diesel-range alkanes over the supported Ni-MoOx catalyst and elucidation of the active phase. Renew. Energy 2020, 162, 2113–2125. [Google Scholar] [CrossRef]
- Wang, J.; Fan, C.; Sun, Q.; Reuter, K.; Jacobi, K.; Scheffler, M.; Ertl, G. Surface coordination chemistry: Dihydrogen versus hydride complexes on RuO2(110). Angew. Chem. Int. Ed. 2003, 42, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Seitsonen, A.P.; Over, H. The atomic geometry of oxygen-rich Ru(0001) surfaces: Coexistence of (1 × 1)O and RuO2(110) domains. Surf. Sci. 2000, 465, 1–8. [Google Scholar] [CrossRef]
- Over, H.; Seitsonen, A.P.; Lundgren, E.; Wiklund, M.; Andersen, J.N. Spectroscopic characterization of catalytically active surface sites of a metallic oxide. Chem. Phys. Lett. 2001, 342, 467–472. [Google Scholar] [CrossRef]
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, NY, USA, 1994. [Google Scholar] [CrossRef]
- Sun, Q.; Reuter, K.; Scheffler, M. Hydrogen adsorption on RuO2(110): Density-functional calculations. Phys. Rev. B 2004, 70, 235402. [Google Scholar] [CrossRef]
- Liang, J.; Chen, T.; Liu, J.; Zhang, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Chemoselective hydrodeoxygenation of palmitic acid to diesel-like hydrocarbons over Ni/MoO2@ Mo2CTx catalyst with extraordinary synergic effect. Chem. Eng. J. 2020, 391, 123472. [Google Scholar] [CrossRef]
- Feng, W.; Yu, M.; Wang, L.; Miao, Y.; Shakouri, M.; Ran, J.; Hu, Y.; Li, Z.; Huang, R.; Lu, Y.; et al. Insights into bimetallic oxide synergy during carbon dioxide hydrogenation to methanol and dimethyl ether over GaZrOx oxide catalysts. ACS Catal. 2021, 11, 4704–4711. [Google Scholar] [CrossRef]






| Catalysts | Ru/wt% | SBET (m2·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) | Vtotal (cm3·g−1) | Total Acid Content (mmol/g) |
|---|---|---|---|---|---|---|
| FAU | - | 506 | 0.217 | 0.013 | 0.23 | 13.53 |
| RuO2@FAU | 1.48 | 424 | 0.177 | 0.104 | 0.281 | 9.91 |
| RuO2@FAU-6 | 3.72 | 446 | 0.211 | 0.059 | 0.27 | 9.84 |
| RuO2@FAU-12 | 1.58 | 493 | 0.235 | 0.04 | 0.275 | 8.29 |
| RuO2@FAU-NH4-1 | 2.22 | 265 | 0.09 | 0.148 | 0.238 | 9.77 |
| RuO2@FAU-NH4-2 | 1.93 | 259 | 0.088 | 0.159 | 0.247 | 11.54 |
| RuO2@FAU-NH4-3 | 1.96 | 243 | 0.063 | 0.16 | 0.223 | 13.56 |
| Catalyst | C a/mol/L | nRu/mmol | S b/% | t c/min | P d/g/L/h/mmol cat. | Ref |
|---|---|---|---|---|---|---|
| RuO2/BaSO4 | 0.01 | 0.05 | 62.0 | 30 | 21.7 | [12] |
| Ru/C | 0.1 | 0.015 | 46.6 | 120 | 161.6 | [10,11] |
| Ru-Mn/Beta | 0.1 | 0.05 | 54.1 | 90 | 73.0 | [13] |
| RuO2@FAU-12 | 0.1 | 0.016 | 39.7 | 30 | 480.3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Xin, Z.; Qin, S.; Huang, Y. Highly Efficient Decarboxylation of L-Lysine to Cadaverine Catalyzed by RuO2 Encapsulated in FAU Zeolite. Catalysts 2022, 12, 733. https://doi.org/10.3390/catal12070733
Ma Z, Xin Z, Qin S, Huang Y. Highly Efficient Decarboxylation of L-Lysine to Cadaverine Catalyzed by RuO2 Encapsulated in FAU Zeolite. Catalysts. 2022; 12(7):733. https://doi.org/10.3390/catal12070733
Chicago/Turabian StyleMa, Zhanling, Zongwu Xin, Shaojie Qin, and Yuhong Huang. 2022. "Highly Efficient Decarboxylation of L-Lysine to Cadaverine Catalyzed by RuO2 Encapsulated in FAU Zeolite" Catalysts 12, no. 7: 733. https://doi.org/10.3390/catal12070733
APA StyleMa, Z., Xin, Z., Qin, S., & Huang, Y. (2022). Highly Efficient Decarboxylation of L-Lysine to Cadaverine Catalyzed by RuO2 Encapsulated in FAU Zeolite. Catalysts, 12(7), 733. https://doi.org/10.3390/catal12070733