Abstract
Copper-catalyzed enantioselective borylative cyclization with various electrophiles via difunctionalization of unsaturated hydrocarbons is a powerful tool for the generation of interesting boron-containing carbocycles and heterocycles processes involving a chiral organocopper intermediate. Alkenes, allenes, and alkynes are versatile and easily accessible substrates that can be subjected to a wide range of reactions to produce densely functionalized, enantioenriched products. In this chapter, I discuss copper-catalyzed alkenes, allenes, and alkynes borofunctionalization and enantioselective cyclization via chiral organocopper intermediate. Copper-catalyzed enantioselective borylative cyclization and regiodivergent functionalization of alkenes, allenes, and alkynes, as well as the current mechanistic understanding of such processes, are given special attention in this review.
1. Introduction
Copper-catalyzed diastereo- and enantioselective borylative coupling represent a powerful tool to build complex, functionalized molecular architecture rapidly and efficiently from simple precursors in modern organic chemistry [1,2,3,4,5]. These methods have found a sustainable future in catalytic reaction for exploring new regions of chemical space including step- and atom-economical processes [6]. In the past few decades, numerous transition metal catalysts have been utilized for the functionalization of alkenes [7,8], alkynes [9], allenes, [10] and many other hydrocarbons [11] for dense functionalization of the desired chemical reaction delivering high-value, multifunctionalized, enantioenriched, stereodefined products [12]. In recent years, copper catalysts have been shown to achieve this goal in this field because they are more abundant and less toxic than other noble transition metal catalysts [13,14]. Copper-catalyzed functionalization of unsaturated hydrocarbons has proven to be an effective method [15]. Indeed, a wide range of unsaturated hydrocarbons is suitable for access to functionalized organocopper reagents, resulting in products with widely disubstituted functional molecules [16]. Alkenes, alkynes, and enynes, in particular, are extremely versatile and robust feedstocks. These chemicals have been used in a variety of effective catalytic applications to produce a wide range of useful densely bifunctionalized products. In particular, an enantioselective intramolecular borylative cyclization of a diverse range of hydrocarbons with various electrophiles such as carbonyl, imine, and electron-withdrawing alkene for the construction of boron-containing carbocycles and heterocycles using copper catalysis is emerging as a key theme in modern synthesis [10,15,17]. Since the first report of copper-catalyzed borylative intramolcular cyclization of allylic carbonates by Ito and Sawamura [18], the cyclization reaction has grown in popularity and been extensively studied [19]. The aim of this review is to highlight the recent development of copper-catalyzed borylative cyclization of unsaturated hydrocarbons with various electrophiles (Figure 1).
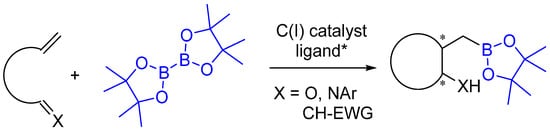
Figure 1.
Copper-catalyzed intramolecular borylative coupling. (ligand* = chiral ligand).
2. Copper-Catalyzed Intramolecular Borylative Coupling with Imines
2.1. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Imines
The copper-catalyzed enantioselective borylative cyclization of aldimine derivative of styrenes was disclosed by You and co-workers in 2018, affording 2,3-disubstituted indolines [20]. Following this report, Xiong groups simultaneously developed a similar enantioselective intramolecular borylative coupling of aldimine of 2-aminostyrene derivatives 1 to access boron-containing indolines [21]. A broad range of styrene derivatives were reacted smoothly. A general mechanism for borylative reactions was shown in Scheme 1C. Initially, the active species (L1Cu-Bpin) is generated after reaction with B2pin2, ligand and base. Then, the active species (L1Cu-Bpin) react with styrene to furnish an organocopper intermediate 1a, which equilibrium to 1a’ followed by intramolecular cyclization gives boron-containing 2,3-disubstituted indolines. The active catalyst L1Cu-Bpin is regenerated in presence of base and B2pin2. An enantioselective coupling reaction was reported by both groups using (S,S)-Ph-BPE as a chiral ligand. A variety of aryl substituted imines were well tolerated and gave high enantioselectivities (Scheme 1B). This methodology offers a simple way to potentially lead regio-, diastereo-, and enantioselective chiral 2,3-disubstituted indolines with the versatile boron functional group. At nearly the same time, Shen, Xu, and co-workers unveiled another borylative diastereoselective coupling of aldimine of 2-aminostyrene derivatives using dppf L2 as a ligand (Scheme 2) [22]. The ligand dppf was found to be the best for diastereoselective reaction. Borylative indoles were used for further application to access indoline-derived scaffolds. Interestingly, cis-tetrahydroindenoindole was synthesized through a palladium-catalyzed intramolecular Suzuki coupling of 2-bromoaryl part of cis-2,3 disubstituted indolines.
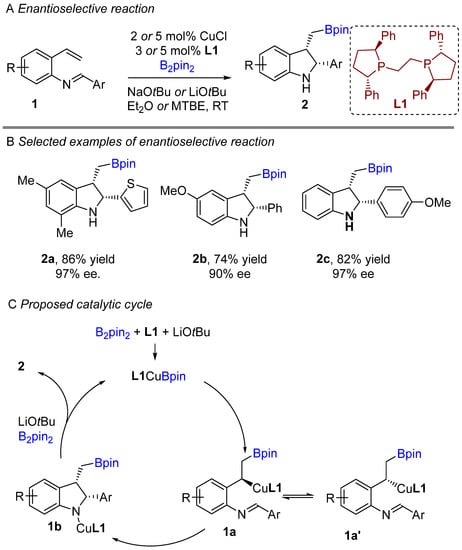
Scheme 1.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of 2,3-disubstituted indolines.
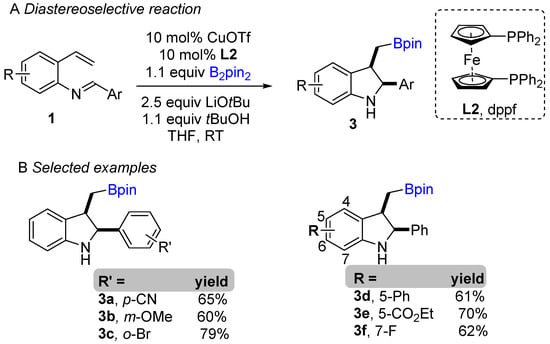
Scheme 2.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of 2,3-disubstituted indolines.
2.2. Copper-Catalyzed Intramolecular Borylative Cyclization with 1,3-Dienes and Imines
Following previous works on copper-catalyzed intramolecular cyclization alkenes, the Yun group reported a graceful intramolecular copper-catalyzed borylative coupling of 1-dienylarenes 4 with tethered imines in 2018 (Scheme 3A) [23]. A range of aryl and heterocyclic aldimines as well as various ketimines were well tolerated, providing rapid access to 2,3-disubstituted cis-1-benzo[b]azepines with high diastereoselectivity. Interestingly, a mixture of (E/Z)-dienyl arenes, instead of pure dienyl arene could be used to obtain high diastereoselectivity. Following the racemic reaction, asymmetric reactions were developed using the N-heterocyclic carbene (NHC) ligand (L3). Chiral NHC ligands are well known for copper-catalyzed borylative coupling (Scheme 3C). However, a conceivable drawback with this method is that lower enantioselectivity (41% ee) was achieved after using pure (E)-dienyl arene bearing aldimine ((E)-4). The suggested mechanism proposed by the authors involves the initial formation of the active LCuBpin species by reaction with B2pin2, base and copper salt. Then, the active species undergoes 1,4-borylcupration with the mixture of (E/Z)-1,3-dienes to furnish (Z)-σ-allylcopper intermediate 4a (Scheme 4). The allylcopper 4a attacks the imine intramolecularly, generating an intermediate 4b via a favored 6-membered ring transition state TS-A with a large group (R3) in the less hindered equatorial position.
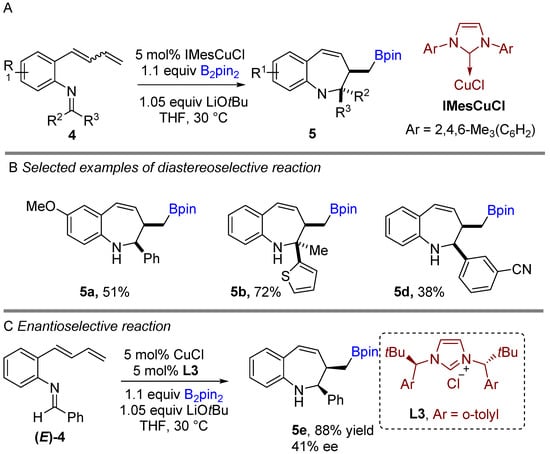
Scheme 3.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of 2,3-disubstituted cis-1-benzo[b]azepines.
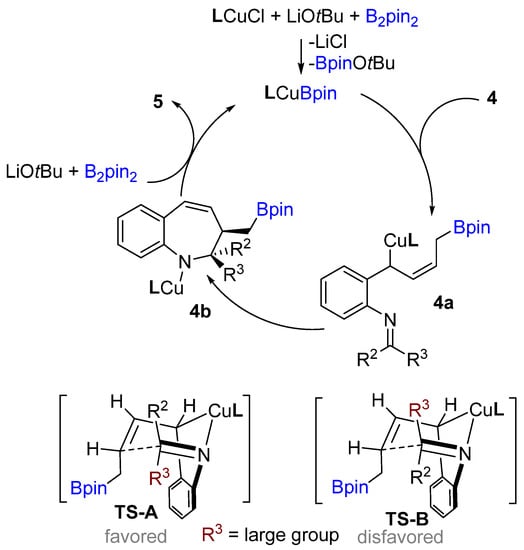
Scheme 4.
A. Proposed catalytic cycle.
2.3. Copper-Catalyzed Intramolecular Borylative Cyclization with Conjugated Alkenes and Imines
An enantioselective copper-catalyzed conjugate intramolecular borylation/cyclization of electron-deficient alkenes with aldimines was developed by Lautens and co-workers using bis (pinacolato)diboronreagent (Bi2pin2) as boron sources to produce enantioenriched tetrahydroquinoline scaffolds (Scheme 5A) [24]. Initially, the active species (L4Cu-Bpin) generate in situ after transmetallation of L4Cu-OtBu with B2pin2 followed by 1,4-addition into the alkene to produce organocopper intermediate 5a. The organocopper species then cyclizes with imine to afford borylated tetrahydroquinoline, followed by routine oxidation with NaBO3•4H2O furnish alcohol-containing products. The authors screened several bisphospine ligands. Josiphos proved to be the best ligand in terms of enantio- and diastereoselectivity. This method worked well with a wide range of electron-deficient alkenes and delivered excellent enantioselectivities. Substrates bearing various aryl imines were well tolerated with representative procedures for the preparation of enantioenrich tetrahydroquinoline scaffolds. The established method was further applied to composing a variety of valuable building blocks.
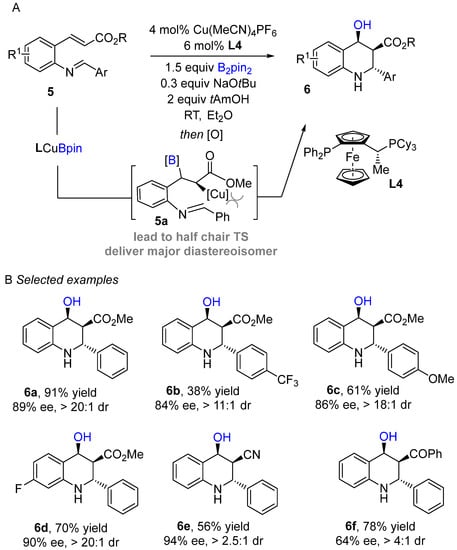
Scheme 5.
Copper-catalyzed intramolecular borylation cyclization for the synthesis of tetrahydroquinoline.
2.4. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Amidines
In 2021, Procter and co-workers demonstrated a copper-catalyzed borylative intramolecular coupling of quinazolinone bearing alkenes to prepare boron-containing heterocycles (Scheme 6A) [25]. By far, this was the introductory report on enantioselective borylative coupling of aliphatic alkenes as well as substituted styrenes with amidines to obtain boron-bearing quinazoline scaffolds. This method proceeds very smoothly with high enantiocontrol and diastereocontrol. Interestingly, ligand L6 was found to be finest when unsubstituted alkene (R1 H) was used for the coupling reaction. The use of the ligand L5 for intramolecular borylative coupling with aryl-substituted alkenes (R1 aryl) provided excellent diastereo- and enantioselectivities. Various substitutions on the aryl ring of the amidine component were well tolerated, delivered pyrroloquinazolines featuring quaternary stereocenters with high enantio- and diastereocontrol. Various aryl-substituted alkenes were successfully used in the borylative cyclization and formation of two adjacent stereocentres to yield pyrroloquinazolinones with very good to excellent enantio- and diastereocontrol. This method demonstrates the use of amidine derivatives for the borylative coupling in following transformations to access more riveting complex structures.
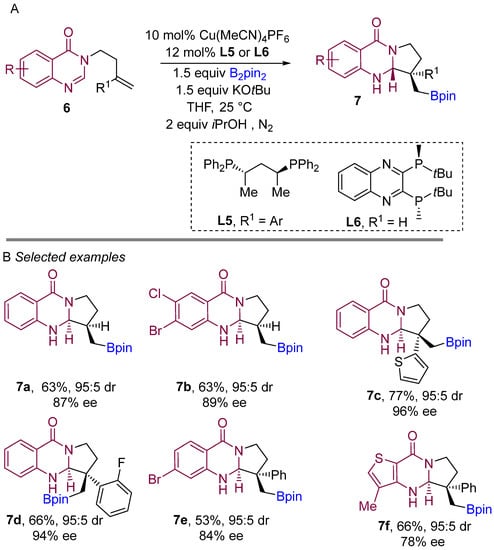
Scheme 6.
Copper-catalyzed borylative cyclization for the synthesis of quinazolinones.
According to the proposed mechanism by the authors, an active LCu-Bpin intermediate is formed initially by reacting with B2pin2, base, and copper salt. Then, the active species (LCu-Bpin) undergo enantioselective borocupration across the double bond of the alkene to generate intermediate 6a (Scheme 7). The authors proposed stereochemical models for forming the most favorable enantiomers. Then, the LCu-Bpin intermediate interacts with alkenes in which the phenyl group of the substrate is oriented toward the ligand’s less hindered position, resulting in a major enantiomer A. The intermediate 6b is then formed by diastereoselective cyclization of the amidine carbon-nitrogen double bond via TS-1b. Finally, the active catalyst (LCu-Bpin) is regenerated in the presence of base, alcohol, and B2pin2.
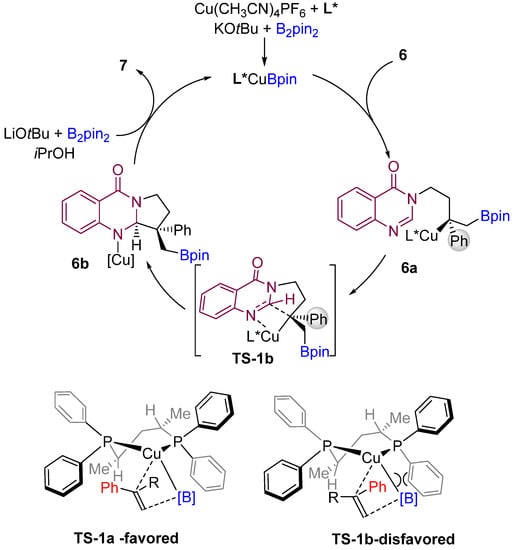
Scheme 7.
Proposed mechanism and model for the origin of stereocontrol. (L* = chiral ligand).
3. Copper-Catalyzed Intramolecular Borylative Coupling with Carbonyls as an Electrophile
3.1. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Ketones
In 2018, Fernández and co-workers developed an exquisite approach, a copper-catalyzed borylation of conjugate alkenes followed by intramolecular cyclization with ketones to enable the construction of boron-containing bicyclic and tricyclic scaffolds such as the azulene core. (Scheme 8A) [26]. At the beginning, a diastereoselective method was discovered using a dppf (L2) as a ligand to achieve borylated bicyclic compounds. Later, established an enantioselective process for the synthesis of boron-bearing cyclic scaffolds. The borylative coupling well functioned with monodentate phosphine as well as bidentate phosphine ligand. Following the racemic reaction, an enantioselective method was developed with a chiral phosphine ligand. Moderate to excellent regio-, diastereo- and enantioselectivity (ee > 90%) was achieved using Josiphos (L4) as a chiral ligand. Cyclodec-2-en-1,6-diones bearing heterocycles, alkenyl and alkynyl substituents were compatible in the reaction. This method can be used to build complex bicyclic and tricyclic scaffolds in a simple and straight forward way. A probable mechanism was explained by the authors. The L4Cu-Bpin is generated in situ before 1,4-addition with an alkene to produce the alkoxy-copper intermediate 10. Intermediate 10 is then subjected to an intramolecular transannular aldol reaction, resulting in borylated bicyclic and tricyclic compounds. The authors speculate that high diastereoselectivity was achieved by forming a preferred chelated (Z)-configured cyclic copper enolate transition state. (TS-8a).
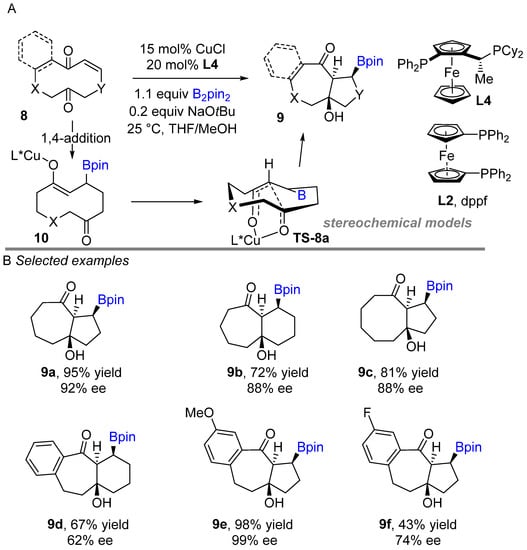
Scheme 8.
Copper-catalyzed intramolecular borylation cyclization for the synthesis of azulene. (L* = chiral ligand).
3.2. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Carbamoyl Chlorides
Pioneering work on copper-catalyzed intramolecular borylative cyclization method to obtain chiral 3,3-disubstituted oxindoles, could be attributed to Lautens et al. [27], who trapped carbamoyl chloride intramolecularly as an electrophile with the benzylic copper intermediate produced by borylcupration (Scheme 9A). Initially, benzylic copper intermediate 13 generate after reacting with alkene and L5Cu-Bpin which subsequently undergoes cyclization with carbamoyl chloride to give product 12. In this coupling reaction, bisphosphine (L5) was found to be the best choice as a ligand. The developed technique demonstrated good enantiocontrol, which was successfully applied to various aryl substituted alkene inputs to produce borylated quaternary oxindole products with an impressive functional group tolerance from good to excellent yields. Notably, the developed method was applied for further functionalization to access various target building blocks, such as arylation of the boron moiety and oxidation of the boron component.
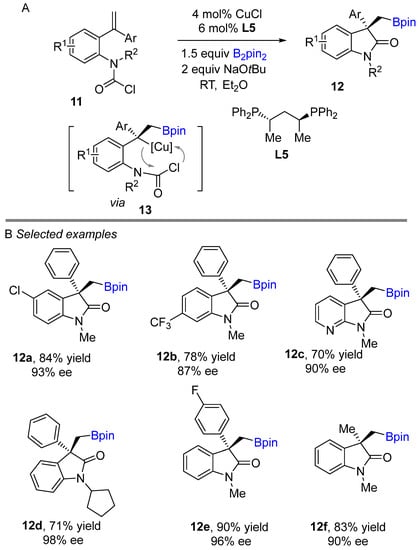
Scheme 9.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of 3,3-disubstituted oxindoles.
3.3. Copper-Catalyzed Intramolecular Borylative Cyclization with Styrene Derivatives and Ketones
Recently, Lautens and his team refined an efficient copper-catalyzed asymmetric borylative nucleophilic addition to carbonyls, allowing for the effective construction of highly substituted boryl-functionalized cyclobutanols (Scheme 10A) [28]. Further, a plausible mechanism was suggested that a copper-boron (L5Cu-Bpin) intermediate generate after transmetalation of preformed Cu(I)-OtBu species that undergoes borocupration of alkene to produce organocopper intermediate 16, which cyclizes with the tethered ketone to afford cyclobutanol. According to the authors’ report, the major diastereomer comes from less steric interaction between the two phenyl groups upon transposition during the cyclization step. This nucleophilic attack occurs via a 4-membered transition state. The versatility of boryl-functionalized cyclobutanols was well demonstrated and showed excellent diastereo- and enantioselectivities with a wide range of alkenes. The method shows an impressive functional group tolerance and provides good to excellent yields. Notably, the developed method was applied for further manipulation to access various interesting building blocks.
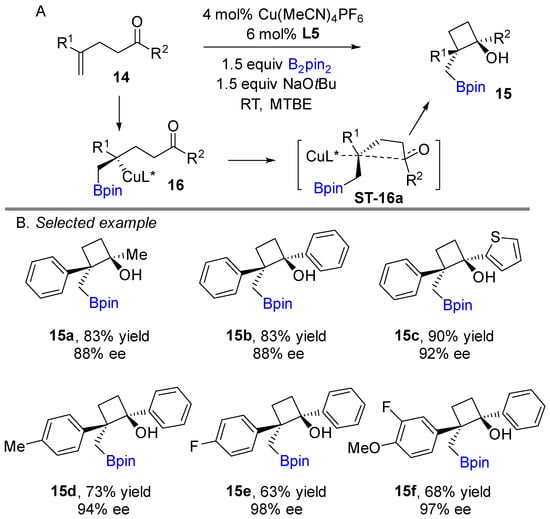
Scheme 10.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of cyclobutanols.
3.4. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Carbamoyl Chlorides for γ-Lactams Synthesis
As a further development, Lautens and co-workers successfully executed a copper-catalyzed intramolecular highly stereoselective borylative cyclization to generate polyfunctionalized γ-lactams (Scheme 11A) [29]. The reaction proceeds via the formation of an achiral-phosphine alkyl copper(I) intermediate, which allows for cyclization of carbamoyl chlorides. Copper-catalyzed diastereoselective intramolecular borylacylation of 1,2-disubstituted alkenes provide to generate 3,4-disubstituted γ-lactams in excellent yields. Later, enantioselective process was developed using 1,1-disubstituted olefins under a copper-catalyzed enantioselective method employing chiral bisphosphine ligand to access chiral 3,3- disubstituted γ-lactams in excellent yield and moderate to excellent enantioselectivity (Scheme 11C). The method shows an impressive functional group tolerance and provides good to excellent yields. In addition, their strategy was then successfully applied to access various target building blocks.
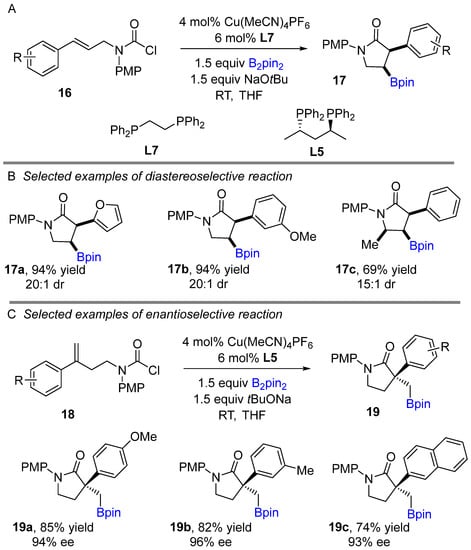
Scheme 11.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of polyfunctionalized γ-lactams.
4. Copper-Catalyzed Intramolecular Borylative Coupling with Carbon as Electrophile
4.1. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkynes and Cyclohexadienones
In 2013, the Lin group reported a copper-catalyzed asymmetric borylative intramolecular cyclization of cyclohexadienones bearing 1,6-enyne moieties to access boron-containing carbo- and heterocycles (Scheme 12A) [30]. Phosphoramidite ligand L8 was found to be best in this reaction. A wide range of alkyne derivatives worked well and proceeded with high regio- and enantioselective control to yield cis-dihydrobenzofuran derivatives. Overall, the reaction had moderate to good yields of the cis-product exclusively with high enantioselectivity. The developed process shows good functional group tolerance, delivering generally very good enantioselectivities (Scheme 12B). Dissecting the mechanism revealed that the steric hindrance of the neighboring substituent (R2) decreased conjugate addition after the borylation of alkyne. Alkyne propargylic ether is crucial for controlling regioselectivity of alkyne borylation through coordination with active Cu-X (X Bpin and SiMe2Ph). Following borylative cyclization reaction, later Lin and co-workers developed a silylative cyclization of cyclohexadienones bearing 1,6-enyne motifs using Suginome’s reagent (PhMe2Si-Bpin) and ligand L9 (Scheme 13) [31]. The reaction worked well with alkyl and aryl-substituted groups and provided moderated to good yields albeit limited enantioselectivity.
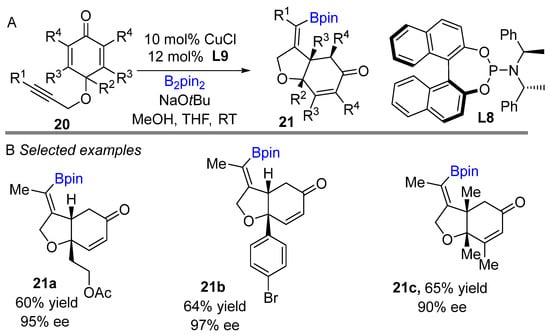
Scheme 12.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of cis-hydrobenzofuran.
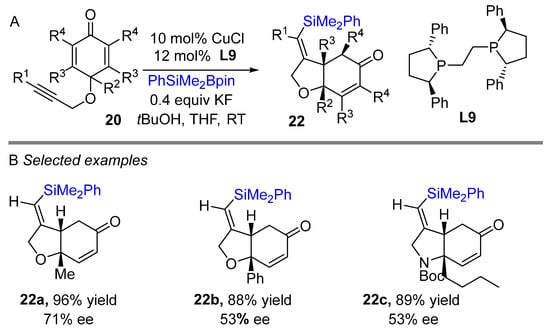
Scheme 13.
Copper-catalyzed intramolecular silylative cyclization for the synthesis of cis-hydrobenzofuran.
4.2. Copper-Catalyzed Intramolecular Borylative Cyclization with Alkenes and Enone-Tethered Cyclohexadienones
In 2022, Chegondi and co-workers reported the first enantioselective Cu(I)-catalyzed borylative cyclization of enone-tethered cyclohexadienones bearing electron-deficient alkenes using bis(pinacolato)diboron as boron source (Scheme 14A) [32]. The (S)-SEGPHOS (L10) was found to be the prime choice for this reaction after screening several chiral bisphosphine ligands. It was emphasized in this study that enantioselectivity highly depends on the temperature as the yield dramatically improved with lowering the temperature to −78 °C. The process worked exceptionally well and afforded the corresponding cyclic borylative products in good yields and with good-excellent enantioselectivities. In this reaction, initial borocupration of enone via 1,2-addition produces the organocopper(I) intermediate (Scheme 14A). Subsequently, the organocopper intermediate undergoes 1,3-copper migration to generate the oxacopper species 25, which undergoes C–C bond formation via a six-membered chair transition state TS-25a.
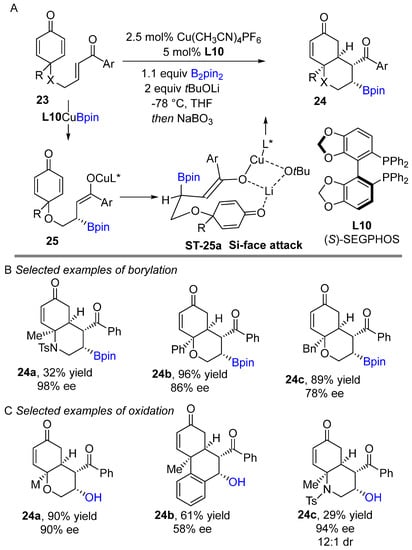
Scheme 14.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of bicyclic pyran and hydroquinoline scaffolds.
4.3. Copper-Catalyzed Intramolecular Borylative Cyclization of Allylic Carbonates
Ito and Sawamura’s group pioneered a copper-catalyzed borylative cyclization of allylic carbonates 26 with a diboron derivative in 2008, producing boron-silicon bifunctional cyclopropanes 27 (Scheme 15A) [18]. After screening several bisphosphine ligands, they found that (R,R)-quinoxP* and (R)-SEGPHOS (L10) promoted the borylative cyclization of allylic carbonates in good yields and excellent enantioselectivities to access bifunctional cyclopropanes. The reaction proceeds with good to excellent enantioselectivities under mild conditions. The authors suggested that borylative cyclopropantion occurs via 1,2-borofunctionalization of alkene to generate organocopper intermediate 28 through the most favored transition state 26a. Then, intramolecular stereospecific nucleophilic substitution between the C-Cu and C-O bonds would afford trans-cyclopropane with (R,R)-quinoxP* (L6) ligand. By comparing the transition states during the addition of the Cu-B bond across the C-C double bond, the stereochemical outcome of copper(I)-catalyzed reactions of (Z)-alkene was explained. The proposed favored transition state 26a by authors is shown in Scheme 15A. Steric repulsion between the substituents of (Z)-alkene and the tert-butyl groups of the quinoxP* ligand is lowered, resulting in trans-cyclopropane as the major product. Similar results were observed when a chiral (R)-SEGPHOS was used in the reaction. Furthermore, the bifunctional cyclopropane was used for selective boron coupling to achieve arylated products via Suzuki–Miyaura coupling. Following Tamao oxidation of the silicon group, the corresponding alcohol was obtained in 73% yield with retention of enantioselectivity and diastereoselectivity of the cyclopropane.
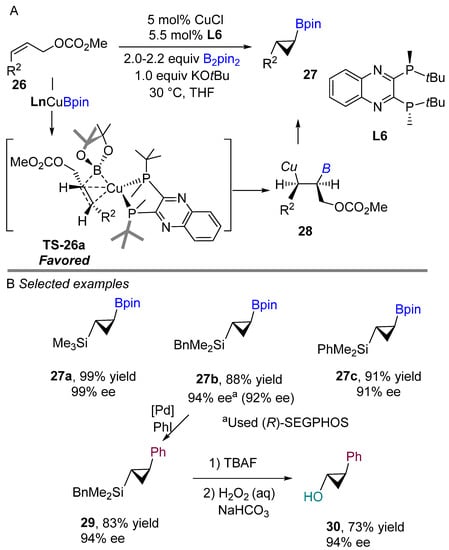
Scheme 15.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of bifunctional cyclopropanes.
4.4. Copper-Catalyzed Intramolecular Borylative Cyclization of Homoallylic Sulfonates
Following the development of bifunctional cyclopropane, Ito and Sawamura’s group reported a stereospecific synthesis of cyclobutylboronates in 2010 using copper(I)-catalyzed borylative cyclization of homoallylic sulfonates and bis(pinacolato) diboron as a boron source (Scheme 16A) [33]. Good to excellent yields and high diastereoselectivities were obtained across a wide range of aryl and silyl substituted alkenes when a racemic bisphosphine ligand (L11) was used in the reaction. This reaction proceeds in a stereospecific and diastereocontrolled manner with various (E)- and (Z)-homoallylic sulfonates. Interestingly, cis-cyclobutylboronates were obtained exclusively when (Z)-alkenes were used in the coupling reaction. In contrast, (E)-alkenes deliver major trans-cyclobutane products in the process. Mechanistically, it was proposed that after borofunctionalization of alkene produces a more stable intermediate 33, in which the fine Cu-C bond is stabilized by the electronic effect of the adjacent silyl- or aryl- group (Scheme 16A). The first high alkene LUMO intermediate 33 is rotated for a low alkene LUMO intermediate 34, allowing for intramolecular nucleophilic substitution to generate Cu(III)-metallacycle intermediate 35, according to the authors. Then, reductive elimination of Cu(III) in the metallacycle intermediate, deliver cyclobutane and Cu(I). The active catalyst (L11Cu-Bpin) is regenerated in the presence of B2pin2 and KOtBu.
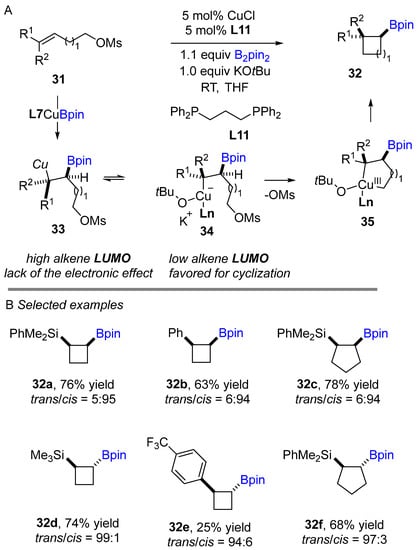
Scheme 16.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of cyclobutylboronates.
4.5. Copper-Catalyzed Intramolecular Borylative Cyclization of Alkylhadide Bearing Terminal Allenes
The Ito group recently extended the intramolecular cylization process to use alkyl halide bearing terminal allenes, resulting in a four-membered ring structure with high regio- and diastereocontrol (Scheme 17) [34]. The reaction tolerated a wide range of functionalized terminal allenes and produced moderate to good yields. Through DFT calculations, the group explained that the products are formed by kinetic control selectivities. The proposed catalytic cycle is shown in Scheme 17C. Mechanically, it was proposed by the authors that the selective addition of the LCu-Bpin intermediate to the allene rather than the alkyl halide to give the allylcopper intermediate 39 or intermediate 42 via borocupration of terminal double of allene. The intermediates 40 and 43 are formed when the allylcopper(I) species 39 and 42 undergo reversible coordination changes with an alkoxide. Then, cupracycles 41 and 44 are produced by a subsequent intramolecular oxidative addition with an alkyl halide. It was proposed by the authors that the five-membered intermediate 41 is favored kinetically over the seven-membered cupracycle. Finally, reductive elimination of Cu(III) intermediates (41 and 44) gives the product and LCu-OR. The active catalyst (LCu-Bpin) is regenerated by the reaction of diboron reagent with LCu-OR.
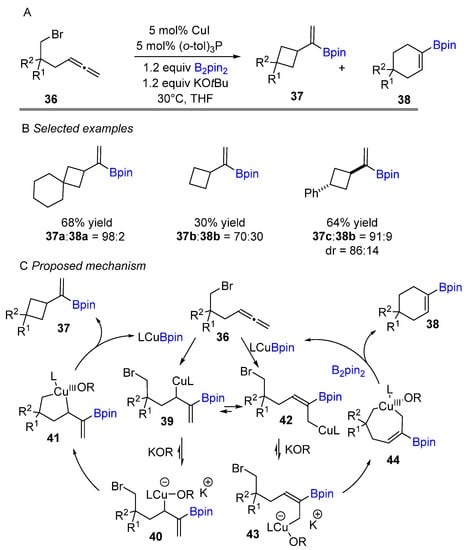
Scheme 17.
Copper-catalyzed intramolecular borylative cyclization for the synthesis of cyclobutales.
5. Conclusions and Outlook
In recent years, copper-catalyzed borofunctionalization of unsaturated hydrocarbons and intramolecular cyclization with various electrophiles have allowed the construction of densely difunctionalized synthetically useful enantioenriched molecules from readily available materials. Because of their importance, notable efforts have been made towards the development of copper-catalyzed borofunctionalization of various hydrocarbon inputs. Even though many elegant methods have been developed in the field, several challenges remain. The general reaction of hydrocarbons proceeds via a key organocopper intermediate that undergoes a subsequent coupling reaction with various coupling reagents. The organocopper species reacts with various electrophiles via a 6-membered cyclic transition state to produce a boron-containing product. The elegant enantioselective transformations were carried out with a variety of phosphine ligands as chiral inductors.
More diversified copper-catalyzed borofunctionalization of unsaturated hydrocarbons to incorporate different electrophilic coupling partners will bridge the gap in this chemical space in the future. New methodologies for enantioselective borofunctionalization of enynes, alkenes, alkynes, allenes, and dienes will surely serve as a potent platform for the construction of complex molecular architecture. Shortly, more difficult enantioselective multicomponent conversions with a wider range of electrophiles as feedstocks will be realized for natural products and bioinspired molecules synthesis.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
S.M. thanks Prof. Jan-Erling Bäckvall for his generous support. S.M. sincerely thanks Dr. Kumar Bhaskar Pal and Dr. Shobhan Mondal for insightful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ms | Methanesulfonyl |
| B2pin2 | Bis(pinacolato)diboron |
| Bpin | Pinacol boron |
| THF | Tetrahydrofuran |
| dppp | 1,3-Bis(diphenylphosphino)propane |
| PMP | p-Methoxyphenyl |
| MTBE | Methyl tertiary-butyl ether |
| tAmOH | tert-Amyl alcohol |
| IMes | 1,3-Bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene |
| Ts | Tosyl |
| (S,S)-Ph-BPE | (+)-1,2-Bis((2S,5S)-2,5-diphenylphospholano)ethane |
| NHC | N-Heterocyclic Carbene |
| Dppe | 1,2-Bis(diphenylphosphino)ethane |
References
- Manna, S.; Dherbassy, Q.; Perry, G.J.P.; Procter, D.J. Enantio- and Diastereoselective Synthesis of Homopropargyl Amines by Copper-Catalyzed Coupling of Imines, 1,3-Enynes, and Diborons. Angew. Chem. Int. Ed. 2020, 59, 4879–4882. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Y.; Shi, Y.; del Pozo, J.; Torker, S.; Hoveyda, A.H. Copper–Hydride-Catalyzed Enantioselective Processes with Allenyl Boronates. Mechanistic Nuances, Scope, and Utility in Target-Oriented Synthesis. J. Am. Chem. Soc. 2019, 141, 12087–12099. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Haeffner, F.; Hoveyda, A.H. Diastereo- and Enantioselective Reactions of Bis(pinacolato)diboron, 1,3-Enynes, and Aldehydes Catalyzed by an Easily Accessible Bisphosphine–Cu Complex. J. Am. Chem. Soc. 2014, 136, 11304–11307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Torker, S.; Li, X.; del Pozo, J.; Hoveyda, A.H. Racemic Vinylallenes in Catalytic Enantioselective Multicomponent Processes: Rapid Generation of Complexity through 1,6-Conjugate Additions. Angew. Chem. Int. Ed. 2019, 58, 2685–2691. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, H.; Hoveyda, A.H. Vicinal Diboronates in High Enantiomeric Purity through Tandem Site-Selective NHC−Cu-Catalyzed Boron−Copper Additions to Terminal Alkynes. J. Am. Chem. Soc. 2009, 131, 18234–18235. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Zhang, F.; Xie, P.-P.; Zhang, S.-Q.; Wang, Y.-F.; Li, Q.-H.; Tian, P.; Hong, X.; Lin, G.-Q. Rhodium(III)-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Dienes: An Experimental and DFT Study. J. Am. Chem. Soc. 2019, 141, 12770–12779. [Google Scholar] [CrossRef]
- Pardo-Rodríguez, V.; Buñuel, E.; Collado-Sanz, D.; Cárdenas, D.J. Pd-catalyzed borylative cyclisation of 1,7-enynes. Chem. Commun. 2012, 48, 10517–10519. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Hong, Y.-C.; Yang, C.-M.; Mannathan, S.; Cheng, C.-H. Nickel-catalyzed highly chemo- and stereoselective borylative cyclization of 1,6-enynes with bis(pinacolato)diboron. Org. Chem. Front. 2017, 4, 1615–1619. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Tang, X.-Q.; Tao, J.-C.; Tian, P.; Lin, G.-Q. Efficient access to cis-decalinol frameworks: Copper(i)-catalyzed borylative cyclization of allene cyclohexanediones. Org. Biomol. Chem. 2016, 14, 4400–4404. [Google Scholar] [CrossRef]
- Wu, X.; Lin, H.-C.; Li, M.-L.; Li, L.-L.; Han, Z.-Y.; Gong, L.-Z. Enantioselective 1,2-Difunctionalization of Dienes Enabled by Chiral Palladium Complex-Catalyzed Cascade Arylation/Allylic Alkylation Reaction. J. Am. Chem. Soc. 2015, 137, 13476–13479. [Google Scholar] [CrossRef] [PubMed]
- Kanti Das, K.; Manna, S.; Panda, S. Transition metal catalyzed asymmetric multicomponent reactions of unsaturated compounds using organoboron reagents. Chem. Commun. 2021, 57, 441–459. [Google Scholar] [CrossRef]
- Talbot, F.J.T.; Dherbassy, Q.; Manna, S.; Shi, C.; Zhang, S.; Howell, G.P.; Perry, G.J.P.; Procter, D.J. Copper-Catalyzed Borylative Couplings with C−N Electrophiles. Angew. Chem. Int. Ed. 2020, 59, 20278–20289. [Google Scholar] [CrossRef]
- Dherbassy, Q.; Manna, S.; Talbot, F.J.T.; Prasitwatcharakorn, W.; Perry, G.J.P.; Procter, D.J. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020, 11, 11380–11393. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622. [Google Scholar] [CrossRef]
- He, C.-Y.; Li, Q.-H.; Wang, X.; Wang, F.; Tian, P.; Lin, G.-Q. Copper-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Dienes. Adv. Synth. Catal. 2020, 362, 765–770. [Google Scholar] [CrossRef]
- Yoon, W.S.; Han, J.T.; Yun, J. Divergent Access to Benzocycles through Copper-Catalyzed Borylative Cyclizations. Adv. Synth. Catal. 2021, 363, 4953–4959. [Google Scholar] [CrossRef]
- Ito, H.; Kosaka, Y.; Nonoyama, K.; Sasaki, Y.; Sawamura, M. Synthesis of Optically Active Boron–Silicon Bifunctional Cyclopropane Derivatives through Enantioselective Copper(I)-Catalyzed Reaction of Allylic Carbonates with a Diboron Derivative. Angew. Chem. Int. Ed. 2008, 47, 7424–7427. [Google Scholar] [CrossRef]
- Iwamoto, H.; Ozawa, Y.; Hayashi, Y.; Imamoto, T.; Ito, H. Conformationally Fixed Chiral Bisphosphine Ligands by Steric Modulators on the Ligand Backbone: Selective Synthesis of Strained 1,2-Disubstituted Chiral cis-Cyclopropanes. J. Am. Chem. Soc. 2022, 144, 10483–10494. [Google Scholar] [CrossRef]
- Li, D.; Kim, J.; Yang, J.W.; Yun, J. Copper-Catalyzed Asymmetric Synthesis of Borylated cis-Disubstituted Indolines. Chem. Asian J. 2018, 13, 2365–2368. [Google Scholar] [CrossRef]
- Zhang, G.; Cang, A.; Wang, Y.; Li, Y.; Xu, G.; Zhang, Q.; Xiong, T.; Zhang, Q. Copper-Catalyzed Diastereo- and Enantioselective Borylative Cyclization: Synthesis of Enantioenriched 2,3-Disubstituted Indolines. Org. Lett. 2018, 20, 1798–1801. [Google Scholar] [CrossRef]
- Wang, H.-M.; Zhou, H.; Xu, Q.-S.; Liu, T.-S.; Zhuang, C.-L.; Shen, M.-H.; Xu, H.-D. Copper-Catalyzed Borylative Cyclization of Substituted N-(2-Vinylaryl)benzaldimines. Org. Lett. 2018, 20, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Park, Y.; Yun, J. Copper-Catalyzed Regioselective and Diastereoselective Synthesis of Borylated 1-Benzo[b]azepines. Org. Lett. 2018, 20, 7526–7529. [Google Scholar] [CrossRef] [PubMed]
- Larin, E.M.; Loup, J.; Polishchuk, I.; Ross, R.J.; Whyte, A.; Lautens, M. Enantio- and diastereoselective conjugate borylation/Mannich cyclization. Chem. Sci. 2020, 11, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Dherbassy, Q.; Manna, S.; Shi, C.; Prasitwatcharakorn, W.; Crisenza, G.E.M.; Perry, G.J.P.; Procter, D.J. Enantioselective Copper-Catalyzed Borylative Cyclization for the Synthesis of Quinazolinones. Angew. Chem. Int. Ed. 2021, 60, 14355–14359. [Google Scholar] [CrossRef]
- Sendra, J.; Manzano, R.; Reyes, E.; Vicario, J.L.; Fernández, E. Catalytic Stereoselective Borylative Transannular Reactions. Angew. Chem. Int. Ed. 2020, 59, 2100–2104. [Google Scholar] [CrossRef]
- Whyte, A.; Burton, K.I.; Zhang, J.; Lautens, M. Enantioselective Intramolecular Copper-Catalyzed Borylacylation. Angew. Chem. Int. Ed. 2018, 57, 13927–13930. [Google Scholar] [CrossRef]
- Whyte, A.; Mirabi, B.; Torelli, A.; Prieto, L.; Bajohr, J.; Lautens, M. Asymmetric Synthesis of Boryl-Functionalized Cyclobutanols. ACS Catal. 2019, 9, 9253–9258. [Google Scholar] [CrossRef]
- Torelli, A.; Whyte, A.; Polishchuk, I.; Bajohr, J.; Lautens, M. Stereoselective Construction of γ-Lactams via Copper-Catalyzed Borylacylation. Org. Lett. 2020, 22, 7915–7919. [Google Scholar] [CrossRef]
- Liu, P.; Fukui, Y.; Tian, P.; He, Z.-T.; Sun, C.-Y.; Wu, N.-Y.; Lin, G.-Q. Cu-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Enynes. J. Am. Chem. Soc. 2013, 135, 11700–11703. [Google Scholar] [CrossRef]
- He, C.-Y.; Xie, L.-B.; Ding, R.; Tian, P.; Lin, G.-Q. Copper-catalyzed asymmetric silylative cyclization of cyclohexadienone-containing 1,6-enynes. Tetrahedron 2019, 75, 1682–1688. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Dash, S.R.; Maurya, S.; Nanubolu, J.B.; Vanka, K.; Chegondi, R. Enantioselective Cu(I)-catalyzed borylative cyclization of enone-tethered cyclohexadienones and mechanistic insights. Nat. Commun. 2022, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Toyoda, T.; Sawamura, M. Stereospecific Synthesis of Cyclobutylboronates through Copper(I)-Catalyzed Reaction of Homoallylic Sulfonates and a Diboron Derivative. J. Am. Chem. Soc. 2010, 132, 5990–5992. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Iwamoto, H.; Ito, H. Copper(i)-catalysed regio- and diastereoselective intramolecular alkylboration of terminal allenes via allylcopper(i) isomerization. Chem. Commun. 2018, 54, 4991–4994. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).