Recent Developments in Nanocatalyzed Green Synthetic Protocols of Biologically Potent Diverse O-Heterocycles—A Review
Abstract
:1. Introduction
2. Nanocatalyzed Green Synthesis of Various O-Heterocycles
2.1. Synthesis of Furans
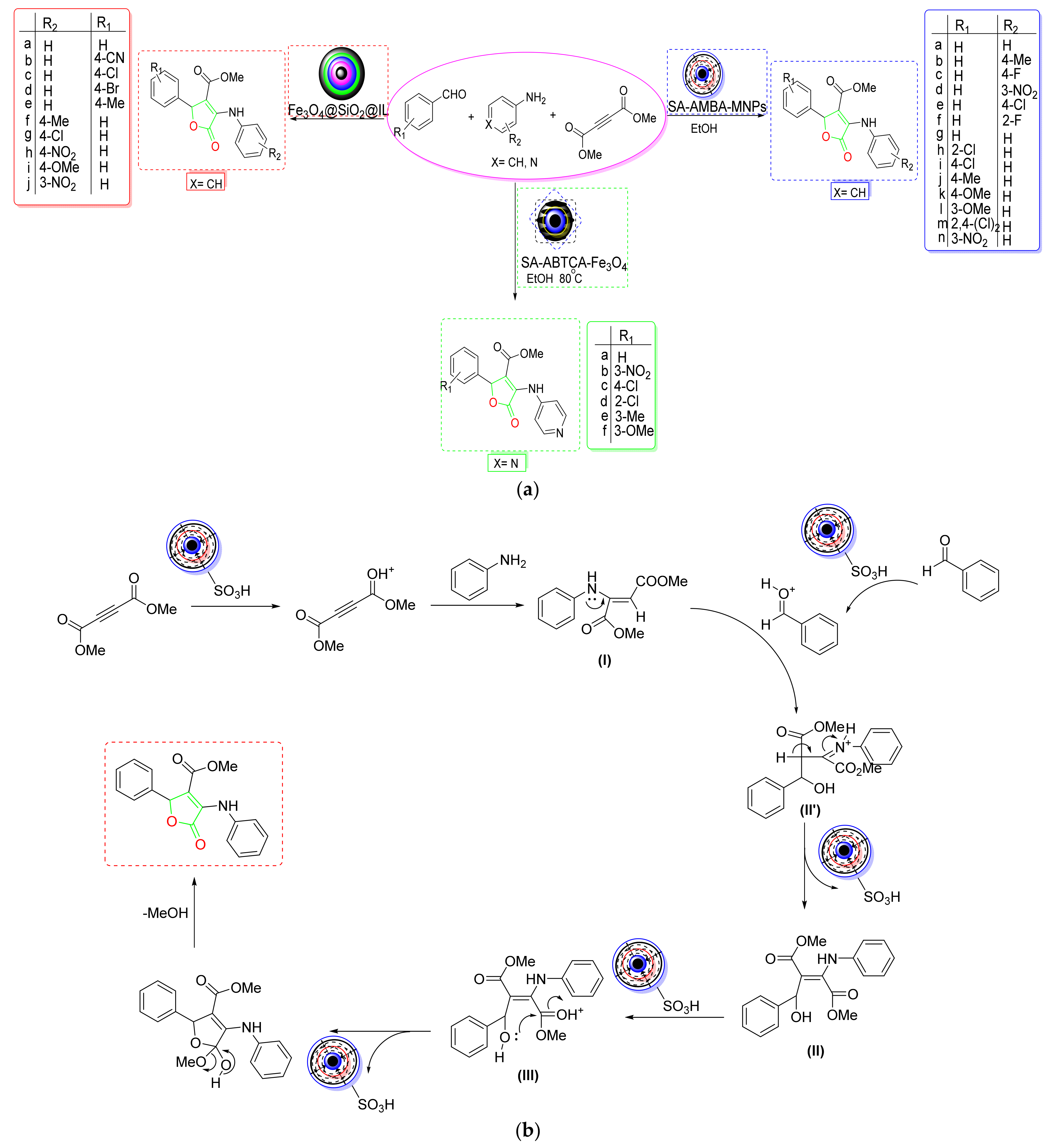
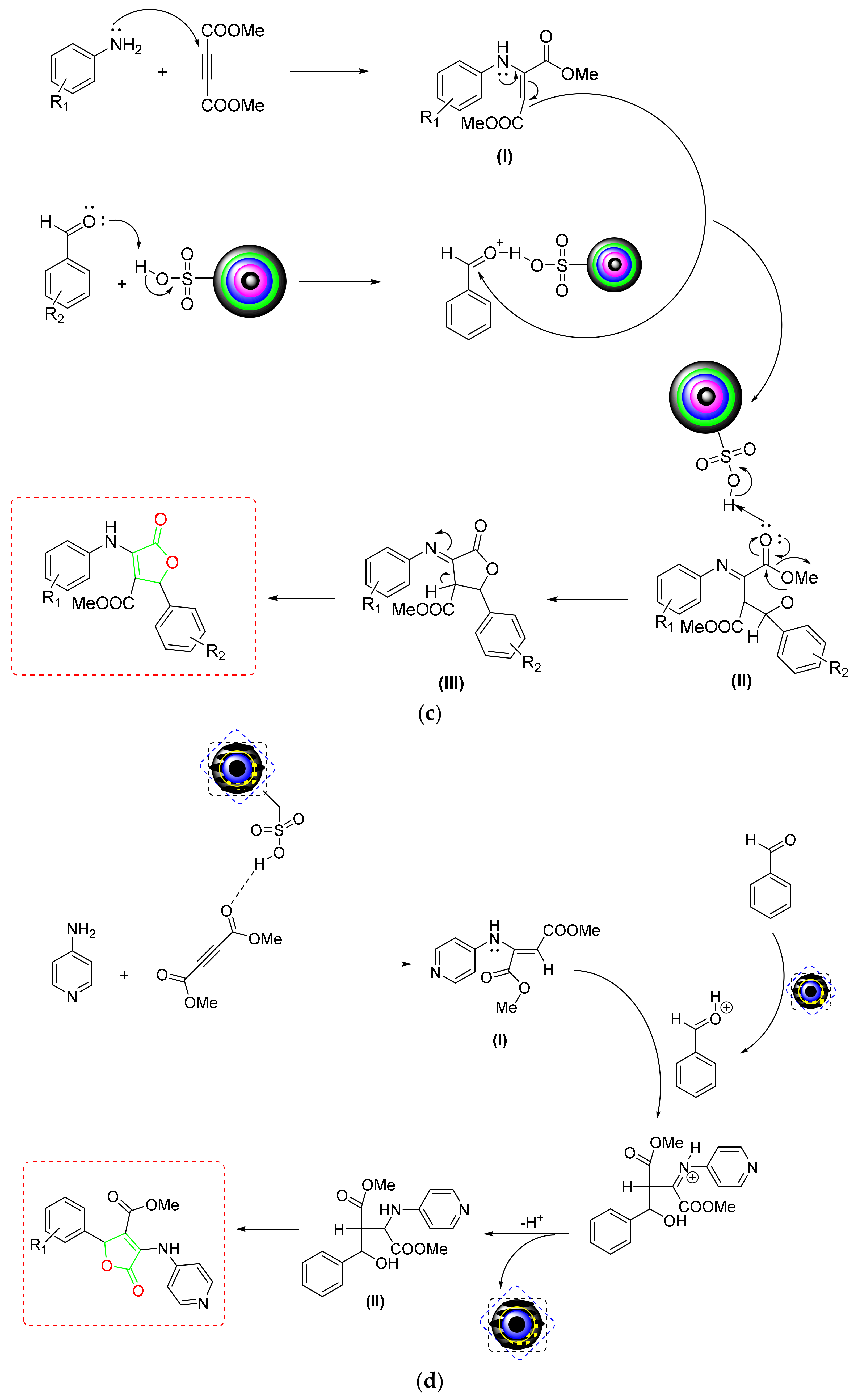
- Khodaei et al. developed 4-carboxybenzyl sulfamic acid functionalized Fe3O4 NPs as a new magnetic five-fold recyclable NC for the rapid one-pot production of furan-2(5H)-ones [57] (Scheme 5a). According to substrate scope data, the proposed MNC displayed outstanding bearing strength against diverse substituents on aldehydes or anilines. According to the postulated mechanism, the acetylic carbonyl was protonated and then attacked by aniline to generate an enamine (I). The protonated aldehyde then interacted with the created enamine, forming an intermediate (II) by rearrangement. After dehydration, the carbonyl group of this newly created intermediate (III) was protonated to cyclized, yielding the desired product (Scheme 5b).
- Shirzaei et al. developed a silica-coated magnetic iron NC doped with thiocarbohydrazide for the quick production of furan derivatives [58] (Scheme 5a). According to a substrate scope investigation, the type of substituents affects the product yield and reaction speed. According to the findings, aromatic aldehydes containing ERG (electron-releasing groups) produced better yields in less time than EWGs (electron-withdrawing groups). The mechanistic research showed that an intermediate (I) was formed via the condensation of aniline and acetylene, which then condensed with activated aldehydes to produce a new intermediate (II). The required derivative of furan was obtained via an intramolecular Michael addition and proton removal of this newly formed intermediate (III) (Scheme 5c). The five-time reusability and high degree of activity make the proposed NC superior to other nonmagnetic catalysts.
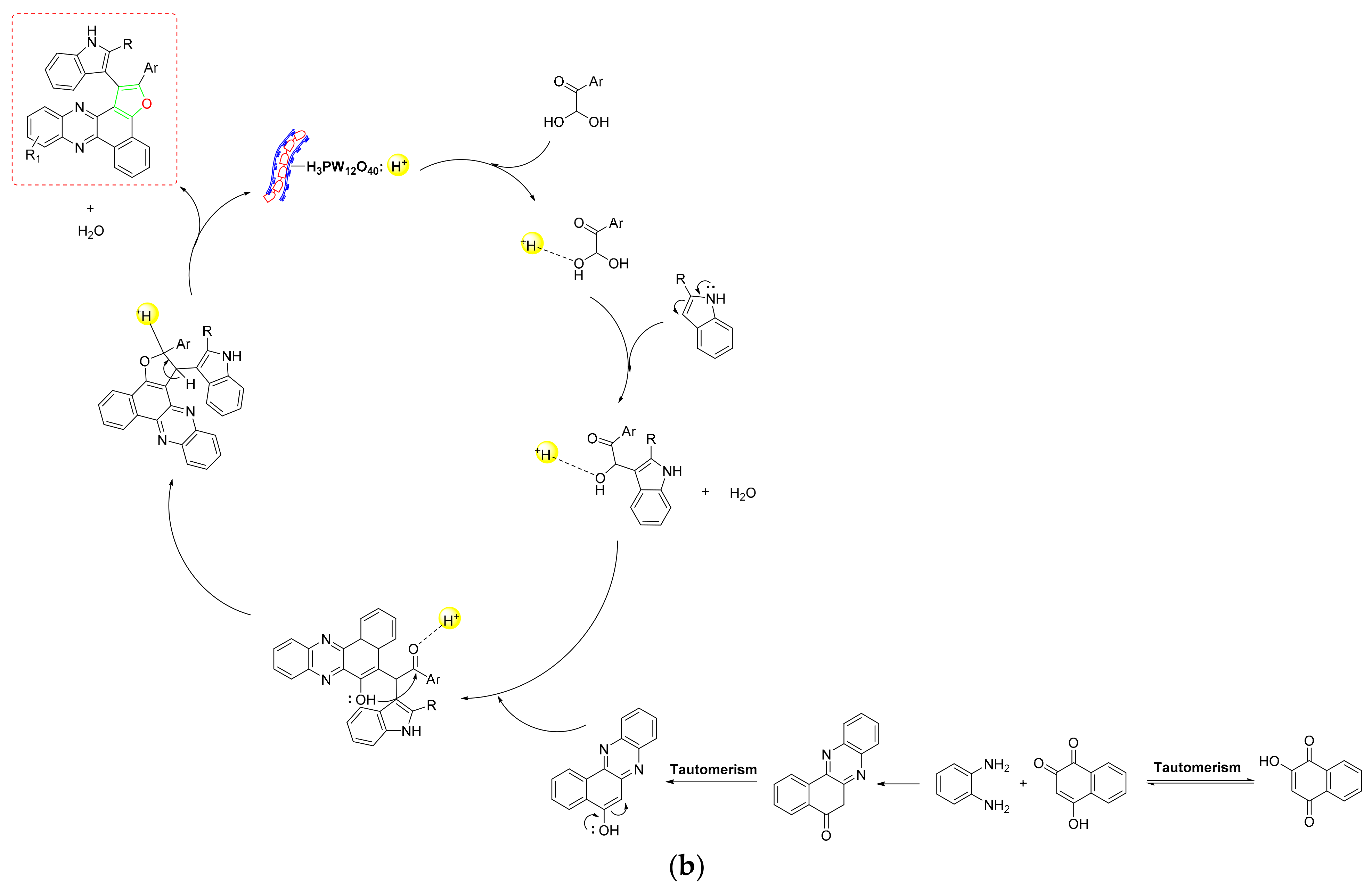
- Using sulfamic-acid-2-Aminobenzothiazole-6-carboxylic-acid-adorned Fe3O4 NPs, Hao et al. created a novel organic–inorganic hybrid NC (SA-ABTCA-Fe3O4) that allowed for effective production of 3,4,5-trisubstituted furan-2(5H)-ones [59] (Scheme 5a). The NC was four times recoverable and showed good tolerance for the substituent pattern. According to mechanistic results, 4-aminopyridine and DMAD first created an enamine (I), which then interacted with protonated benzaldehyde to form an intermediate (II). The produced intermediate was subsequently cyclized after undergoing a proton exchange to provide the required product (Scheme 5d). This green NC demonstrated strong reversibility and allowed for a clean operation in a short amount of time, making the protocol more practical and cost-effective.
2.2. Synthesis of Chalcones—The Heterocyclic Intermediate
- Aryan et al. created a series of unique nanocomposites by employing manganese ferrite (MnFe2O4 NPs) catalyst to modify the surface of natural clinoptilolite [67]. In the aldol-type Claisen–Schmidt process to produce chalcones (Scheme 8a), one of the nanocatalysts demonstrated strong catalytic performance. The proposed synthesis was made faster and more efficient due to robust catalytic synergy between MnFe2O4 NPs and the natural clinoptlolite interface. The substrate scope revealed that the suggested NC was highly tolerant of reactant substituents. According to the mechanistic proof, the acetophenone and benzaldehyde were activated by the nanocatalyst’s dual Lewis acidic and Bronsted basic features. The presence of O2− in the NC made the enolate formed from acetophenone more nucleophilic, which initiated the attack on benzaldehyde, resulting in the formation of an intermediate (I) that, following dehydration, supplied the desired product (Scheme 8b). The NC was readily filtered out of the reaction mixture and reused up to four times without losing any catalytic performance.
- Borade and his colleagues employed a sol-gel autoignition approach to produce the zinc ferrite (ZnFe2O4 NPs) which they used as a green fuel and as an efficient NC for manufacturing chalcones (Scheme 8a) [68]. The proposed nanocatalyst’s Lewis acidic site aided in the enolization of aryl ketone and activated the benzaldehyde carbonyl group for nucleophilic attack (Scheme 8c). Overall, the proposed approach was shown to be efficient due to its effective MW assistance, solvent-free condition, eco-friendliness, shortened reaction time, fast recovery, and continuous five-cycle reuse of NC.
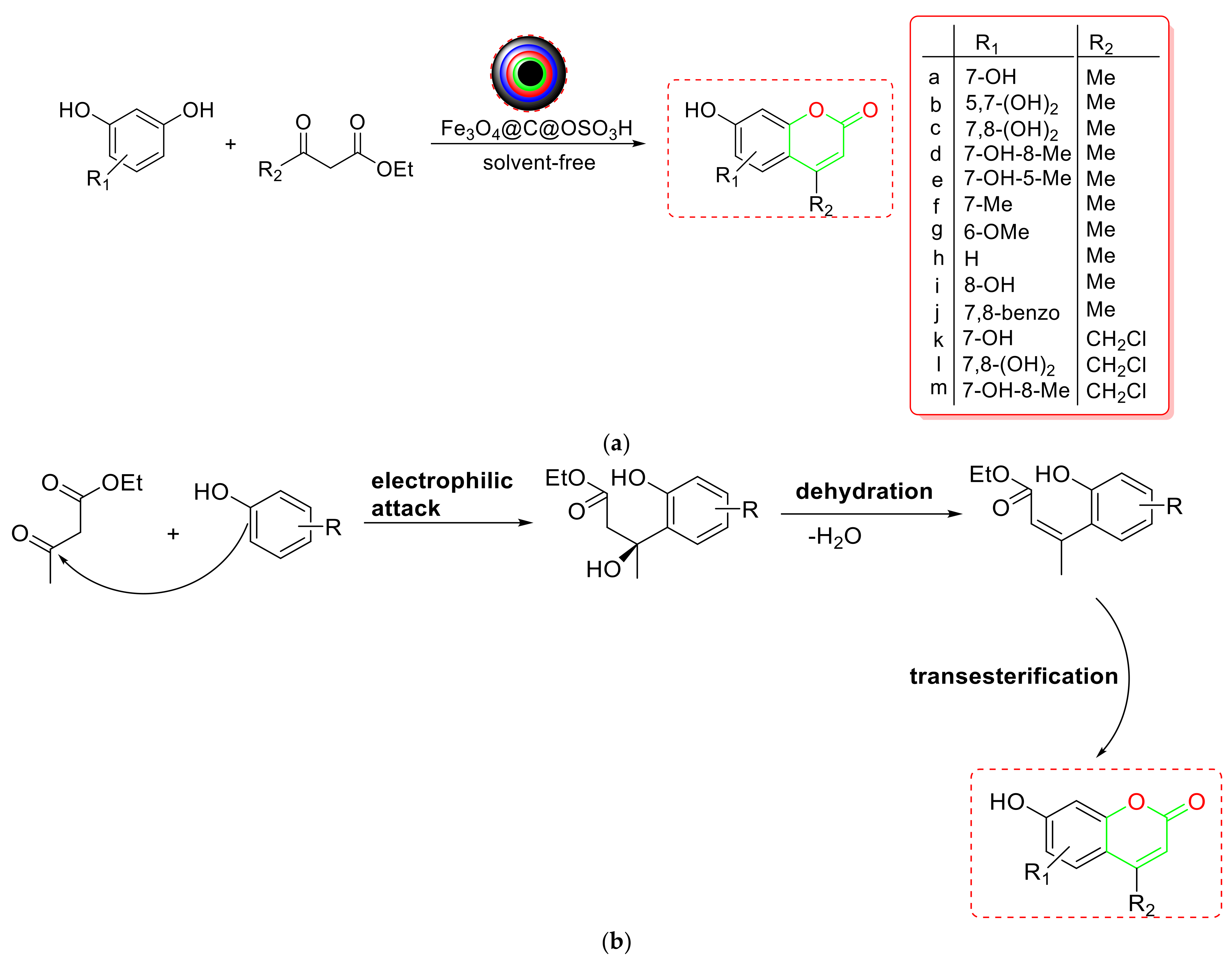
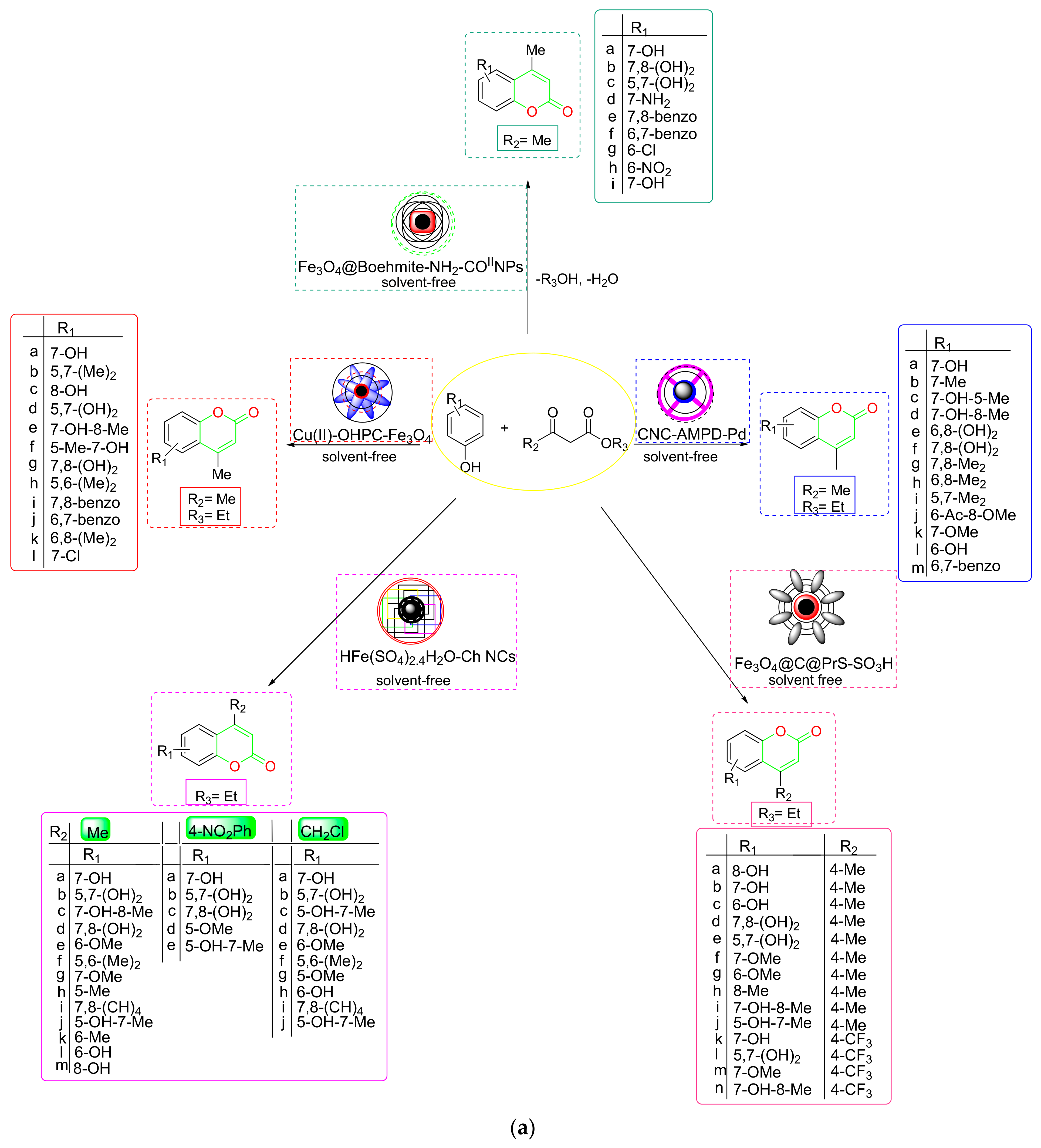
2.3. Synthesis of Coumarins
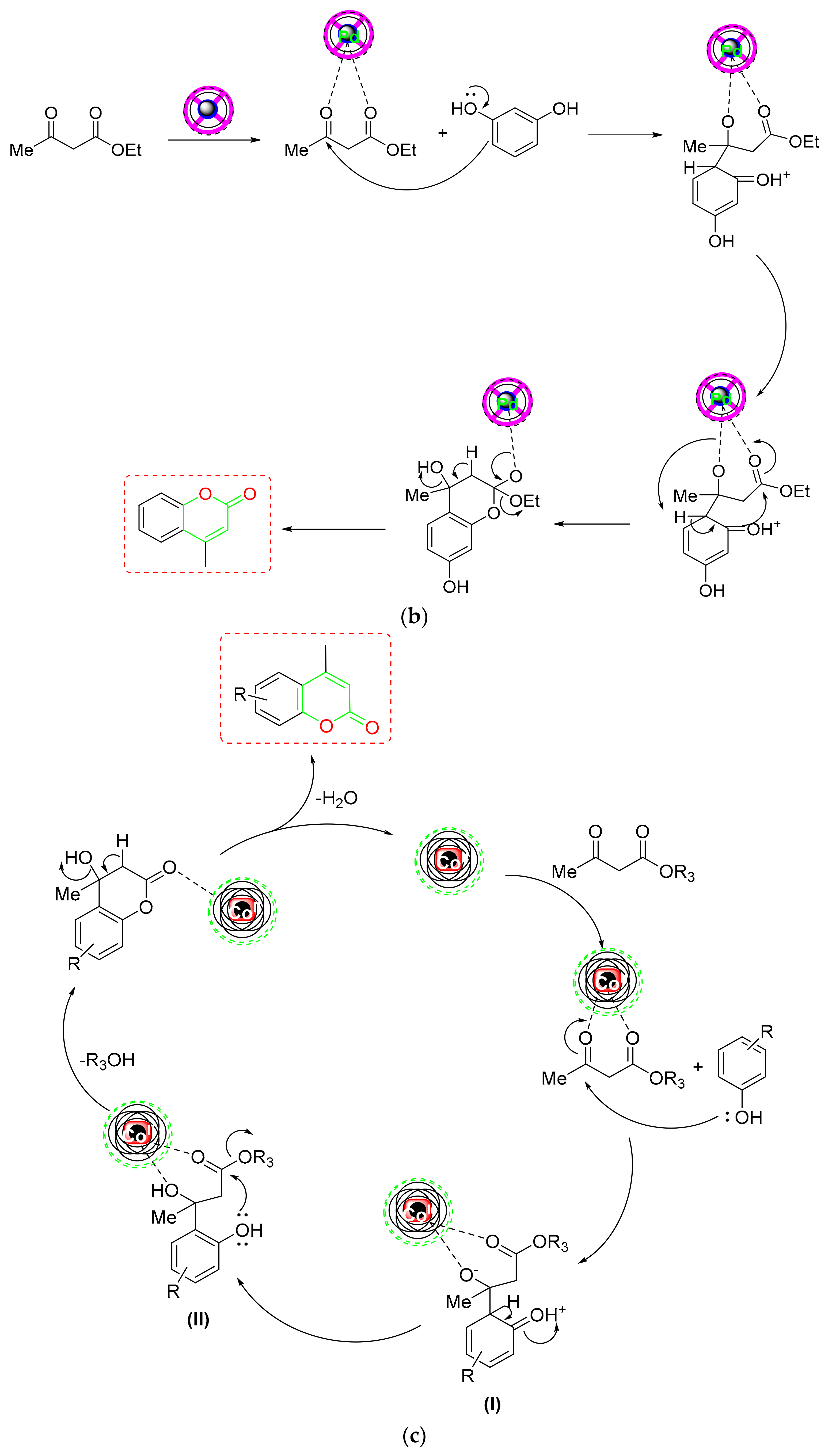
- Mirosanloo et al. fabricated a novel biosupported (CNC-AMPD-Pd) NC on palladium NPs utilizing 2-Amino pyrimidine nanocellulose as a support and tested its catalytic performance in Pechmann condensation to generate coumarin derivatives [76] (Scheme 11a). The proposed NC could be easily recycled and reused up to four times without losing any catalytic activity. According to the proposed mechanism, the CNC-AMPD-Pd catalyzed Pechmann condensation generated an olefinic bond by dehydration while concurrently eliminating ethanol to obtain the required coumarin (Scheme 11b). The proposed technique has the advantages of a reusable catalyst, solvent-free environment, no Pd leaching into the reaction solution, faster reaction times, and simple setup.
- Pakdel et al. proposed a six-time-recyclable magnetic-core-shell-like Fe3O4@Boehmite-NH2-CoII NPs for solvent-free Pechmann condensation [77] (Scheme 11a). The reliance of suggested NC on the phenol substitution pattern was revealed by the substrate scope discovery. The results showed that phenols with ERG-generated high yields of the intended product, whereas EWG were found to be less reactive or unreactive, as the presence of ERG was actually responsible for the nucleophilic addition of phenol to the carbonyl group of β-ketoester. Mechanistic findings showed that the β-ketoester was first activated and was then attacked by substituted phenols, resulting in the formation of an intermediate (I). Then, after the rearomatization of (I), a new intermediate (II) was formed, of which simultaneous transesterification and ring closure finally produced coumarin after dehydration (Scheme 11c).
- To enable effective US-assisted coumarin production (Scheme 11a), Zarei et al. produced a new magnetic HFe(SO4)2·4H2O-chitosan nanocomposite [78] (HFe(SO4)2·4H2O-Ch NCs). According to substrate scope data, phenols containing ERG produced coumarin at a faster rate and with a higher yield than phenols containing EWG. Due to steric hindrance and strong electronegativity, EAA reacted faster than ethyl-4-nitroacetoacetate and 4-chloroacetoacetate. The reported catalyst is easily separated by an external magnetic field, and it can be recycled and reused up to seventeen times. These characteristics make the NCs the most efficient catalyst.
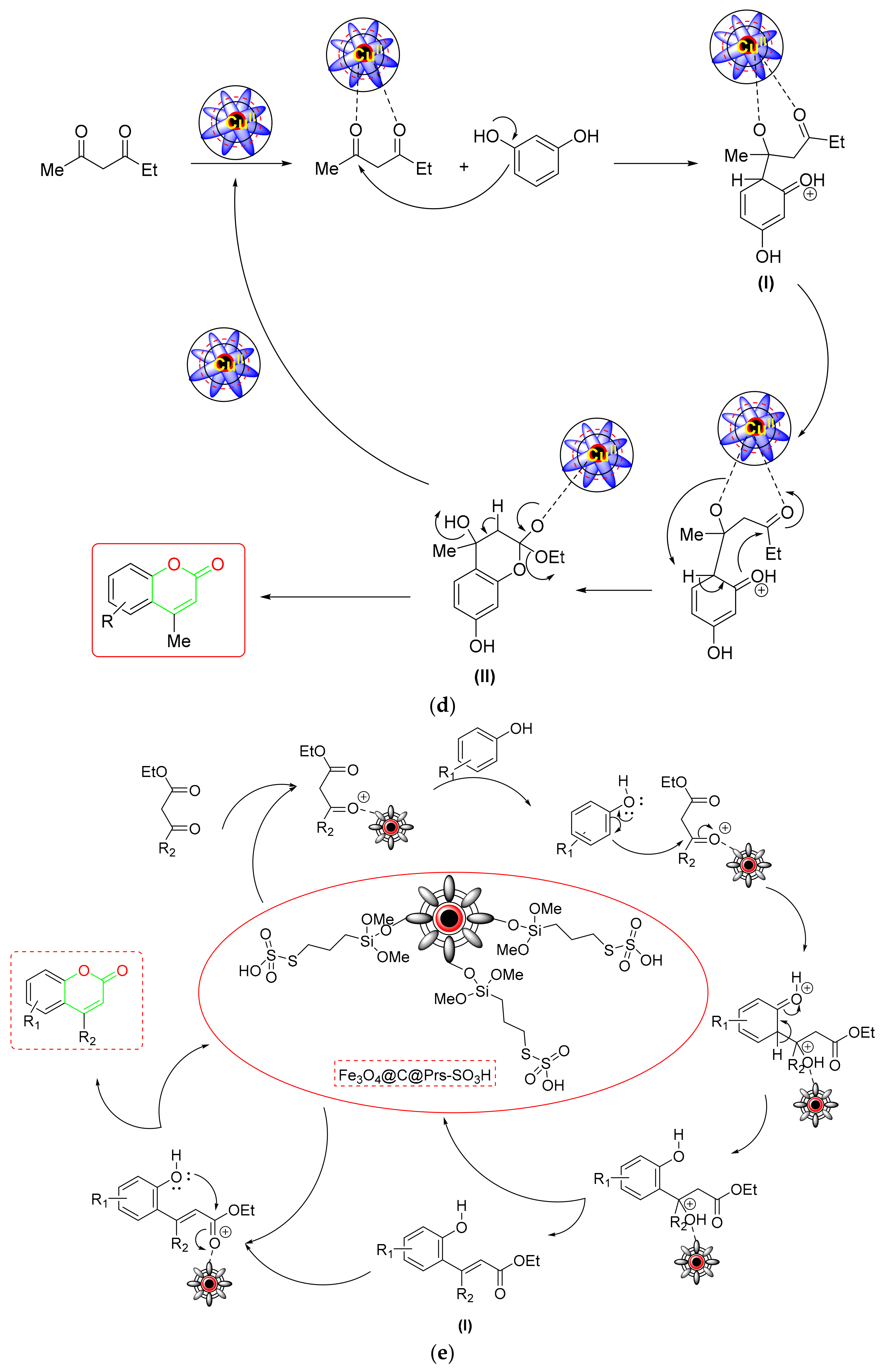
- Yuan et al. developed a copper-supported 5-oxo-4,5-dihydropyrrole-3-carboxylic-acid-functionalized Fe3O4 NPs (Cu(II)-OHPC-Fe3O4) as a new magnetic NC for green, solvent-free coumarin synthesis via Pechmann condensation [79] (Scheme 11a). According to the mechanistic findings, the activated EAA first encountered a nucleophilic attack from phenol, forming an intermediate (I) that underwent intramolecular cyclization. After removing the ethanol, the appropriately generated cyclic intermediate (II) yielded the required coumarin (Scheme 11d). The nanocatalyst’s large surface area, chemical stability, four-time recyclability, low leaching into the environment, and superior accessibility make it more appealing.
- For the US-assisted green synthesis of coumarin derivatives [80] (Scheme 11a), Bonab et al. developed a new magnetic core-shell of Fe3O4@c@PeS-SO3H NC. During the investigation of the substrate scope, it was discovered that the type and position of phenol substituents had a significant impact on the catalyst’s performance. In contrast to the EWG, the phenols with ERG promoted synthesis in a shorter reaction time. According to the mechanistic protocol, the carbonyl group of the β-ketoester was first protonated with the proposed NC to render it vulnerable to phenol’s nucleophilic assault. Next, electrophilic phenol substitution is followed by dehydration to produce intermediate (I). The NC protonated the generated intermediate (I), which subsequently underwent intermolecular esterification, ethanol elimination, and ring closure to yield the appropriate coumarin (Scheme 11e).
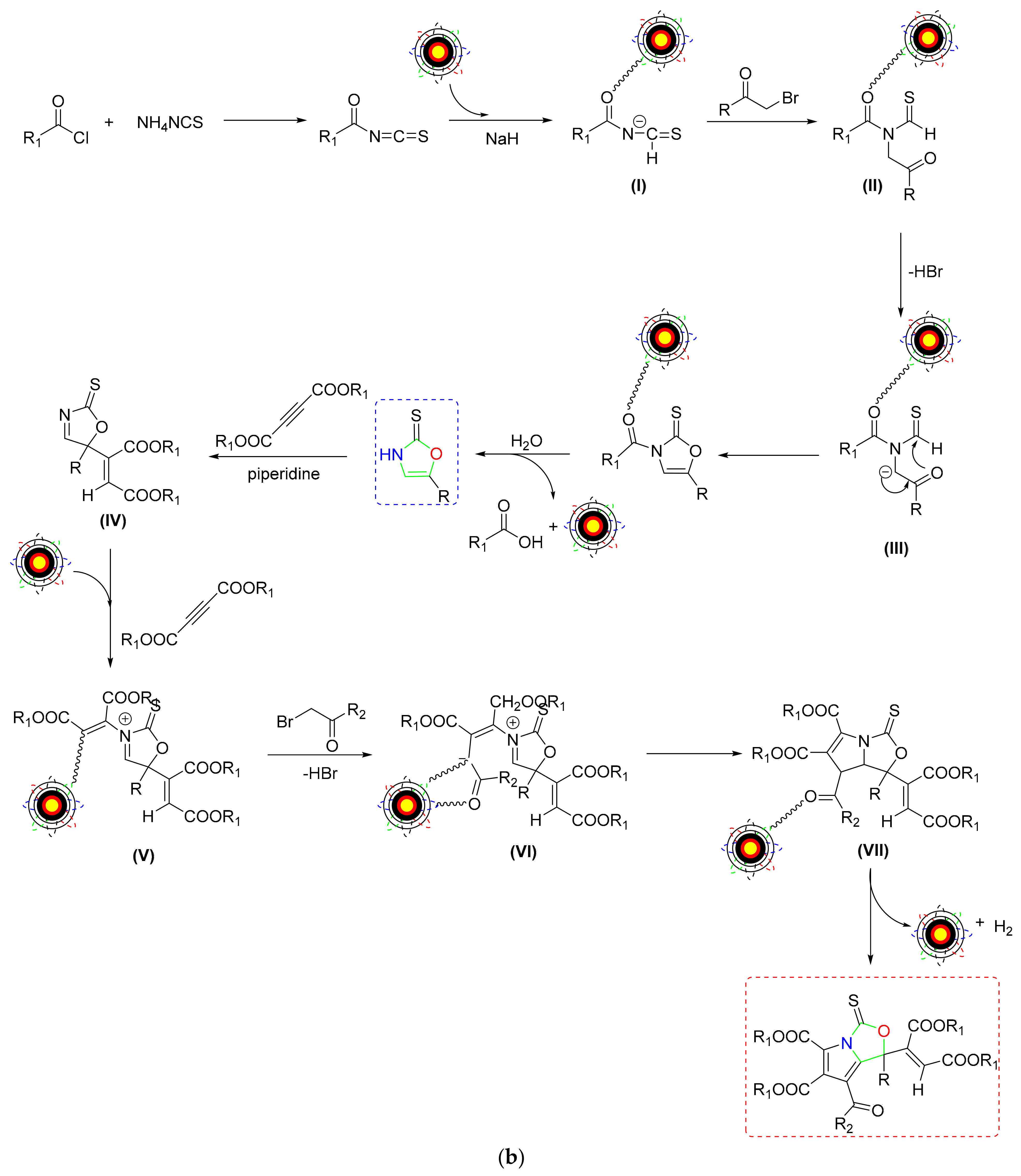
2.4. Synthesis of Oxazole/Isoxazoles
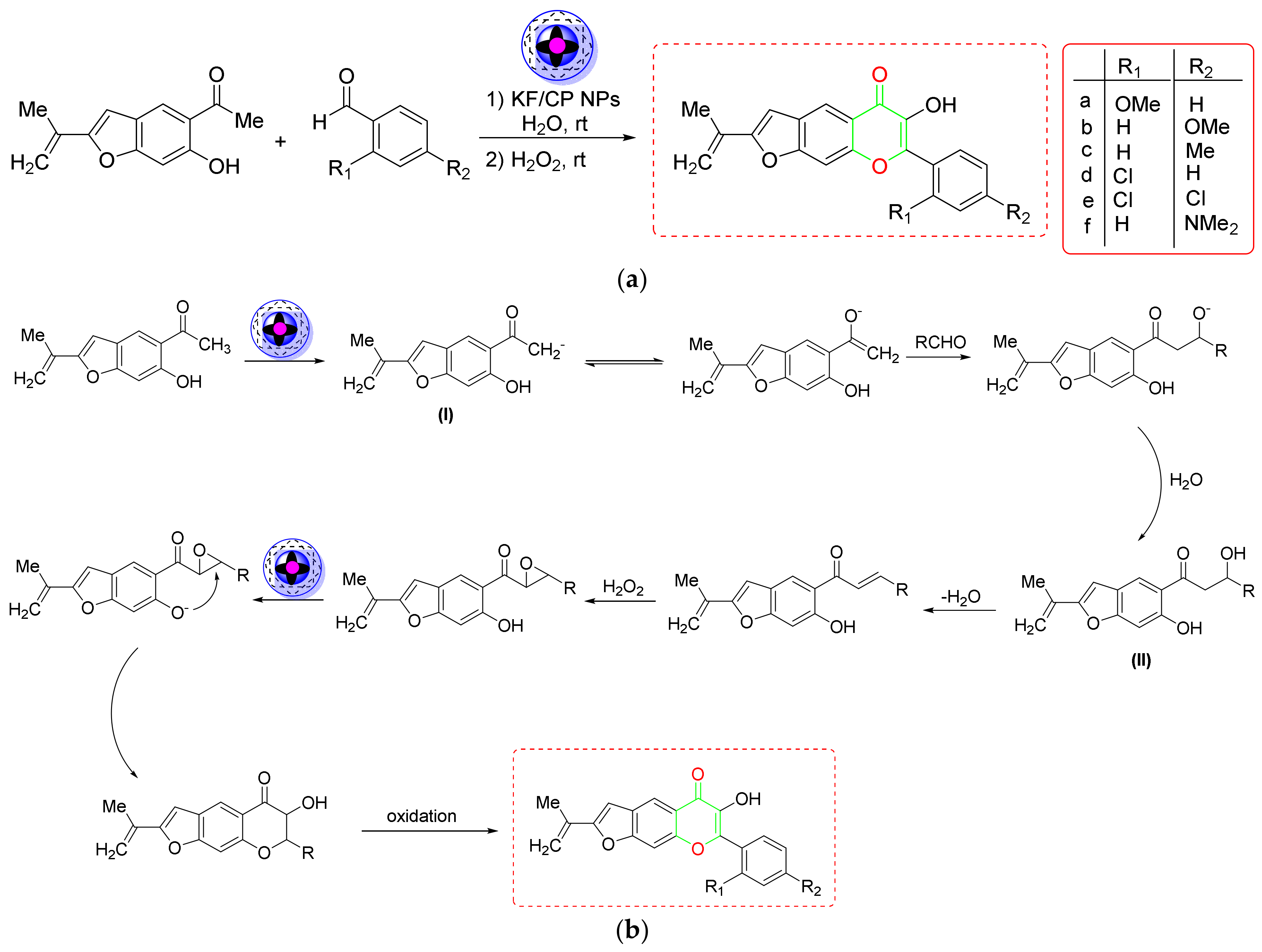
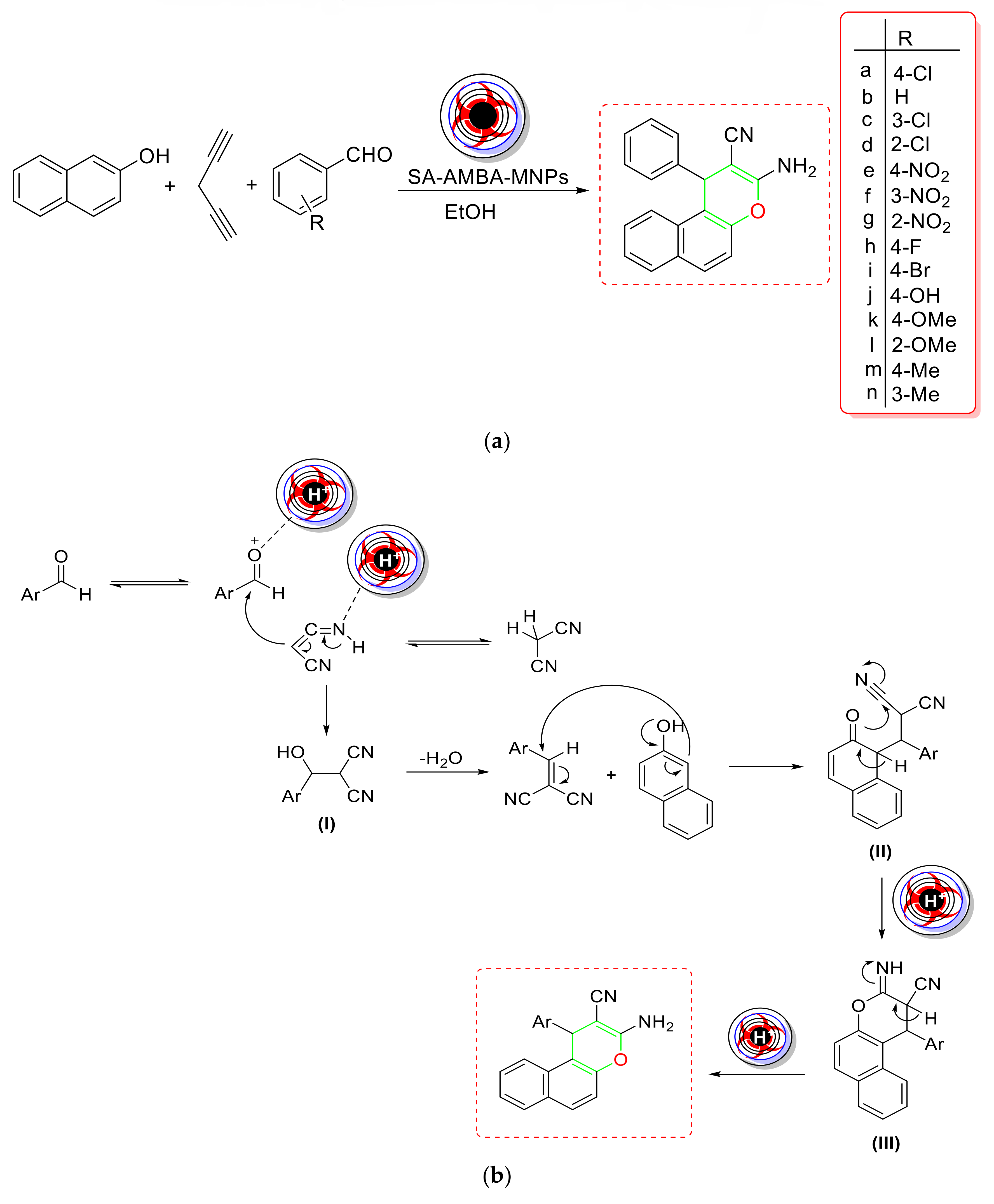
2.5. Synthesis of Pyrans
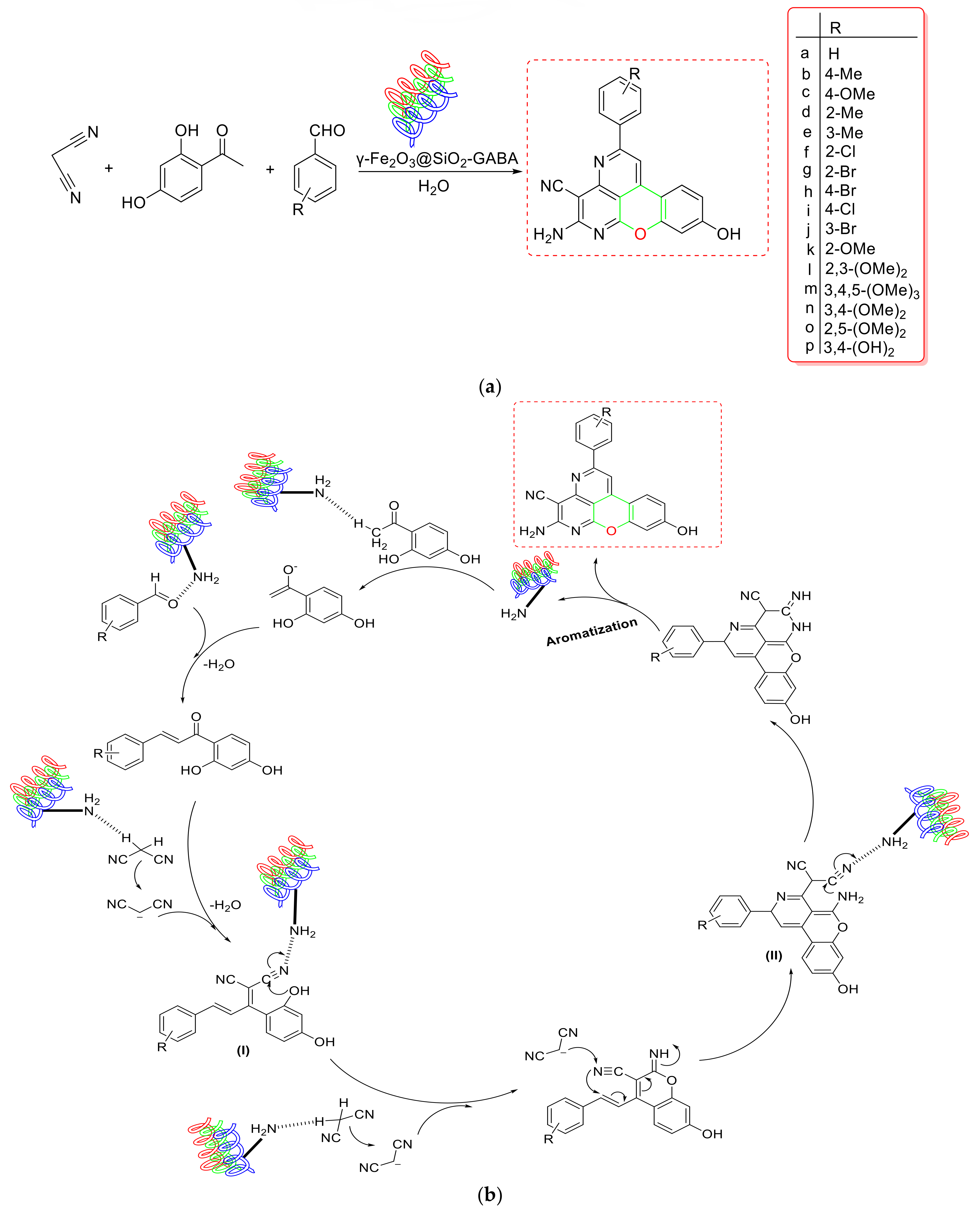
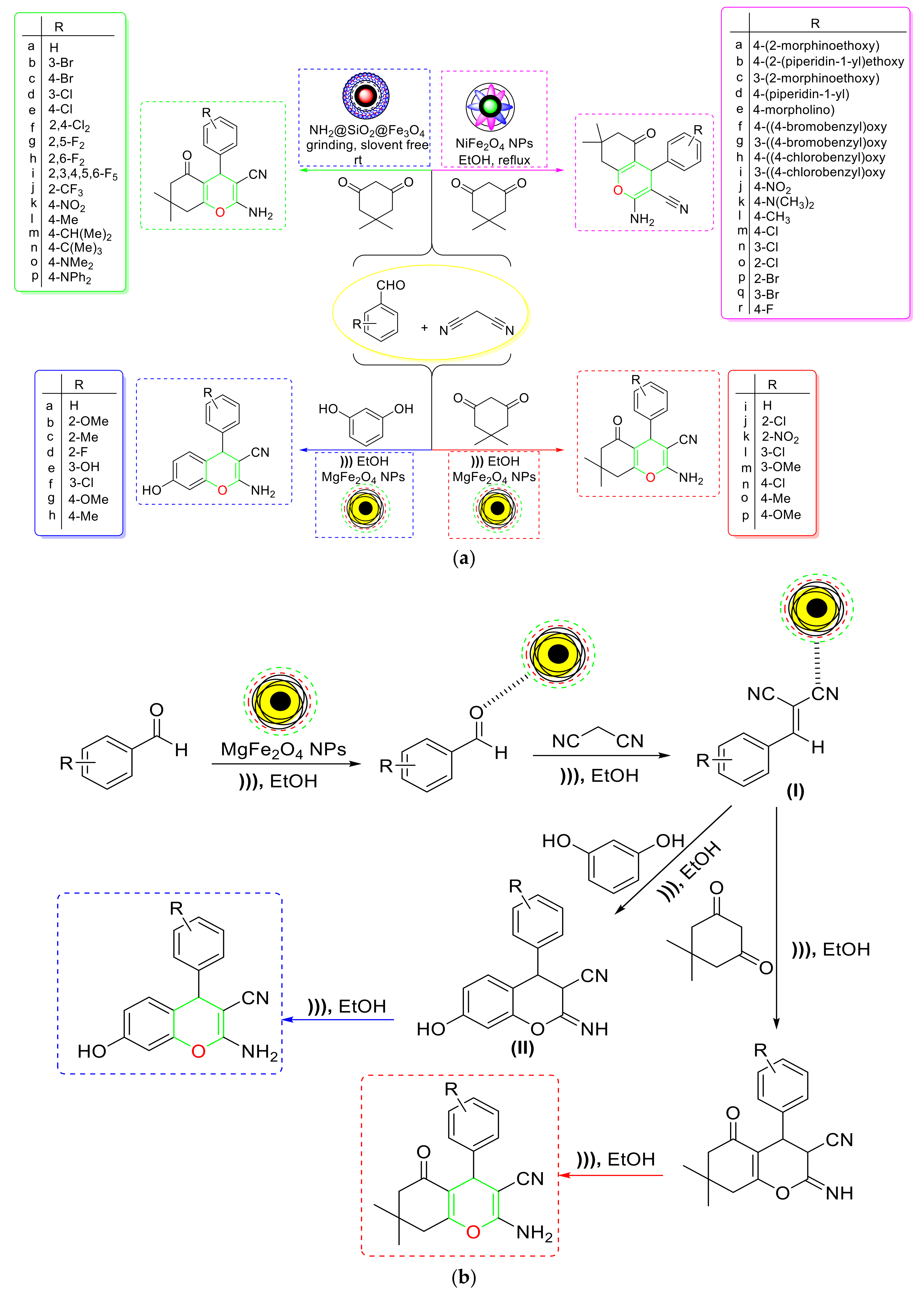
- Eshtehardian et al. used a green and simple method to produce MgFe2O4 NC, which they tested for the synthesis of 2-amino-7-hydroxy-4H-chromene and tetrahydrobenzo[b]pyran derivatives [103] (Scheme 20a). The proposed NC could be magnetically separated and reused four times without losing its catalytic activity. Based on mechanistic research, it was rationally assumed that Knoevenagel condensation of aldehyde and malononitrile formed an intermediate (I), which then proceeded through Michael addition with resorcinol to produce the adduct (II). To produce the corresponding product, this adduct might have undergone intramolecular cyclization followed by [1,3] H-shift (Scheme 20b). As a result, MgFe2O4’s Lewis acidic feature enabled Knoevenagel condensation and Michael addition by interacting with the aldehydic carbonyl O-atom and the cyanide group, respectively, and allowed the reaction to proceed successfully. As a result, the Lewis acidic feature of MgFe2O4 NC enhanced the Knoevenagel condensation and Michael addition by interacting with the aldehydic carbonyl O-atom and the cyanide group, respectively, and allowed the reaction to take place over its large surface area.
- Using a six-time-recoverable nickel ferrite NiFe2O4 NC, Pourshojaei et al. developed a one-pot cascade synthesis of 4H-chromenes [104] (Scheme 20a). The nanocatalyst’s amphoteric Lewis feature makes it useful for the fast synthesis of 4H-chromenes. According to a substrate scope investigation, the type of substituents on benzaldehyde had a significant impact on the effectiveness of the NC. The catalytic activity of the EWG was found to be higher than that of the ERG. According to the mechanistic findings, the Knoevenagel condensation between activated aldehydes and malononitrile first formed an intermediate (I), which then interacted with the activated dimedone and produced a new intermediate (II) after losing a water molecule. The newly created intermediate (II) was then subjected to intramolecular cyclization before being converted to the desired product via imine–enamine tautomerism (Scheme 20c).
- Singh et al. developed a magnetically retrievable amine-decorated SiO2@Fe3O4 hybrid NC (NH2@SiO2@Fe3O4) to carry out a solvent-free multicomponent synthesis of 2-amino-4H-benzo[b]pyran derivatives [105] (Scheme 20a). According to the data from the substrate scope study, the designed NC was tolerant of a wide variety of functional groups. According to mechanistic results, the proposed multicomponent method was initiated by the basic amino sites of the NC. The reaction began with the Knoevenagel condensation of malononitrile and aldehyde to produce arylidiene malononitrile, which was then Michael added to dimedone to produce an intermediate (I). To produce the intended product, this produced intermediate (I) was cyclized intramolecularly and then protonated (Scheme 20d). The hybrid NC has numerous notable characteristics, including durability, reusability, and recyclability for up to three reaction cycles, as well as a short reaction time and milder reaction conditions.
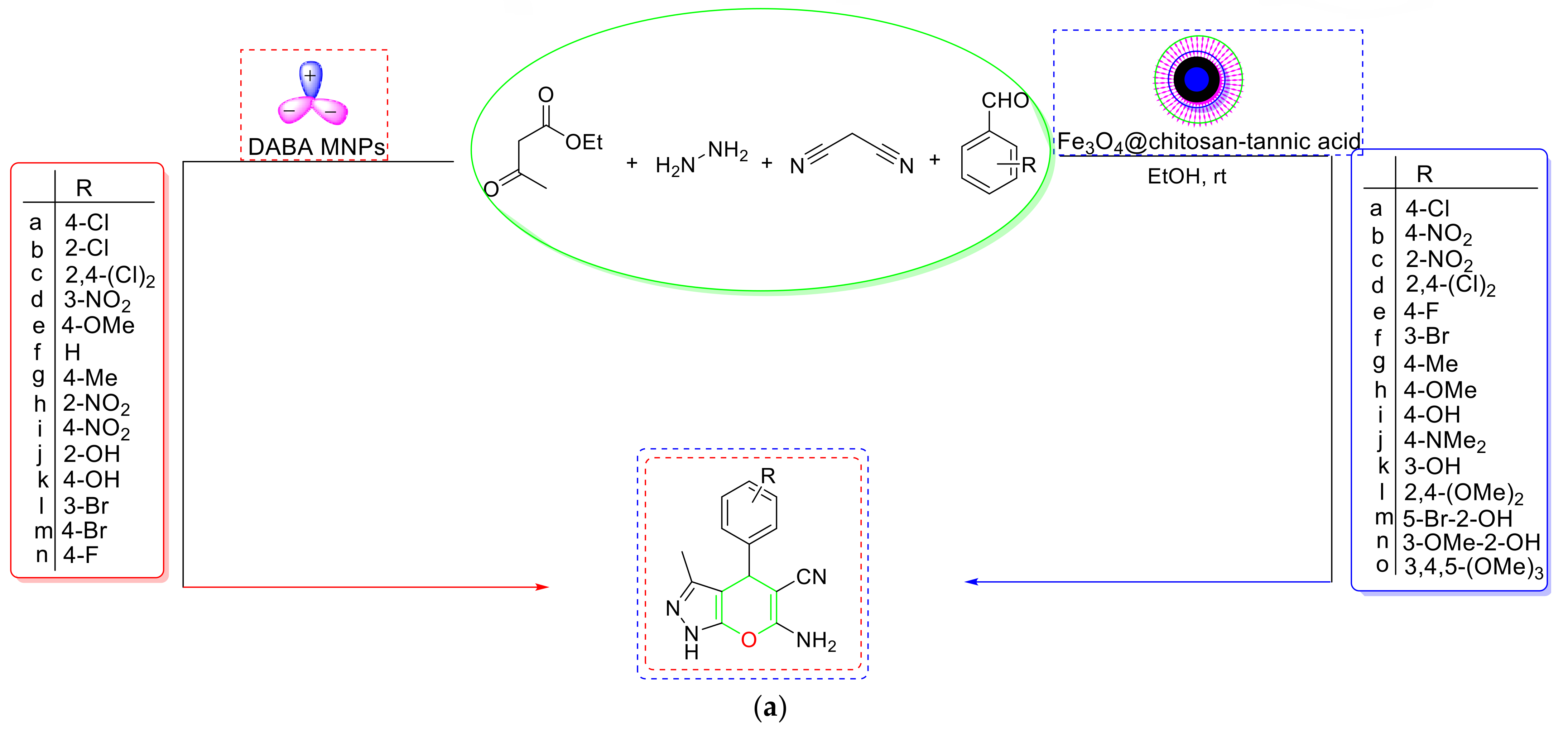
- Karami et al. developed a magnetic bifunctional (Fe3O4@SiO2@PTS-DABA) hybrid NC that was found to be catalytically effective for the synthesis of dihydropyranopyrazole [107] (Scheme 22a). According to the mechanistic findings, a pyrazolone ring was first generated by nanocatalyzed condensation between activated EAA and hydrazine hydrate, followed by dehydration and tautomerization. The Knoevenagel condensation of aldehyde and malononitrile created an intermediate (I), which interacted with pyrazolone to form the desired product by 6-exo-dig cyclization and tautomerization. It was discovered that the catalytic surface’s acidic and basic sites promoted intramolecular electrophilic cyclization via tautomerization (Scheme 22b). The features of the proposed process, such as quick synthesis, environmentally acceptable solvent, a five-time-recyclable catalyst, and the avoidance of caustic reagents, all make this technique appealing.
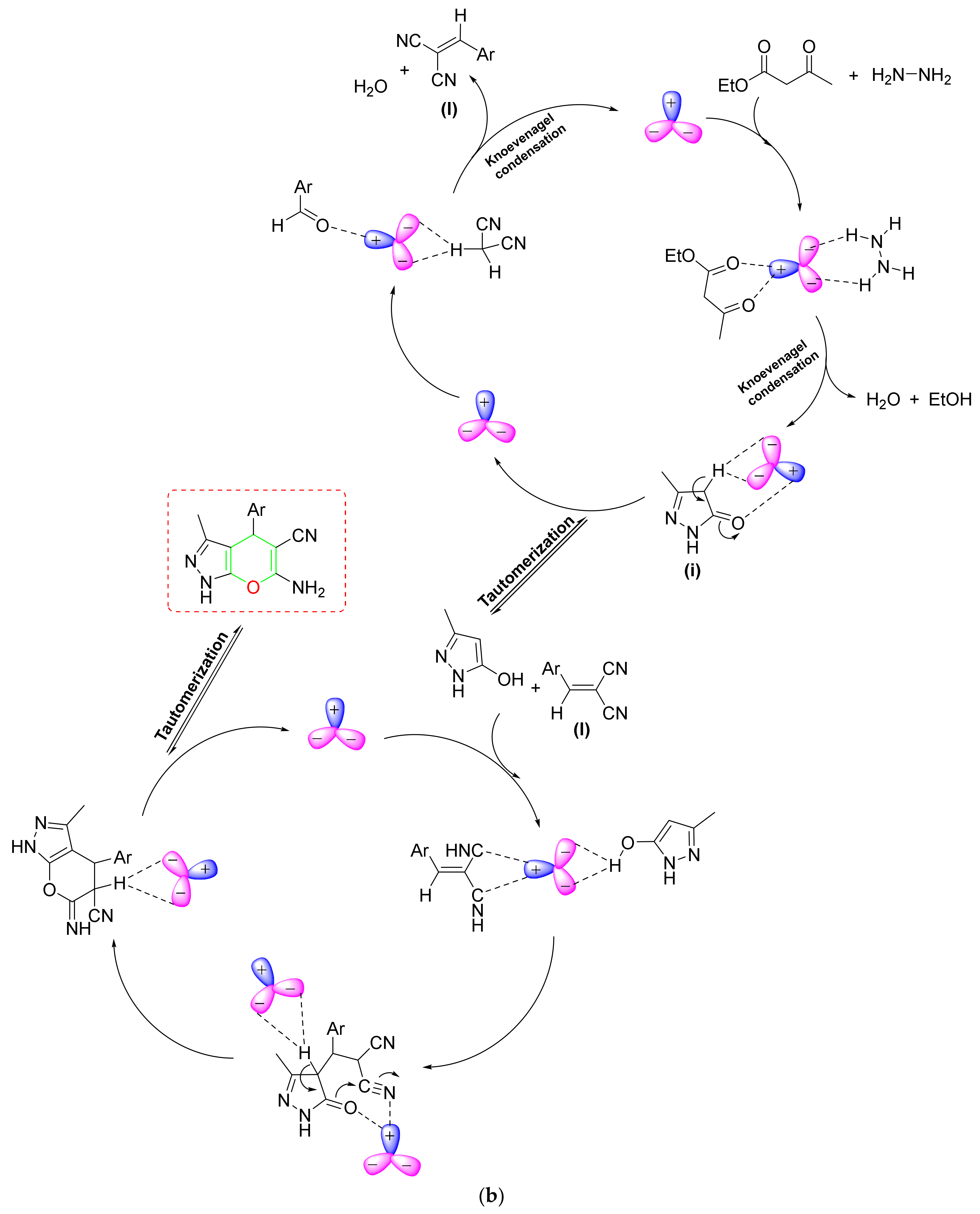
- In line with sustainable chemistry, Kamalzare et al. created a new magnetic biocomposite with chitosan and tannic acid (Fe3O4@chitosan-tannic acid) and used it in a cascade pyranopyrazole synthesis [108] (Scheme 22a). The observed hybrid NC had a high tolerance power for the benzaldehyde substituent pattern. According to one hypothesized mechanism, the proposed hybrid NC activated the carbonyl group of EAA and went through a nucleophilic assault of hydrazine to generate an intermediate, which was then subsequently attacked by another hydrazine molecule and eventually formed the pyrazolone ring (i) after losing water. The Knoevenagel condensation of aldehydes and malononitrile produced a novel intermediate (II), which was subsequently reacted with enolic pyrazolone to produce the desired product after significant intramolecular cyclization and tautomerization (Scheme 22c).
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef] [PubMed]
- Al-Mulla, A. A review: Biological importance of heterocyclic compounds. Der Pharma Chemica 2017, 9, 141–147. [Google Scholar]
- Bansal, P.; Gupta, S.; Christopher, A.; Gupta, V. Tragedies in clinical trials—A history wrapped up. Int. J. Clin. Pharmacol. Toxicol. 2015, 4, 169–178. [Google Scholar]
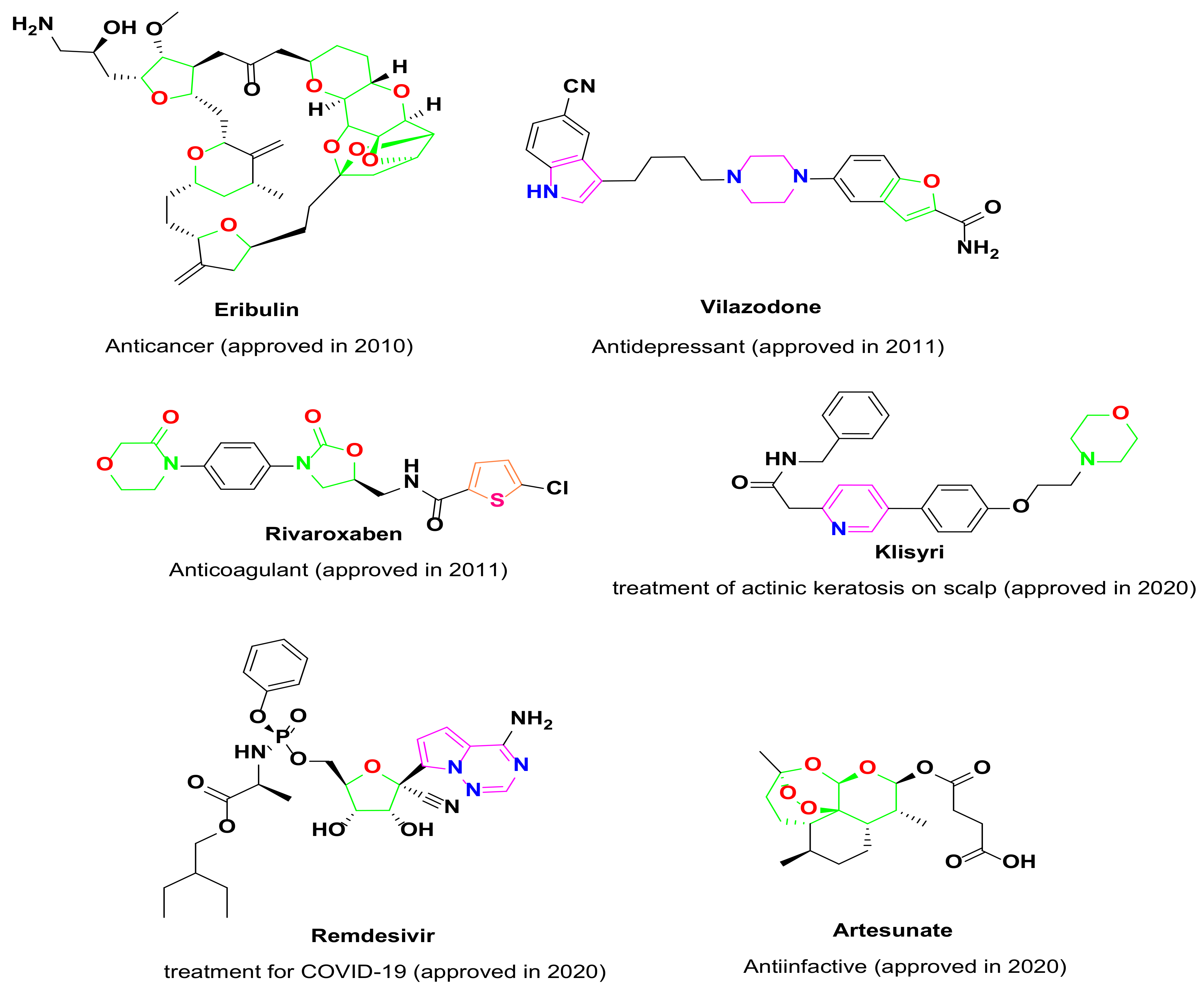
- Delost, M.D.; Smith, D.T.; Anderson, B.J.; Njardarson, J.T. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018, 61, 10996–11020. [Google Scholar] [CrossRef]
- Saroha, B.; Kumar, G.; Kumar, R.; Kumari, M.; Kumar, S. A minireview of 1,2,3-triazole hybrids with O-heterocycles as leads in medicinal chemistry. Chem. Biol. Drug Des. 2021, 1–27. [Google Scholar] [CrossRef]
- Singh, P.K.; Silakari, O. The Current Status of O-Heterocycles: A Synthetic and Medicinal Overview. ChemMedChem 2018, 13, 1071–1087. [Google Scholar] [CrossRef]
- Kumar, G.; Saroha, B.; Kumar, R.; Kumari, M.; Kumar, S. Recent Advances in Synthesis and Biological Assessment of Quinoline-Oxygen Heterocycle Hybrids. ChemistrySelect 2021, 6, 5148–5165. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Y.; Zhang, T.; Li, Y.; Han, L.; Liang, G. Characterization of Oxygenated Heterocyclic Compounds and in vitro Antioxidant Activity of Pomelo Essential Oil. Drug Des. Devel. Ther. 2021, 15, 937–947. [Google Scholar] [CrossRef]
- Avula, S.K.; Das, B.; Csuk, R.; Al-Harrasi, A. Naturally Occurring O-heterocycles as Anticancer Agents. Anticancer Agents Med. Chem. 2021, 21, 1. [Google Scholar] [CrossRef]
- Singh, D.; Hembrom, S. Neuroprotective effect of flavonoids: A systematic review. Int. J. Aging Res. 2019, 2, 26. [Google Scholar]
- Krishnapriya; Sasikumar, P.; Aswathy, M.; Prem, P.T.; Radhakrishnan, K.V.; Baby Chakrapani, P.S. Plant derived bioactive compounds and their potential to enhance adult neurogenesis. Phytomedicine Plus 2022, 2, 100191. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; Baaiu, B.S.; El-Sawy, E.R. Reactions and Antibacterial Activity of 6-Bromo-3-(2-Bromoacetyl)-2H-Chromen-2-One. Polycycl. Aromat. Compd. 2021, 1–10. [Google Scholar] [CrossRef]
- Nicolaou, K.C. The Emergence and Evolution of Organic Synthesis and Why It Is Important to Sustain It as an Advancing Art and Science for Its Own Sake. Isr. J. Chem. 2018, 58, 104–113. [Google Scholar] [CrossRef]
- Saroha, B.; Kumar, G.; Kumar, S.; Kumari, M.; Rani, M.; Raghav, N.; Sahoo, P.K.; Ghosh, S.; Mahata, S.; Nasare, V.D. Ultrasound assisted a one pot multicomponent and greener synthesis of 1, 2, 3-triazole incorporated aurone hybrids: Cathepsin B inhibition, anti-cancer activity against AGS cell line, and in-silico docking evaluation. Curr. Res. Green Sustain. Chem. 2022, 5, 100295. [Google Scholar] [CrossRef]
- Molnar, M.; Loncaric, M.; Kovac, M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Rawal, M.; Singh, A.; Amiji, M.M. Quality-by-Design Concepts to Improve Nanotechnology-Based Drug Development. Pharm. Res. 2019, 36, 153. [Google Scholar] [CrossRef]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green Nanotechnology-Driven Drug Delivery Assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef] [Green Version]
- Sindhwani, S.; Chan, W.C.W. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021, 290, 486–498. [Google Scholar] [CrossRef]
- Antonii, F. Panacea Aurea-Auro Potabile; Bibliopolio Frobeniano: Hamburg, Germany, 1618. [Google Scholar]
- Faraday, M. LIX. Experimental relations of gold (and other metals) to light—The bakerian lecture. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1857, 14, 512–539. [Google Scholar] [CrossRef]
- Rampino, L.D.; Nord, F.F. Preparation of Palladium and Platinum Synthetic High Polymer Catalysts and the Relationship between Particle Size and Rate of Hydrogenation. J. Am. Chem. Soc. 1941, 63, 2745–2749. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V. Recyclable Nanostructured Catalytic Systems in Modern Environmentally Friendly Organic Synthesis. Molecules 2010, 15, 4792–4814. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, B.A.; Rechelo, B.S.; Totoli, E.G.; Kogawa, A.C. Evolution of green chemistry and its Multidimentional Impacts: A Review. Saudi Pharm. J. 2018, 27. [Google Scholar] [CrossRef]
- Madan, Y. A mini review study on “the generation of bioactive heterocyclic motifs by using sustainable metal nanocatalysed organic synthetic routes”. Mater. Today Proc. 2020, 30, 197–202. [Google Scholar] [CrossRef]
- Rai, P.; Gupta, D. Magnetic nanoparticles as green catalysts in organic synthesis—A review. Synth. Commun. 2021, 51, 3059–3083. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Rahaman, H. Chapter 16—Noble Metal–Manganese Oxide Hybrid Nanocatalysts. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Woodhead Publishing: Sawston, UK, 2019; pp. 313–340. [Google Scholar] [CrossRef]
- Naapuri, J.M.; Losada-Garcia, N.; Deska, J.; Palomo, J.M. Synthesis of silver and gold nanoparticles–enzyme–polymer conjugate hybrids as dual-activity catalysts for chemoenzymatic cascade reactions. Nanoscale 2022, 14, 5701–5715. [Google Scholar] [CrossRef]
- Gulati, U.; Chinna Rajesh, U.; Rawat, D.S.; Zaleski, J.M. Development of magnesium oxide–silver hybrid nanocatalysts for synergistic carbon dioxide activation to afford esters and heterocycles at ambient pressure. Green Chem. 2020, 22, 3170–3177. [Google Scholar] [CrossRef]
- Niu, W.; Xu, G. Crystallographic control of noble metal nanocrystals. Nano Today 2011, 6, 265–285. [Google Scholar] [CrossRef]
- Wu, B.; Zheng, N. Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 2013, 8, 168–197. [Google Scholar] [CrossRef]
- Kahsar, K.R.; Schwartz, D.K.; Medlin, J.W. Control of Metal Catalyst Selectivity through Specific Noncovalent Molecular Interactions. J. Am. Chem. Soc. 2014, 136, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Somwanshi, S.B.; Somvanshi, S.B.; Kharat, P.B. Nanocatalyst: A Brief Review on Synthesis to Applications. J. Phys. Conf. Ser. 2020, 1644, 012046. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Singh, G. Nanocatalysts: Potential burning rate modifier for composite solid propellants. Mater. Focus 2014, 3, 81–91. [Google Scholar] [CrossRef]
- Elezovic, N.R.; Radmilovic, V.R.; Krstajic, N.V. Platinum nanocatalysts on metal oxide based supports for low temperature fuel cell applications. RSC Adv. 2016, 6, 6788–6801. [Google Scholar] [CrossRef]
- Hostert, L.; Blaskievicz, S.F.; Fonsaca, J.E.S.; Domingues, S.H.; Zarbin, A.J.G.; Orth, E.S. Imidazole-derived graphene nanocatalysts for organophosphate destruction: Powder and thin film heterogeneous reactions. J. Catal. 2017, 356, 75–84. [Google Scholar] [CrossRef]
- Zuliani, A.; Ivars, F.; Luque, R. Advances in Nanocatalyst Design for Biofuel Production. ChemCatChem 2018, 10, 1968–1981. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, X.; Huo, M.; Wang, L.; Chen, Y.; Chen, W.; Wang, B. Tumor-responsive copper-activated disulfiram for synergetic nanocatalytic tumor therapy. Nano Res. 2021, 14, 205–211. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. Highlights of the Nanocatalysis in Organic Chemistry. Catalysts 2021, 11, 213. [Google Scholar] [CrossRef]
- Arabmarkadeh, A.; Javahershenas, R.; Kazemi, M. Nanomaterials: Catalysis in synthesis of highly substituted heterocycles. Synth. Commun. 2021, 51, 880–903. [Google Scholar] [CrossRef]
- Atish, R.; Bahe, A.K.; Chanderiya, A.; Dangi, H.; Mishra, P.; Mishra, A.K.; Ratnesh, D. Synthesis of nitrogen and oxygen containing heterocyclic compounds using nano catalyst: A review. J. Turk. Chem. Soc. A Chem. 2021, 8, 851–862. [Google Scholar]
- Nishanth Rao, R.; Jena, S.; Mukherjee, M.; Maiti, B.; Chanda, K. Green synthesis of biologically active heterocycles of medicinal importance: A review. Environ. Chem. Lett. 2021, 19, 3315–3358. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, S.; Lamba, B.Y.; Patra, J.; Mahindroo, N. Nanocatalysts: Applications in synthesis of chalcones—A review. Synth. Commun. 2021, 51, 1–12. [Google Scholar] [CrossRef]
- Shiri, P. An overview on the copper-promoted synthesis of five-membered heterocyclic systems. Appl. Organomet. Chem. 2020, 34, e5600. [Google Scholar] [CrossRef]
- Shiri, P.; Aboonajmi, J. A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions. Beilstein J. Org. Chem. 2020, 16, 551–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Pourteymour Fard Tabrizi, F.; Kamari, N. Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Dhara, S.; Chakraborty, K. Turbinafuranone A–C, new 2-furanone analogues from marine macroalga Turbinaria ornata as prospective anti-hyperglycemic agents attenuate tyrosine phosphatase-1B. Med. Chem. Res. 2021, 30, 1635–1648. [Google Scholar] [CrossRef]
- Gouda, M.A.; Abd El-Ggani, G.E.; Berghot, M.A.; Khalil, A.E.G.M. Synthesis and antioxidant activity of some novel nicotinonitrile derivatives bearing a furan moiety. J. Heterocycl. Chem. 2019, 56, 2036–2045. [Google Scholar] [CrossRef]
- Silva Mendes, F.R.; da Silva, A.W.; Amâncio Ferreira, M.K.; de Lima Rebouças, E.; Marinho, E.M.; Marinho, M.M.; Bandeira, P.N.; Rodrigues Teixeira, A.M.; de Menezes, J.E.S.A.; de Siqueira, E.A.; et al. GABAA receptor participation in anxiolytic and anticonvulsant effects of (E)-3-(furan-2-yl)-1-(2hydroxy-3,4,6-trimethoxyphenyl)prop-2-en-1-one in adult zebrafish. Neurochem. Int. 2022, 155, 105303. [Google Scholar] [CrossRef]
- Lu, D.; Zhou, Y.; Li, Q.; Luo, J.; Jiang, Q.; He, B.; Tang, Q. Synthesis, in vitro antitumor activity and molecular mechanism of novel furan derivatives and their precursors. Anti-Cancer Agents Med. Chem. 2020, 20, 1475–1486. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, M.; Beretta, G.; Gelain, A.; Pini, E.; Sammartino, J.C.; Stelitano, G.; Barlocco, D.; Costantino, L.; Lapillo, M.; et al. New insight into structure-activity of furan-based salicylate synthase (MbtI) inhibitors as potential antitubercular agents. J. Enzyme Inhib. Med. Chem. 2019, 34, 823–828. [Google Scholar] [CrossRef] [Green Version]
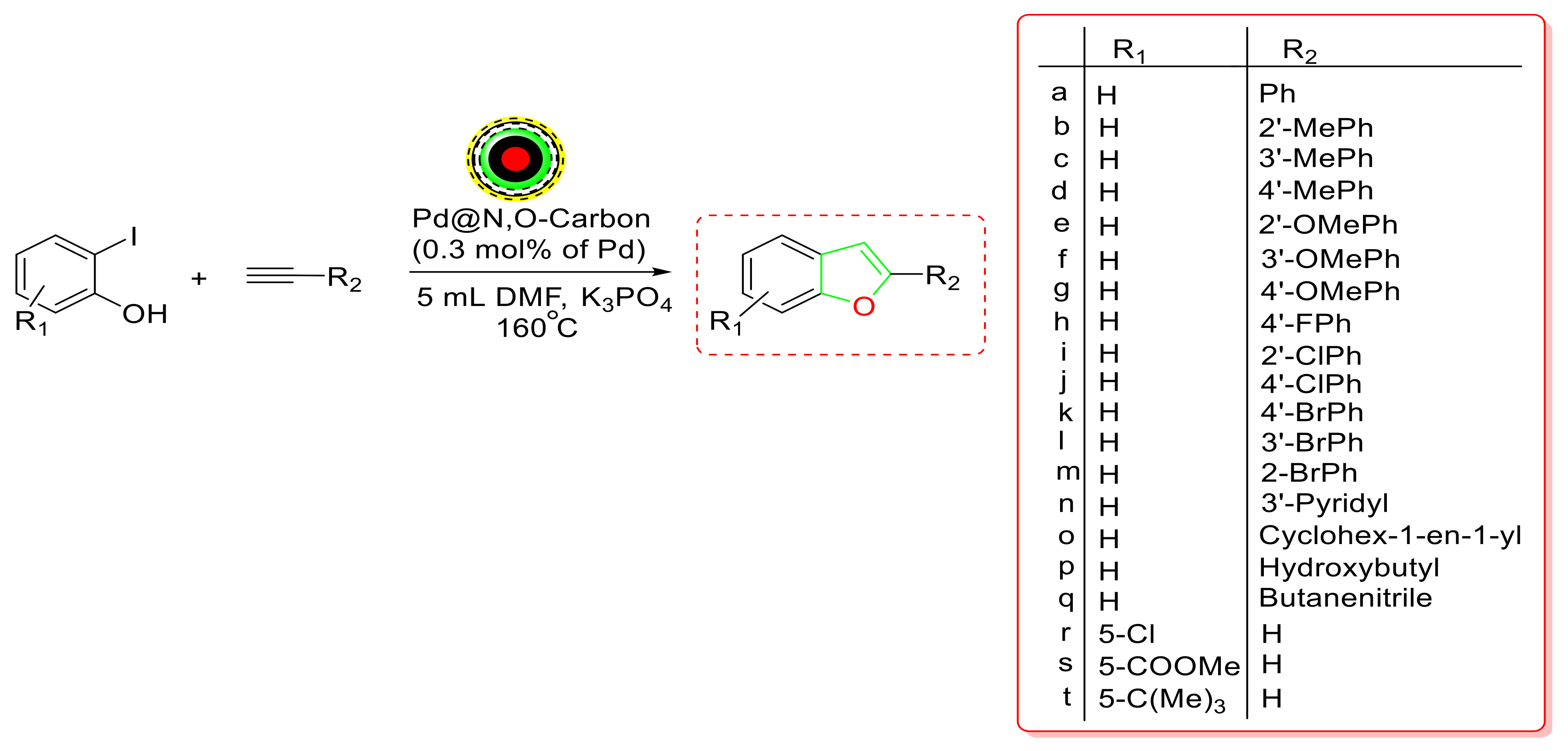
- Ji, G.; Duan, Y.; Zhang, S.; Yang, Y. Synthesis of benzofurans from terminal alkynes and iodophenols catalyzed by recyclable palladium nanoparticles supported on N,O-dual doped hierarchical porous carbon under copper- and ligand-free conditions. Catal. Today 2019, 330, 101–108. [Google Scholar] [CrossRef]
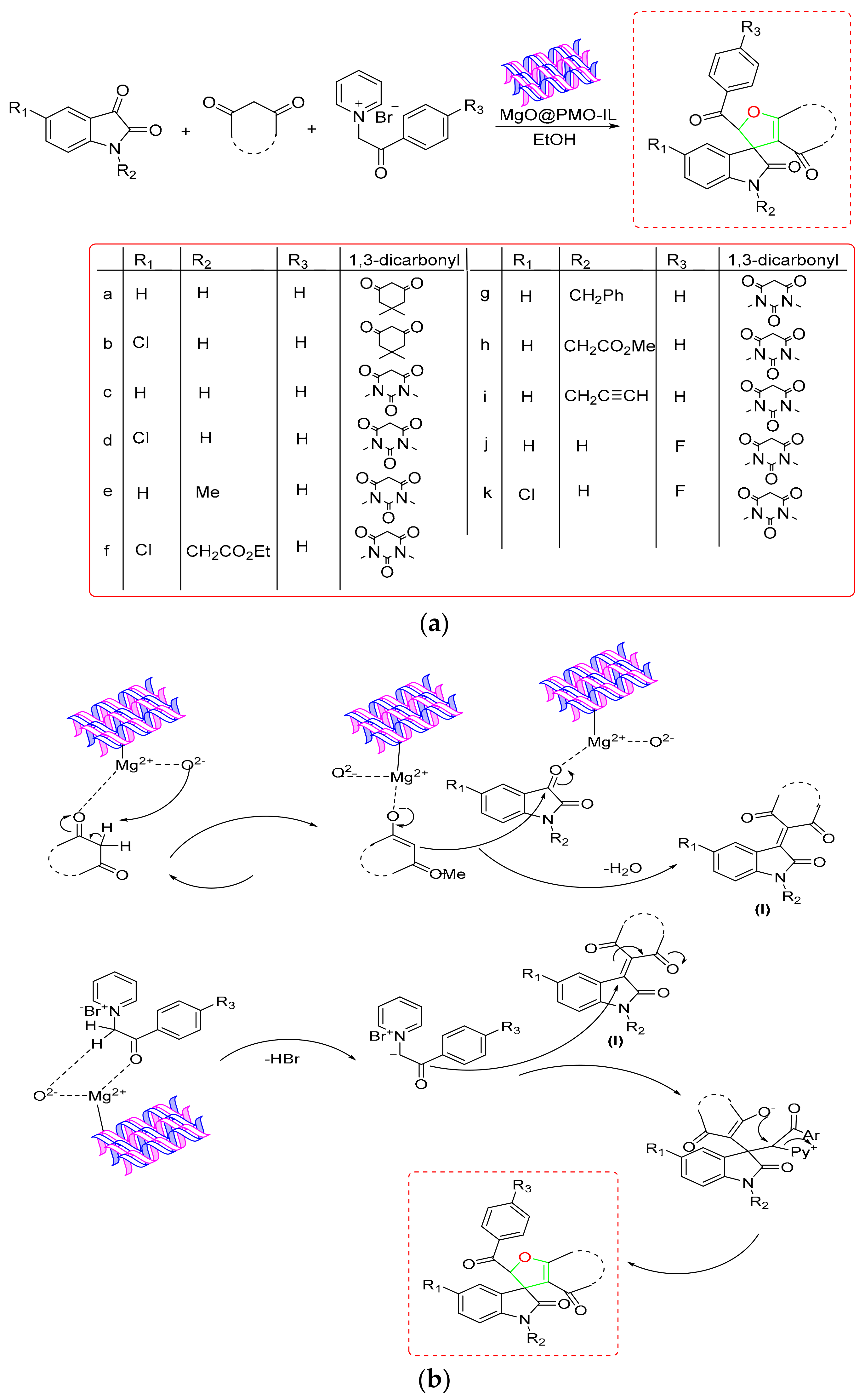
- Baharfar, R.; Zareyee, D.; Allahgholipour, S.L. Synthesis and characterization of MgO nanoparticles supported on ionic liquid-based periodic mesoporous organosilica (MgO@ PMO-IL) as a highly efficient and reusable nanocatalyst for the synthesis of novel spirooxindole-furan derivatives. Appl. Organomet. Chem. 2019, 33, e4805. [Google Scholar] [CrossRef]
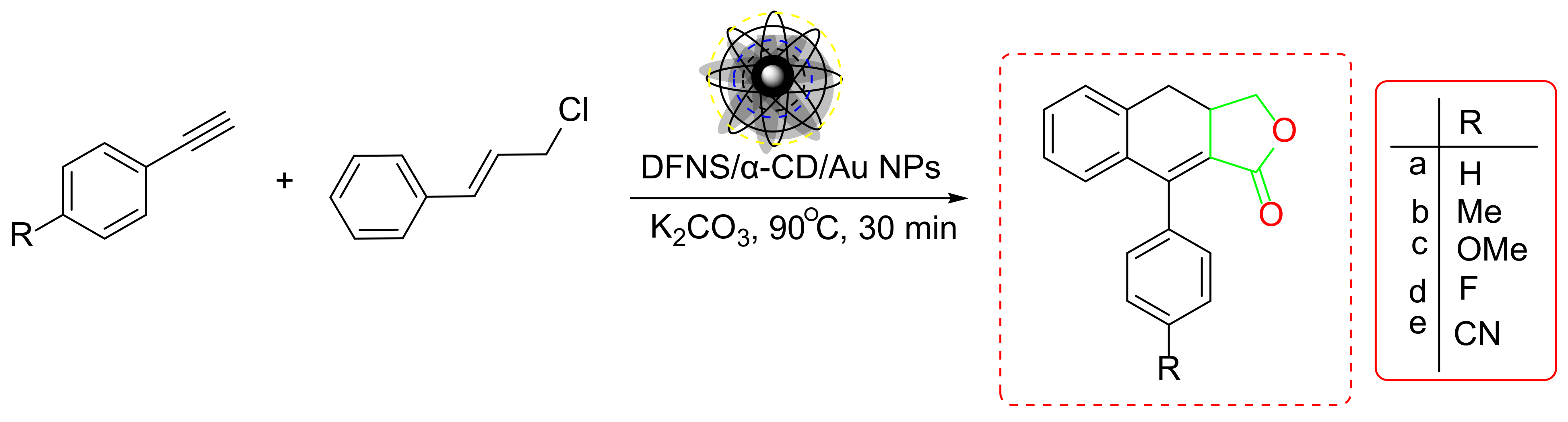
- Wang, Z.; Li, X.; Feng, L.; Liu, B.; Shamsa, F. DFNS/α-CD/Au as a Nanocatalyst for Interpolation of CO2 into Aryl Alkynes Followed by SN2 Coupling with Allylic Chlorides. Catal. Lett. 2021, 151, 1911–1922. [Google Scholar] [CrossRef]
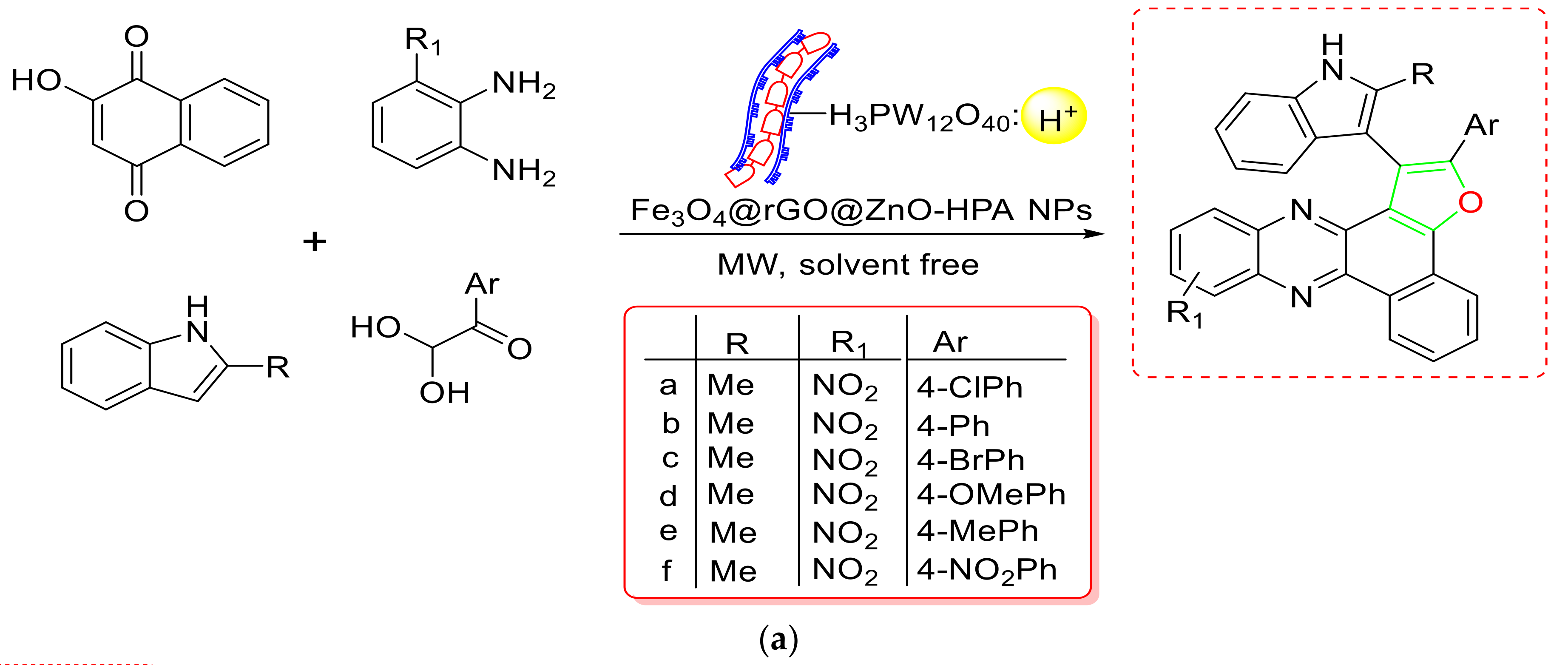
- Taheri, M.; Mohebat, R.; Moslemin, M.H. Microwave-Assisted Multi-Component Green Synthesis of Benzo[α]furo[2, 3-c]phenazine Derivatives via a Magnetically-Separable Fe3O4@rGO@ZnO-HPA Nanocatalyst under Solvent-Free Conditions. Polycycl. Aromat. Compd. 2021, 1–11. [Google Scholar] [CrossRef]
- Khodaei, M.M.; Alizadeh, A.; Haghipour, M. Supported 4-carboxybenzyl sulfamic acid on magnetic nanoparticles as a recoverable and recyclable catalyst for synthesis of 3,4,5-trisubstituted furan-2(5H)-one derivatives. J. Organomet. Chem. 2018, 870, 58–67. [Google Scholar] [CrossRef]
- Shirzaei, M.; Mollashahi, E.; Taher Maghsoodlou, M.; Lashkari, M. Novel synthesis of silica-coated magnetic nano-particles based on acidic ionic liquid, as a highly efficient catalyst for three component system leads to furans derivatives. J. Saudi Chem. Soc. 2020, 24, 216–222. [Google Scholar] [CrossRef]
- Hao, F.; Wang, X.; Mohammadnia, M. Preparation and Characterization of a Novel Magnetic Nano Catalyst for Synthesis and Antibacterial Activities of Novel Furan-2(5H)-Ones Derivatives. Polycycl. Aromat. Compd. 2021, 1–15. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K.P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg. Chem. 2021, 108, 104681. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, M.; Gaur, R.; Gupta, R.; Arora, G.; Rana, P.; Srivastava, A.; Sharma, R.K. Fabrication of copper-based silica-coated magnetic nanocatalyst for efficient one-pot synthesis of chalcones via A3 coupling of aldehydes-alkynes-amines. ChemCatChem 2020, 12, 2488–2496. [Google Scholar] [CrossRef]
- Akbarzadeh, Z.; Safaei-Ghomi, J. Ultrasound assisted eco-friendly synthesis of 3-cinnamoyl coumarins using N,N′-(1,2-phenylene)bis(2-aminobenzamide) dichloro cobalt immobilized on mesoporous Al-SBA-15 as a new and recyclable catalyst. Green Chem. Lett. Rev. 2020, 13, 141–154. [Google Scholar] [CrossRef]
- Aryan, R.; Mir, N.; Beyzaei, H.; Kharade, A. Design and synthesis of novel natural clinoptilolite-MnFe2O4 nanocomposites and their catalytic application in the facile and efficient synthesis of chalcone derivatives through Claisen-Schmidt reaction. Res. Chem. Intermed. 2018, 44, 4245–4258. [Google Scholar] [CrossRef]
- Borade, R.M.; Somvanshi, S.B.; Kale, S.B.; Pawar, R.P.; Jadhav, K.M. Spinel zinc ferrite nanoparticles: An active nanocatalyst for microwave irradiated solvent free synthesis of chalcones. Mater. Res. Express 2020, 7, 016116. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [Green Version]
- Ranjan Sahoo, C.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Kumar Sahu, P.; Dehury, B.; Nath Padhy, R.; Kumar Paidesetty, S. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 2021, 150, 104863. [Google Scholar] [CrossRef]
- Ghomi, J.S.; Akbarzadeh, Z. Ultrasonic accelerated Knoevenagel condensation by magnetically recoverable MgFe2O4 nanocatalyst: A rapid and green synthesis of coumarins under solvent-free conditions. Ultrason. Sonochem. 2018, 40, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Samiei, Z.; Soleimani-Amiri, S.; Azizi, Z. Fe3O4@C@OSO3H as an efficient, recyclable magnetic nanocatalyst in Pechmann condensation: Green synthesis, characterization, and theoretical study. Mol. Divers. 2021, 25, 67–86. [Google Scholar] [CrossRef]
- Mirosanloo, A.; Zareyee, D.; Khalilzadeh, M.A. Recyclable cellulose nanocrystal supported Palladium nanoparticles as an efficient heterogeneous catalyst for the solvent-free synthesis of coumarin derivatives via von Pechmann condensation. Appl. Organomet. Chem. 2018, 32, e4546. [Google Scholar] [CrossRef]
- Pakdel, S.; Akhlaghinia, B.; Mohammadinezhad, A. Fe3O4@Boehmite-NH2-CoII NPs: An Environment Friendly Nanocatalyst for Solvent Free Synthesis of Coumarin Derivatives Through Pechmann Condensation Reaction. Chemistry Africa 2019, 2, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Zarei, F.; Soleimani-Amiri, S.; Azizi, Z. Heterogeneously Catalyzed Pechmann Condensation Employing the HFe(SO4)2·4H2O-Chitosan Nano-Composite: Ultrasound-Accelerated Green Synthesis of Coumarins. Polycycl. Aromat. Compd. 2021, 1–18. [Google Scholar] [CrossRef]
- Yuan, J.; Mohammadnia, M. Preparation of a novel, efficient, and recyclable magnetic catalyst, Cu(II)-OHPC-Fe3O4 nanoparticles, and a solvent-free protocol for the synthesis of coumarin derivatives. J. Coord. Chem. 2021, 74, 2327–2343. [Google Scholar] [CrossRef]
- Feizpour Bonab, M.; Soleimani-Amiri, S.; Mirza, B. Fe3O4@C@PrS-SO3H: A Novel Efficient Magnetically Recoverable Heterogeneous Catalyst in the Ultrasound-Assisted Synthesis of Coumarin Derivatives. Polycycl. Aromat. Compd. 2022, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Zhao, Z.-L.; Zhou, C.-H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef]
- Zhu, J.; Mo, J.; Lin, H.-z.; Chen, Y.; Sun, H.-p. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef]
- Asha Bhanu, P.; China Raju, B.; Jayavardhana Rao, Y.; Narasimha, G.; Kesava Rao, B. Facile synthesis and docking studies of 7-hydroxyflavanone isoxazoles and acrylates as potential anti-microbial agents. Med. Chem. Res. 2020, 29, 217–228. [Google Scholar] [CrossRef]
- Chiacchio, M.A.; Lanza, G.; Chiacchio, U.; Giofrè, S.V.; Romeo, R.; Iannazzo, D.; Legnani, L. Oxazole-based compounds as anticancer agents. Curr. Med. Chem. 2019, 26, 7337–7371. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; O’Connor, P.D.; Blaser, A.; Yardley, V.; Maes, L.; Gupta, S.; Launay, D.; Martin, D.; Franzblau, S.G.; Wan, B.; et al. Repositioning Antitubercular 6-Nitro-2,3-dihydroimidazo[2,1-b][1,3]oxazoles for Neglected Tropical Diseases: Structure–Activity Studies on a Preclinical Candidate for Visceral Leishmaniasis. J. Med. Chem. 2016, 59, 2530–2550. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, H.M.; Al-Ostoot, F.H.; Vivek, H.K.; Khanum, S.A. Synthesis, characterization, DFT, docking studies and molecular dynamics of some 3-phenyl-5-furan isoxazole derivatives as anti-inflammatory and anti-ulcer agents. J. Mol. Struct. 2022, 1250, 131812. [Google Scholar]
- Algethami, F.K.; Saidi, I.; Abdelhamid, H.N.; Elamin, M.R.; Abdulkhair, B.Y.; Chrouda, A.; Ben Jannet, H. Trifluoromethylated Flavonoid-Based Isoxazoles as Antidiabetic and Anti-Obesity Agents: Synthesis, In Vitro α-Amylase Inhibitory Activity, Molecular Docking and Structure–Activity Relationship Analysis. Molecules 2021, 26, 5214. [Google Scholar] [CrossRef] [PubMed]
- Yatam, S.; Jadav, S.S.; Gundla, R.; Gundla, K.P.; Reddy, G.M.; Ahsan, M.J.; Chimakurthy, J. Design, Synthesis and Biological Evaluation of 2 (((5-aryl-1, 2, 4-oxadiazol-3-yl) methyl) thio) benzo [d] oxazoles: New Antiinflammatory and Antioxidant Agents. ChemistrySelect 2018, 3, 10305–10310. [Google Scholar] [CrossRef]
- Abdolmohammadi, S.; Hossaini, Z. Fe3O4 MNPs as a green catalyst for syntheses of functionalized [1, 3]-oxazole and 1H-pyrrolo-[1, 3]-oxazole derivatives and evaluation of their antioxidant activity. Mol. Divers. 2019, 23, 885–896. [Google Scholar] [CrossRef]
- Fekri, L.Z.; Nikpassand, M.; Shariati, S.; Aghazadeh, B.; Zarkeshvari, R.; Norouz pour, N. Synthesis and characterization of amino glucose-functionalized silica-coated NiFe2O4 nanoparticles: A heterogeneous, new and magnetically separable catalyst for the solvent-free synthesis of 2,4,5–trisubstituted imidazoles, benzo[d]imidazoles, benzo[d] oxazoles and azo-linked benzo[d]oxazoles. J. Organomet. Chem. 2018, 871, 60–73. [Google Scholar]
- Aboonajmi, J.; Sharghi, H.; Aberi, M.; Shiri, P. Consecutive Oxidation/Condensation/Cyclization/Aromatization Sequences Catalyzed by Nanostructured Iron(III)-Porphyrin Complex towards Benzoxazole Derivatives. Eur. J. Org. Chem. 2020, 2020, 5978–5984. [Google Scholar] [CrossRef]
- Yu, C.; Guo, X.; Shen, B.; Xi, Z.; Li, Q.; Yin, Z.; Liu, H.; Muzzio, M.; Shen, M.; Li, J.; et al. One-pot formic acid dehydrogenation and synthesis of benzene-fused heterocycles over reusable AgPd/WO2.72 nanocatalyst. J. Mater. Chem. A 2018, 6, 23766–23772. [Google Scholar] [CrossRef]
- Shanshak, M.; Budagumpi, S.; Małecki, J.G.; Keri, R.S. Green synthesis of 3, 4-disubstituted isoxazol-5 (4H)-ones using ZnO@ Fe3O4 core–shell nanocatalyst in water. Appl. Organomet. Chem. 2020, 34, e5544. [Google Scholar] [CrossRef]
- SUTHAR, M.; KUMBHANI, J.; BHATT, K.D. Pyran Heterocyclic Compound as the Prosperous Scaffolds for Biological Sensor (A-Review). Orient. J. Chem. 2021, 37, 1280–1286. [Google Scholar] [CrossRef]
- Safari, F.; Hosseini, H.; Bayat, M.; Ranjbar, A. Synthesis and evaluation of antimicrobial activity, cytotoxic and pro-apoptotic effects of novel spiro-4 H-pyran derivatives. RSC Adv. 2019, 9, 24843–24851. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-J.; Gong, Q.-T.; Wang, Y.; Yu, Y.; Liu, Y.-H.; Wang, N.; Yu, X.-Q. Biocatalytic tandem multicomponent reactions for one-pot synthesis of 2-Amino-4H-Pyran library and in vitro biological evaluation. Mol. Catal. 2020, 491, 110983. [Google Scholar] [CrossRef]
- Sirous, H.; Chemi, G.; Gemma, S.; Butini, S.; Debyser, Z.; Christ, F.; Saghaie, L.; Brogi, S.; Fassihi, A.; Campiani, G.; et al. Identification of Novel 3-Hydroxy-pyran-4-One Derivatives as Potent HIV-1 Integrase Inhibitors Using in silico Structure-Based Combinatorial Library Design Approach. Front. Chem. 2019, 7, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arustamyan, Z.S.; Margaryan, R.E.; Aghekyan, A.A.; Panosyan, G.A.; Muradyan, R.E.; Tumajyan, A.E. Synthesis and Anti-Inflammatory Properties of Substituted 5-(Tetrahydro-4-phenyl-2H-pyran-4-yl)-4H-1,2,4-triazole-3-thiols. Russ. J. Org. Chem. 2021, 57, 195–202. [Google Scholar] [CrossRef]
- Bhat, Z.S.; Lah, H.U.; Rather, M.A.; Maqbool, M.; Ara, T.; Ahmad, Z.; Yousuf, S.K. Synthesis and in vitro evaluation of substituted 3-cinnamoyl-4-hydroxy-pyran-2-one (CHP) in pursuit of new potential antituberculosis agents. MedChemComm 2018, 9, 165–172. [Google Scholar] [CrossRef]
- Sajjadi-Ghotbabadi, H.; Javanshir, S.; Rostami-Charati, F.; Zahra Sayyed-Alangi, S. Eco-compatible synthesis of novel 3-hydroxyflavones catalyzed by KF-impregnated mesoporous natural zeolite clinoptilolite. Chem. Heterocycl. Compd. 2018, 54, 508–513. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.S.; Akhlaghinia, B. γ-Fe2O3@ SiO2-EC-ZnII: A Magnetic Recyclable Nanocatalyst for the Synthesis of Spiro [indoline-3, 9′-xanthene] trione Derivatives in Aqueous Media. ChemistrySelect 2018, 3, 3161–3170. [Google Scholar] [CrossRef]
- Mohammadi, H.; Shaterian, H.R. γ-Fe2O3@SiO2-γ-aminobutyric acid as a novel superparamagnetic nanocatalyst promoted green synthesis of chromeno[4,3,2-de][1,6]naphthyridine derivatives. Monats. Chem. 2019, 150, 327–337. [Google Scholar] [CrossRef]
- Eshtehardian, B.; Rouhani, M.; Mirjafary, Z. Green protocol for synthesis of MgFe2O4 nanoparticles and study of their activity as an efficient catalyst for the synthesis of chromene and pyran derivatives under ultrasound irradiation. J. Iran. Chem. Soc. 2020, 17, 469–481. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Zolala, F.; Eskandari, K.; Talebi, M.; Morsali, L.; Amiri, M.; Khodadadi, A.; Shamsimeymandi, R.; Faghih-Mirzaei, E.; Asadipour, A. Nickel ferrite (NiFe2O4) nanoparticles as magnetically recyclable nanocatalyst for highly efficient synthesis of 4H-chromene derivatives. J. Nanosci. Nanotechnol. 2020, 20, 3206–3216. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Yadav, P.; Mishra, A.; Awasthi, S.K. Green and Mechanochemical One-Pot Multicomponent Synthesis of Bioactive 2-amino-4H-benzo[b]pyrans via Highly Efficient Amine-Functionalized SiO2@Fe3O4 Nanoparticles. ACS Omega 2020, 5, 4223–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lati, M.P.; Shirini, F.; Alinia-Asli, M.; Rezvani, M.A. Fe3O4@ SiO2-Sultone: A Novel and Recyclable Magnetic Nanocatalyst for the Efficient Synthesis of 3, 4 Dihydropyrano [c] Chromenes and 2-Amino-4H-Chromene Derivatives. J. Nanosci. Nanotechnol. 2020, 20, 973–982. [Google Scholar] [CrossRef]
- Karami, S.; Dekamin, M.G.; Valiey, E.; Shakib, P. DABA MNPs: A new and efficient magnetic bifunctional nanocatalyst for the green synthesis of biologically active pyrano[2,3-c]pyrazole and benzylpyrazolyl coumarin derivatives. New J. Chem. 2020, 44, 13952–13961. [Google Scholar] [CrossRef]
- Kamalzare, M.; Ahghari, M.R.; Bayat, M.; Maleki, A. Fe3O4@chitosan-tannic acid bionanocomposite as a novel nanocatalyst for the synthesis of pyranopyrazoles. Sci. Rep. 2021, 11, 20021. [Google Scholar] [CrossRef]
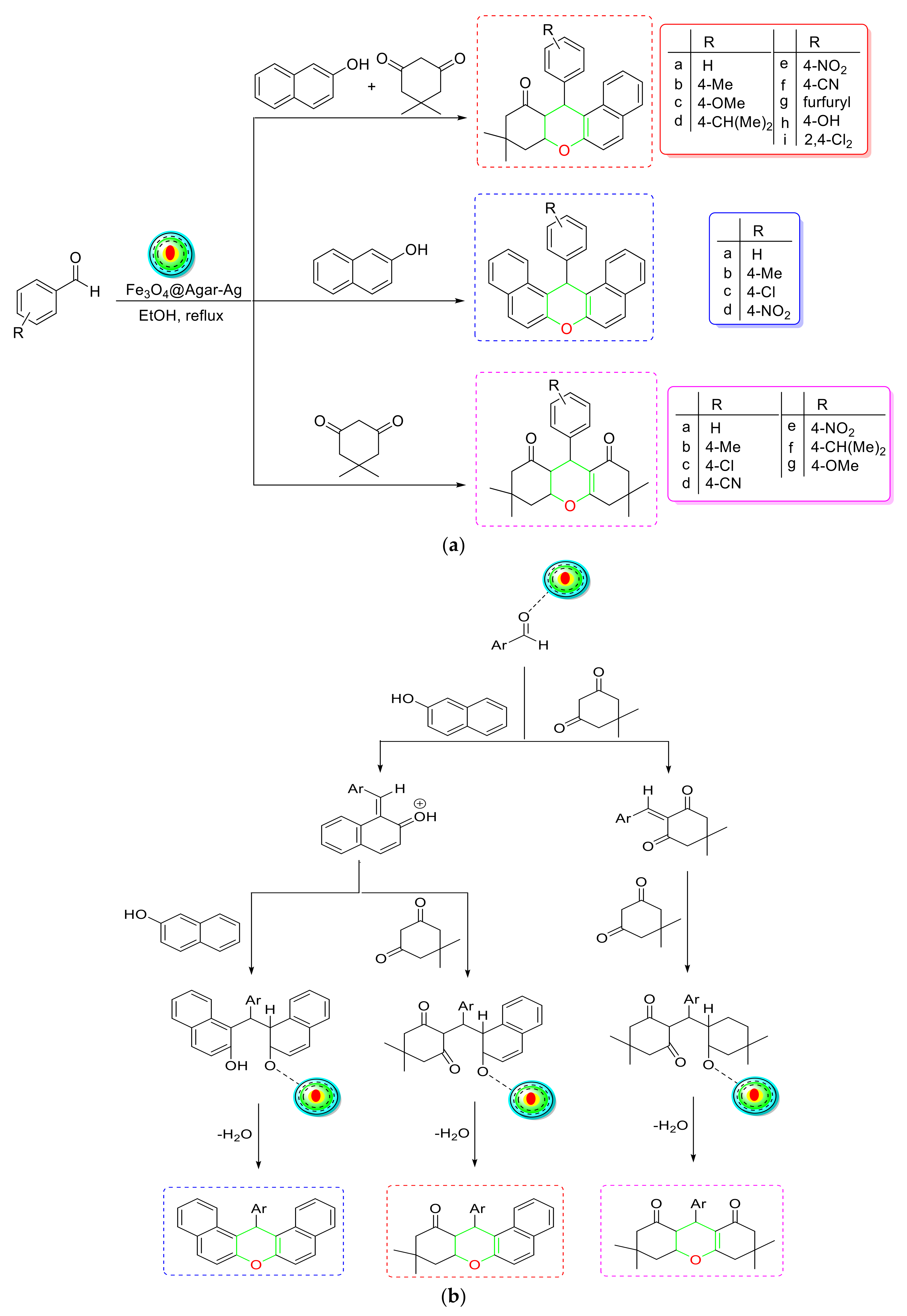
- Hoseinzade, K.; Mousavi-Mashhadi, S.A.; Shiri, A. An efficient and green one-pot synthesis of tetrahydrobenzo[a]xanthenes, 1,8-dioxo-octahydroxanthenes and dibenzo[a,j]xanthenes by Fe3O4@Agar-Ag as nanocatalyst. Mol. Divers. 2022. [Google Scholar] [CrossRef]
- Tanuraghaj, H.M.; Farahi, M. A novel protocol for the synthesis of pyrano[2,3-h]coumarins in the presence of Fe3O4@SiO2@(CH2)3OCO2Na as a magnetically heterogeneous catalyst. New J. Chem. 2019, 43, 4823–4829. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Saroha, B.; Kumar, G.; Lathwal, E.; Kumar, S.; Parshad, B.; Kumari, M.; Kumar, N.; Mphahlele-Makgwane, M.M.; Makgwane, P.R. Recent Developments in Nanocatalyzed Green Synthetic Protocols of Biologically Potent Diverse O-Heterocycles—A Review. Catalysts 2022, 12, 657. https://doi.org/10.3390/catal12060657
Kumar S, Saroha B, Kumar G, Lathwal E, Kumar S, Parshad B, Kumari M, Kumar N, Mphahlele-Makgwane MM, Makgwane PR. Recent Developments in Nanocatalyzed Green Synthetic Protocols of Biologically Potent Diverse O-Heterocycles—A Review. Catalysts. 2022; 12(6):657. https://doi.org/10.3390/catal12060657
Chicago/Turabian StyleKumar, Suresh, Bhavna Saroha, Gourav Kumar, Ekta Lathwal, Sanjeev Kumar, Badri Parshad, Meena Kumari, Naveen Kumar, Mabel M. Mphahlele-Makgwane, and Peter R. Makgwane. 2022. "Recent Developments in Nanocatalyzed Green Synthetic Protocols of Biologically Potent Diverse O-Heterocycles—A Review" Catalysts 12, no. 6: 657. https://doi.org/10.3390/catal12060657
APA StyleKumar, S., Saroha, B., Kumar, G., Lathwal, E., Kumar, S., Parshad, B., Kumari, M., Kumar, N., Mphahlele-Makgwane, M. M., & Makgwane, P. R. (2022). Recent Developments in Nanocatalyzed Green Synthetic Protocols of Biologically Potent Diverse O-Heterocycles—A Review. Catalysts, 12(6), 657. https://doi.org/10.3390/catal12060657