Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC
Abstract
:1. Introduction
2. De-Alloyed Treatment
| Sample | Initial Composition | Pt [wt. %] | Composition after Acid Treatment | Treatment Conditions | Electrochemical Characteristics | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acid, Molarity | Duration | Temperature | Features | ESA, m2g−1 (Pt) | Activity in ORR, A mg−1 (Pt) | |||||
| PtNi/C | Pt25Ni75 | 28 | Pt55Ni45 | 0.5 M H2SO4 | 24 h | room | 0.5 g of catalyst—100 mL of acid with stirring, air atmosphere | 30 | 740 | [39] |
| PtNi/C | PtNi3 | 25 | Pt34Ni66 | 0.5 g of catalyst—100 mL of acid with stirring, N2 | 28 | 760 | ||||
| PtCu/C | Pt0.3Cu | - | Pt1.8Cu | 1 M HNO3 | 2 days | room | - | 50 | 290 | [33] |
| PtCu/C | PtCu0.84 | 14.8 | PtCu0.62 | 1.5 M H2SO4 | 3 h | room | - | 57.5 | 200 | [34] |
| PtCu/C | PtCu2.86 | 10.12 | PtCu0.76 | 22.8 | 501 | |||||
| PtCu/C | PtCu4.65 | 7.2 | PtCu0.53 | 46.7 | 407 | |||||
| Cu@Pt/C | Pt78Cu22 | - | Pt89Cu11 | 1 M HNO3 | 2 h | room | Accompanied by ultrasonic processing | 62.86 | 230 | [23] |
| Hollow PtNi/C | Pt50Ni50 | - | Pt92Ni8 | 1 M H2SO4 | 22 h | 20 °C | - | - | 500 | [42] |
| Hollow PtCo/C | Pt81Co19±5 | - | Pt81Co4.1±3 | 1 M H2SO4 | 24 h | room | - | - | 150 (µA cm−2) | [43] |
| PtCu/C | PtCu2.20 | 14 | PtCu0.76 | 1 M HNO3 | 6 h | room | - | 103 | 200 | [36] |
| Nanoporous PtNi/C | Pt0.36Ni0.64 | - | Pt0.79Ni0.21 | Conc. HNO3 | 1 min | room | Particles without a support were processed under the influence of ultrasound | 85.6 | - | [41] |
| PtCu/C | PtCu0.67 | 22.2 | PtCu0.36 | 1 M HNO3 | 3 h | room | - | 48 | 332 | [45] |
| PtCu/C | Pt19Cu81 | 6.5 | Pt47Cu53 | 1 M CH3COOH | 2 h | room | 4-fold acid washing with CO atmosphere for 30 min per stage | 91 | 1870 | [40] |
| PtFe/C | - | - | Pt84Fe16 | 0.5 M H2SO4 | 12 h | 60 °C | - | 54 | 159 | [35] |
| PtNi/Pt | - | 2-15 | - | 0.01 M HCl | 1 h | room | - | 52.65 | 800 | [37] |
| Sample | Initial Composition | Pt [wt. %] | Composition after Activation | Treatment Conditions | Electrochemical Characteristics | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrolyte, Molarity | Potential Range | Sweep Speed, mV s−1 | The Number of Cycles | ESA, m2 g−1 (Pt) | Activity, MA A mg−1 (Pt) | Activity, SA mA cm−2 | |||||
| Nanowire PtCu/C | Pt/Cu 0.59 | 20 | Pt/Cu 1.43 | 0.5 M H2SO4 or 0.1 M HClO4 | −0.2 to 0.9 V vs. Ag/AgCl | 400 | 400 | 63.5 | 3.15 | - | [32] |
| PtCu/C | 1:3, Pt25Cu75 | 22 | (Pt79Cu21) pure surface Pt (Pt93Cu7) | 0.1 M HClO4 | 0.06 to 1.2 V vs. RHE | 1000 | 200 | 89 | 0.52 | 0.5 | [46] |
| PtCu/CA | PtCu | 20 | - | 0.1 M HClO4 | 0 to 1.1 V vs. RHE | 50 | 300 | 72.4 | 0.054 | 0.075 | [52] |
| PtCu/C | PtCu | 21 | - | 0.1 M HClO4 | 0 to 1.1 V vs. RHE | 50 | 300 | 46.3 | 0.113 | 0.244 | |
| PtNi/C | Ni:Pt (72:28) 1:3 Pt: Ni | - | Ni:Pt (22:78) | 0.1M HClO4 | Step 1: 0.05 to 1.2 V vs. RHE | 0.5 | 200 | - | - | 2.6 | [53] |
| Step 2: 0.06 to 1.2 V vs. RHE | 100 | 3 | |||||||||
| PtNi/C | Ni75Pt25 | - | - | 0.1M H2SO4 | 0.05 to 1.2 V vs. RHE | 250 | >50 | 41 | 1.16 ± 0.035 mA μgPt−1 | 2.98 ± 0.12 | [54] |
| PtCu/C | Cu3Pt | 26 | 56% Cu | 0.1 M HClO4 | 0.05 to 1.2 V vs. RHE | 200 | 500 | 23 | 0.58 | 2.5 | [55] |
| PtCu/C | Cu3Pt | 26 | 80% Cu | 0.1 M HClO4 | holding the potential at 1.2 V (15 min or 2 h) | - | - | 32 | 0.77 | 2.4 | |
| PtCo/C | Pt37±2Co63±2 | - | Pt80±2Co20±2 | 0.1 M HClO4 | 0.06 to 1.0 V vs. RHE | 100 | 3 | 45 | 0.38 | 0.804 | [59] |
| 500 | 200 | ||||||||||
| 100 | 3 | ||||||||||
| PtCu/C | Pt35±2Cu65±2 | - | Pt65±2Cu35±2 | 0.1 M HClO4 | 0.06 to 1.0 V vs. RHE | 100 | 3 | 47 | 0.41 | 0.873 | |
| 500 | 200 | ||||||||||
| 100 | 3 | ||||||||||
| PtCu/C | 0.1 M HClO4 | 0.05 to 1.35 V vs. RHE | 300 | [60] | |||||||
2.1. Acid Treatment
2.2. Electrochemical Activation
3. Thermal Treatment
3.1. Pt/C Catalysts
3.2. PtM/C Catalysts
3.3. PtCo/C Catalysts
3.4. PtNi/C Catalysts
3.5. PtCu/C Catalysts
3.6. PtCr/C Catalysts
3.7. Comparison of Catalysts Heat Treatment Alloying with Various D-Metals
4. Combination of Various Types of Pre-Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FC | Fuel cells |
| PEMFC | Proton exchange membrane fuel cells |
| MEA | Membrane-electrode assembly |
| CV | Cyclic voltammogram |
| ESA | Electrochemically active surface area |
| ORR | Oxygen reduction reaction |
| RHE | Reversible hydrogen electrode |
| ICP | Inductively Coupled Plasma |
| EDX | Energy dispersive X-ray |
| HAADF STEM | High-angle annular dark-field scanning transmission electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| TEM | Transmission electron microscopy |
| FCC | Face centered cubic |
| FCT | Face centered tetragonal |
| BCC | Body-centered cubic |
| HSAC | High surface area carbon |
| EXAFS | Extended X-ray absorption fine structure |
| FCNTs | Functionalized multi-walled carbon nanotubes |
References
- Kongkanand, A.; Mathias, M.F. the priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Jankovic, J.; Susac, D.; Saha, M.S.; Tam, M.; Yang, H.; Ko, F. Electrospun carbon nanofiber catalyst layers for polymer electrolyte membrane fuel cells: Fabrication and optimization. J. Mater. Sci. 2018, 53, 11633–11647. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, D.; Zhang, Q.; Sun, Y.; Zhang, S.; Du, F.; Jin, X. Synthesis of PtCu–based nanocatalysts: Fundamentals and emerging challenges in energy conversion. J. Energy Chem. 2022, 64, 583–606. [Google Scholar] [CrossRef]
- Wang, C.; Spendelow, J.S. recent developments in Pt–Co catalysts for proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2021, 28, 100715. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Luo, F.; Liao, S. Core–shell-structured low-platinum electrocatalysts for fuel cell applications. Electrochem. Energy Rev. 2018, 1, 324–387. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Dobrovolsky, Y.A.; Shaglaeva, N.S.; Frolova, L.A.; Gerasimova, E.V.; Sanginov, E.A. Nanostructured materials for low-temperature fuel cells. Russ. Chem. Rev. 2012, 81, 191–220. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. a comprehensive review of pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef]
- Strasser, P. Free electrons to molecular bonds and back: Closing the energetic oxygen reduction (ORR)–Oxygen evolution (OER) cycle using core—Shell Nanoelectrocatalysts. Acc. Chem. Res. 2016, 49, 2658–2668. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Hong, Y.; Kim, B.; Choi, S.-I.; Lee, K. Pt–Cu based nanocrystals as promising catalysts for various electrocatalytic reactions. J. Mater. Chem. A 2019, 7, 17183–17203. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gullón, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen reduction reaction on nanostructured pt-based electrocatalysts: A review. Int. J. Hydrogen Energy 2020, 45, 31775–31797. [Google Scholar] [CrossRef]
- Mølmen, L.; Eiler, K.; Fast, L.; Leisner, P.; Pellicer, E. Recent advances in catalyst materials for proton exchange membrane fuel cells. APL Mater. 2021, 9, 040702. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, A.; Luo, L.; Wang, C.; Yang, F.; Wei, G.; Xia, G.; Yin, J.; Zhang, J. The asymmetric effects of Cu 2+ contamination in a proton exchange membrane fuel cell (PEMFC). Fuel Cells 2020, 20, 196–202. [Google Scholar] [CrossRef]
- Moriau, L.J.; Hrnjić, A.; Pavlišič, A.; Kamšek, A.R.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Pavko, L.; Šelih, V.S.; Bele, M.; et al. Resolving the nanoparticles’ structure-property relationships at the atomic level: A study of pt-based electrocatalysts. iScience 2021, 24, 102102. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuna, E.; Mrdenovic, D.; Jönsson-Niedziółka, M.; Pieta, P.; Pieta, I.S. Bimetallic nanocatalysts supported on graphitic carbon nitride for sustainable energy development: The shape-structure–activity relation. Nanoscale Adv. 2021, 3, 1342–1351. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Y.; Mao, X.; Li, D.; Li, G.; Zhuang, L.; Wang, X.; Yang, D.; Wang, Q.; Du, A.; et al. Gradient-concentration design of stable core–shell nanostructure for acidic oxygen reduction electrocatalysis. Adv. Mater. 2020, 32, 2003493. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Belenov, S.V.; Menshikov, V.S.; Tabachkova, N.Y.; Safronenko, O.I.; Moguchikh, E.A. Pt/C electrocatalysts based on the nanoparticles with the gradient structure. Int. J. Hydrogen Energy 2018, 43, 3676–3687. [Google Scholar] [CrossRef]
- Lim, T.; Kim, O.-H.; Sung, Y.-E.; Kim, H.-J.; Lee, H.-N.; Cho, Y.-H.; Kwon, O.J. Preparation of onion-like Pt-terminated Pt–Cu bimetallic nano-sized electrocatalysts for oxygen reduction reaction in fuel cells. J. Power Sources 2016, 316, 124–131. [Google Scholar] [CrossRef]
- Herron, J.A.; Jiao, J.; Hahn, K.; Peng, G.; Adzic, R.R.; Mavrikakis, M. Oxygen reduction reaction on platinum-terminated “onion-structured” alloy catalysts. Electrocatalysis 2012, 3, 192–202. [Google Scholar] [CrossRef]
- Ge, X.; Chen, L.; Kang, J.; Fujita, T.; Hirata, A.; Zhang, W.; Jiang, J.; Chen, M. A core-shell nanoporous Pt-Cu catalyst with tunable composition and high catalytic activity. Adv. Funct. Mater. 2013, 23, 4156–4162. [Google Scholar] [CrossRef]
- Luo, M.; Wei, L.; Wang, F.; Han, K.; Zhu, H. Gram-level synthesis of core–shell structured catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2014, 270, 34–41. [Google Scholar] [CrossRef]
- Chandran, R.; Dharmalingam, S. Facile synthesis and characterization of PtCu core–shell and alloy nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 6957–6964. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, A.A.; Belenov, S.V.; Menshikov, V.S.; Guterman, V.E. Pt(Cu)/C electrocatalysts with low platinum content. Russ. J. Electrochem. 2018, 54, 415–425. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Pakharev, A.Y.; Min, M.; Tabachkova, N.Y.; Mikheykina, E.B.; Vysochina, L.L.; Lastovina, T.A. Pt-M/C (M = Cu, Ag) electrocatalysts with an inhomogeneous distribution of metals in the nanoparticles. Int. J. Hydrogen Energy 2016, 41, 1609–1626. [Google Scholar] [CrossRef]
- Sarkar, A.; Manthiram, A. Synthesis of Pt@Cu core−shell nanoparticles by galvanic displacement of Cu by Pt 4+ ions and their application as electrocatalysts for oxygen reduction reaction in fuel cells. J. Phys. Chem. C 2010, 114, 4725–4732. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Wu, Z.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.; et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef]
- Bezerra, C.W.B.; Zhang, L.; Liu, H.; Lee, K.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. J. Power Sources 2007, 173, 891–908. [Google Scholar] [CrossRef]
- Sang, Q.; Hao, S.; Han, J.; Ding, Y. Dealloyed nanoporous materials for electrochemical energy conversion and storage. EnergyChem 2022, 4, 100069. [Google Scholar] [CrossRef]
- Weissmüller, J.; Sieradzki, K. Dealloyed nanoporous materials with interface-controlled behavior. MRS Bull. 2018, 43, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Xue, H.; Bao, A.; Wang, Z.; Sun, J.; Song, T.; Ge, X.; Zhang, W.; Huang, K.; He, F.; et al. Achieving superior electrocatalytic performance by surface copper vacancy defects during electrochemical etching process. Angew. Chem. Int. Ed. 2020, 59, 13778–13784. [Google Scholar] [CrossRef] [PubMed]
- Dutta, I.; Carpenter, M.K.; Balogh, M.P.; Ziegelbauer, J.M.; Moylan, T.E.; Atwan, M.H.; Irish, N.P. Electrochemical and structural study of a chemically dealloyed PtCu oxygen reduction catalyst. J. Phys. Chem. C 2010, 114, 16309–16320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, Y.; Park, J.H.; Kim, P.; Joo, J.B. Dealloyed PtCu catalyst as an efficient electrocatalyst in oxygen reduction reaction. Curr. Appl. Phys. 2015, 15, 993–999. [Google Scholar] [CrossRef]
- Gan, L.; Yu, R.; Luo, J.; Cheng, Z.; Zhu, J. Lattice strain distributions in individual dealloyed Pt–Fe catalyst nanoparticles. J. Phys. Chem. Lett. 2012, 3, 934–938. [Google Scholar] [CrossRef]
- Kirakosyan, S.A.; Alekseenko, A.A.; Guterman, V.E.; Novomlinskii, I.N.; Men’shchikov, V.S.; Gerasimova, E.V.; Nikulin, A.Y. De-Alloyed PtCu/C Catalysts of Oxygen Electroreduction. Russ. J. Electrochem. 2019, 55, 1258–1268. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, D.; Jang, I.; Kim, N.D.; Yoo, S.J.; Kim, P. Synthesis of hollow structured PtNi/Pt core/shell and Pt-Only nanoparticles via galvanic displacement and selective etching for efficient oxygen reduction reaction. J. Ind. Eng. Chem. 2022. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Niu, Z.; Chen, P.; Zhou, G.; Li, Y. A strategy for designing a concave Pt-Ni Alloy through controllable chemical etching. Angew. Chem. Int. Ed. 2012, 51, 12524–12528. [Google Scholar] [CrossRef]
- Gan, L.; Heggen, M.; O’Malley, R.; Theobald, B.; Strasser, P. Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 2013, 13, 1131–1138. [Google Scholar] [CrossRef]
- Gatalo, M.; Moriau, L.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Grom, M.; Galun, T.; Jovanovič, P.; Pavlišič, A.; Bele, M.; et al. CO-assisted ex-situ chemical activation of Pt-Cu/C oxygen reduction reaction electrocatalyst. Electrochim. Acta 2019, 306, 377–386. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, P.; Li, Y. General preparation for Pt-Based alloy nanoporous nanoparticles as potential nanocatalysts. Sci. Rep. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubau, L.; Lopez-Haro, M.; Durst, J.; Maillard, F. Atomic-scale restructuring of hollow PtNi/C electrocatalysts during accelerated stress tests. Catal. Today 2016, 262, 146–154. [Google Scholar] [CrossRef]
- Lopez-Haro, M.; Dubau, L.; Guétaz, L.; Bayle-Guillemaud, P.; Chatenet, M.; André, J.; Caqué, N.; Rossinot, E.; Maillard, F. Atomic-scale structure and composition of Pt3Co/C nanocrystallites during real pemfc operation: A STEM–EELS study. Appl. Catal. B Environ. 2014, 152–153, 300–308. [Google Scholar] [CrossRef]
- Menshchikov, V.; Alekseenko, A.; Guterman, V.; Nechitailov, A.; Glebova, N.; Tomasov, A.; Spiridonova, O.; Belenov, S.; Zelenina, N.; Safronenko, O. Effective platinum-copper catalysts for methanol oxidation and oxygen reduction in proton-exchange membrane fuel cell. Nanomaterials 2020, 10, 742. [Google Scholar] [CrossRef] [Green Version]
- Pavlets, A.; Alekseenko, A.; Nikulin, A. Influence of acid treatment on the functional characteristics of PtCu/C electrocatalysts. In Springer Proceedings in Materials; Springer: Cham, Switzerland, 2021; Volume 10, pp. 25–35. [Google Scholar] [CrossRef]
- Koh, S.; Strasser, P. Electrocatalysis on bimetallic surfaces: Modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Am. Chem. Soc. 2007, 129, 12624–12625. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Yu, C.; Strasser, P. In-situ, In-layer De-alloying of Pt-M intermetallics for high performance PEMFC electrode layers: Mea activity and durability studies. ECS Trans. 2007, 11, 933–939. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Neyerlin, K.C.; Srivastava, R.; Yu, C.; Strasser, P. Electrochemical activity and stability of dealloyed Pt–Cu and Pt–Cu–Co electrocatalysts for the oxygen reduction reaction (ORR). J. Power Sources 2009, 186, 261–267. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Strasser, P. Dealloyed Pt−Cu core−shell nanoparticle electrocatalysts for use in PEM fuel cell cathodes. J. Phys. Chem. C 2008, 112, 2770–2778. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovič, P.; Ruiz-Zepeda, F.; Pavlišič, A.; Robba, A.; Bale, M.; Dražić, G.; Gaberšček, M.; Hodnik, N. Insights into electrochemical dealloying of Cu out of Au-doped Pt-alloy nanoparticles at the sub-nano-scale. J. Electrochem. Sci. Eng. 2018, 8, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Barim, S.B.; Bozbag, S.E.; Deljoo, B.; Aindow, M.; Erkey, C. Highly active carbon supported PtCu electrocatalysts for PEMFCs by in Situ supercritical deposition coupled with electrochemical dealloying. Fuel Cells 2020, 20, 285–299. [Google Scholar] [CrossRef]
- Baldizzone, C.; Gan, L.; Hodnik, N.; Keeley, G.P.; Kostka, A.; Heggen, M.; Strasser, P.; Mayrhofer, K.J.J. Stability of dealloyed porous Pt/Ni nanoparticles. ACS Catal. 2015, 5, 5000–5007. [Google Scholar] [CrossRef]
- Snyder, J.; McCue, I.; Livi, K.; Erlebacher, J. Structure/processing/properties relationships in nanoporous nanoparticles as applied to catalysis of the cathodic oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 8633–8645. [Google Scholar] [CrossRef] [PubMed]
- Jeyabharathi, C.; Hodnik, N.; Baldizzone, C.; Meier, J.C.; Heggen, M.; Phani, K.L.N.; Bele, M.; Zorko, M.; Hocevar, S.; Mayrhofer, K.J.J. Time evolution of the stability and oxygen reduction reaction activity of PtCu/C nanoparticles. ChemCatChem 2013, 5, 2627–2635. [Google Scholar] [CrossRef]
- Yang, T.; Pukazhselvan, D.; da Silva, E.L.; Santos, M.C.; Meng, L.; Ramasamy, D.; Jothi, S.; Graça, V.; Shi, S. Highly branched Pt Cu nanodandelion with high activity for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 174–179. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Xin, H.L.; Hovden, R.; Ercius, P.; Mundy, J.A.; Chen, H.; Richard, J.H.; Muller, D.A.; DiSalvo, F.J.; et al. Tuning oxygen reduction reaction activity via controllable dealloying: A model study of ordered Cu 3 Pt/C intermetallic nanocatalysts. Nano Lett. 2012, 12, 5230–5238. [Google Scholar] [CrossRef]
- Garcia-Cardona, J.; Sirés, I.; Alcaide, F.; Brillas, E.; Centellas, F.; Cabot, P.L. Electrochemical performance of carbon-supported Pt(Cu) electrocatalysts for low-temperature fuel cells. Int. J. Hydrogen Energy 2020, 45, 20582–20593. [Google Scholar] [CrossRef]
- Oezaslan, M.; Heggen, M.; Strasser, P. Size-dependent morphology of dealloyed bimetallic catalysts: Linking the nano to the macro scale. J. Am. Chem. Soc. 2012, 134, 514–524. [Google Scholar] [CrossRef]
- Ruiz-Zepeda, F.; Gatalo, M.; Pavlišič, A.; Dražić, G.; Jovanovič, P.; Bele, M.; Gaberšček, M.; Hodnik, N. Atomically resolved anisotropic electrochemical shaping of nano-electrocatalyst. Nano Lett. 2019, 19, 4919–4927. [Google Scholar] [CrossRef] [Green Version]
- Pavlets, A.; Alekseenko, A.; Menshchikov, V.; Belenov, S.; Volochaev, V.; Pankov, I.; Safronenko, O.; Guterman, V. Influence of electrochemical pretreatment conditions of PtCu/C alloy electrocatalyst on its activity. Nanomaterials 2021, 11, 1499. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Pavlets, A.S.; Belenov, S.V.; Safronenko, O.I.; Pankov, I.V.; Guterman, V.E. The electrochemical activation mode as a way to exceptional ORR performance of nanostructured PtCu/C materials. Appl. Surf. Sci. 2022, 595, 153533. [Google Scholar] [CrossRef]
- Cai, X.; Lin, R.; Shen, D.; Zhu, Y. Gram-scale synthesis of well-dispersed shape-controlled Pt−Ni/C as high-performance catalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2019, 11, 29689–29697. [Google Scholar] [CrossRef]
- Niu, H.-J.; Chen, H.-Y.; Wen, G.-L.; Feng, J.-J.; Zhang, Q.-L.; Wang, A.-J. One-pot solvothermal synthesis of three-dimensional hollow PtCu alloyed dodecahedron nanoframes with excellent electrocatalytic performances for hydrogen evolution and oxygen reduction. J. Colloid Interface Sci. 2019, 539, 525–532. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovič, P.; Petek, U.; Šala, M.; Šelih, V.S.; Ruiz-Zepeda, F.; Bele, M.; Hodnik, N.; Gaberšček, M. Comparison of Pt–Cu/C with benchmark Pt–Co/C: Metal dissolution and their surface interactions. ACS Appl. Energy Mater. 2019, 2, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Cherevko, S.; Kulyk, N.; Mayrhofer, K.J.J. Durability of platinum-based fuel cell electrocatalysts: Dissolution of bulk and nanoscale platinum. Nano Energy 2016, 29, 275–298. [Google Scholar] [CrossRef]
- Topalov, A.A.; Katsounaros, I.; Auinger, M.; Cherevko, S.; Meier, J.C.; Klemm, S.O.; Mayrhofer, K.J.J. Dissolution of platinum: Limits for the deployment of electrochemical energy conversion? Angew. Chem. Int. Ed. 2012, 51, 12613–12615. [Google Scholar] [CrossRef] [Green Version]
- Topalov, A.A.; Cherevko, S.; Zeradjanin, A.R.; Meier, J.C.; Katsounaros, I.; Mayrhofer, K.J.J. Towards a comprehensive understanding of platinum dissolution in acidic media. Chem. Sci. 2014, 5, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–realloying enabled high durability for Pt–Pd-3d-transition metal nanoparticle fuel cell catalysts. Nat. Commun. 2021, 12, 859. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Wang, Z.; Yao, X.; Wei, Y.; Kang, Y.; Du, H.; Li, J.; Gan, L. Mitigating metal dissolution and redeposition of Pt-Co catalysts in PEM fuel cells: Impacts of structural ordering and particle size. J. Electrochem. Soc. 2020, 167, 064520. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Shan, S.; Zang, S.-Q.; Zhong, C.-J. Dynamic core–shell and alloy structures of multimetallic nanomaterials and their catalytic synergies. Acc. Chem. Res. 2020, 53, 2913–2924. [Google Scholar] [CrossRef]
- Oezaslan, M.; Hasché, F.; Strasser, P. PtCu 3, PtCu and Pt 3 Cu alloy nanoparticle electrocatalysts for oxygen reduction reaction in alkaline and acidic media. J. Electrochem. Soc. 2012, 159, B444–B454. [Google Scholar] [CrossRef]
- Yang, W.; Zou, L.; Huang, Q.; Zou, Z.; Hu, Y.; Yang, H. Lattice contracted ordered intermetallic core-shell PtCo@Pt nanoparticles: Synthesis, structure and origin for enhanced oxygen reduction reaction. J. Electrochem. Soc. 2017, 164, H331–H337. [Google Scholar] [CrossRef]
- Su, Y.; Feng, M.; Zhang, C.; Yan, Z.; Liu, H.; Tang, J.; Du, H. Platinum nanowires: Structural and catalytic evolution upon annealing temperature. Electrochim. Acta 2015, 164, 182–186. [Google Scholar] [CrossRef]
- Humphrey, J.J.L.; Sadasivan, S.; Plana, D.; Celorrio, V.; Tooze, R.A.; Fermín, D.J. Surface activation of Pt nanoparticles synthesised by “hot injection” in the presence of oleylamine. Chem. A Eur. J. 2015, 21, 12694–12701. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Moon, Y.; Han, O.; Hwang, K.; Kim, I.; Kim, H. Heat treatment and potential cycling effects on surface morphology, particle size, and catalytic activity of Pt/C catalysts studied by 13C NMR, TEM, XRD and CV. Electrochem. Commun. 2007, 9, 317–324. [Google Scholar] [CrossRef]
- Hasché, F.; Oezaslan, M.; Strasser, P. In situ observation of the thermally induced growth of platinum-nanoparticle catalysts using high-temperature X-Ray diffraction. ChemPhysChem 2012, 13, 828–834. [Google Scholar] [CrossRef]
- Chung, D.Y.; Chung, Y.-H.; Jung, N.; Choi, K.-H.; Sung, Y.-E. Correlation between platinum nanoparticle surface rearrangement induced by heat treatment and activity for an oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 13658. [Google Scholar] [CrossRef]
- Wettergren, K.; Schweinberger, F.F.; Deiana, D.; Ridge, C.J.; Crampton, A.S.; Rötzer, M.D.; Hansen, T.W.; Zhdanov, V.P.; Heiz, U.; Langhammer, C. High sintering resistance of size-selected platinum cluster catalysts by suppressed ostwald ripening. Nano Lett. 2014, 14, 5803–5809. [Google Scholar] [CrossRef]
- Jeon, M.K.; Zhang, Y.; McGinn, P.J. A comparative study of PtCo, PtCr, and PtCoCr catalysts for oxygen electro-reduction reaction. Electrochim. Acta 2010, 55, 5318–5325. [Google Scholar] [CrossRef]
- Kang, Y.S.; Yoo, S.J.; Lee, M.J.; Kim, M.-J.; Lee, S.Y.; Lee, K.-S.; Sung, Y.-E. Facile synthesis of platinum alloy electrocatalyst via aluminum reducing agent and the effect of post heat treatment for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 22952–22962. [Google Scholar] [CrossRef]
- Jung, W.S.; Lee, J. Induced changes of Pt/C in activity and durability through heat-treatment for oxygen reduction reaction in acidic medium. Int. J. Hydrogen Energy 2017, 42, 22830–22840. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, C.; Rusakova, I.A.; Huang, D.; Strasser, P. Synthesis of Pt3Co alloy nanocatalyst via reverse micelle for oxygen reduction reaction in PEMFCs. Top. Catal. 2008, 49, 241–250. [Google Scholar] [CrossRef]
- Valisi, A.N.; Maiyalagan, T.; Khotseng, L.; Linkov, V.; Pasupathi, S. Effects of heat treatment on the catalytic activity and methanol tolerance of carbon-supported platinum alloys. Electrocatalysis 2012, 3, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Lee, C.; Bang, K.; Lim, J.; Lee, H.; Ryu, H.J.; Cho, E.; Lee, H.M. Synthesis of chemically ordered Pt 3 Fe/C intermetallic electrocatalysts for oxygen reduction reaction with enhanced activity and durability via a removable carbon coating. ACS Appl. Mater. Interfaces 2017, 9, 31806–31815. [Google Scholar] [CrossRef]
- Zamanzad Ghavidel, M.R.; Monteverde Videla, A.H.A.; Specchia, S.; Easton, E.B. The relationship between the structure and ethanol oxidation activity of Pt-Cu/C alloy catalysts. Electrochim. Acta 2017, 230, 58–72. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Belenov, S.V.; Shemet, D.B.; Srabionyan, V.V.; Avakyan, L.A.; Volochaev, V.A.; Mikheykin, A.S.; Bdoyan, K.E.; Zizak, I.; Guterman, V.E.; et al. Effect of thermal treatment on the atomic structure and electrochemical characteristics of bimetallic PtCu core–shell nanoparticles in PtCu/C electrocatalysts. J. Phys. Chem. C 2018, 122, 17199–17210. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Belenov, S.V.; Shemet, D.B.; Volochaev, V.A.; Srabionyan, V.V.; Avakyan, L.A.; Tabachkova, N.Y.; Guterman, V.E.; Bugaev, L.A. The effect of thermal treatment on the atomic structure of core–shell PtCu nanoparticles in PtCu/C electrocatalysts. Phys. Solid State 2017, 59, 1666–1673. [Google Scholar] [CrossRef]
- Belenov, S.V.; Menschikov, V.S.; Nevelskaya, A.K.; Rezvan, D.V. Influence of PtCuAu’s nanoparticle structure on its activity in methanol oxidation reaction. Nanotechnol Russ 2019, 14, 557–564. [Google Scholar] [CrossRef]
- Mauer, D.; Belenov, S.; Guterman, V.; Nikolsky, A.; Kozakov, A.; Nikulin, A.; Alexeenko, D.; Safronenko, O. Gram-scale synthesis of CoO/C as base for PtCo/C high-performance catalysts for the oxygen reduction reaction. Catalysts 2021, 11, 1539. [Google Scholar] [CrossRef]
- Konno, N.; Mizuno, S.; Nakaji, H.; Ishikawa, Y. Development of compact and high-performance fuel cell stack. SAE Int. J. Altern. Powertrains 2015, 4, 2015-01-1175. [Google Scholar] [CrossRef]
- Lin, R.; Cao, C.; Zhao, T.; Huang, Z.; Li, B.; Wieckowski, A.; Ma, J. Synthesis and application of core–shell Co@Pt/C electrocatalysts for proton exchange membrane fuel cells. J. Power Sources 2013, 223, 190–198. [Google Scholar] [CrossRef]
- Lin, R.; Zhao, T.; Shang, M.; Wang, J.; Tang, W.; Guterman, V.E.; Ma, J. Effect of heat treatment on the activity and stability of PtCo/C catalyst and application of in-Situ X-Ray absorption near edge structure for proton exchange membrane fuel cell. J. Power Sources 2015, 293, 274–282. [Google Scholar] [CrossRef]
- Oezaslan, M.; Strasser, P. Activity of dealloyed PtCo3 and PtCu3 nanoparticle electrocatalyst for oxygen reduction reaction in polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 5240–5249. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.; Wang, H.; Wang, J.; Tang, Y.; Mao, Z.; Sasaki, K. H 2 -induced thermal treatment significantly influences the development of a high performance low-platinum core-shell PtNi/C alloyed oxygen reduction catalyst. Int. J. Energy Res. 2020, 44, 4773–4783. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Kühl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E.; et al. Tuning the electrocatalytic oxygen reduction reaction activity and stability of shape-controlled Pt–Ni nanoparticles by thermal annealing—Elucidating the surface atomic structural and compositional changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef]
- El-Deeb, H.; Bron, M. Microwave-assisted polyol synthesis of PtCu/carbon nanotube catalysts for electrocatalytic oxygen reduction. J. Power Sources 2015, 275, 893–900. [Google Scholar] [CrossRef]
- Jung, W.S.; Popov, B.N. Effect of pretreatment on durability of Fct-structured Pt-based alloy catalyst for the oxygen reduction reaction under operating conditions in polymer electrolyte membrane fuel cells. ACS Sustain. Chem. Eng. 2017, 5, 9809–9817. [Google Scholar] [CrossRef]
- Kang, Y.S.; Choi, K.-H.; Ahn, D.; Lee, M.J.; Baik, J.; Chung, D.Y.; Kim, M.-J.; Lee, S.Y.; Kim, M.; Shin, H.; et al. Effect of post heat-treatment of composition-controlled PdFe nanoparticles for oxygen reduction reaction. J. Power Sources 2016, 303, 234–242. [Google Scholar] [CrossRef]
- Mai, Y.; Xie, X.; Wang, Z.; Yan, C.; Liu, G. Effect of heat treatment temperature on the Pt3Co binary metal catalysts for oxygen reduced reaction and DFT calculations. J. Fuel Chem. Technol. 2022, 50, 114–121. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Zhang, L.-H.; Wang, J.-B.; Huang, R. Atomic-scale insights into thermal stability of pt3co nanoparticles: A comparison between disordered alloy and ordered intermetallics. J. Alloys Compd. 2019, 776, 629–635. [Google Scholar] [CrossRef]
- Nassr, A.B.A.A.; Sinev, I.; Grünert, W.; Bron, M. PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation. Appl. Catal. B Environ. 2013, 142–143, 849–860. [Google Scholar] [CrossRef]
- Liao, M.; Li, W.; Peng, J.; Zhang, F.; Xu, W.; Huang, Z. Enhancement of anodic oxidation of formic acid on Pd–Fe bimetallic nanoparticles by thermal treatment. Int. J. Hydrogen Energy 2021, 46, 10239–10246. [Google Scholar] [CrossRef]
- Gocyla, M.; Kuehl, S.; Shviro, M.; Heyen, H.; Selve, S.; Dunin-Borkowski, R.E.; Heggen, M.; Strasser, P. Shape stability of octahedral PtNi nanocatalysts for electrochemical oxygen reduction reaction studied by in Situ transmission electron microscopy. ACS Nano 2018, 12, 5306–5311. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Qin, X.; Xu, M.; Zhu, S.; Zhang, L.; Hong, Y.; Choi, S.-I.; Chang, Q.; Xu, Y.; Pan, X.; et al. Impact of heat treatment on the electrochemical properties of carbon-supported octahedral Pt–Ni nanoparticles. ACS Catal. 2019, 9, 11189–11198. [Google Scholar] [CrossRef]
- Ortatatlı, Ş.; Knossalla, J.; Schüth, F.; Weidenthaler, C. Monitoring the formation of PtNi nanoalloys supported on hollow graphitic spheres using in Situ Pair distribution function analysis. Phys. Chem. Chem. Phys. 2018, 20, 8466–8474. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lin, R.; Liu, X.; Zhao, Y. Effect of heat treatment on the surface structure of Pd@Pt–Ni core-shell catalysts for the oxygen reduction reaction. J. Alloys Compd. 2021, 884, 161059. [Google Scholar] [CrossRef]
- Antolini, E. Alloy vs. intermetallic compounds: Effect of the ordering on the electrocatalytic activity for oxygen reduction and the stability of low temperature fuel cell catalysts. Appl. Catal. B Environ. 2017, 217, 201–213. [Google Scholar] [CrossRef]
- Hodnik, N.; Jeyabharathi, C.; Meier, J.C.; Kostka, A.; Phani, K.L.; Rečnik, A.; Bele, M.; Hočevar, S.; Gaberšček, M.; Mayrhofer, K.J.J. Effect of ordering of PtCu 3 nanoparticle structure on the activity and stability for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 13610–13615. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Li, J.; Wang, S.; Su, C.; Jin, H. Structure engineering of PtCu3/C catalyst from disordered to ordered intermetallic compound with heat-treatment for the methanol electrooxidation reaction. Nano Res. 2022, 15, 3866–3871. [Google Scholar] [CrossRef]
- Wu, B.; Du, F.; Wang, H.; Wu, C.; Chu, J.; Wang, X.; Xiong, S. Effects of annealing temperature of PtCu/MWCNT catalysts on their electrocatalytic performance of electrooxidation of methanol. Ionics 2022, 28, 369–382. [Google Scholar] [CrossRef]
- Babu, P.K.; Kim, H.S.; Kuk, S.T.; Chung, J.H.; Oldfield, E.; Wieckowski, A.; Smotkin, E.S. Activation of nanoparticle Pt−ru fuel cell catalysts by heat treatment: A 195 Pt NMR and electrochemical study. J. Phys. Chem. B 2005, 109, 17192–17196. [Google Scholar] [CrossRef]
- Gatalo, M.; Ruiz-Zepeda, F.; Hodnik, N.; Dražić, G.; Bele, M.; Gaberšček, M. Insights into thermal annealing of highly-active PtCu3/C oxygen reduction reaction electrocatalyst: An in-situ heating transmission electron microscopy study. Nano Energy 2019, 63, 103892. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Ju, F.; Wang, H.; Wang, Y.; Li, W. Carbon-supported Pd–Cr bimetallic nanoparticles and their electrocatalytic activity and durability in oxygen reduction reaction. Russ. J. Phys. Chem. A 2017, 91, 2283–2287. [Google Scholar] [CrossRef]
- Sahin, N.E.; Napporn, T.W.; Dubau, L.; Kadirgan, F.; Léger, J.-M.; Kokoh, K.B. Temperature-dependence of oxygen reduction activity on Pt/C and PtCr/c electrocatalysts synthesized from microwave-heated diethylene glycol method. Appl. Catal. B Environ. 2017, 203, 72–84. [Google Scholar] [CrossRef]
- Duan, H.; Xu, C. Nanoporous PdCr alloys as highly active electrocatalysts for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 4166–4173. [Google Scholar] [CrossRef] [PubMed]
- Maniwan, W.; Poochinda, K.; Hunsom, M. Activity and stability of PtxCr/C catalyst for oxygen reduction reaction: Effect of the Pt:Cr ratio and heat treatment atmosphere. Int. J. Hydrogen Energy 2016, 41, 21404–21414. [Google Scholar] [CrossRef]
- Kaewsai, D.; Yeamdee, S.; Supajaroon, S.; Hunsom, M. ORR activity and stability of PtCr/C catalysts in a low temperature/pressure PEM fuel cell: Effect of heat treatment temperature. Int. J. Hydrogen Energy 2018, 43, 5133–5144. [Google Scholar] [CrossRef]
- Min, M.; Kim, H. Performance and stability studies of PtCr/C alloy catalysts for oxygen reduction reaction in low temperature fuel cells. Int. J. Hydrogen Energy 2016, 41, 17557–17566. [Google Scholar] [CrossRef]
- Tsypkin, M.; de la Fuente, J.L.G.; García Rodríguez, S.; Yu, Y.; Ochal, P.; Seland, F.; Safonova, O.; Muthuswamy, N.; Rønning, M.; Chen, D.; et al. Effect of heat treatment on the electrocatalytic properties of nano-structured ru cores with Pt shells. J. Electroanal. Chem. 2013, 704, 57–66. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Shan, S.; Xie, Z.-H.; Kang, N.; Park, K.; Hopkins, E.; Yan, S.; Sharma, A.; Luo, J.; Wang, J.; et al. Revealing the role of phase structures of bimetallic nanocatalysts in the oxygen reduction reaction. ACS Catal. 2018, 8, 11302–11313. [Google Scholar] [CrossRef]
- Belenov, S.V.; Menshchikov, V.S.; Nevelskaya, A.K.; Pryadchenko, V.V.; Shemet, D.B.; Srabionyan, V.V.; Alekseenko, A.A.; Kirakosyan, S.A.; Guterman, V.E. Post-treatment of Pt-M (M = Cu, Co, Ni)/C electrocatalysts with different distribution of metals in nanoparticles: Evolution of structure and activity. In Springer Proceedings in Physics; Springer: Cham, Switzerland, 2017; Volume 207, pp. 65–77. [Google Scholar] [CrossRef]
- Chaisubanan, N.; Maniwan, W.; Hunsom, M. Effect of heat-treatment on the performance of PtM/C (M = Cr, Pd, Co) catalysts towards the oxygen reduction reaction in PEM fuel cell. Energy 2017, 127, 454–461. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.; Xuan, C.; Xiao, W.; Xia, K.; Xia, W.; Lai, C.; Xin, H.L.; Wang, D. Effects of crystal phase and composition on structurally ordered Pt–Co–Ni/C ternary intermetallic electrocatalysts for the formic acid oxidation reaction. J. Mater. Chem. A 2018, 6, 5848–5855. [Google Scholar] [CrossRef]
- Li, B.; Yan, Z.; Xiao, Q.; Dai, J.; Yang, D.; Zhang, C.; Cai, M.; Ma, J. Highly active carbon-supported Pt nanoparticles modified and dealloyed with Co for the oxygen reduction reaction. J. Power Sources 2014, 270, 201–207. [Google Scholar] [CrossRef]
- Beermann, V.; Kühl, S.; Strasser, P. Tuning the catalytic oxygen reduction reaction performance of Pt-Ni octahedral nanoparticles by acid treatments and thermal annealing. J. Electrochem. Soc. 2018, 165, J3026–J3030. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. A highly efficient PtCo/C electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 34484–34491. [Google Scholar] [CrossRef]
- Becknell, N.; Kang, Y.; Chen, C.; Resasco, J.; Kornienko, N.; Guo, J.; Markovic, N.M.; Somorjai, G.A.; Stamenkovic, V.R.; Yang, P. Atomic structure of Pt 3 Ni nanoframe electrocatalysts by in Situ X-ray absorption spectroscopy. J. Am. Chem. Soc. 2015, 137, 15817–15824. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Son, Y.; Kim, D.; Li, D.; Yu, Y.; Niu, Z.; Lei, T.; Sneed, B.T.; More, K.L.; Markovic, N.M.; et al. Control of architecture in rhombic dodecahedral Pt–Ni nanoframe electrocatalysts. J. Am. Chem. Soc. 2017, 139, 11678–11681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: An industrial perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]

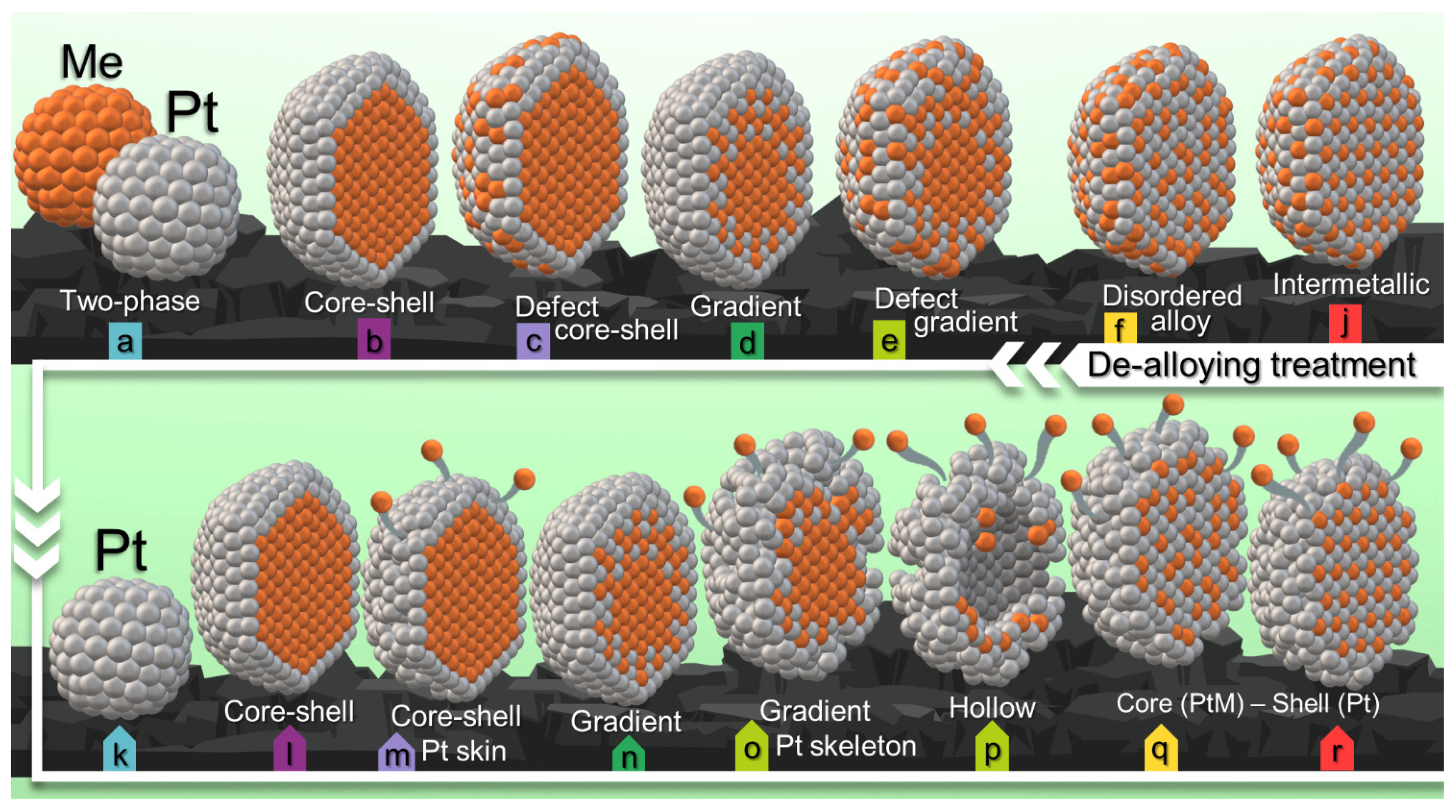
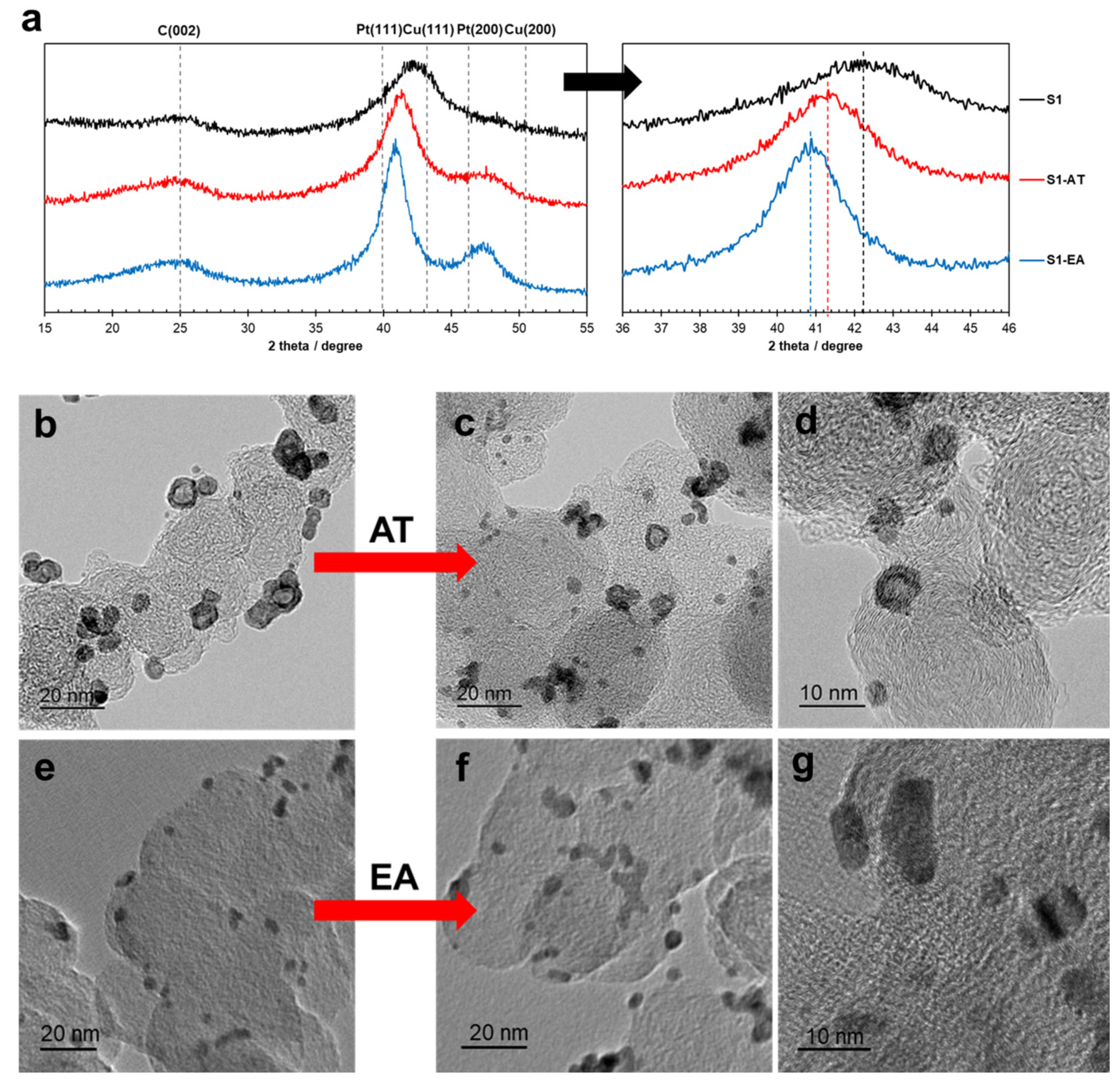
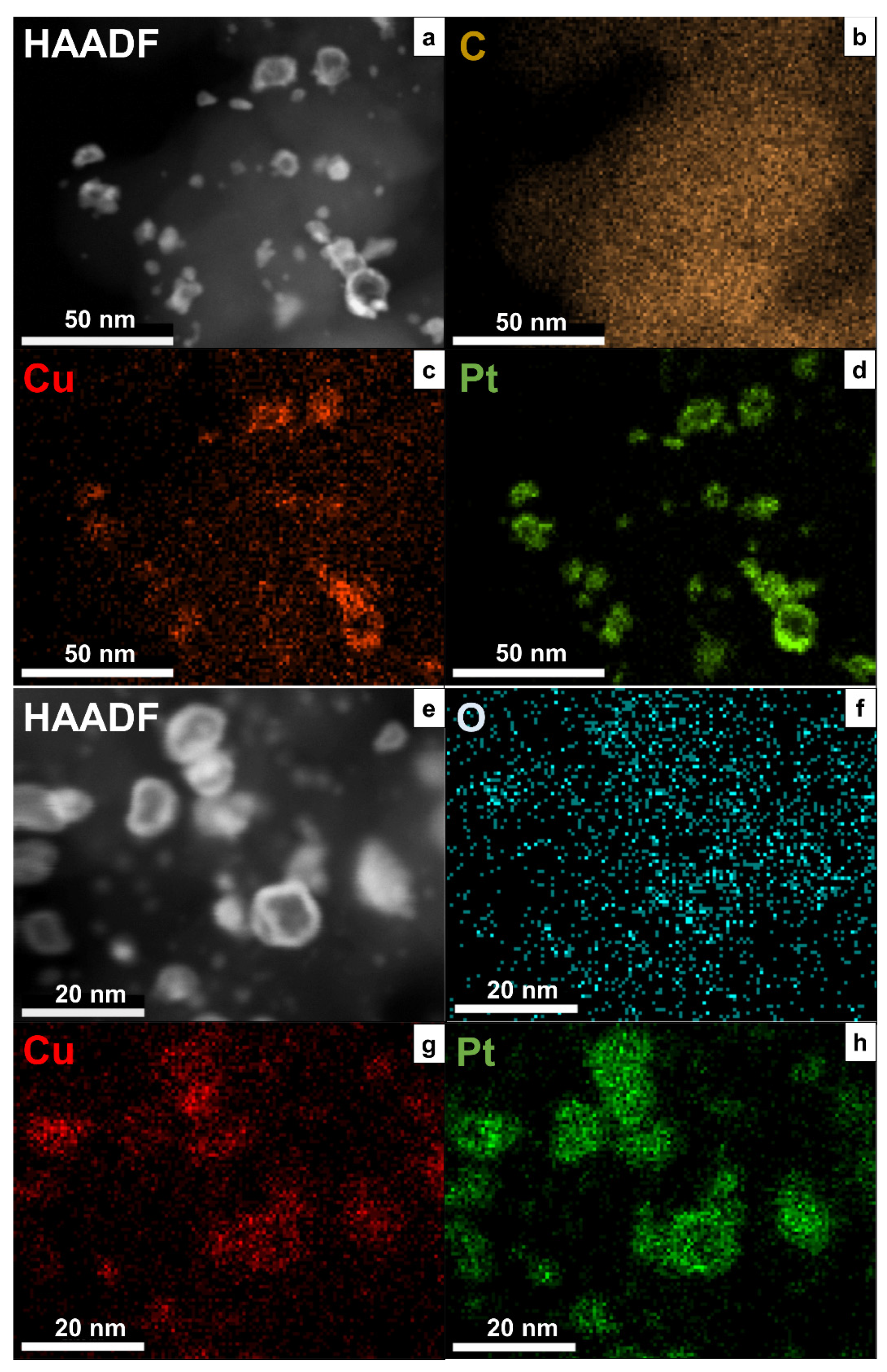

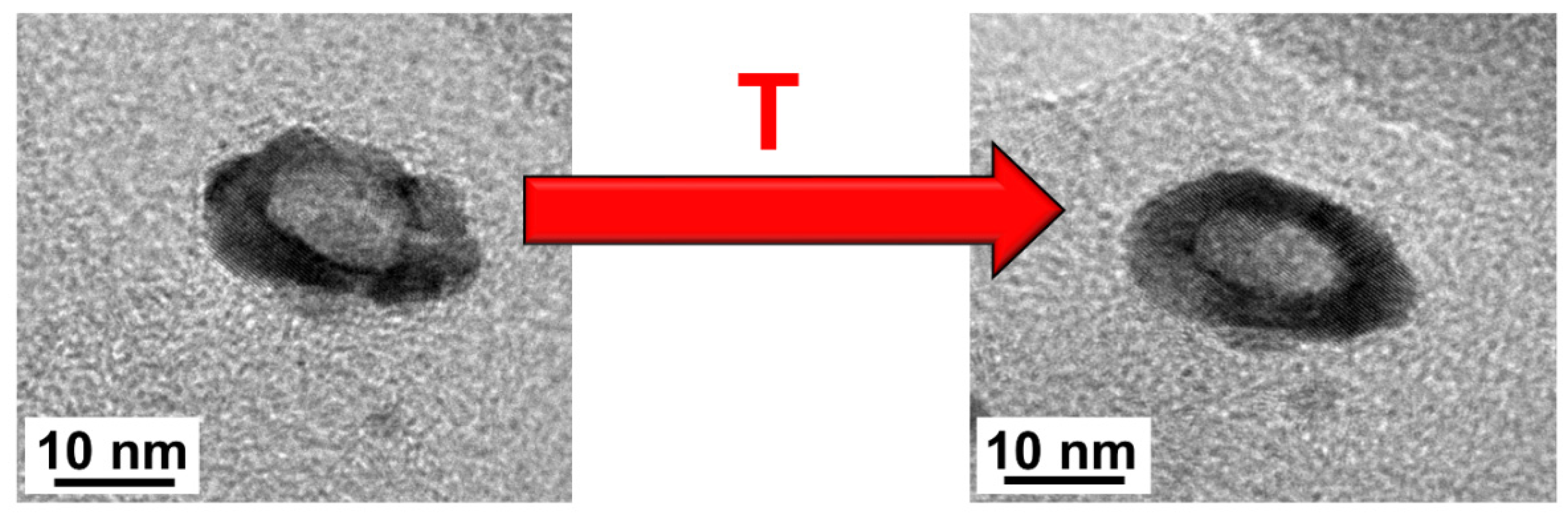
| Sample | Composition | Pt [wt.%] | Type of Support | Heat-Treatment Conditions | Particle Size, nm | Lattice Constant (A) | ESA, m2/g(Pt) | Activity, MA A g−1 at 1600 rpm E = 0.9 V, Unless Otherwise Stated | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Temperature T, °C | Atmosphere | Time Period t (h) | XRD | TEM | ||||||||
| Pt/C | Pt | 20 | Vulcan XC-72R | As-prepared | 2.4 | - | 3.958 | 56.3 | 188 (0.75 V) | [80] | ||
| 900 | H2(5.2 mol.%)/Ar | 1/12 | 3.3 | - | 3.928 | 31.7 | 125 (0.75 V) | |||||
| PtCo/C | Pt30Co70 | 11.7 | Vulcan XC-72R | As-prepared | 1.2 | - | 3.920 | 17.5 | 186 (0.75 V) | |||
| 900 | H2(5.2 mol.%)/Ar | 1/12 | 25.5 | - | 3.720 | 30 | 84 (0.75 V) | |||||
| PtCr/C | Pt30Cr70 | 12.3 | Vulcan XC-72R | As-prepared | 2.7 | - | 3.923 | 26.2 | 158 (0.75 V) | |||
| 900 | H2(5.2 mol.%)/Ar | 1/12 | 12.8 | - | 3.868 | 16.1 | 140 (0.75 V) | |||||
| PtCoCr/C | Pt30Co30Cr40 | 12.1 | Vulcan XC-72R | As-prepared | 2.2 | - | 3.914 | 17.0 | 152 (0.75 V) | |||
| 900 | H2(5.2 mol.%)/Ar | 1/12 | 7.3 | - | 3.774 | 6.27 | 111 (0.75 V) | |||||
| PtCo/C | PtCo3 | 22 | HSAC * | 650 | 4 vol.% H2/96 vol.% Ar, flux 100 mL min−1, | 7 | 1.8 | - | 3.749 | 40 ± 4 | 280 ± 50 | [94] |
| 800 | 2.2 | - | 3.726 | 45 ± 4 | 380 ± 50 | |||||||
| 900 | 3.2 | - | 3.717 | 37 ± 2 | 290 ± 40 | |||||||
| PtCu/C | PtCu3 | 22 | HSAC * | 800 | 3.6 | - | 3.705 | 47 ± 2 | 410 ± 90 | |||
| PtCo/C | Pt3Co | 18.2 | Vulcan XC-72 | As-prepared | 2.7 | 3.0 | 3.924 | 49 | - | [93] | ||
| 300 | H2 (10%)/N2 (90%) | 2 | 3.4 | 3.2 | 3.911 | 41 | - | |||||
| 400 | 3.8 | 3.5 | 3.912 | 66 | - | |||||||
| 500 | 4.3 | 3.6 | 3.912 | 54 | - | |||||||
| 600 | 5.8 | 4.1 | 3.913 | 30 | - | |||||||
| PtCo@Pt/C | PtCo | 60 | Vulcan XC-72 | 360 | H2/N2 | 3 | 5.0 | - | 3.827 | 31.1 | 52.9 | [73] |
| 500 | H2/N2 | 16 | 5.4 | - | 3.803 | 28.4 | 53.6 | |||||
| Pt3Co/C | Pt3Co | ** | Vulcan XC-72 | 400 | H2 | 2 | 4.8 ± 1 | - | 3.873 | 51.6 *** | 160 | [95] |
| 700 | 7.2 ± 1 | - | 3.841 | 47.3 *** | 520 | |||||||
| Co@Pt/C | Pt3Co | 36.3 | Vulcan XC-72 | As-prepared | 2.7 | 2.4 | - | - | 21.2 | [92] | ||
| 300 | H2/N2 (5%/95%), 40 mL s−1 | 2 | 3.8 | 3.0 | - | - | 50.3 | |||||
| Pt3Co/C | Pt3Co | - | Vulcan XC-72 | As-prepared | 2.2 | 2.5 ± 0.3 | 2.24 | 44 | 136 | [83] | ||
| 200 | H2/Ar | 3.2 | 3.0 ± 0.5 | 2.24 | 52 | 129 | ||||||
| 500 | 4.1 | 3.9 ± 1.2 | 2.24 | 36 | 217 | |||||||
| PtNi/C | 6.79 | Vulcan XC-72 | As-prepared | 31.3 | 13.3 | [96] | ||||||
| 350 | H2/N2 (20%/80%) | 6 | - | - | - | 18.7 | 267.6 | |||||
| 700 | H2/N2 (20%/80%) | 2 | - | - | - | 21.6 | 27.5 | |||||
| PtNi/C | Pt34Ni66 | 15 | Vulcan XC-72R | As-prepared | - | 8.4 ± 1.1 | 3.771 | 33 | 1700 | [97] | ||
| 300 | H2/N2 (4%/96%) | 1 | - | 8.3 ± 1.2 | 3.777 | 32 | 2700 | |||||
| 500 | - | 9.4 ± 1.2 | 3.755 | 43 | 700 | |||||||
| PtCu/C | Pt0.8Cu | 21.3 | Vulcan XC-72 | As-prepared | 4.8 | 3.843 | 55 ± 5 | 132 | [87] | |||
| 250 | Ar | 1 | - | - | 3.847 | 58 ± 6 | 116 | |||||
| 280 | - | 5.8 | 3.829 | 56 ± 6 | 151 | |||||||
| 300 | - | 5.9 | 3.798 | 41 ± 4 | 225 | |||||||
| 350 | - | - | 3.777 | 31 ± 3 | 43 | |||||||
| PtCu/C | Pt20Cu80 | 11 | O-CNTS **** | 400 | H2(5%)/Ar | 6 | 4.3 | 2.5 | 3.873 | 83.2 | 49.1 | [98] |
| 500 | 4.3 | 2.7 | 3.882 | 68.9 | 60.8 | |||||||
| 600 | 4.6 | 3.7 | 3.837 | 50.0 | 39.4 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belenov, S.; Alekseenko, A.; Pavlets, A.; Nevelskaya, A.; Danilenko, M. Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC. Catalysts 2022, 12, 638. https://doi.org/10.3390/catal12060638
Belenov S, Alekseenko A, Pavlets A, Nevelskaya A, Danilenko M. Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC. Catalysts. 2022; 12(6):638. https://doi.org/10.3390/catal12060638
Chicago/Turabian StyleBelenov, Sergey, Anastasia Alekseenko, Angelina Pavlets, Alina Nevelskaya, and Maria Danilenko. 2022. "Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC" Catalysts 12, no. 6: 638. https://doi.org/10.3390/catal12060638
APA StyleBelenov, S., Alekseenko, A., Pavlets, A., Nevelskaya, A., & Danilenko, M. (2022). Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC. Catalysts, 12(6), 638. https://doi.org/10.3390/catal12060638








