Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye
Abstract
:1. Introduction
2. Results
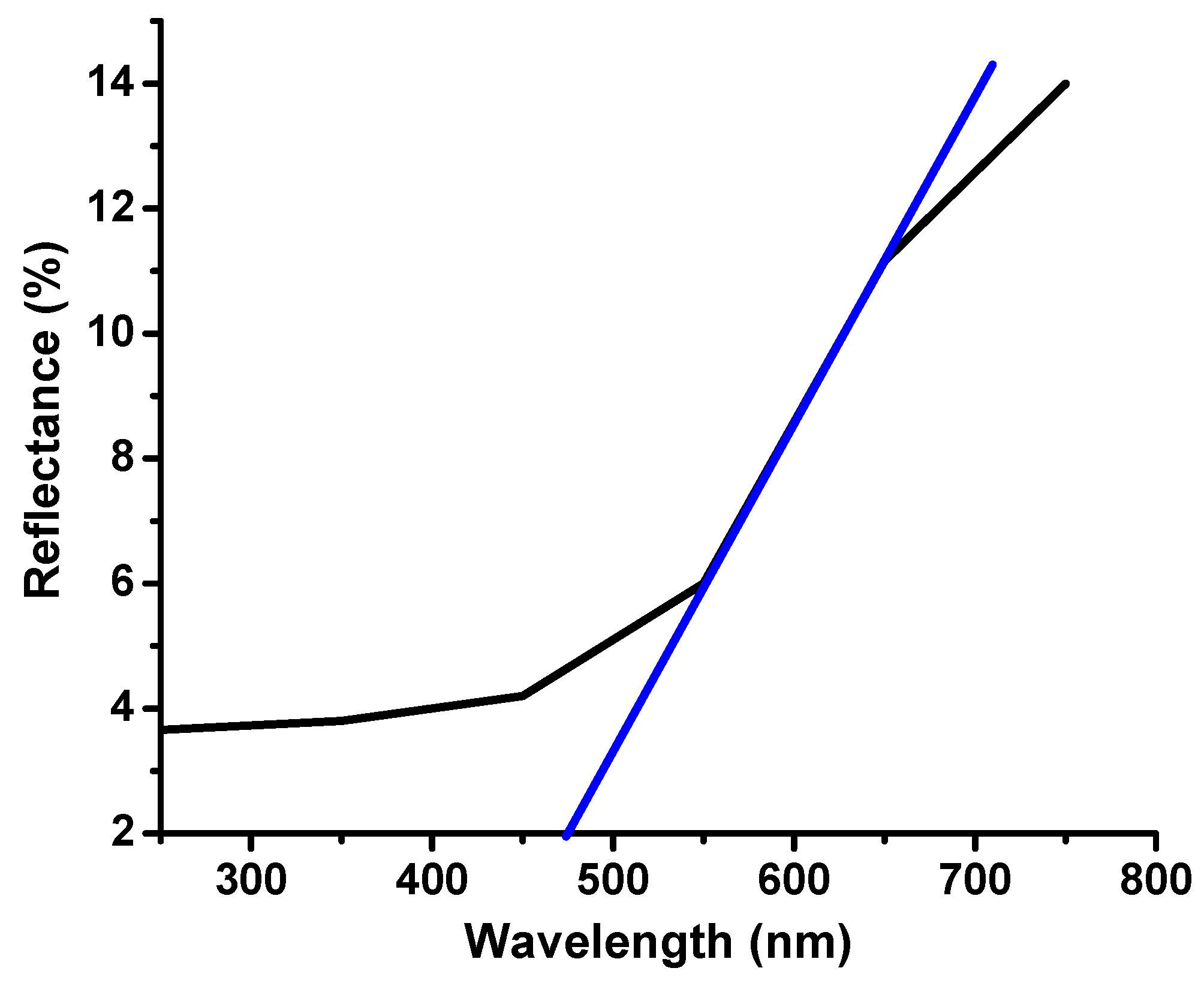
2.1. Structural, Morphological, and Optical Characterization of Cu-ZnO/TiO2 Nanocomposite Photocatalyst
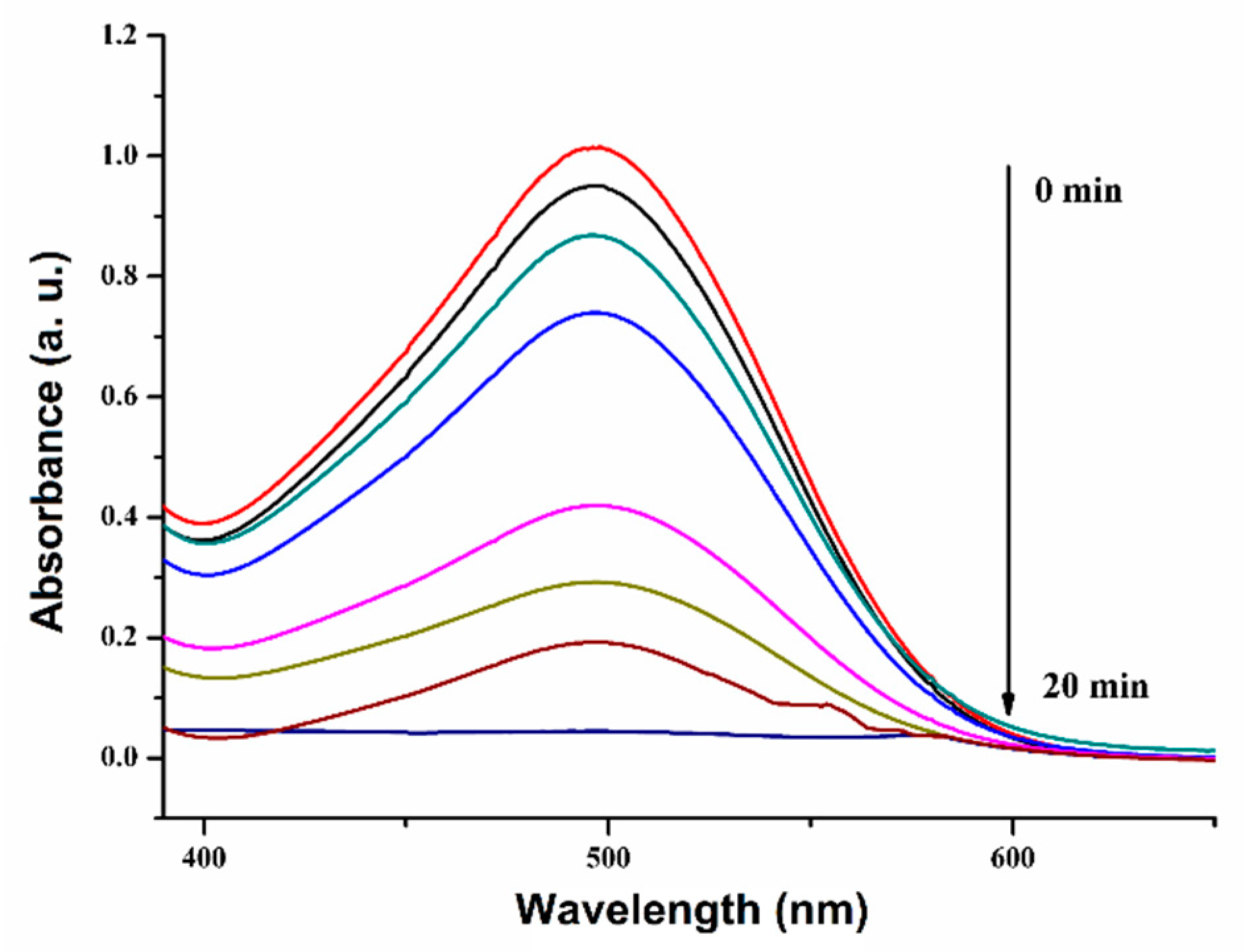
2.2. Assessment of Photocatalytic Efficacy
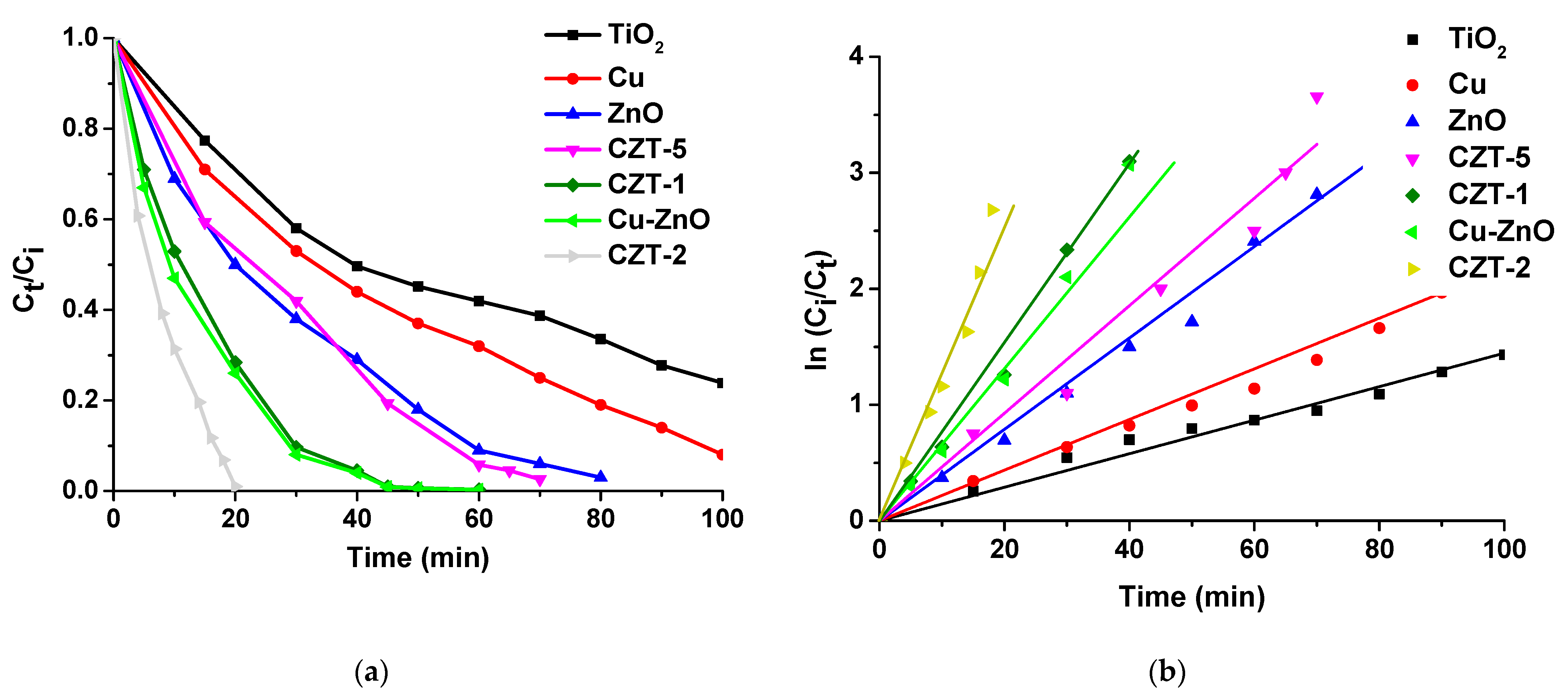
2.2.1. Kinetic Studies of CR Decomposition over Cu-ZnO/TiO2 Nanocomposite Photocatalyst
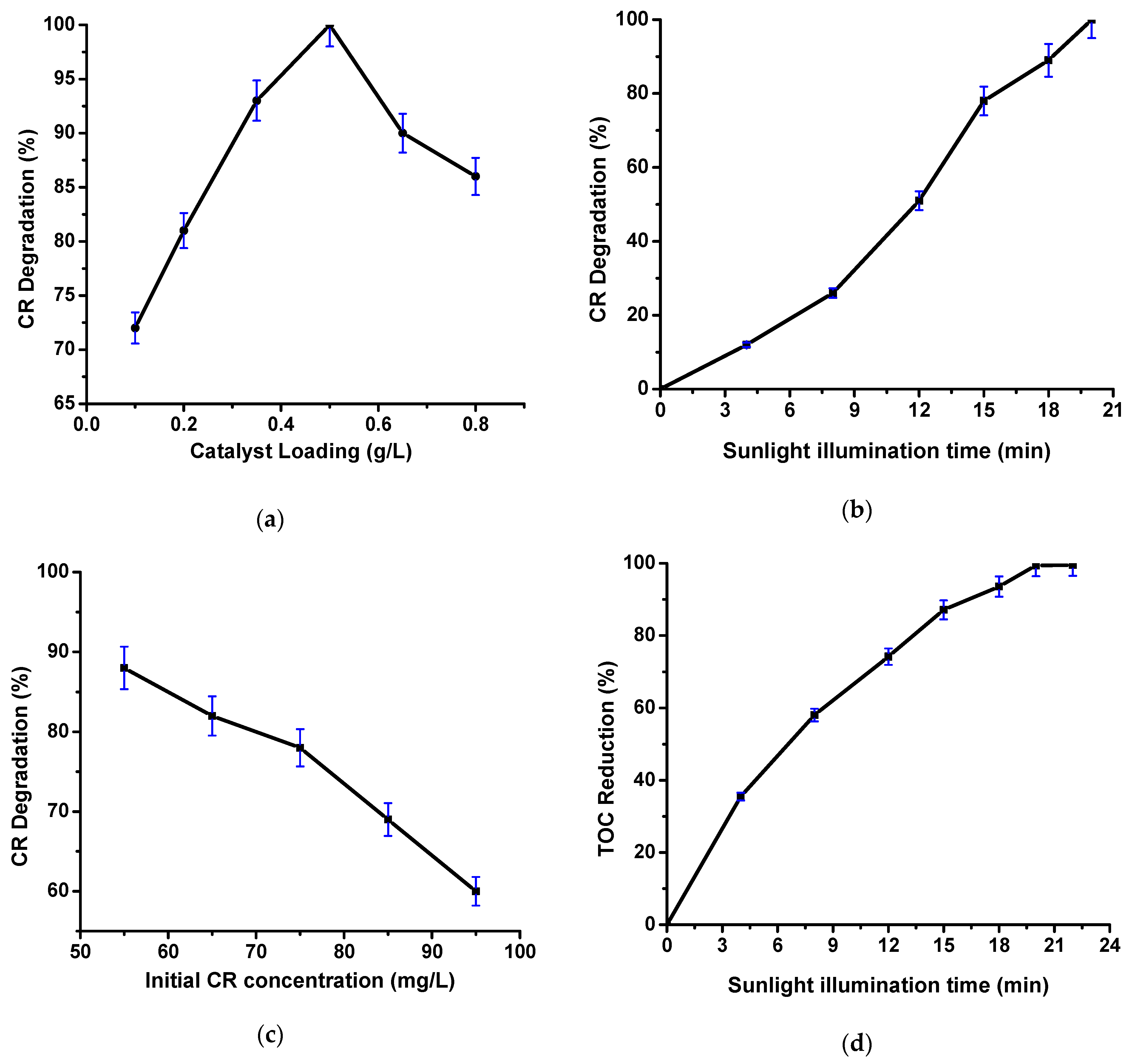
2.2.2. Influence of Catalyst Loading, Sunlight Illumination Time, and Initial CR Concentration on the CR Degradation
2.2.3. Assessment of TOC Abatement during Photocatalytic Degradation of CR Dye
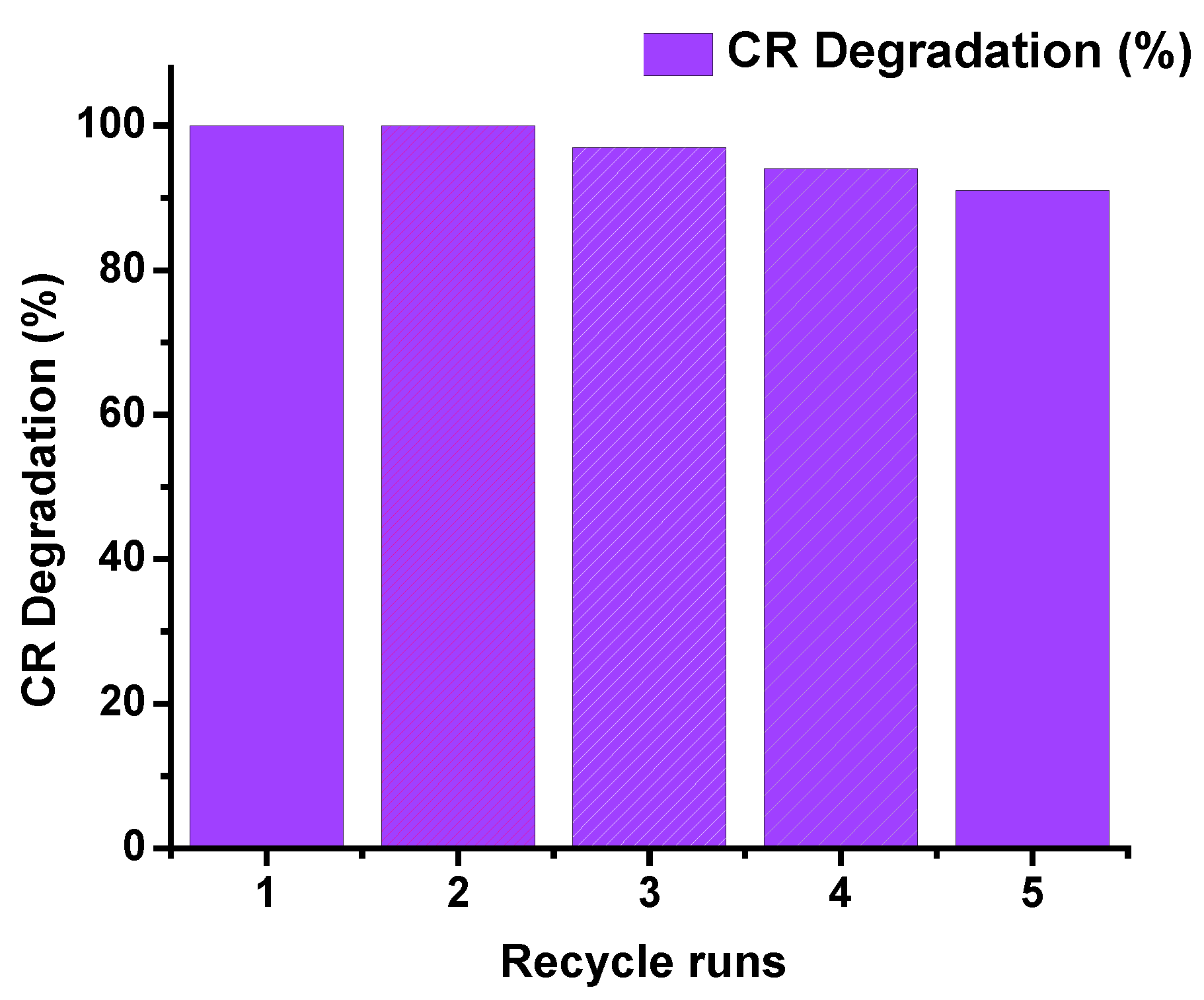
2.2.4. Photocatalyst Stability
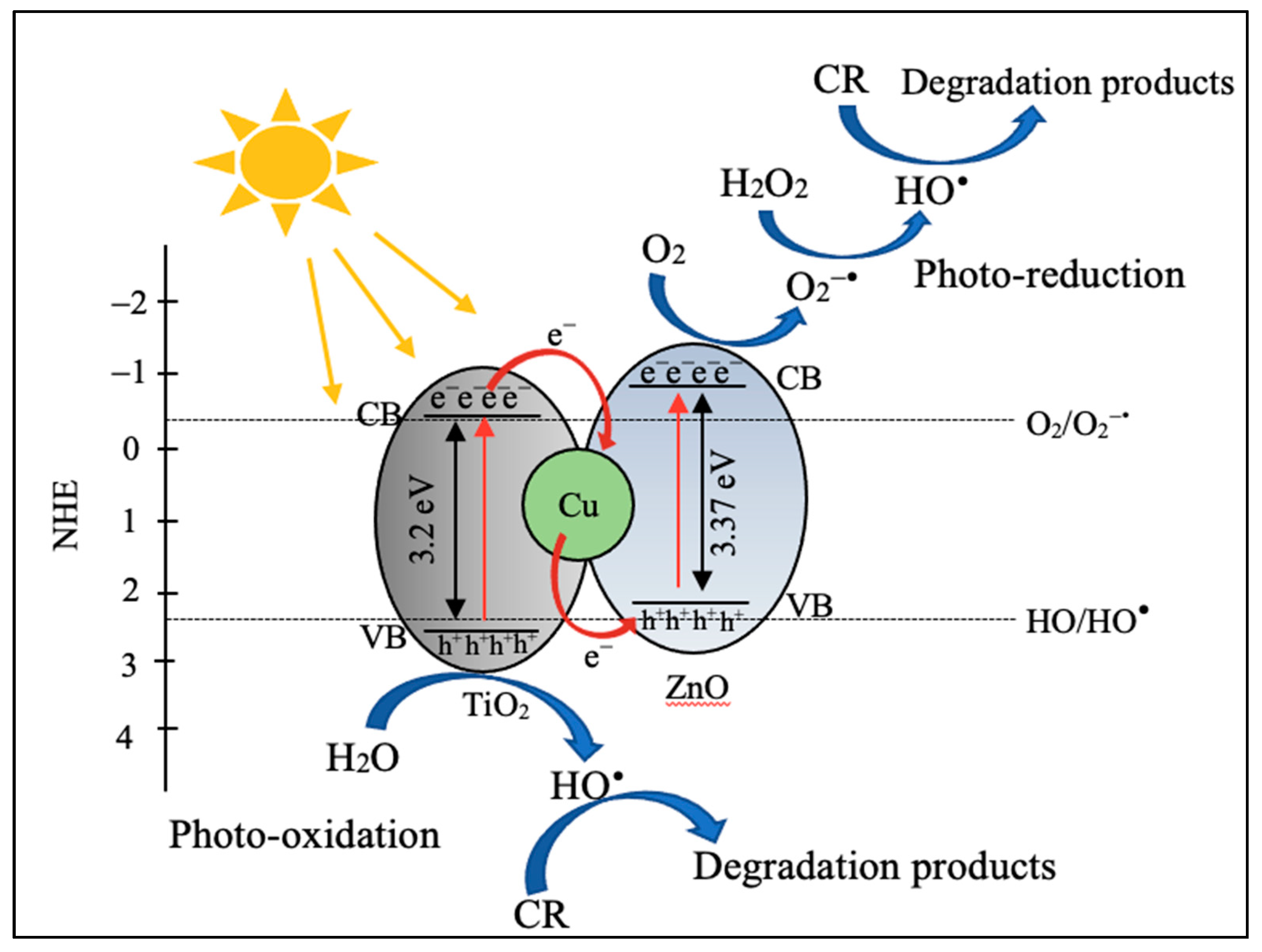
2.2.5. Plausible Mechanism for the Enhanced Photocatalytic Performance of Cu-ZnO/TiO2 Nanocomposite Photocatalyst
3. Materials and Methods
3.1. Reagents
3.2. Sonication-Assisted Synthesis of Cu-ZnO/TiO2 Ternary Z-Scheme Heterojunction Nanocomposite Photocatalyst
3.3. Characterization of the As-Prepared Cu-ZnO/TiO2 Z-Scheme Heterojunction Nanocomposite Photocatalyst
3.4. Photocatalytic Performance of the Sunlight-Driven CR Dye Degradation over Cu-ZnO/TiO2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, M.; Naikoo, G.; Sheikh, M.U.D.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A Chem. 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Moradzadeh, A.; Mahjoub, A.; Sadjadi, M.S.; Sadr, M.H.; Farhadyar, N. Investigation on synthesis, characterization and photo catalytic degradation of congo red by Zn-doped CdTiO3/TiO. Polyhedron 2019, 170, 404–411. [Google Scholar] [CrossRef]
- Pathania, D.; Gupta, D.; Al-Muhtaseb, A.; Sharma, G.; Kumar, A.; Naushad, M.; Ahamad, T.; Alshehri, S.M. Photocatalytic degradation of highly toxic dyes using chitosan-g-poly(acrylamide)/ZnS in presence of solar irradiation. J. Photochem. Photobiol. A Chem. 2016, 329, 61–68. [Google Scholar] [CrossRef]
- Cako, E.; Gunasekaran, K.D.; Soltani, R.D.C.; Boczkaj, G. Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs). Water Resour. Ind. 2020, 24, 100134. [Google Scholar] [CrossRef]
- Patil, S.P.; Bethi, B.; Sonawane, G.; Shrivastava, V.; Sonawane, S. Efficient adsorption and photocatalytic degradation of Rhodamine B dye over Bi2O3 -bentonite nanocomposites: A kinetic study. J. Ind. Eng. Chem. 2016, 34, 356–363. [Google Scholar] [CrossRef]
- Habibi, M.H.; Rahmati, M.H. The effect of operational parameters on the photocatalytic degradation of Congo red organic dye using ZnO–CdS core–shell nano-structure coated on glass by Doctor Blade method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 160–164. [Google Scholar] [CrossRef]
- Khairol, N.F.; Sapawe, N.; Danish, M. Excellent Performance Integrated both Adsorption and Photocatalytic Reaction toward Degradation of Congo Red by CuO/Eggshell. Mater. Today Proc. 2019, 19, 1340–1345. [Google Scholar] [CrossRef]
- Liang, Y.; Li, W.; Wang, X.; Zhou, R.; Ding, H. TiO2–ZnO/Au ternary heterojunction nanocomposite: Excellent antibacterial property and visible-light photocatalytic hydrogen production efficiency. Ceram. Int. 2021, 48, 2826–2832. [Google Scholar] [CrossRef]
- Güy, N.; Çakar, S.; Özacar, M. Comparison of palladium/zinc oxide photocatalysts prepared by different palladium doping methods for congo red degradation. J. Colloid Interface Sci. 2016, 466, 128–137. [Google Scholar] [CrossRef]
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.-I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energy Environ. Sci. 2022, 15, 880–937. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M. Microwave-assisted synthesis, characterization and antibacterial activity of Ag/ZnO nanoparticles supported bentonite clay. J. Hazard. Mater. 2013, 262, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Salehi, K.; Daraei, H.; Teymouri, P.; Shahmoradi, B.; Maleki, A. Cu-doped ZnO nanoparticle for removal of reactive black 5: Application of artificial neural networks and multiple linear regression for modeling and optimization. Desalination Water Treat. 2016, 57, 22074–22080. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Olaru, N.; Samoila, P.; Airinei, A.; Ignat, M.; Sacarescu, L.; Timpu, D. Novel rare earth (RE-La, Er, Sm) metal doped ZnO photocatalysts for degradation of Congo-Red dye: Synthesis, characterization and kinetic studies. J. Environ. Manag. 2019, 239, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Deshmukh, S.; More, K.; Shevale, V.; Mullani, S.; Dhodamani, A.; Delekar, S. Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Mater. Chem. Phys. 2018, 225, 247–255. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [Green Version]
- Yayapao, O.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Synthesis and characterization of highly efficient Gd doped ZnO photocatalyst irradiated with ultraviolet and visible radiations. Mater. Sci. Semicond. Process. 2015, 39, 786–792. [Google Scholar] [CrossRef]
- Das, A.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Hierarchical ZnO-TiO2 nanoheterojunction: A strategy driven approach to boost the photocatalytic performance through the synergy of improved surface area and interfacial charge transport. Appl. Surf. Sci. 2020, 534, 147321. [Google Scholar] [CrossRef]
- Munguti, L.; Dejene, F. Effects of Zn:Ti molar ratios on the morphological, optical and photocatalytic properties of ZnO-TiO2 nanocomposites for application in dye removal. Mater. Sci. Semicond. Process. 2021, 128, 105786. [Google Scholar] [CrossRef]
- Suganthi, N.; Thangavel, S.; Kannan, K. Hibiscus subdariffa leaf extract mediated 2-D fern-like ZnO/TiO2 hierarchical nanoleaf for photocatalytic degradation. FlatChem 2020, 24, 100197. [Google Scholar] [CrossRef]
- Ali, M.M.; Haque, J.; Kabir, M.H.; Kaiyum, M.A.; Rahman, M. Nano synthesis of ZnO–TiO2 composites by sol-gel method and evaluation of their antibacterial, optical and photocatalytic activities. Results Mater. 2021, 11, 100199. [Google Scholar] [CrossRef]
- Shirsath, S.; Pinjari, D.; Gogate, P.; Sonawane, S.; Pandit, A. Ultrasound assisted synthesis of doped TiO2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochemistry 2013, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Pragathiswaran, C.; Smitha, C.; Abbubakkar, B.M.; Govindhan, P.; Krishnan, N.A. Synthesis and characterization of TiO2/ZnO–Ag nanocomposite for photocatalytic degradation of dyes and anti-microbial activity. Mater. Today Proc. 2021, 45, 3357–3364. [Google Scholar] [CrossRef]
- Van Viet, P.; Vinh, T.H.T.; Dung, N.T.N.; Thi, C.M. Facile ball-milling synthesis of TiO2 modified ZnO for efficient photocatalytic removal of atmospheric nitric oxide gas under solar light irradiation. Chem. Phys. Lett. 2021, 775, 138642. [Google Scholar] [CrossRef]
- Hosseini, A.; Karimi, H.; Foroughi, J.; Sabzehmeidani, M.M.; Ghaedi, M. Heterogeneous photoelectro-Fenton using ZnO and TiO2 thin film as photocatalyst for photocatalytic degradation Malachite Green. Appl. Surf. Sci. Adv. 2021, 6, 100126. [Google Scholar] [CrossRef]
- Quang, T.; Viet, Q.; Hoang, V.; Thi, N.; Giang, H. Statistical screening and optimization of photocatalytic degradation of meth-ylene blue by ZnO–TiO2/rGO nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127464. [Google Scholar]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Huerta-Aguilar, C.A.; Palos-Barba, V.; Thangarasu, P.; Koodali, R.T. Visible light driven photo-degradation of Congo red by TiO2ZnO/Ag: DFT approach on synergetic effect on band gap energy. Chemosphere 2018, 213, 481–497. [Google Scholar] [CrossRef]
- Hou, T.; Chen, L.; Xin, Y.; Zhu, W.; Zhang, C.; Zhang, W.; Liang, S.; Wang, L. Porous CuFe for Plasmon-Assisted N2 Photofixation. ACS Energy Lett. 2020, 5, 2444–2451. [Google Scholar] [CrossRef]
- Nie, J.; Patrocinio, A.O.T.; Hamid, S.; Sieland, F.; Sann, J.; Xia, S.; Bahnemann, D.W.; Schneider, J. New insights into the plasmonic enhancement for photocatalytic H2 production by Cu–TiO2 upon visible light illumination. Phys. Chem. Chem. Phys. 2018, 20, 5264–5273. [Google Scholar] [CrossRef]
- Potdar, S.B.; Praveen, B.; Sonawane, S.H. Sonochemical approach for synthesis of zinc oxide-poly methyl methacrylate hybrid nanoparticles and its application in corrosion inhibition. Ultrason. Sonochemistry 2020, 68, 105200. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef] [Green Version]
- An, M.; Li, L.; Wu, Q.; Yu, H.; Gao, X.; Zu, W.; Guan, J.; Yu, Y. CdS QDs modified three-dimensional ordered hollow spherical ZnTiO3-ZnO-TiO2 composite with improved photocatalytic performance. J. Alloy Compd. 2021, 895, 162638. [Google Scholar] [CrossRef]
- Potdar, S.B.; Huang, C.-M.; Praveen, B.; Manickam, S.; Sonawane, S.H. Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach. Catalysts 2022, 12, 78. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, J.; Yu, J.; He, B.; Jin, J.; Wang, H.; Gong, Y. Rational design of all-solid-state TiO2-x/Cu/ZnO Z-scheme heterojunction via ALD-assistance for enhanced photocatalytic activity. J. Colloid Interface Sci. 2022, 607, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Aziz, M.; Ul-Hamid, A.; Salleh, W.N.W.; Yusof, N.; Jaafar, J.; Ismail, A.F. Ultrafast degradation of Congo Red dye using a facile one-pot solvothermal synthesis of cuprous oxide/titanium dioxide and cuprous oxide/zinc oxide p-n heterojunction photocatalyst. Mater. Sci. Semicond. Process. 2021, 122, 105481. [Google Scholar] [CrossRef]
- Joseph, P.D.; Venkateswaran, C. Bandgap engineering in ZnO by doping with 3d transition metal ions. J. At. Mol. Physics. 2011, 2011, 270540. [Google Scholar] [CrossRef] [Green Version]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Yang, Y.; Ali, N.; Khan, A.; Khan, S.; Khan, S.; Khan, H.; Xiaoqi, S.; Ahmad, W.; Uddin, S.; Ali, N.; et al. Chitosan-capped ternary metal selenide nanocatalysts for efficient degradation of Congo red dye in sunlight irradiation. Int. J. Biol. Macromol. 2021, 167, 169–181. [Google Scholar] [CrossRef]
- Khan, S.; Khan, A.; Ali, N.; Ahmad, S.; Ahmad, W.; Malik, S.; Ali, N.; Khan, H.; Shah, S.; Bilal, M. Degradation of Congo red dye using ternary metal selenide-chitosan microspheres as robust and reusable catalysts. Environ. Technol. Innov. 2021, 22, 101402. [Google Scholar] [CrossRef]
- Sarkar, C.; Basu, J.K.; Samanta, A.N. Synthesis of novel ZnO/Geopolymer nanocomposite photocatalyst for degradation of congo red dye under visible light. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100521. [Google Scholar] [CrossRef]
- Shekardasht, M.B.; Givianrad, M.H.; Gharbani, P.; Mirjafary, Z.; Mehrizad, A. Preparation of a novel Z-scheme g-C3N4/RGO/Bi2Fe4O9 nanophotocatalyst for degradation of Congo Red dye under visible light. Diam. Relat. Mater. 2020, 109, 108008. [Google Scholar] [CrossRef]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Photocatalytic Degradation of Congo Red Using PbTiO3 Nanorods Synthesized via a Sonochemical Approach. ChemistrySelect 2018, 3, 11851–11858. [Google Scholar] [CrossRef]
- Ma, C.; Wang, F.; Zhang, C.; Yu, A.; Wei, J.; Yang, Z.; Li, Y.; Li, Z.; Zhu, M.; Shen, L.; et al. Photocatalytic decomposition of Congo red under visible light irradiation using MgZnCr-TiO2 layered double hydroxide. Chemosphere 2017, 168, 80–90. [Google Scholar] [CrossRef]
- Ljubas, D.; Smoljanić, G.; Juretić, H. Degradation of Methyl Orange and Congo Red dyes by using TiO2 nanoparticles activated by the solar and the solar-like radiation. J. Environ. Manag. 2015, 161, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sadollahkhani, A.; Ibupoto, Z.H.; Elhag, S.; Nur, O.; Willander, M. Photocatalytic properties of different morphologies of CuO for the degradation of Congo red organic dye. Ceram. Int. 2014, 40, 11311–11317. [Google Scholar] [CrossRef]
- Khairol, N.F.; Sapawe, N.; Danish, M. Photocatalytic Study of ZnO-CuO/ES on Degradation of Congo Red. Mater. Today Proc. 2019, 19, 1333–1339. [Google Scholar] [CrossRef]
- Yang, J.; Shi, C.; Dong, Y.; Su, H.; Sun, H.; Guo, Y.; Yin, S. Efficient hydrogen generation of vector Z-scheme CaTiO3/Cu/TiO2 photocatalyst assisted by cocatalyst Cu nanoparticles. J. Colloid Interface Sci. 2022, 605, 373–384. [Google Scholar] [CrossRef]
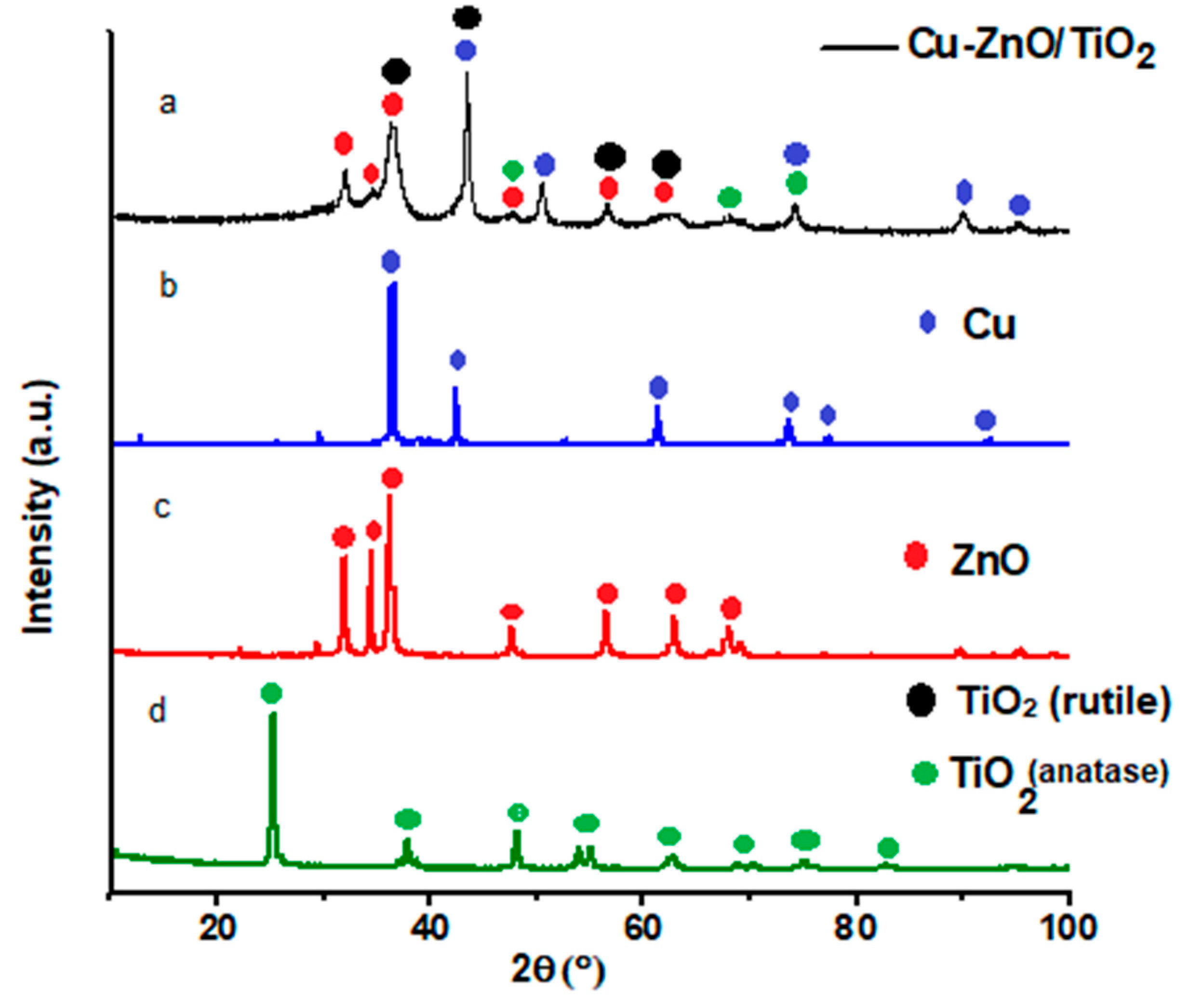

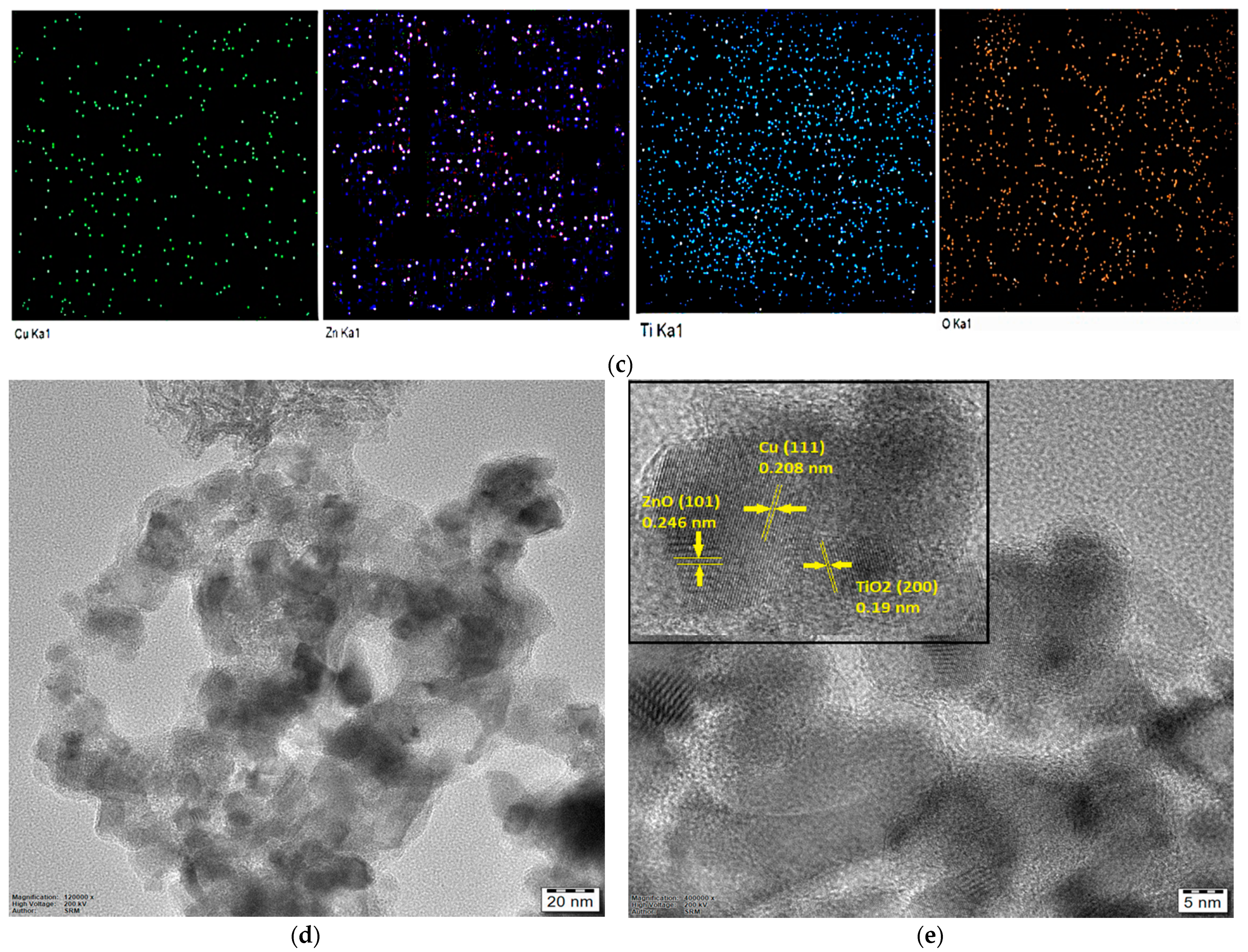
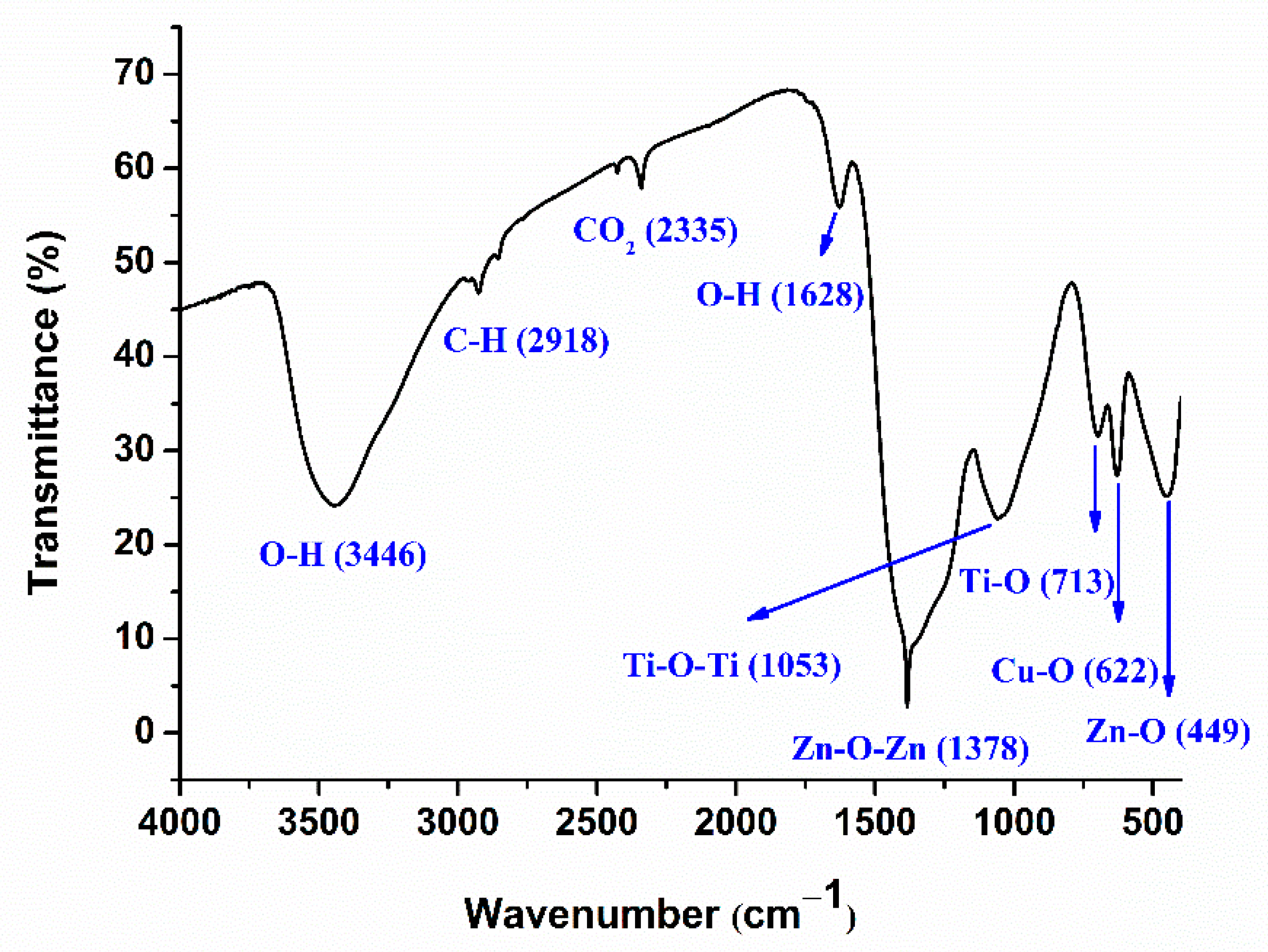
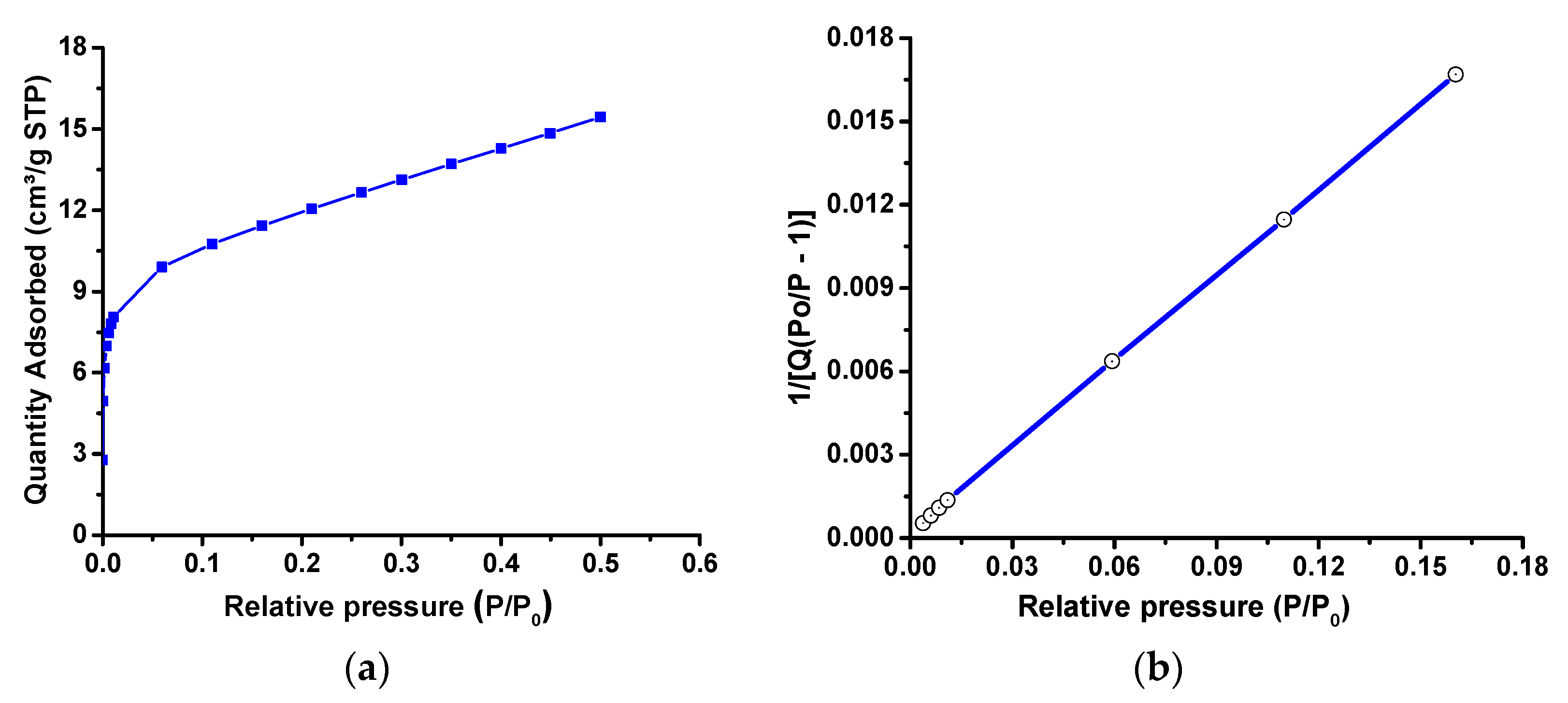
| Nanocomposite | Cu (wt%) | Zn (wt%) | O (wt%) | Ti (wt%) | C (wt%) |
|---|---|---|---|---|---|
| Cu-ZnO/TiO2 | 9.67 | 13.05 | 48.84 | 18.27 | 10.17 |
| Photocatalyst | Degradation Time (min) | Kapp (min−1) | Degradation (%) |
|---|---|---|---|
| TiO2 | 100 | 0.013 | 76 |
| ZnO | 60 | 0.05 | 99 |
| Cu | 70 | 0.02 | 94 |
| CZT-1 | 55 | 0.071 | 93 |
| CZT-2 | 20 | 0.094 | 100 |
| CZT-3 | 40 | 0.075 | 96 |
| CZT-4 | 65 | 0.06 | 91 |
| CZT-5 | 60 | 0.04 | 86 |
| Cu-ZnO | 35 | 0.078 | 96 |
| Photocatalyst | Catalytic Dosage (g) | CR Concentration (mg/L) | Degradation Time (min) | Kapp (min−1) | Degradation (%) | References |
|---|---|---|---|---|---|---|
| ZnO | 0.05 | 16 | 60 | 0.0062 | 53.1 | [9] |
| Pd-ZnO | 0.05 | 16 | 60 | 0.0576 | 100 | [9] |
| TiO2 doped CoFe2O4 | 0.08 | 10 | 120 | - | 85 | [37] |
| FeNiSe-CHM | 0.2 | 60 | 140 | - | 99 | [38] |
| ZBiSe-CM | 0.225 | 40 | 120 | 0.045 | 99.63 | [39] |
| ZnO/Geopolymer | 0.2 | 5 | 60 | 0.048 | 96.97 | [40] |
| g-C3N4/RGO/Bi2Fe4O9 | 0.05 | 10 | 60 | 0.022 | 86.76 | [41] |
| PbTiO3 nanorods | 0.75 | 7 | 150 | 0.017 | 92 | [42] |
| MgZnCr-TiO2 | 0.05 | 100 | 40 | - | 98 | [43] |
| TiO2 | 0.1 | 17.4 | 30 | - | 87 | [44] |
| CuO | 0.05 | 20 | 210 | - | 67 | [45] |
| CuO-ZnO/Egg shell | 0.1 | 10 | 240 | - | 83 | [46] |
| ZnO-TiO2 | 0.1 | 70 | 70 | 0.0065 | 87.5 | [27] |
| Cu-ZnO/TiO2 (CZT-2) | 0.025 | 75 | 20 | 0.094 | 100 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landge, V.K.; Huang, C.-M.; Hakke, V.S.; Sonawane, S.H.; Manickam, S.; Hsieh, M.-C. Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye. Catalysts 2022, 12, 605. https://doi.org/10.3390/catal12060605
Landge VK, Huang C-M, Hakke VS, Sonawane SH, Manickam S, Hsieh M-C. Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye. Catalysts. 2022; 12(6):605. https://doi.org/10.3390/catal12060605
Chicago/Turabian StyleLandge, Vividha Kondba, Chao-Ming Huang, Vikas Sadashiv Hakke, Shirish Hari Sonawane, Sivakumar Manickam, and Ming-Chun Hsieh. 2022. "Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye" Catalysts 12, no. 6: 605. https://doi.org/10.3390/catal12060605
APA StyleLandge, V. K., Huang, C.-M., Hakke, V. S., Sonawane, S. H., Manickam, S., & Hsieh, M.-C. (2022). Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye. Catalysts, 12(6), 605. https://doi.org/10.3390/catal12060605









