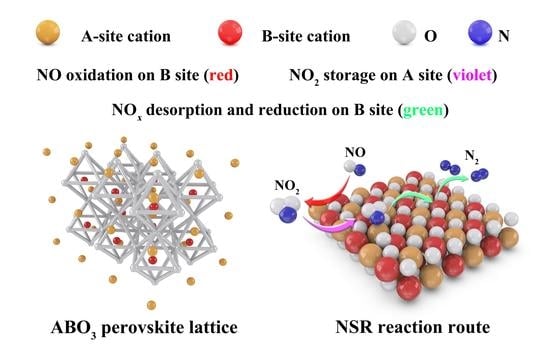
Advances in Designing Efficient La-Based Perovskites for the NOx Storage and Reduction Process
Abstract
:1. Introduction
2. NO Oxidation over La-Based Perovskites
3. NOx Storage
| Catalyst Formulation (Preparation Method) | Reaction Conditions | NOx Storage, μmol g−1 (Temperature, °C) | Ref. |
|---|---|---|---|
| K2O/LaCoO3/ZrTiO4 (impregnation) | 400 ppm NO/5% O2/N2; GHSV = 60,000 mL g−1 h−1 | 325.0 (350) | [57] |
| Pt/La0.5Sr0.5Fe0.5Ti0.5O3/Al2O3 (impregnation) | 500 ppm NO/500 ppm CO/200 ppm C3H6/9% H2O/9% CO2/6.5% O2; GHSV = 80,000 h−1 | 117.0 (250) 150.0 (350) 50.0 (450) | [59] |
| La0.7Sr0.3CoO3 (sol-gel) | 400 ppm NO/ 5% O2/ balanced N2; GHSV = 120,000 mL g−1 h−1 | 967.3 (300) | [67] |
| LaMn0.9Fe0.1O3 (combustion) | 500 ppm NO/8% O2/N2; GHSV = 30,000 h−1 | 371.6 (100) 392.3 (300) 306.3 (400) | [69] |
| LaCoO3 (sol-gel) | 400 ppm NO/5% O2/N2; GHSV = 80,000 mL g−1 h−1 | 93.0 (350) | [73] |
| Mesoporous LaCoO3 (nano-casting) | 400 ppm NO/5% O2/N2; GHSV = 80,000 mL g−1 h−1 | 981.0 (350) | [73] |
| La0.7Sr0.3CoO3 (sol-gel) | 800 ppm NO/5% O2/ N2; GHSV = 80,000 mL g−1 h−1 | 700.0 (300) | [76] |
| La0.7Sr0.3MnO3 (sol-gel) | 800 ppm NO/5% O2/ N2; GHSV = 80,000 mL g−1 h−1 | 170.4 (350) | [77] |
| K2O/LaCoO3/(Y,Ce,Zr)O2 (impregnation) | 400 ppm NO/5% O2/N2; GHSV = 45,000 h−1 | 639.0 (350) | [79] |
| LaCoO3/3% K2O/CeO2 (impregnation) | 400 ppm NO/5% O2/N2; GHSV = 45,000 h−1 | 512.4 (350) | [80] |
| 30% Pd/La0.7Sr0.3CoO3/Al2O3 (impregnation) | 500 ppm NO/6% O2/Ar; GHSV = 123,500 h−1 | 97.3 (350) | [83] |
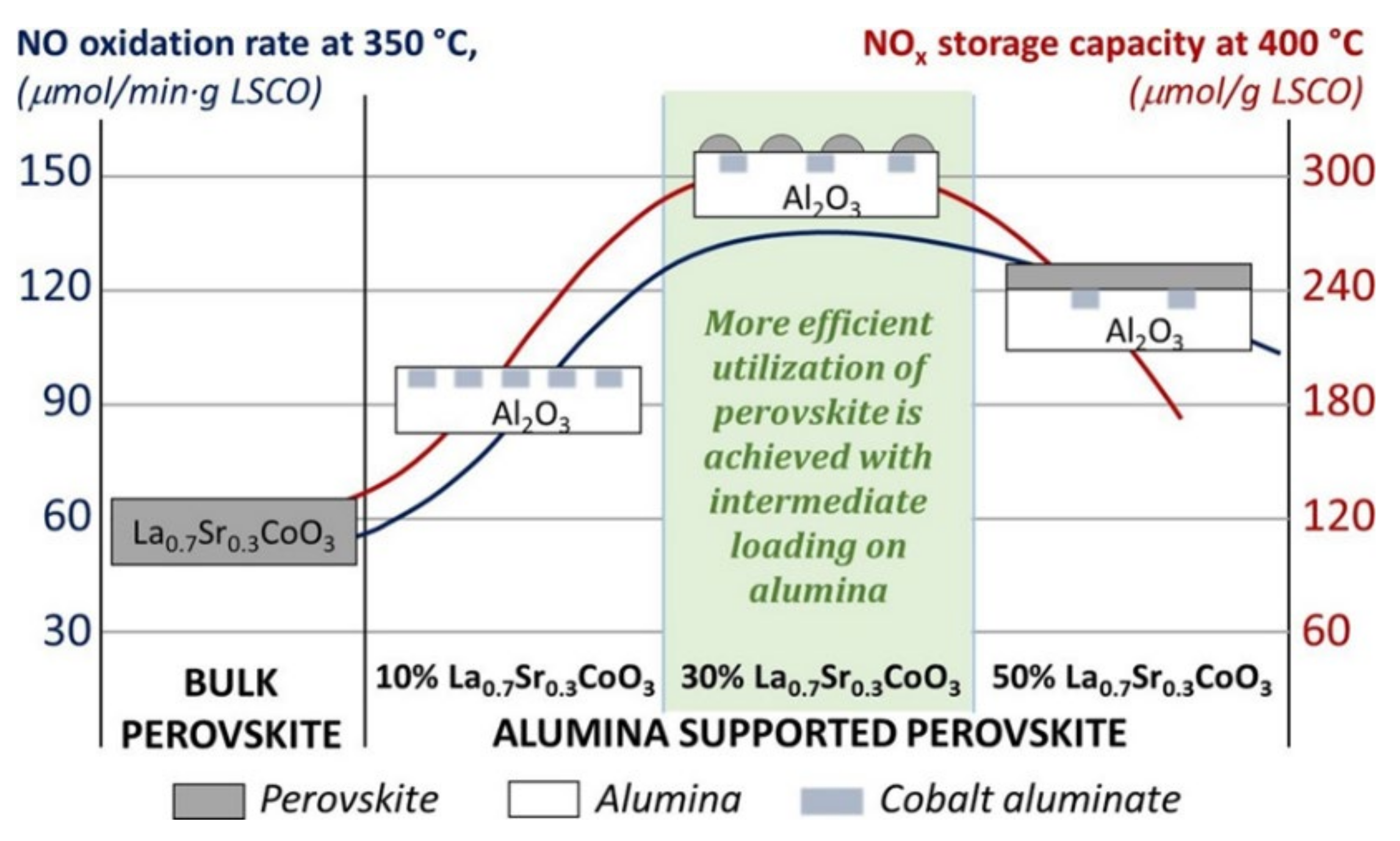
| La0.7Sr0.3CoO3/mesoporous SiO2 (deposition) | 500 ppm NO/ 5% O2/ N2; GHSV = 80,000 mL g−1 h−1 | 5269.2 (300) | [84] |
| La0.7Sr0.3CoO3 (sol-gel) | 500 ppm NO/5% O2/ N2; GHSV = 80,000 mL g−1 h−1 | 2405.0 (300) | [84] |
4. NOx Desorption and Reduction
| Catalyst (Preparation Method) | Reaction Conditions | DeNOx Activity, % (Temperature, °C) | Ref. |
|---|---|---|---|
| La0.5Sr0.3MnO3 (sol-gel) | Lean (50 s): 400 ppm NO/5% O2/ N2; rich: phase (10 s): 1000 ppm C3H6/N2; GHSV = 120,000 mL g−1 h−1 | 43.2 (350) | [31] |
| LaCo0.92Pt0.08O3 (sol-gel) | Lean (120 s): 280 ppm NO/8% O2/8% CO2/N2; rich (30 s): 280 ppm NO/ 8% CO2/3.5% H2/ N2, GHSV = 72,000 h−1 (SO2 treatment: 100 ppm SO2/8% O2/ N2 for 45 min; regeneration: 3.5 vol% H2 at 500 °C for 12 h) | 90.9 (350) 25.5 (350, SO2 treatment) 75.5 (350, regeneration) | [66] |
| La0.7Sr0.3CoO3 (sol-gel) | Lean (50 s): 400 ppm NO/5% O2/ N2; rich (10 s): 1000 ppm C3H6/N2; GHSV = 120,000 mL g−1 h−1 | 51.6 (300) | [67] |
| La0.7Sr0.3Co0.97Pd0.03O3 (sol-gel) | Lean (50 s): 400 ppm NO/ 5% O2/N2; rich (10 s): 1000 ppm C3H6/N2; GHSV = 120,000 mL g−1 h−1 | 74.4 (300) | [67] |
| 1.4% Pd/La0.7Sr0.3CoO3 (impregnation) | Lean (50 s): 400 ppm NO/5% O2/N2; rich (10 s): 1000 ppm C3H6/ N2; GHSV = 120,000 mL g−1 h−1 | 90.4 (300) | [67] |
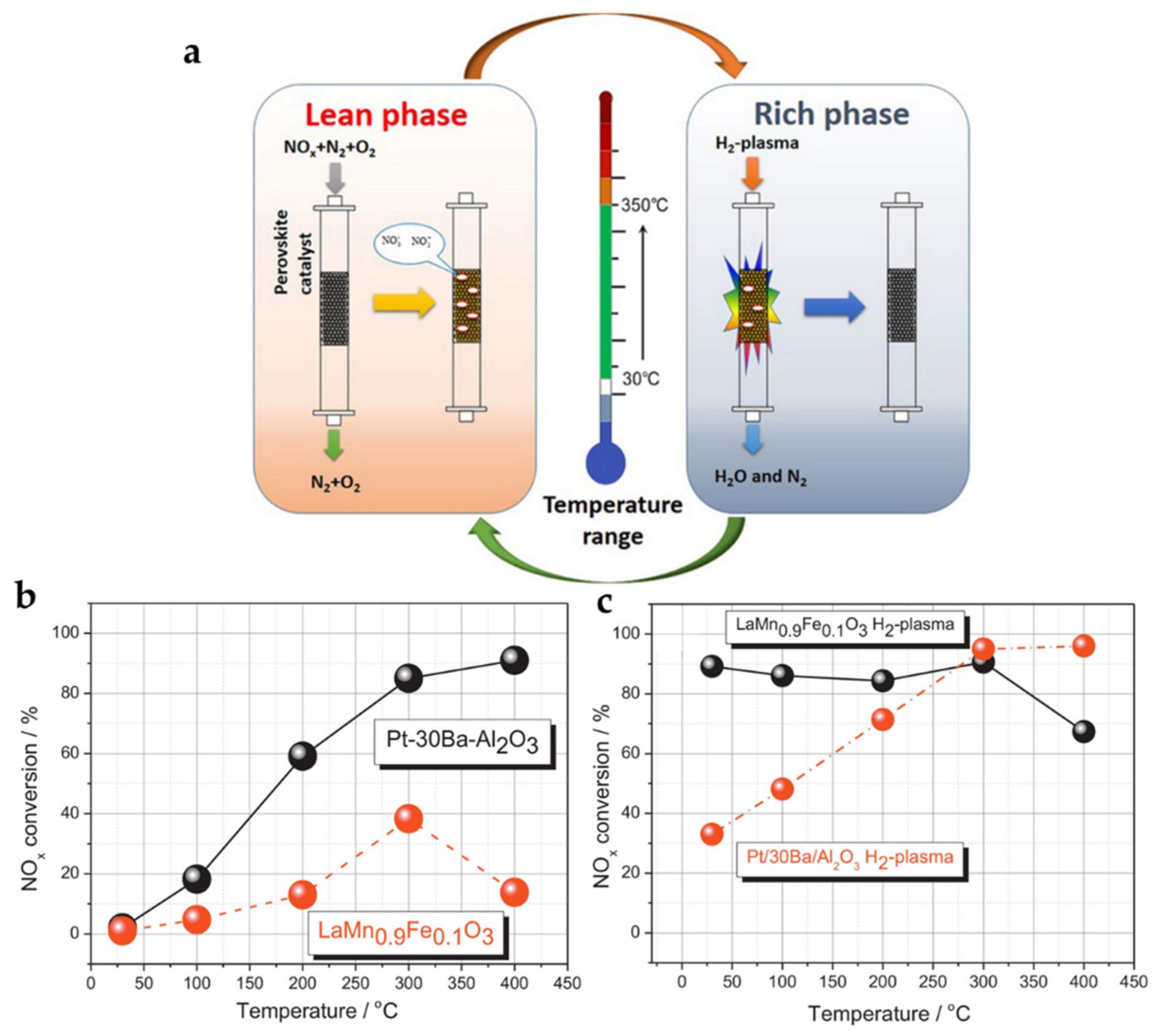
| LaMn0.9Fe0.1O3 (sol-gel) | Lean (10 min): 500 ppm NO/8% O2/N2; rich (2 min): 1% H2/ N2; GHSV = 10,000 h−1, plasma assisted | >80% (30–300) | [69] |
| LaCoO3 (sol-gel) | Lean (3 min): 500 ppm NO/ 1000 ppm C3H6/6.7% O2/N2; rich (1 min): 500 ppm NO/ 1000 ppm C3H6/ N2; GHSV = 80,000 mL g−1 h−1 | 71.4 (300) | [76] |
| 1.5% Pd/30% La0.7Sr0.3CoO3/Al2O3 (impregnation) | Lean (150 s): 500 ppm NO/6% O2/Ar; rich (20 s): 512 ppm NO/ 3% H2/ Ar; GHSV = 123,500 mL g−1 h−1 | 86.2% (350) | [82] |
| La0.7Sr0.3CoO3 (sol-gel, acid wash) | Lean (3 min): 500 ppm NO/5% O2/ N2; rich (1 min): 500 ppm NO/1000 ppm C3H6/ N2; GHSV = 120,000 mL g−1 h−1 | 55.0% (300) | [91] |
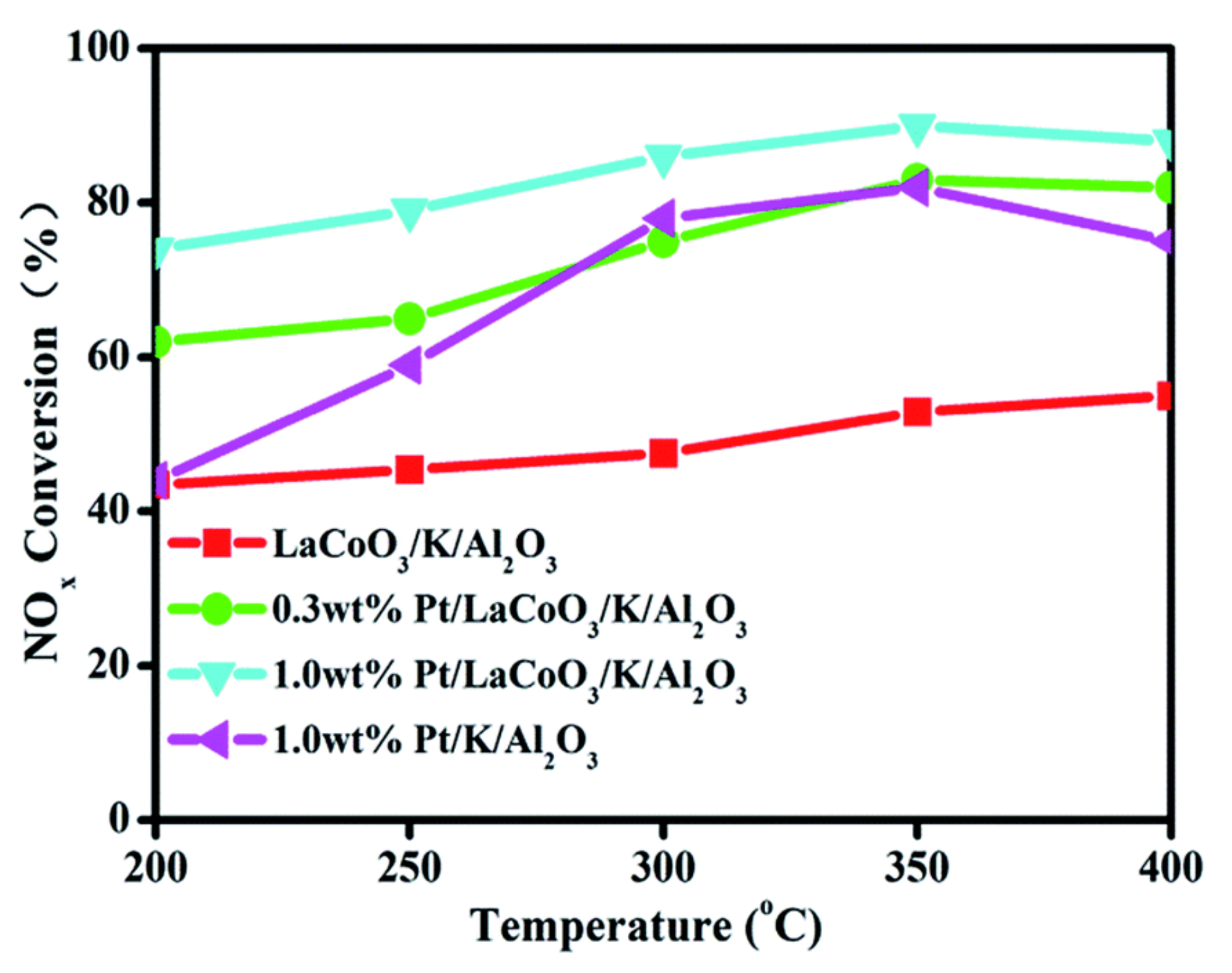
| 0.3% Pt/LaCoO3/K2O/Al2O3 (impregnation) | Lean (120 s): 500 ppm NO/ 8% O2/ Ar2, rich (120 s): 500 ppm NO/3.5%H2/Ar; GHSV = 72,000 mL g−1 h−1 | 80.0 (300) | [93] |
| La0.7Ba0.3Fe0.776Nb0.194Pd0.03O3 (glycine-nitrate method) | Lean (54 s): 512 ppm NO/200 ppm C3H6/10% O2/Ar; rich (6 s): 512 ppm NO/200 ppm C3H6/4% CO/Ar; GHSV = 60,000 mL g−1 h−1 | 47.0 (250) | [97] |
| La0.7Sr0.3Co0.97Pd0.03O3 (sol-gel) | Lean (2 min): 0 or 100 ppm SO2/ 500 ppm NO/ 6.7% O2/ N2; rich (1 min); 0 or 100 ppm SO2/ 500 ppm NO/ 1000 ppm C3H6/ N2; GHSV = 32,000 h−1 | 100 (325) 99.2 (325, with SO2) | [98] |
| 2.1%Pd/La0.7Sr0.3MnO3 (sol-gel) | Lean (50 s): 400 ppm NO/5% O2/ N2; rich: phase (10 s): 1000 ppm C3H6/ N2; GHSV = 120,000 mL g−1 h−1 | 90.1 (350) | [99] |
| 2.1%Pd/La0.7Sr0.3MnO3 (impregnation) | Lean (50 s): 400 ppm NO/5% O2/N2; rich: phase (10 s): 1000 ppm C3H6/N2; GHSV = 120,000 mL g−1 h−1 | 72.0 (350) | [99] |
5. Resistance to Poisoners
6. Conclusions and Perspective
- (1)
- In-depth understanding of the NSR reaction mechanism in perovskites. To date, most mechanism studies concerning the NSR reaction were conducted with a Pt-based model catalyst, while for other types of catalysts, including perovskites, only vague pathways and plausible lines have been proposed. Considering the complexity of the reaction process, more in-depth experimental and theoretical studies should be conducted to unravel the detailed reaction routes, active sites, and intermediates to support the rational design of perovskite formulations with predictable properties.
- (2)
- Comprehensive comparison of NSR performance of La-based perovskites with others. The majority of perovskite-based materials reported for NSR applications over the last decade have been La-based. The obtained data reveals that La3+ provides prominent structural integrity with few impurities and little phase segregation, as well as thermal and chemical stability resistant to phase transformation during NOx storage and reduction. Furthermore, the extensively studied LaCoO3 and LaMnO3 catalytic systems exhibit a good redox property, which is desirable for the NSR reaction. However, the weak basicity of A-site La3+ brings challenges for high-temperature NOx storage, and the usage of La and Co increases environmental burdens from the perspective of sustainability. Some interesting data have been recently reported for Sr- and Ba-based perovskite formulations. On one hand, the stronger basicity of A-site alkaline earth metal provides better high-temperature NOx storage performance, and the +2 oxidation state of the A-site cation increases the B-site oxidation state and/or generates oxygen vacancies desirable for NO oxidation and NOx storage. On the other hand, these strongly basic cations are generally less resistant to CO2, NOx, and SO2 in the reaction atmosphere, and hence are prone to exsolution from the perovskite matrix, leading to the risk of collapse of the perovskite structure, but this tendency may vary depending on specific formulations. At this stage, a comprehensive study of the effects of different A-site cations on the activity and stability of perovskite catalysts in NSR reactions will be rather helpful.
- (3)
- Synergetic/competitive relation between different storage sites. As stated earlier, a practical NSR catalyst should be capable of efficiently trapping and releasing NOx in a wide range of temperatures. This indicates that multiple storage components with different basicity should be used in combination; however, almost no comprehensive research has been devoted to this line. Current research suggests that La-based perovskite is an excellent low-temperature NOx storage component, but whether the combination of perovskite and stronger basic sites efficient for high-temperature NSR reaction can work as they are intended remains unclear.
- (4)
- Redox stability and resistance to H2O, CO2, and SO2 poisoning. Most perovskite formulations are designed and tested under ideal laboratory conditions. The durability of perovskite catalysts in reducing atmosphere or when exposed to the common poisoners in automotive exhaust should be carefully considered and further clarified.
- (5)
- Sustainability has become an essential concern in catalyst design with the guidance of legislation and increasing environmental awareness of the community. However, this is entirely neglected in the currently reviewed publications. The environmental burden of using Pt on a per kilogram basis is calculated to be 3–4 orders of magnitude larger than base metals, in the aspects of global warming potential, cumulative energy demand, terrestrial acidification, freshwater eutrophication, and human toxicity [104], which necessitates the development of environmentally friendly base metal alternatives. Along this line, in perovskite formulation design, the metals at the lower end of the scale of environmental impacts should be preferably considered, such as manganese, iron, and titanium at B-site. These metals also result in decent NSR performance, while the usage of nickel and cobalt should be more prudent. For A-site cations, Sr and Ba are better choices than La.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bai, Z.F.; Zhang, Z.S.; Chen, B.B.; Zhao, Q.; Crocker, M.; Shi, C. Non-thermal plasma enhanced NSR performance over Pt/M/Ba/Al2O3 (M = Mn, Co, Cu) catalysts. Chem. Eng. J. 2017, 314, 688–699. [Google Scholar] [CrossRef] [Green Version]
- Parks, J.E. Less costly catalysts for controlling engine emissions. Science 2010, 327, 1584–1585. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, M.; Palkovits, R. Perovskite-based catalysts for the control of nitrogen oxide emissions from diesel engines. Catal. Sci. Technol. 2019, 9, 2057–2077. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, G.; Yang, X. Advances in De-NOx methods and catalysts for direct catalytic decomposition of NO: A review. Energy Fuels 2021, 35, 6443–6464. [Google Scholar] [CrossRef]
- Ishihara, T.; Ando, M.; Sada, K.; Takiishi, K.; Yamada, K.; Nishiguchi, H.; Takita, Y. Direct decomposition of NO into N2 and O2 over La(Ba)Mn(In)O3 perovskite oxide. J. Catal. 2003, 220, 104–114. [Google Scholar] [CrossRef]
- Zhao, H.W.; Han, L.; Wang, Y.J.; Zheng, J.D. Insight into platinum poisoning effect on Cu-SSZ-13 in selective catalytic reduction of NOx with NH3. Catalysts 2021, 11, 796. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Gao, F. Fe-exchanged small-pore zeolites as ammonia selective catalytic reduction (NH3-SCR) catalysts. Catalysts 2020, 10, 1324. [Google Scholar] [CrossRef]
- Sun, H.; Park, S. Recent advances in MNOx/CeO2-based ternary composites for selective catalytic reduction of NOx by NH3: A review. Catalysts 2021, 11, 1519. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR catalysts: Catalytic activity, stability and interaction among catalytic oxides. Catalysts 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Baiker, A. NOx storage-reduction catalysis: From mechanism and materials properties to storage-reduction performance. Chem. Rev. 2009, 109, 4054–4091. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Liu, Z.; Ihl Woo, S. Recent advances in catalytic DeNOx science and technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today 1996, 27, 63–69. [Google Scholar] [CrossRef]
- Yin, M.X.; Liu, D.S.; Zhao, D.Y.; Ding, T.; Tian, Y.; Li, X.G. Effect of copper doping on lean NOx Trap Performance of Pt/Ba/CuxMg1-xAl2O4 catalysts at high temperatures. Chem. J. Chin. Univ. 2019, 40, 2170–2177. [Google Scholar]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Yi, U. Effects of engine operating conditions on particle emissions of lean-burn gasoline direct-injection engine. Energy 2016, 115, 1148–1155. [Google Scholar] [CrossRef]
- Praveena, V.; Martin, M.L.J. A review on various after treatment techniques to reduce NOx emissions in a CI engine. J. Energy Inst. 2018, 91, 704–720. [Google Scholar] [CrossRef]
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Pérot, G. Hydrotreating catalysts containing zeolites and related materials-mechanistic aspects related to deep desulfurization. Catal. Today 2003, 86, 111–128. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Q.; Zhao, Z.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Surface tuning of noble metal doped perovskite oxide by synergistic effect of thermal treatment and acid etching: A new path to high-performance catalysts for methane combustion. Appl. Catal. B 2018, 239, 373–382. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhao, Z.; Zheng, J.; Zhang, G.; Duan, A.; Jiang, G. Three-dimensionally ordered macroporous LaCoxFe1−xO3 perovskite-type complex oxide catalysts for diesel soot combustion. Catal. Today 2010, 153, 136–142. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Si, W.; Li, J.; Crittenden, J.; Hao, J. Experimental and DFT studies on Sr-doped LaMnO3 catalysts for NOx storage and reduction. Catal. Sci. Technol. 2015, 5, 2478–2485. [Google Scholar] [CrossRef]
- Li, F.; Tang, J.; Ke, Q.; Guo, Y.; Ha, M.N.; Wan, C.; Lei, Z.; Gu, J.; Li, Q.; Nguyen, V.N.; et al. Investigation into enhanced catalytic performance for epoxidation of styrene over LaSrCoxFe2–xO6 double perovskites: The role of singlet oxygen species promoted by the photothermal effect. ACS Catal. 2021, 11, 11855–11866. [Google Scholar] [CrossRef]
- Si, W.; Wang, Y.; Peng, Y.; Li, J. Selective dissolution of A-site cations in ABO3 perovskites: A new path to high-performance catalysts. Angew. Chem. 2015, 127, 8065–8068. [Google Scholar] [CrossRef]
- Gao, Z.N.; Guo, L.H.; Zhao, D.Y.; Li, X.G. Effect of A site-deficiency on the structure and catalytic oxidation activity of the La-Sr-Co-O perovskite. Chem. J. Chin. Univ. 2021, 42, 2869–2877. [Google Scholar]
- Hernández-Giménez, A.; Castelló, D.; Bueno-López, A. Diesel soot combustion catalysts: Review of active phases. Chem. Pap. 2014, 68, 1154–1168. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Thomas, A. Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B 2009, 92, 225–233. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Yang, Y.X.; Gao, Z.N.; Tian, Y.; Zhang, J.; Jiang, Z.; Li, X.G. A-site defects in perovskite-based catalyst promoting NOx storage and reduction for lean-burn exhausts. J. Rare Earth 2021, 39, 959–968. [Google Scholar] [CrossRef]
- Švarcová, S.; Wiik, K.; Tolchard, J.; Bouwmeester, H.J.; Grande, T. Structural instability of cubic perovskite BaxSr1−xCo1−yFeyO3−δ. Solid State Ion. 2008, 178, 1787–1791. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef]
- Li, C.; Soh, K.C.K.; Wu, P. Formability of ABO3 perovskites. J. Alloy Compd. 2004, 372, 40–48. [Google Scholar] [CrossRef]
- Xian, H.; Zhang, X.; Li, X.; Li, L.; Zou, H.; Meng, M.; Li, Q.; Tan, Y.; Tsubaki, N. BaFeO3−x perovskite: An efficient NOx absorber with a high sulfur tolerance. J. Phys. Chem. C 2010, 114, 11844–11852. [Google Scholar] [CrossRef]
- Xian, H.; Zhang, X.W.; Li, X.G.; Zou, H.H.; Meng, M.; Zou, Z.Q.; Guo, L.H.; Tsubaki, N. Effect of the calcination conditions on the NOx storage behavior of the perovskite BaFeO3−x catalysts. Catal. Today 2010, 158, 215–219. [Google Scholar] [CrossRef]
- Xian, H.; Li, F.L.; Li, X.G.; Zhang, X.W.; Meng, M.; Zhang, T.Y.; Tsubaki, N. Influence of preparation conditions to structure property, NOx and SO2 sorption behavior of the BaFeO3−x perovskite catalyst. Fuel Process. Technol. 2011, 92, 1718–1724. [Google Scholar] [CrossRef]
- Ge, C.; Li, L.; Xian, H.; Yan, H.; Meng, M.; Li, X. Effects of Ti-doping on the NOx storage and the sulfur resistance of the BaFe1−xTixO3−y perovskite-type catalysts for lean-burn exhausts. Fuel Process. Technol. 2014, 120, 1–7. [Google Scholar] [CrossRef]
- Hodjati, S.; Vaezzadeh, K.; Petit, C.; Pitchon, V.; Kiennemann, A. Absorption/desorption of NOx process on perovskites: Performances to remove NOx from a lean exhaust gas. Appl. Catal. B 2000, 26, 5–16. [Google Scholar] [CrossRef]
- Hodjati, S.; Petit, C.; Pitchon, V.; Kiennemann, A. Absorption/desorption of NOx process on perovskites: Impact of SO2 on the storage capacity of BaSnO3 and strategy to develop thioresistance. Appl. Catal. B 2001, 30, 247–257. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Lin, P.; Meng, M.; Fu, Y.; Tu, J.; Li, Q. A study of the NOx storage catalyst of Ba-Fe-O complex oxide. Catal. Commun. 2004, 5, 25–28. [Google Scholar] [CrossRef]
- Olsson, L.; Persson, H.; Fridell, E.; Skoglundh, M.; Andersson, B. A kinetic study of NO oxidation and NOx storage on Pt/Al2O3 and Pt/BaO/Al2O3. J. Phys. Chem. B 2001, 105, 6895–6906. [Google Scholar] [CrossRef]
- Bhatia, D.; McCabe, R.W.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic study of NO oxidation on model Pt catalysts. J. Catal. 2009, 266, 106–119. [Google Scholar] [CrossRef]
- Hauff, K.; Tuttlies, U.; Eigenberger, G.; Nieken, U. Platinum oxide formation and reduction during NO oxidation on a diesel oxidation catalyst–experimental results. Appl. Catal. B 2012, 123, 107–116. [Google Scholar] [CrossRef]
- Li, L.D.; Shen, Q.; Cheng, J.; Hao, Z.P. Catalytic oxidation of NO over TiO2 supported platinum clusters. II: Mechanism study by in situ FTIR spectra. Catal. Today 2010, 158, 361–369. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Li, X.B. Catalytic oxidation of nitric oxide (NO) over different catalysts: An overview. Catal. Sci. Technol. 2017, 7, 3440–3452. [Google Scholar] [CrossRef]
- Weiss, B.M.; Iglesia, E. NO oxidation catalysis on Pt clusters: Elementary steps, structural requirements, and synergistic effects of NO2 adsorption sites. J. Phys. Chem. C 2009, 113, 13331–13340. [Google Scholar] [CrossRef] [Green Version]
- Weiss, B.M.; Iglesia, E. Mechanism and site requirements for NO oxidation on Pd catalysts. J. Catal. 2010, 272, 74–81. [Google Scholar] [CrossRef]
- Mulla, S.S.; Chen, N.; Cumaranatunge, L.; Blau, G.E.; Zemlyanov, D.Y.; Delgass, W.N.; Epling, W.S.; Ribeiro, F.H. Reaction of NO and O2 to NO2 on Pt: Kinetics and catalyst deactivation. J. Catal. 2006, 241, 389–399. [Google Scholar] [CrossRef]
- Constantinou, C.; Li, W.; Qi, G.; Epling, W.S. NOx storage and reduction over a perovskite-based lean NOx trap catalyst. Appl. Catal. B 2013, 134, 66–74. [Google Scholar] [CrossRef]
- Mulla, S.S.; Chen, N.; Delgass, W.N.; Epling, W.S.; Ribeiro, F.H. NO2 inhibits the catalytic reaction of NO and O2 over Pt. Catal. Lett. 2005, 100, 267–270. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thévenet, F.; Gravejat, P.; Rousseau, A. Investigation of NO and NO2 adsorption mechanisms on TiO2 at room temperature. Appl. Catal. B 2013, 142, 196–204. [Google Scholar] [CrossRef]
- Epling, W.S.; Parks, J.E.; Campbell, G.C.; Yezerets, A.; Currier, N.W.; Campbell, L.E. Further evidence of multiple NOx sorption sites on NOx storage/reduction catalysts. Catal. Today 2004, 96, 21–30. [Google Scholar] [CrossRef]
- Parker, D.H.; Koel, B.E. Chemisorption of high coverages of atomic oxygen on the Pt (111), Pd (111), and Au (111) surfaces. J. Vac. Sci. Technol. A 1990, 8, 2585–2590. [Google Scholar] [CrossRef]
- Segner, J.; Vielhaber, W.; Ertl, G. Interaction of NO2 with a Pt (111) surface. Isr. J. Chem. 1982, 22, 375–379. [Google Scholar] [CrossRef]
- He, X.; Meng, M.; He, J.; Zou, Z.; Li, X.; Li, Z.; Jiang, Z. A potential substitution of noble metal Pt by perovskite LaCoO3 in ZrTiO4 supported lean-burn NOx trap catalysts. Catal. Commun. 2010, 12, 165–168. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B 2013, 134, 251–257. [Google Scholar] [CrossRef]
- Ecker, S.I.; Dornseiffer, J.; Werner, J.; Schlenz, H.; Sohn, Y.J.; Sauerwein, F.S.; Baumann, S.; Bouwmeester, H.J.M.; Guillon, O.; Weirich, T.E.; et al. Novel low-temperature lean NOx storage materials based on La0.5Sr0.5Fe1-xMxO3-δ/Al2O3 infiltration composites (M = Ti, Zr, Nb). Appl. Catal. B 2021, 286, 119919. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Guo, L.H.; Bo, L.; Li, Y.; Jiang, Z.; Tian, Y.; Li, X.G. Sr doping effect on the structure property and NO oxidation performance of dual-site doped perovskite La(Sr)Co(Fe)O3. Solid State Sci. 2021, 113, 106519. [Google Scholar] [CrossRef]
- Lim, E.; Kim, Y.J.; Kim, J.H.; HoKim, J.; Ryu, T.; Lee, S.; Cho, B.K.; Nam, I.; Choung, J.W.; Yoo, S. NO oxidation activity of Ag-doped perovskite catalysts. J. Catal. 2014, 319, 182–193. [Google Scholar]
- Chen, J.; Shen, M.; Wang, X.; Wang, J.; Su, Y.; Zhao, Z. Catalytic performance of NO oxidation over LaMeO3 (Me = Mn, Fe, Co) perovskite prepared by the sol-gel method. Catal. Commun. 2013, 37, 105–108. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, Y.; Xin, H.; Yang, C.; Chen, L.; Li, X. NO oxidation over Ni-Co perovskite catalysts. Chem. Eng. J. 2015, 275, 351–356. [Google Scholar] [CrossRef]
- Wang, X.; Qi, X.; Chen, Z.; Jiang, L.; Wang, R.; Wei, K. Studies on SO2 tolerance and regeneration over perovskite-type LaCo1-xPtxO3 in NOx storage and reduction. J. Phys. Chem. C 2014, 118, 13743–13751. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, Z.; Xian, H.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Jiang, Z.; Zhang, J.; Zheng, L.R.; et al. Addition of Pd on La0.7Sr0.3CoO3 perovskite to enhance catalytic removal of NOx. Ind. Eng. Chem. Res. 2018, 57, 521–531. [Google Scholar] [CrossRef]
- Epling, W.S.; Yezerets, A.; Currier, N.W. The effects of regeneration conditions on NOx and NH3 release from NOx storage/reduction catalysts. Appl. Catal. B 2007, 74, 117–129. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Z.; Crocker, M.; Xu, L.; Wang, C.Y.; Au, C.; Zhu, A.M. Non-thermal plasma-assisted NOx storage and reduction on a LaMn0.9Fe0.1O3 perovskite catalyst. Catal. Today 2013, 211, 96–103. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Hosokawa, S.; Tamai, K.; Kajino, T.; Yoto, H.; Asakura, H.; Teramura, K.; Tanaka, T. NOx storage performance at low temperature over platinum group metal-free SrTiO3-based material. ACS Appl. Mater. Interfaces 2021, 13, 29482–29490. [Google Scholar] [CrossRef]
- Tamai, K.; Hosokawa, S.; Kato, K.; Asakura, H.; Teramura, K.; Tanaka, T. Low-temperature NO oxidation using lattice oxygen in Fe-site substituted SrFeO3−δ. Phys. Chem. Chem. Phys. 2020, 22, 24181–24190. [Google Scholar] [CrossRef]
- Takamatsu, A.; Tamai, K.; Hosokawa, S.; Tanaka, T.; Ehara, M.; Fukuda, R. Oxidation and storage mechanisms for nitrogen oxides on variously terminated (001) surfaces of SrFeO3−δ and Sr3Fe2O7−δ perovskites. ACS Appl. Mater. Interfaces 2021, 13, 7216–7226. [Google Scholar] [CrossRef]
- Ye, J.; Yu, Y.; Meng, M.; Jiang, Z.; Ding, T.; Zhang, S.; Huang, Y. Highly efficient NOx purification in alternating lean/rich atmospheres over non-platinic mesoporous perovskite-based catalyst K/LaCoO3. Catal. Sci. Technol. 2013, 3, 1915–1918. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. NOx adsorption and reduction over LaMnO3 based lean NOx trap catalysts. Catal. Lett. 2014, 144, 639–647. [Google Scholar] [CrossRef]
- Say, Z.; Dogac, M.; Vovk, E.I.; Kalay, Y.E.; Kim, C.H.; Li, W.; Ozensoy, E. Palladium doped perovskite-based NO oxidation catalysts: The role of Pd and B-sites for NOx adsorption behavior via in-situ spectroscopy. Appl. Catal. B 2014, 154, 51–61. [Google Scholar] [CrossRef]
- Li, X.G.; Dong, Y.H.; Xian, H.; Hernández, W.Y.; Meng, M.; Zou, H.H.; Ma, A.J.; Zhang, T.Y.; Jiang, Z.; Tsubaki, N.; et al. De-NOx in alternative lean/rich atmospheres on La1-xSrxCoO3 perovskites. Energy Environ. Sci. 2011, 4, 3351–3354. [Google Scholar] [CrossRef]
- Dong, Y.H.; Xian, H.; Lv, J.L.; Liu, C.; Guo, L.; Meng, M.; Tan, Y.S.; Tsubaki, N.; Li, X.G. Influence of synthesis conditions on NO oxidation and NOx storage performances of La0.7Sr0.3MnO3 perovskite-type catalyst in lean-burn atmospheres. Mater. Chem. Phys. 2014, 143, 578–586. [Google Scholar] [CrossRef]
- Xie, W.; Xu, G.Y.; Zhang, Y.; Yu, Y.B.; He, H. Mesoporous LaCoO3 perovskite oxide with high catalytic performance for NOx storage and reduction. J. Hazard. Mater. 2022, 431, 128528. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, D.; Meng, M.; Zheng, L.; Zhang, J.; Hu, T. YCeZrO ternary oxide solid solution supported nonplatinic lean-burn NOx trap catalysts using LaCoO3 perovskite as active phase. J. Phys. Chem. C 2014, 118, 25403–25420. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, F.; Meng, M.; Jiang, Z.; Zhang, S.; Huang, Y. A series of ceria supported lean-burn NOx trap catalysts LaCoO3/K2CO3/CeO2 using perovskite as active component. Chem. Eng. J. 2015, 260, 357–367. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Gonzalez-Velasco, J.R. Perovskite-based catalysts as efficient, durable, and economical NOx storage and reduction systems. Catalysts 2020, 10, 208. [Google Scholar] [CrossRef] [Green Version]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Bermejo-Lopez, A.; Caravaca, A.; Vernoux, P.; González-Velasco, J.R. Pd-doped or Pd impregnated 30% La0.7Sr0.3CoO3/Al2O3 catalysts for NOx storage and reduction. Appl. Catal. B 2019, 259, 118052. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Strontium doping and impregnation onto alumina improve the NOx storage and reduction capacity of LaCoO3 perovskites. Catal. Today 2019, 333, 208–218. [Google Scholar] [CrossRef]
- Ding, Q.; Xian, H.; Tan, Y.; Tsubaki, N.; Li, X. Mesoporous SiO2-confined La0.7Sr0.3CoO3 perovskite nanoparticles: An efficient NOx adsorber for lean-burn exhausts. Catal. Sci. Technol. 2013, 3, 1493–1496. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. Pt-free, LaMnO3 based lean NOx trap catalysts. Catal. Today 2012, 184, 72–77. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Caravaca, A.; De-La-Torre, U.; Vernoux, P.; González-Velasco, J.R. Tailoring perovskite surface composition to design efficient lean NOx trap Pd–La1-xAxCoO3/Al2O3-type catalysts (with A = Sr or Ba). Appl. Catal. B 2020, 266, 118628. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Cabrejas, I.; De-La-Torre, U.; González-Velasco, J.R. Ba-doped vs. Sr-doped LaCoO3 perovskites as base catalyst in diesel exhaust purification. Mol. Catal. 2020, 488, 110913. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Urrutxua, M.; De La Torre, U.; González-Velasco, J.R. Boosting NOx removal by perovskite-based catalyst in NSR–SCR diesel aftertreatment systems. Ind. Eng. Chem. Res. 2021, 60, 6525–6537. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Anderson, J.A.; Illán-Gómez, M.J. NOx storage on BaTi0.8Cu0.2O3 perovskite catalysts: Addressing a feasible mechanism. Nanomaterials 2021, 11, 2133. [Google Scholar] [CrossRef]
- Yi, J.; Schroeder, M.; Weirich, T.; Mayer, J. Behavior of Ba (Co, Fe, Nb)O3-δ perovskite in CO2-containing atmospheres: Degradation mechanism and materials design. Chem. Mater. 2010, 22, 6246–6253. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Luo, J.; Su, W.K.; Chang, H.Z.; Li, J.H.; Hao, J.M.; Crittenden, J. Surface tuning of La0.5Sr0.5CoO3 perovskite catalysts by acetic acid for NOx storage and reduction. Environ. Sci. Technol. 2016, 50, 6442–6448. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Wen, W.; Wang, X.; Jin, S.; Wang, R. LaCoO3 perovskite in Pt/LaCoO3/K/Al2O3 for the improvement of NOx storage and reduction performances. RSC Adv. 2016, 6, 74046–74052. [Google Scholar] [CrossRef]
- Ciuparu, D.; Bensalem, A.; Pfefferle, L. Pd–Ce interactions and adsorption properties of palladium: CO and NO TPD studies over Pd–Ce/Al2O3 catalysts. Appl. Catal. B 2000, 26, 241–255. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Tanaka, H.; Uenishi, M.; Kimura, M. Self-regeneration of palladium-perovskite catalysts in modern automobiles. J. Phys. Chem. Solids 2005, 66, 274–282. [Google Scholar] [CrossRef]
- Ueda, A.; Yamada, Y.; Katsuki, M.; Kiyobayashi, T.; Xu, Q.; Kuriyama, N. Perovskite catalyst (La, Ba)(Fe, Nb, Pd)O3 applicable to NOx storage and reduction system. Catal. Commun. 2009, 11, 34–37. [Google Scholar] [CrossRef]
- Li, X.G.; Chen, C.; Liu, C.; Xian, H.; Guo, L.; Lv, J.; Jiang, Z.; Vernoux, P. Pd-doped perovskite: An effective catalyst for removal of NOx from lean-burn exhausts with high sulfur resistance. ACS Catal. 2013, 3, 1071–1075. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Y.; Gao, Z.; Yin, M.; Tian, Y.; Zhang, J.; Jiang, Z.; Yu, X.B.; Li, X. Promoting NOx reduction via in situ activation of perovskite supported Pd catalysts under alternating lean-burn/fuel-rich operating atmospheres. Chin. J. Catal. 2021, 42, 795–807. [Google Scholar] [CrossRef]
- Ma, A.J.; Wang, S.Z.; Liu, C.; Xian, H.; Ding, Q.; Guo, L.; Meng, M.; Tan, Y.S.; Tsubaki, N.; Zhang, J.; et al. Effects of Fe dopants and residual carbonates on the catalytic activities of the perovskite-type La0.7Sr0.3Co1-xFexO3 NOx storage catalyst. Appl. Catal. B 2014, 146, 24–34. [Google Scholar] [CrossRef]
- Artioli, N.; Matarrese, R.; Castoldi, L.; Lietti, L.; Forzatti, P. Effect of soot on the storage-reduction performances of PtBa/Al2O3 LNT catalyst. Catal. Today 2011, 169, 36–44. [Google Scholar] [CrossRef]
- Castoldi, L. An overview on the catalytic materials proposed for the simultaneous removal of NOx and soot. Materials 2020, 13, 3551. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, J.; Tian, G.; Zhu, J.; Song, Q.; Wang, H.; Zhang, C. Efficient catalysts of K and Ce Co-doped LaMnO3 for NOx–soot simultaneous removal and reaction kinetics. ACS Omega 2021, 6, 19836–19845. [Google Scholar] [CrossRef] [PubMed]
- Nuss, P.; Eckelman, M.J. Life cycle assessment of metals: A scientific synthesis. PLoS ONE 2014, 9, e101298. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Song, H.; Liu, J.; Jiang, Q.; Li, X. Advances in Designing Efficient La-Based Perovskites for the NOx Storage and Reduction Process. Catalysts 2022, 12, 593. https://doi.org/10.3390/catal12060593
Zhao D, Song H, Liu J, Jiang Q, Li X. Advances in Designing Efficient La-Based Perovskites for the NOx Storage and Reduction Process. Catalysts. 2022; 12(6):593. https://doi.org/10.3390/catal12060593
Chicago/Turabian StyleZhao, Dongyue, Haitao Song, Jun Liu, Qiuqiao Jiang, and Xingang Li. 2022. "Advances in Designing Efficient La-Based Perovskites for the NOx Storage and Reduction Process" Catalysts 12, no. 6: 593. https://doi.org/10.3390/catal12060593
APA StyleZhao, D., Song, H., Liu, J., Jiang, Q., & Li, X. (2022). Advances in Designing Efficient La-Based Perovskites for the NOx Storage and Reduction Process. Catalysts, 12(6), 593. https://doi.org/10.3390/catal12060593