Impact of Hydrothermally Prepared Support on the Catalytic Properties of CuCe Oxide for Preferential CO Oxidation Reaction
Abstract
:1. Introduction
2. Results
2.1. Morphological Characteristics (SEM)
2.2. Textural Characteristics (N2 Physisorption)
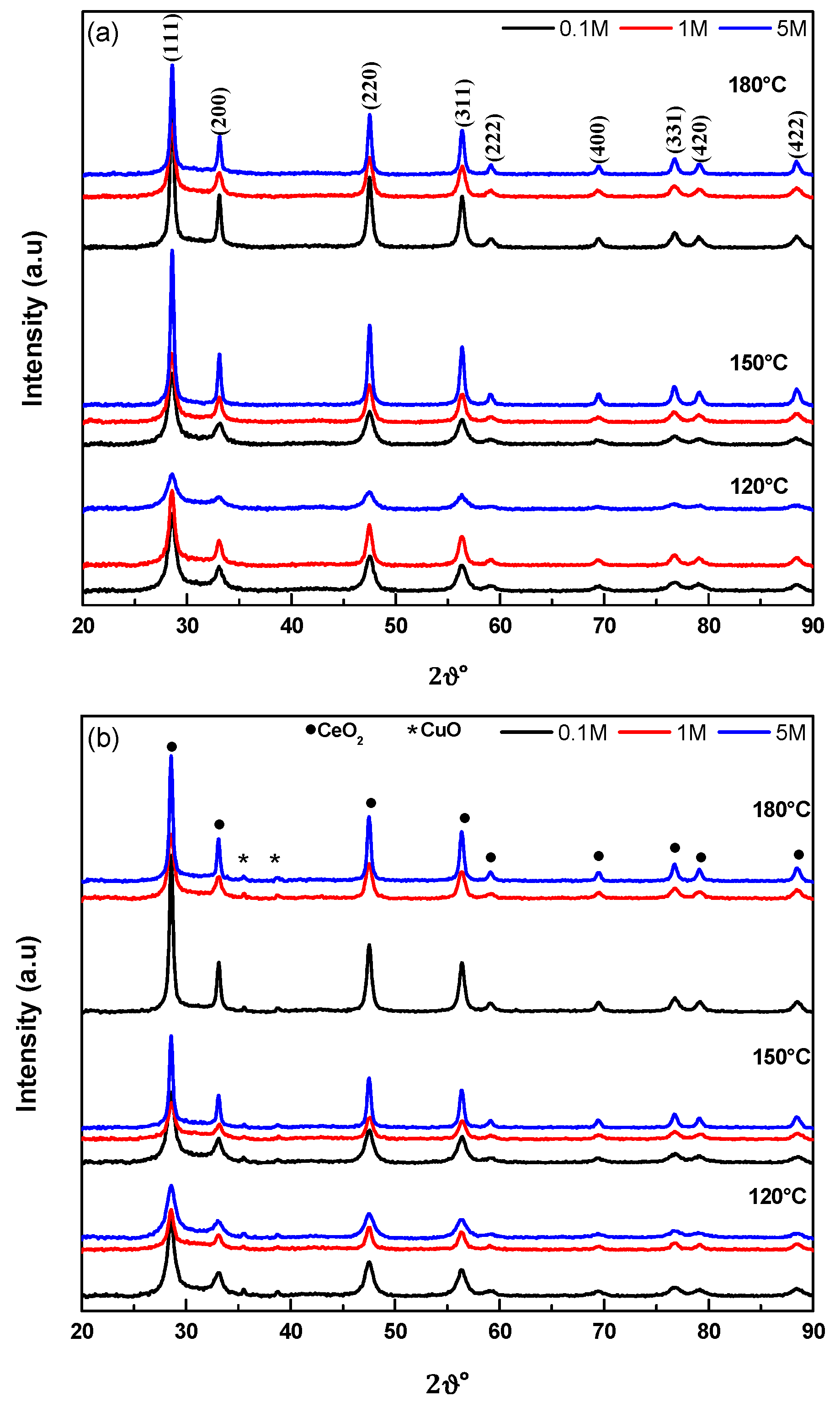
2.3. Structural Characteristics (XRD)
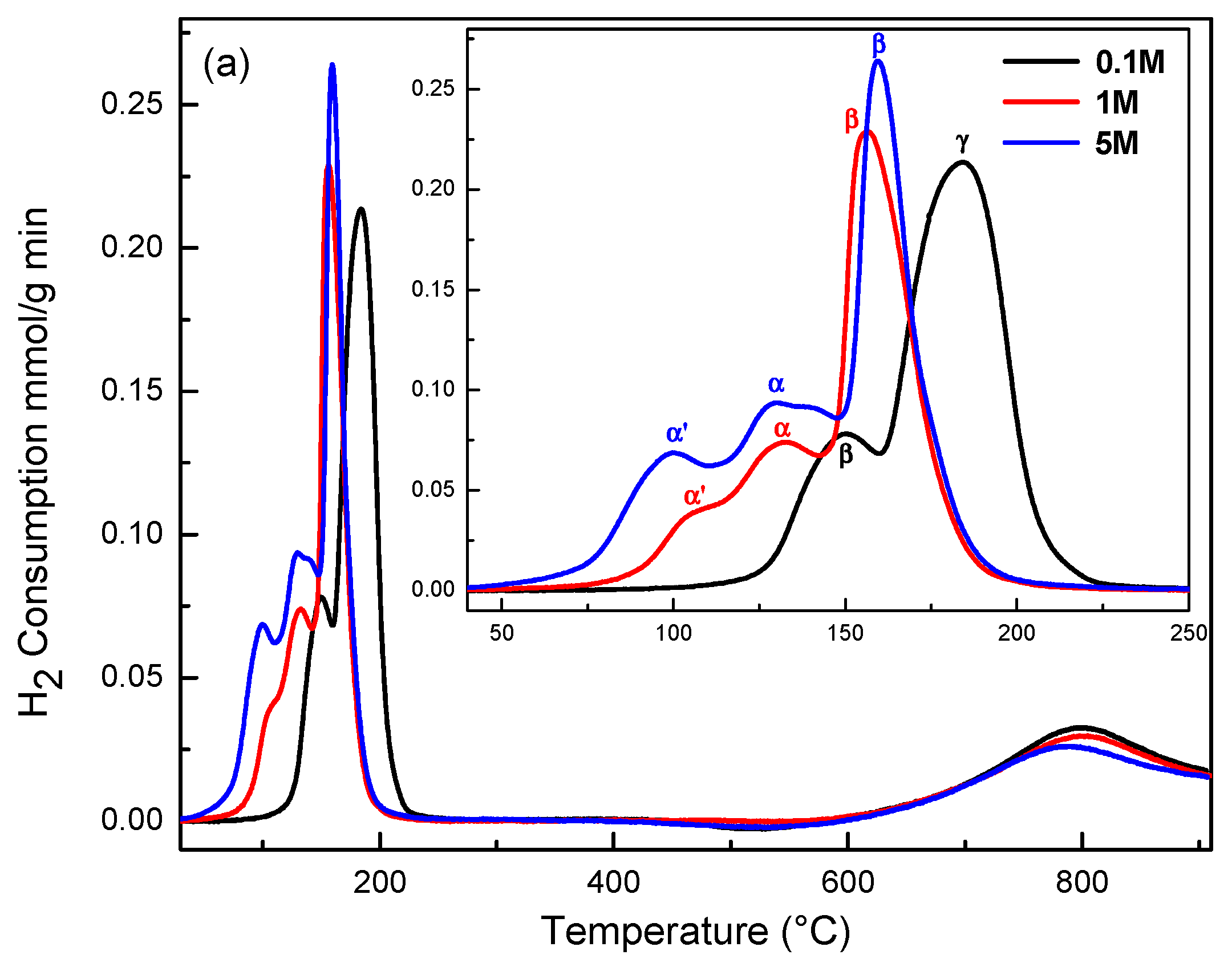
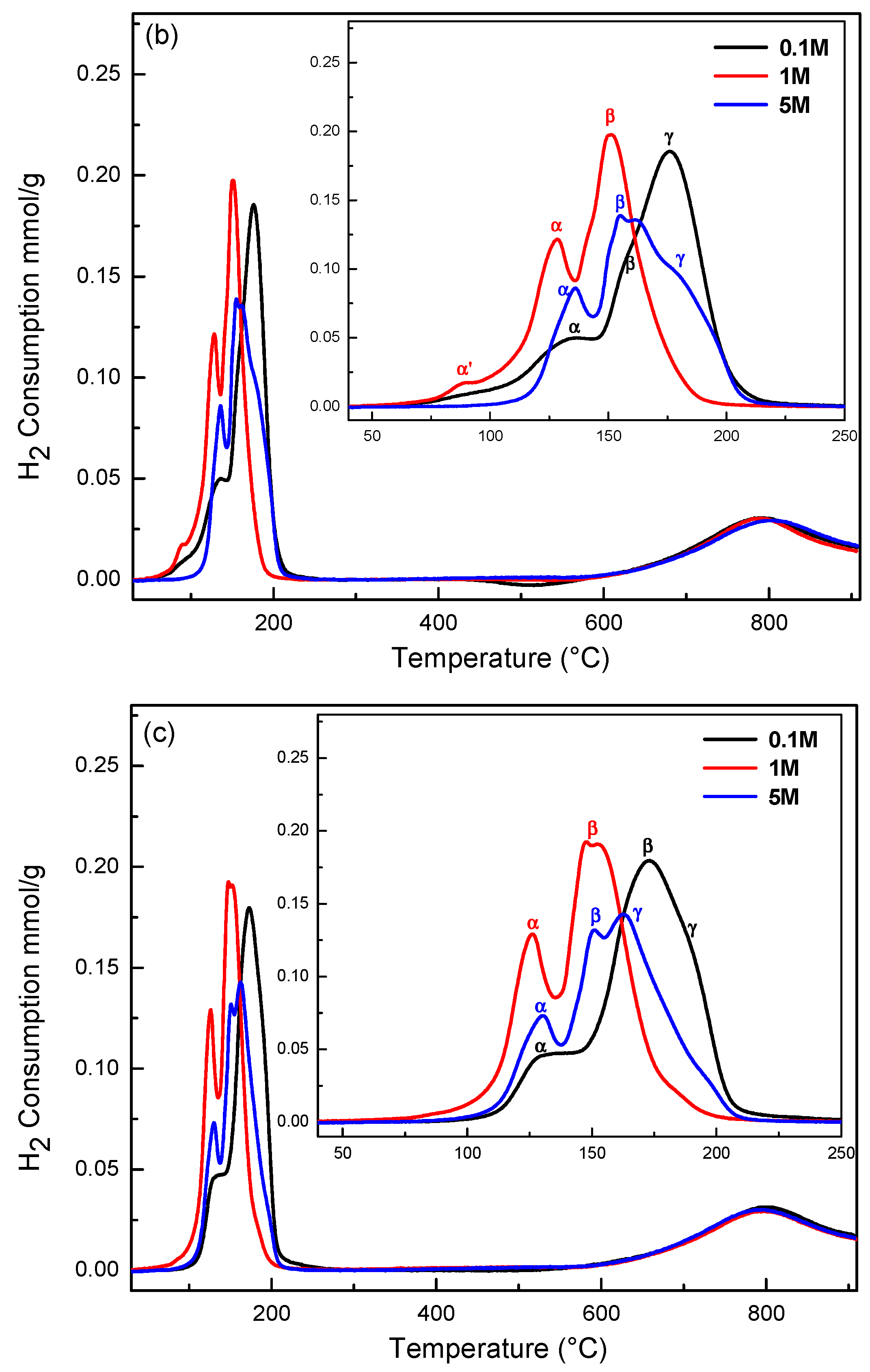
2.4. Redox Properties (H2-TPR)
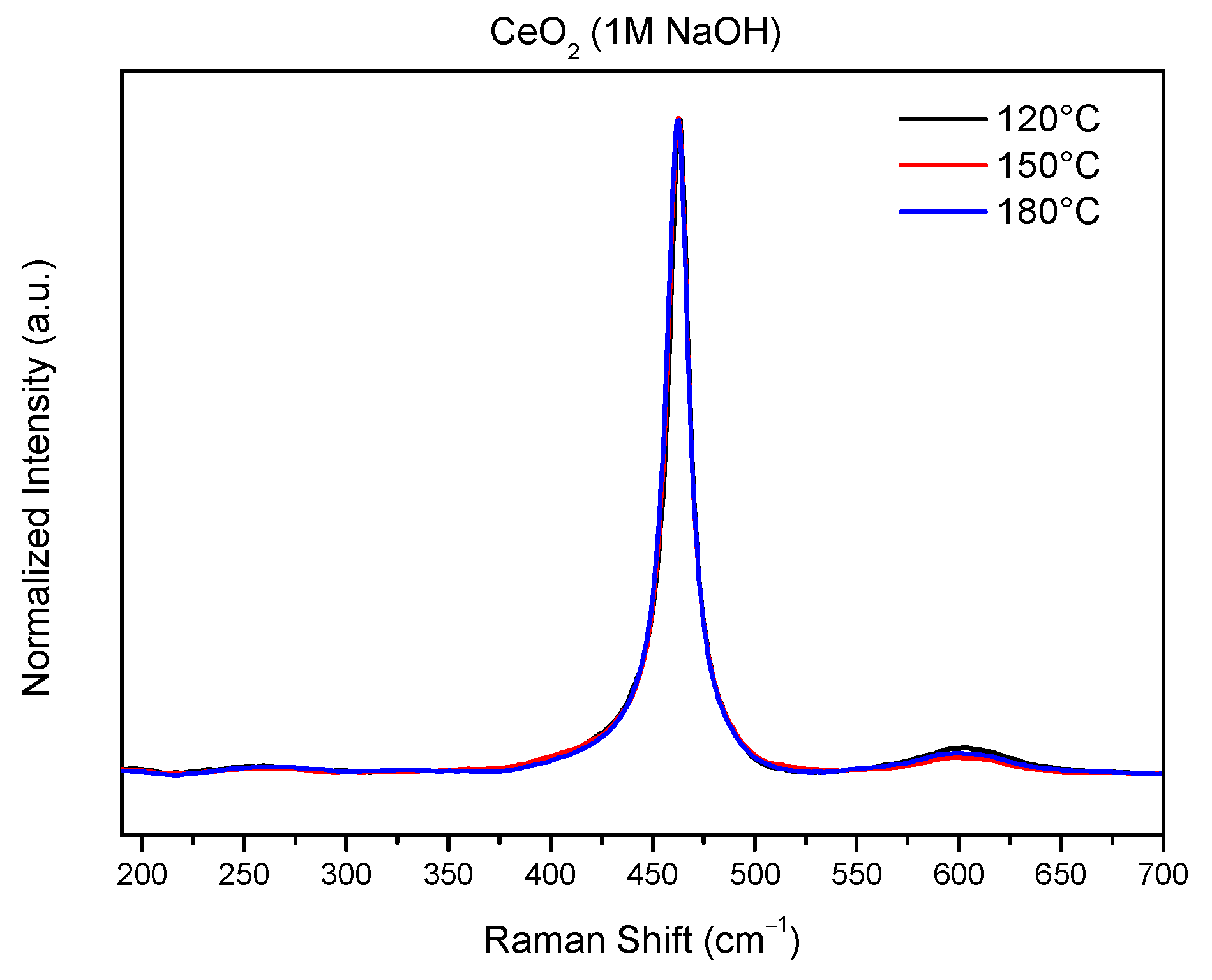
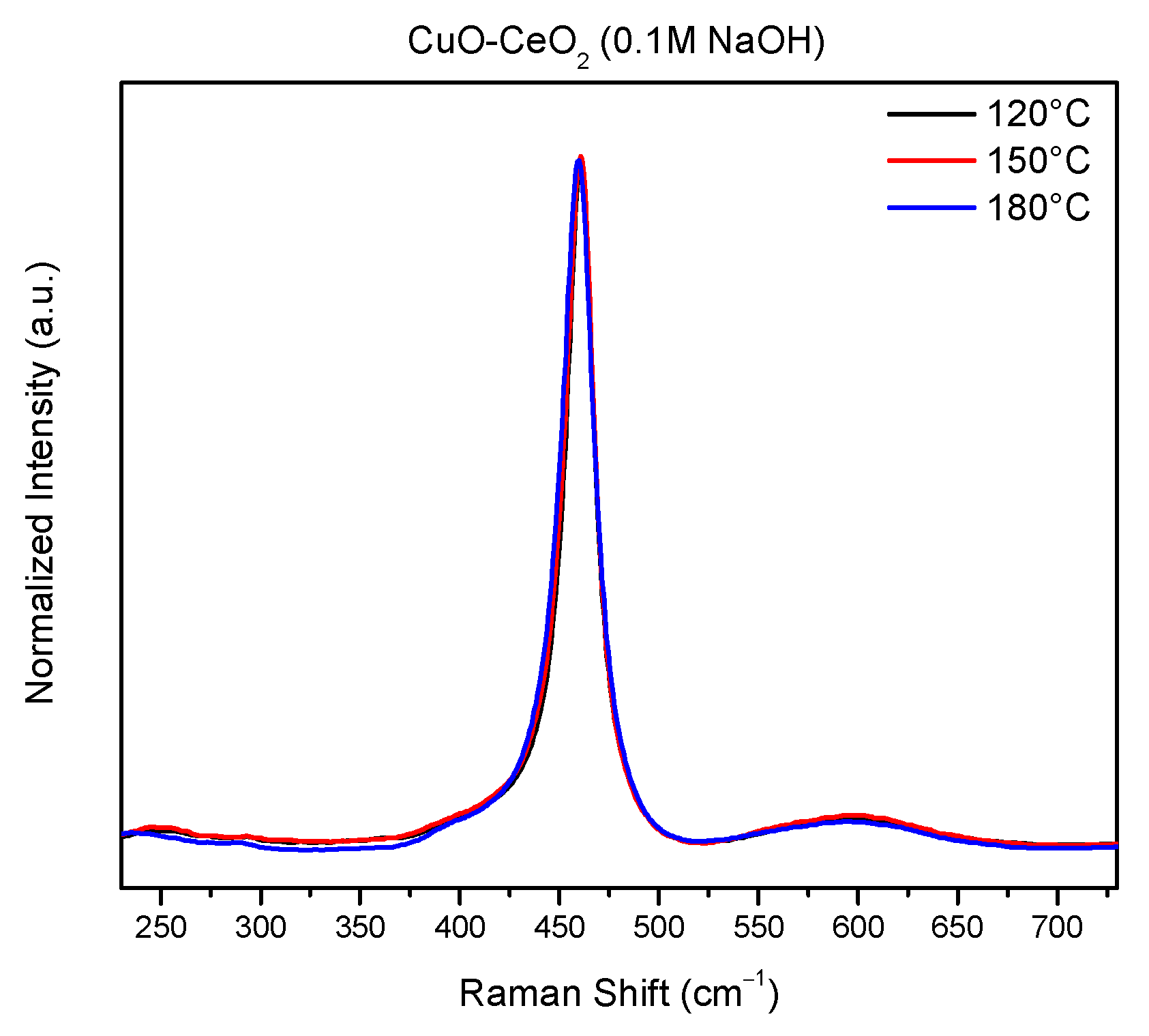
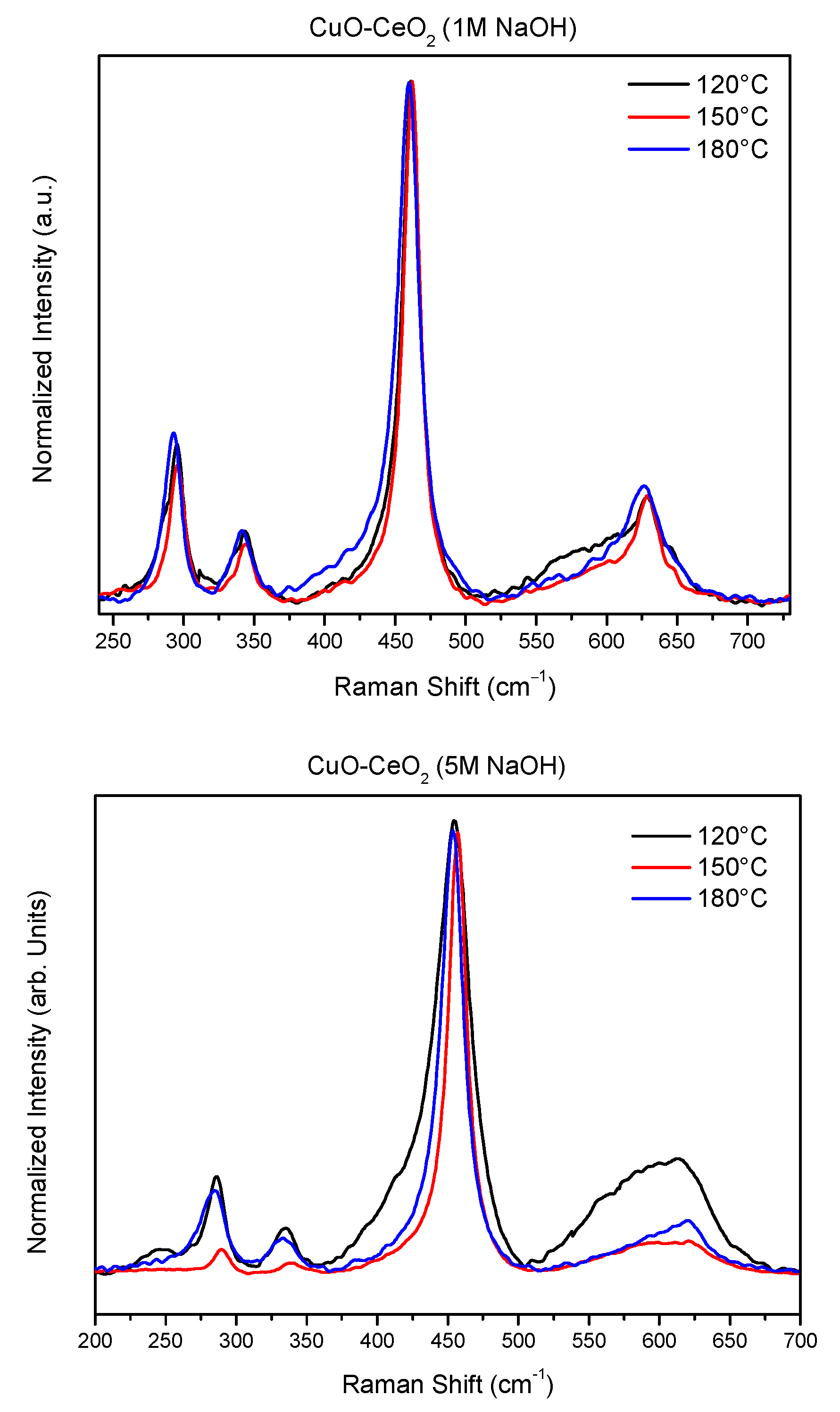
2.5. Raman Analysis
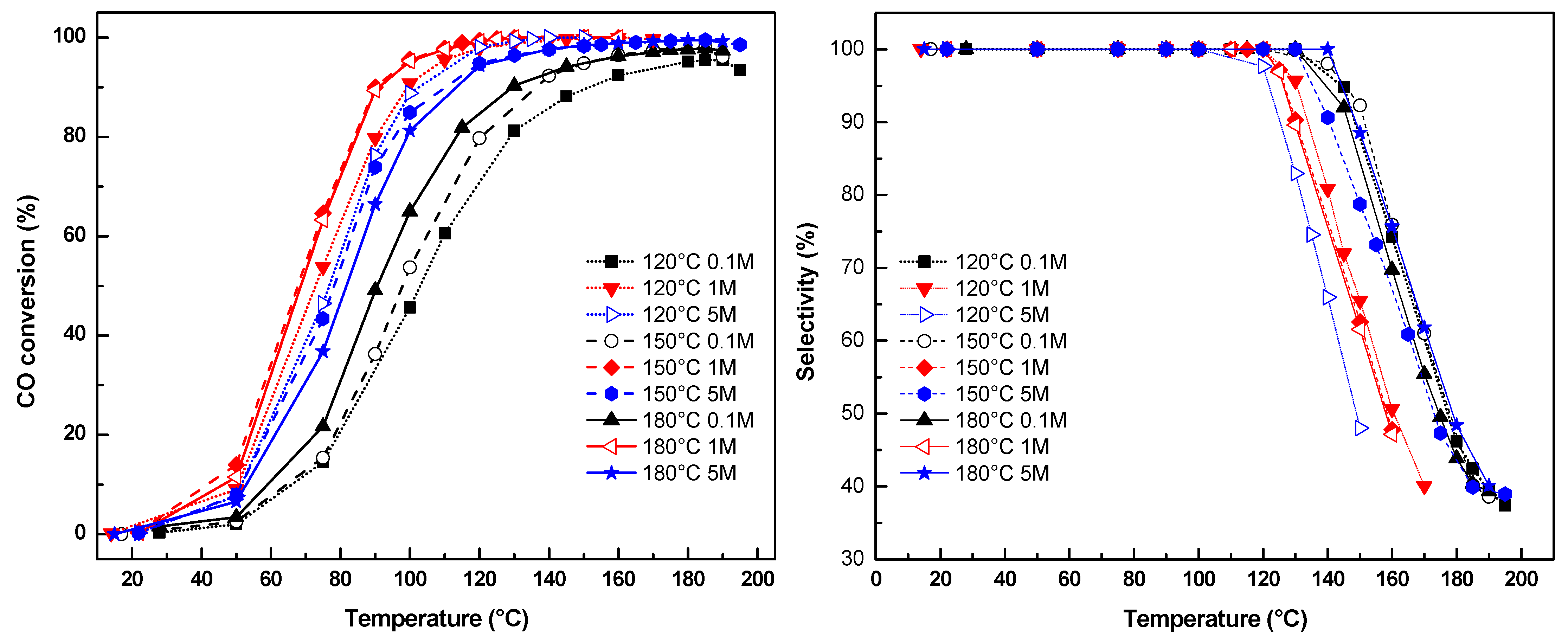
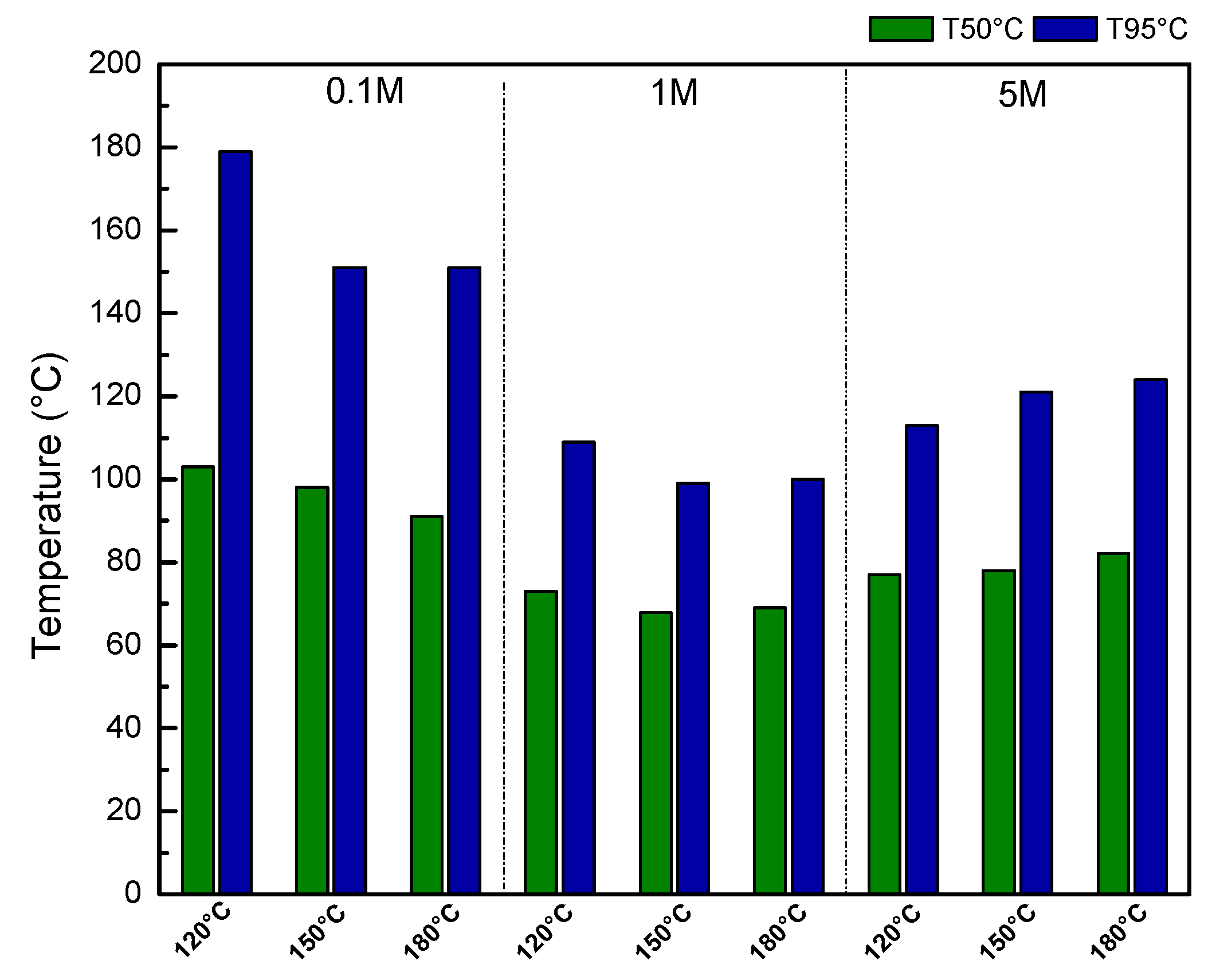
2.6. Preferential Oxidation of CO in Excess H2 (PrOx)
2.7. Correlation of Physicochemical Properties with Catalytic Performance
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opoku, E.E.O.; Dogah, K.E.; Aluko, O.A. The contribution of human development towards environmental sustainability. Energy Econ. 2022, 106, 105782. [Google Scholar] [CrossRef]
- Li, L.; Khodakarami, S.; Yan, X.; Fazle Rabbi, K.; Gunay, A.A.; Stillwell, A.; Miljkovic, N. Enabling Renewable Energy Technologies in Harsh Climates with Ultra-Efficient Electro-Thermal Desnowing, Defrosting, and Deicing. Adv. Funct. Mater. 2022. [Google Scholar] [CrossRef]
- Rogelj, J.; Luderer, G.; Pietzcker, R.C.; Kriegler, E.; Schaeffer, M.; Krey, V.; Riahi, K. Energy system transformations for limiting end-of-century warming to below 1.5 C. Nat. Clim. Change 2015, 6, 519–527. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen fuel cell vehicles; current status and future prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, A.; Fang, B. Electrochemical hydrogen storage: Opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrogen Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Ghenciu, A.F. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. In situ combustion synthesis of structured Cu-Ce-O and Cu-Mn-O catalysts for the production and purification of hydrogen. Appl. Catal. B Environ. 2006, 66, 168–174. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J. Hydrogen generation by steam reforming of methanol over copper-based catalysts for fuel cell applications. Int. J. Hydrogen Energy 2001, 26, 923–933. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F.; Roberge, P.R. Hydrogen generation for fuel-cell power systems by high-pressure catalytic methanol-steam reforming. In Proceedings of the Thirty-Second Intersociety Energy Conversion Engineering Conference, Honolulu, HI, USA, 27 July–1 August 1997. [Google Scholar]
- Joensen, F.; Rostrup-Nielsen, J.R. Conversion of hydrocarbons and alcohols for fuel cells. J. Power Sources 2002, 105, 195–201. [Google Scholar] [CrossRef]
- Laguna, O.H.; Sarria, F.R.; Centeno, M.A.; Odriozola, J.A. Gold supported on metal-doped ceria catalysts (M=Zr, Zn and Fe) for the preferential oxidation of CO (PROX). J. Catal. 2010, 276, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Son, I.H.; Shamsuzzoha, M.; Lane, A.M. Promotion of Pt/γ-Al2O3 by new pretreatment for low-temperature preferential oxidation of CO in H2 for PEM fuel cells. J. Catal. 2002, 210, 460–465. [Google Scholar] [CrossRef]
- Koo, K.Y.; Jung, U.H.; Yoon, W.L. A highly dispersed Pt/γ-Al2O3 catalyst prepared via deposition–precipitation method for preferential CO oxidation. Int. J. Hydrogen Energy 2014, 39, 5696–5703. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Igarashi, H.; Uchida, H.; Suzuki, M.; Sasaki, Y.; Watanabe, M. Removal of carbon monoxide from hydrogen-rich fuels by selective oxidation over platinum catalyst supported on zeolite. Appl. Catal. A Gen. 1997, 159, 159–169. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Manzoli, M.; Boccuzzi, F.; Tabakova, T.; Papavasiliou, J.; Ioannides, T.; Idakiev, V. Catalytic performance and characterization of Au/doped-ceria catalysts for the preferential CO oxidation reaction. J. Catal. 2008, 256, 237–247. [Google Scholar] [CrossRef]
- Manzoli, M.; Avgouropoulos, G.; Tabakova, T.; Papavasiliou, J.; Ioannides, T.; Boccuzzi, F. Preferential CO oxidation in H2-rich gas mixtures over Au/doped ceria catalysts. Catal. Today 2008, 138, 239–243. [Google Scholar] [CrossRef]
- Tabakova, T.; Avgouropoulos, G.; Papavasiliou, J.; Manzoli, M.; Boccuzzi, F.; Tenchev, K.; Ioannides, T. CO-free hydrogen production over Au/CeO2–Fe2O3 catalysts: Part 1. Impact of the support composition on the performance for the preferential CO oxidation reaction. Appl. Catal. B Environ. 2011, 101, 256–265. [Google Scholar] [CrossRef]
- Laguna Espitia, O.H.; Centeno Gallego, M.Á.; Arzamendi, G.; Gandía, L.M.; Romero Sarria, F.; Odriozola Gordón, J.A. (2010). Iron-modified ceria and Au/Ceria catalysts for total and preferential oxidation of CO (TOX and PROX). Catal. Today 2010, 157, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Scirè, S.; Crisafulli, C.; Riccobene, P.M.; Patanè, G.; Pistone, A. Selective oxidation of CO in H2-rich stream over Au/CeO2 and Cu/CeO2 catalysts: An insight on the effect of preparation method and catalyst pretreatment. Appl. Catal. A Gen. 2012, 417, 66–75. [Google Scholar] [CrossRef]
- Fu, Q.; Weber, A.; Flytzani-Stephanopoulos, M. Nanostructured Au–CeO2 catalysts for low-temperature water–gas shift. Catal. Lett. 2001, 77, 87–95. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, X.; Mao, J.; Zhou, R. The catalytic performance of isolated-dispersed Au on nanosized CeO2 for CO preferential oxidation in H2-rich stream. Appl. Surf. Sci. 2019, 481, 1072–1079. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kappis, K.; Papavasiliou, J.; Vakros, J.; Kuśmierz, M.; Gac, W.; Avgouropoulos, G. Copper-promoted ceria catalysts for CO oxidation reaction. Catal. Today 2020, 355, 647–653. [Google Scholar] [CrossRef]
- Kappis, K.; Papadopoulos, C.; Papavasiliou, J.; Vakros, J.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Tuning the catalytic properties of copper-promoted nanoceria via a hydrothermal method. Catalysts 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Xu, W.; Li, X.; Wang, W.; Zhang, L.; Liu, Z. Nature of active sites on Cu–CeO2 catalysts activated by high-temperature thermal aging. ACS Catal. 2020, 10, 12385–12392. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, Z.; Mao, J.; Zhou, R. Shape-controlled CuxCe1-xO2 nanorods catalyst and the active components functioned in selective oxidation of CO in hydrogen-rich stream. J. Power Sources 2020, 451, 227757. [Google Scholar] [CrossRef]
- Cruz, A.R.M.; Assaf, E.M.; Gomes, J.F.; Assaf, J.M. Active copper species of co-precipitated copper-ceria catalysts in the CO-PROX reaction: An in situ XANES and DRIFTS study. Catal. Today 2021, 381, 42–49. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Gamarra, D.; Hungría, A.B.; Fernández-García, M.; Munuera, G.; Hornés, A.; Bera, P.; Conesa, J.C.; Cámara, A.L. Characterization of Active Sites/Entities and Redox/Catalytic Correlations in Copper-Ceria-Based Catalysts for Preferential Oxidation of CO in H2-Rich Streams. Catalysts 2013, 3, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Liu, B.; Kang, B.; Xu, G.; Wang, Q.; Jia, C.; Zhang, J. Boosting Cu-Ce interaction in CuxO/CeO2nanocube catalysts forenhanced catalytic performance of preferential oxidation of CO in H2-rich gases. Mol. Catal. 2017, 436, 90–99. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, L.; Lu, J.; Chen, R.; Lei, Y.; Chen, K.; Han, C.; He, S.; Wan, G.; Luo, Y. A solvent-free method to rapidly synthesize CuO-CeO2 catalysts to enhance their CO preferential oxidation: Effects of Cu loading and calcination temperature. Mol. Catal. 2017, 443, 241–252. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, Y.; Jin, X.; Fang, J.; Li, D.; Li, K.; Lu, J.; Luo, Y. Ceria-nano supported copper oxide catalysts for CO preferential oxidation: Importance of oxygen species and metal-support interaction. Appl. Surf. Sci. 2019, 494, 1166–1176. [Google Scholar] [CrossRef]
- Papavasiliou, J. Interaction of atomically dispersed gold with hydrothermally prepared copper-cerium oxide for preferential CO oxidation reaction. Catal. Today 2020, 357, 684–693. [Google Scholar] [CrossRef]
- Dong, L.; Yao, X.; Chen, Y. Interactions among supported copper-based catalyst components and their effects on performance: A review. Chin. J. Catal. 2013, 34, 851–864. [Google Scholar] [CrossRef]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen Defects and Surface Chemistry of Ceria: Quantum Chemical Studies Compared to Experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Skorodumova, N.V.; Baudin, M.; Hermansson, K. Surface properties of CeO2 from first principles. Phys. Rev. B 2004, 69, 075401. [Google Scholar] [CrossRef]
- Baudin, M.; Wójcik, M.; Hermansson, K. Dynamics, structure and energetics of the (111), (011) and (001) surfaces of ceria. Surf. Sci. 2000, 468, 51–61. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Huebner, W. Size-induced lattice relaxation in CeO2 nanoparticles. Appl. Phys. Lett. 2001, 79, 3512–3514. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea–nitrate combustion method. Appl. Catal. A 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Terribile, D.; Trovarelli, A.; Llorca, J.; de Leitenburg, C.; Dolcetti, G. The Synthesis and Characterization of Mesoporous High-Surface Area Ceria Prepared Using a Hybrid Organic/Inorganic Route. J. Catal. 1998, 178, 299–308. [Google Scholar] [CrossRef]
- Shan, W.; Guo, H.; Liu, C.; Wang, X. Controllable preparation of CeO2 nanostructure materials and their catalytic activity. J. Rare Earths 2012, 30, 665–669. [Google Scholar] [CrossRef]
- Wu, N.-C.; Shi, E.-W.; Zheng, Y.-Q.; Li, W.-J. Effect of pH of Medium on Hydrothermal Synthesis of Nanocrystalline Cerium(IV) Oxide Powders. J. Am. Ceram. Soc. 2002, 85, 2462–2468. [Google Scholar] [CrossRef]
- Lin, M.; Fu, Z.Y.; Tan, H.R.; Tan, J.P.Y.; Ng, S.C.; Teo, E. Hydrothermal Synthesis of CeO2 Nanocrystals: Ostwald Ripening or Oriented Attachment? Cryst. Growth Des. 2012, 12, 3296–3303. [Google Scholar] [CrossRef]
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1827. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L. CTAB assisted hydrothermal synthesis, controlled conversion and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J. Solid State Chem. 2008, 181, 1298–1306. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Chen, L.; Yuan, F.; Zhu, Y. Soot Combustion over Nanostructured Ceria with Different Morphologies. Sci. Rep. 2016, 6, 29062. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Zhang, M.; Ohno, T. Morphology control and characterization of broom-like porous CeO2. Chem. Eng. J. 2015, 260, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Maensiri, S.; Masingboon, C.; Laokul, P.; Jareonboon, W.; Promarak, V.; Anderson, A.P.L.; Seraphin, S. Egg White Synthesis and Photoluminescence of Platelike Clusters of CeO2 Nanoparticles. Cryst. Growth Des. 2007, 7, 950–955. [Google Scholar] [CrossRef]
- Hailstone, R.K.; DiFrancesco, A.G.; Leong, J.G.; Allston, T.D.; Reed, K.J. A Study of Lattice Expansion in CeO2 Nanoparticles by Transmission Electron Microscopy. J. Phys. Chem. C 2009, 113, 15155–15159. [Google Scholar] [CrossRef]
- Zhang, F.; Chan, S.-W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Zhang, D.; Shi, L.; Fang, J. Template-Free Synthesis, Controlled Conversion, and CO Oxidation Properties of CeO2 Nanorods, Nanotubes, Nanowires, and Nanocubes. Eur. J. Inorg. Chem. 2008, 2008, 2429–2436. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Rawski, M.; Vakros, J.; Avgouropoulos, G. Avgouropoulos, A Novel Post-Synthesis Modification of CuO-CeO2 Catalysts: Effect on Their Activity for Selective CO Oxidation. ChemCatChem 2018, 10, 2096–2106. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Skaf, M.; Hany, S.; Cousin, R.; Aouad, S.; Labaki, M.; Abi-Aad, E. A comparative study of Cu, Ag and Au doped CeO2 in the total oxidation of volatile organic compounds (VOCs). Mater. Chem. Phys. 2016, 177, 570–576. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. Identification of the nano/microstructure of CeO2(rod) and the essential role of interfacial copper-ceria interaction in CuCe(rod) for selective oxidation of CO in H2-rich streams. J. Power Sources 2017, 361, 39–53. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.B.; Figueiredo, J.L. Gold supported on metal oxides for carbon monoxide oxidation. Nano Res. 2011, 4, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Si, R.; Raitano, J.; Yi, N.; Zhang, L.; Chan, S.-W.; Flytzani-Stephanopoulos, M. Structure sensitivity of the low-temperature water-gas shift reaction on Cu–CeO2 catalysts. Catal.Today 2012, 180, 68–80. [Google Scholar] [CrossRef]
- Rao, K.N.; Bharali, P.; Thrimurthulu, G.; Reddy, B.M. Supported copper–ceria catalysts for low temperature CO oxidation. Catal. Commun. 2010, 11, 863–866. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Wang, X.; Wang, S.; Wu, S. Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation. Appl. Catal. A Gen. 2005, 295, 142–149. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Matralis, H. Influence of the preparation method on the performance of CuO–CeO2 catalysts for the selective oxidation of CO. Appl. Catal. B 2005, 56, 87–93. [Google Scholar] [CrossRef]
- Du, P.-P.; Wang, W.-W.; Jia, C.-J.; Song, Q.-S.; Huang, Y.-Y.; Si, R. Effect of strongly bound copper species in copper–ceria catalyst for preferential oxidation of carbon monoxide. Appl. Catal. A Gen. 2016, 518, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; Dong, C.; Li, M.; Zhou, Y.; Shen, W. Dispersion of copper oxide species on nanostructured ceria. J. Chem. Phys. 2020, 152, 094708. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinovic, P.; Erjavec, B.; Dražić, G.; Pintar, A. Small CuO clusters on CeO2 nanospheres as active species for catalytic N2O decomposition. Appl. Catal. B Environ. 2015, 163, 113–122. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J. Enhanced CO oxidation activity of CuO/CeO2 catalyst prepared by surfactant-assisted impregnation method. J. Rare Earths 2015, 33, 1268–1274. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, I.H.M.; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Gamarra, D.; Belver, C.; Fernández-García, M.; Martínez-Arias, A. Selective CO Oxidation in Excess H2 over Copper−Ceria Catalysts: Identification of Active Entities/Species. J. Am. Chem. Soc. 2007, 129, 12064–12065. [Google Scholar] [CrossRef]
- Arango-Díaz, A.; Moretti, E.; Talon, A.; Storaro, L.; Lenarda, M.; Núñez, P.; Marrero-Jerez, J.; Jiménez-Jiménez, J.; Jiménez-López, A.; Rodríguez-Castellón, E. Preferential CO oxidation (CO-PROX) catalyzed by CuO supported on nanocrystalline CeO2 prepared by a freeze-drying method. Appl. Catal. A Gen. 2014, 477, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Mock, S.A.; Zell, E.T.; Hossain, S.T.; Wang, R. Effect of Reduction Treatment on CO Oxidation with CeO2 Nanorod-Supported CuOx Catalysts. ChemCatChem 2018, 10, 311–620. [Google Scholar] [CrossRef]
- Moretti, E.; Lenarda, M.; Storaro, L.; Talon, A.; Frattini, R.; Polizzi, S.; Rodríguez-Castellón, E.; Jiménez-López, A. Catalytic purification of hydrogen streams by PROX on Cu supported on an organized mesoporous ceria-modified alumina. Appl. Catal. B Environ. 2007, 72, 149–156. [Google Scholar] [CrossRef]
- Jia, A.-P.; Jiang, S.-Y.; Lu, J.-Q.; Luo, M.-F. Study of Catalytic Activity at the CuO−CeO2 Interface for CO Oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Yao, S.; Mudiyanselage, K.; Xu, W.; Johnston-Peck, A.C.; Hanson, J.C.; Wu, T.; Stacchiola, D.; Rodriguez, J.A.; Zhao, H.; Beyer, K.A.; et al. Unraveling the Dynamic Nature of a CuO/CeO2 Catalyst for CO Oxidation in Operando: A Combined Study of XANES (Fluorescence) and DRIFTS. ACS Catal. 2014, 4, 1650–1661. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Ge, C.; Tang, C.; Dong, L. Comparative study on the catalytic CO oxidation properties of CuO/CeO2 catalysts prepared by solid state and wet impregnation. Chin. J. Catal. 2014, 35, 1347–1358. [Google Scholar] [CrossRef]
- Gamarra, D.; Cámara, A.L.; Monte, M.; Rasmussen, S.B.; Chinchilla, L.E.; Hungría, A.B.; Munuera, G.; Gyorffy, N.; Schay, Z.; Corberán, V.C.; et al. Preferential oxidation of CO in excess H2 over CuO/CeO2 catalysts: Characterization and performance as a function of the exposed face present in the CeO2 support. Appl. Catal. B Environ. 2013, 130–131, 224–238. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Li, L.; Wang, A.; Zhang, W.; Wang, X.; Zhang, T. Activation of an Ir-in-CeO2 catalyst by pulses of CO: The role of oxygen vacancy and carbonates in CO oxidation. Catal. Today 2012, 180, 155–160. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Papadopoulou, C.; Batista, J.; Hocevar, S.; Matralis, H.K. A comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Today 2002, 75, 157–167. [Google Scholar] [CrossRef]
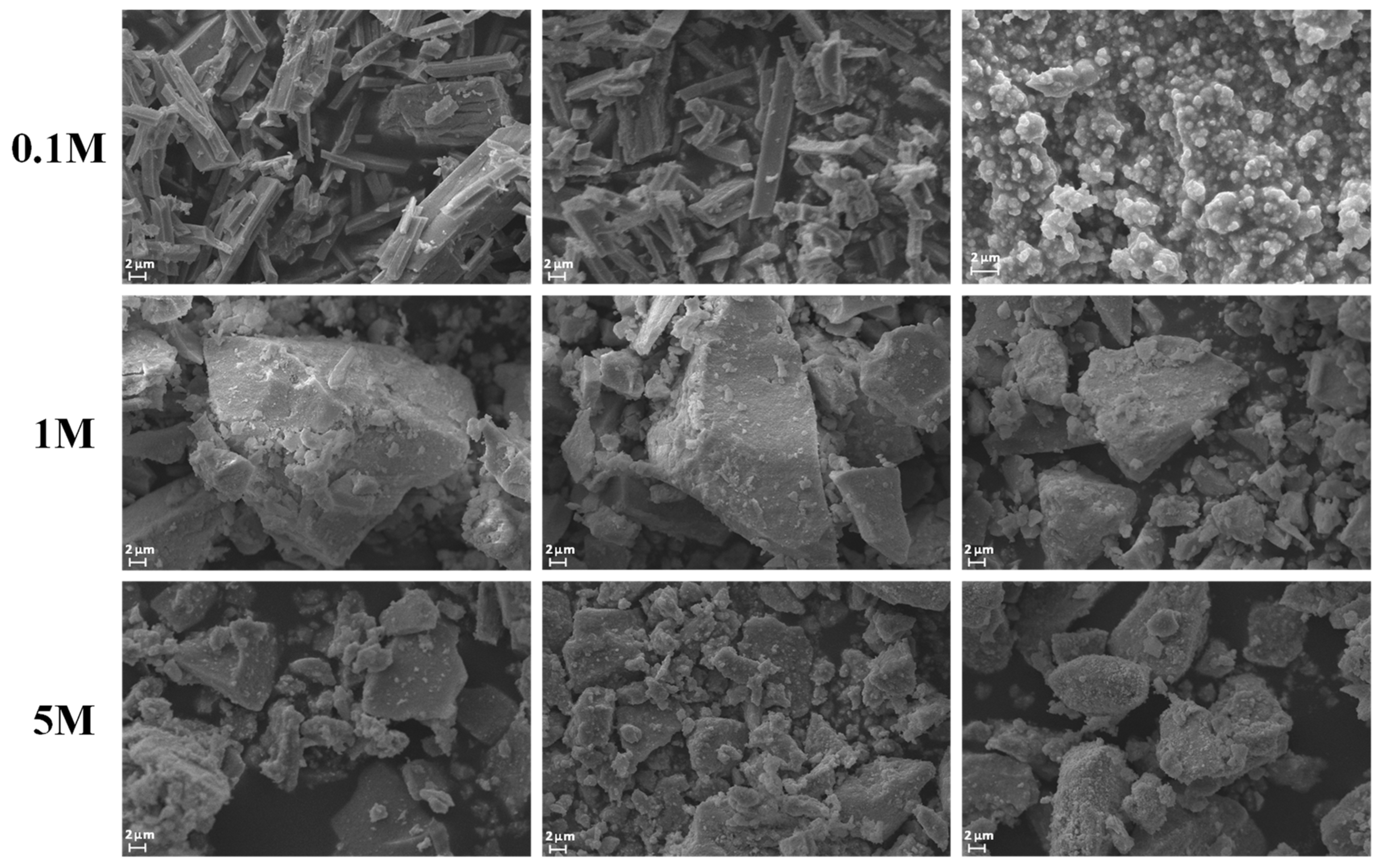
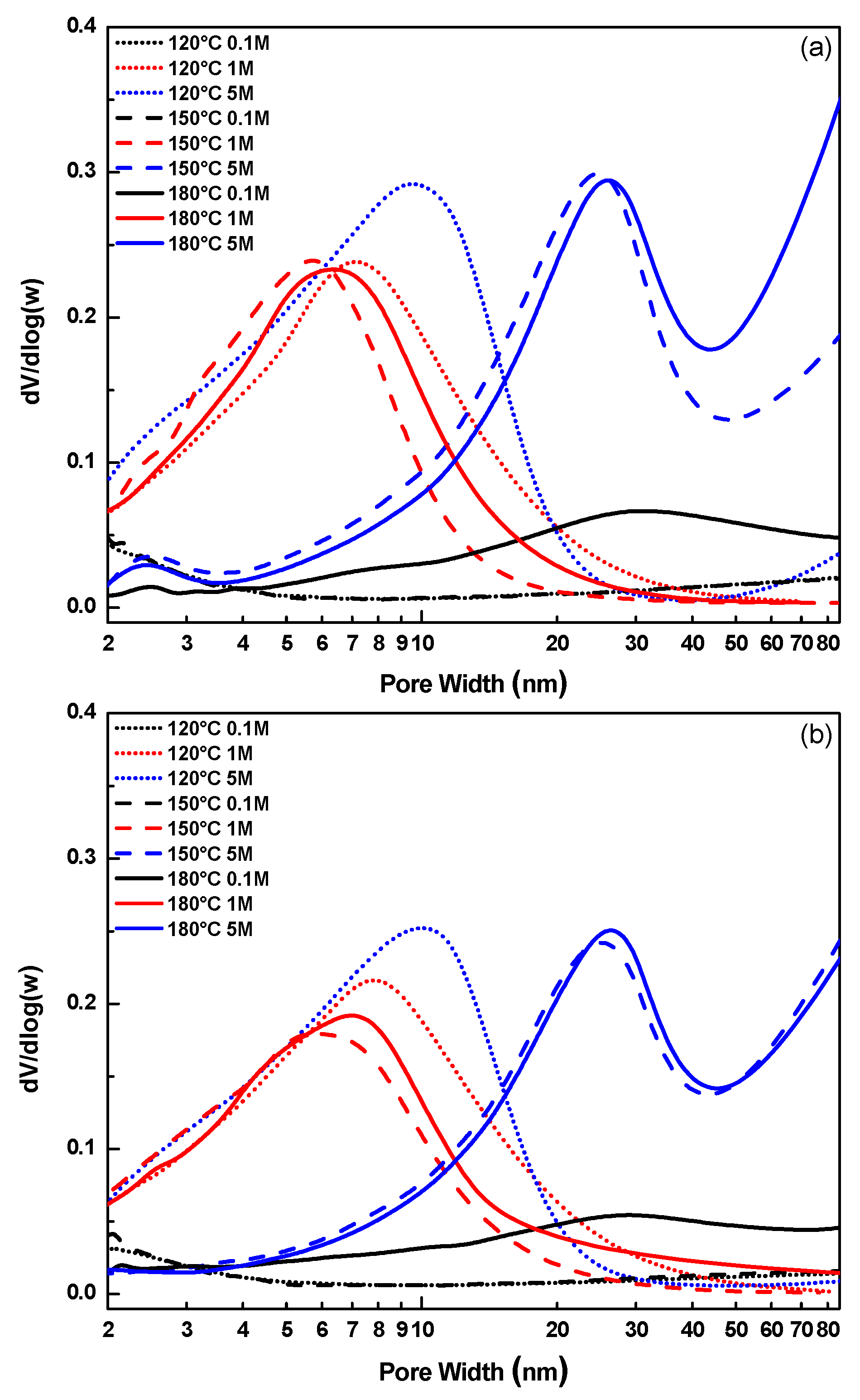
| Sample | CNaOH–T (°C) | SSA (m2 g−1) | MPD (nm) | PV (cm3 g−1) |
|---|---|---|---|---|
| (Cu)Ce-0.1-120 | 0.1M-120 | 29 (20) | 8.6 (8.3) | 0.038 (0.030) |
| (Cu)Ce-0.1-150 | 0.1M-150 | 30 (23) | 8.3 (9.0) | 0.038 (0.035) |
| (Cu)Ce-0.1-180 | 0.1M-180 | 25 (22) | 18.2 (14.6) | 0.087 (0.083) |
| (Cu)Ce-1-120 | 1M-120 | 74 (69) | 4.3 (4.3) | 0.16 (0.15) |
| (Cu)Ce-1-150 | 1M-150 | 75 (70) | 3.8 (3.6) | 0.14 (0.13) |
| (Cu)Ce-1-180 | 1M-180 | 69 (63) | 4.3 (4.0) | 0.15 (0.13) |
| (Cu)Ce-5-120 | 5M-120 | 128 (100) | 6.0 (6.1) | 0.21 (0.18) |
| (Cu)Ce-5-150 | 5M-150 | 42 (38) | 14.2 (16.4) | 0.19 (0.18) |
| (Cu)Ce-5-180 | 5M-180 | 38 (36) | 17.8 (16.6) | 0.22 (0.18) |
| Sample | d111 (nm) 1 | α (nm) 2 |
|---|---|---|
| Ce-0.1-120 | 11.5 | 0.5406 |
| Ce-0.1-150 | 11.1 | 0.5404 |
| Ce-0.1-180 | 28.8 | 0.5408 |
| Ce-1-120 | 14.0 | 0.5412 |
| Ce-1-150 | 12.0 | 0.5410 |
| Ce-1-180 | 12.7 | 0.5410 |
| Ce-5-120 | 8.38 | 0.5410 |
| Ce-5-150 | 21.4 | 0.5407 |
| Ce-5-180 | 21.8 | 0.5407 |
| Sample | Peak α’ (°C) | Peak α (°C) | Peak β (°C) | Peak γ (°C) | H2 Consumption (μmol H2 g−1) |
|---|---|---|---|---|---|
| CuCe-0.1-120 | 150 | 184 | 918.7 | ||
| CuCe-0.1-150 | 134 | 159 | 176 | 880.8 | |
| CuCe-0.1-180 | 130 | 173 | 188 | 842.1 | |
| CuCe-1-120 | 105 | 132 | 157 | 838.2 | |
| CuCe-1-150 | 89 | 128 | 151 | 851.6 | |
| CuCe-1-180 | 126 | 150 | 838.5 | ||
| CuCe-5-120 | 99 | 130 | 160 | 1073.0 | |
| CuCe-5-150 | 136 | 158 | 181 | 737.8 | |
| CuCe-5-180 | 131 | 151 | 163 | 724.7 |
| Samples | F2g Peak Position (cm−1) | ID/IF2g |
|---|---|---|
| CeO2 supports | ||
| Ce-1M-120 | 463.4 | 0.039 |
| Ce-1M-150 | 462.6 | 0.023 |
| Ce-1M-180 | 462.6 | 0.029 |
| CuOCeO2 catalysts | ||
| CuCe-0.1-120 | 460.7 | 0.036 |
| CuCe-0.1-150 | 460.7 | 0.041 |
| CuCe-0.1-180 | 459.6 | 0.034 |
| CuCe-1-120 | 460.9 | 0.107 |
| CuCe-1-150 | 462.1 | 0.070 |
| CuCe-1-180 | 459.6 | 0.080 |
| CuCe-5-120 | 454.5 | 0.243 |
| CuCe-5-150 | 457.0 | 0.067 |
| CuCe-5-180 | 452.6 | 0.087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, C.; Kappis, K.; Papavasiliou, J.; Vakros, J.; Antonelou, A.; Gac, W.; Li, H.; Avgouropoulos, G. Impact of Hydrothermally Prepared Support on the Catalytic Properties of CuCe Oxide for Preferential CO Oxidation Reaction. Catalysts 2022, 12, 674. https://doi.org/10.3390/catal12060674
Papadopoulos C, Kappis K, Papavasiliou J, Vakros J, Antonelou A, Gac W, Li H, Avgouropoulos G. Impact of Hydrothermally Prepared Support on the Catalytic Properties of CuCe Oxide for Preferential CO Oxidation Reaction. Catalysts. 2022; 12(6):674. https://doi.org/10.3390/catal12060674
Chicago/Turabian StylePapadopoulos, Christos, Konstantinos Kappis, Joan Papavasiliou, John Vakros, Aspasia Antonelou, Wojciech Gac, Haibin Li, and George Avgouropoulos. 2022. "Impact of Hydrothermally Prepared Support on the Catalytic Properties of CuCe Oxide for Preferential CO Oxidation Reaction" Catalysts 12, no. 6: 674. https://doi.org/10.3390/catal12060674
APA StylePapadopoulos, C., Kappis, K., Papavasiliou, J., Vakros, J., Antonelou, A., Gac, W., Li, H., & Avgouropoulos, G. (2022). Impact of Hydrothermally Prepared Support on the Catalytic Properties of CuCe Oxide for Preferential CO Oxidation Reaction. Catalysts, 12(6), 674. https://doi.org/10.3390/catal12060674









