Abstract
Waste-activated sludge (WAS) disintegration using peroxydisulfate (PDS) has attracted scientific attention over the past few years. Despite several advantages offered by a sulfate radical-advanced oxidation process, there are still too many downsides of this treatment that limit its facile large-scale application. This study investigated whether modifying nano zero-valent iron (nZVI) with a second metal such as Ag and Cu enhanced the disruption of WAS. The disintegration efficiency was assessed using standard techniques, i.e., soluble chemical oxygen demand, Fourier-transform infrared spectroscopy and a scanning electron microscope. The bimetallics were shown to have an improved disintegration efficiency of > 2.5-fold compared with the untreated sample. Furthermore, nZVI/Ag was found to be more efficient than nZVI/Cu for PDS activation, which was validated by the higher ratio (3 and 2.5 for nZVI/Ag and nZVI/Cu, respectively) between the soluble extracellular polymeric substances and the bound extracellular polymeric substances (S-EPS/B-EPS). Similar conclusions were derived from a SEM analysis. The improved disintegration efficiency could be related to the enhanced electron transfer from nZVI to PDS or the intrinsic properties of silver, which was found to be one of the best activators for PDS under homogeneous conditions. We believe that this study deepens the understanding of PDS heterogeneous activation processes.
1. Introduction
Over the course of several centuries, we have observed an increase in the level of pollution caused by human activity. The population continues to grow, with increased pollution and wastewater production [1]. This problem concerns, among others, high water contamination worldwide. As a result, increasing demand and interest in water purification has led to the development of novel water purification techniques that degrade contaminants more effectively, in less time and consuming less energy.
A wastewater treatment system should operate at maximum efficiency to disinfect and purify the water. Thus, oxidizers such as ozone [2], hydrogen peroxide [3], peracetic acid [4] or potassium permanganate have been extensively studied for the removal of contaminants [5]. Wastewater treatment plants (WWTPs) are usually based on an activated sludge (AS) process to meet the current restrictive requirements [6]. AS is an essential wastewater treatment for converting and neutralizing dangerous contaminants. AS possesses microorganisms used to remove organic matter from wastewater, i.e., in nitrification, denitrification and phosphorus removal processes as well as the oxidation/reduction process for certain compounds [7]. One of the main problems of conducting this process is the increase in biomass. Therefore, the production of waste-activated sludge (WAS) increases as the amount of wastewater discharged intensifies [8]. Furthermore, the microorganisms involved in water purification processes are unable to degrade different types of pollutants [9]. Due to this, there is still a wide variety of harmful chemicals in WAS such as petroleum products, organic compounds, xenobiotics and others [10,11].
Currently, the surplus of WAS is mainly released into the environment via landfill, composting, combustion and methane production [12,13,14]. Therefore, the methods used today for WAS disposal are associated with additional costs. As WAS consists mainly of water, reducing its volume could facilitate the transport and disposal of the sludge. Nowadays, different methods can be used for WAS dehydration such as centrifugation [15], wet milling [16], ultrasound [17], advanced oxidation processes (e.g., the Fenton process [18]), filtering (or belt press), magnetic sedimentation, spinning and compression/filtration [19,20]. Nevertheless, existing disintegration methods remain far from ideal; the main problem is associated with retaining water in the bacteria cells [19,20].
One of the chemical disintegration methods of WAS is a chemical treatment by persulfates. The persulfates—i.e., peroxymonosulfate (PMS) or peroxydisulfate (PDS) anions are highly reactive by themselves because of their chemical structure, which includes a peroxide bond [21,22]. In addition to this reactivity, in combination with several types of activators (such as transition metals and UV radiation), they are able to generate reactive species such as sulfate radicals (SO4•−) and hydroxyl radicals (•OH). The activation is based on the donation of an electron or energy to the peroxide bond with a further SO4•− formation [23]. An example of PDS activation by Fe2+ can be found in Equation (1).
Our research group was one of the first to report WAS disintegration by persulfates [24]. Additionally, it has been found that this treatment can contribute to removing harmful and persistent contaminants in sludge [23,25]. Our group also recently reported that nZVI can be used as a PDS activator in a relatively broad pH spectrum [26] and that modifications can enhance the electron transfer process [27], which (we hypothesized) could improve the formation of sulfate radicals.
To the best of our knowledge, the impact of bimetallic nanoparticles based on nZVI on PDS activation with a subsequent use for WAS disintegration has not been investigated before. Al-Shamsi et al. [28] reported the possibility of enhancing PMS and PDS activation using bimetallic nanoparticles; however, their study did not focus on WAS.
The present work investigates, for the first time, the potential use of nZVI bimetallic nanoparticles for the catalytic activation of PDS in a complex matrix such as sludge. The effect of a second metal (Ag and Cu) in WAS disintegration was also investigated. The efficiency of the process was evaluated by commonly used techniques: soluble chemical oxygen demand (SCOD); extracellular polymeric substance (EPS) determination; Fourier-transform infrared (FTIR) spectroscopy; and scanning electron microscope (SEM) observations. From this, it was found that decorating nZVI with a second metal could enhance the process of PDS catalytic activation. The authors believe this work could help scientists to optimize the heterogeneous activation process of persulfates.
2. Results and Discussion
The most common analytical method used to estimate WAS disintegration efficiency is soluble chemical oxygen demand (SCOD) [29]. The concentration of organic matter is determined by assessing the oxygen consumption for its oxidation [30]. During WAS disintegration by chemical pretreatments, flocs can be destroyed and microorganisms disrupted. As a result, intracellular organic material can be released from the solid particles into the liquid phase, raising the SCOD value. As Figure 1A shows, after 30 min of reaction, the SCOD value was significantly affected by the process. The SCOD value increased from 95 mg/L (R1, untreated sample) to 189, 206, 225, 242 and 241 mg/L for R2, R3, R4, R5 and R6, respectively. The addition of nZVI or bimetallic nanoparticles to PDS increased the SCOD value in all cases. From the reported data, PDS activated by nZVI or bimetallic nanoparticles could improve the breaking down of the cell walls of the bacteria and WAS flocs by the enhanced generation of radicals.
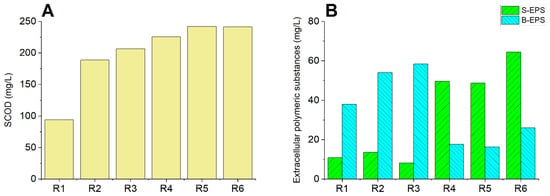
Figure 1.
SCOD and extracellular polymeric substances content: (A) SCOD after 30 min of disintegration by various treatments. (B) Protein fraction of the S-EPS and B-EPS after 30 min of disintegration. R1: blank; R2: nZVI (0.2 g/L); R3: PDS (8.4 mM); R4: PDS (8.4 mM) + nZVI (0.2 g/L); R5: PDS (8.4 mM) + nZVI/Ag (0.2 g/L); R6: PDS (8.4 mM) + nZVI/Cu (0.2 g/L).
The protein concentration changes in the two fractions of EPS are shown in Figure 1B. The samples R1, R2 and R3 had a small fraction of soluble EPS (S-EPS), but a large fraction of the bound ones. The marked increase in the protein concentration of S-EPS in reactors R4, R5 and R6 suggested the partial or complete lysis of the bacterial cells and subsequent release into the liquid phase of the organic matter from inside the cells [31]. Thus, the decrease in the bound EPS (B-EPS) to 17.6 mg/L, 16.3 mg/L (the highest observed in this study) and 26 mg/L for R4, R5 and R6, respectively, confirmed the EPS disintegration. This phenomenon was not observed for R2 and R3. Furthermore, it was noted that in all the PDS-activated systems, the ratio between S-EPS and B-EPS significantly increased. This was an additional confirmation that the PDS was easily activated by the nZVI or bimetallic nanoparticles to form sulfate radicals. Analogous results were obtained by Guo et al. [32], where Fe(II)-activated PDS caused the conversion of B-EPS to S-EPS.
Decorating the nZVI surface with a second metal—in this case, silver—caused a decrease in the B-EPS and S-EPS amount (compared with the PDS/nZVI system). Nonetheless, the ratio between S-EPS/B-EPS was the highest of all the samples in reactor R5 and was equal to 3 (for R4, it was 2.8; for R6, it was 2.5). In the presence of nZVI/Ag, PDS generated the radicals with greater efficiency; this can help to break down cells (releasing organic matter from them), but eventually it also oxidizes organic substances into CO2 + H2O.
Based on the SCOD/EPS results, we decided to analyze samples R1, R4, R5 and R6 by FTIR spectroscopy (Figure 2). This showed the presence of five functional groups (primarily) representing hydrocarbons, proteins, polysaccharides, aromatic amino acids and nucleic acids [33]. The band region from 3650 to 3200 cm−1 showed the O–H groups whereas the peaks at ~2925 and ~2856 cm−1 could be attributed to the C–H, C–H2 and C–H3 groups of organic compounds. The peaks at ~1634, ~1528 and ~1402 cm−1 were attributed to amide I (C–N and C=O stretching), amide II (C–N and N–H stretching) and amide II (C=O symmetric stretching) of the protein fraction, respectively. The peak at ~1026 cm−1 was due to the presence of polysaccharides (C–O–C and C–OH). The peaks at ~906 cm−1 were related to nucleic acids (O–P–O stretching). Of note was the presence of two additional peaks at wavenumbers ~777 and ~696 cm−1. These peaks were ascribed to the presence of aromatic amino acids (C–C and C–OH ring vibrations), as reported by Ruan et al. [34]. As shown in Figure 2, the position of most of the peaks was unchanged or was slightly shifted, which was in agreement with the literature [35,36]. On the other hand, the intensities weakened for most of the peaks, implying that the SO4•− and •OH radicals generated by the nZVI/PDS and nZVI-bimetallic/PDS processes could oxidize the organic substances to CO2 + H2O. The exception was the peaks ascribed to amino acids (777 and 696 cm−1) in which the intensity increased, indicating a release of substances from the disintegrated cells. This agreed with the EPS analysis, suggesting the release of soluble proteins. The largest increase, at ~777 cm−1, was again reported for R5 (Figure 2, insert). The FTIR spectroscopy analysis confirmed the results of the SCOD/protein determination, which indicated an increase in proteins in this reactor; thus, this was the most efficient disintegration.
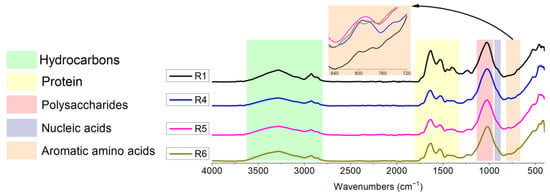
Figure 2.
FTIR spectroscopy analysis of reactors after 30 min of disintegration. Conditions: R1: blank; R4: PDS (8.4 mM) + nZVI (0.2 g/L); R5: PDS (8.4 mM) + nZVI/Ag (0.2 g/L); R6: PDS (8.4 mM) + nZVI/Cu (0.2 g/L).
Figure 3A shows the images of the samples. As the figure shows, it was possible to observe a decrease in the turbidity of the supernatant in R5 and R6 along with an increase in the sedimentation rate. In R5, the supernatant appeared to be clearer compared with the other reactors.
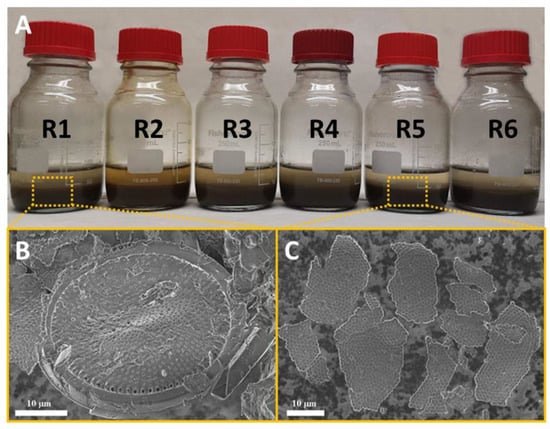
Figure 3.
Reactors after disintegration and SEM analysis: (A) Reactors after disintegration. From left to right: R1: blank; R2: nZVI (0.2 g/L); R3: PDS (8.4 mM); R4: PDS (8.4 mM) + nZVI (0.2 g/L); R5: PDS (8.4 mM) + nZVI/Ag (0.2 g/L); R6: PDS (8.4 mM) + nZVI/Cu (0.2 g/L). (B) SEM observations of WAS in the blank reactor (R1). (C) SEM observations of WAS treated with nZVI/Ag (0.2 g/L) + PDS (8.4 mM) (R5).
A SEM analysis was conducted on the R1 and R5 samples (Figure 3B,C). R1 mostly showed an unaltered structure of the flakes and bacteria (Figure 3B). In this reactor, the shapes were regular, relatively round and smooth, but those treated with metal-activated PDS (Figure 3C) were much less regular and formed a structure with numerous pores and smaller particle sizes. This might have been connected to the improved sedimentation properties of WAS after an efficient disintegration, which our group has repeatedly reported before [37,38]. Changes in the WAS particle size suggested an increase in its liquid value, which aided in improved WAS sedimentation and, at the same time, better dehydration. Wacławek et al. [24] and Sponza [39] arrived at the same conclusion, finding that large flocs (particles) had high sludge volume index values, suggestive of poor settling characteristics.
Based on all the gathered results, the bimetallic nanoparticles were more prone to activating PDS than their monometallic counterparts. The second metal could enhance the electron transfer process needed for breaking the O–O bonds in persulfates [28]. This could be translated into a more efficient generation of sulfate radicals, which could interact faster (compared with other radical species [23]) with the sludge flocs and thus improve the disintegration efficiency. Furthermore, the soluble substances of the sludge were released into the liquid phase, which could be oxidized and mineralized further.
3. Materials and Methods
3.1. Solution and Reagents
The WAS was taken from a Liberec municipality wastewater treatment plant (WWTP) in the Czech Republic. The treatment plant was designed for nutrient removal. The concentration of suspended solids (SS) was, on average, 9.3 g/L and the amount of treated wastewater was about 54,000 m3/d. The solid retention time (SRT) was approximately 25 days and the hydraulic retention time (HRT) was approximately 15.5 h.
All chemicals used in the experiments were of an analytical reagent grade. Iron(III) chloride hexahydrate (≥98%), copper(II) chloride (97%), silver nitrate (≥99%) and sodium borohydride (≥98%) were purchased from Sigma-Aldrich, Czech Republic. Sodium peroxydisulfate–sodium persulfate (99%) was purchased from Lach-Ner, Czech Republic.
In the experiments, deionized water (18.2 MΩ·cm−1, ELGA, Veolia Water, Marlow, UK) was used to prepare all of the reagents.
3.2. Nanoparticle Synthesis and Assessment
Both nZVI and bimetallic nZVI were synthesized following our previous work [27]. Briefly, 0.2 M of Iron(III) was reduced to Fe0 by sodium borohydride (0.5 M) dosed to the reactor at a rate of 1.5 mL/min under a nitrogen atmosphere. After adding the last drop of sodium borohydride, the reaction was carried out for 10 min to ensure the complete reduction of the iron precursor. The manufactured nanoparticles were rinsed three times with ethanol before freeze-drying (for future use). Regarding the bimetallic synthesis, nZVI was activated with 1 M hydrochloric acid for 20 s then 1% (wt% of Me/wt% of nZVI) Ag or Cu metal precursors were added sequentially and mixed for 10 min. The bimetallic nanoparticles were rinsed three times with water.
A full characterization of the monometallic and bimetallic nanoparticles used in this research is available in our previous work [27]. The content of Ag and Cu (in nZVI) was found to be in the range of ~0.5–0.2%.
A leaching test was performed to assess the stability and potential release of the metals during the process. The tests were performed for 24 h at room temperature (25 ± 1 °C) and the concentration of monometallic and bimetallic nanoparticles was 1 g/L. Under these conditions, nZVI released 26.6 mg/L of total iron whereas nZVI/Ag and nZVI/Cu released 25.6 and 15.3 mg/L of total iron, respectively. Regarding the leaching of the second metal, nZVI/Ag released 0.06 mg/L of silver whereas in the case of nZVI/Cu, the Cu concentration in the solution was lower than the detection limit; i.e., < 0.05 mg/L.
3.3. Method of Sludge Disintegration
The WAS disintegration was performed by a metallic/bimetallic chemical disintegration. For the chemical disintegration, PDS was used at a concentration of 8.4 mM in accordance with our previous report [40]; in nZVI and bimetallic nZVI (nZVI/Cu, nZVI/Ag), the concentration was 0.2 g/L [41]. The experiments were performed in six glass 250 mL reactors and continuously mixed: R1: control (no PDS, no nZVI); R2: nZVI (0.2 g/L); R3: PDS (8.4 mM); R4: PDS + nZVI (PDS: 8.4 mM, nZVI: 0.2 g/L); R5: PDS + nZVI/Ag (PDS: 8.4 mM, nZVI/Ag: 0.2 g/L); and R6: PDS + nZVI/Cu (PDS: 8.4 mM, nZVI/Cu: 0.2 g/L). The duration of the experiment was 30 min. All tests were performed in duplicate at room temperature. The average relative standard error for the SCOD and EPS analyses was <5% and <15%, respectively.
3.4. Characterization and Analysis
The SCOD was determined following the standard methods for examining water and wastewater, procedure 5220D [38].
The EPS extraction was performed according to the method developed by Wang et al. [42]. The experimental samples were centrifuged (8000 rpm for 10 min) and the supernatant was collected as S-EPS. A NaCl solution (0.85 wt%, warmed at 70 °C) was used to suspend the bottom sediment. After that, the suspension was stirred for 1 min using a vortex mixer and then centrifuged (8000 rpm for 10 min). The resulting supernatant was kept as B-EPS. A PTFE membrane syringe filter (0.22 μm) was used to filter the S-EPS and B-EPS for further analyses. The determination of protein (as one of the main EPS components) was carried out using the Lowry method [43,44].
The FTIR spectra (resolution of 4 cm−1 at a range of 4000–700 cm−1) were acquired with a germanium ATR crystal (NICOLET IZ10, Thermo Scientific, Waltham, MA, USA) equipped with a single reflection angle 45° horizontal ATR accessory.
A Tescan Vega XMU SEM (TESCAN s.r.o., Brno, Czech Republic) was used to observe the disintegration of the WAS particles.
The dissolved iron, silver and copper concentrations were analyzed by ICP-MS (Elan 6000, Perkin Elmer, Akron, OH, USA).
4. Conclusions
We investigated for the first time the influence of a second metal (Ag and Cu) on an nZVI surface for the catalytic activation of PDS in a complex matrix such as WAS. The bimetallic nanoparticles exhibited superior behavior for the disintegration of WAS by increasing the SCOD from 95 mg/L (untreated WAS) to 242 mg/L (WAS treated by PDS + nZVI/Ag). The quality of the treatment was also confirmed by FTIR spectroscopy, EPS determination and a SEM, which showed an improved decomposition of the WAS.
Such an enhanced catalytic efficiency of bimetallic nanoparticles, especially for nZVI/Ag, might be due to an improved electron transfer from the nZVI core to the second metal by forming a galvanic couple with nZVI, as reported previously by He et al. [45]; silver reacted with the PDS in a different way by which sulfate radicals were produced. The better efficiency of Ag (compared with other transition metals) as an activator for persulfates in homogeneous systems has already been reported by Edwards et al. [46] and Anipsitakis and Dionysiou [47]. We concluded that the intrinsic properties of Ag could also be beneficial in heterogeneous systems (such as presented in this communication), causing an increased formation of free radicals.
To sum up, the experimental results presented an improvement in the disintegration of WAS by bimetallic nZVI (with a slight increase by Ag over Cu). We believe that this can help provide a better comprehensiveness of the heterogeneous activation of PDS.
Author Contributions
S.W. conceptualized the studies; B.S. carried out the data collection; B.S., D.S., K.G. and S.W. analyzed the data; B.S., D.S., K.G. and S.W. wrote the paper; D.S., V.V.T.P., M.D., F.G., M.Č. and S.W. edited the paper; V.V.T.P., M.D., F.G., M.Č. and S.W. corrected the English. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Student Grant Scheme at the Technical University of Liberec through project number SGS-2022-3027. The authors would also like to acknowledge the help of the Ministry of Education, Youth and Sports in the Czech Republic under the Research Infrastructures NanoEnviCz (Project No. LM2018124) and “Inter Excellence—Action Programme” within the framework of the project “Exploring the role of ferrates and modified nano zero-valent iron in the activation process of persulfates “(reg. number: LTAUSA18078). This work was also supported by the Ministry of Education, Youth and Sports of the Czech Republic and the European Union European Structural and Investment Funds in the frames of the Operational Programme Research, Development and Education, project Hybrid Materials for Hierarchical Structures (HyHi, Reg. no. CZ.02.1.01/0.0/0.0/16_019/0000843). Stanisław Wacławek is also grateful for the support provided by Grant Agency of the Czech Republic (GA ČR) GJ20-17028Y, Nano Zero-Valent Iron and Cyclodextrins—Their Synergistic Action for Water Purification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cleland, J. World Population Growth; Past, Present and Future. Env. Resour. Econ 2013, 55, 543–554. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, M.; Xu, L.; Wang, S.; Yang, T.; Wu, M.; Lu, W.; Li, Y.; Yu, D. Unraveling Timescale-Dependent Fe-MOFs Crystal Evolution for Catalytic Ozonation Reactivity Modulation. J. Hazard. Mater. 2022, 431, 128575. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, L.; Fu, K.; Zhu, F.; Yang, T.; Yang, T.; Luo, J.; Wu, M.; Yu, D. Ultrastable MOF-Based Foams for Versatile Applications. Nano Res. 2021, 15, 2961–2970. [Google Scholar] [CrossRef]
- Ao, X.W.; Eloranta, J.; Huang, C.H.; Santoro, D.; Sun, W.J.; Lu, Z.D.; Li, C. Peracetic Acid-Based Advanced Oxidation Processes for Decontamination and Disinfection of Water: A Review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- He, Q.; Wang, H.; Yang, K.; Zou, Z.; Zhong, L. The Use of Potassium Permanganate, Ozone and Associated Coupled Processes for Odor Removal in Drinking Water: Bench and Pilot Scale Tests. J. Water Supply Res. Technol.-AQUA 2017, 66, 249–256. [Google Scholar] [CrossRef]
- Wacławek, S.; Černík, M.; Dionysiou, D.D. The Development and Challenges of Oxidative Abatement for Contaminants of Emerging Concern. In A New Paradigm for Environmental Chemistry and Toxicology; Springer: Singapore, 2020; pp. 131–152. [Google Scholar]
- Ikumi, D.S.; Harding, T.H. Kinetics of Biological and Chemical Processes in Anoxic-Aerobic Digestion of Phosphorus Rich Waste Activated Sludge. Water Res. 2020, 170, 115333. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on Efficiency of Various Techniques in Treatment of Waste and Sewage Water—A Comprehensive Review. Resour. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef] [Green Version]
- To, V.H.P.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. A Review on Sludge Dewatering Indices. Water Sci. Technol. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Filamentous Organisms Observed in Activated Sludge. Water Res. 1975, 9, 365–388. [Google Scholar] [CrossRef]
- Shchegolkova, N.M.; Krasnov, G.S.; Belova, A.A.; Dmitriev, A.A.; Kharitonov, S.L.; Klimina, K.M.; Melnikova, N.V.; Kudryavtseva, A.V. Microbial Community Structure of Activated Sludge in Treatment Plants with Different Wastewater Compositions. Front. Microbiol. 2016, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Jih-Gaw, L.; Chang, C.N.; Chang, S.C. Enhancement of Anaerobic Digestion of Waste Activated Sludge by Alkaline Solubilization. Bioresour. Technol. 1997, 62, 85–90. [Google Scholar] [CrossRef]
- Grubel, K.; Machnicka, A.; Waclawek, S. Impact of Alkalization of Surplus Activated Sludge on Biogas Production. Ecol. Chem. Eng. S 2013, 20, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Grubel, K.; Machnicka, A.; Nowicka, E.; Wacławek, S. Mesophilic-Thermophilic Fermentation Process of Waste Activated Sludge after Hybrid Disintegration. Ecol. Chem. Eng. S 2014, 21, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, H.; Gu, G. Effect of Acid and Surfactant Treatment on Activated Sludge Dewatering and Settling. Water Res. 2001, 35, 2615–2620. [Google Scholar] [CrossRef]
- Baier, U.; Schmidheiny, P. Enhanced Anaerobic Degradation of Mechanically Disintegrated Sludge. Water Sci. Technol. 1997, 36, 137–143. [Google Scholar] [CrossRef]
- Park, N.D.; Helle, S.S.; Thring, R.W. Combined Alkaline and Ultrasound Pre-Treatment of Thickened Pulp Mill Waste Activated Sludge for Improved Anaerobic Digestion. Biomass Bioenergy 2012, 46, 750–756. [Google Scholar] [CrossRef]
- Huang, H.; Guo, G.; Tang, S.; Li, B.; Li, J.; Zhao, N. Persulfate Oxidation for Alternative Sludge Treatment and Nutrient Recovery: An Assessment of Technical and Economic Feasibility. J. Environ. Manag. 2020, 272, 111007. [Google Scholar] [CrossRef]
- Novak, J.T. Dewatering of Sewage Sludge. Dry. Technol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, M.S.; Lee, C. Oxidative Treatment of Waste Activated Sludge by Different Activated Persulfate Systems for Enhancing Sludge Dewaterability. Sustain. Environ. Res. 2016, 26, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, S.A.; Aarons, J.; Hamzah, H.H. Review—Electroreduction of Peroxodisulfate: A Review of a Complicated Reaction. J. Electrochem. Soc. 2018, 165, H785–H798. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbk, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of Persulfates in Water and Wastewater Treatment: A Review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Černík, M. The Impact of Peroxydisulphate and Peroxymonosulphate on Disintegration and Settleability of Activated Sludge. Environ. Technol. 2016, 37, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Grübel, K.; Dennis, P.; Vinod, V.T.P.; Černík, M. A Novel Approach for Simultaneous Improvement of Dewaterability, Post-Digestion Liquor Properties and Toluene Removal from Anaerobically Digested Sludge. Chem. Eng. J. 2016, 291, 192–198. [Google Scholar] [CrossRef]
- Silvestri, D.; Krawczyk, K.; Pawlyta, M.; Krzywiecki, M.; Padil, V.V.T.; Torres-Mendieta, R.; Ghanbari, F.; Dinc, O.; Černík, M.; Dionysiou, D.D.; et al. Influence of Catalyst Zeta Potential on the Activation of Persulfate. Chem. Commun. 2021, 57, 7814–7817. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Silvestri, D.; Wacławek, S.; Ramakrishnan, R.K.; Krawczyk, K.; Saravanan, P.; Pawlyta, M.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. A Comparative Study of the Degradation Efficiency of Chlorinated Organic Compounds by Bimetallic Zero-Valent Iron Nanoparticles. Environ. Sci. Water Res. Technol. 2022, 8, 162–172. [Google Scholar] [CrossRef]
- Al-Shamsi, M.A.; Thomson, N.R.; Forsey, S.P. Iron Based Bimetallic Nanoparticles to Activate Peroxygens. Chem. Eng. J. 2013, 232, 555–563. [Google Scholar] [CrossRef]
- Liu, C.; Wu, B.; Chen, X. Sulfate Radical-Based Oxidation for Sludge Treatment: A Review. Chem. Eng. J. 2018, 335, 865–875. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of Microbial Extracellular Polymeric Substances (EPSs)-Part I: Structural and Ecological Aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.; Wang, D.; Li, X.; Zhong, Y.; Li, X.; Deng, Y.; Wang, L.; Yi, K.; Zeng, G. Enhanced Dewaterability of Waste Activated Sludge by Fe(II)-Activated Peroxymonosulfate Oxidation. Bioresour. Technol. 2016, 206, 134–140. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, L.; Huang, Y.; Huang, X. Insight into the Comparison of Thermally and Fe(II) Activated Persulfate on Sludge Dewaterability and Disintegration. Water Sci. Technol. 2021, 84, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, L.; Zhu, Y.; Yu, Y.; Wang, D.; Yang, G.; Yuan, X.; Liu, X.; Li, H.; Zhang, J. How Does Zero Valent Iron Activating Peroxydisulfate Improve the Dewatering of Anaerobically Digested Sludge? Water Res. 2019, 163, 114912. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Deng, J.; Cai, A.; Chen, S.; Cheng, Y.; Li, J.; Li, Q.; Li, X. Improving Dewaterability of Waste Activated Sludge by Thermally-Activated Persulfate Oxidation at Mild Temperature. J. Environ. Manag. 2021, 281, 111899. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Role of Extracellular Polymeric Substances in Bioflocculation of Activated Sludge Microorganisms under Glucose-Controlled Conditions. Water Res. 2010, 44, 4505–4516. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Zhang, W.; Yang, P.; Du, Y.; Wang, D. Highly Effective Enhancement of Waste Activated Sludge Dewaterability by Altering Proteins Properties Using Methanol Solution Coupled with Inorganic Coagulants. Water Res. 2018, 138, 181–191. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Chład, Z.; Dudziak, M. Impact of Peroxydisulphate on Disintegration and Sedimentation Properties of Municipal Wastewater Activated Sludge. Chem. Pap. 2015, 69, 1473–1480. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Chłąd, Z.; Dudziak, M.; Černík, M. The Impact of Oxone on Disintegration and Dewaterability of Waste Activated Sludge. Water Environ. Res. 2016, 88, 152–157. [Google Scholar] [CrossRef]
- Sponza, D.T. Investigation of Extracellular Polymer Substances (EPS) and Physicochemical Properties of Different Activated Sludge Flocs under Steady-State Conditions. Enzyme Microb. Technol. 2003, 32, 375–385. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Gončuková, Z.; Padil, V.V.T.; Grübel, K.; Černík, M. A New Method for Assessment of the Sludge Disintegration Degree with the Use of Differential Centrifugal Sedimentation. Environ. Technol. 2019, 40, 3086–3093. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres–Mendieta, R.; Pawlyta, M.; Padil, V.V.T.; Filip, J.; Černík, M. Modification of NZVI with a Bio-Conjugate Containing Amine and Carbonyl Functional Groups for Catalytic Activation of Persulfate. Sep. Purif. Technol. 2021, 257, 117880. [Google Scholar] [CrossRef]
- Wang, H.; Cai, W.W.; Liu, W.Z.; Li, J.Q.; Wang, B.; Yang, S.C.; Wang, A.J. Application of Sulfate Radicals from Ultrasonic Activation: Disintegration of Extracellular Polymeric Substances for Enhanced Anaerobic Fermentation of Sulfate-Containing Waste-Activated Sludge. Chem. Eng. J. 2018, 352, 380–388. [Google Scholar] [CrossRef]
- Machnicka, A.; Grübel, K.; Wacławek, S.; Sikora, K. Waste-activated sludge disruption by dry ice: Bench scale study and evaluation of heat phase transformations. Environ. Sci. Pollut. Res. 2019, 26, 26488–26499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Xiao, K.; Liang, P.; Ma, Y.; Huang, X. Improvement on the Modified Lowry Method against Interference of Divalent Cations in Soluble Protein Measurement. Appl. Microbiol. Biotechnol. 2013, 97, 4167–4178. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, Z.; Shi, S.; Xu, W.; Sheng, H.; Gu, Y.; Jiang, Y.; Xi, B. Dechlorination of Excess Trichloroethene by Bimetallic and Sulfidated Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2018, 52, 8627–8637. [Google Scholar] [CrossRef]
- Edwards, J.O.; Gallopo, A.K. Kinetics and Mechanisms of the Spontaneous and Metal-Modified Oxidations of Ethanol by Peroxydisulfate Ion. J. Org. Chem. 1971, 36, 4089–4096. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).