Performance, Reaction Pathway, and Pretreatment of Au Catalyst Precursor in H2/O2 Atmosphere for the Epoxidation of Propylene
Abstract
:1. Introduction
2. Results
2.1. Catalytic Results of Au-TS-1 Catalysts
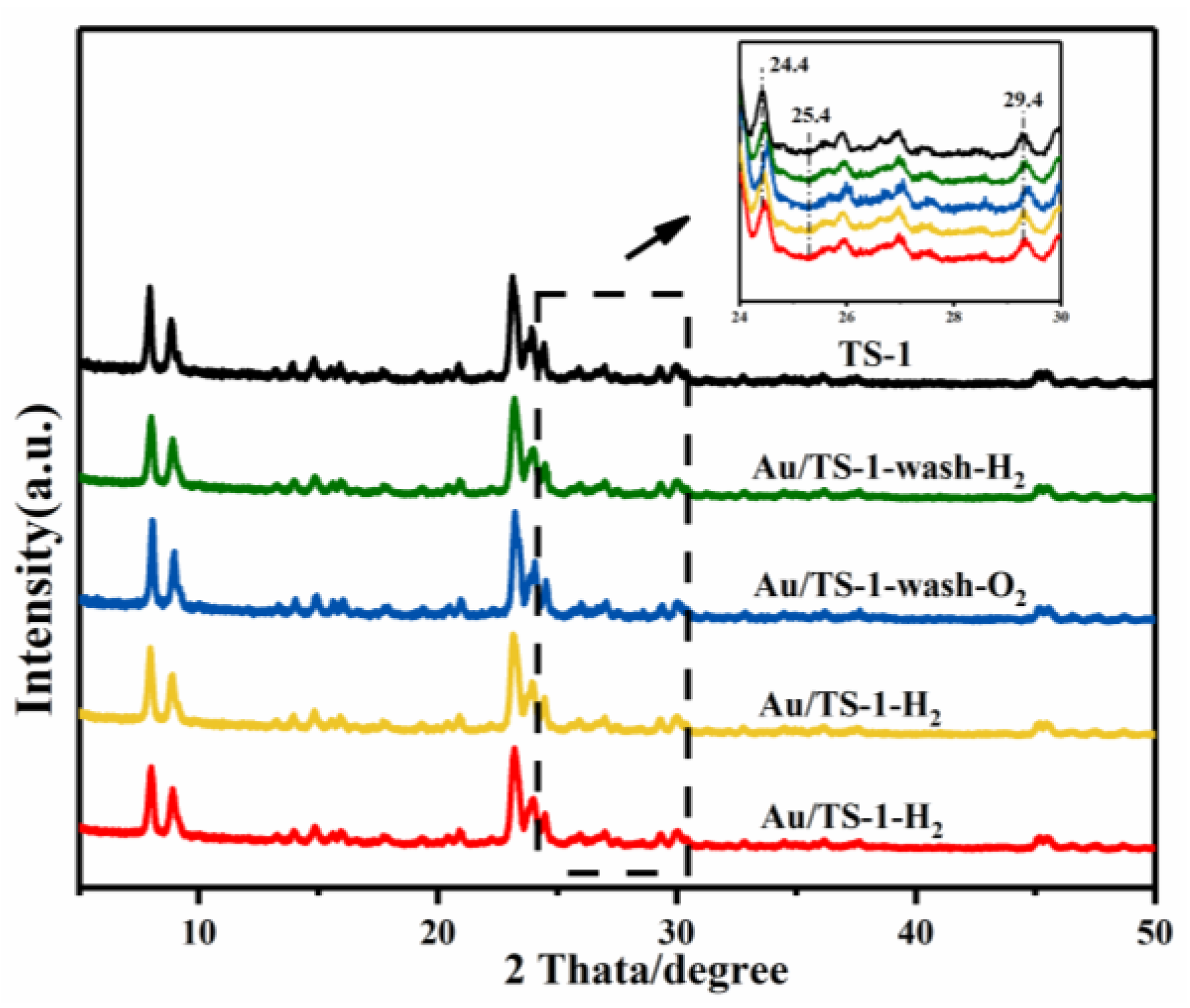
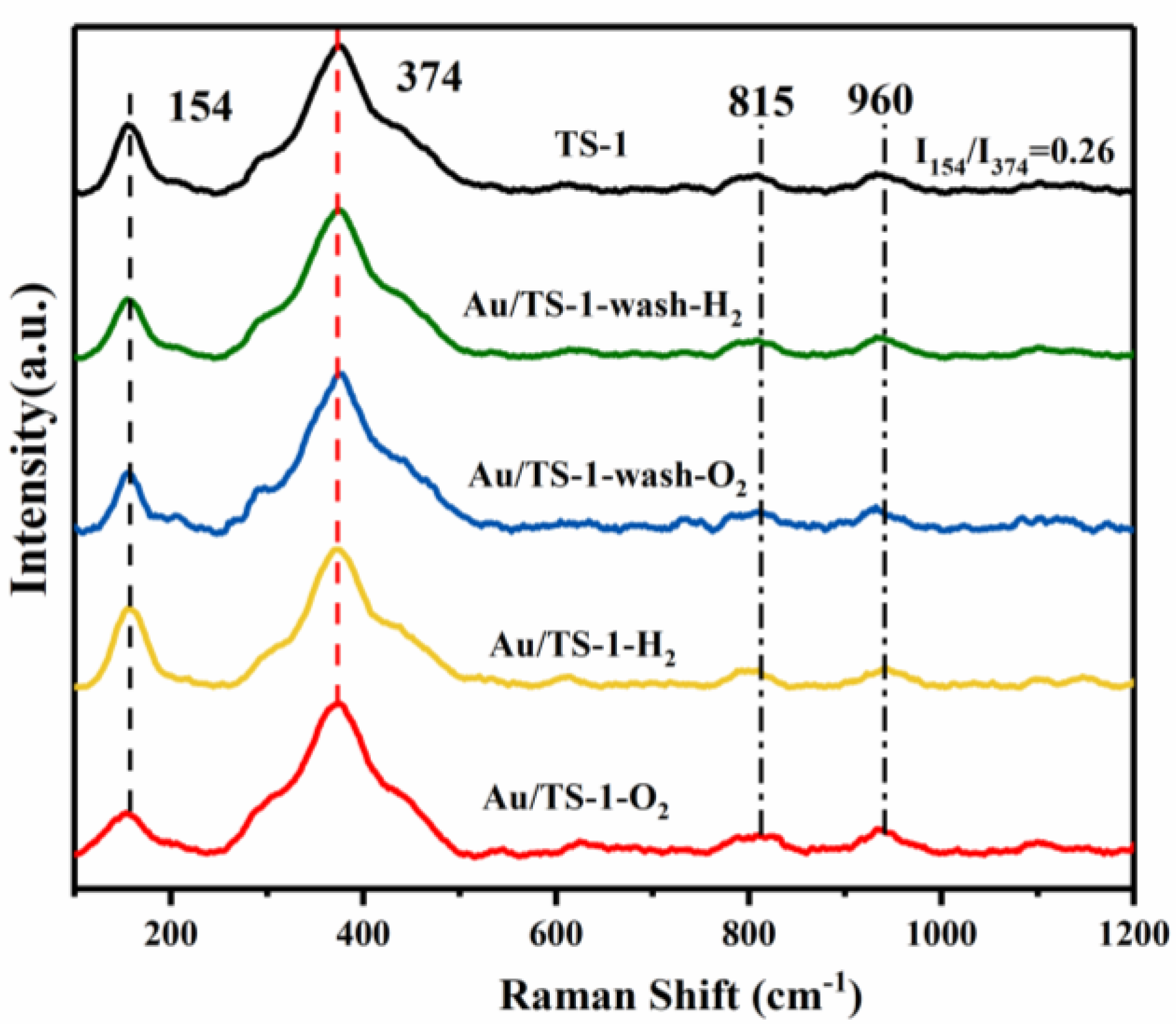
2.2. Structural Analysis of TS-1 Support and Au/TS-1 Catalysts
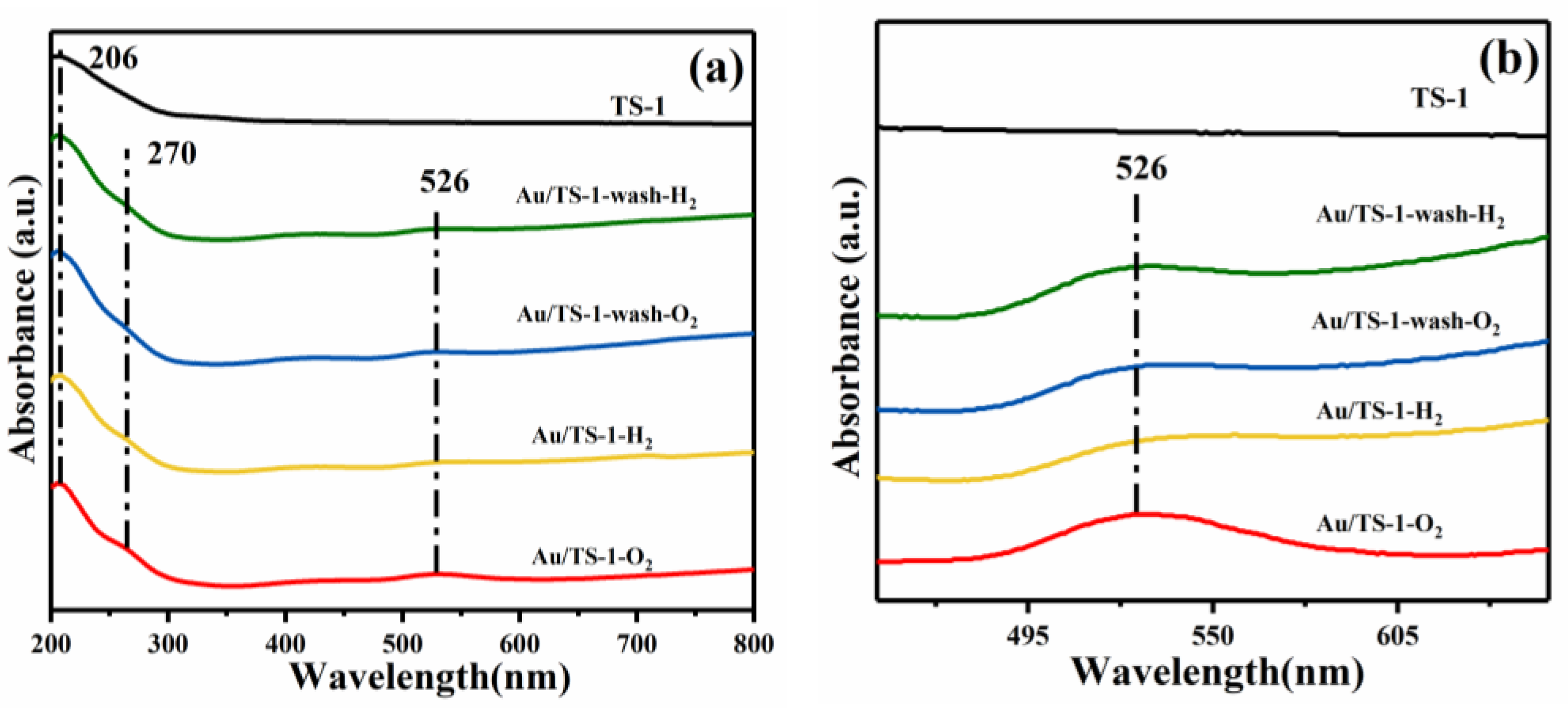
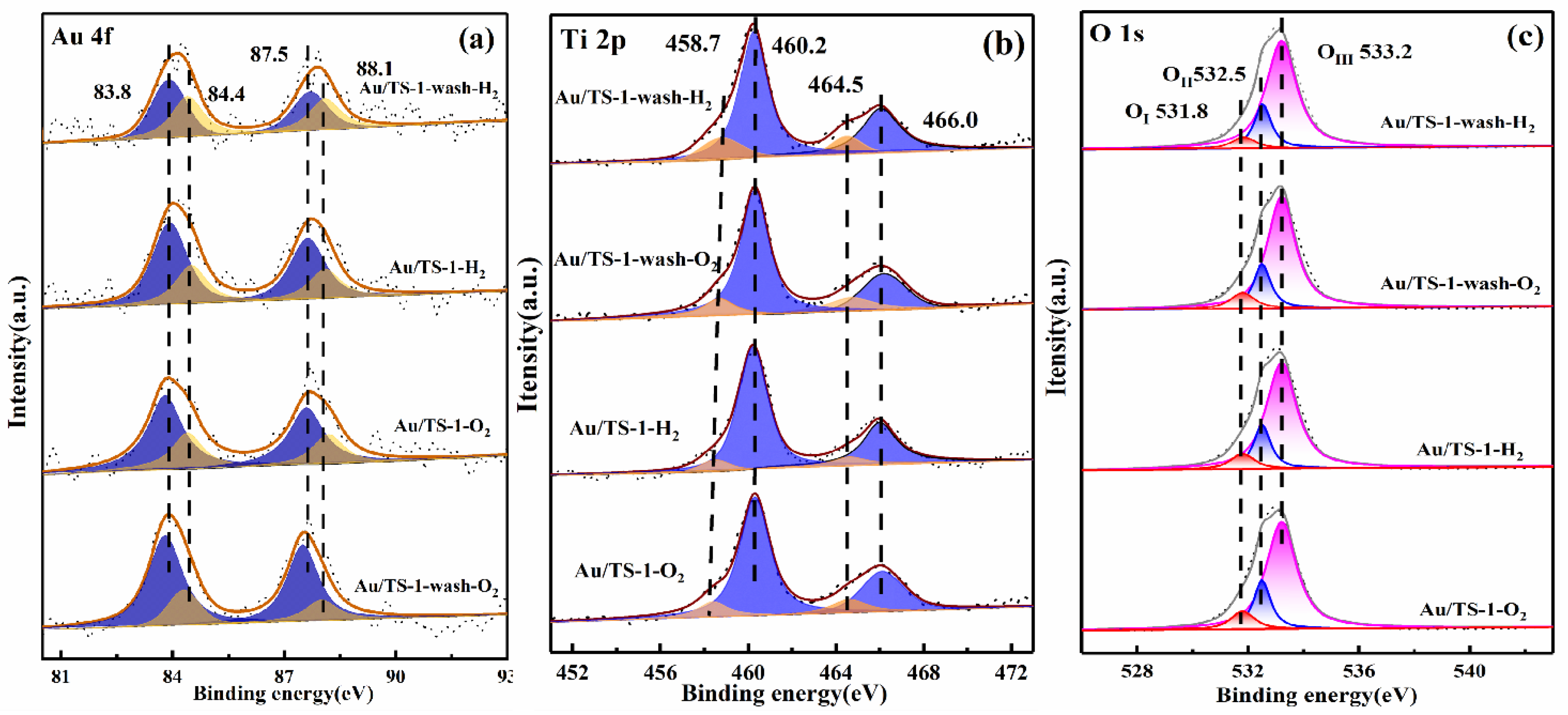
2.3. Chemical State of Au and Concentration of Surface Elements in Au/TS-1 Catalysts
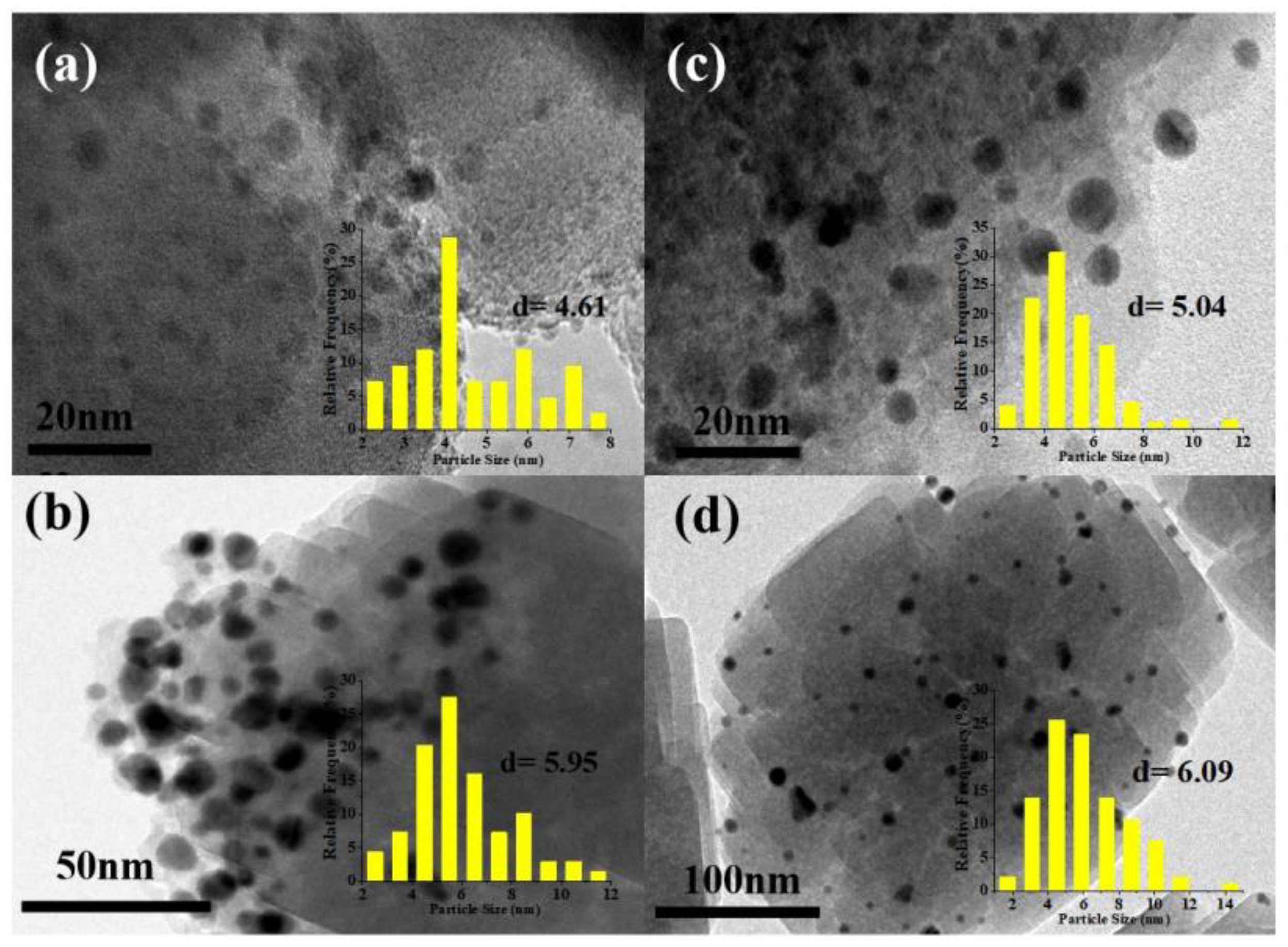
2.4. Dispersion Situation of Gold Particles
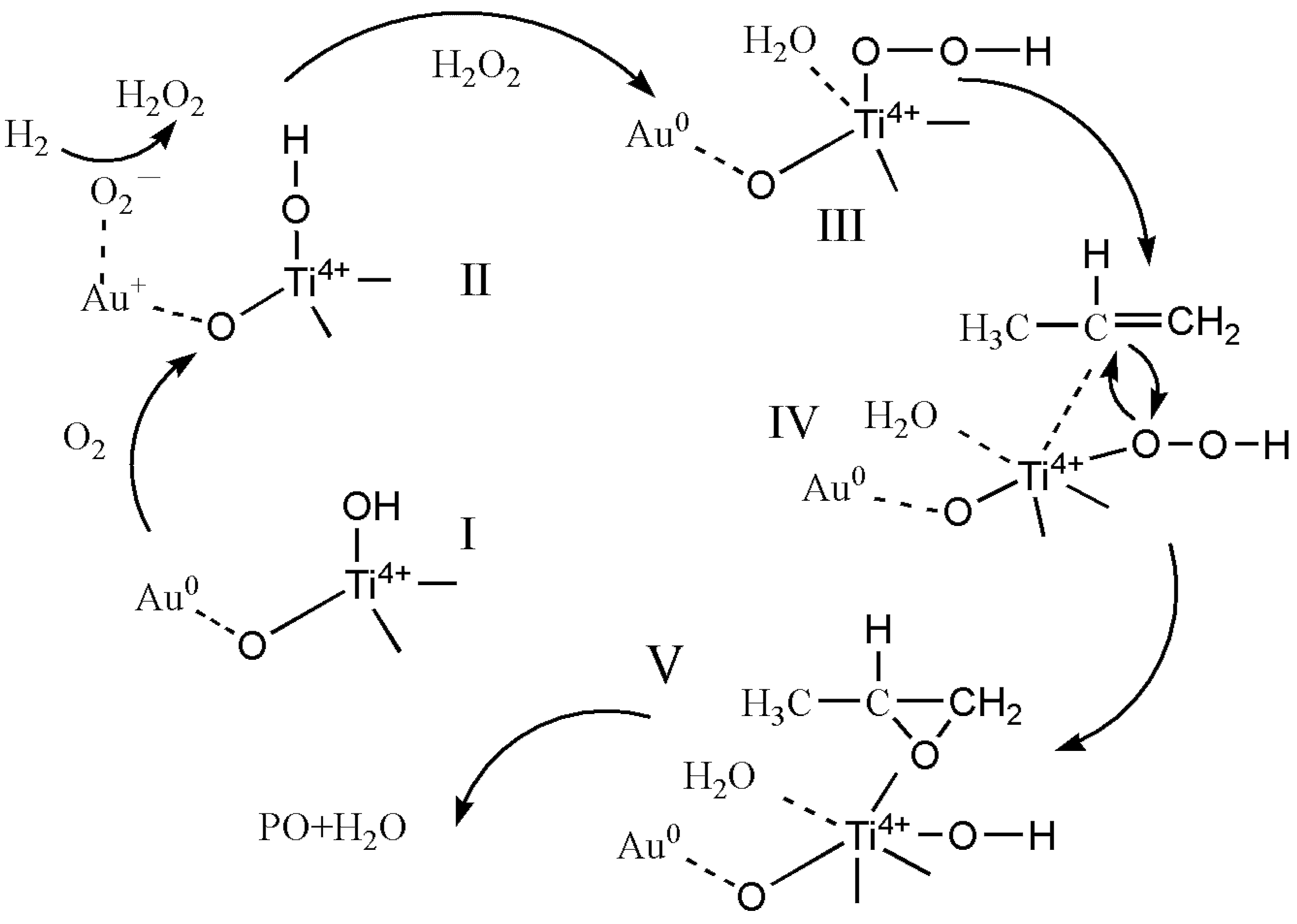
3. Discussion
4. Materials and Methods
4.1. Catalyst Synthesis
4.2. Catalyst Characterization
4.3. Catalytic Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thayer, A. Catalyst suppliers face changing industry. Chem. Eng. News 1992, 70, 27–49. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Direct Oxidation of Propylene to Propylene Oxide with Molecular Oxygen: A Review. Catal. Rev. 2015, 57, 306–344. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Makkee, M.; Moulijn, J.A.; Weckhuysen, B.M. The production of propene oxide: Catalytic processes and recent developments. Ind. Eng. Chem. Res. 2006, 45, 3447–3459. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Tanaka, K.; Haruta, M. Selective vapor-phase epoxidation of propylene over Au/TiO2 catalysts in the presence of oxygen and hydrogen. J. Catal. 1998, 178, 566–575. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Huizinga, B.J.; Makkee, M.; Moulijn, J.A. Direct epoxidation of propene using gold dispersed on TS-1 and other titanium-containing supports. Ind. Eng. Chem. Res. 1999, 38, 884–891. [Google Scholar] [CrossRef]
- Kanungo, S.; Keshri, K.S.; Hoof, A.V.; Angelo, M.F.; Schouten, J.C.; Nijhuis, T.A.; Hensen, E.J.; Chowdhury, B. Silylation enhances the performance of Au/Ti-SiO2 catalysts in direct epoxidation of propene using H2 and O2. J. Catal. 2016, 344, 434–444. [Google Scholar] [CrossRef]
- Uphade, B.S.; Yamada, Y.; Akita, T.; Nakamura, T.; Haruta, M. Synthesis and characterization of Ti-MCM-41 and vapor-phase epoxidation of propylene using H2 and O2 over Au/Ti-MCM-41. Appl. Catal. A-Gen. 2001, 215, 137–148. [Google Scholar] [CrossRef]
- Lu, J.Q.; Zhang, X.M.; Bravosuarez, J.J.; Bando, K.K.; Fujitani, T.; Oyama, S.T. Direct propylene epoxidation over barium-promoted Au/Ti-TUD catalysts with H2 and O2: Effect of Au particle size. J. Catal. 2007, 250, 350–359. [Google Scholar] [CrossRef]
- Feng, X.; Duan, X.Z.; Cheng, H.Y.; Qian, G.; Chen, D.; Yuan, W.K.; Zhou, X.G. Au/TS-1 catalyst prepared by deposition-precipitation method for propene epoxidation with H2/O2: Insights into the effects of slurry aging time and Si/Ti molar ratio. J. Catal. 2015, 325, 128–135. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhao, X.; Wang, G.; Xu, J.L.; Lu, M.K.; Tang, Y.Q.; Fu, W.Z.; Duan, X.Z.; Qian, G.; Chen, D.; et al. Uncalcined TS-2 immobilized Au nanoparticles as a bifunctional catalyst to boost direct propylene epoxidation with H2 and O2. AIChE J. 2019, 66, e16815. [Google Scholar]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Abad, A.; Almela, C.; Corma, A.; García, H. Efficient chemoselective alcohol oxidation using oxygen as oxidant superior performance of gold over palladium catalyst. Tetrahedron 2006, 62, 6666–6672. [Google Scholar] [CrossRef]
- Huang, J.H.; Takei, T.; Akita, T.; Ohashi, H.; Haruta, M. Gold clusters supported on alkaline treated TS-1 for highly efficient propene epoxidation with O2 and H2. Appl. Catal. B-Environ. 2010, 95, 430–438. [Google Scholar] [CrossRef]
- Taylor, B.; Lauterbach, J.; Delgass, W.N. The effect of mesoporous scale defects on the activity of Au/TS-1 for the epoxidation of propylene. Catal. Today 2007, 123, 50–58. [Google Scholar] [CrossRef]
- Qi, C.X.; Okumura, M.; Akita, T.; Haruta, M. Vapor-phase epoxidation of propylene using H2/O2 mixture over gold catalysts supported on non-porous and mesoporous titania-silica: Effect of preparation conditions and pretreatments prior to reaction. Appl. Catal. A-Gen. 2004, 263, 19–26. [Google Scholar] [CrossRef]
- Sinha, A.K.; Seelan, S.; Tsubota, S.; Haruta, M. Catalysis by gold nanoparticles: Epoxidation of propene. Top. Catal. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Stangland, E.E.; Taylor, B.; Andres, R.P.; Delgass, W.N. Direct vapor phase propylene epoxidation over deposition-precipitation gold-titania catalysts in the presence of H2/O2: effects of support, neutralizing agent, and pretreatment. J. Phys. Chem. B 2005, 109, 2321–2330. [Google Scholar] [CrossRef]
- Ren, Y.G.; Huang, J.H.; Lv, Q.; Xie, Y.; Lu, A.H.; Haruta, M. Dual-component gas pretreatment for Au/TS-1: Enhanced propylene epoxidation with oxygen and hydrogen. Appl. Catal. A-Gen. 2019, 584, 117172–117180. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, X.S.; Guo, X.W. Synthesis of titanium silicalite-1 with small crystal size by using mother liquor of titanium silicalite-1 as seeds (II): Influence of synthesis conditions on properties of titanium silicalite-1. Ind. Eng. Chem. Res. 2012, 162, 105–114. [Google Scholar] [CrossRef]
- Prakas, A.M.; Kevan, L. Reducibility and Adsorbate Interactions of Ti in Titanosilicate Molecular Sieve TS-1. J. Catal. 1998, 178, 586–597. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, X.; Zhang, B.; Gan, Z.; Tang, S.; Ma, L.; Wu, T.; Nelson, G.J.; Qin, Y.; Turner, C.H.; et al. Structure and reactivity of single site Ti catalysts for propylene epoxidation. J. Catal. 2019, 377, 419–428. [Google Scholar] [CrossRef]
- Feng, X.; Yang, J.; Duan, X.Z.; Cao, Y.Q.; Chen, B.X.; Lin, D.; Qian, G.; Chen, D.; Yang, C.H.; Zhou, X.G. Enhanced catalytic performance for propene epoxidation with h2 and O2 over bimetallic Au–Ag/uncalcined titanium silicate-1 catalysts. ACS Catal. 2018, 8, 7799–7808. [Google Scholar] [CrossRef]
- Li, C.; Xiong, G.; Liu, J.; Ying, P.L.; Xin, Q.; Feng, Z.C. Identifying framework titanium in TS-1 Zeolite by UV resonance raman spectroscopy. J. Phys. Chem. B 2001, 105, 2993–2997. [Google Scholar] [CrossRef]
- Zhang, T.J.; Chen, X.X.; Chen, G.R.; Chen, M.Y.; Bai, R.S.; Jia, M.G.; Yu, J.H. Synthesis of anatase-free nano-sized hierarchical TS-1 zeolites and their excellent catalytic performance in alkene epoxidation. J. Mater. Chem. A 2018, 6, 9473–9479. [Google Scholar] [CrossRef]
- Cumaranatunge, L.; Delgas, W.N. Enhancement of Au capture efficiency and activity of Au/TS-1 catalysts for propylene epoxidation. J. Catal. 2005, 232, 38–42. [Google Scholar] [CrossRef]
- Sacaliuc-Parvulescu, E.; Friedrich, H.; Palkovits, R.; Weckhuysen, B.M.; Nijhuis, T.A. Understanding the effect of postsynthesis ammonium treatment on the catalytic activity of Au/Ti-SBA-15 catalysts for the oxidation of propene. J. Catal. 2008, 259, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Boyd, D.; Golunski, S.; Hearne, G.R.; Magadzu, T.; Mallick, K.; Raphulu, M.C.; Venugopal, A.; Scurrell, M.S. Reductive routes to stabilized nanogold and relation to catalysis by supported gold. Appl. Catal. A-Gen. 2005, 292, 76–81. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gaudet, J.; Zhang, W.; Su, S.D.; Bando, K.K. Platinum-Like Catalytic behavior of Au+. ChemCatChem 2010, 2, 1582–1586. [Google Scholar] [CrossRef]
- Okumura, M.; Kitagawa, Y.; Haruta, M.; Yamaguchi, K. The interaction of neutral and charged Au clusters with O2, CO and H2. Appl. Catal. A-Gen. 2005, 291, 37–44. [Google Scholar] [CrossRef]
- Javier, G.; Carrettin, S.; Corma, A. Spectroscopic Evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nanocrystalline CeO2. J. Am. Chem. Soc. 2005, 127, 3286–3287. [Google Scholar]
- Moretti, G.; Salvi, A.M.; Guascito, M.R.; Langerame, F. An XPS study of microporous and mesoporous titanosilicates. Surf. Interface Anal. 2004, 36, 1402–1412. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Chen, G.; Fan, J.; Lan, H.; Yang, Y. Ultra-low-gold loading Au/CeO2 catalysts for ambient temperature CO oxidation: Effect of preparation conditions on surface composition and activity. J. Catal. 2010, 273, 167–176. [Google Scholar] [CrossRef]
- Liu, H.; Lu, G.; Hu, H. Synthesis, characterization and catalytic performance of titanium silicalite-1 prepared in the presence of nonionic surfactants. Mater. Chem. Phys. 2006, 100, 162–167. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Liu, J.; Liu, S.; Xu, C.; Duan, A.; Jiang, G. Multifunctional catalysts of three-dimensionally ordered macroporous oxide-supported Au@Pt core-shell nanoparticles with high catalytic activity and stability for soot oxidation. J. Catal. 2014, 317, 62–74. [Google Scholar] [CrossRef]
- Contarinia, S.; Van der Heide, P.A.W.; Prakashc, A.M.; Kevanc, L. Titanium coordination in microporous and mesoporous oxide materials by monochromated X-ray photoelectron spectroscopy and X-ray Auger electron spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 2002, 125, 25–33. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, X.H.; Feng, X.; Sheng, N.; Song, Z.N.; Liu, Y.B.; Chen, X.B.; Cai, Z.P.; Chen, D.; Yang, C.H. Enhancing the dynamic electron transfer of Au species on wormhole-like TS-1 for boosting propene epoxidation performance with H2 and O2. Green Energy Environ. 2020, 5, 433–443. [Google Scholar] [CrossRef]
- Bravo-Suarez, J.J.; Bando, K.K.; Lu, J.; Haruta, M.; Fujitani, T.; Oyama, T. Transient technique for identification of true reaction intermediates: Hydroperoxide species in propylene epoxidation on gold/titanosilicate catalysts by x-ray absorption fine structure spectroscopy. J. Phys. Chem. C 2008, 112, 1115–1123. [Google Scholar] [CrossRef]
- Stangland, E.; Stavens, K.; Andres, R.; Delgass, W. Characterization of gold–titania catalysts via oxidation of propylene to propylene oxide. J. Catal. 2000, 191, 332–347. [Google Scholar] [CrossRef]
- Qi, C.X.; Huang, J.H.; Bao, S.Q.; Su, H.J.; Akita, T.; Haruta, M. Switching of reactions between hydrogenation and epoxidation of propene over Au/Ti-based oxides in the presence of H2 and O2. J. Catal. 2011, 281, 12–20. [Google Scholar] [CrossRef]
- Chen, S.L.; Li, D.; Cao, T.; Huang, W.X. Size-dependent structures and catalytic performances of Au/TiO2-{001} catalysts for propene epoxidation. J. Phys. Chem. C 2020, 124, 15264–15274. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhang, B.S.; Su, D.S.; Huang, W.X. Titania Morphology-dependent gold–titania interaction, structure, and catalytic performance of gold/titania catalysts. ChemCatChem 2015, 7, 3290–3298. [Google Scholar] [CrossRef]
- Du, S.T.; Li, F.; Sun, Q.M.; Wang, N.; Jia, M.J.; Yu, J.H. A green surfactant-assisted synthesis of hierarchical TS-1 zeolites with excellent catalytic properties for oxidative desulfurization. Chem. Commun. 2016, 52, 3368–3371. [Google Scholar] [CrossRef] [PubMed]
- Khomane, R.B.; Kulkarni, B.D.; Paraskar, A.; Sainkar, S.R. Synthesis, characterization and catalytic performance of titanium silicalite-1 prepared in micellar media. Mater. Chem. Phys. 2002, 76, 99–103. [Google Scholar] [CrossRef]

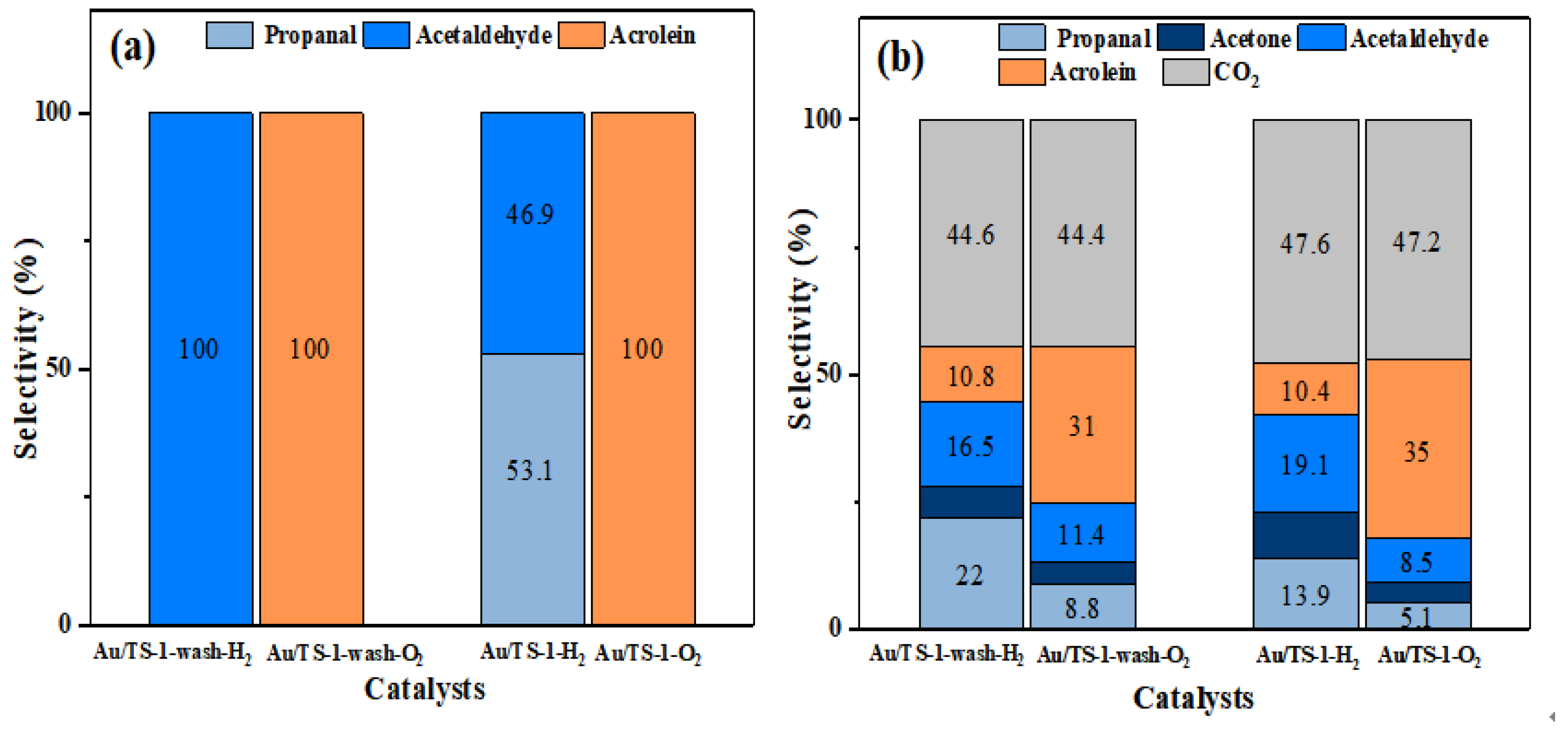
| Sample | T. (°C) | Conv. (%) | Products Selectivity (%) | Carbon Balance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PO | Propanal | Acetone | Acetaldehyde | Acrolein | CO2 | C3- Oxygenates | ||||
| 0.5 wt% Au/TS-1- wash-H2 | 100 150 200 | 0.08 0.89 3.74 | 26.1 31.5 14.6 | 56.7 36.7 45 | 0 8.6 10.3 | 17.2 6.6 8 | 0 3.9 3.3 | 0 12.7 18.8 | 82.8 76.8 69.9 | 0.98 1.00 1.04 |
| 0.5 wt% Au/TS-1-wash-O2 | 100 150 200 | 0.01 1.13 3.87 | 40.1 32.1 20.5 | 59.9 35.5 24.4 | 0 6.7 18.1 | 0 6.5 6 | 0 2.8 1.8 | 0 16.5 29.1 | 100.0 74.3 63.0 | 1.00 1.00 1.03 |
| 0.5 wt% Au/TS-1-H2 | 100 150 200 | 0.05 0.60 2.19 | 33.6 21.8 12.7 | 66.4 46.5 28.6 | 0.0 7.9 14.1 | 0.0 6.5 7.7 | 0.0 4.3 2.8 | 0.0 12.9 34.1 | 100.0 76.2 55.4 | 0.99 0.99 1.04 |
| 0.5 wt% Au/TS-1-O2 | 100 150 200 | 0.09 1.01 3.87 | 54.7 27.9 10.7 | 34.9 35.4 33.1 | 0.0 8.4 19.5 | 10.5 7.7 7.2 | 0.0 2.4 1.7 | 0.0 18.2 27.8 | 100 71.7 63.3 | 0.96 0.98 1.02 |
| Catalyst | Au species (%) | O species (%) | |||
|---|---|---|---|---|---|
| Au0 | Au+ | OI | OII | OIII | |
| 0.5%Au/TS-1-wash-H2 | 68.4 | 31.6 | 6.04 | 17.77 | 76.19 |
| 0.5%Au/TS-1-wash-O2 | 77.6 | 22.4 | 8.72 | 18.03 | 73.25 |
| 0.5%Au/TS-1-H2 | 55.1 | 44.9 | 8.34 | 17.68 | 73.98 |
| 0.5%Au/TS-1-O2 | 71.1 | 28.9 | 9.32 | 18.74 | 71.94 |
| Catalyst | Actual Loading (wt.%) a | Surface Atom Content (%) b | ||
|---|---|---|---|---|
| Au | K | Cl | K | |
| Au/TS-1-wash-H2 | 0.25 | 0.11 | 27.57 | 0.32 |
| Au/TS-1-wash-O2 | 27.13 | 0.33 | ||
| Au/TS-1-H2 | 0.26 | 0.12 | 28.54 | 0.35 |
| Au/TS-1-O2 | 28.31 | 0.39 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Su, H.; Cheng, Y.; Sun, X.; Sun, L.; Zhao, L.; Qi, C. Performance, Reaction Pathway, and Pretreatment of Au Catalyst Precursor in H2/O2 Atmosphere for the Epoxidation of Propylene. Catalysts 2022, 12, 540. https://doi.org/10.3390/catal12050540
Yang Z, Su H, Cheng Y, Sun X, Sun L, Zhao L, Qi C. Performance, Reaction Pathway, and Pretreatment of Au Catalyst Precursor in H2/O2 Atmosphere for the Epoxidation of Propylene. Catalysts. 2022; 12(5):540. https://doi.org/10.3390/catal12050540
Chicago/Turabian StyleYang, Zixuan, Huijuan Su, Yanan Cheng, Xun Sun, Libo Sun, Lijun Zhao, and Caixia Qi. 2022. "Performance, Reaction Pathway, and Pretreatment of Au Catalyst Precursor in H2/O2 Atmosphere for the Epoxidation of Propylene" Catalysts 12, no. 5: 540. https://doi.org/10.3390/catal12050540
APA StyleYang, Z., Su, H., Cheng, Y., Sun, X., Sun, L., Zhao, L., & Qi, C. (2022). Performance, Reaction Pathway, and Pretreatment of Au Catalyst Precursor in H2/O2 Atmosphere for the Epoxidation of Propylene. Catalysts, 12(5), 540. https://doi.org/10.3390/catal12050540







