Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress
Abstract
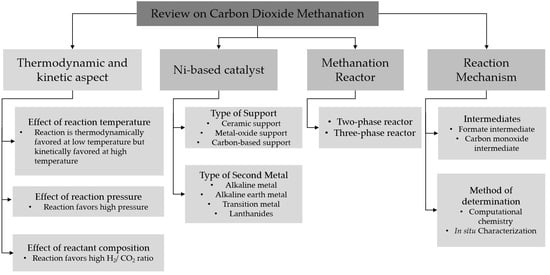
:1. Introduction
2. Thermodynamics and Kinetic Aspect of CO2 Methanation
2.1. Effect of Reaction Temperature
2.2. Effect of Reaction Pressure
2.3. Effect of Reactant Composition
3. Ni-Based Catalyst
| Reference | Second Metal | Support | Synthesis Method | Optimum Temperature (°C) | CO2 Conversion (%) |
|---|---|---|---|---|---|
| [52] | - | Al2O3 | Wetness Impregnation | 450 | ~65.0 |
| [53] | Fe | Al2O3 | Co-Precipitation | 220 | 58.50 |
| [46] | - | ZSM-5 | Impregnation | 400 | 76.0 |
| SBA-15 | 73.0 | ||||
| MCM41 | 65.0 | ||||
| Al2O3 | 70.0 | ||||
| SiO2 | 66.0 | ||||
| [47] | SiO2/rGO | Vapor Deposition | 470 | 83.7 | |
| [54] | Zr | Al2O3 | Co-Precipitation | 400 | 77.0 |
| [55] | - | SiO2/Al2O3 | Sol-gel | 350 | 82.38 |
| [56] | La | Zeolite | Wetness Impregnation | 450 | 73.0 |
| [57] | - | SiO2 | Impregnation | 400 | 80.0 |
| [58] | - | ZrO2 | Wetness Impregnation | 450 | 60.0 |
| [27] | - | Al2O3 | Hydrolysis | 350 | 77.0 |
| [59] | K | ZrO2 | Wetness Impregnation | 450 | 60.0 |
| La | 35.0 | ||||
| [60] | Ce | MCM-41 | Precipitation | 380 | 85.6 |
| [61] | Na | CeO2 | Impregnation | 290 | 95.0 |
| [25] | - | Al2O3 | Evaporation-induced assembly | 400 | 60.0 |
| La | 73.0 | ||||
| Ce | 64.0 | ||||
| Sm | 67.0 | ||||
| Pr | 77.0 | ||||
| [45] | - | Al2O3 | Impregnation | 350 | 75.0 |
| Y2O3 | 350 | 77.0 | |||
| ZrO2 | 350 | 76.0 | |||
| CeO2 | 300 | 71.0 | |||
| La2O3 | 400 | 53.0 | |||
| Sm2O3 | 300 | 66.0 | |||
| [62] | - | CeO2 | Impregnation | 250 | 91.0 |
| [63] | - | Al2O3 | 3D-fibre deposition | 400 | 91.0 |
| Wetness Impregnation | 400 | 74.0 | |||
| [64] | Cu | SiO2 | Wetness Impregnation | 350 | 55.0 |
| [65] | - | CeO2 | Hydrothermal | 300 | ~90.0 |
| [66] | - | CeO2 | Sol-gel | 250 | 80.5 |
| [67] | - | rGO | Wetness Impregnation | 240 | 51.0 |
| [68] | - | CeO2 | Impregnation | 300–350 | 90.0 |
| [69] | Cu | Hydrotalcite | Co-precipitation | 350 | 86.0 |
| [52] | V2O5 | MCM-41 | Hydrothermal | 400 | 69.3 |
| [70] | Co | Hydrotalcite | Co-precipitation | 300 | 77.0 |
| [71] | - | Zeolite | Wetness Impregnation | 400 | 85.0 |
| [72] | Cr | Al2O3 | Solid-state | 350 | 80.5 |
| [73] | Y2O3/Mg | MCM-41 | Co-precipitation | 400 | 65.5 |
| [74] | - | Al2O3 | Evaporation-induced sel-assembly | 350 | 83.0 |
| [75] | - | Al2O3 | Hydrothermal | 325 | ~70.0 |
| [76] | Ce | rGO | Impregnation | 350 | 84.5 |
| [77] | - | Phyllosilicate | Hydrothermal | 330 | ~80% |
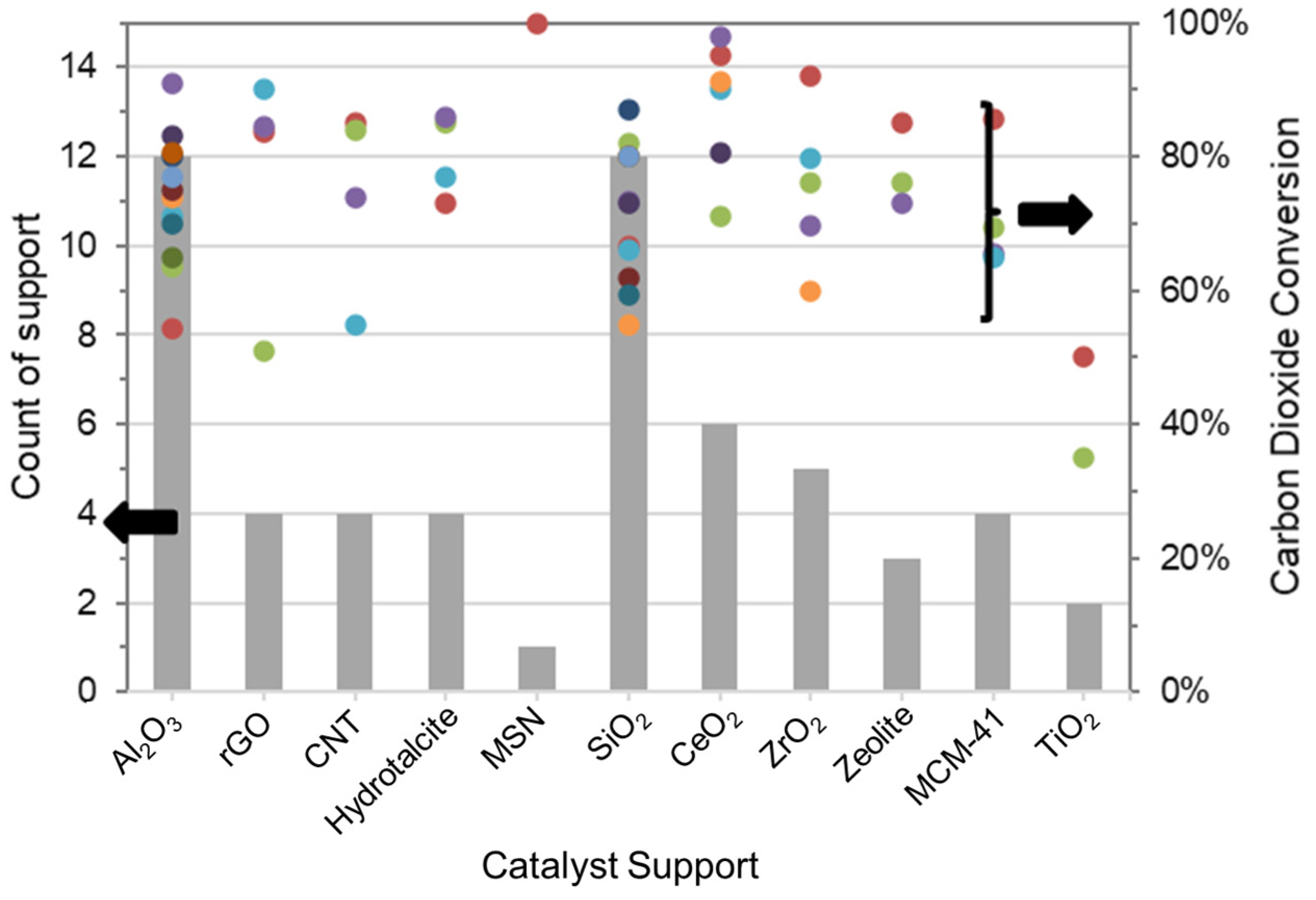
4. Effect of Support
4.1. Ceramic Support
4.2. Metal-Oxide Support
4.3. Carbon-Based Support
5. Effect of Second Metal
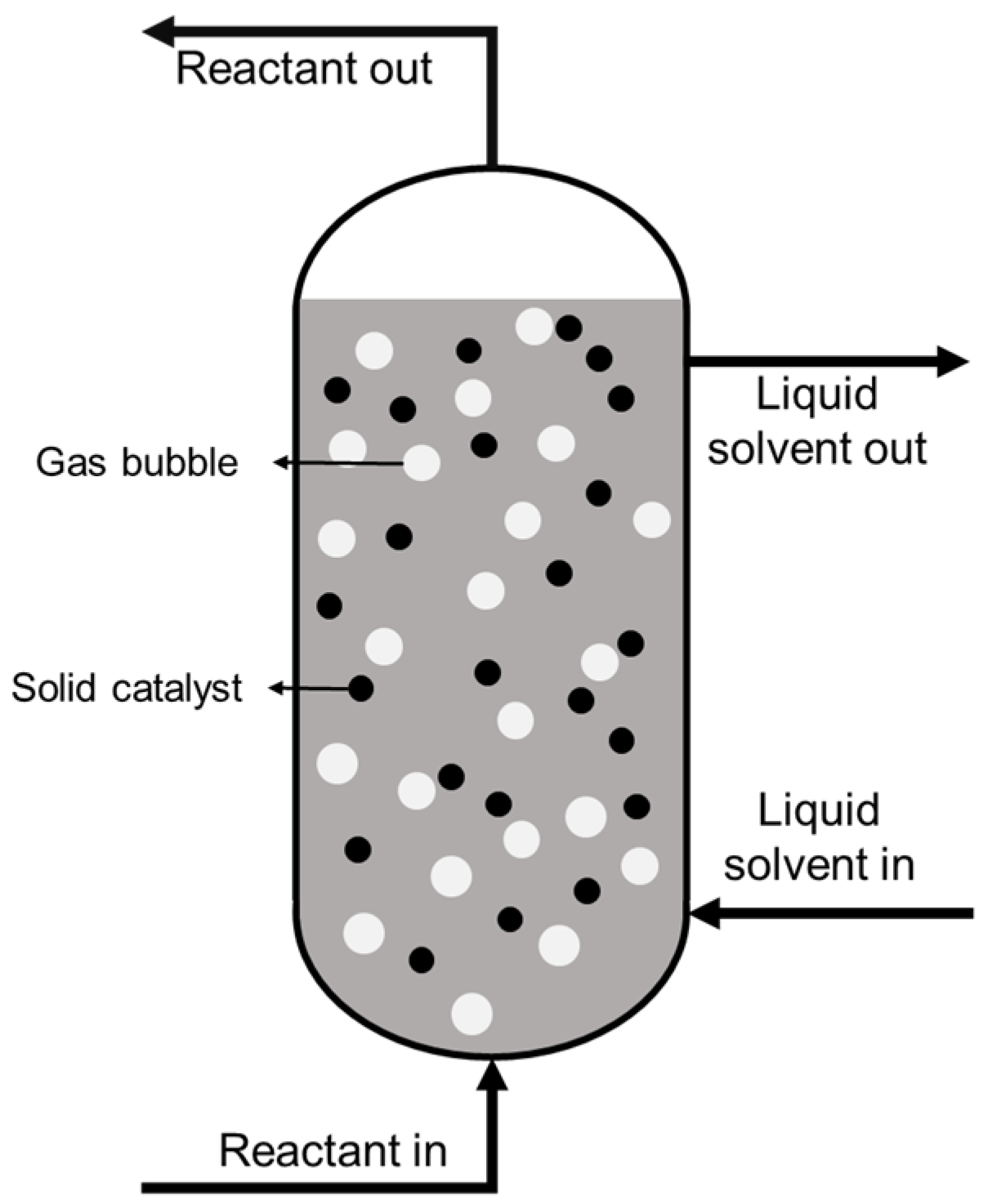
6. Reactor
7. Mechanism of CO2 Methanation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; Al-Mutairi, E.M. A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Cai, M.; Wen, J.; Chu, W.; Cheng, X.; Li, Z. Methanation of carbon dioxide on Ni/ZrO2-Al2O3 catalysts: Effects of ZrO2 promoter and preparation method of novel ZrO2-Al2O3 carrier. J. Nat. Gas Chem. 2011, 20, 318–324. [Google Scholar] [CrossRef]
- Gulzar, A.; Gulzar, A.; Ansari, M.B.; He, F.; Gai, S.; Yang, P. Carbon dioxide utilization: A paradigm shift with CO2 economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Sodeifian, G.; Ardestani, N.S.; Sajadian, S.; Ghorbandoost, S. Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: Experimental, response surface and grey wolf optimization methodology. J. Supercrit. Fluids 2016, 114, 55–63. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Derakhsheshpour, R. CO2 utilization as a supercritical solvent and supercritical antisolvent in production of sertraline hydrochloride nanoparticles. J. CO2 Util. 2022, 55, 101799. [Google Scholar] [CrossRef]
- Sodeifian, G.; Razmimanesh, F.; Sajadian, S. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids 2019, 146, 89–99. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Park, J.H.; Yang, J.; Kim, D.; Gim, H.; Choi, W.Y.; Lee, J.W. Review of recent technologies for transforming carbon dioxide to carbon materials. Chem. Eng. J. 2022, 427, 130980. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.-Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 2021, 9, 105460. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Ma, J. Highly active and stable nano NiO–MgO catalyst encapsulated by silica with a core–shell structure for CO2 methanation. RSC Adv. 2014, 4, 17420–17428. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.; Triwahyono, S.; Mukti, R.; Taufiq-Yap, Y.; Sazegar, M. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.-Z.; Qin, Z.-F.; Guo, H.-L.; Lin, J.-Y.; Li, Z. Methanation of carbon dioxide over Ni–M/ZrO2 (M = Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Hu, F.; Ye, R.; Lu, Z.-H.; Zhang, R.; Feng, G. Structure–Activity Relationship of Ni-Based Catalysts toward CO2 Methanation: Recent Advances and Future Perspectives. Energy Fuels 2021, 36, 156–169. [Google Scholar] [CrossRef]
- Li, L.; Zeng, W.; Song, M.; Wu, X.; Li, G.; Hu, C. Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts 2022, 12, 244. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Y.; Zhang, L.; Hu, S.; Xiang, J.; Wang, Y.; Xu, L.; Liu, Q.; Zhang, S.; Hu, X. Impacts of nickel loading on properties, catalytic behaviors of Ni/γ–Al2O3 catalysts and the reaction intermediates formed in methanation of CO2. Int. J. Hydrogen Energy 2019, 44, 9291–9306. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrogen Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Luo, J.; Kong, X.; Xu, Y.; Ma, G.; Wang, J.; Zhang, C.; Li, Z.; Ding, M. Preparation of Ni based mesoporous Al2O3 catalyst with enhanced CO2 methanation performance. RSC Adv. 2019, 9, 8684–8694. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Younis, M.N.; Shawabkeh, R.; Ahmed, S. Synthesis of lanthanide series (La, Ce, Pr, Eu & Gd) promoted Ni/γ-Al2O3 catalysts for methanation of CO2 at low temperature under atmospheric pressure. Catal. Commun. 2017, 100, 121–126. [Google Scholar] [CrossRef]
- Guo, X.; He, H.; Traitangwong, A.; Gong, M.; Meeyoo, V.; Li, P.; Li, C.; Peng, Z.; Zhang, S. Ceria imparts superior low temperature activity to nickel catalysts for CO2 methanation. Catal. Sci. Technol. 2019, 9, 5636–5650. [Google Scholar] [CrossRef]
- Hamid, M.Y.S.; Jalil, A.A.; Rahman, A.F.A.; Abdullah, T.A.T. Enhanced reactive CO2 species formation via V2O5-promoted Ni/KCC-1 for low temperature activation of CO2 methanation. React. Chem. Eng. 2019, 4, 1126–1135. [Google Scholar] [CrossRef]
- Lefebvre, J.; Bajohr, S.; Kolb, T. A comparison of two-phase and three-phase CO2 methanation reaction kinetics. Fuel 2019, 239, 896–904. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zemansky, M.W.; Dittman, R.H. Heat and Thermodynamics; American Association of Physics Teachers: College Park, MD, USA, 1998. [Google Scholar]
- Esa, Y.A.M.; Sapawe, N. A short review on carbon dioxide (CO2) methanation process. Mater. Today Proc. 2020, 31, 394–397. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Zhang, Q.; Wang, X.; Zhang, T.; Tan, Y.; Han, Y. Low-temperature methanation of syngas in slurry phase over Zr-doped Ni/γ-Al2O3 catalysts prepared using different methods. Fuel 2014, 132, 211–218. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced Investigation of CO Methanation over Ni/Al2O3 Catalysts for Synthetic Natural Gas Production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Park, J.-N.; Mc Farland, E.W. A highly dispersed Pd–Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrog Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Zamani, A.; Ali, R.; Bakar, W.A. The investigation of Ru/Mn/Cu–Al2O3 oxide catalysts for CO2/H2 methanation in natural gas. J. Taiwan Inst. Chem. Eng. 2014, 45, 143–152. [Google Scholar] [CrossRef]
- Kuznecova, I.; Gusca, J. Property based ranking of CO and CO2 methanation catalysts. Energy Procedia 2017, 128, 255–260. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, U.G.; Lee, J.; Baik, J.H.; Koh, D.J.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous nickel–M–alumina (M= Fe, Zr, Ni, Y, and Mg) xerogel catalysts: Effect of second metal. Catal. Lett. 2012, 142, 860–868. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Ma, H.; Ma, K.; Ji, J.; Tang, S.; Liu, C.; Jiang, W.; Yue, H.; Liang, B. Graphene intercalated Ni-SiO2/GO-Ni-foam Catalyst with enhanced reactivity and heat-transfer for CO2 methanation. Chem. Eng. Sci. 2019, 194, 10–21. [Google Scholar] [CrossRef]
- Unwiset, P.; Chanapattharapol, K.C.; Kidkhunthod, P.; Poo-Arporn, Y.; Ohtani, B. Catalytic activities of titania-supported nickel for carbon-dioxide methanation. Chem. Eng. Sci. 2020, 228, 115955. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The difference of roles of alkaline-earth metal oxides on silica-supported nickel catalysts for CO2 methanation. RSC Adv. 2014, 4, 58171–58177. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The effect of impregnation strategy on structural characters and CO2 methanation properties over MgO modified Ni/SiO2 catalysts. Catal. Commun. 2014, 54, 55–60. [Google Scholar] [CrossRef]
- Carenco, S.; Tuxen, A.; Chintapalli, M.; Pach, E.; Escudero, C.; Ewers, T.D.; Jiang, P.; Borondics, F.; Thornton, G.; Alivisatos, A.P.; et al. Dealloying of Cobalt from CuCo Nanoparticles under Syngas Exposure. J. Phys. Chem. C 2013, 117, 6259–6266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Y.; Liu, Q. Green synthesis of MCM-41 derived from renewable biomass and construction of VOx-Modified nickel phyllosilicate catalyst for CO2 methanation. Int. J. Hydrogen Energy 2021, 46, 32003–32016. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, U.G.; Lee, J.; Gil Seo, J.; Baik, J.H.; Koh, D.J.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Ni–Fe–Al2O3 catalysts prepared by a coprecipitation method: Effect of precipitation agent. J. Ind. Eng. Chem. 2013, 19, 2016–2021. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, Y.; Gu, D.; Chen, C.; Jiang, L.; Takehira, K. Ni/Al2O3-ZrO2 catalyst for CO2 methanation: The role of γ-(Al, Zr)2O3 formation. Appl. Surf. Sci. 2018, 459, 74–79. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Synthesis of nanocrystalline mesoporous Ni/Al2O3SiO2 catalysts for CO2 methanation reaction. Int. J. Hydrogen Energy 2018, 43, 19038–19046. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Marcos, J.A.G.; González-Velasco, J.R. Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl. Catal. B Environ. 2018, 238, 393–403. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Słowik, G.; Sienkiewicz, A.; Kierys, A. Nickel catalysts supported on silica microspheres for CO2 methanation. Microporous Mesoporous Mater. 2018, 272, 79–91. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Hu, L.; Urakawa, A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/Zr, O2. J. CO2 Util. 2018, 25, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhu, L.; Liu, Y.; Wang, S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci. Total Environ. 2018, 625, 686–695. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Lefevere, J.; Bedel, L.; Guilet, R.; Marty, P. Efficient CO2 methanation over Ni/Al 2 O 3 coated structured catalysts. Catal. Today 2016, 273, 234–243. [Google Scholar] [CrossRef]
- Dias, Y.R.; Perez-Lopez, O.W. Carbon dioxide methanation over Ni-Cu/SiO2 catalysts. Energy Convers. Manag. 2020, 203, 112214. [Google Scholar] [CrossRef]
- Varvoutis, G.; Lykaki, M.; Stefa, S.; Binas, V.; Marnellos, G.E.; Konsolakis, M. Deciphering the role of Ni particle size and nickel-ceria interfacial perimeter in the low-temperature CO2 methanation reaction over remarkably active Ni/CeO2 nanorods. Appl. Catal. B Environ. 2021, 297, 120401. [Google Scholar] [CrossRef]
- Ye, R.-P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J.; et al. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 2020, 268, 118474. [Google Scholar] [CrossRef]
- Ridzuan, N.D.M.; Shaharun, M.S.; Lee, K.M.; Din, I.U.; Puspitasari, P. Influence of Nickel Loading on Reduced Graphene Oxide-Based Nickel Catalysts for the Hydrogenation of Carbon Dioxide to Methane. Catalysts 2020, 10, 471. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of support composition on the performance of nickel catalysts in CO2 methanation reaction. Catal. Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Summa, P.; Samojeden, B.; Motak, M.; Wierzbicki, D.; Alxneit, I.; Świerczek, K.; Da Costa, P. Investigation of Cu promotion effect on hydrotalcite-based nickel catalyst for CO2 methanation. Catal. Today 2022, 384–386, 133–145. [Google Scholar] [CrossRef]
- Summa, P.; Świrk, K.; Wang, Y.; Samojeden, B.; Rønning, M.; Hu, C.; Motak, M.; Da Costa, P. Effect of cobalt promotion on hydrotalcite-derived nickel catalyst for CO2 methanation. Appl. Mater. Today 2021, 25, 101211. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Słowik, G.; Kuśmierz, M.; Dzwigaj, S. The state of BEA zeolite supported nickel catalysts in CO2 methanation reaction. Appl. Surf. Sci. 2021, 564, 150421. [Google Scholar] [CrossRef]
- Gholami, S.; Alavi, S.M.; Rezaei, M. Synthesis of Cr2O3–Al2O3 powders with various Cr2O3/Al2O3 molar ratios and their applications as support for the preparation of nickel catalysts in CO2 methanation reaction. Int. J. Hydrogen Energy 2021, 46, 5311–5322. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Promoted nickel-based catalysts on modified mesoporous silica support: The role of yttria and magnesia on CO2 methanation. Microporous Mesoporous Mater. 2020, 306, 110455. [Google Scholar] [CrossRef]
- Aljishi, A.; Veilleux, G.; Lalinde, J.A.H.; Kopyscinski, J. The effect of synthesis parameters on ordered mesoporous nickel alumina catalyst for CO2 methanation. Appl. Catal. A Gen. 2018, 549, 263–272. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Zou, H.; Guo, X.; Wang, Z.-J. Ni catalysts supported on nanosheet and nanoplate γ-Al2O3 for carbon dioxide methanation. J. Energy Chem. 2019, 29, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Tong, S.; Lu, K.; Chen, C.-M.; Su, F.-Y.; Zhou, J.; Lu, Z.-H.; Wang, X.; Feng, G.; Zhang, R. Reduced graphene oxide supported Ni-Ce catalysts for CO2 methanation: The support and ceria promotion effects. J. CO2 Util. 2019, 34, 676–687. [Google Scholar] [CrossRef]
- Liao, L.; Chen, L.; Ye, R.; Tang, X.; Liu, J. Robust nickel silicate catalysts with high Ni loading for CO2 methanation. Chem. Asian J. 2021, 16, 678–689. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the Preparation of Coke Resistant Ni-based Catalyst for Steam and CO2 Reforming of Methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.E.; Da Costa, P. Novel Ni-La-hydrotalcite derived catalysts for CO2 methanation. Catal. Commun. 2016, 83, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Abate, S.; Barbera, K.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Synthesis, Characterization, and Activity Pattern of Ni–Al Hydrotalcite Catalysts in CO2 Methanation. Ind. Eng. Chem. Res. 2016, 55, 8299–8308. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Wang, B.; Han, Y.; Xu, Y.; Liang, Z.; Wang, Z. Nickel Nanoparticles Encapsulated in Microporous Graphenelike Carbon (Ni@MGC) as Catalysts for CO2 Methanation. Ind. Eng. Chem. Res. 2019, 58, 20536–20542. [Google Scholar] [CrossRef]
- Riani, P.; Garbarino, G.; Lucchini, M.A.; Canepa, F.; Busca, G. Unsupported versus alumina-supported Ni nanoparticles as catalysts for steam/ethanol conversion and CO2 methanation. J. Mol. Catal. A Chem. 2014, 383–384, 10–16. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl. Catal. B Environ. 2019, 243, 262–272. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, B.; Fan, J.; Yang, J. Cobalt doped Ni based ordered mesoporous catalysts for CO2 methanation with enhanced catalytic performance. Int. J. Hydrogen Energy 2018, 43, 4893–4901. [Google Scholar] [CrossRef]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 methanation over Co Ni bimetal-doped ordered mesoporous Al2O3 catalysts with enhanced low-temperature activities. Int. J. Hydrogen Energy 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Enhanced activity of CO2 methanation over mesoporous nanocrystalline Ni–Al2O3 catalysts prepared by ultrasound-assisted co-precipitation method. Int. J. Hydrogen Energy 2017, 42, 15115–15125. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Copeland, J.R.; Kim, W.G.; Crittenden, J.C.; Sievers, C. Structural changes of γ-Al2O3-supported catalysts in hot liquid water. Acs Catal. 2011, 1, 552–561. [Google Scholar] [CrossRef]
- Bai, X.; Wang, S.; Sun, T.; Wang, S. The sintering of Ni/Al2O3 methanation catalyst for substitute natural gas production. React. Kinet. Mech. Catal. 2014, 112, 437–451. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Magnesium as Promoter of CO2 Methanation on Ni-Based USY Zeolites. Energy Fuels 2017, 31, 9776–9789. [Google Scholar] [CrossRef] [Green Version]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Yoshida, T.; Akiyama, E.; Kawashima, A.; Asami, K.; Hashimoto, K.; Komori, M.; Shimamura, K. Compositional dependence of the CO2 methanation activity of Ni/ZrO2 catalysts prepared from amorphous NiZr alloy precursors. Appl. Catal. A Gen. 1997, 163, 187–197. [Google Scholar] [CrossRef]
- HPabazaki, H. Amorphous iron group metal- valve metal alloy catalysts for hydrogenation of carbon dioxide. Electrochem. Soc. Inc. Corros. Electrochem. Catal. Metastable Met. Intermet. (USA) 1993, 1993, 393–404. [Google Scholar]
- Ren, J.; Li, H.; Jin, Y.; Zhu, J.; Liu, S.; Lin, J.; Li, Z. Silica/titania composite-supported Ni catalysts for CO methanation: Effects of Ti species on the activity, anti-sintering, and anti-coking properties. Appl. Catal. B Environ. 2017, 201, 561–572. [Google Scholar] [CrossRef]
- Jia, C.; Dai, Y.; Yang, Y.; Chew, J.W. Nickel cobalt catalyst supported on TiO2-coated SiO2 spheres for CO2 methanation in a fluidized bed. Int. J. Hydrogen Energy 2019, 44, 13443–13455. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Moioli, E.; Chen, W.; Zhang, M.; Terlingen, B.J.; Zijlstra, B.; Filot, I.A.; Züttel, A.; Pidko, E.A.; Hensen, E.J. Efficient Base-Metal NiMn/TiO2 Catalyst for CO2 Methanation. ACS Catal. 2019, 9, 7823–7839. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chu, W.; Wang, N.; Yang, W.; Jiang, C. Mesoporous nickel catalyst supported on multi-walled carbon nanotubes for carbon dioxide methanation. Int. J. Hydrogen Energy 2016, 41, 967–975. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.; Subbarao, D.; Naeem, A. Synthesis, characterization and activity pattern of carbon nanofibers based copper/zirconia catalysts for carbon dioxide hydrogenation to methanol: Influence of calcination temperature. J. Power Sources 2015, 274, 619–628. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.; Subbarao, D.; Naeem, A.; Hussain, F. Influence of niobium on carbon nanofibres based Cu/ZrO 2 catalysts for liquid phase hydrogenation of CO2 to methanol. Catal. Today 2016, 259, 303–311. [Google Scholar] [CrossRef]
- Deerattrakul, V.; Dittanet, P.; Sawangphruk, M.; Kongkachuichay, P. CO2 hydrogenation to methanol using Cu-Zn catalyst supported on reduced graphene oxide nanosheets. J. CO2 Util. 2016, 16, 104–113. [Google Scholar] [CrossRef]
- Jiménez, V.; Sánchez, P.; Panagiotopoulou, P.; Valverde, J.L.; Romero, A. Methanation of CO, CO2 and selective methanation of CO, in mixtures of CO and CO2, over ruthenium carbon nanofibers catalysts. Appl. Catal. A Gen. 2010, 390, 35–44. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, X.; Han, J.; Wang, H.; Ge, Q. Rhenium-promoted selective CO2 methanation on Ni-based catalyst. J. CO2 Util. 2018, 26, 8–18. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M. Nanocrystalline MgO supported nickel-based bimetallic catalysts for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2010, 35, 10295–10301. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Jung, J.C.; Koh, D.J.; Lim, H.; Byun, C.; Song, I.K. Hydrogenation of carbon monoxide to methane over mesoporous nickel-M-alumina (M=Fe, Ni, Co, Ce, and La) xerogel catalysts. J. Ind. Eng. Chem. 2012, 18, 243–248. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Baik, J.H.; Koh, D.J.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Ni–Fe–Ru–Al2O3 xerogel catalysts: Effect of ruthenium content. J. Ind. Eng. Chem. 2013, 19, 698–703. [Google Scholar] [CrossRef]
- Held, M.; Schollenberger, D.; Sauerschell, S.; Bajohr, S.; Kolb, T. Methanation Concepts for SNG Production at the Engler-Bunte-Institut. Chem. Ing. Tech. 2020, 92, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, J.; Götz, M.; Bajohr, S.; Reimert, R.; Kolb, T. Improvement of three-phase methanation reactor performance for steady-state and transient operation. Fuel Process. Technol. 2015, 132, 83–90. [Google Scholar] [CrossRef]
- Bendjaouahdou, C.; Bendjaouahdou, M.H. Control of the Hot Spot Temperature in an Industrial SO2 Converter. Energy Procedia 2013, 36, 428–443. [Google Scholar] [CrossRef] [Green Version]
- De Swart, J.; Krishna, R. Simulation of the transient and steady state behaviour of a bubble column slurry reactor for Fischer–Tropsch synthesis. Chem. Eng. Process. Process Intensif. 2002, 41, 35–47. [Google Scholar] [CrossRef]
- Basha, O.M.; Morsi, B.I. CFD for the Design and Optimization of Slurry Bubble Column Reactors. In Computational Fluid Dynamics—Basic Instruments and Applications in Science; InTech: London, UK, 2018. [Google Scholar]
- Lefebvre, J.; Trudel, N.; Bajohr, S.; Kolb, T. A study on three-phase CO2 methanation reaction kinetics in a continuous stirred-tank slurry reactor. Fuel 2018, 217, 151–159. [Google Scholar] [CrossRef]
- Seyednejadian, S.; Rauch, R.; Bensaid, S.; Hofbauer, H.; Weber, G.; Saracco, G. Power to Fuels: Dynamic Modeling of a Slurry Bubble Column Reactor in Lab-Scale for Fischer Tropsch Synthesis under Variable Load of Synthesis Gas. Appl. Sci. 2018, 8, 514. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, J. Three-phase CO2 Methanation: Methanation Reaction Kinetics and Transient Behavior of a Slurry Bubble Column Reactor, in Fakultät für Chemieingenieurwesen und Verfahrenstechnik; Karlsruher Instituts für Technologie: Karlsruhe, Germany, 2019. [Google Scholar]
- Ussa, P.A.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyka, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Akamaru, S.; Shimazaki, T.; Kubo, M.; Abe, T. Density functional theory analysis of methanation reaction of CO2 on Ru nanoparticle supported on TiO2 (101). Appl. Catal. A Gen. 2014, 470, 405–411. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Protecting Finite Natural Gas Stores with Green Hydrogen. Green Hydrogen for Methanation. 2022. Available online: https://itm-power.com/markets/hydrogen-for-methanation (accessed on 19 April 2022).
- Audi Opens Power-to-Gas Facility in Werlte/Emsland; e-Gas from Water, Green Electricity and CO2. 2013. Available online: https://www.greencarcongress.com/2013/06/audi-20130625.html (accessed on 19 April 2022).
- HELMETH Integrated High-Temperature ELectrolysis and METHanation for Effective Power to Gas Conversion. Project 2022. Available online: http://www.helmeth.eu/index.php/project (accessed on 19 April 2022).
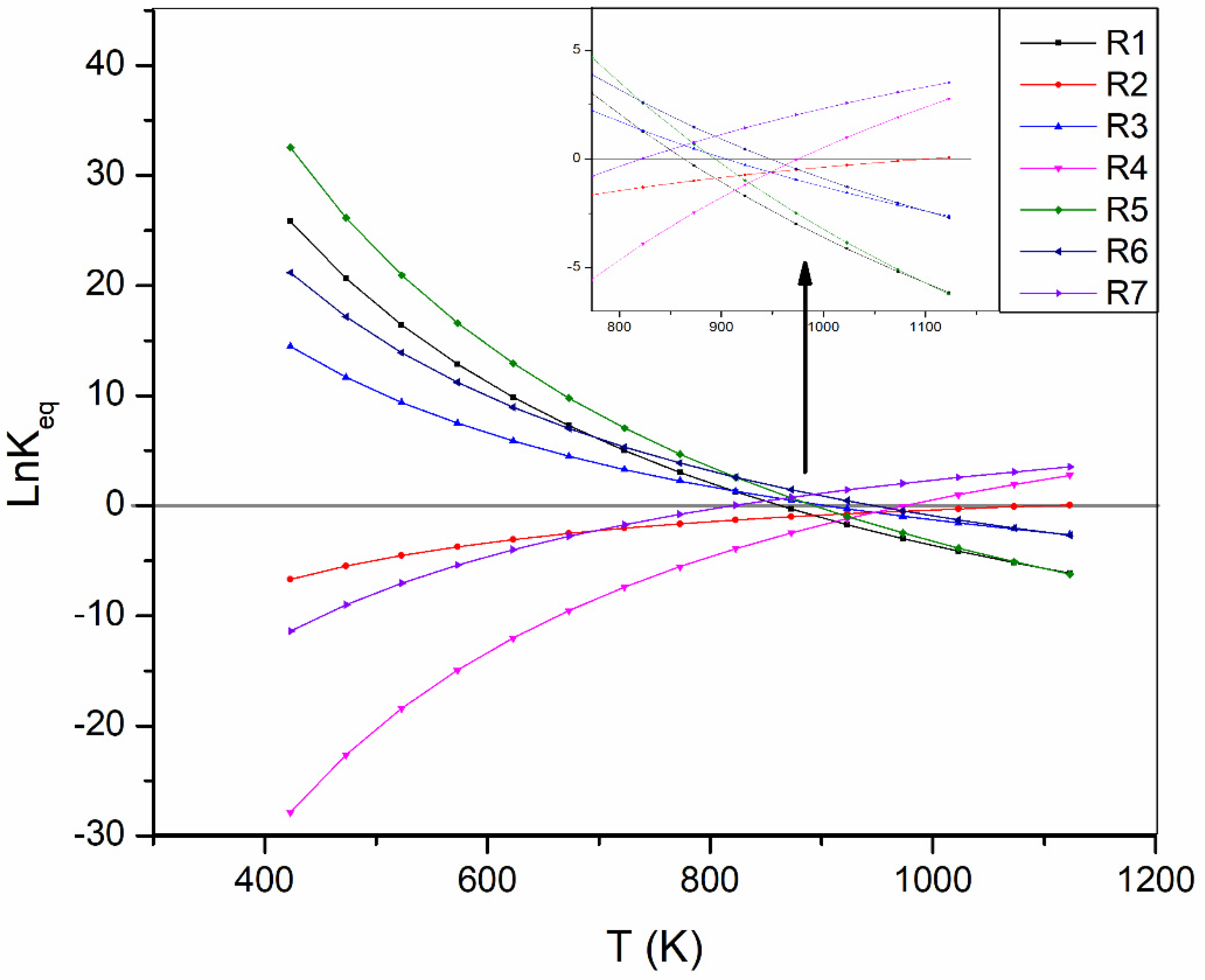



| Equation | Reaction Label | ΔH298K (kJ/mol) | ΔG298K (kJ/mol) |
|---|---|---|---|
| (1) | R1 | −165.01 | −113,618 |
| (2) | R2 | 41.16 | 28,674 |
| (3) | R3 | −90.14 | −90,143.1 |
| (4) | R4 | 172.47 | 120,153.5 |
| (5) | R5 | −206.17 | −142,292 |
| (6) | R6 | −131.3 | −91.48 |
| (7) | R7 | 77.91 | 42.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Ridzuan, N.D.; Shaharun, M.S.; Anawar, M.A.; Ud-Din, I. Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress. Catalysts 2022, 12, 469. https://doi.org/10.3390/catal12050469
Mohd Ridzuan ND, Shaharun MS, Anawar MA, Ud-Din I. Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress. Catalysts. 2022; 12(5):469. https://doi.org/10.3390/catal12050469
Chicago/Turabian StyleMohd Ridzuan, Nur Diyan, Maizatul Shima Shaharun, Mohd Azrizan Anawar, and Israf Ud-Din. 2022. "Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress" Catalysts 12, no. 5: 469. https://doi.org/10.3390/catal12050469
APA StyleMohd Ridzuan, N. D., Shaharun, M. S., Anawar, M. A., & Ud-Din, I. (2022). Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress. Catalysts, 12(5), 469. https://doi.org/10.3390/catal12050469