Transition Metal Dichalcogenides [MX2] in Photocatalytic Water Splitting
Abstract
:1. Introduction
2. Catalysts for Hydrogen Evolution
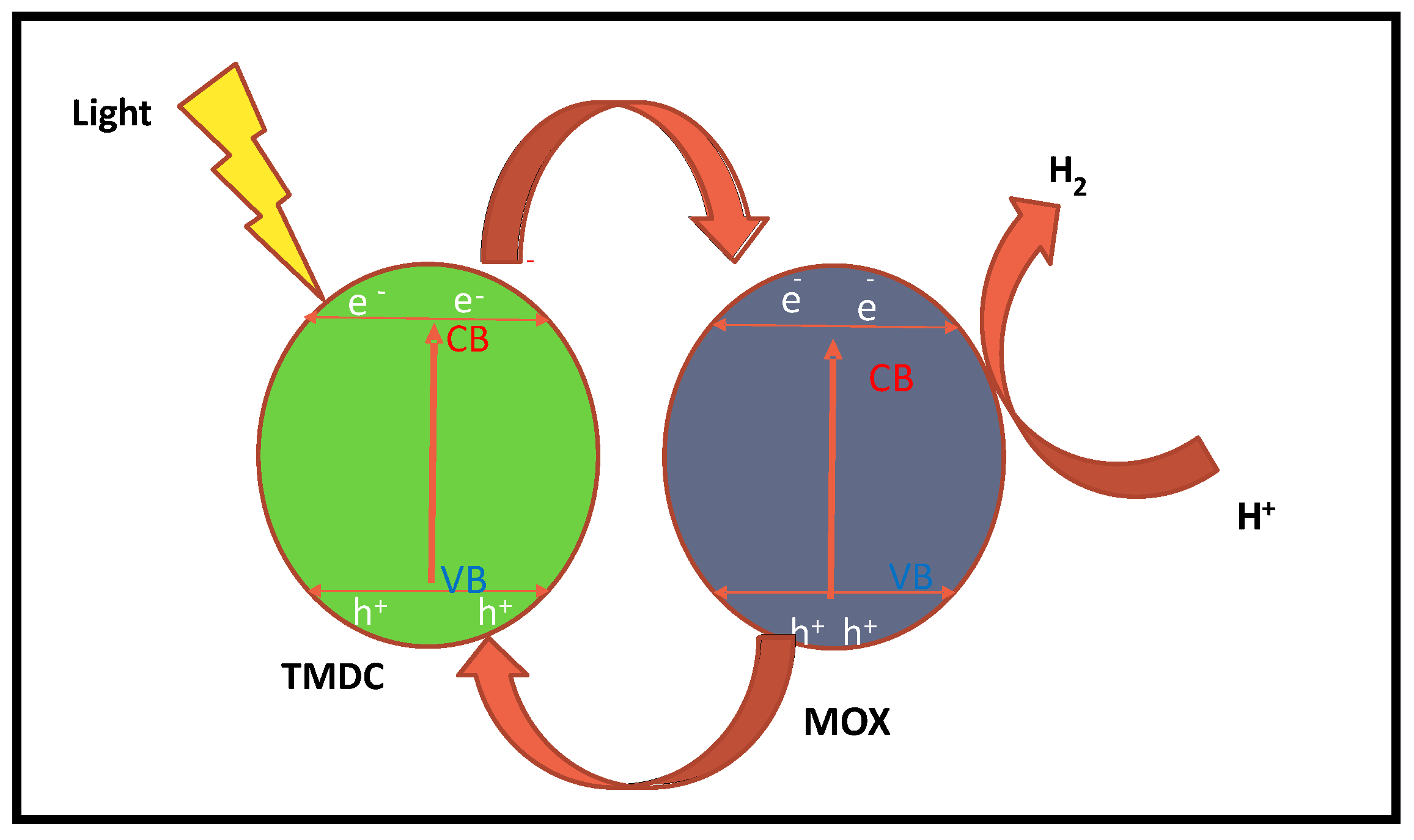
2.1. Basic Principle of Photocatalytic Water Splitting
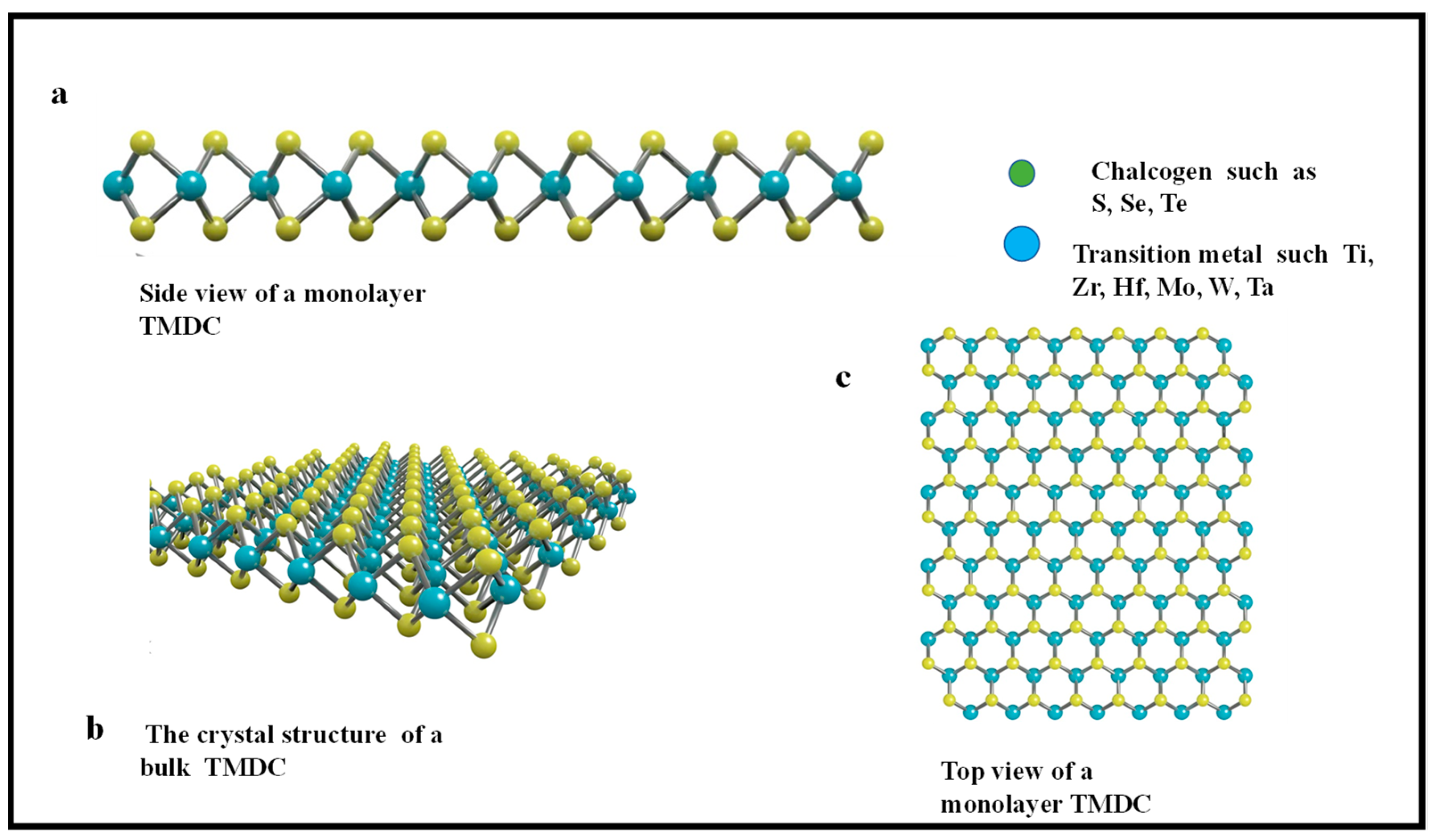
2.2. Transition Metal Dichalcogenides
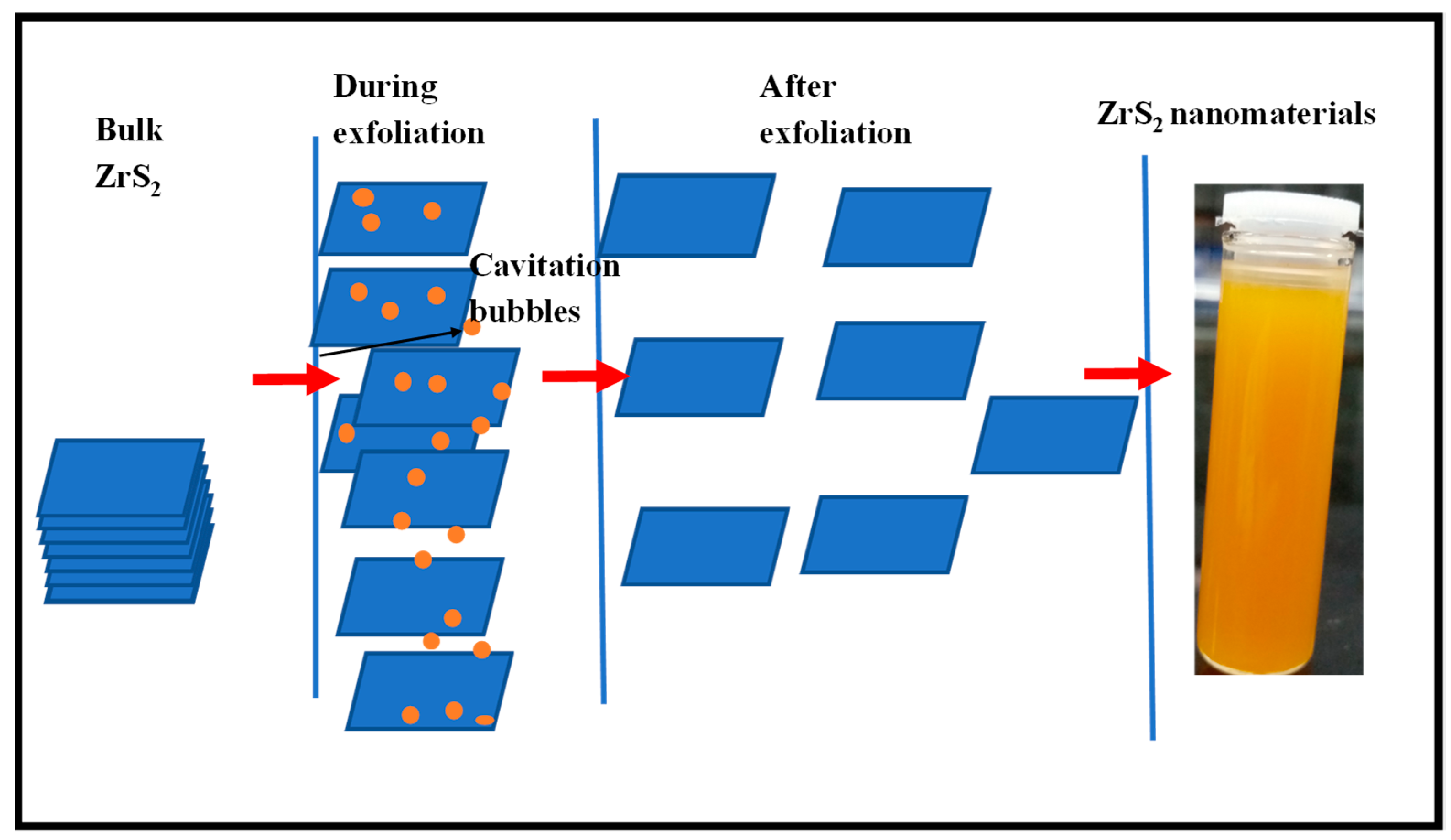
2.2.1. Exfoliation
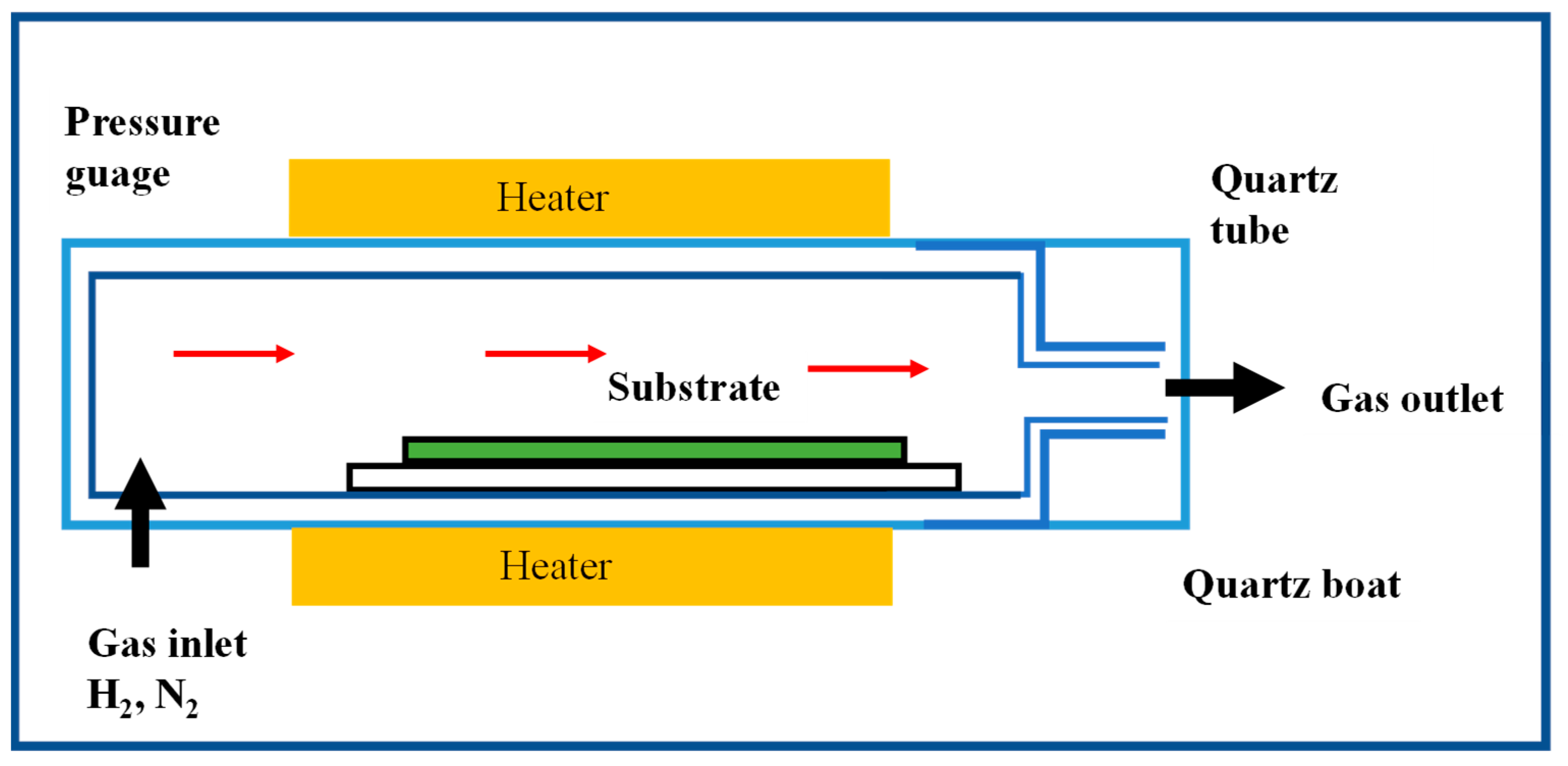
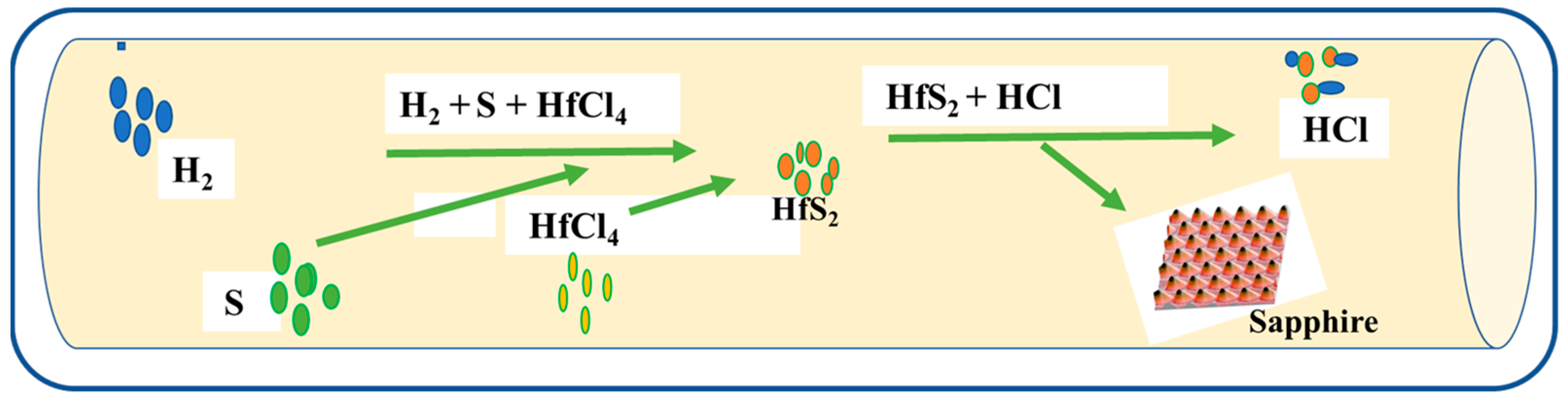
2.2.2. Chemical Vapour Deposition
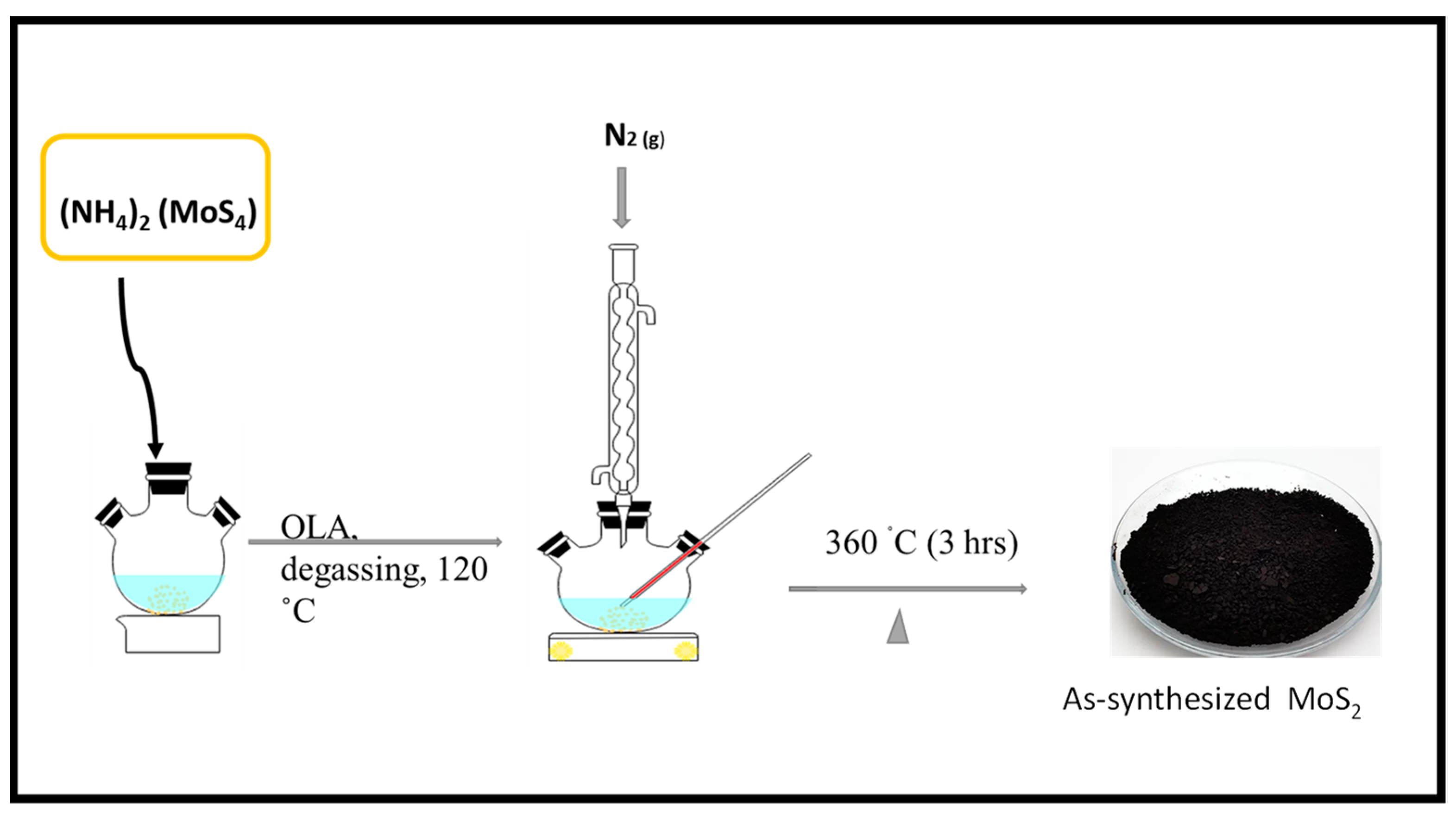
2.2.3. Wet Chemical Synthesis
- (i)
- Colloidal Synthesis
- (ii)
- Hydrothermal or solvothermal synthesis
- (iii)
- Sol-gel method
3. Application of Transition Metal Dichalcogenides in Photocatalytic Hydrogen Evolution
| Catalyst | Synthesis Method | Sacrificial Agent | Light Source | Activity | Ref. |
|---|---|---|---|---|---|
| MoS2-TiO2 | Hydrothermal | 0.35 M Na2SO3 and Na2S | Xe Lamp 300 W | 1600 µmolh−1 | [100] |
| MoS2-CaIn2S4 | Two-step hydrothermal | 0.025M Na2SO3 and Na2S | Xe Lamp 150 W | 602 µmolh−1 | [106] |
| MoS2-CdS | Sonication and stirring | Na2SO3 and Na2S | Xe Lamp 300 W | 1750 µmolh−1 | [120] |
| MoS2-CdS | Impregnation | Methanol and 10% Lactic acid | Xe Lamp 300 W | 532.8 µmolh−1 | [121] |
| MoS2-CdS | Centrifugation | Lactic acid | Xe Lamp 300 W | 259 µmolh−1 | [122] |
| WS2-CdS | Impregnation | 10% Lactic acid | Xe Lamp 300 W | 420 µmolh−1 | [123] |
| CdS-MoS2-WS2 | Hydrothermal | 10% Lactic acid | - | 209.790 µmolh−1 | [124] |
| MoS2-Zn0.2Cd0.8S | Photo deposition | 0.25 M Na2SO3 and 0.25 Na2S | Xe Lamp 300 W | 2 µmolh−1 | [125] |
| MoS2-ZnIn2S4 | Electrostatic self-assembly | Lactic acid | Xe lamp | 4974 µmolh−1 | [126] |
| WS2-ZnIn2S4 | Micro wave | Na2SO3 and Na2S | Xe lamp 150 W | 293.3 µmolh−1 | [127] |
| MoSe2-ZnInS4 | One polyol | 0.35 M Na2SO3 and 0.25 Na2S | Xe Lamp 300 W | 2228 µmolh−1 | [128] |
| MoS2-TiO2 | Hydrothermal | Methanol | Xe Lamp 350 W | 75 µmolh−1 | [129] |
| MoS2-TiO2 | Hydrothermal | 10% Lactic acid | - | 550 µmolh−1 | [130] |
| MoS2-TiO2 | Annealing and impregnation | Triethanolamine | Xe Lamp 350 W | 391.1 µmolh−1 | [131] |
| Mn-CdS-MoS2-TiO2 | Hydrothermal | Methanol | Xe Lamp 300 W | 408.370 µmolh−1 | [132] |
| MoS2-ZnO | Hydrothermal | 0.5M Na2SO4 | Xe Lamp 1000 W | 768 µmolh−1 | [133] |
| MoS2-ZnO | Hydrothermal | 0.10M Na2S | Xe Lamp 300 W | 27,690 µmolh−1 | [134] |
| MoS2-ZnO | Hydrothermal | 0.10 Na2SO4 | Xe Lamp 100 W | 145.6 µmolh−1 | [135] |
| MoS2-SnO2 | Hydrothermal | 0.10 Na2SO4 | Xe Lamp 400 W | 117.2 µmolh−1 | [136] |
| Bi2O3-MoS2 | Two steps hydrothermal | - | Xe Lamp 300 W | 10 µmolh−1 | [137] |
| MoS2-CeO2 | Hydrothermal | Methanol | - | 508.44 µmolh−1 | [138] |
| MoS2-g-C3N4 | Impregnation and sulfidation | 10% lactic acid | Xe Lamp 300 W | 108 µmolh−1 | [139] |
| WS2-g-C3N4 | Impregnation and sulfidation | 10% lactic acid | Xe Lamp 300 W | 20.6 µmolh−1 | [139] |
| MoS2-g-C3N4 | Impregnation | Na2SO4 | Xe Lamp 300 W | 23.10 µmolh−1 | [140] |
| MoS2-g-C3N4 | Sonication and treatment | Triethanolamine | Xe Lamp 300 W | 887.6 µmolh−1 | [142] |
| MoS2-C | Hydrothermal | Na2SO3 and Na2S | Xe Lamp 1000 W | 120 µmolh−1 | [143] |
| MoS2-RGO | Hydrothermal | Triethanolamine | Xe Lamp 400 W | 42,000 µmolh−1 | [147] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Commonwealth of Australia. Hydrogen for Australia’s Future. In A Briefing Paper for the COAG Energy Council; Commonwealth of Australia: Canberra, Australia, 2018. [Google Scholar]
- Burdon, R.; Palmer, G.; Chakraborty, S. National Hydrogen Strategy-Submission; Energy Transition Hub: Canberra, Australia, 2019; pp. 1–23. [Google Scholar]
- Vozniuk, O.; Tanchoux, N.; Millet, J.-M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Chapter 14—Spinel Mixed Oxides for Chemical-Loop Reforming: From Solid State to Potential Application. In Studies in Surface Science and Catalysis; Albonetti, S., Perathoner, S., Quadrelli, E.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 178, pp. 281–302. [Google Scholar]
- Ahmad, I.; Arther, S.; Khan, R. Green hydrogen production potential for developing a hydrogen economy in Pakistan. Int. J. Hydrog. Energy 2018, 43, 6011–6039. [Google Scholar]
- Kickhöfer, B.; Agarwal, A.; Nagel, K. Mind the price gap: How optimal emission pricing relates to the EU CO2 reduction targets. Int. J. Sustain. Transp. 2019, 13, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K.; Kikuchi, S. Photosensitized Electrolytic Oxidation on Semiconducting n- Type TiO2 Electrode. Kogyo Kagaku Zasshi 1969, 72, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Li, Y.; Zhang, F.; Zhang, G.; Fan, X. The Role of Two-Dimensional Transition Metal Dichalcogenides as Cocatalysts in Photocatalytic Hydrogen Evolution and Environmental Remediation. Ind. Eng. Chem. Res. 2017, 56, 4611–4626. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Pan, X.; Yang, M.-Q.; Xu, Y.-J. Constructing Ternary CdS–Graphene–TiO2 Hybrids on the Flatland of Graphene Oxide with Enhanced Visible-Light Photoactivity for Selective Transformation. J. Phys. Chem. C 2012, 116, 18023–18031. [Google Scholar] [CrossRef]
- Du, J.; Yang, M.; Zhang, F.; Cheng, X.; Wu, H.; Qin, H.; Jian, Q.; Lin, X.; Li, K.; Kang, D. Enhanced charge separation of CuS and CdS quantum-dot-cosensitized porous TiO2-based photoanodes for photoelectrochemical water splitting. J. Ceram. Int. 2018, 44, 3099–3106. [Google Scholar] [CrossRef]
- Fu, L.; Wang, F.; Wu, B.; Wu, N.; Huang, W.; Wang, H.; Jin, C.; Zhuang, L.; He, J.; Fu, L.; et al. Van der Waals Epitaxial Growth of Atomic Layered HfS2 Crystals for Ultrasensitive Near-Infrared Phototransistors. Adv. Mater. 2017, 29, 1700439–1700446. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Wang, F.; Liu, Y.; Chen, P.; Au, C.-T.; Yin, S.-F. CdS Nanowires Decorated with Ultrathin MoS2 Nanosheets as an Efficient Photocatalyst for Hydrogen Evolution. ChemSusChem 2016, 9, 624–630. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, T.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, D.; Singh, S.; Vankar, V.D.; Khare, N. ZnO nanoparticles decorated multi-walled carbon nanotubes for enhanced photocatalytic and photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2018, 351, 154–161. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts; Khan, S.B., Akhtar, K., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Gan, X.; Lei, D.; Ye, R.; Zhao, H.; Wong, K.-Y. Transition metal dichalcogenide-based mixed-dimensional heterostructures for visible-light-driven photocatalysis: Dimensionality and interface engineering. Nano Res. 2021, 14, 2003–2022. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Sumesh, C.K.; Peter, S.C. Two-dimensional semiconductor transition metal based chalcogenide based heterostructures for water splitting applications. Dalton Trans. 2019, 34, 12772–12802. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic hydrogen production: Role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Bazzo, A.; Urakawa, A. Understanding synergetic effects of Zn and Rh–Cr promotion to wide-bandgap Ga, Ta and Ti oxides in photocatalytic water splitting. Catal. Sci. Technol. 2016, 6, 4243–4253. [Google Scholar] [CrossRef]
- Panagiotis, L. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar]
- Hendi, A.H.; Osman, A.M.; Khan, I.; Saleh, T.A.; Kandiel, T.A.; Oahtan, T.F.; Hossain, M.K. Visible Light-Driven Photoelectrocatalytic Water Splitting Using Z-Scheme Ag-Decorated MoS2/RGO/NiWO4 Heterostructure. ACS Omega 2020, 5, 31644–31656. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Q.; Han, L.; Zhang, X.; Jiang, L.; Wu, Z.; Lai, Y.; Wang, D.; Liu, F. Construction of In2Se3/MoS2 heterojunction as photoanode toward efficient photoelectrochemical water splitting. Chem. Eng. J. 2019, 358, 752–758. [Google Scholar] [CrossRef]
- Lee, M.; Turan, B.; Becker, J.-P.; Welter, K.; Klingebiel, B.; Neumann, E.; Sohn, Y.J.; Merdzhanova, T.; Kirchartz, T.; Finger, F.; et al. A Bias-Free, Stand-Alone, and Scalable Photovoltaic–Electrochemical Device for Solar Hydrogen Production. Adv. Sustain. Syst. 2020, 4, 2000070. [Google Scholar] [CrossRef]
- Pehlivan, I.B.; Oscarsson, J.; Qiu, Z.; Stolt, L.; Edoff, M.; Edvinsson, T. NiMoV and NiO-based catalysts for efficient solar-driven water splitting using thermally integrated photovoltaics in a scalable approach. iScience 2021, 24, 101910. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Wang, Y.; Rui, Z. Photothermocatalytic water splitting over Pt/ZnIn2S4 for hydrogen production without external heat. Catal. Today, 2022; in press. [Google Scholar] [CrossRef]
- Jeong, S.; Yoo, D.; Jang, J.-T.; Kim, M.; Cheon, J. Well-Defined Colloidal 2-D Layered Transition-Metal Chalcogenide Nanocrystals via Generalized Synthetic Protocols. J. Am. Chem. Soc. 2012, 134, 18233–18236. [Google Scholar] [CrossRef] [PubMed]
- Tedstone, A.; Lewis, D.; O’Brien, P. Synthesis, Properties, and Applications of Transition Metal-Doped Layered Transition Metal Dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Yan, C.; Gan, L.; Zhou, X.; Guo, J.; Huang, W.; Huang, J.; Jin, B.; Xiong, J.; Zhai, T.; Li, Y. Space-Confined Chemical Vapor Deposition Synthesis of Ultrathin HfS2 Flakes for Optoelectronic Application. Adv. Funct. Mater. 2017, 27, 1702918–1702926. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.-J. Synthesis of Transition Metal Dichalcogenides. In 2D Materials: Properties and Devices; Avouris, P., Low, T., Heinz, T.F., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 344–358. [Google Scholar]
- Yan, C.; Gong, C.; Wangyang, P.; Chu, J.; Hu, K.; Li, C.; Wang, X.; Du, X.; Zhai, T.; Li, Y.; et al. 2D Group IVB Transition Metal Dichalcogenides. Adv. Funct. Mater. 2018, 28, 1803305. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Late, D.J.; Kumar, A.; Patel, S.; Singh, J. 2D layered transition metal dichalcogenides (MoS2): Synthesis, applications and theoretical aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Rahmanian, E.; Malekfar, R.; Pumera, M. Nanohybrids of Two-Dimensional Transition-Metal Dichalcogenides and Titanium Dioxide for Photocatalytic Applications. Chem. Eur. J. 2018, 24, 18–31. [Google Scholar] [CrossRef]
- Kagkoura, A.; Skaltsas, T.; Tagmatarchis, N. Transition-Metal Chalcogenide/Graphene Ensembles for Light-Induced Energy Applications. Chem. Eur. J. 2017, 23, 12967–12979. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Guo, G.; Gao, S.; Li, X.; Meng, J.; Yin, Z.; Liu, H.; Gao, M.; Cheng, L.; et al. Large-Area Synthesis of Layered HfS2(1−x)Se2x Alloys with Fully Tunable Chemical Compositions and Bandgaps. Adv. Mater. 2018, 30, e1803285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yu, N.; Wang, J.; Xue, K.-H.; Miao, X. Design lateral heterostructure of monolayer ZrS2 and HfS2 from first principles calculations. Appl. Surf. Sci. 2018, 436, 919–926. [Google Scholar] [CrossRef]
- Kang, M.; Rathi, S.; Lee, I.; Lim, D.; Wang, J.; Li, L.; Khan, M.A.; Kim, G.-H. Electrical characterization of multilayer HfSe2 field-effect transistors on SiO2 substrate. Appl. Phys. Lett. 2015, 106, 143108. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Kumar, A.; Ahuja, R. 2D-HfS2as an efficient photocatalyst for water splitting. Catal. Sci. Technol. 2016, 6, 6605–6614. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Qiu, H. Single- and few-layer ZrS2 as efficient photocatalysts for hydrogen production under visible light. Int. J. Hydrog. Energy 2015, 40, 15503–15509. [Google Scholar] [CrossRef]
- Cruz, A.; Mutlu, Z.; Ozkan, M.; Ozkan, C.S. Raman investigation of the air stability of 2H polytype HfSe2 thin films. MRS Commun. 2018, 8, 1191–1196. [Google Scholar] [CrossRef]
- Brooks, D.J.; Douthwaite, R.E.; Brydson, R.; Calvert, C.; Measures, M.G.; Watson, A. Synthesis of inorganic fullerene (MS2, M = Zr, Hf and W) phases using H2S and N2/H2 microwave-induced plasmas. Nanotechnology 2006, 17, 1245–1250. [Google Scholar] [CrossRef]
- Chae, S.H.; Jin, Y.; Kim, T.S.; Chung, D.S.; Na, H.; Nam, H.; Kim, H.; Perello, D.J.; Jeong, H.Y.; Ly, T.H.; et al. Oxidation Effect in Octahedral Hafnium Disulfide Thin Film. ACS Nano 2016, 10, 1309–1316. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Wang, F.; Huang, Y.; Wang, F.; Yin, L.; Jiang, C.; He, J. Ultrasensitive Phototransistors Based on Few-Layered HfS2. Adv. Mater. 2015, 27, 7881–7887. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, T.; Amemiya, T.; Upadhyaya, V.; Ishikawa, A.; Tsuruta, K.; Tanaka, T.; Miyamoto, Y. Performance Improvement of HfS2 Transistors by Atomic Layer Deposition of HfO2. IEEE Trans. Nanotechnol. 2017, 16, 582–587. [Google Scholar] [CrossRef]
- Li, L.; Fang, X.; Zhai, T.; Liao, M.; Gautam, U.K.; Wu, X.; Koide, Y.; Bando, Y.; Golberg, D. Electrical Transport and High-Performance Photoconductivity in Individual ZrS2 Nanobelts. Adv. Mater. 2010, 22, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, G.; McGeough, C.; Schmidt, M.; McCarthy, E.; Monaghan, S.; Povey, I.; Mccarthy, M.; Gity, F.; Nagle, R.; Hughes, G.; et al. Air sensitivity of MoS2, MoSe2, MoTe2, HfS2, and HfSe2. J. App. Phys. 2016, 120, 125102. [Google Scholar] [CrossRef] [Green Version]
- Mattinen, M.; Popov, G.; Vehkamäki, M.; King, P.J.; Mizohata, K.; Jalkanen, P.; Räisänen, J.; Leskelä, M.; Ritala, M. Atomic Layer Deposition of Emerging 2D Semiconductors, HfS2 and ZrS2, for Optoelectronics. Chem. Mater. 2019, 31, 5713–5724. [Google Scholar] [CrossRef] [Green Version]
- Mleczko, M.J.; Zhang, C.; Lee, H.R.; Kuo, H.-H.; Magyari-Köpe, B.; Moore, R.G.; Shen, Z.-X.; Fisher, I.R.; Nishi, Y.; Pop, E. HfSe2 and ZrSe2: Two-dimensional semiconductors with native high-κ oxides. Sci. Adv. 2017, 3, e1700481. [Google Scholar] [CrossRef] [Green Version]
- Mañas-Valero, S.; García-López, V.; Cantarero, A.; Galbiati, M. Raman Spectra of ZrS2 and ZrSe2 from Bulk to Atomically Thin Layers. Appl. Sci. 2016, 6, 264. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, T.; Amemiya, T.; Ishikawa, A.; Upadhyaya, V.; Tsuruta, K.; Tanaka, T.; Miyamoto, Y. Few-layer HfS2 transistors. Sci. Rep. 2016, 6, 22277. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Grayfer, E.D.; Kozlova, M.N.; Fedorov, V. Colloidal 2D nanosheets of MoS2 and other transition metal dichalcogenides through liquid-phase exfoliation. Adv. Colloid Interface Sci. 2017, 245, 40–61. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Sharda, K.; Grotta, C.; Ranalli, J.; Sokolikova, M.S.; Pesci, F.M.; Palczynski, P.; Bemmer, V.L.; Mattevi, C. Thickness-Dependent Characterization of Chemically Exfoliated TiS2 Nanosheets. ACS Omega 2018, 3, 8655–8662. [Google Scholar]
- Jana, M.K.; Rao, C.N.R. Transition Metal Dichalcogenides and Other Layered Materials. In 2D Inorganic Materials beyond Graphene; World Scientific: Singapore, 2017; pp. 1–65. [Google Scholar]
- Han, J.H.; Kwak, M.; Kim, Y.; Cheon, J. Recent Advances in the Solution-Based Preparation of Two-Dimensional Layered Transition Metal Chalcogenide Nanostructures. Chem. Rev. 2018, 118, 6151–6188. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Jung, H.; Huh, J.-S.; Yu, J.-B.; Yang, W.-C. Efficient exfoliation of MoS2 with volatile solvents and their application for humidity sensor. J. Nanosci. Nanotechnol. 2014, 14, 8518–8522. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Yadav, S.; Srivastava, A.K.; Singh, N.; Rath, S.; Schneider, J.J.; Sinha, O.P. High-yield synthesis and liquid-exfoliation of two-dimensional belt-like hafnium disulphide. Nano Res. 2017, 11, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-Layer Semiconducting Nanosheets: High-Yield Preparation and Device Fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sun, T.; Zhu, J.; Huang, X.; Yin, Z.; Lu, G.; Fan, Z.; Yan, Q.; Hng, H.H.; Zhang, H. An Effective Method for the Fabrication of Few-Layer-Thick Inorganic Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 9052–9056. [Google Scholar] [CrossRef]
- Jeong, S.; Yoo, D.; Ahn, M.; Miró, P.; Heine, T.; Cheon, J. Tandem intercalation strategy for single-layer nanosheets as an effective alternative to conventional exfoliation processes. Nat. Commun. 2015, 6, 5763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-Processed Two-Dimensional MoS2 Nanosheets: Preparation, Hybridization, and Applications. Angew. Chem. Int. 2016, 55, 8816–8838. [Google Scholar] [CrossRef]
- O’Brien, M.; McEvoy, N.; Hanlon, D.; Hallam, T.; Coleman, J.; Duesberg, G.S. Mapping of Low-Frequency Raman Modes in CVD-Grown Transition Metal Dichalcogenides: Layer Number, Stacking Orientation and Resonant Effects. Sci. Rep. 2016, 6, 19476. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Y.; Wang, X.; Feng, Q.; Qiao, S.; Wen, W.; Chen, Y.; Cui, M.; Zhang, J.; Cai, C.; et al. Controlled Synthesis of ZrS2 Monolayer and Few Layers on Hexagonal Boron Nitride. J. Am. Chem. Soc. 2015, 137, 7051–7054. [Google Scholar] [CrossRef]
- Shimazu, Y.; Fujisawa, Y.; Arai, K.; Iwabuchi, T.; Suzuki, K. Front Cover: Synthesis and Characterization of Zirconium Disulfide Single Crystals and Thin-Film Transistors Based on Multilayer Zirconium Disulfide Flakes (ChemNanoMat 10/2018). ChemNanoMat 2018, 4, 1021. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Fu, Y.; Wang, Y.; Feng, Y.; Liu, H.; Wan, X.; Zhou, W.; Wang, B.; Shao, L.; Ho, C.-H.; et al. Integrated digital inverters based on two-dimensional anisotropic ReS2 field-effect transistors. Nat. Commun. 2015, 6, 6991. [Google Scholar] [CrossRef]
- Pawbake, A.S.; Pawar, M.S.; Jadkar, S.R.; Late, D.J. Large area chemical vapor deposition of monolayer transition metal dichalcogenides and their temperature dependent Raman spectroscopy studies. Nanoscale 2016, 8, 3008–3018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, P.; Hong, M.; Jiang, S.; Zhao, G.; Shi, J.; Xie, Q.; Zhang, Y. Recent progress in the controlled synthesis of 2D metallic transition metal dichalcogenides. Nanotechnology 2019, 30, 182002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Wang, X.; Zhang, M.; Cai, C.; Xie, L. Thickness and temperature dependent electrical properties of ZrS2 thin films directly grown on hexagonal boron nitride. Nano Res. 2016, 9, 2931–2937. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, S. Low temperature synthesis of ZrS2 nanoflakes and their catalytic activity. RSC Adv. 2015, 5, 66082–66085. [Google Scholar] [CrossRef]
- Muhammad, Z.; Mu, K.; Lv, H.; Wu, C.; Rehman, Z.U.; Habib, M.; Sun, Z.; Wu, X.; Song, L. Electron doping induced semiconductor to metal transitions in ZrSe2 layers via copper atomic intercalation. Nano Res. 2018, 11, 4914–4922. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Hossain, D.; Luo, Z. Synthesis of 2D transition metal dichalcogenides by chemical vapor deposition with controlled layer number and morphology. Nano Converg. 2018, 5, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Tenne, R. Advances in the Synthesis of Inorganic Nanotubes and Fullerene-Like Nanoparticles. Angew. Chem. Int. Ed. 2003, 42, 5124–5132. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, Z.; Qi, F.; Wang, X.; Yu, B.; Zhang, W.; Chen, Y. CVD growth of large-area and high-quality HfS2 nanoforest on diverse substrates. Appl. Surf. Sci. 2018, 435, 563–567. [Google Scholar] [CrossRef]
- Gao, Z.; Ji, Q.; Shen, P.-C.; Han, Y.; Leong, W.S.; Mao, N.; Zhou, L.; Su, C.; Niu, J.; Ji, X.; et al. In Situ-Generated Volatile Precursor for CVD Growth of a Semimetallic 2D Dichalcogenide. ACS Appl. Mater. Interfaces 2018, 10, 34401–34408. [Google Scholar] [CrossRef] [Green Version]
- Parvaz, M.; Ahmed, S.; Khan, M.; Johari, R.; Ahmad, S.; Khan, Z. Synthesis of TiS2 nanodiscs for supercapacitor application. AIP Conf. Proc. 2018, 1953, 030121. [Google Scholar]
- Hu, Z.; Tai, Z.; Liu, Q.; Wang, S.; Jin, H.; Wang, S.; Lai, W.-H.; Chen, M.; Li, L.; Chen, L.; et al. Ultrathin 2D TiS2 Nanosheets for High Capacity and Long-Life Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803210. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Wet-chemical synthesis and applications of non-layer structured two-dimensional nanomaterials. Nat. Commun. 2015, 6, 7873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, A.; Semagina, N. Colloidal Synthesis Protocol of Shape- and Dimensionally-Controlled Transition-Metal Chalcogenides and Their Hydrodesulfurization Activities. ACS Appl. Nano Mater. 2018, 1, 4408–4412. [Google Scholar] [CrossRef]
- Razgoniaeva, N.; Yang, M.; Garrett, P.; Kholmicheva, N.; Moroz, P.; Eckard, H.; Romero, L.R.; Porotnikov, D.; Khon, D.; Zamkov, M. Just Add Ligands: Self-Sustained Size Focusing of Colloidal Semiconductor Nanocrystals. Chem. Mater. 2018, 30, 1391–1398. [Google Scholar] [CrossRef]
- Gqoba, S.; Airo, M.; Ntholeng, N.; Machogo, L.; Sithole, R.; Moloto, M.J.; van Wyk, J.; Moloto, N. Evolution of In2S3 Nanoplates with Time. Mater. Today Proc. 2015, 2, 3901–3908. [Google Scholar] [CrossRef]
- van Embden, J.; Chesman, A.S.R.; Jasieniak, J.J. The Heat-Up Synthesis of Colloidal Nanocrystals. Chem. Mater. 2015, 27, 2246–2285. [Google Scholar] [CrossRef]
- Qiao, L.; Swihart, M.T. Solution-phase synthesis of transition metal oxide nanocrystals: Morphologies, formulae, and mechanisms. Adv. Colloid Interface Sci. 2017, 244, 199–266. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, J.; Li, J.; Wang, R.; Huang, H.; Su, B.; Lei, Z. Highly Efficient Photocatalytic Hydrogen Production of Flower-like Cadmium Sulfide Decorated by Histidine. Sci. Rep. 2015, 5, 13593. [Google Scholar] [CrossRef] [Green Version]
- Prabakar, S.; Bumby, C.; Tilley, R. Liquid-Phase Synthesis of Flower-like and Flake-like Titanium Disulfide Nanostructures. Chem. Mater. 2009, 21, 1725–1730. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, J.; Kim, H.J.; Oh, D.; Ahn, J.R.; Son, S.U. Unstable Single-Layered Colloidal TiS2Nanodisks. Small 2008, 4, 945–950. [Google Scholar] [CrossRef]
- Jang, J.-T.; Jeong, S.; Seo, J.-W.; Kim, M.-C.; Sim, E.; Oh, Y.; Nam, S.; Park, B.; Cheon, J. Ultrathin Zirconium Disulfide Nanodiscs. J. Am. Chem. Soc. 2011, 133, 7636–7639. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Kim, M.; Jeong, S.; Han, J.; Cheon, J. Chemical Synthetic Strategy for Single-Layer Transition-Metal Chalcogenides. J. Am. Chem. Soc. 2014, 136, 14670–14673. [Google Scholar] [CrossRef]
- Sekar, P.; Greyson, E.C.; Barton, J.E.; Odom, T.W. Synthesis of Nanoscale NbSe2 Materials from Molecular Precursors. J. Am. Chem. Soc. 2005, 127, 2054–2055. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kim, H.K.; Baek, B.; Han, J.; Ahn, H.S.; Baik, M.-H.; Cheon, J. Activation of the Basal Plane in Two Dimensional Transition Metal Chalcogenide Nanostructures. J. Am. Chem. Soc. 2018, 140, 13663–13671. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Zhu, J.; Zhang, C.; Liu, T. Self-Templated Growth of Vertically Aligned 2H-1T MoS2 for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 31702–31708. [Google Scholar] [CrossRef] [PubMed]
- Kiriya, D.; Lobaccaro, P.; Nyein, H.Y.Y.; Taheri, P.; Hettick, M.; Shiraki, H.; Sutter-Fella, C.M.; Zhao, P.; Gao, W.; Maboudian, R.; et al. General Thermal Texturization Process of MoS2 for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 4047–4053. [Google Scholar] [CrossRef]
- Mahler, B.; Hoepfner, V.; Liao, K.; Ozin, G.A. Colloidal Synthesis of 1T-WS2 and 2H-WS2 Nanosheets: Applications for Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 14121–14127. [Google Scholar] [CrossRef]
- Geisenhoff, J.Q.; Tamura, A.K.; Schimpf, A.M. Using ligands to control reactivity, size and phase in the colloidal synthesis of WSe2 nanocrystals. Chem. Commun. 2019, 55, 8856–8859. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Wu, J.; Xu, Z.; Huang, Y.; Zhang, C. Colloidal synthesis of MoS2 quantum dots: Size-dependent tunable photoluminescence and bioimaging. New J. Chem. 2015, 39, 8492–8497. [Google Scholar] [CrossRef]
- Altavilla, C.; Sarno, M.; Ciambelli, P. A Novel Wet Chemistry Approach for the Synthesis of Hybrid 2D Free-Floating Single or Multilayer Nanosheets of MS2@oleylamine (M=Mo, W). Chem. Mater. 2011, 23, 3879–3885. [Google Scholar] [CrossRef]
- Antunez, P.D.; Webber, D.H.; Brutchey, R.L. Solution-Phase Synthesis of Highly Conductive Tungsten Diselenide Nanosheets. Chem. Mater. 2013, 25, 2385–2387. [Google Scholar] [CrossRef]
- Jung, W.; Lee, S.; Yoo, D.; Jeong, S.; Miró, P.; Kuc, A.; Heine, T.; Cheon, J. Colloidal Synthesis of Single-Layer MSe2 (M = Mo, W) Nanosheets via Anisotropic Solution-Phase Growth Approach. J. Am. Chem. Soc. 2015, 137, 7266–7269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of Few-Layer MoS2Nanosheet-Coated TiO2Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef]
- Rao, G.; Mukherjee, D.; Reddy, B. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy: Synthesis, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–36. ISBN 978-0-323-46142-9. [Google Scholar]
- Chen; Fan, R. Low-Temperature Hydrothermal Synthesis of Transition Metal Dichalcogenides. Chem. Mater. 2001, 13, 802–805. [Google Scholar] [CrossRef]
- Rajamathi, M.; Seshadri, R. Oxide and chalcogenide nanoparticles from hydrothermal/solvothermal reactions. Curr. Opin. Solid State Mater. Sci. 2003, 6, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wang, S.; Run, Z.; Zhang, G.; Du, W.; Pang, H. Hydrothermal Synthesis of Nickel Phosphate Nanorods for High-Performance Flexible Asymmetric All-Solid-State Supercapacitors. Part. Part. Syst. Charact. 2015, 32, 880–885. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Shi, G.-W.; Liu, Y.-M. Hydrothermal synthesis of molybdenum disulfide nanosheets as supercapacitors electrode material. Electrochim. Acta 2014, 132, 397–403. [Google Scholar] [CrossRef]
- Swain, G.; Sultana, S.; Moma, J.; Parida, K. Fabrication of Hierarchical Two-Dimensional MoS2 Nanoflowers Decorated upon Cubic CaIn2S4 Microflowers: Facile Approach to Construct Novel Metal-Free p–n Heterojunction Semiconductors with Superior Charge Separation Efficiency. Inorg. Chem. 2018, 57, 10059–10071. [Google Scholar] [CrossRef]
- Jang, J.W.; Kim, H.G.; Joshi, U.A.; Lee, J.S. Fabrication of CdS nanowires decorated with TiO2 nanoparticles for photocatalytic hydrogen production under visible light irradiation. Int. J. Hydrog. Energy 2008, 33, 5975–5980. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 2015, 7, 1806–1811. [Google Scholar]
- Therese, H.A.; Li, J.; Kolb, U.; Tremel, W. Facile large scale synthesis of WS2 nanotubes from WO3 nanorods prepared by a hydrothermal route. Solid State Sci. 2005, 7, 67–72. [Google Scholar] [CrossRef]
- Zheng, W.; Lin, J.; Feng, W.; Xiao, K.; Qiu, Y.; Chen, X.; Liu, G.; Cao, W.; Pantelides, S.T.; Zhou, W.; et al. Patterned Growth of P-Type MoS2 Atomic Layers Using Sol–Gel as Precursor. Adv. Funct. Mater. 2016, 26, 6371–6379. [Google Scholar] [CrossRef]
- Marco Vittorio, N.; Timpel, M.; Ligorio, G.; Morales, N.; Chiappini, A.; Toccoli, T.; Verucchi, R.; Ceccato, R.; Pasquali, L.; List-Kratochvil, E.; et al. Versatile and Scalable Strategy To Grow Sol–Gel Derived 2H-MoS2 Thin Films with Superior Electronic Properties: A Memristive Case. ACS Appl. Mater. Interfaces 2018, 10, 34392–34400. [Google Scholar]
- Firmiano, E.G.S.; Cordeiro, M.A.L.; Rabelo, A.C.; Dalmaschio, C.J.; Pinheiro, A.N.; Pereira, E.C.; Leite, E.R. Graphene oxide as a highly selective substrate to synthesize a layered MoS2 hybrid electrocatalyst. Chem. Commun. 2012, 48, 7687–7689. [Google Scholar] [CrossRef] [PubMed]
- Ramsha, K.; Adeel, R.; Sofia, J.; Rahim, J.; Muhammad, A.A.; Mohammad, M. Synthesis and characterization of MoS2/TiO2 nanocomposites for enhanced photocatalytic degradation of methylene blue under sunlight irradiation. In Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2018; Volume 778, pp. 137–143. [Google Scholar]
- Guo, X.; Yin, P.; Wang, Z.; Yang, H. Template-assisted sol–gel synthesis of porous MoS2/C nanocomposites as anode materialsfor lithium-ion batteries. J. Sol-Gel Sci. Technol. 2017, 85, 140–148. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Anandhavelu, S.; Vikraman, D. Facile Methodology of Sol-Gel Synthesis for Metal Oxide Nanostructures. In Recent Applications in Sol-Gel Syntheses; Books on Demand: Norderstedt, Germany, 2017; ISBN 978-953-51-3245-5. [Google Scholar]
- Rosman, N.N.; Yunus, R.M.; Minggu, L.J.; Arifin, K.; Salehmin, M.N.I.; Mohamed, M.A.; Kassim, M.B. Photocatalytic properties of two-dimensional graphene and layered transition-metal dichalcogenides based photocatalyst for photoelectrochemical hydrogen generation: An overview. Int. J. Hydrog. Energy 2018, 43, 18925–18945. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Peng, W. 2D Transition Metal Dichalcogenides and Graphene-Based Ternary Composites for Photocatalytic Hydrogen Evolution and Pollutants Degradation. Nanomaterials 2017, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic.and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2015, 28, 1917–1933. [Google Scholar] [CrossRef]
- Li, Y.; Kang, J.; Li, J. Indirect-to-direct band gap transition of the ZrS2 monolayer by strain: First-principles calculations. RSC Adv. 2014, 4, 7396–7401. [Google Scholar] [CrossRef]
- Ma, S.; Xie, J.; Wen, J.; He, K.; Li, X.; Liu, W.; Zhang, X. Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2016, 391, 580–591. [Google Scholar] [CrossRef]
- Chang, K.; Li, M.; Wang, T.; Ouyang, S.; Li, P.; Liu, L.; Ye, J. Drastic Layer-Number-Dependent Activity Enhancement in Photocatalytic H2 Evolution over nMoS2/CdS (n ≥ 1) Under Visible Light. Adv. Energy Mater. 2015, 10, 1402279. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of Photocatalytic H2 Evolution on CdS by Loading MoS2 as Cocatalyst under Visible Light Irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 Evolution on CdS Loaded with WS2 as Cocatalyst under Visible Light Irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2Ultrathin Nanosheets Integrated on CdS Nanorods to Promote Charge Separation and Migration and Improve Solar-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 1563–1570. [Google Scholar] [CrossRef]
- Nguyen, M.; Tran, P.D.; Pramana, S.S.; Lee, R.L.; Batabyal, S.K.; Mathews, N.; Wong, L.H.; Graetzel, M. In situ photo-assisted deposition of MoS2 electrocatalyst onto zinc cadmium sulphide nanoparticle surfaces to construct an efficient photocatalyst for hydrogeneration. Nanoscale 2013, 5, 1479–1482. [Google Scholar] [CrossRef]
- Huang, L.; Han, B.; Huang, X.; Liang, S.; Deng, Z.; Chen, W.; Peng, M.; Deng, H. Ultrathin 2D/2D ZnIn2S4/MoS2 hybrids for boosted photocatalytic hydrogen evolution under visible light. J. Alloys Compd. 2019, 798, 553–559. [Google Scholar] [CrossRef]
- Pudkon, W.; Kaowphong, S.; Pattisson, S.; Miedziak, P.J.; Bahruji, H.; Davies, E.D.; Morgan, D.J.; Hutchings, G.J. Microwave synthesis of ZnIn2S4/WS2 composites for photocatalytic hydrogen production and hexavalent chromium reduction. Catal Sci. Technol. 2019, 20, 5698–5711. [Google Scholar] [CrossRef]
- Zeng, D.; Xiao, L.; Ong, W.J.; Wu, P.; Zheng, H.; Chen, Y.; Peng, D.L. Hierarchical ZnIn2S4/MoSe2 Nanoarchitectures for Efficient Noble-Metal-Free Photocatalytic Hydrogen Evolution under Visible Light. ChemSusChem 2017, 10, 4624–4631. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, Y.; Chen, G.; Sang, Y.; Jiang, H.; He, J.; Li, X.; Liu, H. Few-layered MoS2 nanosheets wrapped ultrafine TiO2 nanobelts with enhanced photocatalytic property. Nanoscale 2016, 8, 6101–6109. [Google Scholar] [CrossRef]
- Zhang, P.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Efficient charge separation on 3D architectures of TiO2 mesocrystals packed with a chemically exfoliated MoS2 shell in synergetic hydrogen evolution. Chem. Commun. 2015, 51, 7187–7190. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, J.; Xiao, W.; Wang, J.; Li, C. The MoS2 Quantum Modified Hollow TiO2 Nano-Heterojunction for Enhanced Hydrogen Evolution. IOP Conf. Ser. Earth Environ. Sci. 2018, 189, 032042. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, W.; Zhang, X.; Zhang, S.; Liu, B.; Zhen, D. Synthesis of Z-scheme Mn-CdS/MoS2/TiO2 ternary photocatalysts for high-efficiency sunlight-driven photocatalysis. Adv. Compos. Lett. 2019, 28. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.-J.; Wang, F.; Hu, B.; Lu, H.-W.; Yu, Z.-T.; Zou, Z.-G. Significant enhancement in photocatalytic hydrogen evolution from water using a MoS2 nanosheet-coated ZnO heterostructure photocatalyst. Dalton Trans. 2015, 44, 10997–11003. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, W.; Zhang, J.; Xi, J.; Du, G.; Ji, Z. MoS2 nanosheet/ZnO nanowire hybrid nanostructures for photoelectrochemical water splitting. J. Am. Ceram. Soc. 2018, 101, 3989–3996. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, Y.W.; Lu, M.Y. Construction of MoS2/ZnO heterostructures as highly efficient photocatalysts for enhanced visible-light decomposition of methylene blue and hydrogen evolution. Mater. Chem. Phys. 2021, 266, 124560. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, Y.W. MoS2@SnO2 core-shell sub-microspheres for high efficient visible-light photodegradation and photocatlytic hydrogen production. Mater. Res. Bull. 2020, 129, 110912. [Google Scholar] [CrossRef]
- Khalid, N.R.; Israr, Z.; Tahir, M.B.; Iqbal, T.H. Highly efficient Bi2O3/MoS2 p-n heterojunction photocatalyst for H2 evolution from water splitting. Int. J. Hydrog. Energy 2020, 15, 8479–8489. [Google Scholar] [CrossRef]
- Swain, G.; Sultana, S.; Naik, B.; Parida, K. Coupling of Crumpled-Type Novel MoS2 with CeO2 Nanoparticles: A Noble-Metal-Free p–n Heterojunction Composite for Visible Light Photocatalytic H2 Production. ACS Omega 2017, 2, 3745–3753. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Laursen, A.B.; Zhang, J.; Zhang, G.; Zhu, Y.; Wang, X.; Dahl, S.; Chorkendorff, I. Layered Nanojunctions for Hydrogen Evolution Catalysis. Angew. Chem. Int. Ed. 2013, 52, 3621–3625. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L. Synthesis and characterization of composite visible light active photocatalysts MoS2–g-C3N4 with enhanced hydrogen evolution activity. Int. J. Hydrog. Energy 2013, 38, 6960–6969. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Z.; Huang, J.; Li, Q.; Yang, J. Enhanced photocatalytic mechanism for the hybrid g-C3N4/MoS2 nanocomposite. J. Mater. Chem. A 2014, 2, 7960–7966. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zhang, J.; Zhang, H.; Tian, W.; Li, X.; Tade, M.; Sun, H.; Wang, S. Flower-like MoS2 on graphitic carbon nitride for enhanced photocatalytic and electrochemical hydrogen evolutions. Appl. Catal. B Environ. 2018, 239, 334–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Gao, W.; Chen, P.; Cui, H.; Fan, Y.; Shi, X.; Zhao, Y.; Cui, G.; Tang, B. MoS2 Nanosheets Assembled on Three-Way Nitrogen-Doped Carbon Tubes for Photocatalytic Water Splitting. Front. Chem. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Rao, C.N.R. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Kochuveedu, S.T. Photocatalytic and Photoelectrochemical Water Splitting on TiO2 via Photosensitization. J. Nanomater. 2016, 10, 4073142. [Google Scholar]
- Zong, X.; Na, Y.; Wen, F.; Ma, G.; Yang, J.; Wang, D.; Ma, Y.; Wang, M.; Sun, L.; Li, C. Visible light driven H2 production in molecular systems employing colloidal MoS2 nanoparticles as catalyst. Chem. Commun. 2009, 30, 4536–4538. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Lu, G. Sites for High Efficient Photocatalytic Hydrogen Evolution on a Limited-Layered MoS2 Cocatalyst Confined on Graphene Sheets―The Role of Graphene. Phys. Chem. C 2012, 48, 25415–25424. [Google Scholar] [CrossRef]
- Uttam, G.; Naidu, B.S.; Maitra, U.; Singh, A.; Shirodkar, S.N.; Waghmare, U.V.; Rao, C.N. Characterization of few-layer 1T-MoSe2 and its superior performance in the visible-light induced hydrogen evolution reaction. APL Mater. 2014, 9, 4892976. [Google Scholar]
- Rao, T.; Wang, H.; Zeng, Y.; Guo, Z.; Zhang, H.; Liao, W. Phase Transitions and Water Splitting Applications of 2D Transition Metal Dichalcogenides and Metal Phosphorous Trichalcogenides. Adv. Sci. 2021, 8, 2002284. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadojutimi, P.O.; Gqoba, S.S.; Tetana, Z.N.; Moma, J. Transition Metal Dichalcogenides [MX2] in Photocatalytic Water Splitting. Catalysts 2022, 12, 468. https://doi.org/10.3390/catal12050468
Fadojutimi PO, Gqoba SS, Tetana ZN, Moma J. Transition Metal Dichalcogenides [MX2] in Photocatalytic Water Splitting. Catalysts. 2022; 12(5):468. https://doi.org/10.3390/catal12050468
Chicago/Turabian StyleFadojutimi, Paul O., Siziwe S. Gqoba, Zikhona N. Tetana, and John Moma. 2022. "Transition Metal Dichalcogenides [MX2] in Photocatalytic Water Splitting" Catalysts 12, no. 5: 468. https://doi.org/10.3390/catal12050468
APA StyleFadojutimi, P. O., Gqoba, S. S., Tetana, Z. N., & Moma, J. (2022). Transition Metal Dichalcogenides [MX2] in Photocatalytic Water Splitting. Catalysts, 12(5), 468. https://doi.org/10.3390/catal12050468








