Impact of Black Body Material Enhanced Gas Movement on CO2 Photocatalytic Reduction Performance
Abstract
:1. Introduction
2. Results and Discussion
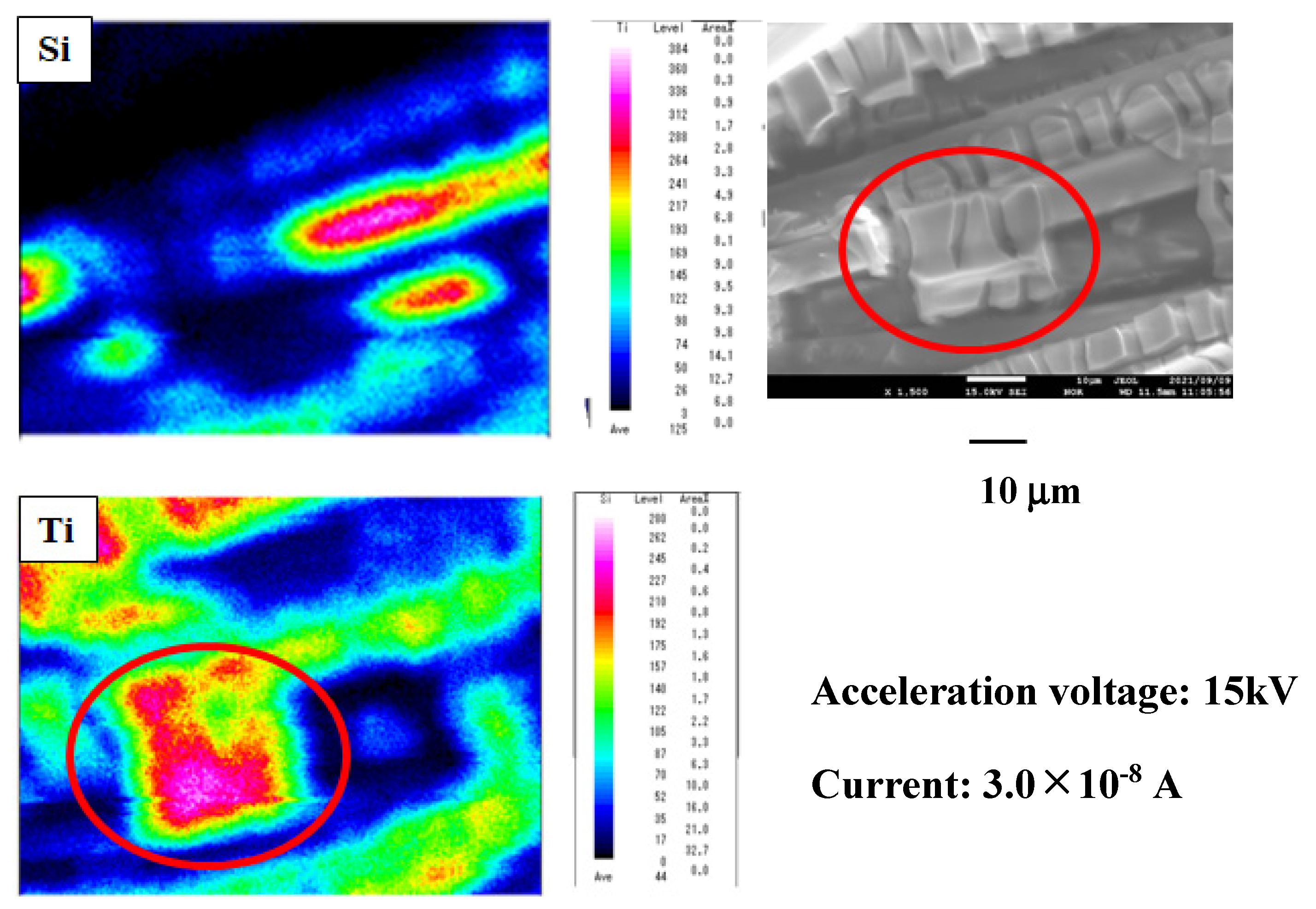
2.1. The Characterization of TiO2 Film
2.2. The CO2 Reduction Performance with and without Black Body Material
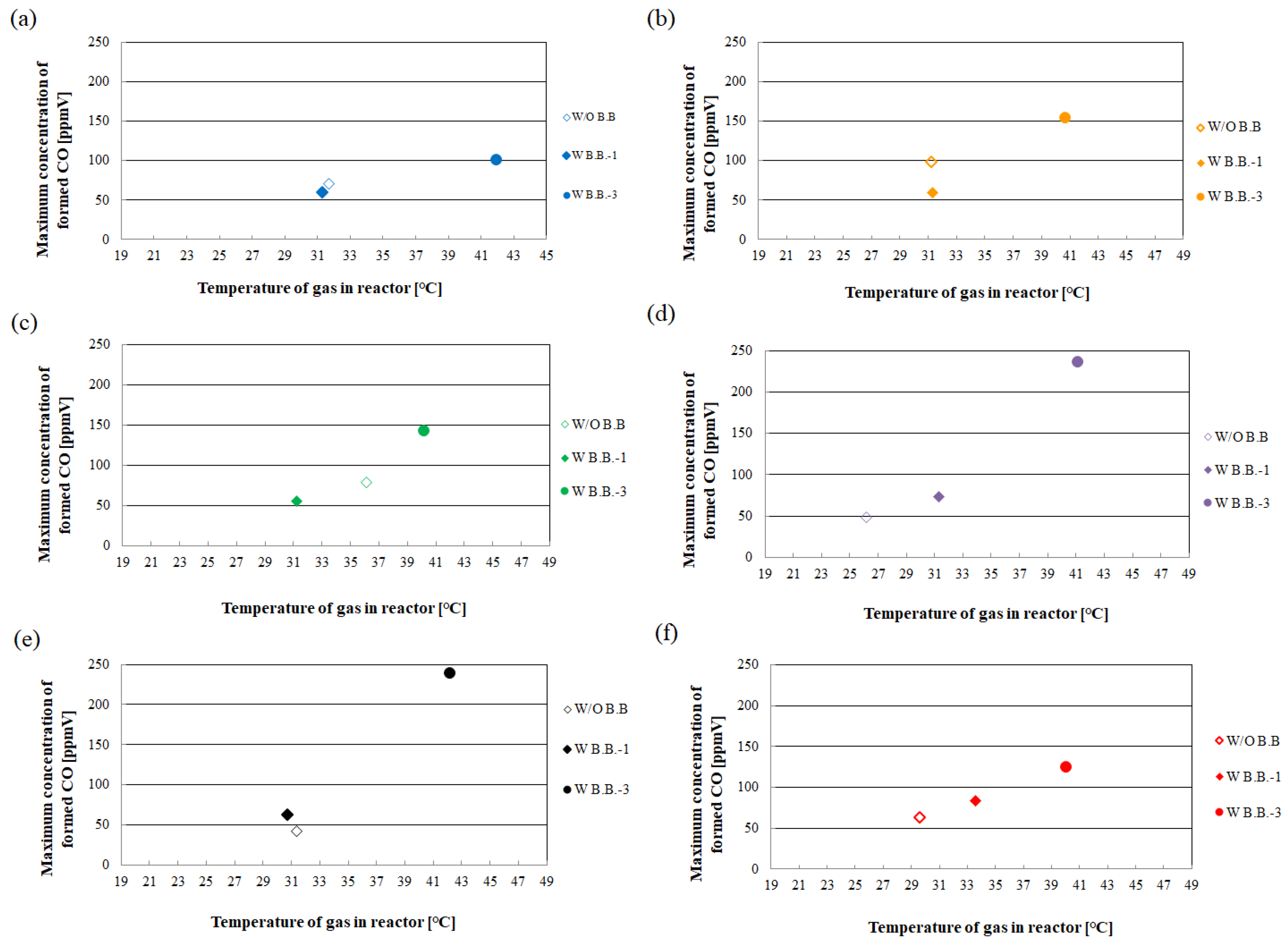
2.3. Relationship between Temperature of Gas in Reactor and Concentration of Formed CO
3. Experiments
3.1. The Preparation Procedure of TiO2 Film
3.2. The Preparation Procedure of Black Body Material
3.3. The Characterization Procedure of TiO2 Film
3.4. The Experimental Procedure of CO2 Reduction
4. Conclusions
- (i)
- The mismatch between the theoretical molar ratio to produce CO and the optimum molar ratio obtained with W/O B.B. as well as W B.B.-1 is confirmed. As the products near the photocatalyst surface might remain, the photocatalytic CO2 reduction performance is smaller under the investigated conditions with W/O B.B. and W B.B.-1 in this study.
- (ii)
- The highest concentration of formed CO occurring in the case of CO2:NH3 = 3:2 with W B.B.-3 is higher than that with W B.B.-1 by 175 ppmV. It is revealed that the impact of the natural thermosiphon movement of gasses around the TiO2 photocatalyst created by black body material on CO2 reduction performance is larger with W B.B.-3.
- (iii)
- There is almost no difference between the W B.B.-1 case and the W/O B.B. case in terms of gas temperature and the maximum concentration of formed CO.
- (iv)
- The temperature of the gas in the reactor with W B.B.-3 is approximately 10 °C higher than that with W/O B.B. The maximum concentration of formed CO with W B.B.-3 is two to five times as large as that with W/O B.B.
- (v)
- Comparing the heat capacity of black body material with that of the mixed gas of CO2 and NH3, it is revealed that one black body material is not enough to heat the mixed gas of CO2 and NH3 in the reactor. Three black body materials are needed to heat up the mixed gas of CO2 and NH3 in the reactor.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Monitoring Laboratory. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 8 March 2022).
- Jesic, D.; Jurkovic, L.D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering Photocatalytic and Photoelectrocatalytic CO2 Reduction Reactions: Mechanisms, Intrinsic Kinetics, Mass Transfer Resistances, Reactors and Multi-scale Modeling Simulations. Chem. Eng. J. 2021, 407, 126799. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, S.; Asif, M.; In, S.I. Layered Double Hydroxide (LDH) Based Photocatalysts: An Outstanding Strategy for Efficient Photocatalytic CO2 Reduction. Catalysts 2020, 10, 1185. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Soukhakian, S.; Jazebizadeh, M.H.; Moussavi, M.; Hojjati, M.R. On Engineering Strategies for Photoselective CO2 Reduction—A through Review. Appl. Mater. Today 2020, 18, 100499. [Google Scholar] [CrossRef]
- Remiro-Buenamanana, S.; Garcia, H. Photoassisted CO2 Conversion into Fuels. Chem. Cat Chem. Minirev. 2019, 11, 342–356. [Google Scholar]
- Zhu, S.; Chen, X.; Li, Z.; Ye, X.; Liu, Y.; Chen, Y.; Yang, L.; Chen, M.; Zhang, D.; Li, G.; et al. Cooperation between Inside and Outside of TiO2: Lattice Cu+ Accelerates Carrier Migration to the Surface of Metal Copper for Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2020, 264, 118515. [Google Scholar] [CrossRef]
- She, H.; Zhao, Z.; Bai, W.; Huang, J.; Wang, L.; Wang, Q. Enhanced Performance of Photocatalytic CO2 Reduction via Synergetic Effect between Chitosan and Cu: TiO2. Mater. Res. Bull. 2020, 124, 110758. [Google Scholar] [CrossRef]
- Chen, X.; Ye, X.; He, J.; Pan, L.; Xu, S.; Xiong, C. Preparation of Fe3+-doped TiO2 Aerogels for Photocatalytic Reduction of CO2 to Methanol. J. Sol.-Gel. Sci. Technol. 2020, 95, 353–359. [Google Scholar] [CrossRef]
- Su, K.Y.; Chen, C.Y.; Wu, R.J. Preparation of Pd/TiO2 Nanowires for the Photoreduction of CO2 into Renewable Hydrocarbon Fuels. J. Taiwan Inst. Chem. Eng. 2019, 96, 409–418. [Google Scholar] [CrossRef]
- Camarillo, R.; Toston, S.; Martinez, F.; Jimenez, C.; Rincon, J. Enhancing the Photocatalytic Reduction of CO2 through Engineering of Catalysts with High Pressure Technology: Pd/TiO2 Photocatalysts. J. Supercrit. Fluids 2017, 123, 18–27. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, X.; Zhao, Y.; Wang, L.; Zhao, Z.; Huang, X.; Liu, J.; Li, J. Efficient Photocatalyst of TiO2 Nanocrystal-supported PtRu Alloy Nanoparticles for CO2 Reduction with H2O: Synergistic Effect of Pt-Ru. Appl. Catal. B Environ. 2018, 236, 445–457. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 Photocatalysts with Enhanced Photogenerated Charges Separation and Reactant Adsorption for High Selective Photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Toston, S.; Camarillo, R.; Martinez, F.; Jimenez, C.; Rincon, J. Supercritical Synthesis of Platinum-modified Titanium Dioxide for Solar Fuel Production from Carbon Dioxide. Chin. J. Catal. 2017, 38, 636–650. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yuan, Z.; Chen, J.; Huang, B.; Dionysios, D.D.; Yang, G. Enhanced Photocatalytic CO2 Reduction via the Synergistic Effect between Ag and Activated Carbon in TiO2/AC-Ag Ternary Composite. Chem. Eng. J. 2018, 348, 592–598. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y.; Zhao, Z.; Duan, A.; Liu, J.; Pang, Y.; Li, J.; Jiang, G.; Wang, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B Environ. 2017, 209, 228–239. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Cheng, B.; Zhang, J.; Zhang, L.; Yu, J. In-situ Preparation of TiO2/N-doped Graphene Hollow Sphere Photocatalyst with Enhanced Photocatalytic CO2 Reduction Performance. Chin. J. Catal. 2021, 42, 1648–1658. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wan, Q.; Shi, Y.Z.; Wang, H.; Kang, Y.Y.; Zhu, S.Y.; Liu, S.; Wu, L. Selective Photocatalytic Reduction CO2 to CH4 on Ultrathin TiO2 Nanosheet via Coordination Activation. Appl. Catal. B Environ. 2021, 288, 120000. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.; Xiong, Z.; Sun, M.; Muruganganthan, M.; Zhang, Y. Enhanced photocatalytic CO2 reduction with defective TiO2 nanotubes modified by single-atom binary metal components. Environ. Res. 2021, 198, 111176. [Google Scholar] [CrossRef]
- Baniamer, M.; Aroujalian, A.; Sharifnia, S. Photocatalytic Membrane Reactor for Simultaneous Separation and Photoreduction of CO2 to Methanol. Int. J. Energy Res. 2020, 45, 2353–2366. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. The Evolution of Photocatalytic Membrane Reactors over the Last 20 Years: A State of the Art Perspective. Catalysts 2021, 11, 775. [Google Scholar] [CrossRef]
- Nishimura, A.; Komatsu, N.; Mitsui, G.; Hirota, M.; Hu, E. CO2 Reforming into Fuel Using TiO2 Photocatalyst and Gas Separation Membrane. Catal. Today 2009, 148, 341–349. [Google Scholar] [CrossRef]
- Nishimura, A.; Sakakibara, Y.; Koshio, A.; Hu, E. The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O. Catalysts 2021, 11, 610. [Google Scholar] [CrossRef]
- Nishimura, A.; Shimada, R.; Sakakibara, Y.; Koshio, A.; Hu, E. Comparison of CO2 Reduction Performance with NH3 and H2O between Cu/TiO2 and Pd/TiO2. Molecules 2021, 26, 2904. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Abu, H.H. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials 2017, 10, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, M.; Amin, N.S. Advances in Visible Light Responsive Titanium Oxide Based Photocatalysts for CO2 Conversion to Hydrocarbon Fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Goren, Z.; Willner, I.; Nelson, A.J. Selective Photoreduction of CO2/HCO3- to formate by aqueous suspensions and colloids of Pd-TiO2. J. Physic. Chem. 1990, 94, 3784–3790. [Google Scholar] [CrossRef]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 Using Sol-gel Derived Titania and Titania-supported Copper Catalysts. Appl. Catal. B 2002, 37, 37–38. [Google Scholar] [CrossRef]
- Izumi, Y. Recent Advances in the Photocatalytic Conversion of Carbon Dioxide to Fuels with Water and/or Hydrogen Using Solar Energy and Beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.C.; Hung, C.H.; Yuan, C.S.; Wu, J.F. Photoreduction of Carbon Dioxide with H2 and H2O over TiO2 and ZrO2 in a Circulated Photocatalytic Reactor. Sol. Energy Mater. Sci. 2007, 91, 1765–1774. [Google Scholar] [CrossRef]
- Nishimura, A.; Ishida, N.; Tatematsu, D.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Effect of Fe Loading Condition and Reductants on CO2 Reduction Performance with Fe/TiO2 Photocatalyst. Int. J. Photoenergy 2017, 2017, 1625274. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, A.; Sakakibara, Y.; Inoue, T.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Impact of Molar Ratio of NH3 and H2O on CO2 Reduction Performance over Cu/TiO2 Photocatalyst. Phys. Astron. Int. J. 2019, 3, 176–182. [Google Scholar]
- Nemoto, J.; Goken, N.; Ueno, K. Photodecomposition of Ammonia to Dinitrogen and Dihydrogen on Platinized TiO2 Nanoparticles in an Aqueous Solution. J. Photochem. Photobiol. A Chem. 2007, 185, 295–300. [Google Scholar] [CrossRef]
- Japan Society of Mechanical Engineering. Heat Transfer Hand Book, 1st ed.; Maruzen: Tokyo, Japan, 1993; pp. 238, 367–369. [Google Scholar]
- Abe, T.; Tanizawa, M.; Watanabe, K.; Taguchi, A. CO2 Methanation Property of Ru Nanoparticle-loaded TiO2 Prepared by a Polygonal Barrel-sputtering Method. R. Soc. Chem. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Photocatalytic Reduction of Carbon Dioxide with Water Vapors over Montmorillonite Modified TiO2 Nanocomposites. Appl. Catal. B Environ. 2013, 142–143, 512–522. [Google Scholar] [CrossRef]
- Anzai, A.; Fukuo, N.; Yamamoto, A.; Yoshida, H. Highly Selective Photocatalytic Reduction of Carbon Dioxide with Water over Silver-loaded Calcium Titanate. Catal. Commun. 2017, 100, 134–138. [Google Scholar] [CrossRef] [Green Version]
- CHINO. Available online: https://www.chino.co.jp/support/technique/thermometers_index/housyaritsu/ (accessed on 12 April 2022).
- List for Physical Properties of Material. Available online: https://www.ohm.jp/media/tech_cooling204.pdf (accessed on 12 April 2022).
- List for Physical Properties of Metal Element. Available online: https://www.nikkin-flux.co.jp/technology/up_img/1338425712-232811.pdf (accessed on 12 April 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Kato, T.; Mae, H.; Hu, E. Impact of Black Body Material Enhanced Gas Movement on CO2 Photocatalytic Reduction Performance. Catalysts 2022, 12, 470. https://doi.org/10.3390/catal12050470
Nishimura A, Kato T, Mae H, Hu E. Impact of Black Body Material Enhanced Gas Movement on CO2 Photocatalytic Reduction Performance. Catalysts. 2022; 12(5):470. https://doi.org/10.3390/catal12050470
Chicago/Turabian StyleNishimura, Akira, Takaharu Kato, Homare Mae, and Eric Hu. 2022. "Impact of Black Body Material Enhanced Gas Movement on CO2 Photocatalytic Reduction Performance" Catalysts 12, no. 5: 470. https://doi.org/10.3390/catal12050470
APA StyleNishimura, A., Kato, T., Mae, H., & Hu, E. (2022). Impact of Black Body Material Enhanced Gas Movement on CO2 Photocatalytic Reduction Performance. Catalysts, 12(5), 470. https://doi.org/10.3390/catal12050470







