Carbon Formation in the Reforming of Simulated Biomass Gasification Gas on Nickel and Rhodium Catalysts
Abstract
:1. Introduction
2. Results and Discussion
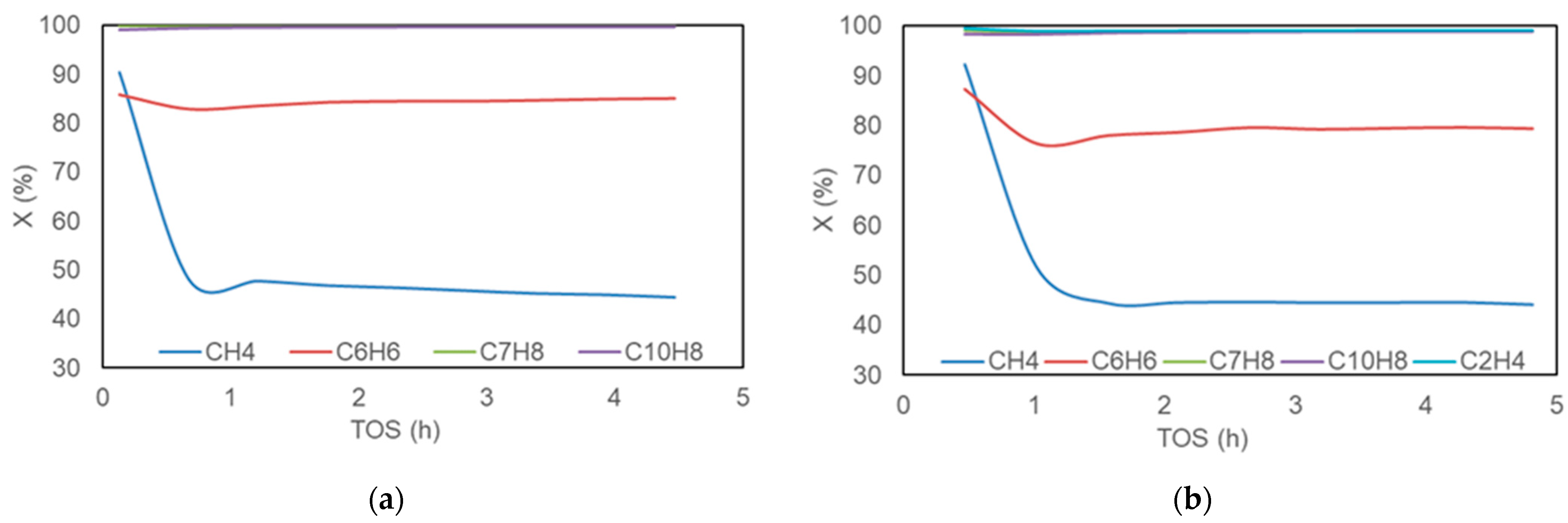
2.1. Conversions and Stabilities
2.2. Effect of Ethylene on Carbon Formation
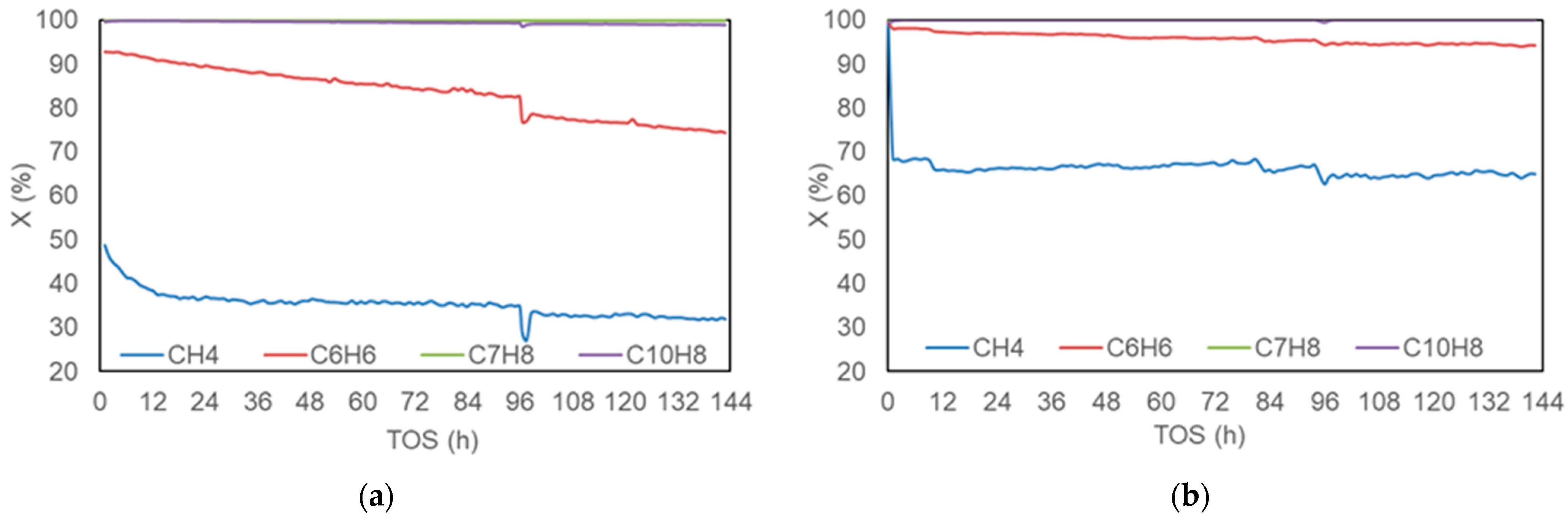
2.3. Effect of Reaction Time on Carbon Formation
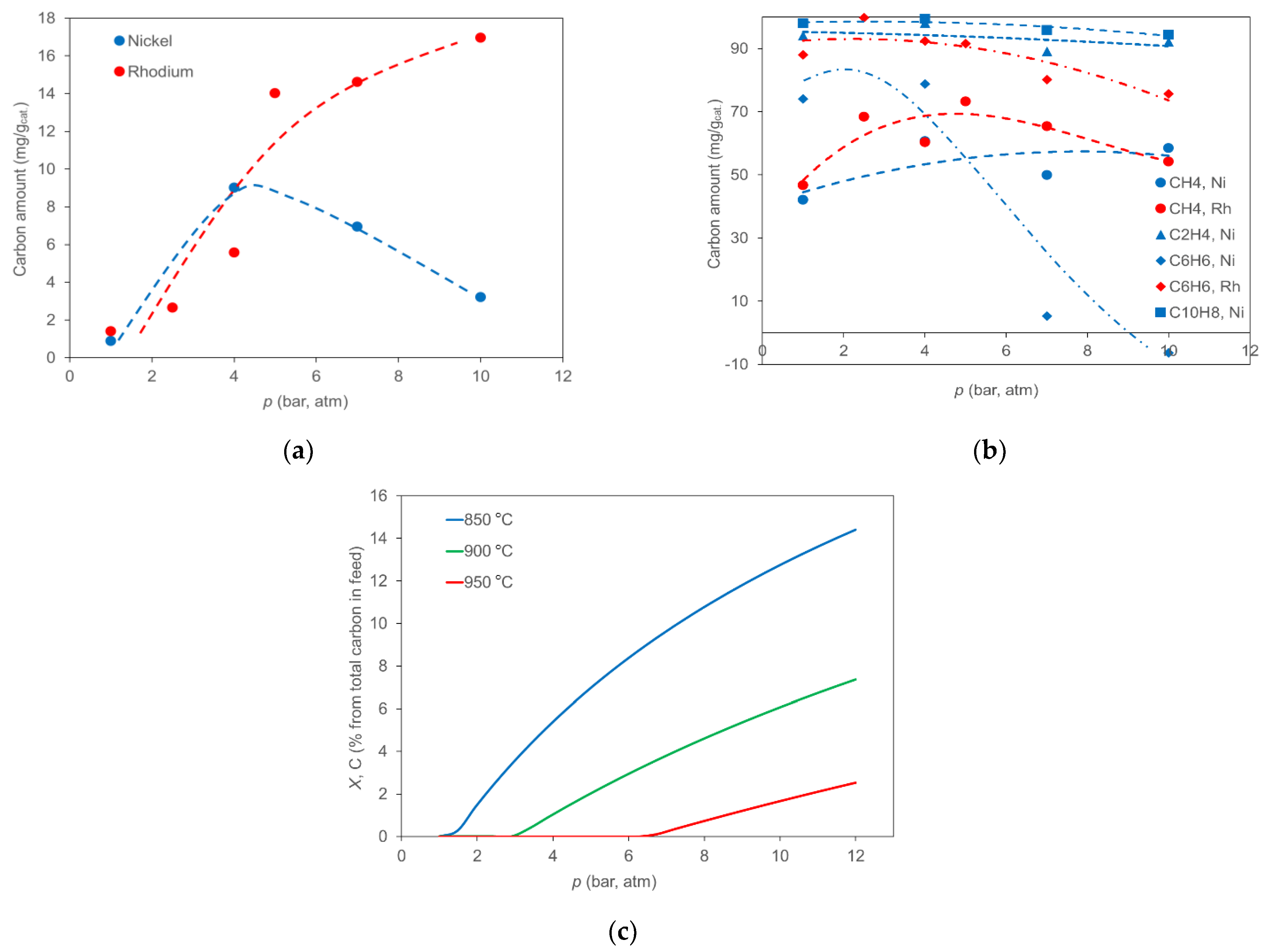
2.4. Effect of Pressure on Carbon Formation
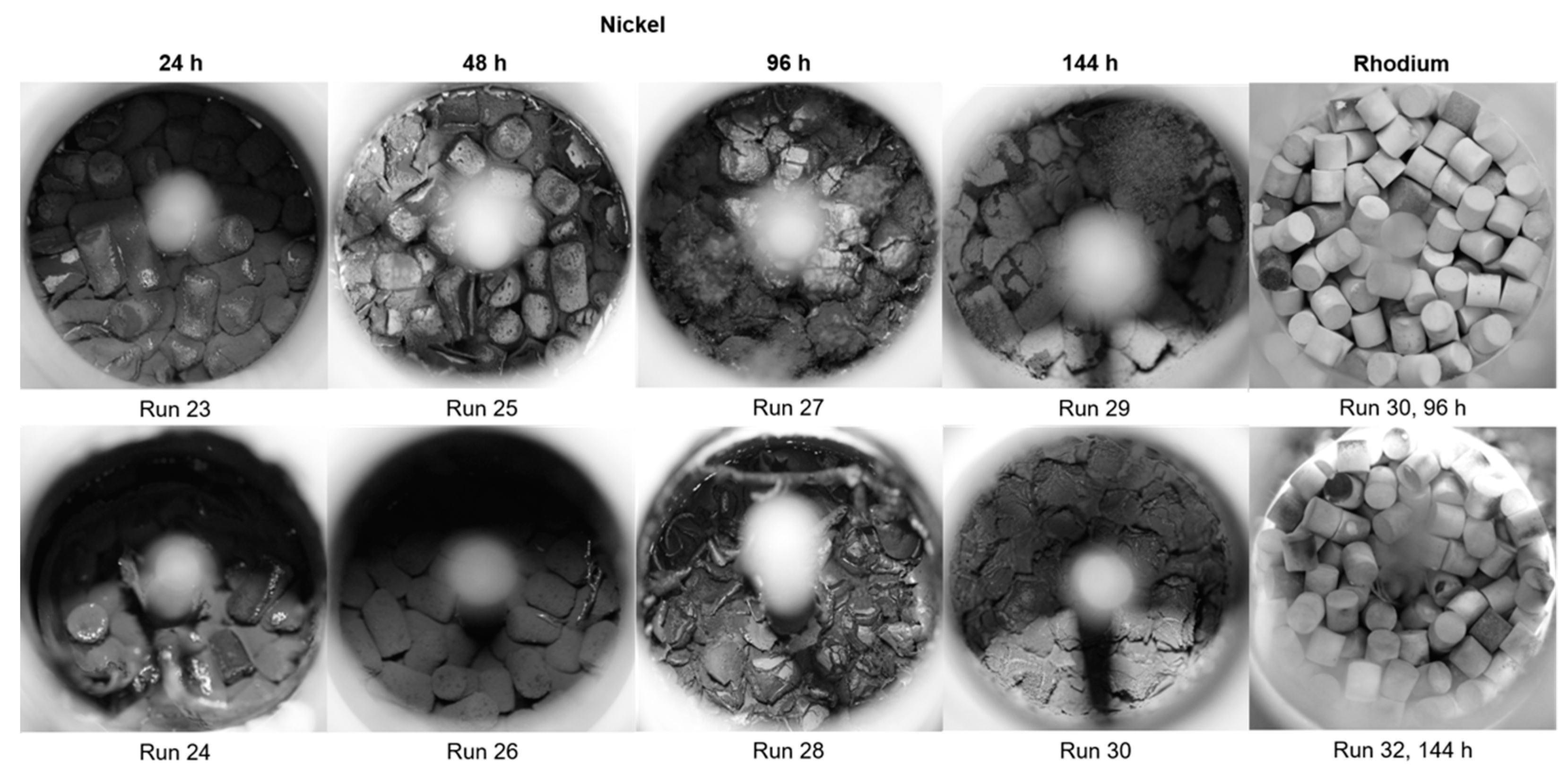
2.5. Carbon Characterization
3. Materials and Methods
3.1. Catalysts
3.2. Experimental Setup
3.3. Gas Analysis System
3.4. Carbon Oxidation
3.5. Calculation Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannula, I.; Kurkela, E. Liquid Transportation Fuels via Large-Scale Fluidised-Bed Gasification of Lignocellulosic Biomass; VTT Technology 91; VTT Technical Research Centre of Finland: Espoo, Finland, 2012; p. 114. [Google Scholar]
- Frilund, C.; Tuomi, S.; Kurkela, E.; Simell, P. Small-to medium-scale deep syngas purification: Biomass-to-liquids multi-contaminant removal demonstration. Biomass-Bioenergy 2021, 148, 106031. [Google Scholar] [CrossRef]
- Maier, S.; Tuomi, S.; Kihlman, J.; Kurkela, E.; Dietrich, R.-U. Techno-economically-driven identification of ideal plant configurations for a new biomass-to-liquid process—A case study for Central-Europe. Energy Convers. Manag. 2021, 247, 114651. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Kurkela, E.; Kurkela, M.; Hiltunen, I. Steam–oxygen gasification of forest residues and bark followed by hot gas filtration and catalytic reforming of tars: Results of an extended time test. Fuel Process. Technol. 2016, 141, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Simell, P.; Hannula, I.; Tuomi, S.; Nieminen, M.; Kurkela, E.; Hiltunen, I.; Kaisalo, N.; Kihlman, J. Clean syngas from biomass—process development and concept assessment. Biomass Conv. Bioref. 2014, 4, 357–370. [Google Scholar] [CrossRef]
- Gruber, H.; Groß, P.; Rauch, R.; Reichhold, A.; Zweiler, R.; Aichering, C.; Müller, S.; Ataimisch, N.; Hofbauer, H. Fischer-Tropsch products from biomass-derived syngas and renewable hydrogen. Biomass Conv. Bioref. 2019, 11, 2281–2292. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef] [Green Version]
- Rostrup-Nielsen, J.; Christiansen, L. Concepts in Syngas Manufacture; Imperial College Press: London, UK, 2011; p. 379. [Google Scholar]
- Knight, R. Experience with raw gas analysis from pressurized gasification of biomass. Biomass-Bioenergy 2000, 18, 67–77. [Google Scholar] [CrossRef]
- Hepola, J.; Simell, P.; Kurkela, E.; Ståhlberg, P. Sulphur poisoning of nickel catalysts in catalytic hot gas cleaning conditions of biomass gasification. Stud. Surf. Sci. Catal. 1994, 88, 499–506. [Google Scholar] [CrossRef]
- Hepola, J.; Simell, P. Sulphur poisoning of nickel-based hot gas cleaning catalysts in synthetic gasification gas: I. Effect of different process parameters. Appl. Catal. B Environ. 1997, 14, 287–303. [Google Scholar] [CrossRef]
- Hepola, J.; Simell, P. Sulphur poisoning of nickel-based hot gas cleaning catalysts in synthetic gasification gas: II. Chemisorption of hydrogen sulphide. Appl. Catal. B Environ. 1997, 14, 305–321. [Google Scholar] [CrossRef]
- Kaisalo, N.; Koskinen-Soivi, M.-L.; Simell, P.; Lehtonen, J. Effect of process conditions on tar formation from thermal reactions of ethylene. Fuel 2015, 153, 118–127. [Google Scholar] [CrossRef]
- Jackson, S.D.; Thomson, S.J.; Webb, G. Carbonaceous deposition associated with the catalytic steam-reforming of hydrocarbons over nickel alumina catalysts. J. Catal. 1981, 70, 249–263. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Catalytic Steam Reforming. In Catalysis: Science and Technology; Springer: Berlin, Germany, 1984; Volume 5, p. 117. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Bourtsalas, A.C.; Chi, Y.; Yan, J. Effect of Operating Conditions on the Coke Formation and Nickel Catalyst Performance During Cracking of Tar. Waste Biomass-Valorization 2019, 10, 155–165. [Google Scholar] [CrossRef]
- Dong, G.L.; Hüttinger, K.J. Consideration of reaction mechanisms leading to pyrolytic carbon of different textures. Carbon 2002, 40, 2515–2528. [Google Scholar] [CrossRef]
- Norinaga, K.; Deutschmann, O.; Hüttinger, K.J. Analysis of gas phase compounds in chemical vapor deposition of carbon from light hydrocarbons. Carbon 2006, 44, 1790–1800. [Google Scholar] [CrossRef]
- Norinaga, K.; Janardhanan, V.M.; Deutschmann, O. Detailed Chemical Kinetic Modeling of Pyrolysis of Ethylene, Acetylene, and Propylene at 1073–1373 K with a Plug-Flow Reactor Model. Int. J. Chem. Kinet. 2008, 40, 199–208. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energ. Combust. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Norinaga, K.; Deutschmann, O.; Saegusa, N.; Hayashi, J. Analysis of pyrolysis products from light hydrocarbons and kinetic modeling for growth of polycyclic aromatic hydrocarbons with detailed chemistry. J. Anal. Appl. Pyrol. 2009, 86, 148–160. [Google Scholar] [CrossRef]
- Kaisalo, N. Tar Reforming in Biomass Gasification Gas Cleaning; 132/2017, VTT Science 160. Doctoral Dissertations, Aalto University, Espoo, Finland, 2017. [Google Scholar]
- Meng, J.; Zhao, Z.; Wang, X.; Zheng, A.; Zhang, D.; Huang, Z.; Zhao, K.; Wei, G.; Li, H. Comparative study on phenol and naphthalene steam reforming over Ni-Fe alloy catalysts supported on olivine synthesized by different methods. Energ. Convers. Manag. 2018, 168, 60–73. [Google Scholar] [CrossRef]
- Lahaye, J. Particulate Carbon from the Gas Phase. Carbon 1992, 30, 309–314. [Google Scholar] [CrossRef]
- Qin, G.; Hao, K.-R.; Yan, Q.-B.; Hu, M.; Su, G. Exploring T-carbon for energy applications. Nanoscale 2019, 11, 5798–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihlman, J.; Kaisalo, N.; Koskinen-Soivi, M.-L.; Simell, P.; Niemelä, M.; Lehtonen, J. Whisker carbon formation in catalytic steam reforming of biomass gasification gas. Appl. Catal. A Gen. 2018, 564, 133–141. [Google Scholar] [CrossRef]
- Kihlman, J.; Sucipto, J.; Kaisalo, N.; Simell, P.; Lehtonen, J. Carbon formation in catalytic steam reforming of natural gas with SOFC anode off-gas. Int. J. Hydrogen Energy 2015, 40, 1548–1558. [Google Scholar] [CrossRef]
- Fan, Z.; Xiao, W. Electrochemical Splitting of Methane in Molten Salts to Produce Hydrogen. Angew. Chem. Int. Ed. 2021, 60, 7664–7668. [Google Scholar] [CrossRef]
- Ratso, S.; Walke, P.R.; Mikli, V.; Ločs, J.; Šmits, K.; Vītola, V.; Šutka, A.; Kruusenberg, I. CO2 turned into a nitrogen doped carbon catalyst for fuel cells and metal–air battery applications. Green Chem. 2021, 23, 4435. [Google Scholar] [CrossRef]
- Palmer, C.; Upham, D.C.; Smart, S.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 2020, 3, 83–89. [Google Scholar] [CrossRef]
- Anderson, P.W. Absence of Diffusion in Certain Random Lattices. Phys. Rev. 1958, 109, 1492. [Google Scholar] [CrossRef]
- Jäger, C.; Mutschke, H.; Henning, T.; Huisken, F. From PAHs to Solid Carbon. In PAHs and the Universe; Joblin, C., Tielens, A.G.G.M., Eds.; EAS Publications Series; EAS Publications: Les Ulis, France, 2011; Volume 46, pp. 293–304. [Google Scholar] [CrossRef]
- Hess, W.; Herd, C. Microstructure, Morphology And General Physical Properties. In Carbon Black; Donnet, J., Bansal, R., Wang, M., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1993; pp. 89–174. [Google Scholar]
- Kaisalo, N.; Kihlman, J.; Hannula, I.; Simell, P. Reforming solutions for biomass-derived gasification gas—Experimental results and concept assessment. Fuel 2015, 147, 208–220. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Liu, J.; Chen, D. Catalytic characteristics of innovative Ni/slag catalysts for syngas production and tar removal from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 11848–11860. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sutthisripok, W.; Charojrochkul, S.; Assabumrungrat, S. Development of Ni–Fe bimetallic based catalysts for biomass tar cracking/reforming: Effects of catalyst support and co-fed reactants on tar conversion characteristics. Fuel Process. Technol. 2014, 127, 26–32. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Watanabe, H.; Xu, Y.; Nakagawa, Y.; Tomishige, K. Catalytic performance and characterization of Ni–Co catalysts for the steam reforming of biomass tar to synthesis gas. Fuel 2013, 112, 654–661. [Google Scholar] [CrossRef]
- Li, D.; Koike, M.; Chen, J.; Nakagawa, Y.; Tomishige, K. Preparation of Ni–Cu/Mg/Al catalysts from hydrotalcite-like compounds for hydrogen production by steam reforming of biomass tar. Int. J. Hydrogen Energy 2014, 39, 10959–10970. [Google Scholar] [CrossRef]
- Daorattanachai, P.; Laosiripojana, W.; Laobuthee, A.; Laosiripojana, N. Catalytic activity of sewage sludge char supported Re-Ni bimetallic catalyst toward cracking/reforming of biomass tar. Renew. Energy 2018, 121, 644–651. [Google Scholar] [CrossRef]
- de Castro, T.P.; Silveira, E.B.; Rabelo-Neto, R.C.; Borges, L.E.P.; Noronha, F.B. Study of the performance of Pt/Al2O3 and Pt/CeO2/Al2O3 catalysts for steam reforming of toluene, methane and mixtures. Catal. Today 2018, 299, 251–262. [Google Scholar] [CrossRef]
- Świerczyński, D.; Libs, S.; Courson, C.; Kiennemann, A. Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl. Catal. B Environ. 2007, 74, 211–222. [Google Scholar] [CrossRef]
- Zou, X.; Chen, T.; Zhang, P.; Chen, D.; He, J.; Dang, Y.; Ma, Z.; Chen, Y.; Toloueinia, P.; Zhu, C.; et al. High catalytic performance of Fe-Ni/Palygorskite in the steam reforming of toluene for hydrogen production. Appl. Energy 2018, 226, 827–837. [Google Scholar] [CrossRef]
- He, L.; Hu, S.; Jiang, L.; Liao, G.; Chen, X.; Han, H.; Xiao, L.; Ren, Q.; Wang, Y.; Su, S.; et al. Carbon nanotubes formation and its influence on steam reforming of toluene over Ni/Al2O3 catalysts: Roles of catalyst supports. Fuel Process. Technol. 2018, 176, 7–14. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Ashok, J.; Kawi, S. NiCo@NiCo phyllosilicate@CeO2 hollow core shell catalysts for steam reforming of toluene as biomass tar model compound. Energy Convers. Manag. 2019, 180, 822–830. [Google Scholar] [CrossRef]
- Ashok, J.; Das, S.; Yeo, T.Y.; Dewangan, N.; Kawi, S. Incinerator bottom ash derived from municipal solid waste as a potential catalytic support for biomass tar reforming. Waste Manag. 2018, 82, 249–257. [Google Scholar] [CrossRef]
- Koike, M.; Li, D.; Nakagawa, Y.; Tomishige, K. A Highly Active and Coke-Resistant Steam Reforming Catalyst Comprising Uniform Nickel–Iron Alloy Nanoparticles. ChemSusChem 2012, 5, 2312–2314. [Google Scholar] [CrossRef] [PubMed]
- Josuinkas, F.M.; Quitete, C.P.B.; Ribeiro, N.F.P.; Souza, M.M.V.M. Steam reforming of model gasification tar compounds over nickel catalysts prepared from hydrotalcite precursors. Fuel Process. Technol. 2014, 121, 76–82. [Google Scholar] [CrossRef]
- Quitete, C.P.B.; Bittencourt, R.C.P.; Souza, M.M.V.M. Steam Reforming of Tar Model Compounds Over Nickel Catalysts Supported on Barium Hexaaluminate. Catal. Lett. 2015, 145, 541–548. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, Z.; Wang, X.; Chen, J.; Zheng, A.; Huang, Z.; Wei, G.; Li, H. Steam reforming and carbon deposition evaluation of phenol and naphthalene used as tar model compounds over Ni and Fe olivine-supported catalysts. J. Energ. Inst. 2019, 92, 1765–1778. [Google Scholar] [CrossRef]
- Li, D.; Lu, M.; Aragaki, K.; Koike, M.; Nakagawa, Y.; Tomishige, K. Characterization and catalytic performance of hydrotalcite-derived Ni-Cu alloy nanoparticles catalysts for steam reforming of 1-methylnaphthalene. Appl. Catal. B Environ. 2016, 192, 171–181. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Bak Hansen, J.-H. CO2-reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Lobo, L.S.; Trimm, D.L. Carbon formation from light hydrocarbons on nickel. J. Catal. 1973, 29, 15–19. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Coking on Nickel Catalysts for Steam Reforming of Hydrocarbons, J. Catal. 1974, 33, 184–201. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Hyrogen via Steam Reforming of Naphtha. Chem. Eng. Prog. 1977, 73, 87–92. [Google Scholar]
- Kuvshinova, G.G.; Popovb, M.V.; Tonkodubovb, S.E.; Kuvshinov, D.G. Effect of Pressure on the Efficiency of Nickel and Nickel-Copper Catalysts in Decomposition of Methane. Russ. J. Appl. Chem. 2016, 89, 1777–1785. [Google Scholar] [CrossRef]
- Hazra, M.; Croiset, E.; Hudgins, R.R.; Silveston, P.L.; Elkamel, A. Experimental investigation of the catalytic cracking of methane over a supported Ni catalyst. Can. J. Chem. Eng. 2009, 87, 99–105. [Google Scholar] [CrossRef]
- Snoeck, J.-W.; Froment, G.F.; Fowles, M. Kinetic Study of the Carbon Filament Formation by Methane Cracking on a Nickel Catalyst. J. Catal. 1997, 169, 250–262. [Google Scholar] [CrossRef]
- Andersen, N. Statistical models for ensemble control by alloying and poisoning of catalysts I. Mathematical assumptions and derivations. J. Catal. 1987, 104, 454–465. [Google Scholar] [CrossRef]
- Basagiannis, A.; Verykios, X. Reforming reactions of acetic acid on nickel catalysts over a wide temperature range. Appl. Catal. A Gen. 2006, 308, 182–193. [Google Scholar] [CrossRef]
- Chen, X.; Yik, E.; Butler, J.; Schwank, J.W. Gasification characteristics of carbon species derived from model reforming compound over Ni/Ce–Zr–O catalyst. Catal. Today 2014, 233, 14–20. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Tan, B.; Ozkan, U. Effect of preparation method on structural characteristics and propane steam reforming performance of Ni–Al2O3 catalysts. J. Mol. Catal. A Chem. 2009, 297, 26–34. [Google Scholar] [CrossRef]
- Duprez, D.; DeMicheli, M.; Marecot, P.; Barbier, J.; Ferretti, O.; Ponzi, E. Deactivation of steam-reforming model catalysts by coke formation: I. kinetics of the formation of filamentous carbon in the hydrogenolysis of cyclopentane on Ni/Al2O3 catalysts. J. Catal. 1990, 124, 324–335. [Google Scholar] [CrossRef]
- Bampenrat, A.; Meeyoo, V.; Kitiyanan, B.; Rangsunvigit, P.; Rirksomboon, T. Naphthalene steam reforming over Mn-doped CeO2–ZrO2 supported nickel catalysts. Appl. Catal. A Gen. 2010, 373, 154–159. [Google Scholar] [CrossRef]
- Kurkela, E.; Simell, P.; McKeough, P.; Kurkela, M. Synteesikaasun ja Puhtaan Polttokaasun Valmistus; VTT Publications, No. 682; VTT Technical Research Centre of Finland: Espoo, Finland, 2008; p. 59. Available online: https://publications.vtt.fi/pdf/publications/2008/P682.pdf (accessed on 4 March 2022).
- Kivelä, V. Filtration of Biomass-Based Gasification Gas at Elevated Temperatures. Master’s Thesis, Aalto University, Espoo, Finland, 2018; p. 89. [Google Scholar]
- Simell, P.; Kurkela, E.; Ståhlberg, P.; Hepola, J. Catalytic hot gas cleaning of gasification gas. Catal. Today 1996, 27, 55–62. [Google Scholar] [CrossRef]









| Run | Catalyst | Variable | Conversion X (%) | mcarbon (mg) | ||||
|---|---|---|---|---|---|---|---|---|
| C2H4 (vol-ppm) | CH4 | C6H6 | C10H8 | C7H8 | C2H4 | |||
| 1 | Rh | 0 | 50 | 94 | 100 | 100 | - | 4 |
| 2 | Rh | 0 | 54 | 93 | 100 | 100 | - | 17 |
| 3 | Rh | 5000 | 36 | 89 | 100 | 100 | 100 | 17 |
| 4 | Rh | 10,000 | 48 | 90 | 100 | 100 | 100 | 7 |
| 5 | Rh | 20,000 | 47 | 88 | 100 | 100 | 100 | 31 |
| 6 | Rh | 35,000 | 55 | 90 | 100 | 100 | 100 | 45 |
| 7 | Rh | 50,000 | 45 | 85 | 100 | 100 | 100 | 174 |
| 8 | Ni1 | 0 | 50 | 89 | 99 | 99 | - | 31 |
| 9 | Ni1 | 5000 | 41 | 78 | 98 | 97 | 98 | 3 |
| 10 | Ni1 | 20,000 | 42 | 74 | 98 | 98 | 94 | 19 |
| 11 | Ni1 | 35,000 | 35 | 62 | 96 | 94 | 97 | 61 |
| 12 | Ni1 | 50,000 | 44 | 80 | 99 | 99 | 99 | 330 |
| 13 | Ni1 | 50,000 | 44 | 76 | 98 | 98 | 99 | 354 |
| 14 | Ni1 | 50,000 | 56 | 87 | 99 | 99 | 99 | 937 |
| p (bara) | ||||||||
| 15 | Rh | 2.5 | 68 | 100 | 100 | 100 | 100 | 60 |
| 16 | Rh | 4 | 60 | 92 | 100 | 100 | 100 | 126 |
| 17 | Rh | 5 | 73 | 92 | 100 | 100 | 100 | 317 |
| 18 | Rh | 7 | 65 | 80 | 100 | 100 | 100 | 330 |
| 19 | Rh | 10 | 54 | 76 | 100 | 100 | 100 | 384 |
| 20 | Ni1 | 4 | 61 | 79 | 99 | 100 | 98 | 195 |
| 21 | Ni1 | 7 | 50 | 5 | 96 | 100 | 89 | 150 |
| 22 | Ni1 | 10 | 58 | -6 | 94 | 100 | 92 | 69 |
| TOS (h) | ||||||||
| 23 | Ni2 | 24 | 63 | 97 | 100 | 100 | 100 | 509 |
| 24 | Ni2 | 24 | 51 | 96 | 100 | 100 | 100 | 670 |
| 25 | Ni2 | 48 | 74 | 95 | 100 | 100 | 100 | 596 |
| 26 | Ni2 | 48 | 35 | 79 | 99 | 100 | 99 | 918 |
| 27 | Ni2 | 96 | 76 | 97 | 100 | 100 | 100 | 1152 |
| 28 | Ni2 | 96 | 71 | 96 | 100 | 100 | 100 | 844 |
| 29 | Ni2 | 144 | 69 | 91 | 100 | 100 | 100 | 1448 |
| 30 | Ni2 | 144 | 65 | 96 | 100 | 100 | 100 | 1168 |
| 31 | Rh | 144 | 35 | 84 | 100 | 100 | 100 | 39 |
| 32 | Rh | 96 | 29 | 80 | 99 | 100 | 99 | 28 |
| Gas | CO (vol%) | CO2 (vol%) | H2 (vol%) | CH4 (vol%) | O2 (vol%) | N2 (vol%) | C2H4 1 (vol%) | H2O (vol%) | NH3 (vol-ppm) | H2S (vol-ppm) | Tar (vol-ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.9 | 11.9 | 20.9 | 6 | 0 | 4.7 | 1.2 | 40 | 1200 | 60 | 2900 |
| 2 2 | 7.2 | 18.7 | 21.6 | 5 | 2 | 3.4 | 1.5 | 40 | 1200 | 60 | 4400 |
| Run | Catalyst | Feed Gas | mcatalyst (g) | p (bara) | T (°C) | τ (s) | TOS (h) | S/C Molar | C2H4 (vol-ppm, Dry Gas) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Rh | Gas1 | 22.0 | 1 | 900 | 0.07 | 5 | 6.4 | 0 |
| 2 | Rh | Gas1 | 22.6 | 1 | 900 | 0.07 | 5 | 6.4 | 0 |
| 3 | Rh | Gas1 | 22.6 | 1 | 900 | 0.07 | 5 | 6.1 | 5000 |
| 4 | Rh | Gas1 | 22.6 | 1 | 900 | 0.07 | 5 | 5.8 | 10,000 |
| 5 | Rh | Gas1 | 22.6 | 1 | 900 | 0.07 | 5 | 5.4 | 20,000 |
| 6 | Rh | Gas1 | 22.5 | 1 | 900 | 0.07 | 5 | 4.8 | 35,000 |
| 7 | Rh | Gas1 | 22.6 | 1 | 900 | 0.07 | 5 | 4.3 | 50,000 |
| 8 | Ni1 | Gas1 | 22.9 | 1 | 900 | 0.07 | 5 | 6.4 | 0 |
| 9 | Ni1 | Gas1 | 21.6 | 1 | 900 | 0.07 | 5 | 6.1 | 5000 |
| 10 | Ni1 | Gas1 | 21.6 | 1 | 900 | 0.07 | 5 | 5.4 | 20,000 |
| 11 | Ni1 | Gas1 | 21.6 | 1 | 900 | 0.07 | 5 | 4.8 | 35,000 |
| 12 | Ni1 | Gas1 | 23.0 | 1 | 900 | 0.07 | 5 | 4.3 | 50,000 |
| 13 | Ni1 | Gas1 | 22.9 | 1 | 900 | 0.07 | 5 | 4.3 | 50,000 |
| 14 | Ni1 | Gas1 | 22.9 | 1 | 960 | 0.07 | 5 | 4.3 | 50,000 |
| 15 | Rh | Gas1 | 22.6 | 2.5 | 900 | 0.17 | 5 | 5.4 | 20,000 |
| 16 | Rh | Gas1 | 22.6 | 4 | 900 | 0.27 | 5 | 5.4 | 20,000 |
| 17 | Rh | Gas1 | 22.6 | 5 | 900 | 0.34 | 5 | 5.4 | 20,000 |
| 18 | Rh | Gas1 | 22.6 | 7 | 900 | 0.48 | 5 | 5.4 | 20,000 |
| 19 | Rh | Gas1 | 22.6 | 10 | 900 | 0.68 | 5 | 5.4 | 20,000 |
| 20 | Ni1 | Gas1 | 21.6 | 4 | 900 | 0.27 | 5 | 5.4 | 20,000 |
| 21 | Ni1 | Gas1 | 21.5 | 7 | 900 | 0.48 | 5 | 5.4 | 20000 |
| 22 | Ni1 | Gas1 | 21.6 | 10 | 900 | 0.68 | 5 | 5.4 | 20,000 |
| 23 | Ni2 | Gas2 | 25.0 | 1 | 950 | 0.06 | 24 | 3.6 | 24,500 |
| 24 | Ni2 | Gas2 | 25.1 | 1 | 950 | 0.06 | 24 | 3.6 | 24,500 |
| 25 | Ni2 | Gas2 | 25.1 | 1 | 950 | 0.06 | 48 | 3.6 | 24,500 |
| 26 | Ni2 | Gas2 | 25.0 | 1 | 950 | 0.06 | 48 | 3.6 | 24,500 |
| 27 | Ni2 | Gas2 | 25.1 | 1 | 950 | 0.06 | 96 | 3.6 | 24,500 |
| 28 | Ni2 | Gas2 | 24.9 | 1 | 950 | 0.06 | 96 | 3.6 | 24,500 |
| 29 | Ni2 | Gas2 | 25.0 | 1 | 950 | 0.06 | 144 | 3.6 | 24,500 |
| 30 | Ni2 | Gas2 | 25.0 | 1 | 950 | 0.06 | 144 | 3.6 | 24,500 |
| 31 | Rh | Gas2 | 22.8 | 1 | 950 | 0.06 | 144 | 3.6 | 24,500 |
| 32 | Rh | Gas2 | 24.1 | 1 | 950 | 0.06 | 96 | 3.6 | 24,500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kihlman, J.; Simell, P. Carbon Formation in the Reforming of Simulated Biomass Gasification Gas on Nickel and Rhodium Catalysts. Catalysts 2022, 12, 410. https://doi.org/10.3390/catal12040410
Kihlman J, Simell P. Carbon Formation in the Reforming of Simulated Biomass Gasification Gas on Nickel and Rhodium Catalysts. Catalysts. 2022; 12(4):410. https://doi.org/10.3390/catal12040410
Chicago/Turabian StyleKihlman, Johanna, and Pekka Simell. 2022. "Carbon Formation in the Reforming of Simulated Biomass Gasification Gas on Nickel and Rhodium Catalysts" Catalysts 12, no. 4: 410. https://doi.org/10.3390/catal12040410
APA StyleKihlman, J., & Simell, P. (2022). Carbon Formation in the Reforming of Simulated Biomass Gasification Gas on Nickel and Rhodium Catalysts. Catalysts, 12(4), 410. https://doi.org/10.3390/catal12040410







