Abstract
Polyhydroxyalkanoates, or PHAs, belong to a class of biopolyesters where the biodegradable PHA polymer is accumulated by microorganisms as intracellular granules known as carbonosomes. Microorganisms can accumulate PHA using a wide variety of substrates under specific inorganic nutrient limiting conditions, with many of the carbon-containing substrates coming from waste or low-value sources. PHAs are universally thermoplastic, with PHB and PHB copolymers having similar characteristics to conventional fossil-based polymers such as polypropylene. PHA properties are dependent on the composition of its monomers, meaning PHAs can have a diverse range of properties and, thus, functionalities within this biopolyester family. This diversity in functionality results in a wide array of applications in sectors such as food-packaging and biomedical industries. In order for PHAs to compete with the conventional plastic industry in terms of applications and economics, the scale of PHA production needs to grow from its current low base. Similar to all new polymers, PHAs need continuous technological developments in their production and material science developments to grow their market opportunities. The setup of end-of-life management (biodegradability, recyclability) system infrastructure is also critical to ensure that PHA and other biobased biodegradable polymers can be marketed with maximum benefits to society. The biobased nature and the biodegradability of PHAs mean they can be a key polymer in the materials sector of the future. The worldwide scale of plastic waste pollution demands a reformation of the current polymer industry, or humankind will face the consequences of having plastic in every step of the food chain and beyond. This review will discuss the aforementioned points in more detail, hoping to provide information that sheds light on how PHAs can be polymers of the future.
1. Introduction
1.1. What Are PHAs
Polyhydroxyalkanoates (PHAs) belong to a class of biopolyesters, first discovered by Lemoigne in 1925 [1]. PHA is accumulated as intracellular granules (carbonosomes, Figure 1), in various Gram-positive and Gram-negative bacteria [2]. These bacteria can accumulate PHA using a wide range of carbon rich growth substrates [3,4,5,6,7]. Generally limitation of an inorganic nutrient (e.g., nitrogen) in the growth medium is needed to stimulate PHA accumulation from the carbon source which should be in excess [8]. However, Zinn et al., have reported PHA accumulation in continuous cultures grown under both carbon and nitrogen limitation [9].

Figure 1.
Transmission electron micrograph showing granules of PHA accumulated in the bacterium P. putida CA-3 from styrene [10].
Several enzyme driven pathways are used by microorganisms to metabolise carbon-based substrates and channel the carbon in those substrates towards PHA accumulation. These pathways will be described later in this review. Microorganisms can use a variety of carbon sources for growth and PHA accumulation [4] many of these carbon sources originate from waste or low value biomass [11]. The use of such carbon sources instead of higher cost starting materials makes PHA production via this route cheaper. The use of waste resources to make PHAs presents possibilities in a circular economy and an alternative to fossil-based resources to make conventional plastics. However, the heterogenous nature of waste materials as well as the security of supply provide challenges for industrial processes.
The most common PHAs are branched polymers with the carbon chains branching or extending out away from the 2-carbon repeating unit backbone of the polymer, as seen in Figure 2 [6].
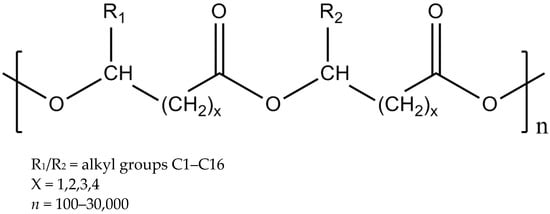
Figure 2.
General chemical structure of poly-3-hydroxyalkanoates (PHAs) [6].
Bacteria are capable of synthesising different subgroups of PHAs namely short-chain-length (scl), medium-chain-length (mcl) and long-chain-length (lcl) PHA [12]. scl and mcl-PHA groups are the most studied polymer chain lengths, with 3–5 carbons for scl-PHAs and 6–14 carbons for mcl-PHAs [13]. lcl-PHAs (>14 carbon atoms per monomer unit) are the least common occurring chain length of PHAs, with limited studies on their production and applications [14]. The molecular weight and type of PHA monomers determine the physical and thermal properties of the biopolymers; the branched nature of the polymer carbon means that the side chain interferes with crystallinity. Furthermore, the interaction of the side chains affects other properties such as tensile strength, elongation to break ratio, glass transition temperature and melting points [15]. Currently, PHAs and their blends are used in various applications. It is important to assess these composites’ mechanical, barrier, and thermal properties for optimal application. PHAs and blends/composites with other polymers have been spun into fibres but also processed into trays and films with high gas and liquid barrier capabilities [13]. PHA properties are often comparable to conventional fossil fuel plastics, with authors suggesting PHAs as a sustainable alternative to plastics originating from fossil-based resources [16,17].
PHB (scl-PHA) is made via a three-step pathway once acetyl-CoA is made in the cell. While mcl-PHAs are made in microorganisms through two major pathway β-oxidation, starting from fatty acids, and de novo fatty acid synthesis using acetyl-CoA. Acetyl-CoA is a central metabolite produced by microorganisms when they metabolise sugars and other carbon substrates [18,19]. Within bacterial species, a small number of strains have been studied in depth and brought to an industrial scale for production. Various factors such as carbon source cost, efficiency of bacterial growth, PHA accumulation levels, properties of the PHA, and downstream processing costs, directly affects the scale-up potential of polymer production [12]. Pseudomonas putida (P. putida), Pseudomonas oleovorans, Burkholderia sacchari and Cupriavidus necator are some of these promising bacterial strains that have been studied extensively for their efficacy in PHA production.
PHAs are considered to be bioplastics, meaning they are biobased (originating from renewable sources) and biodegradable (can be broken down naturally through biological processes) (European Bioplastics, 2016). PHA’s potentially offer more end of life options when compared to non-biodegradable biobased materials such as bio-polyethylene (bio-PE) and bio-terephthalate (bio-PET). Although PHA’s look like a genuine green alternative to other plastics, much research still needs to be carried out to employ more control over the quality, quantity, and general economics for the production of PHA’s [20]. The synthesis, properties, applications, and biodegradability of PHAs will be discussed in more detail in the following sections of this review.
1.2. The Synthesis of PHAs—Types of Microorganisms That Accumulate PHA and the Processes Within
PHAs are macromolecules, allowing for the storage of carbon and energy in PHA producing microorganisms [4]. In nature, PHA synthesis can occur through a number of different metabolic pathways, with these pathways determined by the microorganism producing it and the conditions in which this microorganism lives [20]. The synthesis of PHAs is regulated through three genes and three enzymes and is one of the simplest biosynthetic pathways [4]. One of the most common, and shorter pathways for PHA production is that of polyhydroxybutyrate (PHB) (Figure 3). Out of the 150 plus monomers that can be produced by PHA producing bacteria, only a small number of these, under natural conditions, can be formed into the homopolymers and copolymers required for the formation of PHAs [21]. Due to this natural bottleneck for inhibiting polymer formation, scl-PHAs such as PHB and P(3HB:HV) are more commonly found in more typical physiological conditions due to their shorter metabolic pathways [22]. With over 300 microorganisms capable of producing PHA, there are relatively few that can synthesize PHA at a level that is sufficient for scaling up to an industrial level. There are several bacterial species that can produce PHA at sufficient levels, though (Table 1). Although PHA can be formed by bacteria, fungi, and microalgae, there is significantly more research and promise for the efficiency of bacteria to produce PHA [23].
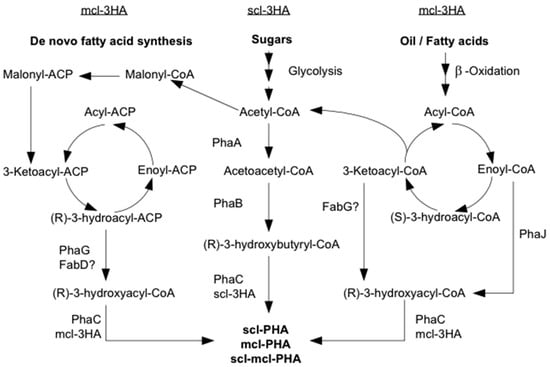
Figure 3.
Biosynthesis pathways of short chain length (scl) PHA, medium chain length (mcl) PHA and short-medium-chain length (scl-mcl) PHA from sugars and oils. PhaA, β-ketothiolase; PhaB, NADPH-dependent acetoacetyl-CoA reductase; PhaC, PHA synthase; PhaG, 3-hydroxyacyl-ACP-CoA transferase; PhaJ, (R)-enoyl-CoA hydratase; FabD, malonyl-CoA-ACP transacylase; FabG, 3-ketoacyl-CoA reductase [19].

Table 1.
Microorganisms extensively studied for their ability to accumulate PHA.
P. putida strain KT2440, is one of the most studied bacterial strains in the pseudomonas genus. P. putida KT2440 is a Gram-negative bacterium that has been extensively studied due to its ability to degrade aromatic compounds [28] and accumulate mcl-PHA. It is one of the most studied bacteria strains as it can synthesize high quantities of polymer per cell mass, amounts up to 75% w/w make it an attractive strain for production scaleup of PHA [8]. P. putida KT2440 also has advantageous features for biotechnology due to it metabolic diversity and genetic tractability [18]. It is capable of utilizing a wide range of carbon sources such as glucose, glycerol and fatty acids [29]. PHA accumulation in KT2440 from sugars requires nitrogen limitation but high levels of PHA can be accumulated, without nitrogen limitation while utilising fatty acids as the carbon source [29]. P. putida KT2440 has two main metabolic pathways that provide precursor molecules for the synthesis of mcl-PHA’s (Figure 3) namely de novo fatty acid synthesis when using substrates such as glucose and glycerol and β-oxidation when using fatty acids. Another mcl-PHA producing bacterial strain that has been studied extensively is Pseudomonas oleovorans ATCC 29347. This strain type can grow on substrates such as n-alkane, n-alkene and long-chain fatty acids to accumulate copolymers of mcl-PHA [19]. Monomer units ranging from C6 to C14 are produced via β-oxidation of acyl-CoA [30], with monomers below C6 bypassing the PHA producing phase with these monomers converted into acetyl-CoA. These monomers below C6 are then harnessed via the TCA cycle for energy and as a carbon source for cell growth [19].
Burkholderia sacchari (stain IPT101 in the case of UCD), is a Gram-negative bacterium that was originally isolated from sugarcane crops in Brazil [31]. It is capable of utilizing the components of lignocellulose (cellulose, hemicellulose, lignin) to produce high-value chemicals and products such as poly-3-hydroxybutyrate [P(3HB)] [32]. The capability of B. sacchari to produce such high-value products from the most abundant renewable resource worldwide (lignocellulose) was the driving force for its use as a strain for the industrial scale-up of PHA production processes [32,33]. This is particularly apparent in studies that have shown this bacterium can accumulate P(3HB) up to 80% of its cell dry weight from sucrose [34]. It also grows efficiently on glucose, glycerol, organic acids and hydrolysed straw [35]. Another well studied PHB producing bacterial strain is Cupriavidus necator H16, previously known as Ralstonia eutropha. This bacterium is considered a model organism due to the availability of its complete genome sequence as well as its genetic tractability [36,37]. On top of its metabolic diversity, C. necator can achieve high cell densities aerobically under both heterotrophic and chemolithoautotrophic growth conditions. Under heterotrophic conditions C. necator can utilize substrates such as fructose, fatty acids and N-acetylglucosamine in tandem with oxygen. Under chemolithoautotrophic conditions C. necator can utilize carbon dioxide as it’s substrate in tandem with hydrogen and oxygen [36]. When carbon dioxide is the single available source of carbon C. necator can assimilate via the reductive pentose phosphate cycle, otherwise known as the Calvin-Benson-Bassham cycle [37]. Due to its capability to produce a variety of valuable chemicals and polymer from an array of sources, C. necator is considered a good candidate for biotechnological processes [36,38].
1.3. Substrates Utilised by Microorganisms to Accumulate PHA
Microorganisms use a wide variety of carbon based substrates for growth and PHA accumulation. Monosaccharides such as glucose and fructose, disaccharides such as sucrose, and lactose have been used by microorganisms to accumulate PHA [31]. Sucrose can be generated from sugar beet and sugar cane while lactose is a major product and by-product of the dairy industry [33,39]. Complex carbohydrates such as cellulose and starch can be hydrolysed to produce glucose, arabinose, xylose and other monosaccharides which can subsequently be supplied to microorganisms for PHA accumulation [39,40]. Fatty acids can also be utilized as the sole carbon source for the production of PHA allowing for the use of waste, low-value or renewable oils for PHA production in place of other higher valued food-chain crops [41]. Examples such as palm kernel oil (PKO), where lauric acid is the pre-dominant 12-carbon saturated fatty acid of PKO, has shown promise a substrate for the production of mcl-PHA with strains of P. putida [41,42]. In order for PHA production to compete with that of petroleum based polymers in the world today, the substrates must originate from a biomass source that is considered either of low value or from a waste stream, to make the process as economically attractive as possible. The following sub-sections will cover the wide array of substrates that are utilised by microorganisms in the accumulation of PHA in more detail.
1.4. Sugar Containing Feedstocks for PHA Accumulation (Including Wastes)
1.4.1. Dextrose from Corn
Corn grain and corn stover (leaves, stalks) have been investigated as a carbon source for PHA production, focussing on the 1st generation substrates such as dextrose, corn oil and corn gluten [43]. The consistency and quantity in the production of the sugar dextrose from agricultural corn grain farming provide a reliable feedstock for PHA production, but with this comes the overlapping and competition with the demand for corn grain in the food chain. A past example of commercial-scale production of PHA from corn can be seen from the U.S. biotech company Metabolix, Inc. in Cambridge, MA, USA. Metabolix opened their first commercial-scale plant in Iowa in 2010, where they produced a corn-syrup based PHA up to 50,000 tons per year [44]. The commercial-scale production of PHA from 1st generation substrates of corn is possible, but it’s viability is still uncertain due to the environmental burdens associated with corn cultivation [45]. For example, when compared to polystyrene production, PHA production does not improve overall environmental impacts regarding photochemical smog, acidification and eutrophication [43]. In order for corn to PHA production to be viable, PHA fermentation technologies must improve in tandem with increased integrated systems involved with corn cultivation.
1.4.2. Sucrose from Sugar Beet/Cane
After reviewing the viability of corn to PHA, a clear path to making commercial-scale production of PHA viable is to obtain the substrate from a plentiful low-value or waste carbon source stream. An example of this is low-grade mollases, which is a residual syrup by-product of sugar refining mills that is high in sucrose but unsuitable for food products [46]. Using such as low-value or waste stream has potential, but most studies show that cell production and polymer accumulation within are still not commercially viable [47]. Although studies by Chaudhry et al. [48] using Pseudomonas species growing on sugar wastes have shown little promise with maximum cell dry weights of of 12.53 g L−1 and PHA content of 35.63%, other studies by Kulpreecha et al. [49] with Bacillus megaterium on sugar wastes have shown more promise with cell dry weights of up to 72.7 g L−1 and PHA content of 42% achieved over a 24 h period. The more promising latter study indicates that variables such as microorganism strain, carbon source, carbon source production and pre-treatments as well as fermentation conditions all play a role in optimizing PHA production from low-value and waste streams for industrial scaleup.
1.4.3. Whey to PHA
Dairy whey is another promising food waste stream that has the potential for use as a commercial-scale carbon source for PHA production. Whey is a by-product of the cheese making industry comprised of lactose, proteins, fats, water-soluble vitamins, mineral salts and other key nutrients that microorganisms require to grow [47]. Up to 1.60 × 108 tons of whey produced worldwide, surpassing the quantities needed for whey powder production [50]. Whey is currently a problematic by-product for the dairy industry as only ~50% is utilised for the production of lactose, casein and protein powder with the remainder either disposed of or inefficiently treated and processed for animal feed [51]. For these reasons, whey has potential to be a reliable and cheap carbon source stream for PHA production via microbial fermentation. Whey has the additional benefit of not requiring any enzymatic or chemical pre-treatments prior to being used as a carbon source. Life cycle assessments also suggest that the whey to PHA production is comparable to the ecological footprint of existing petroleum based plastics [47,50]. However, there are some disadvantages involved with using whey for PHA production. The fermentation process energy requirements, minimal PHA accumulation yields per whey input as well as the inability of typical PHA accumulating microorganisms to directly metabolise whey have hindered the development for the commercial scale-up of the whey to PHA process [47]. Some studies have altered PHA producing bacterial strains to combat the metabolic issue, with the transformation of Cupriavidus necator DSM 545 to include genes lacZ, lac and lacO from of E. coli to produce C. necator mRePT that can directly metabolize whey to produce PHA [52]. Whey is a promising carbon source stream with many benefits for PHA production, but much development of the fermentation process and microorganism strain manipulation are still required to improve current energy inefficacies, output yields (per kg of input) and the quality of the polymer produced before whey to PHA is commercially viable across the world.
1.4.4. Grass to PHA
Many researchers are investigating the short-circuiting of the anaerobic digestion (AD) process to produce fatty acids such as acetic, propionic and butyric acid [53]. We have previously reported on the conversion of grass via AD generated fatty acids to PHA production by Pseudomonas strains which is comparable to the same strains on laboratory fatty acids [54]. The availability of glucose rich hydrolysates from pre-treatment and de-lignification of perennial grasses also allowed the production of PHA.
1.4.5. Waste Cooking Oils (WCO) as Substrates for PHA Accumulation
Waste cooking oils could be used as cheap substrates for microbial production of PHA. Waste cooking oil is comprised of animal and/or vegetable matter that’s been previously used in the frying of foods both in the service industry and domestic households [55]. In the EU, up to 60% of 1.748 millions tonnes of WCO is improperly disposed of annually [56]. Some WCOs can be utilized as biofuels, but it is advantageous to apply this carbon to materials rather than for energy due to its carbon-rich composition. Deep fat frying food has become more common in households and the food industry, leaving up to 29 million tonnes of inedible oils needing disposal of per year [57]. This leaves a carbon rich waste product with a poor end of life management system in place. However, one can keep the cooking oil in the materials cycle by converting it into a material, burning it for energy or converting it to biofuels (biodiesel). The conversion of WCO to the biodegradable polymer PHA is a promising route given that WCO is rich in fatty acids (conjugated to glycerol) and fatty acids are well known substrates for PHA accumulation. The conversion of waste cooking oils into PHAs has been reported for a number of bacterial species (Table 2). Plant oils (triacylglycerols) can support growth and PHA production in some mcl-PHA producing strains and could lead to polymers with altered properties to those produced using pure fatty acids as substrates [58]. Few of the well characterized, commonly used, mcl-PHA accumulating bacterial strains have the ability to grow and produce PHA directly on triacylglycerols [59].

Table 2.
Bacterial strains growing on different plant oils and waste substrates to produce PHA.
Lipase mediated hydrolysis of plant oils or WCO results in glycerol and fatty acid production, both of which have been shown to be converted to PHA via de novo fatty acid synthesis and β-oxidation respectively [66]. The general structure of triglycerides and their hydrolysis products can be seen in Figure 4. Mcl-PHA has been produced by Pseudomonas aeruginosa from palm oil [67], Brassica carinta oil [68] and waste frying oil [65]. However, this species is an opportunistic pathogen and not suitable for PHA production at an industrial level. Pseudomonas chlororaphis was previously used to produce mcl-PHA from palm oil [69]. Comamonas testosteroni produced mcl-PHA from caster seed oil, coconut oil, mustard oil, cottonseed oil, groundnut oil, olive oil and sesame oil [70]. Pseudomonas resinovorans used lard, butter oil, olive oil, sunflower oil, coconut oil and soybean oil to produce mcl-PHA [58] and Pseudomonas saccharophilia used soybean oil and sunflower oil to produce mcl-PHA [60]. Genetic engineering has also been used to express lipase genes in well-known PHA producing strains such as P. putida, P. oleovorans and P. corrugata to allow them to grow directly on oils [61,71]. Ruiz et al. (2019) demonstrated high cell density (100 g CDW L−1) using WCO but with a modest PHA accumulation level (25–30% CDW) [56] compared to PHA accumulated from fatty acids where cells can accumulate between 60 and 70% PHA. In addition to this, Ruiz et al. (2019) demonstrated that hydrolysed waste cooking oil could be utilised by P. putida KT2440 to achieve biomass of 159.4 g L−1 with 36.4% of this CDW composed of PHA [72].
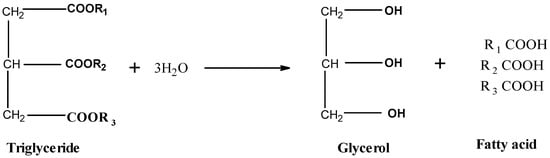
Figure 4.
General schematic showing the general structure of triglycerides present in plant oils and the hydrolysis products of these triglycerides (glycerol and fatty acids).
1.4.6. PHA from Gases
Utilising gases, such as CH4, as a carbon feedstock is one interesting option as it provides a cheap and accessible substrate, removes or prevents greenhouse gases from entering our atmosphere and at the same time produces biobased biodegradable polymer [73]. Industrial waste gases such as methane (CH4) from biogas, carbon dioxide (CO2) and syngas (CO2 + H2) can be utilized as substrates for PHA production [17]. Biogas is a renewable resource that is comprised mostly of CH4 and CO2 with trace amounts of nitrogen (N2), oxygen (O2) and hydrogen sulfide (H2S) [74]. It’s a renewable resource produced through the anaerobic digestion of organic substrates, this process is found at waste-water treatment plants where sewage (the organic substrate) is broken down to produce biogas [75]. There are also dedicated sites that use biomass such as agricultural crops and food waste to produce biogas. Due to the widespread production of biogas across the world, it is estimated a total of 58.7 billion Normal cubic metres (Nm3) of biogas was produced in 2014 (World Bioenergy Association, 2017). These significant quantities of biogas can be harnessed for energy through combined heat and power production (CHP) gas engines that utilise the majority component CH4 to produce electric and thermal energy [75]. Although CHP gas engines can harness more than 80% of the potential energy from biogas, the economic feasibility is challenged due to factors such as initial high capital investment, operation and maintenance costs, and depreciation/outdating of the CHP gas engines themselves [76]. For these reasons, an alternative method for harnessing this abundant, renewable and carbon rich biogas is highly sought after.
Through a bacterial fermentation process, it is possible for microorganisms to use gases such as CO2, O2 and CH4 as feedstocks to produce PHA [77]. These are methanotrophic bacteria that have been used by biotech start-up Mango Materials, who use biogas as their sole carbon source in tandem with O2 for the production of PHB (National Science Foundation, 2014). After a life cycle assessment, the production of PHB from methane originating from wastewater treatment plants and landfills requires >10% less energy when compared to other substrates such as corn-derived sugars [78]. Mango Materials currently use the excess biogas from a CHP gas engine system in a waste-water treatment plant for the production of PHB, that would otherwise be wasted through flare burning. On top of this, the use of this biogas to PHA technology closes the carbon cycle through sequestration of the carbon within the final polymer produced [77]. The abundance of wastewater treatment plants and landfills, current inefficient use of biogas, benefits of PHA production and carbon sequestering all make biogas to PHA a good candidate for commercial scale-up across much of the world.
2. PHA Fermentation Production Processes
There are three common fermentation strategies for the cultivation of bacteria to produce PHA: batch; fed-batch and continuous, all having shared goals for the control and optimization of factors such as carbon to nitrogen ratio, length of the fermentation run, temperature, pH and others [79]. The scale-up of PHA production processes and the downstream processing of fermentation harvests will be discussed in this section also.
2.1. Batch Cultivation
In a batch cultivation the media for the microbial culture (including the carbon source) is provided at the beginning of the fermentation, with no addition or removal of these throughout the process. Cells are harvested at the end of the batch run and the removal of the PHA cell mass at the end of the process [80]. This batch fermentation strategy can then be broken into two types based on the form of bacterial growth, a one stage process and two stage process [81]. The one-stage process involves the simultaneous growth of biomass and PHA in the closed fermentation system. The two-stage process is divided into a biomass growth phase with a subsequent PHA accumulation phase [82]. Although there is simplicity and cost effectiveness for the batch cultivation process, it does present issues regarding the optimisation of yields. Final PHA yields can be low when conditions such as the exhaustion of the carbon source occur, ultimately resulting in PHA within the cell mass being depolymerised to provide carbon and energy for the growth of bacteria [9]. The growth of the bacterial strain Azohydromonas australica DSM 1124 with sucrose as the carbon source in a batch fermentation resulted in a final biomass of 8.71 g L−1 CDW with PHB accumulation of 6.24 g L−1 over a 36 h period [83]. This means a growth rate of only 0.17 g L−1 h−1 was achieved and points to the limitations of batch fermentation processes which is not efficient and suited for industrial scale-up.
2.2. Fed-Batch Cultivation
Fed-batch cultivations are the most common biotechnological processes [84] including PHA production due to the ability to control nutrient and carbon input which allows for the avoidance of carbon limitations throughout the process. Controlling these conditions allows for a more efficient process resulting in an increased cell density and PHA accumulation within the process [81]. The real-time monitoring and regulation of substrate concentration and the adjustment of conditions within the process presents challenges in maintaining a standardised repeatable process but once the predicted growth rate and nutrient consumption are controlled/maintained then reproducible processes can be achieved. Some high cell density fed-batch operations involving P. putida and B. sacchari have shown growth of up to 159.4 g L−1 and 221 g L−1 respectively, with % PHA of this CDW at 51% and 45%, respectively [12,72,85,86]. P. putida KT2440 produced a biomass of 141 g L−1 CDW and PHA (mcl) accumulation of 72.6 g L−1 after 38 hrs when growing on oleic acid achieving a PHA accumulation rate of 1.91 g L−1 h−1 [12]. Ramsay and co-workers reported on a number of fed batch fermentation strategies to produce high cell density cultures with a high content of PHA achieving up to 71 g L−1 and 56% PHA and 1.44 g PHA L−1 h−1 [87]. Thus fed batch fermentations are far more productive compared to the batch cultivation mentioned above in Section 2.1—making fed-batch cultivations a far more suitable fermentation production process for scaling up.
2.3. Continuous Cultivation
Continuous cultivations are very different when compared to batch and fed-batch cultivations as once the desired PHA and biomass accumulation rates are reached, these conditions are kept constant making for a chemostat culture [9,88,89]. The harvesting of PHA accumulating cells is continuous. This fermentation strategy is considered to be beneficial in that it allows for a consistent production of PHA with near identical quantities and qualities from start to finish of the continuous process [90]. Even though this process aims to establish long-term genetic stability of the PHA producing strain, the very nature of the long lasting continuous process can result in the contamination of the culture and genetic changes over time [91]. Another challenge is the large volume of spent fermentation media that is produced on a daily basis. This has to be cleaned up and the water re-used. An example of continuous cultivation was demonstrated using Pseudomonas oleovorans ATCC 29347 on single carbon substrates such as citrate, hexanoate and octanoate. This involved a constant concentration of nitrogen with a step-wise increase in carbon concentrations which allowed for the production of mcl-PHA during the growth phase on fatty acids and the nitrogen limitation phase once carbon was present [92].
2.4. Scale up of PHA Fermentation Processes
Although PHAs are accepted as a promising biopolymer for a green economy compared to petrochemical polymers, their production at an industrial scale does present challenges. The production of PHA’s at a commercial scale is made possible through the manipulation and optimization of the process with modern scientific and engineering practices to achieve high cell densities with a higher percentage of PHA within the cell [21]. The cost of producing PHA is dependent upon the cost of the fermentation substrate, which can account for up to 50% of the overall production costs, the volumetric productivity of the process (g·L−1 h−1) and the level of PHA in the cell (% of cell dry weight) as the efficiency of the downstream process is higher with higher PHA content in the bacterial cells [4]. Choi and Lee estimated that at 100 kt scale, PHA production costs between USD 2.6–6.7 $ kg−1, depending on the PHA producing microorganism [93]. Compared to plastics such as polyethylene (USD 0.9–1.0 kg−1), production costs are up to 7 times higher for PHA and some reports indicate as much as 10 times higher [4]. One method for reducing this ten-fold cost difference is the scale-up of the fermentation process. In Austria, PHB was produced at 1000 kg/week in a 15 m3 fermenter using bacterial species Alcaligenes latus which grew rapidly on sucrose [94]. Up to 90% of the CDW from this process contained PHB which allowed for the high productivity, although the carbon source is still in direct competition with the food chain [95]. PHBHHx was produced in a 20 m3 using glucose and lauric acid achieving 50 g L−1 and 50% of CDW was PHBHHx, using the strain Aeromonas hydrophila [96]. The major issue for this scale-up was the cost of DSP, specifically extraction, where the use of ethyl acetate and hexane increased overall production costs dramatically. Other research has looked at the production of PHAs by halophilic, salt requiring microorganisms. The use of such microorganisms is advantageous as they can grow optimally under conditions of high salt concentrations, with such high salt concentrations capable of reducing the chance of microbial contamination to a large extent, and the inherent low value of substrates that can be utilised from these high salinity environments [97,98]. However, it has been reported by the company BluePHA in China that the production of PHB and PHBV using Halomonas species can present problems when scaling up. The high salinity causes difficulties with the downstream processing effluent, corrosion among fermentation equipment, and the lack of well-defined genetic and system engineering, making PHA production using halophiles challenging [98,99]. Despite this BluePHA appear to be operating at an industrial scale and producing PHAs. The use of methanotrophic (methane consuming) microorganisms to produce PHB is also of high interest, as utilising a renewable and potent greenhouse gas and creating a strong carbon capture technology within the polymer itself is highly advantageous [100]. Even with this double-barrel benefit, the use of methane (often fed in the form of scrubbed biogas) as a substrate for PHA producing microorganisms does present significant challenges. The low solubility of methane in aqueous solutions under atmospheric pressure (22 mg L−1) results in a low mass transfer, which in turn can result in low cell growth and density [100,101]. Methane can be sourced from anaerobic digesters or landfills, so scaling up fermentation systems using this carbon substrate is feasible [102]. The cost of PHB production using methane can range from $4.1–8.5/kg, depending on production capacity [103,104]. These are not economically competitive prices and the market may require incentives for methane to PHB production facilities in tandem with rising fossil fuel prices, in order to be a viable technology [100]. Even with these challenges, some biotech start-ups such as Mango Materials in the San Francisco Bay Area are scaling up their methane to PHB process. They have most recently implemented their ‘Launch Facility’ which includes a 5000 L fermentation tower system with complete DSP setup at the Silicon Valley Clean Water waste water treatment plant in California.
Another important issue for biopolymer production is the sourcing of a sustainable carbon feedstock, which should not compete with food-chain feedstocks [105,106] as this creates competition with food supply and land use consequently increasing the cost of that raw material affecting both food prices and PHA production costs [85]. Waste substrates are a good alternative to food based resources but the availability, heterogeneity and quality of the starting material are challenges that need to be addressed.
Although the upstream costs for the scale-up of PHA production is vitally important (e.g., carbon feed cost) to make it competitive with other fossil fuel polymer production processes, an important research and development focus for successful up-scaling is on the downstream processing that ensures a reproducible polymer with desired physical and chemical properties. The drying of bacterial cells, the method of extracting the polymer from those cells affects the molecular weight of the PHA. The properties of polymers are dependent on the monomeric composition and molecular weight of the polymer and this affects the types of applications the polymer can be utilised for, hence the biotechnological and downstream processes need to economically produce a polymer with a consistent and desirable molecular weight.
2.5. Downstream Processing to Harvest PHA
The downstream processing of PHA and the extraction methods involved are critical for the commercial scale-up of PHA production, with many of these technologies currently in the development stage [50]. Many downstream processing recovering methods have advantages or disadvantages in regard to economics, ecological impacts, safety, recovery yields, production purity/quality and difficult scalability [83]. Some of the more well-known recovery methods involve the use of solvents to obtain a good quality product, but solvents such as chloroform should have no place in the PHA production chain on a larger scale [107]. The extraction of PHA using the halogenated solvent chloroform is the performance benchmark for the extraction process of PHB and PHB co-polymers from its cell biomass, particularly at a laboratory scale [108]. It is the benchmark as studies by Rebocho et al. showed that their PHBHV/3HB polymer grown from halophilic yeast Pichia kudriavzevi VIT-NN02 from agricultural waste materials has a yield recovery of 99.99% using this method [109]. Solvent extraction using the likes of chloroform then require an antisolvent (usually at low freezing temperatures) to precipitate the polymer out of solution. This increases the resource input thus increasing waste potential that requires even further significant energy input to separate the solvent and antisolvent for reuse [108]. For these reasons the commercial scaling of such a process is not feasible. The extraction of mcl-PHA using acetone is the most common solvent extraction method for this type of polymer, with precipitation induced most prominently by the anti-solvents methanol and ethanol (typically at freezing temperatures) [110]. It is possible to obtain a pure polymer with a high molecular weight, while removing the bacterial endotoxin using this method [9,111]. Although a portion of the utilised acetone can be recycled, the environmental impacts and capital costs for using solvents in such extraction processes is not recommended for industrial scale-up [9]. Larger scale studies (medium scale @ ~200L) on cells produced by P. Putida involved heat pre-treatment at 121 °C, followed by digestion using Alcalase, EDTA and SDS with an optional final chloroform extraction to increase PHA purity from 95% to 99% [111,112]. This method is preferable as the quantity of chloroform used it significantly reduced. The used of SDS and enzymes will be discussed in more detail in the following paragraphs.
The use of sodium hypochlorite (halogenated) in tandem with anionic surfactant SDS (irritant) for the removal of PHA from cell mass goes against the theory of a greener plastic production process [83,113]. The use of sodium hypochlorite has also been reported to reduce the molecular weight of the polymer by half, even post optimization of the digestion time and parameter conditions [83,114].
Other reported methods for the extraction of PHA from biomass involves the use of enzymes to breakdown the outer non-PHA component of the biomass [83]. This non-PHA part of the biomass usually makes up <10% of the total cell dry weight, with the use of enzymatic catalysts for this step highly beneficial compared to chemicals such as sodium hypochlorite. Although enzymes have advantages in theory, the methods are still in their infancy and not cost effective due to the slow reaction rates of enzymes and low final product purities [34]. For these reasons, enzyme cocktails are often used in tandem with oxidants such as hydrogen peroxide to achieve the yields and purities required for commercial-scale up [115].
3. Properties of PHAs
Conventional fossil fuel derived plastics are used across every facet of life today, from the home to nearly all industries due to their properties allowing for their manipulation into convenient, durable and specialised products [4]. The formation of such products has paved the way for applications that would not otherwise be possible. However, in recent decades it has become apparent that this has come at a high cost with little or no sign of this cost subsiding. Common fossil derived plastics such as polyethylene, polypropylene and nylon are xenobiotic. Their release into the environment is resulting in environmental pollution that is further complicated by their mechanical degradation into microplastics [4,116]. The environmental damage and unsightly nature of non-biodegradable polymers must be halted but the dependency that the modern world has on these plastics is strong with little sign of subsiding. Plastics have many positive attributes that the world needs but a lack of waste management safety nets to capture the waste plastic contributes to a world where plastic pollution is taking place unabated. Society is calling for the reduction in plastic use and the use of a suitable replacement. A number of polymers such as polylactic acid and thermoplastic starch are in the market and their production is increasing. PHAs (Figure 5) show great promise but have limited market share [4].

Figure 5.
General structure of short chain length and medium chain length PHA [4].
The replacement of fossil plastics with biobased plastics addresses the issue of renewable carbon but not end of life management. The biodegradability of biobased plastics can help to address end of life management by offering more options. Biodegradable plastics should not be produced so that they can be released into the environment but rather that they increase the end of life management options for plastic and increase the chances of the plastic being collected and managed. Plastic products could be designed with emergency biodegradation in the event that they are accidentally released into the environment but this should not be the driver for plastic resource management as it could promote bad behaviour and continue to perpetuate plastic pollution.
3.1. General Properties of PHA
The properties of PHAs, such as processability, mechanical properties, UV resistance, are similar to commercially available thermoplastic fossil-based polymers such as polypropylene (PP) and polystyrene (PS). Depending on the composition of its monomers, with over 150 types of PHA structures reported in the scientific literature, an array of different properties and functionalities exist within this biopolyester family [44]. One universal property of PHAs is that they are thermoplastic, with other properties varying depending upon their chemical structure [117]. The number of carbon atoms present in the monomer dictates their molecular structure, along with the chain length of these associated monomers, which results in the diverse and multifaceted characteristics of PHAs [118]. The thermal, mechanical, and barrier properties are the most important and studied features of PHAs. Thermal properties such as glass transition temperature (Tg) and melting temperature (Tm), along with mechanical properties such as tensile strength (MPa) and extension to break (%) are key for determining the appropriate use of PHA polymers for various practical applications [119]. The thermal properties of PHAs depend on the polymer’s chain length, and these attributes directly affect the polymer’s mechanical properties. As mentioned earlier, based on the length of side hydrocarbon chain, PHAs can be broadly classified into three categories; scl-PHA, mcl-PHA and lcl-PHA. The subsequent section of this review will cover mechanical, thermal, and other functional properties like PHA’s gas barrier properties and its structure-property relationships. The discussion will primarily focus on scl-PHA and mcl-PHA; however, lcl-PHA is less common for applications due to its inferior properties, so it will not be discussed.
3.2. Properties of Scl-PHAs
The scl-PHAs are a class of PHAs with a number of side chain carbon atoms ranging from 3 to 5. For example, poly(3-hydroxybutyrate) (PHB), poly(3-hydroxyvalerate) (PHV) and their copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) are typical examples of short-chain-length PHAs [120]. The properties of these polymers vary depending on their substrate type, length of side chain, co-monomer and co-polymer composition. In general, PHA polymers produced by bacteria are semicrystalline, and crystallinity can influence the polymer properties. PHB homopolymer is known to develop high crystallinity (>60%), making it stiff and brittle [121]. Despite PHB having mechanical properties close to commercial polypropylene (PP), the challenge in melt processing PHB into products is one of the major factors restricting its broad adoption in commodity applications. The thermal properties, mechanical properties and other physical properties of PHB are discussed here in this sub-section.
3.2.1. Thermal Properties
The scl-PHA polymers are thermoplastics and differ in their thermal properties depending on their chemical composition. PHB homopolymer has a melting temperature (Tm) around 180 °C, close to its decomposition temperature [122]. Incorporating co-monomers such as 3-hydroxyvalerate (3HV) into the polymer chain decreases Tm and crystallinity of the polymer [123]. The glass transition temperature (Tg) is linked to the segmental mobility of polymer chains, which play a critical role in dictating the thermal and mechanical properties of the polymer. Previous studies have indicated that the number of carbons has a direct effect on the glass transition (Tg) and melting temperature (Tm), and crystallization temperature (Tc), of PHAs [119]. The Tg and Tm are essential thermal properties for various applications of PHAs. PHB is one scl-PHA that is highly explored for its properties. The linear chain structure of PHB leads to a predominantly crystalline phase, which is interconnected through the amorphous sections creating a semicrystalline polymer. A large number of reports suggest the range of Tg is −15 °C to 9 °C, depending upon the structure of PHB and its copolymers [124,125,126,127]. Similarly, the melting point (Tm) of PHB is reported to be between 160 °C to 180 °C, which depends upon the type of its crystalline phase [128]. Isostatic PHB displays a high melting temperature close to 175 °C and the Tg is close to room temperature (21 °C), resulting in a very narrow processing temperature window for PHB [129].
The thermal stability and degradation mechanism are important as they affect processability and mechanical properties. Thermal decomposition of PHB takes place just above melting temperature, making it vulnerable to degradation during processing at 180 °C. A degradation temperature of 210–220 °C of PHB limits the melt processability of PHB due to a narrow temperature window between melt and degradation temperatures. Temperature-induced chain scission in PHB can result in a rapid decrease in molecular weight [130]. Thermal analysis of melt-processed PHB indicates a double melting peak due to bimodal distribution of crystallite size resulting from changes in molecular weight due to random scission of PHB during the melt processing [131]. Various approaches have been implemented to improve the melt processibility of the PHB polymers. The addition of nucleating agents, plasticizers, blending with other polymers, co-polymerisation, nano additives etc. have been evaluated to improve the processability of PHB [132,133,134,135]. The addition of plasticizers reduces the interchain entanglement within the polymer and promotes PHB processing without thermal degradation [136]. Also, the inclusion of nucleating agents increases the spherulite counts due to the formation of numerous small and imperfect crystallites, leading to the lowering of Tg and the overall decrease in crystallinity of the polymer [137,138,139]. However, it is important to mention that some of the approaches to improve thermal stability and processability of PHAs can affect biodegradation [140].
3.2.2. Mechanical Properties
PHB polymers are traditionally stiff and brittle due to their high crystallinity. The mechanical properties of PHBs are similar to polypropylene; however, the lower elongation and high stiffness prevent PHB polymers from replacing fossil-based polymers such as polypropylene. Tensile strength measures the amount of force required to pull a material until it breaks and is typically within a range of 8.8 to 50 MPa for PHB synthesized from different raw materials. Similarly, Young’s modulus presents the stiffness characteristics, and scl-PHAs show a modulus of 3.5 × 103 MPa [141]. Elongation at break is the measure of the material’s ability to be stretched until it breaks, and it is expressed in the percentage of the original length of the material. The scl-PHAs show percentage elongation at break ranging very low to 15% to >200% based on its chemical and physical structure [142]. Pure isotactic PHB is brittle that show a tensile strength of 30–35 MPa, elongation at break around 15% and modulus of 1.2 GPa [117,143]. The primary reason for the high brittleness of PHB is due to slow secondary crystallization that occurs within the amorphous phase of PHB [117].
Though PHB has poor ductile properties, the addition of co-monomers, plasticizers, various polymers and fillers significantly enhance its elongation and tensile strength by reducing the crystallinity of the polymer [144,145]. The mechanical properties of the PHB polymers are altered by incorporating co-monomer units, side chains, and bulky functional groups by controlling the overall crystallinity of the polymers. Mangeon et al., reported a 6.5 fold improvement in percentage elongation at break values of natural terpene based plasticized PHB [145]. Table 3 below presents the effect of copolymerization on the mechanical properties of PHB.

Table 3.
Mechanical properties of various types of PHB.
Thus, the rigidity of PHB can be well-tuned by introducing some co-monomers into the backbone, which increases the flexibility to a significant extent. For example, the introduction of 3-hydroxyhexanoate (3HHx) comonomer to PHB by 17 mol% increased the ductility in poly-3-hydroxybutyrate-co-3-hydroxyhexanoate, P(3HB-co-3HHx) [148]. The physical blending of PHB with P(3HB-co-3HHx) through melt or solvent mixing lead to a similar improvement in flexibility of the blend system [151].
The ageing process largely impacts the mechanical properties of PHA overtime during the shelf-life of the polymer. The secondary crystallization phenomenon in the amorphous phase of PHB reduces flexibility and makes the material brittle. As a consequence of ageing in PHB, the tensile moduli increase while stress at break remains stable [152]. Further discussion on the ageing process for PHB is discussed in the next section.
3.2.3. Ageing of PHB
One major drawback with PHB materials is their slow ageing phenomenon over time, decreasing mechanical properties. Ageing of PHB occurs by slow changes in its amorphous and crystalline phases, resulting in either hardening or softening of the material. The ageing phenomenon in PHB primarily occurs through secondary crystallization as well as physical ageing [153]. At the first stage, after melt processing, PHB cools down without undergoing any crystallization; this is followed by the second stage of autocatalytic crystallization that occurs at a high rate. In the final stage, secondary crystallization occurs at a very slow rate within the amorphous regions of PHB [154]. The secondary crystallization that usually occurs within the amorphous phase leads to the formation of imperfect crystals along with the interlamellar spherulitic spaces. As a result, the polymer chain mobility in the amorphous phase becomes restricted, making the polymer more brittle [129]. The physical factors of ageing in polymers are related to the relaxation of polymer chains below its Tg due to the residual mobility of polymer chains in its glass phase [155]. Overall, the ageing of PHB leads to deterioration of mechanical and physical performances. Biddlestone et al., reported that the embrittlement effect of ageing in PHB can be reduced satisfactorily by annealing the PHB samples at 77 °C prior to storage. Another method to control the secondary crystallization of PHB is the addition of nucleating agents and then anneal is at 146 °C. Such annealed PHB samples are reported to retain their ductility during complete storage period [156].
3.2.4. Gas Barrier Properties
PHAs have an excellent barrier to air and moisture, making them suitable for packaging applications. The gas barrier property of polymers has been considered an essential property in packaging applications due to its associated advantages of lightweight, easy processing, and forming characteristics. Most biodegradable polymers have similar oxygen barrier properties to conventional petroleum based polymers, however, the barrier properties of biodegradable polymers decreases with the increase in humidity. PHB show better moisture and oxygen gas barrier properties than polypropylene (PP) and polyethylene terephthalate (PET), respectively [157]. The lamellar structure in the PHB crystalline phase contributes to its superior aroma barrier properties and other gas and moisture barrier properties [158]. Unlike amorphous polymers, the crystalline phase of PHB restricts the passage of gas molecules, making it more relevant to food packaging applications. Given the advantage of the high barrier properties of PHB, it can be blended with other biodegradable polymers and obtain a polymer blend with improved gas barrier properties suitable for food packaging applications. Thellen et al., reported extruded PHB and PHBV films with OTR (0% relative humidity (RH) and 23 °C) and WVTR (100% RH, 23 °C) values ranging from 193–410 cc-mil × m−2 × day−1 and 114–217 g-mil × m−2 × day−1, respectively [159]. However, further developments are needed to improve PHB barrier properties to replace fossil-based polymers with PHB for packaging applications.
3.3. Properties of Mcl-PHA
The mcl-PHA polymers typically have more than 5 side-chain carbon atoms and the physical nature varies from semicrystalline to an amorphous nature. The long side-chains of PHA affect the crystallisation phenomenon and lead to a reduction in thermal and mechanical properties. Also, the variation in monomer composition and bulky functional groups influence the elastomeric properties of mcl-PHA [160]. The typical examples of mcl-PHA are poly(3-hydroxyoctanoate) (PHO) and poly(3-hydroxynonanoate) (PHN), which are primarily formed as copolymers with 3-hydroxyhexanoate (HHx), 3-hydroxyheptanoate (HH) and/or 3-hydroxydecanoate (HD). Wide variation in properties of mcl-PHAs has been reported in the literature due to the lack of commercial polymer availability, variation in the production methods, type of carbon source that is used for production of mcl-PHA polymers [161]. A discussion on the thermal and mechanical properties of mcl-PHA are detailed below.
3.3.1. Thermal Properties of Mcl-PHA
The glass transition temperature (Tg) is closely associated with the polymer chains’ segmental mobility, which dictates the toughness and other physical properties of the PHA polymers. The mcl-PHAs have a Tg value ranging between −65 °C and −25 °C with a melting temperature between 40–70 °C [140]. An increase of 4 to 7 carbons in the side chain of PHAs, increases the melting temperature (Tm) from 45 °C to 69 °C due to increased crystallisation upon the participation of both the main chain and side chain carbons in the smectic structure [162]. The Tg of mcl-PHAs decreases with an increase in the carbon chain length and presence of pendant groups. The mcl-PHA and its co-polymers have lower thermal properties due to large and irregular side chains that inhibit polymer chains’ close packaging to crystalline structures [163]. Several other literatures support the tendency of side chains of mcl-PHA to crystallize and thus alter the thermal properties [164]. In the case of mcl-PHA with a large fraction of 3-hydroxydodecanoate (3HDD) and 3-hydroxytetradecanoate (3HTD), these show two melting peaks separated by a cold crystallization peak [165]. This dual-mode crystallization has been attributed to the presence of two distinct crystalline phases, which have different crystallization kinetics due to the variation in side chains lengths. PHA co-polymers containing 12–15 mol% aromatic side chains have shown increased Tg due to the rigidness of the side groups in the copolymers [166]. However, some co-polymers of mcl-PHA polymers do not crystalise due to the disorder introduced by the side chains present in the polymers [56,167]. The crystallization phenomenon of PHO is prolonged and requires several days to weeks to complete at room temperature; however, lower temperatures are more favourable to achieve maximum degree of crystallisation [168]. PHO polymers can attain 30% crystallinity (max) and having a Tg close to −35 °C and Tm nearly at 61 °C [30]. The crystalline regions act as physical crosslinks between amorphous phases of PHO and hence the polymer behaves like thermoplastic elastomer [167]. In isothermal crystallization studies using DSC, it is understood that the melting peak of PHO becomes more defined and increases at higher crystallization temperatures. It refers to the ability of the polymer chains to reorganize in more ordered crystalline domains at higher crystallization temperatures [169]. Copolymerization of mcl-PHA alters thermal properties like Tg, Tm, Tc and heat of fusion (ΔHm). In particular, the side chain carbon length of co-monomers has a greater influence on the crystallization mechanism and thermal properties of mcl-PHA [170]. For example, as the number of side-chain carbon atoms increases from 3 to 4 or higher, the layered crystal structure of packing with both main and side chains is developed, increasing Tg and Tm [164]. As the number of -side chain carbon becomes 7 or more, smectic liquid crystalline phases form at low temperatures and leads to cold crystallization [162]. Hence, considering its thermal characteristics are more relevant to elastomers, PHO has gained significant importance as an additive in blending with low ductile biodegradable polymers.
3.3.2. Mechanical Properties of mcl-PHA
Mechanical properties are critical when selecting PHA polymers for a specific application. In general, mcl-PHAs are more flexible and suitable for food packaging and tissue engineering, where biodegradability and biocompatibility are required [141]. The mcl-PHA co-polymers containing 3-hydroxyalkonate are much more flexible due to their lower crystallinity and close to properties that of LLDPE polymer [171]. The crystalline parts of mcl-PHA act as the physical crosslinks, contributing to its mechanical strength. Marchessault et al., reported the modulus and elongation at break of PHO is 17 MPa and 250–320%, respectively [170]. In another report (Gagnon et al.) mcl-PHA with 86% of 3HO presents modulus and elongation to break of 9.3 ± 1.4 MPa and 380 ± 40%, respectively [168]. The elongation at break for mcl-PHA is also reported to be as high as 1000%, which varies with the side chain’s length and chemical nature [88]. Table 4 presents the mechanical properties of various mcl-PHA polymers.

Table 4.
Mechanical properties of various types of mcl-PHA.
From Table 4, it is evident that there is a change in the mechanical properties of the PHA with the addition of one or more co-monomers into the PHA main chain backbone. For example, the copolymer containing 4.6 mol% of 3HHx improves tensile strength and modulus to a greater extent, while the elongation to break is reduced by 97–99% relative to PHO. In general, it can be understood that the high elasticity of mcl-PHA is relevant to flexible packaging applications. At the same time, the poor mechanical strength and low melt temperature limit its widespread acceptance. Further, an emphasis has been made to modify mcl-PHA adopting different techniques discussed later sections in the review.
3.4. PHA Modification
PHAs are a type of polymer that is derived from bacteria and are degraded by bacteria. Hence, this polymer holds great potential for the circular bioeconomy. Though PHAs possess a wide variety of properties, they are difficult to use on their own in certain applications [117]. In this regard, modification of PHAs is an essential prerequisite to make them suitable for various applications. In this section, a detailed discussion on PHA modification will be covered.
In the broad sense, PHA modification is classified into three primary sections as presented in Figure 6:
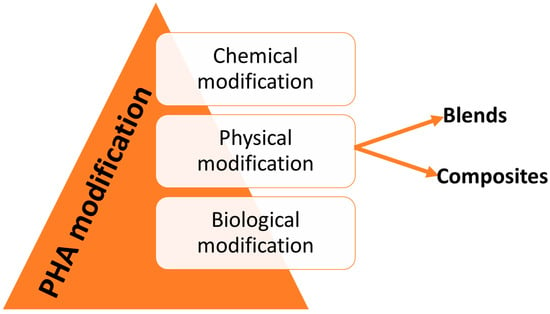
Figure 6.
Different modifications of PHA.
- Chemical modification
- Physical modification
- Biological modification
Each section contains multiple subsections that will be discussed individually, along with the underlying mechanism associated with the modification process.
3.4.1. Chemical Modification of PHA
The primary reason for chemical modification is introducing functional groups to PHA that add valuable attributes that biotechnological conversion processes cannot easily achieve. These chemically modified PHAs, possessing improved properties, can be utilized as multifunctional materials [174]. The actual processing temperatures of PHAs are essentially determined by their crystalline melting points (Tm) because of their poor thermal stability during melt conditions. Larsson et al., performed a stability study on both the PHAs after Soxhlet extraction with CHCl3 followed by washing with aq. HCl solution, respectively. An increase in thermal decomposition temperature of 50 °C was reported for PHA washed with an aqueous solution of HCl acid [175]. In another attempt of mcl-PHA modification, Nerkar et al. used Lauroyl peroxide (L-231) as a crosslink agent of PHO, before melt blending with PHB [176]. Due to the variation in the melt viscosity between PHB and PHO there is poor compatibility between the two polymers during the melt mixing. However, the chain extension of PHO using peroxide prior to melt mixing with PHB enhances the viscosity of PHO, which improves both modulus and elongation to break. In similar context, Xiang et al., modified PHBV using DCP as crosslinking agent and the resultant long-chain branched copolymeric poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (LCB-PHBV) showed an accelerated crystallization rate [177]. Gopi et al., reported improvement in crystallization characteristics of PHO upon chemical modification with DCP and triallyl trimesate coagent [178]. Bhatia et al., modified PHBV co-polymer with ascorbic acid using Candida antarctica lipase B mediated esterification [179]. The ascorbic-modified PHBV showed a lower degree of crystallinity (99.6%), making it more ductile and easy to process. Also, the thermal degradation temperature increases to 294.9 °C and biodegradability enhanced by 1.6 fold compared to unmodified PHBV. PHA can be modified by chemical treatment with para-toluene sulfonic acid monohydrate (APTS) catalysed by acid, which leads to PHA linear chains forming with one end hydroxyl group and the other end carboxyl group [180]. These monohydroxyl terminated PHA act as an initiator for ring-opening polymerization of lactones. Polyurethanes based on different PHAs like P(3HB), P(4HB) etc. were synthesized by melt polymerization of PHA with Hexamethylene diisocyanate (HDI) under relatively mild processing condition [181]. Similarly, dihydroxyl terminated PHA is synthesized by the reaction of PHA with 1,4-butanediol in the presence of p-toluenesulfonic acid catalyst [182]. Diblock and triblock blends were prepared by reacting dehydroxylated PHA with polyethylene glycol (PEG), which show improvement in properties [183]. A range of literature is available on PHA chemical modification, and some of the methods can be adopted for large scale industrial applications.
3.4.2. Physical Modification of PHA
The blending approach and incorporation of additives are widely adopted methods to modify the individual polymers’ deficiencies. Moreover, the blending approach is more economical than co-polymerisation and other chemical modification methods. The goal for developing biodegradable polymer blends and composites is to improve the adhesion between the individual polymers components, reduce the interfacial tension between these components, and generate desired phase morphology and improved performance [3]. However, three factors that limit the blending with various polymers is; morphology of the biphasic system, degree of miscibility, and extent of compatibility between the phases. At thermodynamic equilibrium, the mixture of two polymers in an amorphous state exists as a single-phase; hence, the blend is treated as compatible, improving the physical and mechanical properties of the resulting blend polymer. The blend systems reviewed below focus on PHA based blend composites suitable for packaging film applications.
The selection of plastic packaging materials for food packaging largely depends on the type of food and shelf life of the product. The plastic film materials targeted for food packaging need to be versatile enough to withstand the handling process and should be able to maintain the physical and chemical integrity while retaining the aroma and keeping the food products fresh [184]. For example, stereoregular poly(L-lactic acid) [PLLA] cannot withstand high-temperature beverages; however, stereo complex PLA can be high heat resistant and suitable for high-temperature beverage applications [185]. While PLA alone does not provide good gas and moisture barrier properties [186], specific grades of PHA with a high degree of crystallinity can provide better barrier properties than PLA [152]. Therefore, biodegradable polymer blends are desirable to produce products similar to fossil-based polymer products.
PHA–PLA Blends: PLA is one of the most extensively used biobased and biodegradable plastics to date and has already gained attention for use in food packaging applications. The difference in theoretically calculated solubility parameters of PLA and PHB is very low and this represents possibility for good miscibility between the two polymers [187]. However, it is not always the case since the miscibility between PLA and PHB is also dependent on the molecular weight of both polymers, processing temperature and composition of blends. One component with low molecular weight in these blend systems leads to better miscibility, whereas PLA and PHB are higher in molecular weight and distinctively phase-separated [188]. Melt mixing of PHB with PLA in different compositions while keeping PLA as the matrix phase leads to a blend system with a higher degree of crystallinity and improved thermal properties [189]. The better mechanical property of PLA/PHB at 75/25 ratio is attributed to the fact that the added PHB acted as a nucleating agent in the PLA matrix. It is speculated that the synergistic effect of PLA/PHB blend system within the range of 75/25 ratio refers to good compatibility between the two phases [190]. The major difficulty experienced during film extrusion of PLA/PHB blends is due to its high brittleness and poor processing characteristics [191]. To improve these blends’ processability, blending the third component like a plasticizer or compatibilizer enhances the material flexibility and makes it suitable for film extrusion [188]. Numerous biocompatible and biobased plasticizers, used both for PHB and PLA, improve the flexibility of PLA and PHB [192]. Using acetyl tri-n-butyl citrate (ATBC) and polyethylene glycol as plasticizers in PLA/PHB blend led to improved thermal stability, which was studied under isothermal TGA analysis at 180 °C for less than 6 min [187]. Also, adding additives like cellulose nanocrystals (CNC) in the PLA/PHB system retain thermal stability while enhancing the mechanical properties significantly [193]. Apart from cellulosic fillers, the mechanical properties PLA/PHB blends can be improved by the addition of fillers like catechin, and nanofillers like, organically modified nanoclay etc. [194].
PHA holds great promise to be used in blends due to its high elongation properties [195,196]. The blending of PLA with PHA can be beneficial as it improves both polymers’ mechanical performance, in terms of reducing their brittle nature [196]. Most PHA/PLA blends are formed through conventional melt processing. Noda et al. (2004) reported melt-processed blends of poly(L-lactic acid) PLLA with Nodax™, a family of mcl-PHA, poly(3-hydroxybutyrate)-co-(3-hydroxyalkanote) bacterial copolyesters. It was found that a wide range of thermal and mechanical properties could be obtained by varying the type and quantity of mcl-PHA [197]. They reported that the elongation to break ratio was increased as well as improved energy at break was observed in solvent cast PLA-PHB and PLA-PHBV blends. Melt mixing of mcl-PHA with PLA leads to a reduction in brittleness of PLA while improving the ductility in the blend system. The addition of 5–15% of mcl-PHA into PLA reduces modulus of the blend compared to neat PLA, which is attributed to the plasticization effect of low molecular weight PHA and lubrication effect imparted by the high molecular weight mcl-PHA chains. Hence, the material’s ductility, which is usually inverse of modulus, increases in the blend system upon increasing the mcl-PHA content. Subsequently, the formability of these blends into the desired shape also enhances [187]. There is evidence of improved miscibility between PLA and mcl-PHA phases in blends where PHA fraction is higher than 50% [198].
PHA-PCL Blends: To improve their properties, PHA polymers can be blended with fossil-based biodegradable polymers such as polycaprolactone (PCL). PHB blended with PCL to overcome the limitation of brittleness, though several studies reflect the that PHB is immiscible with PCL [199,200,201]. Another member of the PHA family is poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HH)], which shows better ductility compared to PHB; however, the brittleness induced by the slow ageing of P(3HB-co-3HH) limits its application [202].The inclusion of 2.5–20 wt% of PCL into P(3HB-co-3HH) and PHB leads to an appreciable improvement in the ductility of the resultant blend, which is attributed to the fine dispersion of PCL phase within the PHA matrix [203,204]. In the same study, it is observed that there is a severe issue with the embrittlement of PCL/PHA blends with time due to the development of cracks or voids in PHA phase due to secondary crystallisation over a period of time [203]. Garcia et al., studied PHB-PCL blends along the entire composition with 25 wt% increment and found the blends are immiscible at all compositions [205]. Further, in the study, PCL was found to act as an impact modifier for PHB as the elongation to break changed from 11.2% for neat PHB to 1000% for blend with 75% PCL [205]. Nonetheless, the selective solvent etching of one phase of the these blends readily shows immiscibility in scanning electron micrographs, which is supported by two Tgs Przybysz et al., reported improved compatibilization between PCL and PHB through reactive extrusion using di-(2-tert-butyl-peroxyisopropyl)-benzene (BIB) as peroxide crosslinking agent. Compatibilized PCL-PHB (75/25) blend system with 0.5 wt% BIB show elongation to break 305 ± 14%; whereas the un-crosslinked PCL-PHB (75/25) blend show 125 ± 5%. Such improvement in properties is attributed to partial cross-linking/branching of studied blends confirmed by the melt flow rate and gel fraction measurements [206].
PHA-PBAT Blends: PBAT has good melt processability and shows ductile behaviour suitable for film extrusion and high thermal stability. It is chosen as one of the desired biodegradable polymers to blend with PHA. However, PBAT is not miscible or compatible with any of the PHAs, and requires compatibilizers to produce miscible blends with improved properties [207]. The addition of compatibilizers, such as organically modified natural Fibres, clay etc. are some of the preffered approaches to improve compatibility. Though scl-PHA like PHB or PHBV shows high barrier properties due to its high crystallinity, blending of PBAT diminishes its mechanical strength and gas barrier property due to the amorphous nature of PBAT [208]. However, co-polymers such as, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene adipate-co-terephthalate) (PBAT) blend (weight ratio of PHBV:PBAT is 30:70) modified with silane treated 10 wt% of recycled wood Fibre (RWF) improved mechanical morphological and thermal properties of PHBV-PBAT blends [209]. Nagarajan et al., observed improved interphase compatibility in PHBV-PBAT blends upon adding 0.75 phr poly diphenylmethane diisocyanate (pMDI) as compatibilizer in association with switchgrass Fibre loading of up to 30 wt% [210,211]. Larsson et al., carrier out reactive extrusion study of PHB (ENMAT Y3000P)/PBAT (Ecoflex) blend across the complete range of composition using dicumyl peroxide (DCP) as crosslinking agent. The peroxide free radical initiator DCP provided compatibility between the two constituent polymers and this is evident from the improved dynamic shear modulus, an apparent increase in interfacial adhesion, an increase in tensile storage modulus [175].
PHA-PBS Blends: Polybutylene succinate (PBS) is a thermoplastic biodegradable polymer synthesized by polycondensation reaction of 1,4-butanediol with succinic acid [212]. The advantages of PBS over PHB are its high flexibility and impact strength, thermal degradation stability, and chemical resistance makes it a good candidate for blending with scl-PHA [213]. At the first instance, it is reported that PHBV/PBS blends are immiscible, as evidenced by the decreasing crystallization rate of PHBV upon the addition of PBS [214]. In-situ compatibilization of PHBV-PBS blends using DCP as crosslinking agent improved the interfacial adhesion between PBHV and PBS phases due to formation of PHBV-g-PBS copolymers which subsequently acted as compatibilizer and partially cross-linked networks in the blends. An addition of 0.5 wt% DCP in PHBV/PBS (80:20) blend, the elongations at break increased from <10% (for neat blend) to 400% and the un-notched Izod impact toughness values increased from 10 kJ·m−2 (for neat blend) to 50 kJ·m−2 [215]. The increased rate of crystallization of PHBV in the presence of PBS indicated the developed miscibility induced by the crosslinking. Recently, Righetti et al., studied thermodynamically immiscible blends of poly(3-hydroxybutyrate) (PHB)/poly(butylene succinate) (PBS) and PBSA. The resultant blends showed improved ductility with minimal reduction in elastic modulus due to successive solidification of crystalline phases of the individual polymers [216].
PHA-Natural Polymer Blends: Natural polymers are attractive in designing biodegradable polymer blends based on PHA due to their degradability and abundant availability. Poly(3-hydroxybutyrate-co-3-hydroxy valerate) PHBV and cellulose acetate were blended with plasticiser and chain extending agent [217] to improve the ductility of the composites. However, the resultant blends had significantly reduced mechanical and thermal properties due to poor miscibility between the polymers. PHA blended with cellulose acetate butyrate (CAB) is reported to be miscible blends as evidenced by single Tg of PHB [218]. The improved compatibility was due to reduced spherulite growth of PHB in the presence of CAB, leading the amorphous phase of PHB. The morphology investigation of the blend through small-angle X-ray scattering (SAXS) indicates the presence of a homogeneous amorphous phase situated mainly in the interlamellar regions of crystalline PHB [219]. Chiulan et al., carried out a systematic cytocompatibility studies using L929 cell line of a triblend system comprising poly(3-hydroxybutyrate) (PHB), polyhydroxyalkanoate (PHA), predominantmy poly(3-hydroxyoctanoate) with high amorphous content and bacterial cellulose (BC) [220]. The blends show high surface roughness, medium hydrophobicity and enhanced cytocompatibility, making it suitable for biomedical applications. Blending natural rubber (NR) with mcl-PHA was reported to widen the application range of medium chain length polyhydroxyalkanoates by altering its thermal properties while reducing the overall cost of polymer. Also, it was observed that the degradation behaviour of the blend could be further tailored based on the blend composition [221]. The potential applications of these blends include flexible conventional and barrier packaging films, medical materials such as absorbable surgical sutures, matrices for drug delivery systems, and biodegradable moulded goods, paper coatings, non-woven fabrics, adhesives, films and performance additives [219,222,223].
Starch and thermoplastic starch are home compostable and mostly soluble in water, whereas PHAs are industrially compostable and hydrophobic. Hence, blending both the polymers not only eliminates the limitations related to starch films for packaging applications and aids flexible film processing of the blend system [224]. Lai et al., reported a compatible blend of modified corn starch-PHB with a single Tg at 37 °C [225]. However, the large difference in polarity between starch and PHA makes the two systems incompatible. Therefore, crosslinking or a compatibilizer are required to improve the compatibility between the two phases [226]. Sun et al. developed acid crosslinked blend of hydroxypropyl di-starchphosphate (HPDSP)/PHA. Different acids like citric acid, adipic acid, and boric acid were used as a crosslink agent. The superior thermal degradation stability of the crosslinked blends represented effectively is claimed to be due to intermolecular interactions of grafting or cross-linking between starch and PHA. Further, the citric acid crosslinked blend showed 45–72% improvement in Oxygen permeability (OP) and 45.6–270% improvement in water vapour permeability (WVP) in comparison to the control starch/PHA blend. Improved crystallinity and the compact and uniform crystal microstructure in crosslinked crystalline blend systems is the primary reason of increased gas barrier property and light transmission property of the blends.
PHA Bionanocomposites: Many researchers have studied composites of PHA, and a large variety of natural biofibres used as reinforcing fillers in PHA and the resultant composites are termed ‘Bionanocomposites’. For example, plant-based natural fibres like flax fibre [227,228], jute fibre [229], wheat [230], rice straw fibre [220], cellulose fibre [221], wood fibre [222], pineapple fibre [223], and bamboo fibre [231], kenaf fibre [232] etc. are reported to have improved mechanical properties of PHAs. PHA biocomposites with 40% kenaf fibre lost their flexural modulus and strength under the condition of aqueous exposure for 60 days. However, incorporating a suitable compatibilizer to the composite system suggested improvement in the homogeneity between the two phases of polymer and kenaf fibre [233]. Short abaca fibres were melt mixed with PHA and subsequently, injection moulded to demonstrate products. These biofibres were surface modified with butyric anhydride, and the effect of surface treatment of these fibres, fibre length, fibre content were thoroughly investigated by Shibata et al. [234]. The surface treatment of abaca fibres improved the flexural properties of the PHA/abaca fibre biocomposites and 5 mm length is the appropriate length to obtain best mechanical properties. Sisal fibres were added to PHA under inert and oxidative condition resulting in improved thermal stability [235]. Hemp fibres were embedded in PHA polymer matrix and the crystallization studies were conducted to understand the behaviour of the fibres in the PHA matrix [236]. Transcrystallization took place at the surface of the hemp fibres indicating nucleation and improvement in the crystallization process. Wheat starch granules were melt mixed with PHBV at 160 °C and the tensile strength of the composite was found to reduce from 18 MPa to 8 MPa, whereas the Young’s modulus has increased by ~63%, making the material useful within the range of flexible film packaging applications. As part of an interesting observation of this biocomposite, the degree of biodegradation significantly improved upon addition of starch into the PHA matrix. While 20 days were required to degrade 100% pure, 100 micron thick PHBV film, the biocomposite of PHBV with 50 wt% starch granules took only 8 days to completely disappear under aerobic conditions [237]. Wood celluloses are one of the cost-effective biofillers in polymer matrices and incorporation of these fillers into PHBV enhance the stiffness, brittleness and other mechanical properties. The reduced percentage elongation at break, due to the addition of the cellulose, was mitigated by the increased HV content of PHBV [238]. Posidonia oceanica (PO), an abundant Mediterranean seagrass, was used as biofiller for PHBV. The resultant biocomposites were analysed for their physical properties and biodegradability in the marine environment separate to its routine performance analysis [239]. The addition of 10 wt% of acetyl tributyl citrate (ATBC) as a plasticizer along with 30 wt% of PO fibres enhance the processibility of the composite. An increase in PO fibre content from 0 wt% to 30 wt% results increase in tensile strength and Charpy’s impact energy from 2 GPa to 2.4 GPa and 3.6 kJ m−2 to 4.4 kJ m−2, respectively at the cost of a moderate reduction in the elongation to break from 3.2% to 1.9%. Also, biocomposites with 20 wt% PO fibre caused 10% rise in the biodegradation rate of the polymeric matrix after 216 days. Under real-time marine environment, higher degradation of composites was observed compared to neat PHBV film. It was understood that the PO fibres were capable of developing biofilm consisting of bacteria and fungi on the surface after only 3 months of incubation in marine sediments [239].
Inorganic fillers are also used as additives in PHA primarily to enhance its functional properties while maintaining biodegradation characteristics. It is essential to use non-toxic and biocompatible minerals as additives and hence unlike biofibres, selection of mineral additives is challenging. Limited studies were made on layered silicate based PHA composites and Maiti et al., which reported melt extrusion technique to develop PHB/organically modified montmorillonite (MMT) clay composites [240]. These composites have shown improvement in mechanical properties like storage modulus by 40% at 2 wt% filler concentration compared to pristine PHB. Furthermore, the modified clay particles were found to act as strong nucleating agents of PHB and improved thermal stability. The time for 30% biodegradation at 60 °C is 9 and 6 weeks for pristine PHB and PHB with 2 wt% clay particles, respectively [240]. In a similar line, Choi et al., reported improvement in tensile properties like Young’s modulus from 480 MPa for neat PHBV to >790 MPa of PHBV upon addition of intercalated MMT clays. Similarly, from thermogravimetric analysis of the composites, an increase of 11 °C in degradation temperature corresponding to 3% weight loss (T3%) of the composites were observed [241]. Bordes et al. reported to have improved the elongation at the break by 21% and 85% for PHB and PHBV while retaining modulus (>1884 MPa) and tensile strength (>24 MPa) upon adding an optimum of 1 wt% of organically modified montmorillonite (OMMT) due to high degree of nanolayer dispersion within PHB and PHBV matrices [242]. Xu et al., presented the work to develop high-performance PHA-based nanocomposites with long alkyl chain quaternary salt (LAQ) functionalized graphene oxide (GO-g-LAQ). The obtained nanocomposites show improvement in tensile strength and storage modulus from neat PHA by 60% and 140%, respectively. Incorporation of 5 wt% of GO-g-LAQ reduced oxygen permeability of the nanocomposite (0.21 cm3 × m−2 × d × atm) by 86% from neat PHA film (0.21 cm3 × m−2 × d × atm). These nanocomposites also provide inherent antibacterial performance against Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria [243]. The tensile strength, storage modulus at room temperature improved by 60% and 140%, respectively, and it is suggested to have the potential for food packaging applications [243]. Other biobased inorganic fillers like silicon dioxide (SiO2) [244], titanium dioxide (TiO2) [245], calcium carbonate (CaCO3) [246], polyhedral oligomeric silsesquioxane (POSS) [247] etc. have great potential for improvement in multiple properties of PHA based composites.
3.4.3. Biological Modification of PHA
PHAs can be biologically modified in a number ways; simultaneous addition of two substrates in the culture medium and; feeding of substrates containing functional groups [248].
Simultaneous addition of two substrates in culture medium: Many PHA formations can be modified through the co-feeding of substrates in the bacterial fermentation [248]. Halomonas bluephagenesis can produce a PHBV copolymer depending on the concentrations of glucose and propionic acid (or other propiongenic substrates) supplied within the fermentation media [98]. A study by Xu et al. showed that the co-metabolism of glycerol and lignin derivates simultaneously improved cell dry weight and biosynthesis of PHA for P. putida KT2440 [249].
Feeding of substrates containing functional groups: The feeding of different but specific substrates with functional groups is a method for biologically modifying PHA [248]. For example, P. putida can be grown with the presence of ω-phenoxyalkanoates in its culture medium, producing PHA with phenoxy groups in the side chains [250]. Another study found that the introduction of functional groups to PHA through fatty acids allowed for the formation of functional PHA for further grafting [251]. This opens the door to widening PHA diversity further through functional PHA site chains that can be modified through controllable homopolymerization, random copolymerization, block copolymerization and grafting [252].
4. Applications of PHAs
4.1. Food Packaging Applications
The use of plastics in food packaging is ubiquitous as plastics provide physical, mechanical, chemical and microbial protection from the external environment. Plastics are lightweight and have good transparency, making them highly convenient to use as display products. Conventional petroleum-based plastics have advantages like good gas and moisture barrier properties, thermal seal-ability, easy processing, non-toxic, low price, and readily available. Most plastics used in food packaging are non-polar in characteristics and are non-biodegradable e.g., polyethylene’s (PE), polypropylenes (PP), polystyrenes (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET) etc. These non-biodegradable plastics are becoming the primary source of plastic waste that can and are causing severe environmental pollution. The single-use culture of these non-biodegradable plastics represents a severe global ecological problem, also commonly known as “White pollution” [253,254]. In recent years, biodegradable polymers produced from renewable resources are emerging as alternative packaging materials to mitigate the environmental consequences caused by packaging waste. Examples of such biodegradable polymers include starch-based plastics, polylactic acid, polyhydroxyalkanoates, Polybutylene Succinate, cellulose esters etc. However, the major drawback of biodegradable plastics used in food packaging is their poor mechanical, thermal, and barrier properties.
Among commercially available biodegradable polymers, PHA polymers are promising candidates for packaging applications due to their similar barrier and mechanical properties compared to fossil-based plastics such as LDPE and polypropylene [255]. Table 5 presents the barrier properties of some of the biodegradable polymers.

Table 5.
Water vapor transmission rate [WVTR] and oxygen transmission rate [OTR] of certain biodegradable plastics [256,257,258,259,260].
Comparing the moisture barrier property among the mentioned polymers in the Table 5, it is realized that PHBV and PBS exhibit better moisture barrier properties compared to PLA and other commercial bioplastics. Similarly, the PHBV shows the lowest (best) oxygen gas barrier properties, contributing to its valued addition for packaging applications. In a similar context, it is worth mentioning that Thellen et al., observed ten-times higher oxygen gas barrier property in PHA with high degree of crystallinity compared to PLA [159]. PHAs show good gas barrier properties, and it is the appealing point of this bioplastic in numerous packaging applications. Keskin et al., reported having better moisture and gas barrier properties for PHA than its commercial counterparts PLA and PCL [261]. Water vapour permeability (WVP) of commercial PHA like Enmat Y1000P from Tianan and Mirel F1006, Mirel F3002 from Telles show intermediate water vapour barrier properties compared to commercial PP and is much better than PLA [152]. However, PHA polymer containing processing aids such as plasticizers has inferior barrier properties due to decreased crystallinity. Crystallinity is the factor that dictates the gas barrier property of PHAs. scl-PHAs show better barrier performance compared to mcl-PHAs due to their high crystallinity. The side chain length naturally affects the barrier properties of PHA by affecting the crystallinity of the polymer. In the list of biodegradable plastics that are 100% biobased, PHA is rated at the top in terms of gas barrier properties, which makes it a suitable candidate to blend with other bioplastics to improve their mechanical properties.
PHAs are thermosensitive at temperatures generally considered not high in the polymer processing industry and have inferior mechanical properties to conventional fossil-based polymers. Also, widening the applications of PHAs as commodity plastics requires a reduction in the cost of production. Comparatively high production costs of PHA to conventional plastics present problems in its competitiveness for production in the global market [3,262]. Therefore, blending PHA with commercial biodegradable polymers could be an viable approach to PHA polymers for various applications.
4.2. Biomedical Applications
The biomedical applications of PHA and their blends have been widely reported [255]. The properties of these biodegradable, biocompatible and non-toxic blends makes their use in medical applications such as wound management, drug delivery carriers, biodegradable implants (vascular system, orthopaedy), tissue engineering and anticancer agents an attractive option. Some of these applications found in Table 6 will be reviewed in this section.

Table 6.
Applications of PHA in medicine [9].
A high biocompatibility is necessary when exposing the human or animal body to an object that isn’t naturally occurring to that organism [9]. This biocompatibility of a foreign object used in a human or animal is determined by factors such as shape, surface porosity, material chemistry and the environment in which the object is in-situ (tissue) [9]. This is why PHAs have high potential in biomedical applications, as tests have shown that components of PHA, such as (R)-3-hydroxybutyric acid from PHB, are already present in human blood [263]. This ultimately reduces the potential for the immune system to reject an implant that is comprised of natural occurring elements. The natural occurring properties of PHAs are a good starting point for their use in medical applications, but the potential and optimization of such applications requires improvement of properties through the blending of polymers. Raza et al. (2017) reported that mice fibroblast cell growth was increased when they were grown on a PHB/PHBHHx polymer blend when compared to the individual PHB and PHBHHx components. The monomeric units of PHAs can reduce bacterial infection like that from Staphylocuccus aureus with P3HB/P4HB known for aiding in the enhancement of angiogenic properties of the skin and wound healing [255]. Along with increased biocompatibility and regenerative benefits, there is also a significant increase in the tensile strength of blended films when the PHBHHx content of the blend is increased [264,265]. As PHA is so biocompatible and biodegradable it’s also a promising candidate for use as a drug carrier. It was shown by Kassab et al. (1997) that microspheres of PHB can be formed in a range of sizes, these spheres being loaded with rifampicin, allowing for the release of the drug over a 24 h period. This allowed for the rate of the drug released to be increased or decreased depending on the width of the PHB sphere [266]. As well as a wound management and as a drug carrier tool, PHA can also be utilized as a scaffold material in tissue engineering [9]. This is a complex application as it requires the biopolymer scaffold to serve a wide array of functions: biocompatibility; support of cell growth and cell adhesion; guide and organize cells; allow ingrowth of cells and flow of nutrients/wastes and biodegrade in a non-toxic manner [267]. PHA production costs are currently high and so cannot compete with petroleum-based polymers in most applications today, so using PHAs in these complex biomedical applications can warrant the initial production cost.
5. Biodegradability of PHAs and Composites
The detrimental impact of the continuous accumulation and physical degradation of petroleum-based plastics to form microplastics is putting unprecedented pressure on our natural Environment [268]. The total cumulative global plastic production reached 7.82 billion metric tons by 2015 [269]. Increased efforts are required to prevent the release of plastics into the environment, to increase the rate of plastics recycling and to find alternatives to these fossil based recalcitrant plastics. The two major movements circular economy and bioeconomy seek to address resource efficiency (circular economy) and the switch to the use of biobased resources (bioeconomy). The latter makes up one half of the circular economy. Thus, society is shifting towards efficiency of resource use, increased recycling and the use of biobased and biodegradable polymers. Not all biobased polymers are biodegradable and not all biodegradable polymers are biobased. For example there are technologies emerging to produce bio-based equivalents to fossil based plastics such as poly(ethylene) and poly(ethylene terephthalate) [270]. There are also polymers such as Polybutylene adipate terephthalate (PBAT) and polycaprolactone (PCL) that are fossil based and biodegradable. Biobased polyethylene can be manufactured from sugar cane through a multi-step process involving fermentation, to produce ethanol, and chemical catalysis to produce ethylene and subsequently polyethylene [271], refer to ‘Figure 7’ below for more details on this multi-step process. PET has two monomers terephthalate and ethylene glycol. Terephthalate is made from oxidation of para-xylene (1,4 dimethylbenzene) which can be synthesised from 2,5-dimethylfuran (DMF) and ethylene or 4-methyl-3-cyclohexene-1-carboxaldehyde (4-MCHCA). Ethylene glycol, having a similar to structure to glycerol is normally produced from ethylene (fossil fuel based) but can also be produced from biomass such as sugars via microorganisms such as Pseudomonas syringae [272]. Ethylene glycol can then be synthesized from p-xylene (originating from biomass sugars), polymerized with terephthalic acid from biomass to form PET [273]. DMF can be synthesised from fructose [274] while 4-MCHCA can be produced from acrolein and isoprene. Acrolein can be produced through the dehydration of glycerol, a major component of plant oils and a by-product of biodiesel production [275]. Biobased Isoprene can be derived from plants and more recently genetically engineered microbes using glucose as the carbon substrate [276].
Figure 7.
Production routes for fully biobased polyethylene terephthalate (PET) and polytri-methylene terephthalate (PTT). Biomass-derived substrates are marked by white boxes, biobased synthesis of monomers is highlighted in light gray, key monomers in dark gra y, key monomers in dark gray. Abbreviations: MEG, monoethylene glycol; PDO, 1,3-propanediol; TPA, terephthalic acid [277].
Biodegradable polymers such as PHAs are compostable in industrial composting facilities. PHB is also home compostable and a particularly good example of a highly degradable polymer in multiple environments i.e Marine pelagic, fresh water aerobic, anaerobic aquatic, and soil. However PHO, a medium chain length PHA is not home compostable and degraded slowly in aerobic marine and fresh water environments. Furthermore, it exhibited very poor biodegradation in anaerobic aquatic and soil environments [278]. The biodegradation profile of PHO in managed environments is similar in many ways to polylactic acid which is industrially compostable but not home compostable. However, PLA shows no biodegradation in marine fresh water, or soil. Thermoplastic starch (TPS) is the single biggest selling biobased polymer while poly(lactic acid) is the fastest growing. PHA production remains low but its growth potential remains high [279].
The use of PHAs in polymer composites can improve the mechanical and thermal properties of the final biobased products, while also improving the biodegradability properties of the overall biocomposite product [280]. This section will review PHA biodegrading microorganisms and their extracellular biodegradation of PHA, the properties of PHAs affecting biodegradation and the biodegradation of PHA blends and their additives.
Short and medium chain-length PHAs can be degraded by an array of bacteria and fungi under both aerobic and anaerobic conditions. Some of the most effective PHA biodegrading bacteria belong to genera’s such as Pseudomonas, Bacillus and Burkholderia with fungi in the Ascomycota and Zygomycotina genera’s proven to be effective and sometimes even more effective PHA degraders than bacteria [281,282]. Enzymes called PHA depolymerases are mediators of the extracellular biodegradation of PHA, where they breakdown the polymer into shorter chains through hydrolytic depolymerisation to form oligomers with further biodegradation into trimer and dimer units either through the action of PHA polymerase [280] or through lipases and hydrolases [283]. PHB is a common PHA that is broken down in this manner, as shown in Figure 8 below. This figure depicts the protein structure of the extracellular PHB depolymerases where there are three domains: binding, which is responsible for absorption and spreading across the polymer; linker, which is responsible for linking the binding domain to the catalytic domain and; catalytic, which cleaves the PHA and any available dimers and trimers [280].
Figure 8.
A Single PHB crystal enzymatic degradation by PHB depolymerase [280].
Although there are three domains involved with the enzyme attachment to the PHB surface, the enzymatic hydrolysis of PHA’s is a two-step process. The first being the absorption of enzymes onto the polymer surface and the second being the hydrolytic cleavage of PHA bonds induced by the absorbed enzyme [284]. This process favours less crystalline polymers that have amorphous surface crystals as the hydrolytic enzymes can access the less ordered structure more easily [285]. The by-products and efficiency of this enzymatic degradation are dependent on the type of PHA. PHA biodegradation rate or efficiency depends on the physical and chemical properties of the polymer such as crystallinity, types of copolymers, and the copolymeric structure [280,286]. For example, PHBV has a greater amorphous region than PHB due to the presence of 3-hydroxyvalerate meaning it has a reduced water barrier, increasing absorption which allows for an easier access for the enzyme catalytic domain to cleave the PHA [287]. Other factors such as unknown enzymes, PHA side chain length and frequency, environmental conditions (e.g., water temperature) and molecular weight can affect the rate of a polymers biodegradation [280,288].
6. Recycling of PHAs
Recycling is the most desired option to manage plastic waste and associated environmental pollution and greenhouse gas emissions. There is increasing use of biobased and biodegradable plastics to replace fossil-based plastics in various applications to curb the ever-growing plastic pollution and terrestrial water littering. As a result, there is considerable growth in biodegradable plastics in packaging and other commodity products due to more comprehensive end-of-life options available with biodegradable plastics like PLA, PHA, PBS, TPS, and other biodegradable plastics. Figure 9 shows the end-of-life options available for biodegradable polymers. Different recycling processes recycle only 9% of total plastics produced [289].
Figure 9.
End-of-life options for biodegradable plastics.
Mechanical recycling is the most used recycling process, which involves collecting, sorting, washing, and grinding the plastics to obtain homogeneous material for reuse and further processing [149]. However, not all the biodegradable polymers can be recycled in this way as they could degrade due to the reprocessing (thermal-mechanical degradation) and factors during lifetime (exposure to environmental factors like heat, oxygen, light, moisture) [290]. Chemical recycling involves the degradation of polymer into their chemical constituents that can be polymerized to the original product or converted into other valuable products [291]. Even though mechanical recycling is the most preferred methodology, chemical recycling can produce value-added materials and fully represent the circular polymer production economy [292]. Indeed the use of chemical and biological technologies to both depolymerise plastics and convert the individual monomers to value added products including biodegradable plastics is being investigated in two joint EU-China projects i.e Mix-up (https://www.mix-up.eu accessed on 20 January 2022)and BIOICEP (https://www.bioicep.eu accessed on 20 January 2022) Overall, the lack of an ample quantity of biodegradable polymer waste makes recycling less economically attractive than conventional plastics. Chemical recycling is possible with biodegradable polymers; however, the current processes are expensive and not practised on a commercial scale. Apart from traditional recycling techniques, biodegradable polymers can be recycled by organic recycling under industrial composting conditions, which is a unique advantage with biodegradable polymers compared to fossil based plastics [293]. In polymers that cannot be recycled, energy recovery is the preferred option through an incineration process.
PHA polymers have the fastest market growth rate since 2014, and its production reached about 100,000 tonnes in 2020 [294]. A variety of PHA polymers produced on an industrial scale have shown similar fossil-based plastics performances for various applications. Mechanical recycling of PHA polymers is not recommended for PHA polymers due to their poor thermal properties. Generally, PHA polymers are recycled by the industrial composting process, with very few studies in terms of PHA recycling available [295]. Poly(hydroxybutyrate) (PHB) recycling via extrusion studied by [296], showed a significant reduction in the physical properties after two extrusion cycles and 50% decrease in the tensile strength at complete degradation after the third recycling cycle. However, the degraded products of PHB can be used as plasticizer for making PLA based composites [297].
Furthermore, PHA based polymers are often blended with other materials to reinforce their properties, and consequently, mechanical recycling system becomes more complex and expensive. However, it was reported that by blending a small amount of PLA with PHB, PLA could act as a stabiliser by improving the stability of the PHB up to 5 cycles of recycling with a slight decrease in mechanical properties [298,299]. Furthermore, chemical recycling of PHA is reported through the pyrolysis process, leading to the production of crotonic acid monomers [300]. These resultant monomers can be used to produce other polymers, plasticizers, herbicides and cosmetic products. Table 7 shows the recycling pathways of PHA polymer by different recycling and associated products. Currently, PHA polymers not separated from mixed plastic waste streams due to their low volume and non-existence of an eco-system for the recovery of PHA polymers.

Table 7.
Recycling Methods for various PHAs and its blends.
The introduction of biodegradable plastics into the market has given rise to the need for more efficient recycling techniques. Currently, the traditional recycling methods are not compatible with most of the biodegradable polymers. A further problem in the recycling of biodegradable plastics comes from the separation of different types of plastics. Therefore, further efforts and investments are required to improve the segregation of biodegradable plastics to re-use the biodegradable material most efficiently.
7. Conclusions
The current economic viability for PHA production is still challenging when competing with fossil-based plastics sold into high-volume, low-value applications. Given that starting materials to make PHAs is a significant cost factor in their production, waste or low-value substrates are of interest in this review and continue to be across much of the research and development on PHAs. The fermentation production processes, scale-up of these processes, and the downstream processing involved are key addressable pillars of the PHA production chain. An in-depth knowledge of the polymers’ properties and how these relate to applications is critical in steering the production of PHAs in the right direction. While the biodegradability of PHAs and composites is a critical characteristic, the performance of these polymers in products will also be a major factor affecting their market uptake. Thus, research into substrates, production processes, properties, applications, modifications, biodegradability, and even recyclability of PHAs are areas that require further research and development.
In general, biodegradable plastic can reduce plastic littering and return clean organic matter to the soil in the form of high-quality compost by avoiding contamination with persistent plastics. Products based on biodegradable polymers are designed to safeguard our ecosystem and minimise plastic pollution while improving the circularity of resources used to produce the polymers. At the same time, creating circular products, such as biodegradable polymers, provides solutions to environmental issues and supports the creation of new jobs and infrastructures that unlock the potential for local agricultural and forestry value chains. Currently, the share of biodegradable plastic products is tiny (1–2%) and is expected to grow 5–8% in the next 5 years. However, the entry of biodegradable-based plastic products into the plastic market is slow due to high cost, nonavailability of polymers, and widespread confusion among the consumers regarding biodegradable plastic products. Promoting biodegradable-based plastics requires further investments and improving the scale of manufacturin and availability of these polymers to use in various applications. Also, appropriate regulatory policies are necessary to minimise barriers and create end-of-life infrastructure to promote products based on biodegradable polymers.
Further, an appropriate labelling system is needed to distinguish the products based on biodegradable plastic products to create proper recycling and disposal infrastructure to capture the valuable biodegradable plastics entering into mixed-plastic waste streams. In addition, to realise the true environmental benefits of biodegradable plastics, correctly verified biodegradation standards are essential to boost the use of biodegradable polymers. Therefore, biodegradable plastics such as PHA and their composites can contribute to a sustainable circular plastic economy and reduce unavoidable plastic littering.
Author Contributions
Conceptualization, K.O. and R.B.P.; investigation, B.D., P.B. and J.D.M.; writing—original draft preparation, B.D., P.B. and J.D.M.; writing—review and editing, K.O. and R.B.P.; funding acquisition, R.B.P. and K.O. All authors have read and agreed to the published version of the manuscript.
Funding
The researchers were funded by Environmental Protection Agency, grant number 2019-RE-LS-4 and Science Foundation Ireland (BiOrbic Bioeconomy SFI research centre 16/RC/3889). R.B. would like to acknowledge funding from the European Union’s Horizon 2020 research innovation program under grant agreement No. 870292 (BIOICEP). KOC would like to acknowledge funding from the European Union’s Horizon 2020 research innovation program under grant agreement No. 870292 (870294).
Data Availability Statement
Data can be shared with permission from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alamgeer, M. Polyhydroxyalkanoates (PHA) genes database. Bioinformation 2019, 15, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoates in gram-positive bacteria: Insights from the genera Bacillus and Streptomyces. Antonie Leeuwenhoek 2006, 91, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G.W. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; Connor, K.E. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200. [Google Scholar]
- Le Meur, S.; Zinn, M.; Egli, T.; Thöny-Meyer, L.; Ren, Q. Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. BMC Biotechnol. 2012, 12, 53. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Ward, P.G.; de Roo, G.; O’Connor, K.E. Accumulation of polyhydroxyalkanoate from styrene and phenylacetic acid by Pseudomonas putida CA-3. Appl. Environ. Microbiol. 2005, 71, 2046–2052. [Google Scholar] [CrossRef]
- Bhuwal, A.K.; Singh, G.; Aggarwal, N.K.; Goyal, V.; Yadav, A. Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Int. J. Biomater. 2013, 2013, 752821. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Wong, H.H.; Choi, J.; Han, C.S. Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol. Bioeng. 2000, 68, 466–470. [Google Scholar] [CrossRef]
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phospate limitation. Biotechnol. Bioeng. 1997, 55, 28–32. [Google Scholar] [CrossRef]
- Licciardello, G.; Catara, A.F.; Catara, V. Production of polyhydroxyalkanoates and extracellular products using Pseudomonas corrugata and P. mediterranea: A review. Bioengineering 2019, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Munir, R.I.; Blunt, W.; Dartiailh, C.; Cheng, J.; Charles, T.C.; Levin, D.B. Synthesis and physical properties of polyhydroxyalkanoate polymers with different monomer compositions by recombinant Pseudomonas putida LS46 expressing a novel PHA SYNTHASE (PhaC116) enzyme. Appl. Sci. 2017, 7, 242. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and petroleum-based plastics: Strengths and weaknesses. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’Hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for food packaging applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Salvachúa, D.; Rydzak, T.; Auwae, R.; de Capite, A.; Black, B.A.; Bouvier, J.T.; Cleveland, N.S.; Elmore, J.; Furches, A.; Huenemann, J.; et al. Metabolic engineering of Pseudomonas putida for increased polyhydroxyalkanoate production from lignin. Microb. Biotechnol. 2020, 13, 290–298. [Google Scholar] [CrossRef]
- Yu, J. Microbial production of bioplastics from renewable resources. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 585–610. [Google Scholar]
- Reddy, C.; Ghai, R.; Kalia, V. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2016, 29, 1213–1232. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, M.; Mehariya, S.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Ecobiotechnological approach for exploiting the abilities of bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J. Microbiol. 2014, 54, 151–157. [Google Scholar] [CrossRef]
- Bernard, M. Industrial potential of polyhydroxyalkanoate bioplastic: A brief review. USURJ Univ. Sask. Undergrad. Res. J. 2014, 1, 1–14. [Google Scholar] [CrossRef]
- Ankenbauer, A.; Schäfer, R.A.; Viegas, S.C.; Pobre, V.; Voß, B.; Arraiano, C.M.; Takors, R. Pseudomonas putida KT2440 is naturally endowed to withstand industrial-scale stress conditions. Microb. Biotechnol. 2020, 13, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Durner, R.; Witholt, B.; Egli, T. Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth with octanoate in continuous culture at different dilution rates. Appl. Environ. Microbiol. 2000, 66, 3408–3414. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, T.; Gomez, J.; Buffoni, E.; Rodriguez, R.J.S.; Schripsema, J.; Lopes, M.; Silva, L. Exploring the potential of Burkholderia sacchari to produce polyhydroxyalkanoates. J. Appl. Microbiol. 2014, 116, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing ‘Knallgas’ bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Pazdernik, N.J. Basics of Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–31. [Google Scholar]
- Wang, Q.; Nomura, C.T. Monitoring differences in gene expression levels and polyhydroxyalkanoate (PHA) production in Pseudomonas putida KT2440 grown on different carbon sources. J. Biosci. Bioeng. 2010, 110, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [CrossRef]
- Guamán, L.P.; Barba-Ostria, C.; Zhang, F.; Oliveira-Filho, E.R.; Gomez, J.G.C.; Silva, L.F. Engineering xylose metabolism for production of polyhydroxybutyrate in the non-model bacterium Burkholderia sacchari. Microb. Cell Factories 2018, 17, 74. [Google Scholar] [CrossRef]
- Raposo, R.S.; de Almeida, M.C.M.; de Oliveira, M.D.C.M.; da Fonseca, M.M.; Cesário, M.T. A Burkholderia sacchari cell factory: Production of poly-3-hydroxybutyrate, xylitol and xylonic acid from xylose-rich sugar mixtures. New Biotechnol. 2017, 34, 12–22. [Google Scholar] [CrossRef]
- Chen, H. Brief introduction to the biotechnology of lignocellulose. In Biotechnology of Lignocellulose; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2014; pp. 1–24. [Google Scholar]
- Nonato, R.; Mantelatto, P.; Rossell, C. Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar]
- De Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-batch synthesis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from sucrose and 4-hydroxybutyrate precursors by Burkholderia sacchari strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Windhorst, C.; Gescher, J. Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Henne, A.; Cramm, R.; Eitinger, T.; Friedrich, B.; Gottschalk, G. Complete nucleotide sequence of pHG1: A Ralstonia eutropha H16 megaplasmid encoding key enzymes of H2-based lithoautotrophy and anaerobiosis. J. Mol. Biol. 2003, 332, 369–383. [Google Scholar] [CrossRef]
- Krieg, T.; Sydow, A.; Faust, S.; Huth, I.; Holtmann, D. CO2 to terpenes: Autotrophic and electroautotrophic α-humulene production with Cupriavidus necator. Angew. Chem. Int. Ed. 2018, 57, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Modelska, M.; Berłowska, J.; Kregiel, D.; Cieciura-Włoch, W.; Antolak, H.; Tomaszewska, J.; Binczarski, M.; Szubiakiewicz, E.; Witońska, I.A. Concept for recycling waste biomass from the sugar industry for chemical and biotechnological purposes. Molecules 2017, 22, 1544. [Google Scholar] [CrossRef] [PubMed]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef]
- Munawar, K.M.M.; Simarani, K.; Annuar, M.S.M. Bioconversion of mixed free fatty acids to poly-3-hydroxyalkanoates by Pseudomonas putida BET001 and modeling of its fermentation in shake flasks. Electron. J. Biotechnol. 2016, 19, 50–55. [Google Scholar] [CrossRef]
- Annuar, M.; Tan, I.; Ibrahim, S.; Ramachandran, K. Production of medium-chain-length poly(3-hydroxyalkanoates) from crude fatty acids mixture by Pseudomonas putida. Food Bioprod. Process. 2007, 85, 104–119. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B. Life cycle assessment study of biopolymers (polyhydroxyalkanoates)—Derived from no-tilled corn (11 pp). Int. J. Life Cycle Assess. 2004, 10, 200–210. [Google Scholar] [CrossRef]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Gerngross, T.U. Can biotechnology move us toward a sustainable society? Nat. Biotechnol. 1999, 17, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.G.; Méndez, B.S.; Nikel, P.I.; Pettinari, M.J.; Prieto, M.A.; Silva, L.F. Making green polymers even greener: Towards sustainable production of polyhydroxyalkanoates from agroindustrial by-products. Adv. Appl. Biotechnol. 2012, 3, 41–62. [Google Scholar] [CrossRef]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Jamil, N.; Ali, I.; Ayaz, M.H.; Hasnain, S. Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann. Microbiol. 2011, 61, 623–629. [Google Scholar] [CrossRef]
- Kulpreecha, S.; Boonruangthavorn, A.; Meksiriporn, B.; Thongchul, N. Inexpensive fed-batch cultivation for high poly(3-hydroxybutyrate) production by a new isolate of Bacillus megaterium. J. Biosci. Bioeng. 2009, 107, 240–245. [Google Scholar] [CrossRef]
- Koller, M.; Sandholzer, D.; Salerno, A.; Braunegg, G.; Narodoslawsky, M. Biopolymer from industrial residues: Life cycle assessment of poly(hydroxyalkanoates) from whey. Resour. Conserv. Recycl. 2013, 73, 64–71. [Google Scholar] [CrossRef]
- Pescuma, M.; de Valdez, G.F.; Mozzi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef]
- Povolo, S.; Toffano, P.; Basaglia, M.; Casella, S. Polyhydroxyalkanoates production by engineered Cupriavidus necator from waste material containing lactose. Bioresour. Technol. 2010, 101, 7902–7907. [Google Scholar] [CrossRef]
- Alves, M.M.; Pereira, M.A.; Sousa, D.Z.; Cavaleiro, A.; Picavet, M.; Smidt, H.; Stams, A. Waste lipids to energy: How to optimize methane production from long-chain fatty acids (LCFA). Microb. Biotechnol. 2009, 2, 538–550. [Google Scholar] [CrossRef]
- Cerrone, F.; Choudhari, S.K.; Davis, R.; Cysneiros, D.; O’flaherty, V.; Duane, G.; Casey, E.; Guzik, M.W.; Kenny, S.T.; Babu, R.P.; et al. Medium chain length polyhydroxyalkanoate (mcl-PHA) production from volatile fatty acids derived from the anaerobic digestion of grass. Appl. Microbiol. Biotechnol. 2014, 98, 611–620. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Ruiz, C.; Kenny, S.T.; Narancic, T.; Babu, R.; Connor, K.O. Conversion of waste cooking oil into medium chain polyhydroxyalkanoates in a high cell density fermentation. J. Biotechnol. 2019, 306, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. Influence of fatty acids in waste cooking oil for cleaner biodiesel. Clean Technol. Environ. Policy 2017, 19, 859–868. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A.; Liu, C.-K. Glucose/lipid mixed substrates as a means of controlling the properties of medium chain length poly(hydroxyalkanoates). Biomacromolecules 2001, 2, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, D.K.Y.; Ashby, R.D.; Hotchkiss, A.T.; Foglia, T.A. Biosynthesis of medium-chain-length poly(hydroxyalkanoates) from soy molasses. Biotechnol. Lett. 2006, 28, 157–162. [Google Scholar] [CrossRef]
- Solaiman, D.K.; Ashby, R.D.; Foglia, T.A. Medium-chain-length poly(beta-hydroxyalkanoate) synthesis from triacylglycerols by Pseudomonas saccharophila. Curr. Microbiol. 1999, 38, 151–154. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A. Production of polyhydroxyalkanoates from intact triacylglycerols by genetically engineered Pseudomonas. Appl. Microbiol. Biotechnol. 2001, 56, 664–669. [Google Scholar] [CrossRef]
- Haba, H.; Lavaud, C.; Harkat, H.; Magid, A.A.; Marcourt, L.; Benkhaled, M. Diterpenoids and triterpenoids from Euphorbia guyoniana. Phytochemistry 2007, 68, 1255–1260. [Google Scholar] [CrossRef]
- Song, J.H.; Jeon, C.O.; Choi, M.H.; Yoon, S.C.; Park, W. Polyhydroxyalkanoate (PHA) production using waste vegetable oil by Pseudomonas sp. strain DR2. J. Microbiol. Biotechnol. 2008, 18, 1408–1415. [Google Scholar]
- Costa, S.G.; Lépine, F.; Milot, S.; Déziel, E.; Nitschke, M.; Contiero, J. Cassava wastewater as a substrate for the simultaneous production of rhamnolipids and polyhydroxyalkanoates by Pseudomonas aeruginosa. J. Ind. Microbiol. Biotechnol. 2009, 36, 1063–1072. [Google Scholar] [CrossRef]
- Fernández, D.; Rodríguez, E.; Bassas, M.; Viñas, M.; Solanas, A.; Llorens, J.; Marqués, A.; Manresa, A. Agro-industrial oily wastes as substrates for PHA production by the new strain Pseudomonas aeruginosa NCIB 40045: Effect of culture conditions. Biochem. Eng. J. 2005, 26, 159–167. [Google Scholar] [CrossRef]
- Magdouli, S.; Brar, S.; Blais, J.; Tyagi, R. How to direct the fatty acid biosynthesis towards polyhydroxyalkanoates production? Biomass Bioenergy 2015, 74, 268–279. [Google Scholar] [CrossRef]
- Marsudi, S.; Unno, H.; Hori, K. Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 78, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Impallomeni, G.; Ballistreri, A.; Carnemolla, G.M.; Guglielmino, S.P.; Nicolò, M.S.; Cambria, M.G. Synthesis and characterization of poly(3-hydroxyalkanoates) from Brassica carinata oil with high content of erucic acid and from very long chain fatty acids. Int. J. Biol. Macromol. 2011, 48, 137–145. [Google Scholar] [CrossRef]
- Sun, Y.; Do Young, K.I.M.; Chung, W.C.; Hyung, W.K.; Young, K.Y.; Young, H.R. Characterization of a tacky poly(3-hydroxyalkanoate) produced by Pseudomonas chlororaphis HS21 from palm kernel oil. J. Microbiol. 2003, 13, 64–69. [Google Scholar]
- Thakor, N.; Trivedi, U.; Patel, K. Biosynthesis of medium chain length poly(3-hydroxyalkanoates) (mcl-PHAs) by Comamonas testosteroni during cultivation on vegetable oils. Bioresour. Technol. 2005, 96, 1843–1850. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A. Physiological characterization and genetic engineering of Pseudomonas corrugata for medium-chain-length polyhydroxyalkanoates synthesis from triacylglycerols. Curr. Microbiol. 2002, 44, 189–195. [Google Scholar] [CrossRef]
- Ruiz, C.; Kenny, S.T.; Babu, P.R.; Walsh, M.; Narancic, T.; O’Connor, K.E. High cell density conversion of hydrolysed waste cooking oil fatty acids into medium chain length polyhydroxyalkanoate using Pseudomonas putida KT2440. Catalysts 2019, 9, 468. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Rusbandi, E. Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Pérez, V.; Mota, C.R.; Muñoz, R.; Lebrero, R. Polyhydroxyalkanoates (PHA) production from biogas in waste treatment facilities: Assessing the potential impacts on economy, environment and society. Chemosphere 2020, 255, 126929. [Google Scholar] [CrossRef] [PubMed]
- Wellinger, A.; Murphy, J.D.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Strong, P.J.; Laycock, B.; Mahamud, S.N.S.; Jensen, P.D.; Lant, P.A.; Tyson, G.; Pratt, S. The opportunity for high-performance biomaterials from methane. Microorganisms 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, K.H. Understanding Methanotrophic Polyhydroxybutyrate (PHB) Production across Scale: Life Cycle Assessment, Pure Culture Experimentation, and Pathway/Genome Database Development; Stanford University: Stanford, CA, USA, 2012. [Google Scholar]
- Lee, W.-H.; Azizan, M.N.; Sudesh, K. Effects of culture conditions on the composition of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Comamonas acidovorans. Polym. Degrad. Stab. 2004, 84, 129–134. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Szacherska, K.; Marciniak, P. Pseudomonas species as producers of eco-friendly polyhydroxyalkanoates. J. Polym. Environ. 2019, 27, 1151–1166. [Google Scholar] [CrossRef]
- Chee, J.Y.; Yoga, S.S.; Lau, N.S.; Ling, S.C.; Abed, R.M.; Sudesh, K. Bacterially produced polyhydroxyalkanoate (PHA): Converting renewable resources into bioplastic. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1395–1404. [Google Scholar]
- Lee, S.Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996, 14, 431–438. [Google Scholar] [CrossRef]
- Koller, M. A review on established and emerging fermentation schemes for microbial production of polyhydroxyalkanoate (PHA) biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, S. Fed-batch fermentation—Design strategies. In Comprehensive Biotechnology; Academic Press: Cambridge, MA, USA, 2011; pp. 515–526. [Google Scholar]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef]
- Rocha, R.C.S.; da Silva, L.F.; Taciro, M.K.; Pradella, J.G.C. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) P(3HB-co-3HV) with a broad range of 3HV content at high yields by Burkholderia sacchari IPT 189. World J. Microbiol. Biotechnol. 2008, 24, 427–431. [Google Scholar] [CrossRef]
- Sun, Z.; Ramsay, J.; Guay, M.; Ramsay, B. Enhanced yield of medium-chain-length polyhydroxyalkanoates from nonanoic acid by co-feeding glucose in carbon-limited, fed-batch culture. J. Biotechnol. 2009, 143, 262–267. [Google Scholar] [CrossRef]
- Zinn, M. Biosynthesis of medium-chain-length poly[(R)-3-hydroxyalkanoates]. In Plastics from Bacteria; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010; pp. 213–236. [Google Scholar]
- Zinn, M.; Egli, T.; Herwig, C.; Narang, A. Editorial: Recent advances in continuous cultivation. Front. Bioeng. Biotechnol. 2021, 9, 641249. [Google Scholar] [CrossRef]
- Lillo, J.G.; Rodriguez-Valera, F. Effects of culture conditions on poly(beta-hydroxybutyric acid) production by Haloferax mediterranei. Appl. Environ. Microbiol. 1990, 56, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Durner, R.; Zinn, M.; Witholt, B.; Egli, T. Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth in batch and chemostat culture with different carbon sources. Biotechnol. Bioeng. 2001, 72, 278–288. [Google Scholar] [CrossRef]
- Choi, J.-I.; Lee, S.Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol. Bioeng. 1999, 62, 546–553. [Google Scholar] [CrossRef]
- Chen, G.-Q. Industrial production of PHA. In Plastics from Bacteria: Natural Functions and Applications; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2009; pp. 121–132. [Google Scholar] [CrossRef]
- Chen, G.-Q. Production of Poly-D(-)-3-Hydroxybutyrate and Poly-D(-)-3-Hydroxyvalerate by strains of Alcaligenes latus. Antonie Leeuwenhoek 1991, 60, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, G.; Park, S.; Lee, S. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 2001, 57, 50–55. [Google Scholar] [PubMed]
- Quillaguamán, J.; Guzmán, H.; Van-Thuoc, D.; Hatti-Kaul, R. Synthesis and production of polyhydroxyalkanoates by halophiles: Current potential and future prospects. Appl. Microbiol. Biotechnol. 2010, 85, 1687–1696. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Factories 2020, 19, 86. [Google Scholar] [CrossRef]
- Liu, C.; Baffoe, D.K.; Zhan, Y.; Zhang, M.; Li, Y.; Zhang, G. Halophile, an essential platform for bioproduction. J. Microbiol. Methods 2019, 166, 105704. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Xie, G.-J.; Xing, D.-F.; Liu, B.-F.; Ding, J.; Ren, N.-Q. Biological conversion of methane to polyhydroxyalkanoates: Current advances, challenges, and perspectives. Environ. Sci. Ecotechnol. 2020, 2, 100029. [Google Scholar] [CrossRef]
- Lee, J.; Yasin, M.; Park, S.; Chang, I.S.; Ha, K.S.; Lee, E.Y.; Lee, J.; Kim, C. Gas-liquid mass transfer coefficient of methane in bubble column reactor. Korean J. Chem. Eng. 2015, 32, 1060–1063. [Google Scholar] [CrossRef]
- Rostkowski, K.H.; Criddle, C.S.; Lepech, M.D. Cradle-to-gate life cycle assessment for a cradle-to-cradle cycle: Biogas-to-bioplastic (and back). Environ. Sci. Technol. 2012, 46, 9822–9829. [Google Scholar] [CrossRef] [PubMed]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—The case for thermophilic bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- Listewnik, H.-F.; Wendlandt, K.-D.; Jechorek, M.; Mirschel, G. Process design for the microbial synthesis of poly-β-hydroxybutyrate (PHB) from natural gas. Eng. Life Sci. 2007, 7, 278–282. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S. Production of poly(3-hydroxybutyrate) from inexpensive substrates. Enzym. Microb. Technol. 2000, 27, 774–777. [Google Scholar] [CrossRef]
- Jiang, G.; Johnston, B.; Townrow, D.E.; Radecka, I.; Koller, M.; Chaber, P.; Adamus, G.; Kowalczuk, M. Biomass extraction using non-chlorinated solvents for biocompatibility improvement of polyhydroxyalkanoates. Polymers 2018, 10, 731. [Google Scholar] [CrossRef]
- Ramsay, J.A.; Berger, E.; Voyer, R.; Chavarie, C. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol. Tech. 1994, 8, 589–594. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Freitas, F.; Reis, M.A. Preparation and characterization of films based on a natural P(3HB)/mcl-PHA blend obtained through the co-culture of cupriavidus necator and pseudomonas citronellolis in apple pulp waste. Bioengineering 2020, 7, 34. [Google Scholar] [CrossRef]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.-W.; Wei, Y.-H.; Wu, H.-S.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- De Koning, G.J.M.; Kellerhals, M.; van Meurs, C.; Witholt, B. A process for the recovery of poly(hydroxyalkanoates) from Pseudomonads part 2: Process development and economic evaluation. Bioprocess Biosyst. Eng. 1997, 17, 15–21. [Google Scholar] [CrossRef]
- Marudkla, J.; Patjawit, A.; Chuensangjun, C.; Sirisansaneeyakul, S. Optimization of poly(3-hydroxybutyrate) extraction from Cupriavidus necator DSM 545 using sodium dodecyl sulfate and sodium hypochlorite. Agric. Nat. Resour. 2018, 52, 266–273. [Google Scholar] [CrossRef]
- Berger, E.; Ramsay, B.A.; Chavarie, C.; Braunegg, G. PHB recovery by hypochlorite digestion of non-PHB biomass. Biotechnol. Tech. 1989, 3, 227–232. [Google Scholar] [CrossRef]
- Holmes, P.A.; Lim, G.B. Separation Process. U.S. Patent 4,910,145, 20 March 1990. [Google Scholar]
- Mondal, S.; Subramaniam, C. Xenobiotic contamination of water by plastics and pesticides revealed through real-time, ultrasensitive, and reliable surface-enhanced raman scattering. ACS Sustain. Chem. Eng. 2020, 8, 7639–7648. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Álvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Pötter, M.; Steinbüchel, A. Biogenesis and structure of polyhydroxyalkanoate granules. In Inclusions in Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2006; pp. 109–136. [Google Scholar]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial polyhydroxyalkanoates (PHAs): Efficient replacement of synthetic polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Rigouin, C.; Lajus, S.; Ocando, C.; Borsenberger, V.; Nicaud, J.M.; Marty, A.; Avérous, L.; Bordes, F. Production and characterization of two medium-chain-length polydroxyalkanoates by engineered strains of Yarrowia lipolytica. Microb. Cell Factories 2019, 18, 99. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; di Serio, M. In vivo and post-synthesis strategies to enhance the properties of PHB-based materials: A review. Front. Bioeng. Biotechnol. 2021, 8, 1454. [Google Scholar] [CrossRef]
- Modi, S.J. Assessing the Feasibility of Poly-(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) and Poly-(Lactic Acid) for Potential Food Packaging Applications. Ph.D. Dissertation, The Ohio State University, Columbus, OH, USA, 2010. [Google Scholar]
- Wei, L.; Stark, N.M.; Mc Donald, A.G. Interfacial improvements in biocomposites based on poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastics reinforced and grafted with α-cellulose fibers. Green Chem. 2015, 17, 4800–4814. [Google Scholar] [CrossRef]
- Pradhan, S.; Dikshit, P.K. Production, ultrasonic extraction, and characterization of poly(3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym. Adv. Technol. 2018, 29, 2392–2400. [Google Scholar] [CrossRef]
- Sharma, R.; Ray, A.R. Polyhydroxybutyrate, its copolymers and blends. J. Macromol. Sci. Part C 1995, 35, 327–359. [Google Scholar] [CrossRef]
- Srubar, W.; Wright, Z.; Tsui, A.; Michel, A.; Billington, S.; Frank, C. Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. Polym. Degrad. Stab. 2012, 97, 1922–1929. [Google Scholar] [CrossRef]
- Mc Adam, B.; Fournet, M.B.; Mc Donald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- El-Hadi, A.M.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly(3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Rosengart, A.; Cesário, M.; de Almeida, C.D.; Raposo, R.S.; Espert, A.; de Apodaca, E.D.; da Fonseca, M.M. Efficient P(3HB) extraction from Burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Zhu, C.; Nomura, C.T.; Perrotta, J.A.; Stipanovic, A.J.; Nakas, J.P. The effect of nucleating agents on physical properties of poly-3-hydroxybutyrate (PHB) and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (PHB-co-HV) produced by Burkholderia cepacia ATCC 17759. Polym. Test. 2012, 31, 579–585. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Effect of two different plasticizers on the properties of poly(3-hydroxybutyrate) binary and ternary blends. J. Appl. Polym. Sci. 2018, 135, 46016. [Google Scholar] [CrossRef]
- Barouti, G.; Guillaume, S.M. Polyhydroxybutyrate (PHB)-based triblock copolymers: Synthesis of hydrophobic PHB/poly(benzyl β-malolactonate) and amphiphilic PHB/poly(malic acid) analogues by ring-opening polymerization. Polym. Chem. 2016, 7, 4603–4608. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent developments in food packaging based on nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Savenkova, L.; Gercberga, Z.; Nikolaeva, V.; Dzene, A.; Bibers, I.; Kalnin, M. Mechanical properties and biodegradation characteristics of PHB-based films. Process Biochem. 2000, 35, 573–579. [Google Scholar] [CrossRef]
- Puente, J.A.S.; Esposito, A.; Chivrac, F.; Dargent, E. Effect of boron nitride as a nucleating agent on the crystallization of bacterial poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2013, 128, 2586–2594. [Google Scholar] [CrossRef]
- Androsch, R. Surface structure of folded-chain crystals of poly(R-3-hydroxybutyrate) of different chain length. Polymer 2008, 49, 4673–4679. [Google Scholar] [CrossRef]
- Wunderlich, B. Reversible crystallization and the rigid–amorphous phase in semicrystalline macromolecules. Prog. Polym. Sci. 2002, 28, 383–450. [Google Scholar] [CrossRef]
- Sauvageau, D.; Cooper, D.G.; Nicell, J.A. Relative rates and mechanisms of biodegradation of diester plasticizers mediated by Rhodococcus rhodochrous. Can. J. Chem. Eng. 2009, 87, 499–506. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef]
- Chen, G.G.Q. Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Choi, J.S.; Park, W.H. Effect of biodegradable plasticizers on thermal and mechanical properties of poly(3-hydroxybutyrate). Polym. Test. 2004, 23, 455–460. [Google Scholar] [CrossRef]
- Mangeon, C.; Michely, L.; de Anda, A.R.; Thevenieau, F.; Renard, E.; Langlois, V. Natural terpenes used as plasticizers for poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar] [CrossRef]
- Lee, E.Y.; Jendrossek, D.; Schirmer, A.; Choi, C.Y.; Steinbüchel, A. Biosynthesis of copolyesters consisting of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids from 1, 3-butanediol or from 3-hydroxybutyrate by Pseudomonas sp. A33. Appl. Microbiol. Biotechnol. 1995, 42, 901–909. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Hartmann, R.; Hany, R.; Pletscher, E.; Ritter, A.; Witholt, B.; Zinn, M. Tailor-made olefinic medium-chain-length poly[(R)-3-hydroxyalkanoates] by Pseudomonas putida GPo1: Batch versus chemostat production. Biotechnol. Bioeng. 2006, 93, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of bioplastics: Routes and benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Mirjalili, F.; Chuah, L.; Salahi, E. Mechanical and morphological properties of polypropylene/nano α-Al2O3 composites. Sci. World J. 2014, 2014, 718765. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.-Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- De Koning, G.; Lemstra, P.; Hill, D.; Carswell, T.; O’Donnell, J. Ageing phenomena in bacterial poly[(R)-3-hydroxybutyrate]: 1. A study on the mobility in poly[(R)-3-hydroxybutyrate] powders by monitoring the radical decay with temperature after γ-radiolysis at 77 K. Polymer 1992, 33, 3295–3297. [Google Scholar] [CrossRef][Green Version]
- Sawyer, L.C.; Grubb, D.T.; Meyers, G.F. (Eds.) Polymer Microscopy, 3rd ed.; Springer: New York, NY, USA, 2008; 540p, ISBN 978-0-387-72627-4. [Google Scholar]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Biddlestone, F.; Harris, A.; Hay, J.N.; Hammond, T. The physical ageing of amorphous poly(hydroxybutyrate). Polym. Int. 1996, 39, 221–229. [Google Scholar] [CrossRef]
- Hankermeyer, C.R.; Tjeerdema, R.S. Polyhydroxybutyrate: Plastic made and degraded by microorganisms. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1999; pp. 1–24. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.B.; Pereira, J.R.; Marreiros, B.C.; Reis, M.A.; Freitas, F. Microbial production of medium-chain length polyhydroxyalkanoates. Process Biochem. 2021, 102, 393–407. [Google Scholar] [CrossRef]
- Abe, H.; Ishii, N.; Sato, S.; Tsuge, T. Thermal properties and crystallization behaviors of medium-chain-length poly(3-hydroxyalkanoate)s. Polymer 2012, 53, 3026–3034. [Google Scholar] [CrossRef]
- Sánchez, R.J.; Schripsema, J.; da Silva, L.F.; Taciro, M.K.; Pradella, J.G.; Gomez, J.C. Medium-chain-length polyhydroxyalkanoic acids (PHAmcl) produced by Pseudomonas putida IPT 046 from renewable sources. Eur. Polym. J. 2003, 39, 1385–1394. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, A.; Chen, G.-Q. Synthesis of medium-chain-length polyhydroxyalkanoate homopolymers, random copolymers, and block copolymers by an engineered strain of Pseudomonas entomophila. Adv. Health Mater. 2017, 6, 1601017. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Q.; Wang, H.; Zhu, B.; Yu, F.; Chen, G.-Q.; Inoue, Y. Polymorphic crystallization of fractionated microbial medium-chain-length polyhydroxyalkanoates. Polymer 2009, 50, 4378–4388. [Google Scholar] [CrossRef]
- Mizuno, S.; Hiroe, A.; Fukui, T.; Abe, H.; Tsuge, T. Fractionation and thermal characteristics of biosynthesized polyhydoxyalkanoates bearing aromatic groups as side chains. Polym. J. 2017, 49, 557–565. [Google Scholar] [CrossRef]
- Gross, R.A.; de Mello, C.; Lenz, R.W.; Brandl, H.; Fuller, R.C. The biosynthesis and characterization of poly(β-hydroxyalkanoates) produced by Pseudomonas oleovorans. Macromolecules 1989, 22, 1106–1115. [Google Scholar] [CrossRef]
- Gagnon, K.D.; Lenz, R.W.; Farris, R.J.; Fuller, R.C. Crystallization behavior and its influence on the mechanical properties of a thermoplastic elastomer produced by Pseudomonas oleovorans. Macromolecules 1992, 25, 3723–3728. [Google Scholar] [CrossRef]
- Larrañaga, A.; Fernández, J.; Vega, A.; Etxeberria, A.; Ronchel, C.; Adrio, J.; Sarasua, J. Crystallization and its effect on the mechanical properties of a medium chain length polyhydroxyalkanoate. J. Mech. Behav. Biomed. Mater. 2014, 39, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Marschessault, R.; Monasterios, C.; Morin, F.; Sundararajan, P. Chiral poly(β-hydroxyalkanoates): An adaptable helix influenced by the alkane side-chain. Int. J. Biol. Macromol. 1990, 12, 158–165. [Google Scholar] [CrossRef]
- Posen, I.D.; Jaramillo, P.; Landis, A.E.; Griffin, W.M. Greenhouse gas mitigation for U.S. plastics production: Energy first, feedstocks later. Environ. Res. Lett. 2017, 12, 034024. [Google Scholar] [CrossRef]
- Plackett, D.; Siró, I. Polyhydroxyalkanoates (PHAs) for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 498–526. [Google Scholar]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Biosynthesis and properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Loh, X.J. Polyhydroxyalkanoates: Chemical modifications toward biomedical applications. ACS Sustain. Chem. Eng. 2014, 2, 106–119. [Google Scholar] [CrossRef]
- Larsson, M.; Markbo, O.; Jannasch, P. Melt processability and thermomechanical properties of blends based on polyhydroxyalkanoates and poly(butylene adipate-co-terephthalate). RSC Adv. 2016, 6, 44354–44363. [Google Scholar] [CrossRef]
- Nerkar, M.; Ramsay, J.A.; Ramsay, B.A.; Kontopoulou, M. Melt compounded blends of short and medium chain-length poly-3-hydroxyalkanoates. J. Polym. Environ. 2014, 22, 236–243. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, W.; Chen, Z.; Sun, B.; Zhu, M. Significant accelerated crystallization of long chain branched poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with high nucleation temperature under fast cooling rate. Compos. Sci. Technol. 2017, 142, 207–213. [Google Scholar] [CrossRef]
- Gopi, S.; Kontopoulou, M.; Ramsay, B.A.; Ramsay, J.A. Manipulating the structure of medium-chain-length polyhydroxyalkanoate (MCL-PHA) to enhance thermal properties and crystallization kinetics. Int. J. Biol. Macromol. 2018, 119, 1248–1255. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Wadhwa, P.; Hong, J.W.; Hong, Y.G.; Jeon, J.-M.; Lee, E.S.; Yang, Y.-H. Lipase mediated functionalization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with ascorbic acid into an antioxidant active biomaterial. Int. J. Biol. Macromol. 2019, 123, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Timbart, L.; Renard, E.; Tessier, M.; Langlois, V. Monohydroxylated poly(3-hydroxyoctanoate) oligomers and its functionalized derivatives used as macroinitiators in the synthesis of degradable diblock copolyesters. Biomacromolecules 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cheng, S.; Xu, K. Block poly(ester-urethane)s based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxyhexanoate-co-3-hydroxyoctanoate). Biomaterials 2009, 30, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Saad, G.R.; Lee, Y.J.; Seliger, H. Synthesis and characterization of biodegradable poly(ester-urethanes) based on bacterial poly(R-3-hydroxybutyrate). J. Appl. Polym. Sci. 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Ravenelle, F.; Marchessault, R.H. Self-assembly of poly([R]-3-hydroxybutyric acid)-block-poly(ethylene glycol) diblock copolymers. Biomacromolecules 2003, 4, 856–858. [Google Scholar] [CrossRef]
- Mrkić, S.; Galić, K.; Ivanković, M.; Hamin, S.; Ciković, N. Gas transport and thermal characterization of mono- and di-polyethylene films used for food packaging. J. Appl. Polym. Sci. 2006, 99, 1590–1599. [Google Scholar] [CrossRef]
- Luo, F.; Fortenberry, A.; Ren, J.; Qiang, Z. Recent Progress in enhancing poly(lactic acid) stereocomplex formation for material property improvement. Front. Chem. 2020, 8, 688. [Google Scholar] [CrossRef]
- Gupta, A.; Mulchandani, N.; Shah, M.; Kumar, S.; Katiyar, V. Functionalized chitosan mediated stereocomplexation of poly(lactic acid): Influence on crystallization, oxygen permeability, wettability and biocompatibility behavior. Polymer 2018, 142, 196–208. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Lopez, J.; Jiménez, A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Loureiro, N.C.; Ghosh, S.; Viana, J.C.; Esteves, J.L. Thermal characterization of polyhydroxyalkanoates and poly(lactic acid) blends obtained by injection molding. Polym. Technol. Eng. 2015, 54, 350–356. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Loureiro, N.; Esteves, J.L.; Viana, J.C.; Ghosh, S. Mechanical characterization of polyhydroxyalkanoate and poly(lactic acid) blends. J. Thermoplast. Compos. Mater. 2015, 28, 195–213. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT 2015, 64, 980–988. [Google Scholar] [CrossRef]
- Arrieta, M.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-Lopez, M.D.M.; Rayόn, E.; Barral-Losada, L.F.; Lόpez-Vilariño, J.M.; Lόpez, J.; González-Rodríguez, M.V. Plasticized poly(lactic acid)–poly(hydroxybutyrate) (PLA–PHB) blends incorporated with catechin intended for active food-packaging applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef]
- Noda, I.; Green, P.R.; Satkowski, M.M.; Schechtman, L.A. Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 2005, 6, 580–586. [Google Scholar] [CrossRef]
- Schreck, K.M.; Hillmyer, M.A. Block copolymers and melt blends of polylactide with Nodax, TM microbial polyesters: Preparation and mechanical properties. J. Biotechnol. 2007, 132, 287–295. [Google Scholar] [CrossRef]
- Noda, I.; Satkowski, M.M.; Dowrey, A.E.; Marcott, C. Polymer alloys of nodax copolymers and poly(lactic acid). Macromol. Biosci. 2004, 4, 269–275. [Google Scholar] [CrossRef]
- Botta, L.; Mistretta, M.C.; Palermo, S.; Fragala, M.; Pappalardo, F. Characterization and processability of blends of polylactide acid with a new biodegradable medium-chain-length polyhydroxyalkanoate. J. Polym. Environ. 2015, 23, 478–486. [Google Scholar] [CrossRef]
- Gassner, F.; Owen, A. Physical properties of poly(β-hydroxybutyrate)-poly(ε-caprolactone) blends. Polymer 1994, 35, 2233–2236. [Google Scholar] [CrossRef]
- Antunes, M.C.M.; Felisberti, M.I. Blends of poly(hydroxybutyrate) and poly(ε-caprolactone) obtained from melting mixture. Polímeros 2005, 15, 134–138. [Google Scholar] [CrossRef]
- Vogel, C.; Wessel, E.; Siesler, H.W. FT-IR imaging spectroscopy of phase separation in blends of poly(3-hydroxybutyrate) with poly(l-lactic acid) and poly(ϵ-caprolactone). Biomacromolecules 2008, 9, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Watanabe, T.; Wang, Y.; Kichise, T.; Fukuchi, T.; Chen, G.-Q.; Doi, Y.; Inoue, Y. Studies on comonomer compositional distribution of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)s and thermal characteristics of their factions. Biomacromolecules 2002, 3, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, K.; Saito, T.; Yu, F.; Nakamura, N.; Inoue, Y. The toughening effect of a small amount of poly(ɛ-caprolactone) on the mechanical properties of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/PCL blend. Polym. J. 2011, 43, 484–492. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Hayakawa, Y.; Ogura, T.; Ito, Y.; Nishida, M. Multi-scale instrumental analyses of plasticized polyhydroxyalkanoates (PHA) blended with polycaprolactone (PCL) and the effects of crosslinkers and graft copolymers. RSC Adv. 2019, 9, 1551–1561. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; Lopez-Martinez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef]
- Przybysz, M.; Marć, M.; Klein, M.; Saeb, M.R.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.-S. Processing and characterization of microcellular PHBV/PBAT blends. Polym. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive extrusion of sustainable PHBV/PBAT-based nanocomposite films with organically modified nanoclay for packaging applications: Compression moulding vs. cast film extrusion. Compos. Part B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Lee, J.; Pilla, S.; Clemons, C.; Gong, S.; Turng, L.-S. Processing and characterization of solid and microcellular PHBV/PBAT blend and its RWF/nanoclay composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 982–990. [Google Scholar] [CrossRef]
- Nagarajan, V.; Misra, M.; Mohanty, A.K. New engineered biocomposites from poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene adipate-co-terephthalate) (PBAT) blends and switchgrass: Fabrication and performance evaluation. Ind. Crop. Prod. 2013, 42, 461–468. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Chieng, B.W.; Yunus, W.M.Z.W. Morphology, thermal and mechanical properties of biodegradable poly(butylene succinate)/poly(butylene adipate-co-terephthalate)/clay nanocomposites. Polym. Plast. Technol. Eng. 2010, 49, 1571–1580. [Google Scholar] [CrossRef]
- Kennouche, S.; le Moigne, N.; Kaci, M.; Quantin, J.C.; Caro-Bretelle, A.S.; Delaite, C.; Lopez-Cuesta, J.M. Morphological characterization and thermal properties of compatibilized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene succinate) (PBS)/halloysite ternary nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.; Bhattacharya, S.; Choi, H. Compatibility of biodegradable poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) blends for packaging application. Korea-Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and crystallization behaviour of biodegradable blends of two aliphatic polyesters. poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(butylene succinate) blends. Polymer 2003, 44, 7519–7527. [Google Scholar] [CrossRef]
- Ma, P.P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via in situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol. Mater. Eng. 2011, 297, 402–410. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Aliotta, L.; Bianchi, E.; Tricoli, F.; Seggiani, M.; Lazzeri, A. Immiscible PHB/PB S and PHB/PBSA blends: Morphology, phase composition and modelling of elastic modulus. Polym. Int. 2021, 71, 47–56. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Pal, A.K.; Misra, M.; Mohanty, A.K. Sustainable PHBV/cellulose acetate blends: Effect of a chain extender and a plasticizer. ACS Omega 2020, 5, 14221–14231. [Google Scholar] [CrossRef]
- Tomasi, G.; Scandola, M. Blends of bacterial poly(3-hydroxybutyrate) with cellulose acetate butyrate in activated sludge. J. Macromol. Sci. Part A 1995, 32, 671–681. [Google Scholar] [CrossRef]
- El-Shafee, E.; Saad, G.R.; Fahmy, S.M. Miscibility, crystallization and phase structure of poly(3-hydroxybutyrate)/cellulose acetate butyrate blends. Eur. Polym. J. 2001, 37, 2091–2104. [Google Scholar] [CrossRef]
- Chiulan, I.; Panaitescu, D.M.; Frone, A.N.; Teodorescu, M.; Nicolae, C.A.; Căşărică, A.; Tofan, V.; Sălăgeanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, andin vitroevaluation. J. Biomed. Mater. Res. Part A 2016, 104, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Shah, D.; Patel, K.; Trivedi, U. PHA–rubber blends: Synthesis, characterization and biodegradation. Bioresour. Technol. 2008, 99, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Hilliou, L.; Escuin, J.; Cabedo, L.; Nevo, Y.; Prieto, C.; Lagaron, J. High-oxygen-barrier multilayer films based on polyhydroxyalkanoates and cellulose nanocrystals. Nanomaterials 2021, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Dhar, P.; Kumar, A.; Katiyar, V. Polyhydroxyalkanoates (PHA)-cellulose based nanobiocomposites for food packaging applications. In Food Additives and Packaging; Komolprasert, V., Turowski, P., Eds.; ACS Publications: Washington, DC, USA, 2014; Volume 1162, pp. 275–314. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Preparation and properties of polyhydroxybutyrate blended with different types of starch. J. Appl. Polym. Sci. 2009, 116, 688–694. [Google Scholar] [CrossRef]
- Lai, S.-M.; Sun, W.-W.; Don, T.-M. Preparation and characterization of biodegradable polymer blends from poly(3-hydroxybutyrate)/poly(vinyl acetate)-modified corn starch. Polym. Eng. Sci. 2015, 55, 1321–1329. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of various cross-linking agents on the physicochemical properties of starch/PHA composite films produced by extrusion blowing. Food Hydrocoll. 2018, 77, 964–975. [Google Scholar] [CrossRef]
- Wong, S.; Shanks, R.; Hodzic, A. Properties of poly(3-hydroxybutyric acid) composites with flax fibres modified by plasticiser absorption. Macromol. Mater. Eng. 2002, 287, 647–655. [Google Scholar] [CrossRef]
- Barkoula, N.; Garkhail, S.; Peijs, T. Biodegradable composites based on flax/polyhydroxybutyrate and its copolymer with hydroxyvalerate. Ind. Crop. Prod. 2010, 31, 34–42. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, K.M.I.; Hinrichsen, G.; Kopp, C.; Kropke, S. Study on physical and mechanical properties of biopol-jute composite. Polym. Technol. Eng. 1999, 38, 99–112. [Google Scholar] [CrossRef]
- Avella, M.; la Rota, G.; Martuscelli, E.; Raimo, M.; Sadocco, P.; Elegir, G.; Riva, R. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and wheat straw fibre composites: Thermal, mechanical properties and biodegradation behaviour. J. Mater. Sci. 2000, 35, 829–836. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.K.; Sugie, T.; Takai, Y.; Hamada, H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos. Part A Appl. Sci. Manuf. 2008, 39, 875–886. [Google Scholar] [CrossRef]
- Persico, P.; Acierno, D.; Carfagna, C.; Cimino, F. Mechanical and thermal behaviour of ecofriendly composites reinforced by kenaf and caro fibers. Int. J. Polym. Sci. 2011, 2011, 841812. [Google Scholar] [CrossRef]
- Joyyi, L.; Thirmizir, M.Z.A.; Salim, M.S.; Han, L.; Murugan, P.; Kasuya, K.-I.; Maurer, F.H.; Arifin, M.I.Z.; Sudesh, K. Composite properties and biodegradation of biologically recovered P(3HB-co-3HHx) reinforced with short kenaf fibers. Polym. Degrad. Stab. 2017, 137, 100–108. [Google Scholar] [CrossRef]
- Shibata, M.; Takachiyo, K.-I.; Ozawa, K.; Yosomiya, R.; Takeishi, H. Biodegradable polyester composites reinforced with short abaca fiber. J. Appl. Polym. Sci. 2002, 85, 129–138. [Google Scholar] [CrossRef]
- Moliner, C.; Badia, J.D.; Bosio, B.; Arato, E.; Kittikorn, T.; Strömberg, E.; Juanes, R.T.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Thermal and thermo-oxidative stability and kinetics of decomposition of PHBV/sisal composites. Chem. Eng. Commun. 2017, 205, 226–237. [Google Scholar] [CrossRef]
- Hermida Élida, B.; Mega, V.I. Transcrystallization kinetics at the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/hemp fibre interface. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1387–1394. [Google Scholar] [CrossRef]
- Ramsay, B.A.; Langlade, V.; Carreau, P.J. Biodegradability and mechanical properties of poly-(beta-hydroxybutyrate-co-beta-hydroxyvalerate)-starch blends. Appl. Environ. Microbiol. 1993, 59, 1242–1246. [Google Scholar] [CrossRef]
- Gatenholm, P.; Kubát, J.; Mathiasson, A. Biodegradable natural composites. I. Processing and properties. J. Appl. Polym. Sci. 1992, 45, 1667–1677. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Balestri, E.; Mallegni, N.; Stefanelli, E.; Rossi, A.; Lardicci, C.; Lazzeri, A. Novel sustainable composites based on poly(hydroxybutyrate-co-hydroxyvalerate) and seagrass beach-CAST fibers: Performance and degradability in marine environments. Materials 2018, 11, 772. [Google Scholar] [CrossRef]
- Maiti, P.; Batt, C.A.; Giannelis, E.P. New biodegradable polyhydroxybutyrate/layered silicate nanocomposites. Biomacromolecules 2007, 8, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.M.; Kim, T.W.; Park, O.O.; Chang, Y.K.; Lee, J.W. Preparation and characterization of poly(hydroxybutyrate-co-hydroxyvalerate)-organoclay nanocomposites. J. Appl. Polym. Sci. 2003, 90, 525–529. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Bourbigot, S.; Avérous, L. Structure and properties of PHA/clay nano-biocomposites prepared by melt intercalation. Macromol. Chem. Phys. 2008, 209, 1473–1484. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Tamiya, T.; Hsu, Y.-I.; Asoh, T.-A.; Uyama, H. Improvement of interfacial adhesion between poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and silica particles. Ind. Eng. Chem. Res. 2020, 59, 13595–13602. [Google Scholar] [CrossRef]
- Jaques, N.; Silva, I.D.D.S.; Neto, M.D.C.; Diniz, R.K.M.; Wellen, R.M.R.; Canedo, E.L. Comparative study of the effect of TiO2 and ZnO on the crystallization of PHB. Matéria Rio Jan. 2017, 22. [Google Scholar] [CrossRef]
- Kirboga, S.; Öner, M. The properties of PHBV/CaCO3 composites prepared by melt processing. In Proceedings of the 6th International Conference on New Trends in Chemistry, Online, 17–18 October 2020. [Google Scholar]
- Visakh, P.M. Chapter 1. Polyhydroxyalkanoates (PHAs), their blends, composites and nanocomposites: State of the art, new challenges and opportunities. In Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites; Royal Society of Chemistry: London, UK, 2014; pp. 1–17. [Google Scholar]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, C.; Li, X.; Hao, N.; Zhang, T.; Gaffrey, M.J.; Pu, Y.; Cort, J.R.; Ragauskas, A.J.; Qian, W.-J.; et al. Enhancement of polyhydroxyalkanoate production by co-feeding lignin derivatives with glycerol in Pseudomonas putida KT2440. Biotechnol. Biofuels 2021, 14, 11. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.W.; Chung, M.G.; Rhee, Y.H. Biosynthesis, modification, and biodegradation of bacterial medium-chain-length polyhydroxyalkanoates. J. Microbiol. 2007, 45, 87–97. [Google Scholar]
- Chen, G.-Q.; Jiang, X.-R.; Guo, Y. Synthetic biology of microbes synthesizing polyhydroxyalkanoates (PHA). Synth. Syst. Biotechnol. 2016, 1, 236–242. [Google Scholar] [CrossRef]
- Meng, D.-C.; Shen, R.; Yao, H.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Engineering the diversity of polyesters. Curr. Opin. Biotechnol. 2014, 29, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Tian, L.; Liu, Z.; Peng, Z. A new polystyrene–TiO2 nanocomposite film and its photocatalytic degradation. Appl. Catal. A Gen. 2004, 264, 237–242. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Lopez-Rubio, A. Nanotechnology for bioplastics: Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical applications of polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Water vapor and oxygen barrier properties of extrusion-blown poly(lactic acid)/cellulose nanocrystals nanocomposite films. Compos. Part A Appl. Sci. Manuf. 2018, 114, 204–211. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Norrrahim, F.; Ariffin, H.; Hassan, M.; Ibrahim, N.; Nishida, H. Performance evaluation and chemical recyclability of a polyethyene/poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) blend for sustainable packaging. RSC Adv. 2013, 3, 24378. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Galotto, M.J.; Guarda, A.; Bruna, J.E. Modification of cellulose acetate films using nanofillers based on organoclays. J. Food Eng. 2012, 110, 262–268. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Asrar, J.; Gruys, K.J. Biodegradable polymer (Biopol®). Biopolym. Online 2005, 4. [Google Scholar] [CrossRef]
- Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Nanostructured interlayers of zein to improve the barrier properties of high barrier polyhydroxyalkanoates and other polyesters. J. Food Eng. 2014, 127, 1–9. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, K.; Chen, G.-Q. Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials 2002, 23, 1391–1397. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, K.; Zhang, X.-F.; Hu, P.; Chen, G.-Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef]
- Kassab, A.C.; Xu, K.; Denkbas, E.; Dou, Y.; Zhao, S.; Piskin, E. Rifampicin carrying polyhydroxybutyrate microspheres as a potential chemoembolization agent. J. Biomater. Sci. Polym. Ed. 1997, 8, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.; Horowitz, D.M.; Peoples, O.P. PHA applications: Addressing the price performance issue: I. Tissue engineering. Int. J. Biol. Macromol. 1999, 25, 111–121. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic pollution and potential solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Sudesh, K.; Iwata, T. Sustainability of biobased and biodegradable plastics. CLEAN—Soil Air Water 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Yang, S.-T.; Liu, X.; Zhang, Y. Metabolic engineering—Applications, methods, and challenges. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 73–118. [Google Scholar]
- Minteer, S. Biochemical production of other bioalcohols: Biomethanol, biopropanol, bioglycerol, and bioethylene glycol. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2011; pp. 258–265. [Google Scholar]
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, D.W.; Hwang, Y.K.; Lee, U.-H.; Hong, D.-Y.; Chang, J.-S. An integrated process for the production of 2,5-dimethylfuran from fructose. Green Chem. 2015, 17, 3310–3313. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Hezaveh, H. Glycerol for renewable acrolein production by catalytic dehydration. Renew. Sustain. Energy Rev. 2014, 40, 28–59. [Google Scholar] [CrossRef]
- Morais, A.R.; Dworakowska, S.; Reis, A.; Gouveia, L.; Matos, C.T.; Bogdał, D.; Bogel-Łukasik, R. Chemical and biological-based isoprene production: Green metrics. Catal. Today 2015, 239, 38–43. [Google Scholar] [CrossRef]
- Andreeßen, C.; Steinbüchel, A. Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Narancic, T.; Verstichel, S.; Chaganti, S.R.; Morales-Gamez, L.; Kenny, S.T.; de Wilde, B.; Padamati, R.B.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef] [PubMed]
- Storz, H.; Vorlop, K.-D. Bio-based plastics: Status, challenges and trends. Landbauforsch. Volkenrode 2013, 63, 321–332. [Google Scholar]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Prudnikova, S.V.; Filipenko, M.L.; Khrapov, E.A.; Vasil’ev, A.D.; Volova, T.G. Biodegradation of polyhydroxyalkanoates by soil microbial communities of different structures and detection of PHA degrading microorganisms. Appl. Biochem. Microbiol. 2011, 48, 28–36. [Google Scholar] [CrossRef]
- Kim, D.Y.; Rhee, Y.H. Biodegradation of microbial and synthetic polyesters by fungi. Appl. Microbiol. Biotechnol. 2003, 61, 300–308. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uchino, K.; Abe, T.; Yamazaki, Y.; Saito, T. Novel intracellular 3-hydroxybutyrate-oligomer hydrolase in Wautersia eutropha H16. J. Bacteriol. 2005, 187, 5129–5135. [Google Scholar] [CrossRef]
- Mukai, K.; Yamada, K.; Doi, Y. Enzymatic degradation of poly(hydroxyalkanoates) by a marine bacterium. Polym. Degrad. Stab. 1993, 41, 85–91. [Google Scholar] [CrossRef]
- Numata, K.; Abe, H.; Doi, Y. Enzymatic processes for biodegradation of poly(hydroxyalkanoate)s crystals. Can. J. Chem. 2008, 86, 471–483. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Numata, K.; Kikkawa, Y.; Tsuge, T.; Iwata, T.; Doi, Y.; Abe, H. Enzymatic degradation processes of poly[(R)-3-hydroxybutyric acid] and poly[(R)-3-hydroxybutyric acid-co-(R)-3-hydroxyvaleric acid] single crystals revealed by atomic force microscopy: Effects of molecular weight and second-monomer composition on erosion rates. Biomacromolecules 2005, 6, 2008–2016. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Pan, Y.; Zhou, Y.; Jiang, L.; Dan, Y. Effect of NR on the hydrolytic degradation of PLA. Polym. Degrad. Stab. 2013, 98, 943–950. [Google Scholar] [CrossRef]
- Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. J. Field Actions 2019, 19, 12–21. [Google Scholar]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing biobased recyclable polymers for plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Arikan, E.B.; Ozsoy, H.D. A review: Investigation of bioplastics. J. Civ. Eng. Archit. 2015, 9, 188–192. [Google Scholar]
- Prieto, A. To be, or not to be biodegradable… That is the question for the bio-based plastics. Microb. Biotechnol. 2016, 9, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. Recycling of biopolymers—The patent perspective. Eur. Polym. J. 2019, 114, 464–475. [Google Scholar] [CrossRef]
- Rivas, L.F.; Casarin, S.A.; Nepomuceno, N.C.; Alencar, M.I.; Agnelli, J.A.M.; de Medeiros, E.S.; Neto, A.D.O.W.; Oliveira, M.; de Medeiros, A.M.; Santos, A.S.F. Reprocessability of PHB in extrusion: ATR-FTIR, tensile tests and thermal studies. Polímeros 2017, 27, 122–128. [Google Scholar] [CrossRef]
- Yang, X.; Clénet, J.; Xu, H.; Odelius, K.; Hakkarainen, M. Two step extrusion process: From thermal recycling of PHB to plasticized PLA by reactive extrusion grafting of PHB degradation products onto PLA chains. Macromolecules 2015, 48, 2509–2518. [Google Scholar] [CrossRef]
- Zembouai, I.; Bruzaud, S.; Kaci, M.; Benhamida, A.; Corre, Y.M.; Grohens, Y. Mechanical recycling of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polylactide based blends. J. Polym. Environ. 2014, 22, 449–459. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Vu, D.; Åkesson, D.; Taherzadeh, M.J.; Ferreira, J.A. Recycling strategies for polyhydroxyalkanoate-based waste materials: An overview. Bioresour. Technol. 2020, 298, 122393. [Google Scholar] [CrossRef]
- Zaverl, M.; Seydibeyoğlu, M.Ö.; Misra, M.; Mohanty, A. Studies on recyclability of polyhydroxybutyrate-co-valerate bioplastic: Multiple melt processing and performance evaluations. J. Appl. Polym. Sci. 2012, 125, E324–E331. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Yamashita, K.; Doi, Y. Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(S)-lactide]. Polym. Degrad. Stab. 2002, 76, 53–59. [Google Scholar] [CrossRef]
- Ariffin, H.; Nishida, H.; Shirai, Y.; Hassan, M.A. Highly selective transformation of poly[(R)-3-hydroxybutyric acid] into trans-crotonic acid by catalytic thermal degradation. Polym. Degrad. Stab. 2010, 95, 1375–1381. [Google Scholar] [CrossRef]
- Sato, S.; Ishii, N.; Hamada, Y.; Abe, H.; Tsuge, T. Utilization of 2-alkenoic acids for biosynthesis of medium-chain-length polyhydroxyalkanoates in metabolically engineered Escherichia coli to construct a novel chemical recycling system. Polym. Degrad. Stab. 2012, 97, 329–336. [Google Scholar] [CrossRef]
- Yang, X.; Odelius, K.; Hakkarainen, M. Microwave-assisted reaction in green solvents recycles PHB to functional chemicals. ACS Sustain. Chem. Eng. 2014, 2, 2198–2203. [Google Scholar] [CrossRef]
- Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2018, 29, 30–40. [Google Scholar] [CrossRef]
- Wang, S.; Lydon, K.A.; White, E.M.; Grubbs, J.B.; Lipp, E.K.; Locklin, J.; Jambeck, J.R. Biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) plastic under anaerobic sludge and aerobic seawater conditions: Gas evolution and microbial diversity. Environ. Sci. Technol. 2018, 52, 5700–5709. [Google Scholar] [CrossRef]
- Shogren, R.; Doane, W.; Garlotta, D.; Lawton, J.; Willett, J. Biodegradation of starch/polylactic acid/poly(hydroxyester-ether) composite bars in soil. Polym. Degrad. Stab. 2003, 79, 405–411. [Google Scholar] [CrossRef]
- Mandic, M.; Spasic, J.; Ponjavic, M.; Nikolic, M.S.; Cosovic, V.R.; O’Connor, K.E.; Nikodinovic-Runic, J.; Djokic, L.; Jeremic, S. Biodegradation of poly(ε-caprolactone) (PCL) and medium chain length polyhydroxyalkanoate (mcl-PHA) using whole cells and cell free protein preparations of Pseudomonas and Streptomyces strains grown on waste cooking oil. Polym. Degrad. Stab. 2019, 162, 160–168. [Google Scholar] [CrossRef]
- Sikorska, W.; Musiol, M.; Nowak, B.; Pajak, J.; Labuzek, S.; Kowalczuk, M.; Adamus, G. Degradability of polylactide and its blend with poly[(R,S)-3-hydroxybutyrate] in industrial composting and compost extract. Int. Biodeterior. Biodegrad. 2015, 101, 32–41. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, X.-L.; Wang, Y.-Z. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polym. Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).