Effect of a Metallocene Catalyst Mixture on CNT Yield Using the FC-CVD Process
Abstract
:1. Introduction
2. Experimental Part
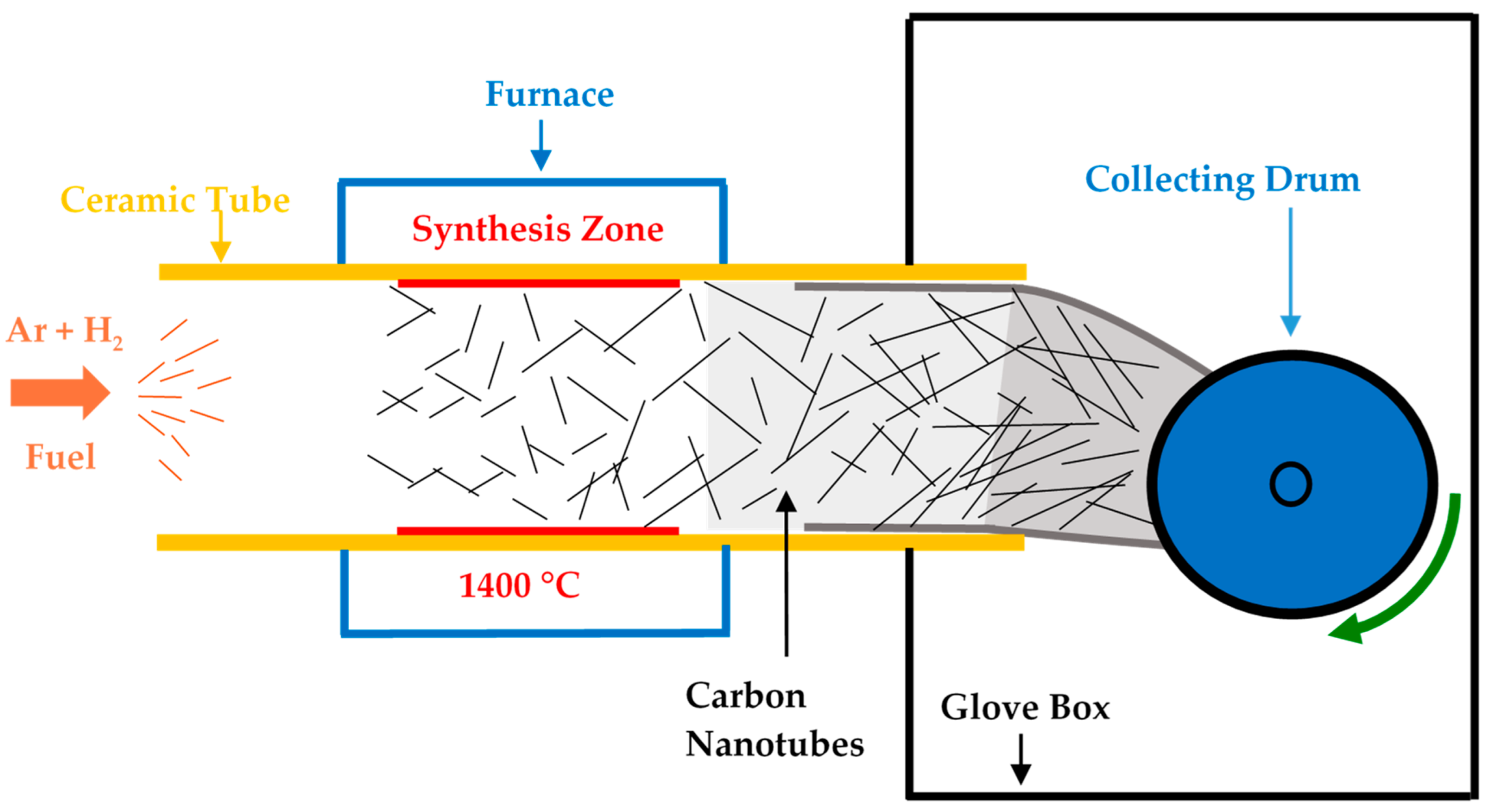
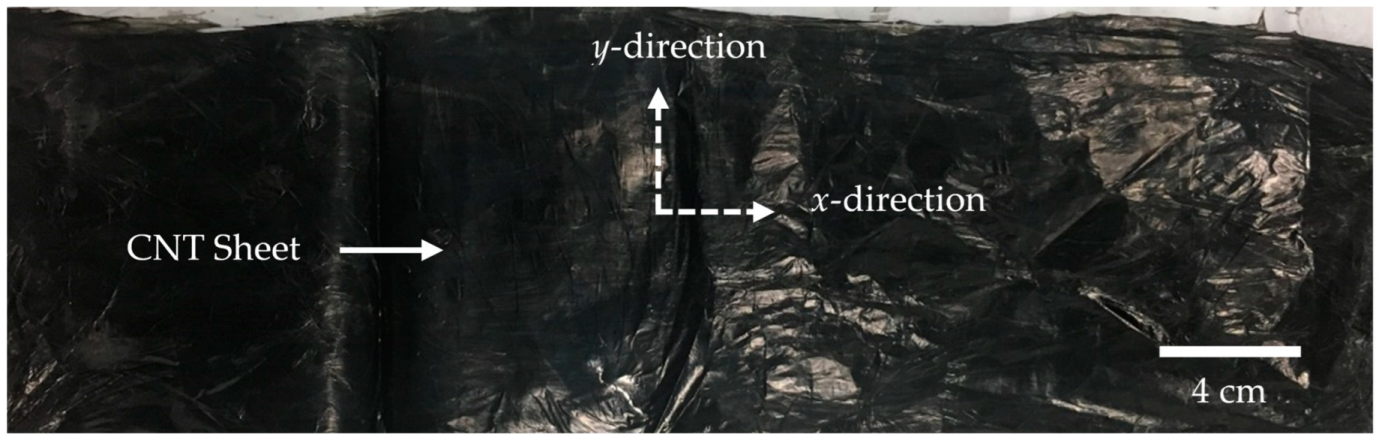
2.1. Carbon Nanotube Sheet Synthesis
2.2. Characterization
2.3. Calculation
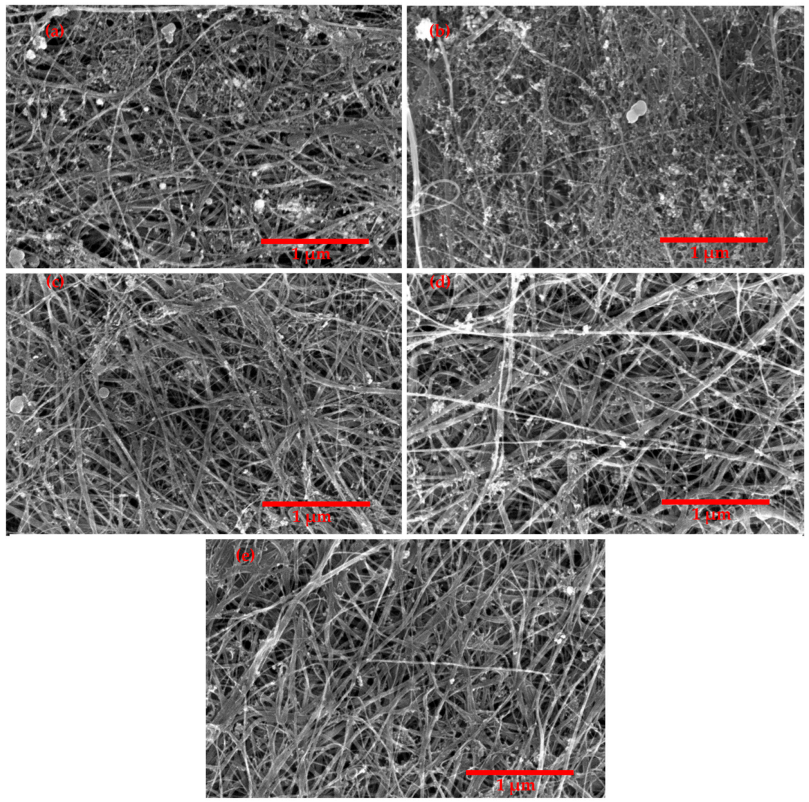
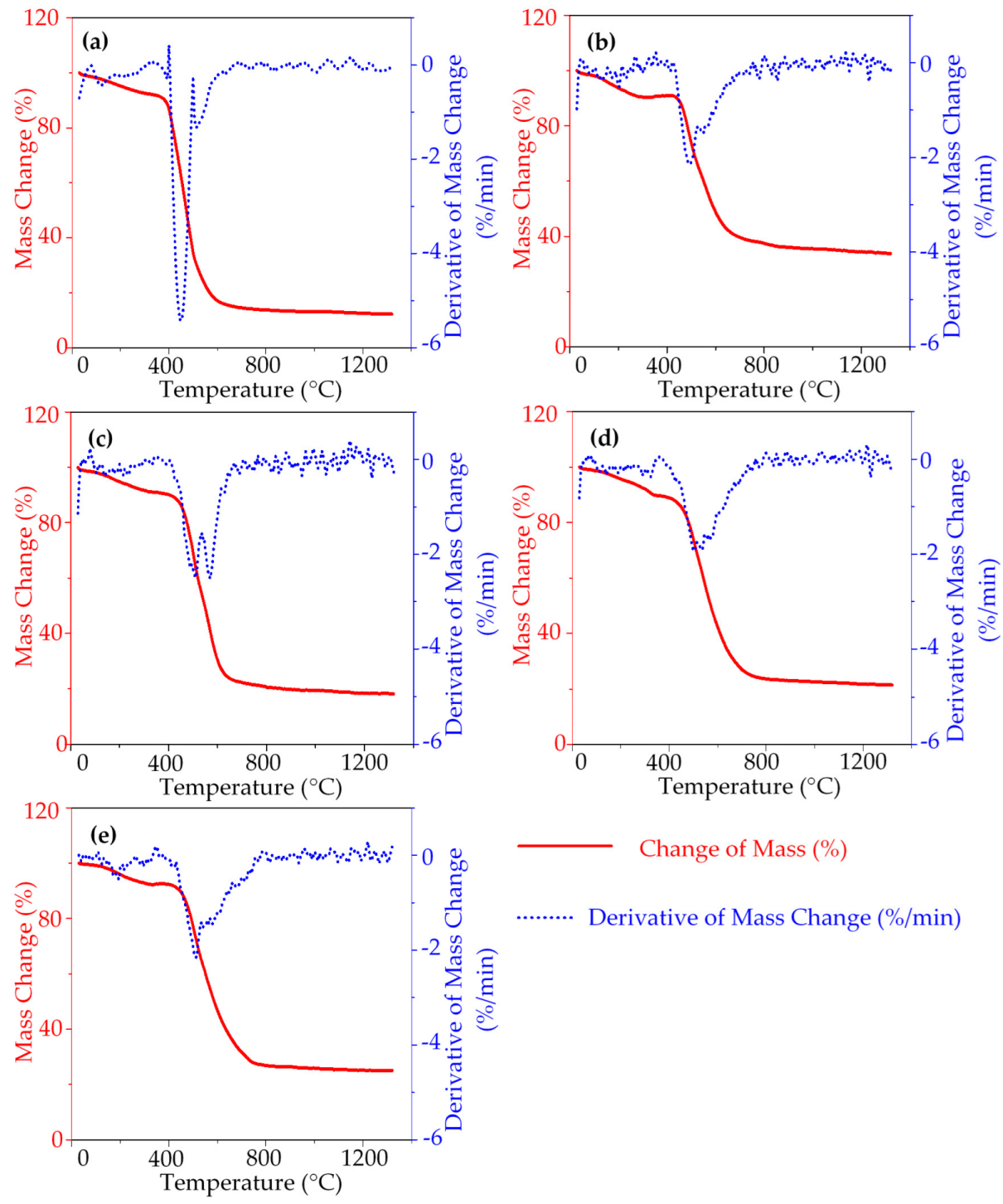
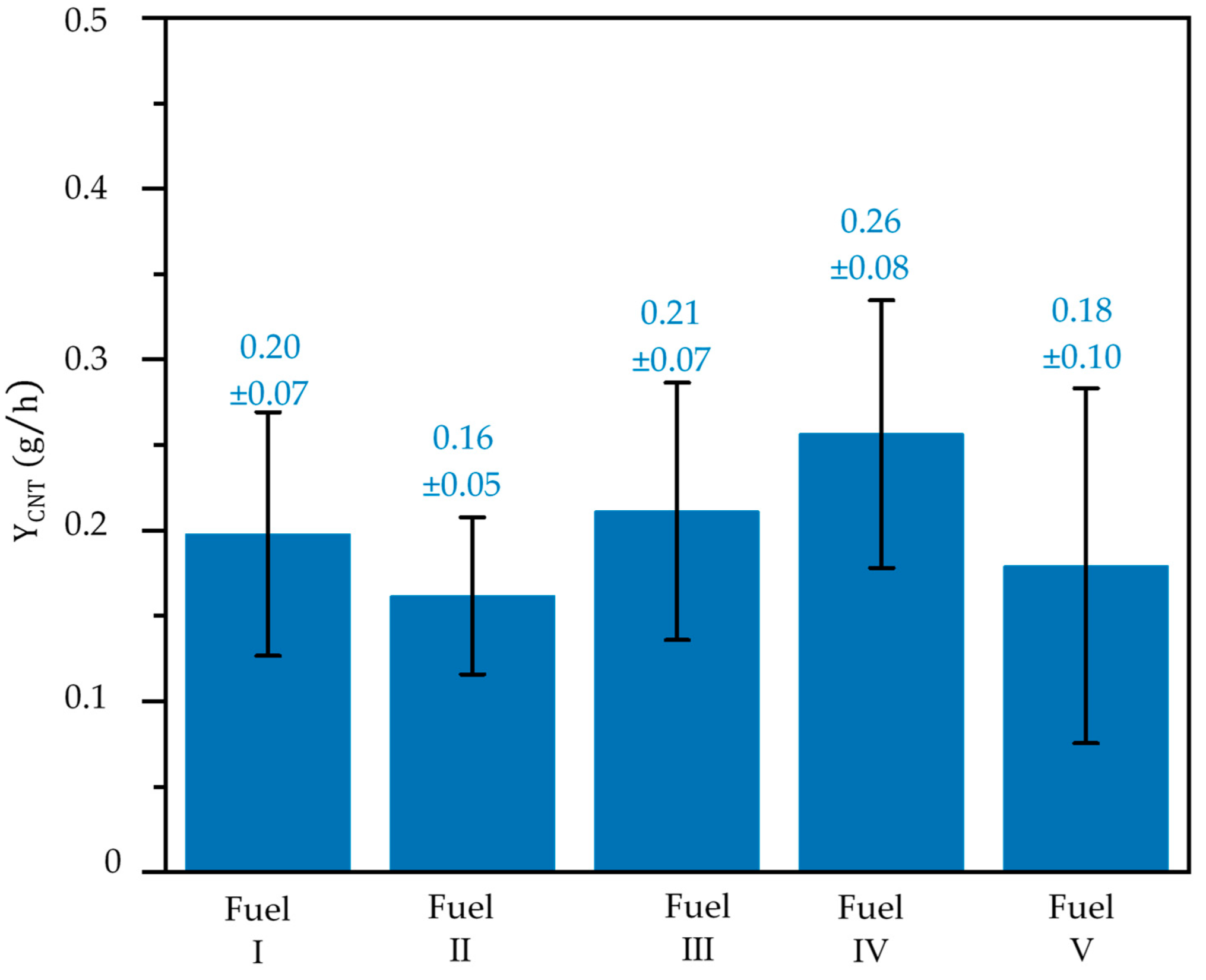
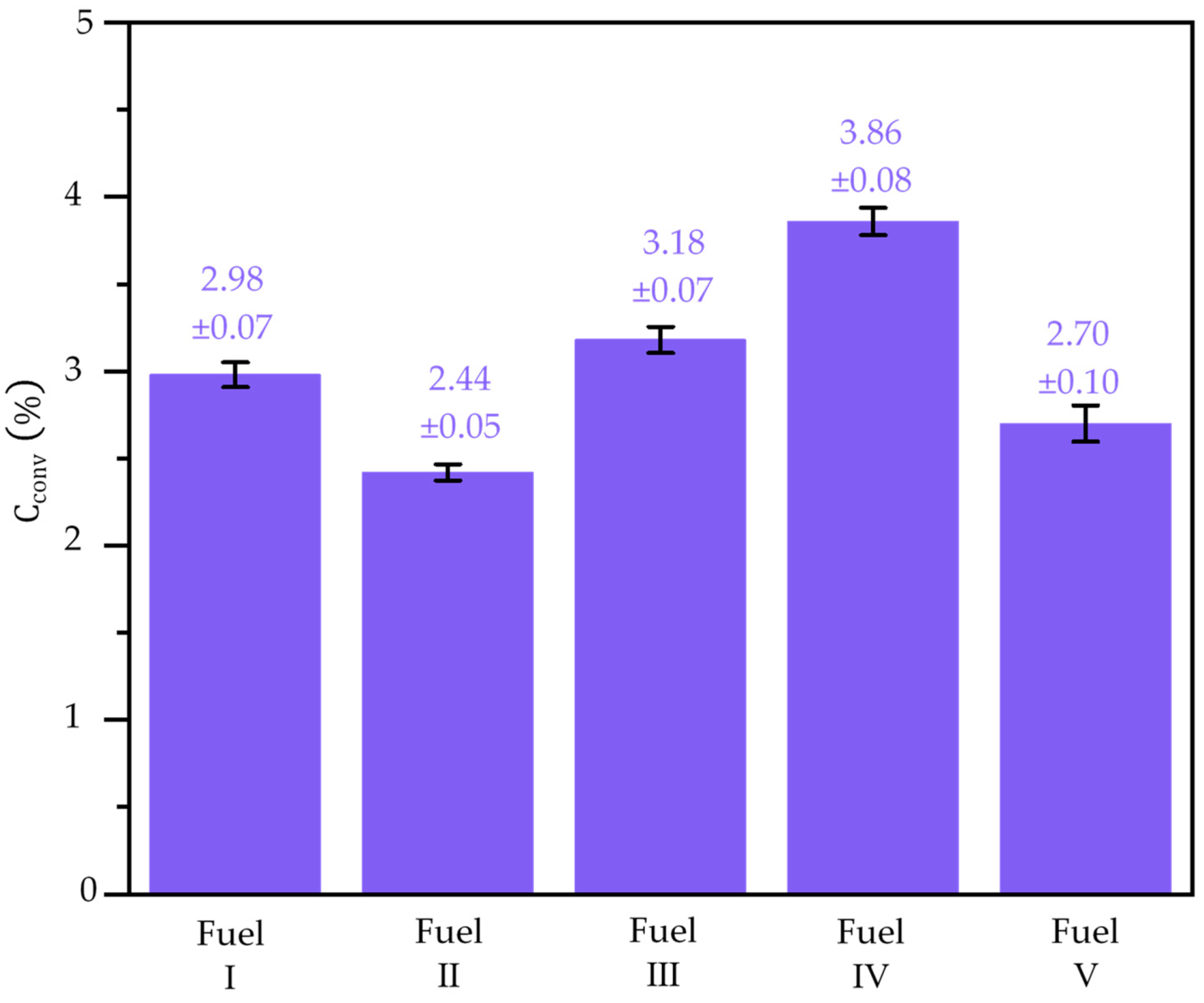
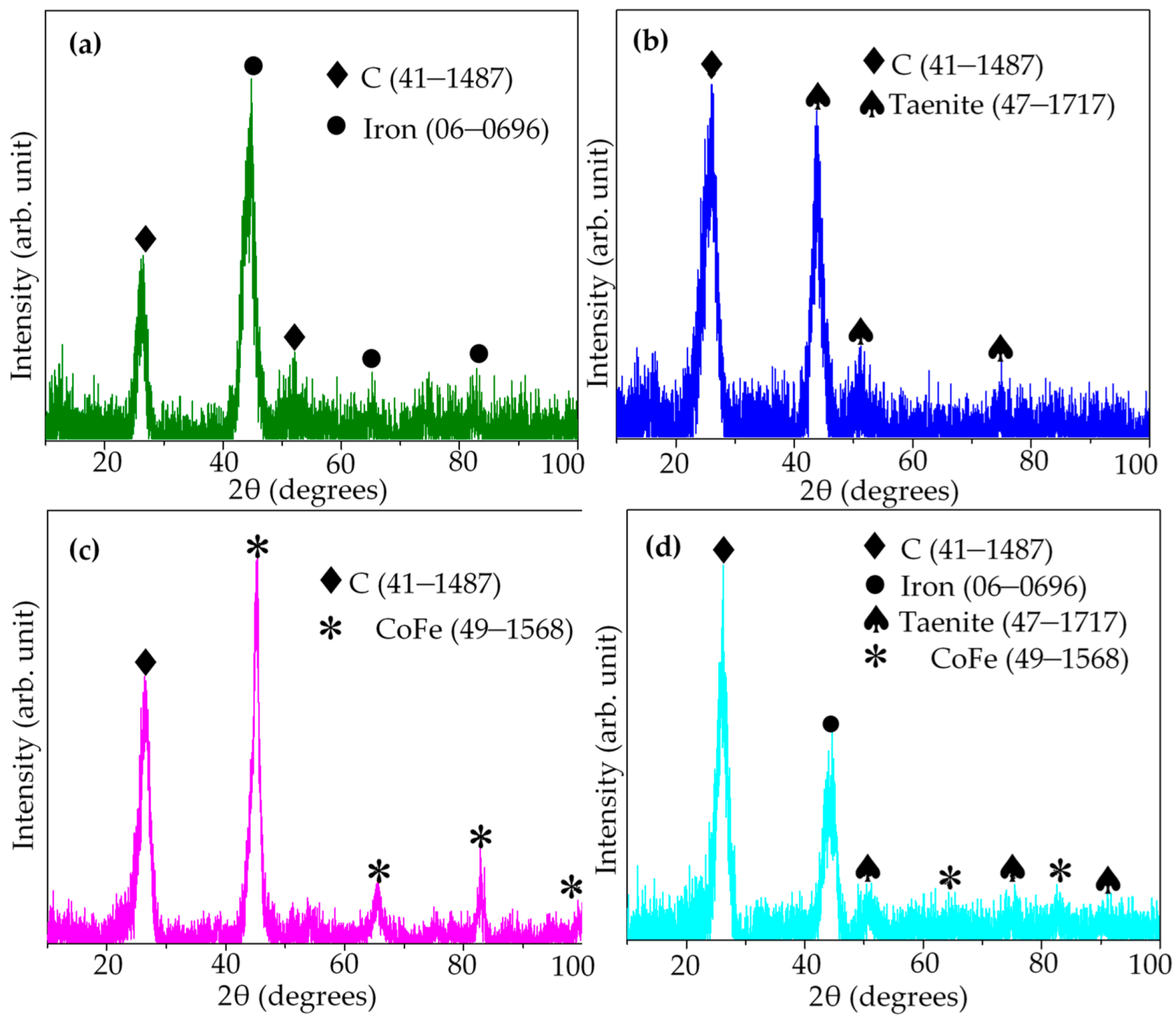
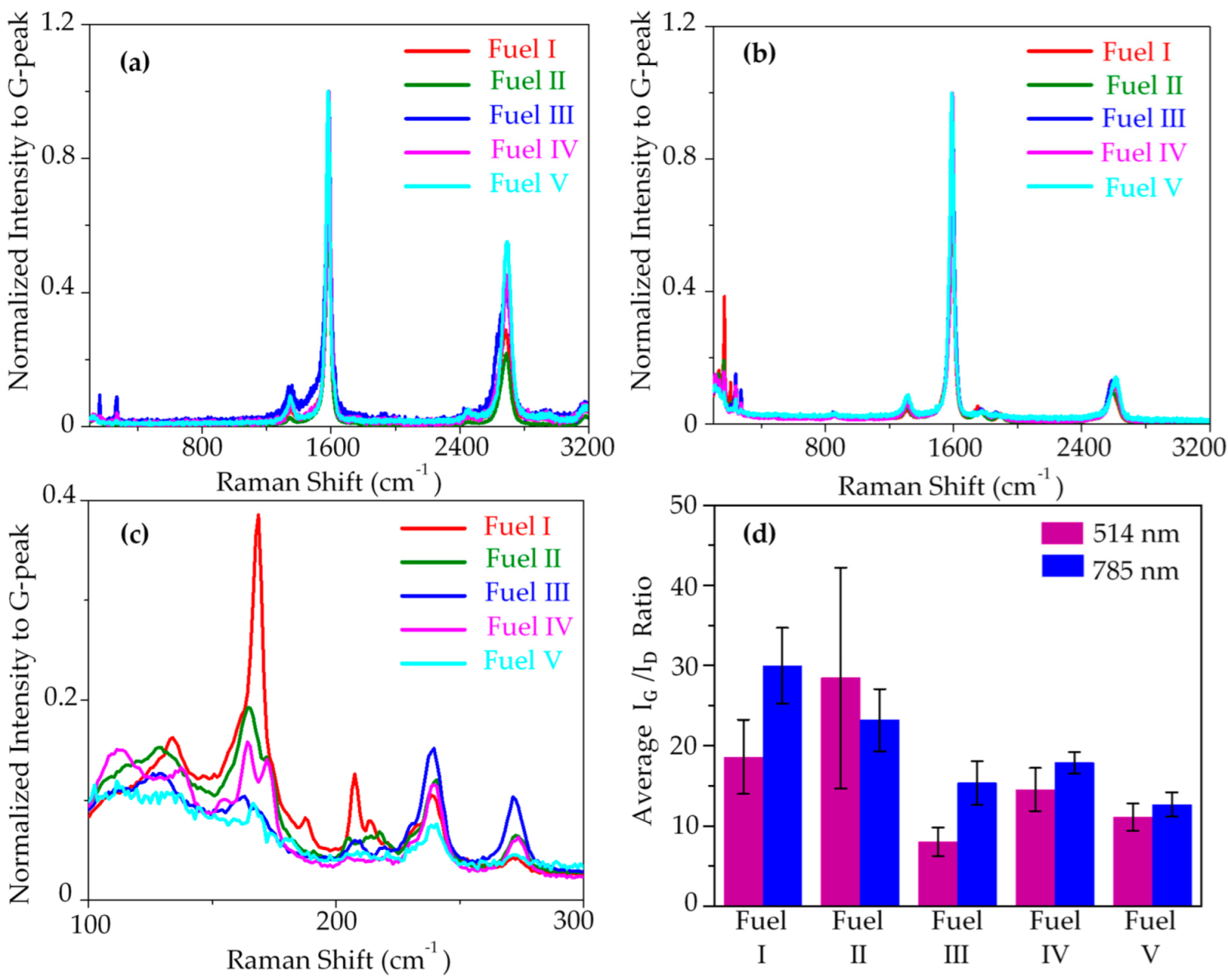
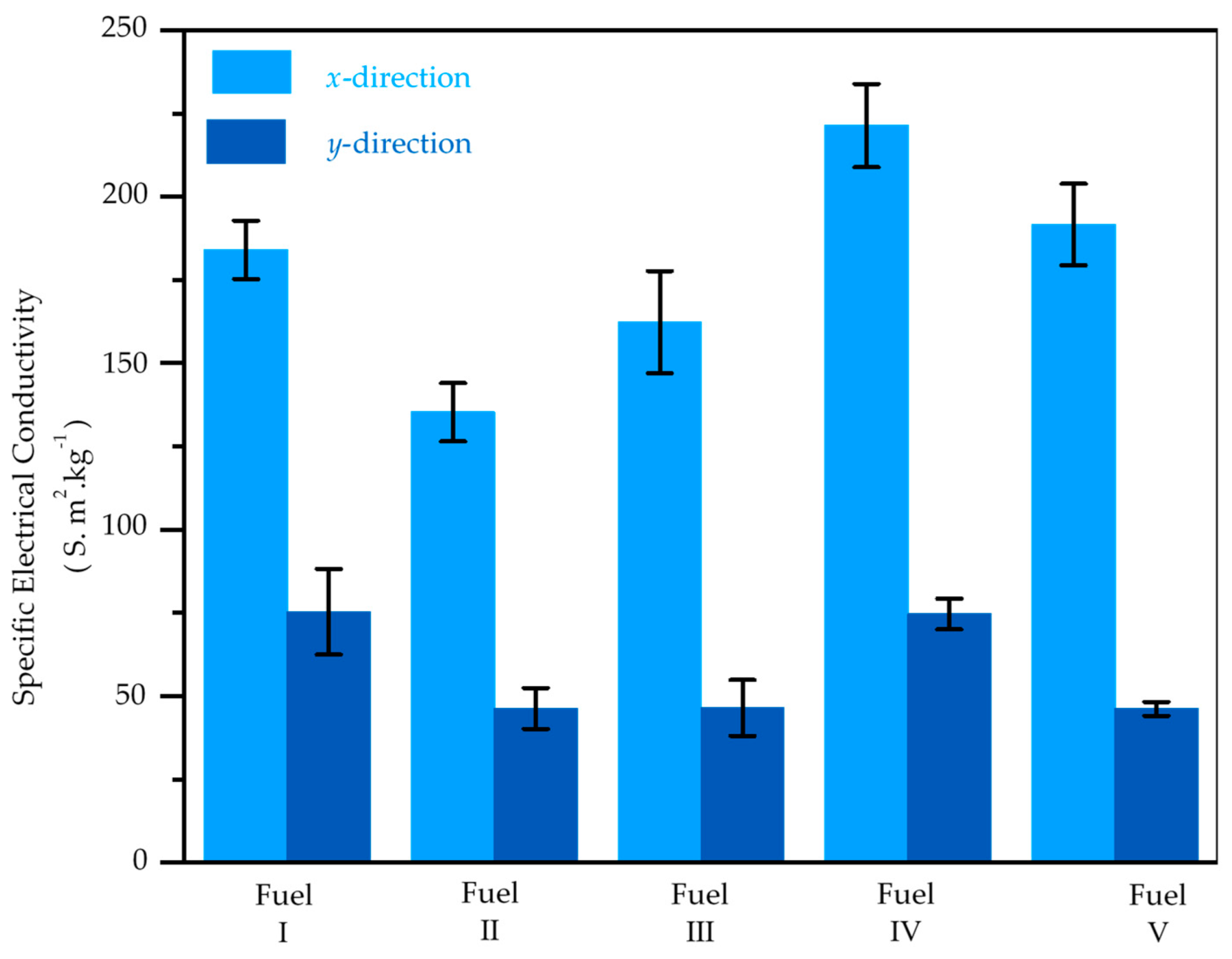
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Carbon Nanotube Synthesis Experiment
| Material | Methanol | n-Hexane | Metallocene | Thiophene |
|---|---|---|---|---|
| Formula | CH3OH | C6H14 | * Metal(C5H5)2 | C4H4S |
| Quantity per hour | 18 mL/h | 2 mL/h | 0.26 g/h | 0.15 mL/h |
| Molar mass (g/mol) | 32.04 | 86.18 | 186.04 | 84.14 |
| Density | 0.792 (g/mL) | 0.655 (g/mL) | 1.11 (g/cm3) | 1.05 (g/mL) |
| Moles per milliliter (mol/mL) | 0.025(mol/mL) | 0.008(mol/mL) | 0.005(mol/g) | 0.012(mol/mL) |
| No. of moles per hour (mol/h) | 0.445 | 0.015 | 0.001 | 0.002 |
| Moles of carbon per hour (mol/h) | 0.445 | 0.091 | 0.014 | 0.007 |
References
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Adusei, P.K.; Gbordzoe, S.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Alvarez, N.T.; Fang, Y.; Johnson, K.; McConnell, C.; Shanov, V. Fabrication and Study of Supercapacitor Electrodes Based on Oxygen Plasma Functionalized Carbon Nanotube Fibers. J. Energy Chem. 2020, 40, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Tan, P.; Wang, D.; Li, Z.; Peng, L.; Hu, Y.; Wang, C.; Zhu, W.; Chen, S.; Chen, W. High-Performance Supercapacitors Based on Electrochemical-Induced Vertical-Aligned Carbon Nanotubes and Polyaniline Nanocomposite Electrodes. Sci. Rep. 2017, 7, 43676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.D.; Zaghloul, A.I.; Shanov, V.; Schulz, M.J.; Mast, D.B.; Alvarez, N.T. Radiation Performance of Polarization Selective Carbon Nanotube Sheet Patch Antennas. IEEE Trans. Antennas Propag. 2014, 62, 48–55. [Google Scholar] [CrossRef]
- Amram Bengio, E.; Senic, D.; Taylor, L.W.; Tsentalovich, D.E.; Chen, P.; Holloway, C.L.; Babakhani, A.; Long, C.J.; Novotny, D.R.; Booth, J.C.; et al. High Efficiency Carbon Nanotube Thread Antennas. Appl. Phys. Lett. 2017, 111, 163109. [Google Scholar] [CrossRef]
- Chauhan, D.; Hou, G.; Ng, V.; Chaudhary, S.; Paine, M.; Moinuddin, K.; Rabiee, M.; Cahay, M.; Lalley, N.; Shanov, V.; et al. Multifunctional Smart Composites with Integrated Carbon Nanotube Yarn and Sheet. In A Tribute Conference Honoring Daniel Inman; Leo, D.J., Tarazaga, P.A., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10172, p. 1017205. [Google Scholar]
- Zhao, M.; Meng, L.; Ma, L.; Ma, L.; Yang, X.; Huang, Y.; Ryu, J.E.; Shankar, A.; Li, T.; Yan, C.; et al. Layer-by-Layer Grafting CNTs onto Carbon Fibers Surface for Enhancing the Interfacial Properties of Epoxy Resin Composites. Compos. Sci. Technol. 2018, 154, 28–36. [Google Scholar] [CrossRef]
- Bhanushali, H.; Bradford, P.D. Woven Glass Fiber Composites with Aligned Carbon Nanotube Sheet Interlayers. J. Nanomater. 2016, 2016, 55. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Chauhan, D.; Hou, G.; Wen, X.; Kattoura, M.; Christine, R.; Vesselin, S. Carbon Nanotube Sheet Reinforced Laminated Composites. In Proceedings of the 31st Annual Technical Conference of the American Society for Composites, Williamsburg, VA, USA, 19–22 September 2016. [Google Scholar]
- Xu, C.; Chauhan, D.; Hou, G.; Ng, V.; Song, Y.; Paine, M. Synthesis of Hybrid Carbon Nanotube Yarn and Sheet and Their Applications. In Nanotube Superfiber Materials; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 897–914. ISBN 9780128126677. [Google Scholar]
- Gbordzoe, S.; Adusei, P.K.; Chauhan, D.; Alvarez, N.T.; Haase, M.R.; Mansari, K.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Shanov, V. A Simple Two-Step Process for Producing Strong and Aligned Carbon Nanotube-Polymer Composites. C 2019, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Statista. Carbon Nanotubes Global Market Value Forecast 2026. Available online: https://www.statista.com/statistics/714463/global-market-value-of-carbon-nanotubes/ (accessed on 27 August 2019).
- Dixit, S.; Shukla, A.K. Raman Studies of Single-Walled Carbon Nanotubes Synthesized by Pulsed Laser Ablation at Room Temperature. Appl. Phys. A 2018, 124, 400. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, Y. New Technique of Synthesizing Single-Walled Carbon Nanotubes from Ethanol Using Fluidized-Bed over Fe–Mo/MgO Catalyst. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2006, 64, 296–300. [Google Scholar] [CrossRef]
- Sari, A.H.; Khazali, A.; Parhizgar, S.S. Synthesis and Characterization of Long-CNTs by Electrical Arc Discharge in Deionized Water and NaCl Solution. Int. Nano Lett. 2018, 8, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Corrias, M.; Caussat, B.; Ayral, A.; Durand, J.; Kihn, Y.; Kalck, P.; Serp, P. Carbon Nanotubes Produced by Fluidized Bed Catalytic CVD: First Approach of the Process. Chem. Eng. Sci. 2003, 58, 4475–4482. [Google Scholar] [CrossRef]
- Öner, D.; Ghosh, M.; Coorens, R.; Bové, H.; Moisse, M.; Lambrechts, D.; Ameloot, M.; Godderis, L.; Hoet, P.H.M. Induction and Recovery of CpG Site Specific Methylation Changes in Human Bronchial Cells after Long-Term Exposure to Carbon Nanotubes and Asbestos. Environ. Int. 2020, 137, 105530. [Google Scholar] [CrossRef] [PubMed]
- Schauer, M.W.; White, M.A. Tailoring Industrial Scale CNT Production to Specialty Markets. MRS Online Proc. Libr. 2015, 1752, 103–109. [Google Scholar] [CrossRef]
- Nanocomp Technologies|Nanotechnology. Available online: https://www.miralon.com/what-are-carbon-nanotubes (accessed on 25 January 2020).
- Chitranshi, M.; Pujari, A.; Ng, V.; Chen, D.; Chauhan, D.; Hudepohl, R.; Saleminik, M.; Kim, S.Y.; Kubley, A.; Shanov, V.; et al. Carbon Nanotube Sheet-Synthesis and Applications. Nanomaterials 2020, 10, 2023. [Google Scholar] [CrossRef]
- Chauhan, D. Manufacturing and Applications of Carbon Nanotube Sheet and Thread. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2018. [Google Scholar]
- Hou, G.; Mast, D.; Kleismit, R.; Chauhan, D.; Xu, C.; Dugre, J.; Ng, V.; Turgut, Z.; Chen, R. Industrializing Nanotube Superfiber Materials. In Nanotube Superfiber Materials; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 573–601. ISBN 9780128126677. [Google Scholar]
- Kim, M.; Park, Y. High Sensitive CNT Imbedded Knit Fabrics for Heat Comfort. Fibers Polym. 2018, 19, 2112–2120. [Google Scholar] [CrossRef]
- Schulz, M.J.; Chauhan, D.; Kanakaraj, S.; Mast, D.; Shanov, V.; Hou, G.; Ng, V.; Xu, C.; Chen, R.D.; Kubley, A.; et al. Carbon Nanotube Hybrid Material Fabric, Composite Fabric, and Personal Protective Apparel and Equipment. U.S. Patent US20200270774A1, 27 August 2020. [Google Scholar]
- Chauhan, D.; Xu, C.; Chen, D.; Kubley, A.; Brandewie, B.; Hou, G.; Li, W.; Ng, V.; Rabiee, M.; Cahay, M.; et al. Introduction to Carbon Nanotube Hybrid Textiles. J. Text. Sci. Fash. Technol. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Kubley, A.; Chauhan, D.; Kanakaraj, S.N.; Xu, C.; Chen, R.; Ng, V.; Bell, G.; Verma, P.; Hou, X.; Chitranshi, M.; et al. Smart Textiles and Wearable Technology Innovation with Carbon Nanotube Technology. In Nanotube Superfiber Materials; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 263–311. ISBN 9780128126677. [Google Scholar]
- Chitranshi, M.; Chauhan, D.; Kubley, A.; Pujari, A.; Xu, C.; Chen, D.; Chaudhary, S.; Hou, G.; Bell, G.; Brandewie, B.; et al. Pioneering Carbon Nanotube Textile Engineering & Fashion Technology. J. Text. Eng. Fash. Technol. 2019, 5, 89–92. [Google Scholar] [CrossRef]
- Chauhan, D.; Chen, R.; Xu, C.; Mast, D.; Kleismit, R.; Kubley, A.; Hou, G.; Chitranshi, M.; Pujari, A.; Devarakonda, S.; et al. Carbon Nanotube Hybrid Fabric and Tape. In Nanotube Superfiber Materials; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 239–261. ISBN 9780128126677. [Google Scholar]
- Chen, X.; Zhou, J.; Chen, S.; Zhang, H. Catalytic Performance of M@Ni (M = Fe, Ru, Ir) Core−shell Nanoparticles towards Ammonia Decomposition for COx-Free Hydrogen Production. J. Nanopart. Res. 2018, 20, 148. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Ghaffar, A.; AbdEl-Salam, N.M. Synthesis of MWCNT Forests with Alumina-Supported Fe2O3 Catalyst by Using a Floating Catalyst Chemical Vapor Deposition Technique. J. Nanomater. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Fuge, R.; Liebscher, M.; Schröfl, C.; Damm, C.; Eckert, V.; Eibl, M.; Leonhardt, A.; Büchner, B.; Mechtcherine, V. Influence of Different Hydrocarbons on the Height of MWCNT Carpets: Role of Catalyst and Hybridization State of the Carbon Precursor. Diam. Relat. Mater. 2018, 90, 18–25. [Google Scholar] [CrossRef]
- Bouanis, F.Z.; Florea, I.; Bouanis, M.; Muller, D.; Nyassi, A.; Le Normand, F.; Pribat, D. Diameter Controlled Growth of SWCNTs Using Ru as Catalyst Precursors Coupled with Atomic Hydrogen Treatment. Chem. Eng. J. 2018, 332, 92–101. [Google Scholar] [CrossRef]
- Weller, L.; Smail, F.R.; Elliott, J.A.; Windle, A.H.; Boies, A.M.; Hochgreb, S. Mapping the Parameter Space for Direct-Spun Carbon Nanotube Aerogels. Carbon 2019, 146, 789–812. [Google Scholar] [CrossRef]
- Mclean, B.; Kauppinen, E.I.; Page, A.J. Initial Competing Chemical Pathways during Floating Catalyst Chemical Vapor Deposition Carbon Nanotube Growth. J. Appl. Phys. 2021, 129, 44302. [Google Scholar] [CrossRef]
- Dyagileva, L.M.; Mar’in, V.P.; Tsyganova, E.I.; Razuvaev, G.A. Reactivity of the First Transition Row Metallocenes in Thermal Decomposition Reaction. J. Organomet. Chem. 1979, 175, 63–72. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Bajada, M.; Pick, M.; Boies, A. Catalyst Nanoparticle Growth Dynamics and Their Influence on Product Morphology in a CVD Process for Continuous Carbon Nanotube Synthesis. Carbon 2016, 96, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Hoecker, C.; Smail, F.; Pick, M.; Weller, L.; Boies, A.M. The Dependence of CNT Aerogel Synthesis on Sulfur-Driven Catalyst Nucleation Processes and a Critical Catalyst Particle Mass Concentration. Sci. Rep. 2017, 7, 14519. [Google Scholar] [CrossRef] [Green Version]
- Kuwana, K.; Saito, K. Modeling CVD Synthesis of Carbon Nanotubes: Nanoparticle Formation from Ferrocene. Carbon 2005, 43, 2088–2095. [Google Scholar] [CrossRef]
- Gökstorp, F.K.A.; Juniper, M.P. Flow Simulations Including Iron Nanoparticle Nucleation, Growth and Evaporation for Floating Catalyst CNT Production. Catalysts 2020, 10, 1383. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Pick, M.; Boies, A. The Influence of Carbon Source and Catalyst Nanoparticles on CVD Synthesis of CNT Aerogel. Chem. Eng. J. 2017, 314, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Hou, G.; Chauhan, D.; Ng, V.; Xu, C.; Yin, Z.; Paine, M.; Su, R.; Shanov, V.; Mast, D.; Schulz, M.; et al. Gas Phase Pyrolysis Synthesis of Carbon Nanotubes at High Temperature. Mater. Des. 2017, 132, 112–118. [Google Scholar] [CrossRef]
- Gspann, T.S.; Smail, F.R.; Windle, A.H. Spinning of Carbon Nanotube Fibres Using the Floating Catalyst High Temperature Route: Purity Issues and the Critical Role of Sulphur. Faraday Discuss. 2014, 173, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Conroy, D.; Moisala, A.; Cardoso, S.; Windle, A.; Davidson, J. Carbon Nanotube Reactor: Ferrocene Decomposition, Iron Particle Growth, Nanotube Aggregation and Scale-Up. Chem. Eng. Sci. 2010, 65, 2965–2977. [Google Scholar] [CrossRef]
- Windle, A. Understanding the Direct Spinning of Cnt Fibers in Terms of the Thermodynamic and Kinetic Landscape: A Personal View. In Nanotube Superfiber Materials: Science, Manufacturing, Commercialization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–184. ISBN 9780128126677. [Google Scholar]
- Reguero, V.; Alemán, B.; Mas, B.; Vilatela, J.J. Controlling Carbon Nanotube Type in Macroscopic Fibers Synthesized by the Direct Spinning Process. Chem. Mater. 2014, 26, 3550–3557. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Windle, A.H. Effect of Carbon Precursors on the Structure and Properties of Continuously Spun Carbon Nanotube Fibers. Sci. Adv. Mater. 2015, 7, 643–653. [Google Scholar] [CrossRef]
- Gohier, A.; Ewels, C.P.; Minea, T.M.; Djouadi, M.A. Carbon Nanotube Growth Mechanism Switches from Tip- to Base-Growth with Decreasing Catalyst Particle Size. Carbon 2008, 46, 1331–1338. [Google Scholar] [CrossRef]
- Xiang, R.; Luo, G.; Qian, W.; Zhang, Q.; Wang, Y.; Wei, F.; Li, Q.; Cao, A. Encapsulation, Compensation, and Substitution of Catalyst Particles during Continuous Growth of Carbon Nanotubes. Adv. Mater. 2007, 19, 2360–2363. [Google Scholar] [CrossRef] [Green Version]
- Zoican Loebick, C.; Podila, R.; Reppert, J.; Chudow, J.; Ren, F.; Haller, G.L.; Rao, A.M.; Pfefferle, L.D. Selective Synthesis of Subnanometer Diameter Semiconducting Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2010, 132, 11125–11131. [Google Scholar] [CrossRef]
- He, M.; Liu, B.; Chernov, A.I.; Obraztsova, E.D.; Kauppi, I.; Jiang, H.; Anoshkin, I.; Cavalca, F.; Hansen, T.W.; Wagner, J.B.; et al. Growth Mechanism of Single-Walled Carbon Nanotubes on Iron–Copper Catalyst and Chirality Studies by Electron Diffraction. Chem. Mater. 2012, 24, 1796–1801. [Google Scholar] [CrossRef]
- Ahmad, S.; Liao, Y.; Hussain, A.; Zhang, Q.; Ding, E.X.; Jiang, H.; Kauppinen, E.I. Systematic Investigation of the Catalyst Composition Effects on Single-Walled Carbon Nanotubes Synthesis in Floating-Catalyst CVD. Carbon 2019, 149, 318–327. [Google Scholar] [CrossRef]
- Karaeva, A.R.; Urvanov, S.A.; Kazennov, N.V.; Mitberg, E.B.; Mordkovich, V.Z. Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes. Nanomaterials 2020, 10, 2279. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Kim, W.S. The Synergistic Effect of a Bimetallic Catalyst for the Synthesis of Carbon Nanotube Aerogels and Their Predominant Chirality. Chem.—Eur. J. 2019, 25, 13635–13639. [Google Scholar] [CrossRef] [PubMed]
- Mayne, M. Pure and Aligned Carbon Nanotubes Produced by the Pyrolysis of Benzene-Based Aerosols. AIP Conf. Proc. 2003, 591, 204–207. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Yu, H.; Gui, X.; Wang, H.; Huang, H.; Peng, F.; Yang, X.; Li, Y.; Yu, H.; et al. Enhanced Catalytic Activity of Carbon Nanotubes for the Oxidation of Cyclohexane by Filling with Fe, Ni, and FeNi Alloy Nanowires. Aust. J. Chem. 2015, 69, 689–695. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.-B.; Lin, G.-D.; Hong, Q.; Tsai, K.R. Growth of Carbon Nanotubes by Catalytic Decomposition of CH4 or CO on a Ni-MgO Catalyst. Carbon 1997, 35, 1495–1501. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Q.; Guo, J.; Zhu, L. Influence of Ni Promotion on Liquid Hydrocarbon Fuel Production over Co/CNT Catalysts. Energy Fuels 2013, 27, 3961–3968. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.; Park, J.H.; Lee, D.M.; Lee, A.; Moon, S.Y.; Lee, S.Y.; Jeong, H.S.; Kim, S.M. Deep-Injection Floating-Catalyst Chemical Vapor Deposition to Continuously Synthesize Carbon Nanotubes with High Aspect Ratio and High Crystallinity. Carbon 2021, 173, 901–909. [Google Scholar] [CrossRef]
- Gspann, T.S.; Juckes, S.M.; Niven, J.F.; Johnson, M.B.; Elliott, J.A.; White, M.A.; Windle, A.H. High Thermal Conductivities of Carbon Nanotube Films and Micro-Fibres and Their Dependence on Morphology. Carbon 2017, 114, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Loebick, C.Z.; Derrouiche, S.; Marinkovic, N.; Wang, C.; Hennrich, F.; Kappes, M.M.; Haller, G.L.; Pfefferle, L.D. Effect of Manganese Addition to the Co-MCM-41 Catalyst in the Selective Synthesis of Single Wall Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 21611–21620. [Google Scholar] [CrossRef]
- Lee, S.; Zoican Loebick, C.; Pfefferle, L.D.; Haller, G.L. High-Temperature Stability of Cobalt Grafted on Low-Loading Incorporated Mo−MCM-41 Catalyst for Synthesis of Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 1014–1024. [Google Scholar] [CrossRef]
- He, M.; Chernov, A.I.; Obraztsova, E.D.; Jiang, H.; Kauppinen, E.I.; Lehtonen, J. Synergistic Effects in FeCu Bimetallic Catalyst for Low Temperature Growth of Single-Walled Carbon Nanotubes. Carbon 2013, 52, 590–594. [Google Scholar] [CrossRef]
- Mattevi, C.; Kim, H.; Chhowalla, M. A Review of Chemical Vapour Deposition of Graphene on Copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Gutierrez-Wing, C.; Miki, M.; Yang, D.Q.; Piyakis, K.N.; Sacher, E. Surface Diffusion and Coalescence of Mobile Metal Nanoparticles. J. Phys. Chem. B 2005, 109, 9703–9711. [Google Scholar] [CrossRef]
- Hawa, T.; Zachariah, M.R. Coalescence Kinetics of Unequal Sized Nanoparticles. J. Aerosol Sci. 2006, 37, 1–15. [Google Scholar] [CrossRef]
- Goudeli, E.; Pratsinis, S.E. Surface Composition and Crystallinity of Coalescing Silver-Gold Nanoparticles. ACS Nano 2017, 11, 11653–11660. [Google Scholar] [CrossRef]
- Lehtinen, K.E.J.; Zachariah, M.R. Energy Accumulation in Nanoparticle Collision and Coalescence Processes. J. Aerosol Sci. 2002, 33, 357–368. [Google Scholar] [CrossRef]
- Zhao, X.; Ando, Y.; Qin, L.-C.; Kataura, H.; Maniwa, Y.; Saito, R. Radial Breathing Modes of Multiwalled Carbon Nanotubes. Chem. Phys. Lett. 2002, 361, 169–174. [Google Scholar] [CrossRef]
- ASM International. Handbook committee. In ASM Handbook: Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 2002; Volume 3, ISBN 9781627080705. [Google Scholar]
- Gromov, D.G.; Gavrilov, S.A. Heterogeneous Melting in Low-Dimensional Systems and Accompanying Surface Effects. In Thermodynamics—Physical Chemistry of Aqueous Systems; InTech: London, UK, 2011; pp. 157–190. [Google Scholar]
- Leonhardt, A.; Hampel, S.; Müller, C.; Mönch, I.; Koseva, R.; Ritschel, M.; Elefant, D.; Biedermann, K.; Büchner, B. Synthesis, Properties, and Applications of Ferromagnetic-Filled Carbon Nanotubes. Chem. Vap. Depos. 2006, 12, 380–387. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Mohan Sankaran, R. Linking Catalyst Composition to Chirality Distributions of As-Grown Single-Walled Carbon Nanotubes by Tuning NixFe1-x Nanoparticles. Nat. Mater. 2009, 8, 882–886. [Google Scholar] [CrossRef]
- Abdullahi, I.; Sakulchaicharoen, N.; Herrera, J.E. Selective Growth of Single-Walled Carbon Nanotubes over Co–MgO Catalyst by Chemical Vapor Deposition of Methane. Diam. Relat. Mater. 2013, 38, 1–8. [Google Scholar] [CrossRef]
- Lolli, G.; Zhang, L.; Balzano, L.; Sakulchaicharoen, N.; Tan, Y.; Resasco, D.E. Tailoring (n,m) Structure of Single-Walled Carbon Nanotubes by Modifying Reaction Conditions and the Nature of the Support of CoMo Catalysts. J. Phys. Chem. B 2006, 110, 2108–2115. [Google Scholar] [CrossRef]
- He, M.; Fedotov, P.V.; Chernov, A.; Obraztsova, E.D.; Jiang, H.; Wei, N.; Cui, H.; Sainio, J.; Zhang, W.; Jin, H.; et al. Chiral-Selective Growth of Single-Walled Carbon Nanotubes on Fe-Based Catalysts Using CO as Carbon Source. Carbon 2016, 108, 521–528. [Google Scholar] [CrossRef]



| Fuel Type | Metal Ratio (%) | Metallocene Mass (g) | Catalyst/S Ratio | ||
|---|---|---|---|---|---|
| Ferrocene | Nickelocene | Cobaltocene | |||
| I | Fe~54 | 0.7 | 0 | 0 | 1.85 |
| II | Fe-100 | 1.3 | 0 | 0 | |
| III | Fe:Ni~54:46 | 0.7 | 0.6 | 0 | |
| IV | Fe-Co~54:46 | 0.7 | 0 | 0.6 | |
| V | Fe:Ni:Co~54:23:23 | 0.7 | 0.3 | 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, D.; Pujari, A.; Zhang, G.; Dasgupta, K.; Shanov, V.N.; Schulz, M.J. Effect of a Metallocene Catalyst Mixture on CNT Yield Using the FC-CVD Process. Catalysts 2022, 12, 287. https://doi.org/10.3390/catal12030287
Chauhan D, Pujari A, Zhang G, Dasgupta K, Shanov VN, Schulz MJ. Effect of a Metallocene Catalyst Mixture on CNT Yield Using the FC-CVD Process. Catalysts. 2022; 12(3):287. https://doi.org/10.3390/catal12030287
Chicago/Turabian StyleChauhan, Devika, Anuptha Pujari, Guangqi Zhang, Kinshuk Dasgupta, Vesselin N. Shanov, and Mark J. Schulz. 2022. "Effect of a Metallocene Catalyst Mixture on CNT Yield Using the FC-CVD Process" Catalysts 12, no. 3: 287. https://doi.org/10.3390/catal12030287
APA StyleChauhan, D., Pujari, A., Zhang, G., Dasgupta, K., Shanov, V. N., & Schulz, M. J. (2022). Effect of a Metallocene Catalyst Mixture on CNT Yield Using the FC-CVD Process. Catalysts, 12(3), 287. https://doi.org/10.3390/catal12030287







