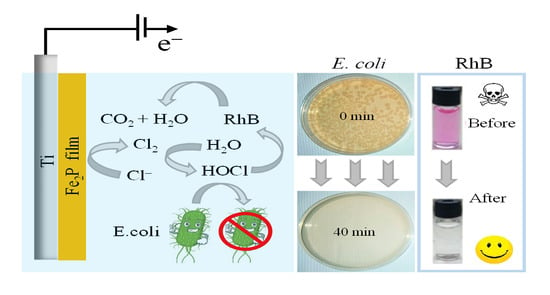
Iron Phosphide Precatalyst for Electrocatalytic Degradation of Rhodamine B Dye and Removal of Escherichia coli from Simulated Wastewater
Abstract
:1. Introduction
2. Results and Discussion
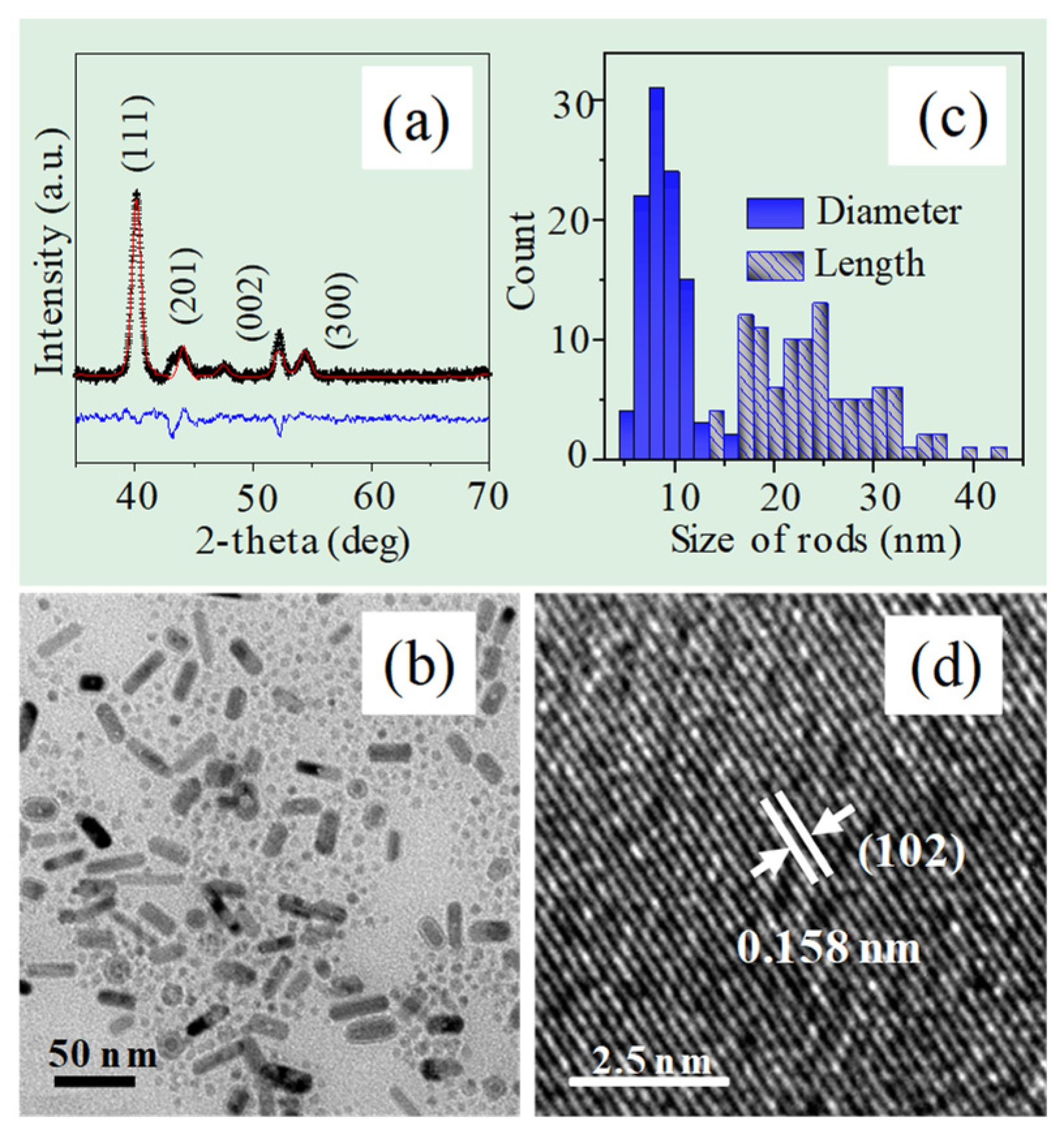
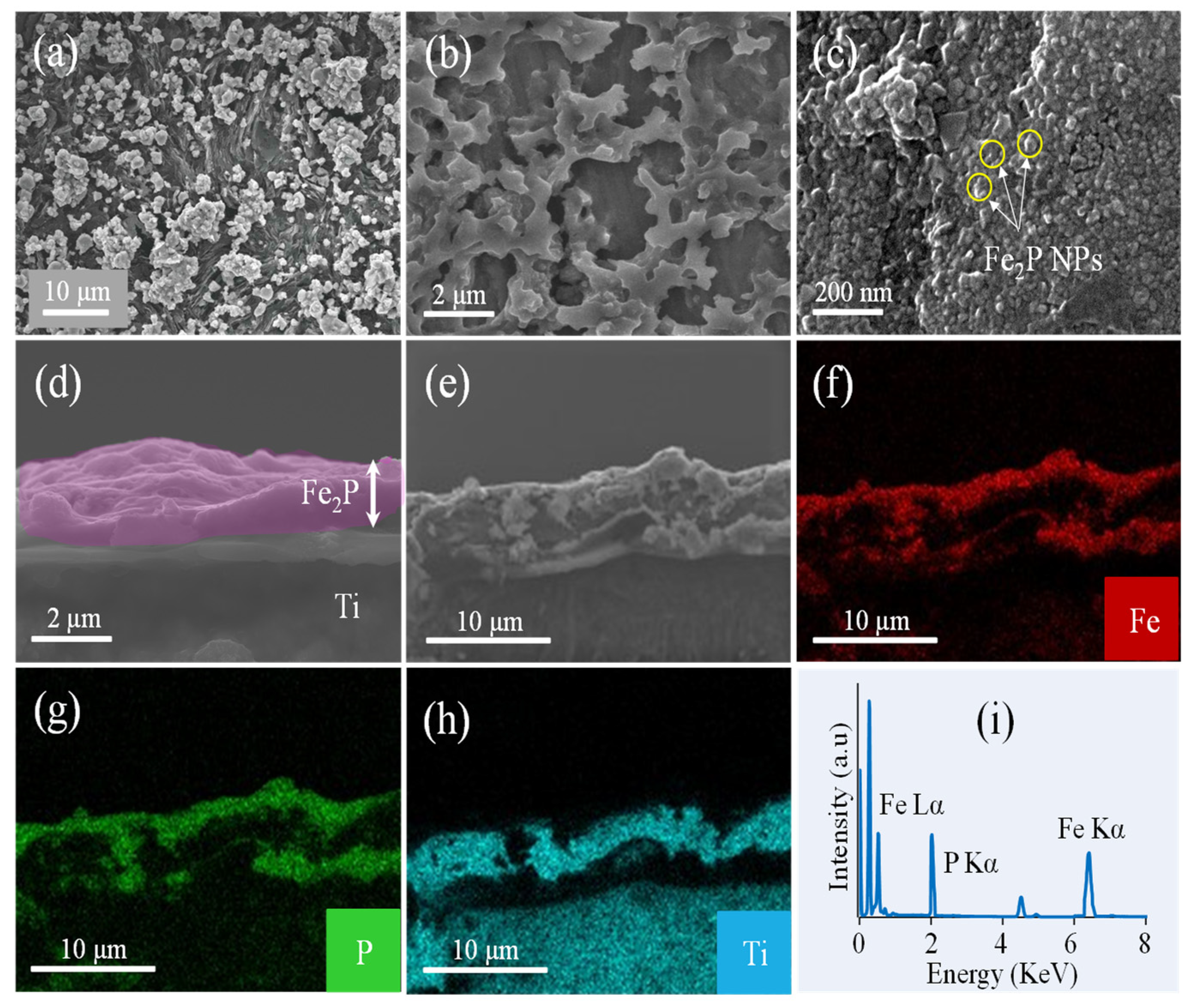
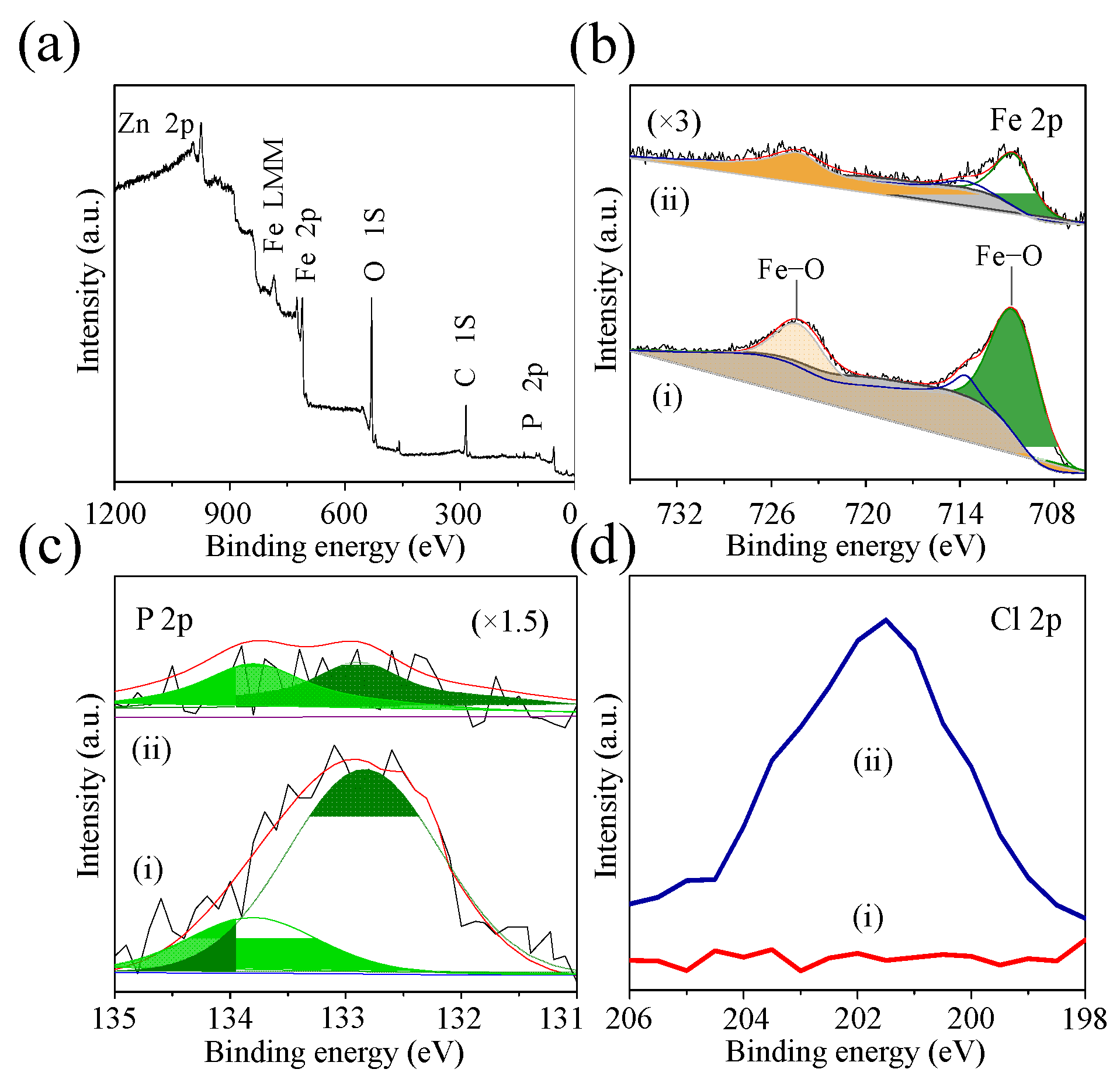
2.1. Characterization of Fe2P Thin Films
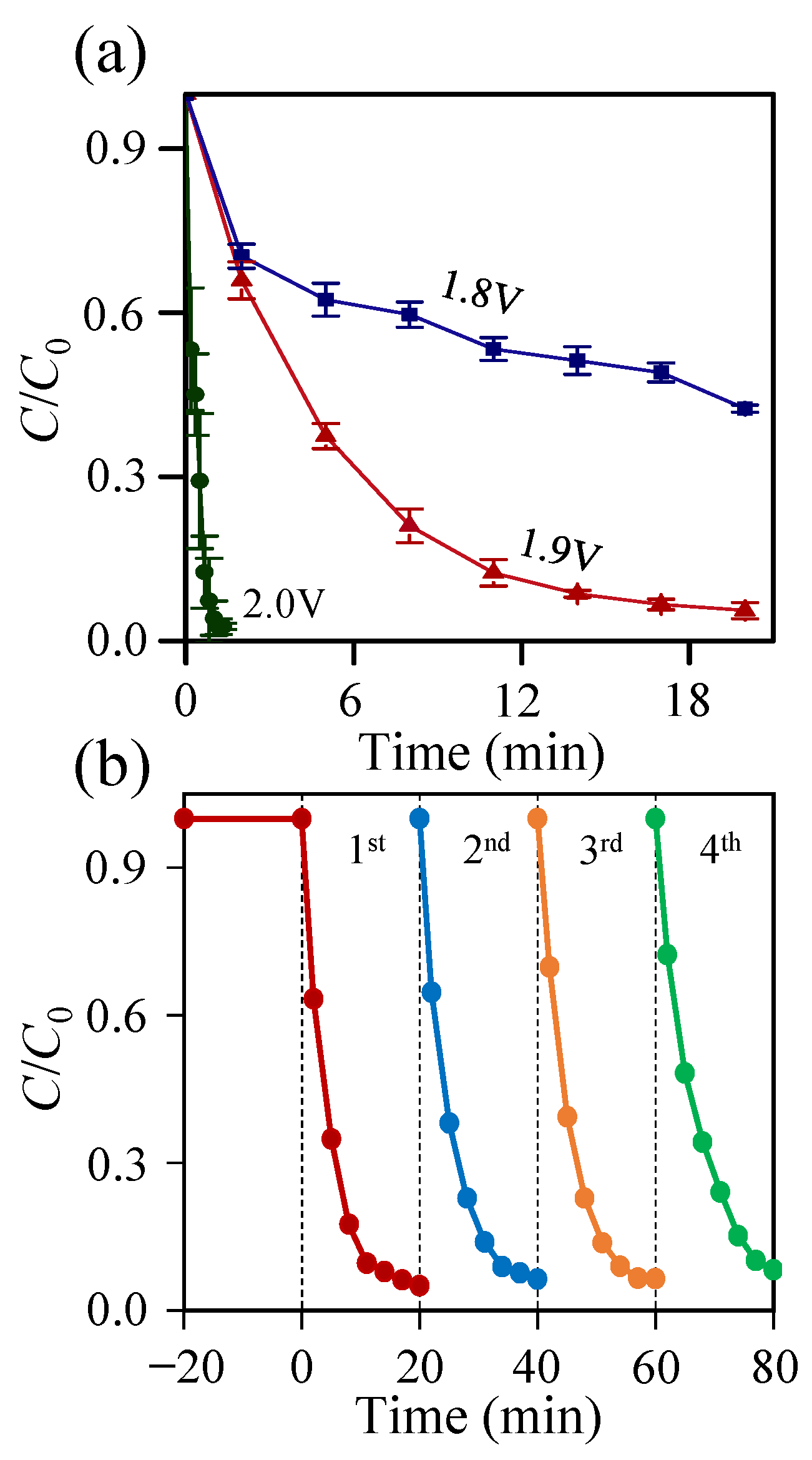
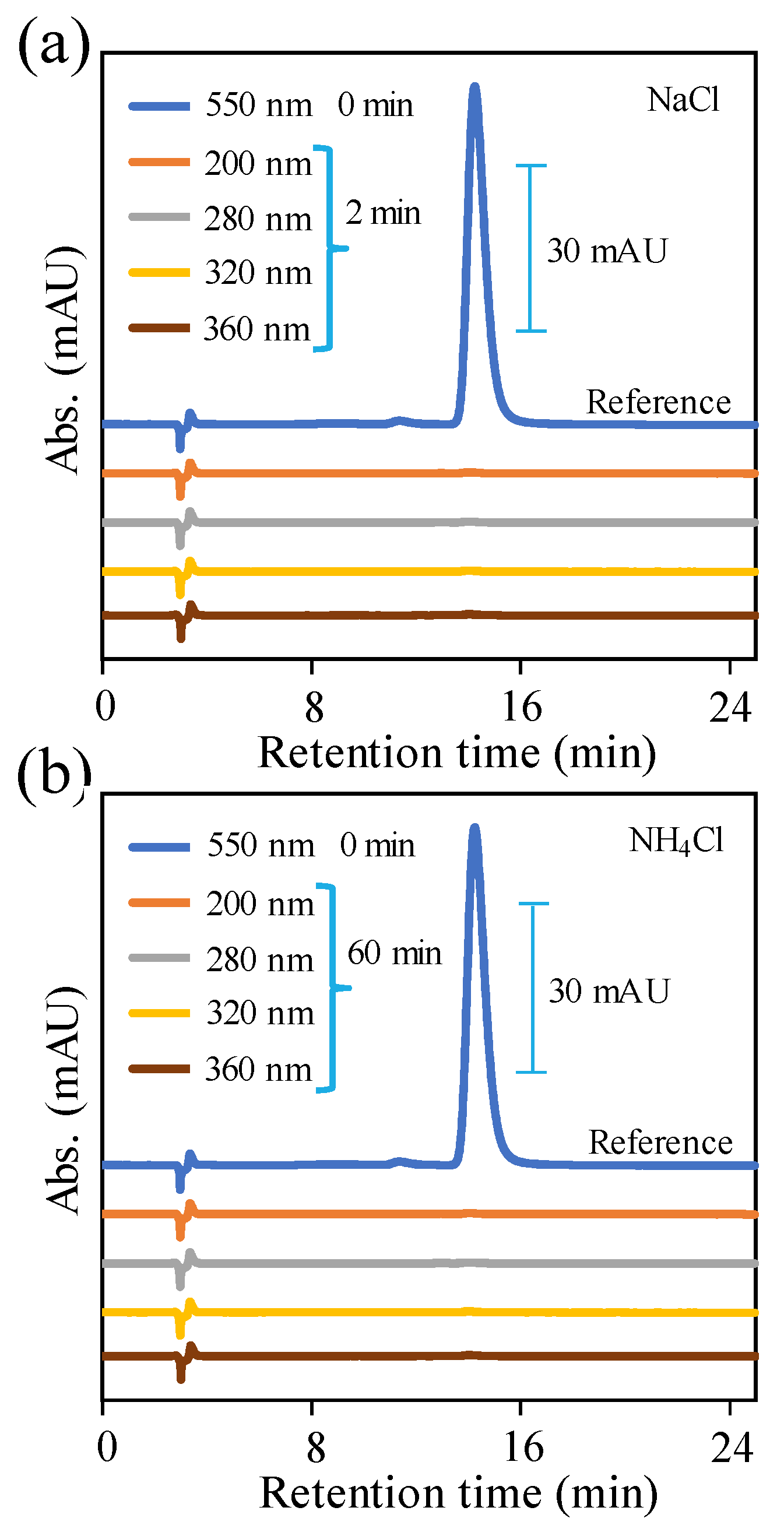
2.2. Electrochemical Studies
2.3. RhB Dye Degradation Studies
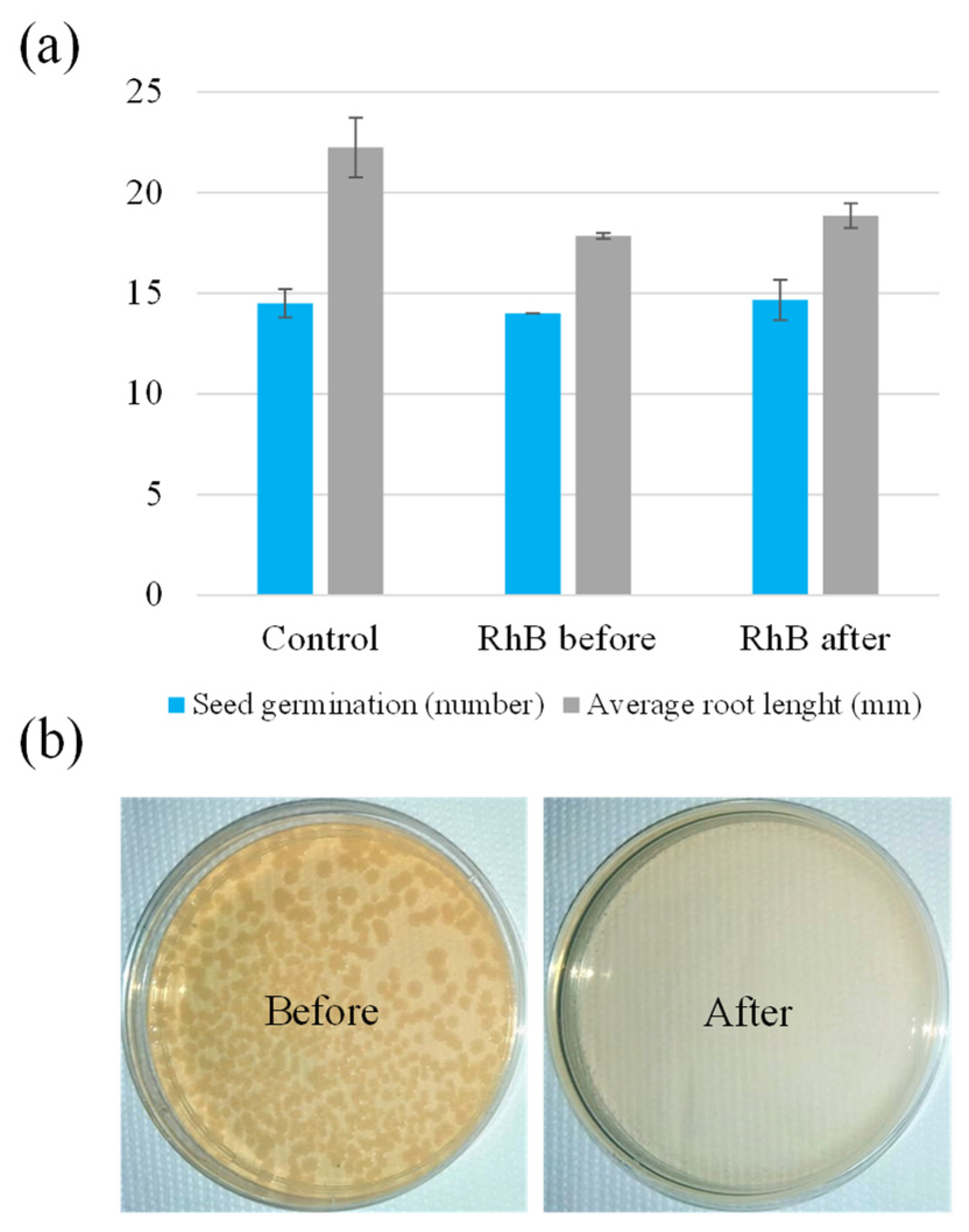
2.4. Phytotoxicity Test of RhB and Electrochemical Inactivation of E. coli Bacteria
2.4.1. Phytotoxicity Test of RhB
2.4.2. Electrochemical Inactivation of E. coli Bacteria
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Fe2P Thin Films
3.3. E. coli Preparation and Evaluation of Removal Efficiency
3.4. Phytotoxicity Test Using Lepidium sativum L.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oturan, M.A. Electrochemical advanced oxidation technologies for removal of organic pollutants from water. Environ. Sci. Pollut. Res. 2014, 21, 8333–8335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faouzi Elahmadi, M.; Bensalah, N.; Gadri, A. Treatment of aqueous wastes contaminated with Congo Red dye by electrochemical oxidation and ozonation processes. J. Hazard. Mater. 2009, 168, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Anas, M.; Han, D.S.; Mahmoud, K.; Park, H.; Abdel-Wahab, A. Photocatalytic degradation of organic dye using titanium dioxide modified with metal and non-metal deposition. Mater. Sci. Semicond. Process. 2015, 41, 209–218. [Google Scholar] [CrossRef]
- Raza, N.; Raza, W.; Gul, H.; Kim, K.H. ZnO–ZnTe hierarchical superstructures as solar-light-activated photocatalysts for azo dye removal. Environ. Res. 2021, 194, 110499. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhou, Y.; Yang, W.; Chen, G.; Yang, F. Electrochemical degradation of aqueous solution of Amaranth azo dye on ACF under potentiostatic model. Dye. Pigment. 2008, 76, 440–446. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Zhao, Z.; Su, Y.; Long, Y.; Zheng, Z.; Cui, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Microwave-assisted hydrothermal assembly of 2D copper-porphyrin metal-organic frameworks for the removal of dyes and antibiotics from water. Environ. Sci. Pollut. Res. 2020, 27, 39186–39197. [Google Scholar] [CrossRef]
- Hashim, K.S.; Kot, P.; Zubaidi, S.L.; Alwash, R.; Al Khaddar, R.; Shaw, A.; Al-Jumeily, D.; Aljefery, M.H. Energy efficient electrocoagulation using baffle-plates electrodes for efficient Escherichia coli removal from wastewater. J. Water Process Eng. 2020, 33, 101079. [Google Scholar] [CrossRef]
- Bensalah, N.; Louhichi, B.; Abdel-Wahab, A. Electrochemical oxidation of succinic acid in aqueous solutions using boron doped diamond anodes. Int. J. Environ. Sci. Technol. 2012, 9, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Green electrochemical process for metronidazole degradation at BDD anode in aqueous solutions via direct and indirect oxidation. Sep. Purif. Technol. 2016, 157, 9–16. [Google Scholar] [CrossRef]
- Patil, S.A.; Hägerhäll, C.; Gorton, L. Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal. Rev. 2014, 1, 71–129. [Google Scholar] [CrossRef]
- Koo, M.S.; Chen, X.; Cho, K.; An, T.; Choi, W.; Chen, X.; Cho, K.; An, T.; Choi, W. In situ photoelectrochemical chloride activation using a WO3 electrode for oxidative treatment with simultaneous H2 evolution under visible light. Environ. Sci. Technol. 2019, 53, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Lemeyonouin, P.; Guillaume, A.; Honoré, K.K.; Albert, T. Influence of chlorides on the electrochemical oxidation of formic acid on thermally prepared platinum electrodes. Int. J. Pure Appl. Sci. Technol. 2013, 14, 33–43. [Google Scholar]
- Baddouh, A.; Bessegato, G.G.; Rguiti, M.M.; El Ibrahimi, B.; Bazzi, L.; Hilali, M.; Zanoni, M.V.B. Electrochemical decolorization of Rhodamine B dye: Influence of anode material, chloride concentration and current density. J. Environ. Chem. Eng. 2018, 6, 2041–2047. [Google Scholar] [CrossRef] [Green Version]
- Babu, B.R.; Venkatesan, P.; Kanimozhi, R.; Basha, C.A. Removal of pharmaceuticals from wastewater by electrochemical oxidation using cylindrical flow reactor and optimization of treatment conditions. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2009, 44, 985–994. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Ganizadeh, F.; Rezaee, A.; Godini, H. Elimination of pathogenic bacteria using electrochemical process containing steel mesh electrode. J. Adv. Environ. Health Res. 2017, 5, 23–28. [Google Scholar] [CrossRef]
- Yang, Y. Recent advances in the electrochemical oxidation water treatment: Spotlight on byproduct control. Front. Environ. Sci. Eng. 2020, 14, 1264–1267. [Google Scholar] [CrossRef]
- Duan, T.; Chen, Y.; Wen, Q.; Duan, Y. Novel composition graded Ti/Ru-Sb-SnO2 electrode synthesized by selective electrodeposition and its application for electrocatalytic decolorization of dyes. J. Phys. Chem. C 2015, 119, 7780–7790. [Google Scholar] [CrossRef]
- Maharana, D.; Niu, J.; Gao, D.; Xu, Z.; Shi, J. Electrochemical degradation of Rhodamine B over Ti/SnO2-Sb electrode. Water Environ. Res. 2015, 87, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.E.H.; Koparal, A.S. Studies on electrochemical disinfectant production using anodes containing RuO2. J. Appl. Electrochem. 2005, 35, 1321–1329. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Electrochemical alternatives for drinking water disinfection. Angew. Chem.-Int. Ed. 2008, 47, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; Abdel-Wahab, A. Electrochemical inactivation of P. Aeruginosa, A. hydrophila, L. pneumophila using boron doped diamond anodes. J. Adv. Oxid. Technol. 2013, 16, 9–15. [Google Scholar] [CrossRef]
- Barashkov, N.N.; Eisenberg, D.; Eisenberg, S.; Shegebaeva, G.S.; Irgibaeva, I.S.; Barashkova, I.I. Electrochemical chlorine-free AC disinfection of water contaminated with Salmonella typhimurium bacteria. Russ. J. Electrochem. 2010, 46, 306–311. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Cho, M.; Choi, W.; Yoon, J. Inactivation of Escherichia coli in the electrochemical disinfection process using a Pt anode. Chemosphere 2007, 67, 652–659. [Google Scholar] [CrossRef]
- Ghasemian, S.; Asadishad, B.; Omanovic, S.; Tufenkji, N. Electrochemical disinfection of bacteria-laden water using antimony-doped tin-tungsten-oxide electrodes. Water Res. 2017, 126, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, T.O.; Babalola, S.O.; Onwudiwe, D.C. Photocatalytic inactivation as a method of elimination of E. coli from drinking water. Appl. Sci. 2021, 11, 1313. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, N.; Bhargava, M. Electrochemical degradation of Rhodamine B dye in textile and paper industries effluent. J. Sci. Ind. Res. 2003, 62, 1138–1144. [Google Scholar]
- Kothari, M.S.; Shah, K.A. Electrochemical oxidation for decolorization of Rhodamine-B dye using mixed metal oxide electrode: Modeling and optimization. Water Sci. Technol. 2020, 81, 720–731. [Google Scholar] [CrossRef]
- Ahmadi, A.; Wu, T. Inactivation of E. coli using a novel TiO2 nanotube electrode. Environ. Sci. Water Res. Technol. 2017, 3, 534–545. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Chen, J.; Meng, F.; Zhang, Y.; Brandl, H.; Lippert, T.; Chen, N. Photocatalytic and electrochemical degradation of methylene blue by titanium dioxide. Chin. Sci. Bull. 2014, 59, 1964–1967. [Google Scholar] [CrossRef]
- Li, H.; Zhu, X.; Ni, J. Inactivation of Escherichia coli in Na2SO4 electrolyte using boron-doped diamond anode. Electrochim. Acta 2010, 56, 448–453. [Google Scholar] [CrossRef]
- Li, X.Y.; Diao, H.F.; Fan, F.X.J.; Gu, J.D.; Ding, F.; Tong, A.S.F. Electrochemical wastewater disinfection: Identification of its principal germicidal actions. J. Environ. Eng. 2004, 130, 1217–1221. [Google Scholar] [CrossRef]
- Vos, J.G.; Koper, M.T.M. Measurement of competition between oxygen evolution and chlorine evolution using rotating ring-disk electrode voltammetry. J. Electroanal. Chem. 2018, 819, 260–268. [Google Scholar] [CrossRef]
- Wang, L.; Qin, M.; Yang, W.; Gao, Y.; Li, Y.Y. Efficient electrochemical water oxidation and oxidative degradation of Rhodamine B: A comparative study using high-purity birnessites containing Li+, Na+ or K+ ions. ChemistrySelect 2017, 2, 5587–5594. [Google Scholar] [CrossRef]
- Chouki, T.; Lazarević, D.; Donkova, B.V.; Emin, S. Synthesis of efficient iron phosphide catalyst for electrocatalytic hydrogen generation. Bulg. Chem. Commun. 2021, 53, 72–77. [Google Scholar] [CrossRef]
- Yao, Y.; Mahmood, N.; Pan, L.; Shen, G.; Zhang, R.; Gao, R.; Aleem, F.E.; Yuan, X.; Zhang, X.; Zou, J.J. Iron phosphide encapsulated in P-doped graphitic carbon as efficient and stable electrocatalyst for hydrogen and oxygen evolution reactions. Nanoscale 2018, 10, 21327–21334. [Google Scholar] [CrossRef]
- Guo, R.; Lai, X.; Huang, J.; Du, X.; Yan, Y.; Sun, Y.; Zou, G.; Xiong, J. Phosphate-based electrocatalysts for water splitting: Recent Progress. ChemElectroChem 2018, 5, 3822–3834. [Google Scholar] [CrossRef]
- Tang, K.; Wang, X.; Wang, M.; Xie, Y.; Zhou, J.; Yan, C. Highly sensitive activity on Ni/Fe ratio of monodispersed ternary nickel iron phosphide for efficient water oxidation. ChemElectroChem 2017, 4, 2150–2157. [Google Scholar] [CrossRef]
- Lei, Z.; Bai, J.; Li, Y.; Wang, Z.; Zhao, C. Fabrication of nanoporous nickel-iron hydroxylphosphate composite as bifunctional and reversible catalyst for highly efficient intermittent water splitting. ACS Appl. Mater. Interfaces 2017, 9, 35837–35846. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Samo, I.A.; Zhang, X.; Wang, Z.; Wang, C.; Li, Y.; Du, Y.; Zhong, Y.; Cheng, C.; et al. Common-ion effect triggered highly sustained seawater electrolysis with additional NaCl production. Research 2020, 2020, 2872141. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zeng, H.C. Promoting electrocatalytic oxygen evolution over transition-metal phosphide-based nanocomposites via architectural and electronic engineering. ACS Appl. Mater. Interfaces 2019, 11, 46825–46838. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zeng, H.C. Bimetallic Ni-Fe phosphide nanocomposites with a controlled architecture and composition enabling highly efficient electrochemical water oxidation. J. Mater. Chem. A 2018, 6, 2231–2238. [Google Scholar] [CrossRef]
- Chouki, T.; Machreki, M.; Emin, S. Solvothermal synthesis of iron phosphides and their application for efficient electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 21473–21482. [Google Scholar] [CrossRef]
- Park, J.; Koo, B.; Hwang, Y.; Bae, C.; An, K.; Park, J.G.; Park, H.M.; Hyeon, T. Novel synthesis of magnetic Fe2P nanorods from thermal decomposition of continuously delivered precursors using a syringe pump. Angew. Chem.-Int. Ed. 2004, 43, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Rasras, A.; Hamdi, R.; Mansour, S.; Samara, A.; Haik, Y. Study of the magnetocaloric effect in single-phase antiferromagnetic GdMnO3. J. Phys. Chem. Solids 2021, 149, 109798. [Google Scholar] [CrossRef]
- Sharif, F.; Roberts, E.P.L. Electrochemical oxidation of an organic dye adsorbed on tin oxide and antimony doped tin oxide graphene composites. Catalysts 2020, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Jothi, P.R.; Thersleff, T.; Budnyak, T.M.; Rokicinska, A.; Yubuta, K.; Dronskowski, R.; Kuśtrowski, P.; Fokwa, B.P.T.; Slabon, A. Nanostructured core-shell metal borides-oxides as highly efficient electrocatalysts for photoelectrochemical water oxidation. Nanoscale 2020, 12, 3121–3128. [Google Scholar] [CrossRef] [Green Version]
- Chouki, T.; Donkova, B.; Aktarla, B.; Stefanov, P.; Emin, S. Growth of MoSe2 electrocatalyst from metallic molybdenum nanoparticles for efficient hydrogen evolution. Mater. Today Commun. 2021, 26, 101976. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Chen, R.; Lin, Y. Boosting the oxygen evolution electrocatalysis performance of iron phosphide via architectural design and electronic modulation. ACS Sustain. Chem. Eng. 2020, 8, 9206–9216. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. Revealing the mechanism of indirect ammonia electrooxidation. Electrochim. Acta 2012, 63, 209–219. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Su, Y.; Yu, H.; Chen, S.; Quan, X.; Yang, F. Photoelectrocatalytic oxidation of aqueous ammonia using TiO2 nanotube arrays. Appl. Surf. Sci. 2014, 311, 851–857. [Google Scholar] [CrossRef]
- Baddouh, A.; El Ibrahimi, B.; Amaterz, E.; Rguiti, M.M.; Bazzi, L.; Hilali, M. Removal of the Rhodamine B dye at Ti/Ru0.3Ti0.7O2 anode using flow cell system. J. Chem. 2019, 2019, 1424797. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Y.; Li, J.; Bai, J.; Chen, S.; Li, L.; Wang, J.; Zhou, T.; Jiang, P.; Guan, X.; et al. Efficient ammonia removal and toxic chlorate control by using BiVO4/WO3 heterojunction photoanode in a self-driven PEC-chlorine system. J. Hazard. Mater. 2021, 402, 123725. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Van Duijne, A.K.; Braakhuis, L.; Saha, P.; Jeremiasse, A.W.; Mei, B.; Mul, G. Comparative analysis of photocatalytic and electrochemical degradation of 4-Ethylphenol in saline conditions. Environ. Sci. Technol. 2019, 53, 8725–8735. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Bai, J.; Li, J.; Luo, T.; Qiao, L.; Zeng, Q.; Zhou, B. Highly selective transformation of ammonia nitrogen to N2 based on a novel solar-driven photoelectrocatalytic-chlorine radical reactions system. Water Res. 2017, 125, 512–519. [Google Scholar] [CrossRef]
- Iguchi, S.; Miseki, Y.; Sayama, K. Efficient hypochlorous acid (HClO) production: Via photoelectrochemical solar energy conversion using a BiVO4 -based photoanode. Sustain. Energy Fuels 2018, 2, 155–162. [Google Scholar] [CrossRef]
- Benvenuti, T.; Gabriel, A.P.; Heberle, A.N.A.; Lucena, M.P.P.; Petter, P.M.H.; Meneguzzi, Á.; Bernardes, A.M. Evaluation of direct photolysis, electrooxidation and photoelectrooxidation for Rhodamine-B degradation. Braz. J. Chem. Eng. 2018, 35, 957–968. [Google Scholar] [CrossRef]
- Norouzi, N.; Choudhury, F.A.; El-Kaderi, H.M. Iron phosphide doped, porous carbon as an efficient electrocatalyst for oxygen reduction reaction. ACS Appl. Energy Mater. 2020, 3, 2537–2546. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Luo, R.; Yang, Y.; Lu, Y.; Guo, Y.; Liu, X.; Cao, S.; Kim, J.K.; Luo, Y. A 3D porous FeP/rGO modulated separator as a dual-function polysulfide barrier for high-performance lithium sulfur batteries. Nanoscale Horiz. 2020, 5, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, D.M.; Sáez, C.; Martínez-Huitle, C.A.; Cañizares, P.; Rodrigo, M.A. Influence of mediated processes on the removal of Rhodamine with conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2015, 166–167, 454–459. [Google Scholar] [CrossRef]
- Machreki, M.; Chouki, T.; Martelanc, M.; Butinar, L.; Vodopivec, B.M.; Emin, S. Preparation of porous α-Fe2O3 thin films for efficient photoelectrocatalytic degradation of Basic blue 41 dye. J. Environ. Chem. Eng. 2021, 9, 105495. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Von Dreele, R.B. Protein crystal structure analysis from high-resolution X-Ray powder-diffraction data. Methods Enzymol. 2003, 368, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Zhu, K.; Ren, X.; Sun, X.; Zhu, L.; Sun, Z. Effect of supporting electrolyte on the surface corrosion and anodic oxidation performance of graphite electrode. Electrocatalysis 2019, 10, 549–559. [Google Scholar] [CrossRef]
- Society, T.E. Water electrolysis using diamond thin film electrodes. J. Electrochem. Soc. 1998, 145, 2358. [Google Scholar]
- Kuo, D.Y.; Paik, H.; Nelson, J.N.; Shen, K.M.; Schlom, D.G.; Suntivich, J. Chlorine evolution reaction electrocatalysis on RuO2(110) and IrO2(110) grown using molecular-beam epitaxy. J. Chem. Phys. 2019, 150, 041726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasekar, N.; Srileka, S.; Samson Arun Prasath, S.; Robinson, S.; Saravanan, K. Kinetic modeling for biosorption of Metylene Blue onto H3PO4 activated acacia arabica. Carbon Lett. 2008, 9, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Mishra, A.; Kainth, S.; Basu, S. Carbon quantum dots/TiO2 nanocomposite for sensing of toxic metals and photodetoxification of dyes with kill waste by waste concept. Mater. Des. 2018, 155, 485–493. [Google Scholar] [CrossRef]
- Oliveira, F.H.; Osugi, M.E.; Paschoal, F.M.M.; Profeti, D.; Olivi, P.; Zanoni, M.V.B. Electrochemical oxidation of an acid dye by active chlorine generated using Ti/Sn(1-x)IrxO2 electrodes. J. Appl. Electrochem. 2007, 37, 583–592. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Urea degradation by electrochemically generated reactive chlorine species: Products and reaction pathways. Environ. Sci. Technol. 2014, 48, 11504–11511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusmierek, E. Semiconductor electrode materials applied in photoelectrocatalytic wastewater treatment—An overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Mañas, P.; De las Heras, J. Phytotoxicity test applied to sewage sludge using Lactuca sativa L. and Lepidium sativum L. seeds. Int. J. Environ. Sci. Technol. 2018, 15, 273–280. [Google Scholar] [CrossRef]
- Andrade, L.S.; Ruotolo, L.A.M.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R.; Iniesta, J.; García-Garcia, V.; Montiel, V. On the performance of Fe and Fe,F doped Ti-Pt/PbO2 electrodes in the electrooxidation of the Blue Reactive 19 dye in simulated textile wastewater. Chemosphere 2007, 66, 2035–2043. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Gao, F.; Yue, P.L. High-performance Ti/BDD electrodes for pollutant oxidation. Environ. Sci. Technol. 2003, 37, 5021–5026. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, Y.; Yang, W.; Chen, G.; Yang, F. Electrochemical degradation of Amaranth aqueous solution on ACF. J. Hazard. Mater. 2006, 137, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Pazos, M.; Sanromán, M.A. Selection of an electrolyte to enhance the electrochemical decolourisation of indigo. Optimisation and scale-up. Chemosphere 2005, 60, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Martins, S.; Morão, A.; Magrinho, M.; Gonçalves, I. Degradation of a textile dye C. I. Direct Red 80 by electrochemical processes. Port. Electrochim. Acta 2004, 22, 279–294. [Google Scholar] [CrossRef]
- Salazar, R.; Ureta-Zañartu, M.S.; González-Vargas, C.; Brito, C.d.N.; Martinez-Huitle, C.A. Electrochemical degradation of industrial textile dye disperse yellow 3: Role of electrocatalytic material and experimental conditions on the catalytic production of oxidants and oxidation pathway. Chemosphere 2018, 198, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, F.; Chen, G. Comparison of Ti/BDD and Ti/SnO2-Sb2O5 electrodes for pollutant oxidation. J. Appl. Electrochem. 2005, 35, 185–191. [Google Scholar] [CrossRef]
- Chang, J.H.; Wang, Y.L.; Dong, C.D.; Shen, S.Y. Electrocatalytic degradation of azo dye by vanadium-doped TiO2 nanocatalyst. Catalysts 2020, 10, 482. [Google Scholar] [CrossRef]
- Ozturk, D.; Yilmaz, A.E. Investigation of electrochemical degradation of Basic Red 13 dye in aqueous solutions based on COD removal: Numerical optimization approach. Int. J. Environ. Sci. Technol. 2020, 17, 3099–3110. [Google Scholar] [CrossRef]
- Hamad, H.; Bassyouni, D.; El-Ashtoukhy, E.S.; Amin, N.; Abd El-Latif, M. Electrocatalytic degradation and minimization of specific energy consumption of synthetic azo dye from wastewater by anodic oxidation process with an emphasis on enhancing economic efficiency and reaction mechanism. Ecotoxicol. Environ. Saf. 2018, 148, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Li, J.; Wang, Z.; Wei, Z.; Chen, C.; Du, K.; Zhao, C.; Yang, G.; Li, Y. Performance and mechanism of methylene blue degradation by an electrochemical process. RSC Adv. 2020, 10, 24712–24720. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Yoon, J. The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environ. Sci. Technol. 2006, 40, 6117–6122. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, I.C.C.P.; Moraes, P.B.; Bidoia, E.D. Studies on the electrochemical disinfection of water containing Escherichia coli using a dimensionally stable anode. Braz. Arch. Biol. Technol. 2010, 53, 1235–1244. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouki, T.; Machreki, M.; Topić, J.; Butinar, L.; Stefanov, P.; Jez, E.; Summers, J.S.; Valant, M.; Fait, A.; Emin, S. Iron Phosphide Precatalyst for Electrocatalytic Degradation of Rhodamine B Dye and Removal of Escherichia coli from Simulated Wastewater. Catalysts 2022, 12, 269. https://doi.org/10.3390/catal12030269
Chouki T, Machreki M, Topić J, Butinar L, Stefanov P, Jez E, Summers JS, Valant M, Fait A, Emin S. Iron Phosphide Precatalyst for Electrocatalytic Degradation of Rhodamine B Dye and Removal of Escherichia coli from Simulated Wastewater. Catalysts. 2022; 12(3):269. https://doi.org/10.3390/catal12030269
Chicago/Turabian StyleChouki, Takwa, Manel Machreki, Jelena Topić, Lorena Butinar, Plamen Stefanov, Erika Jez, Jack S. Summers, Matjaz Valant, Aaron Fait, and Saim Emin. 2022. "Iron Phosphide Precatalyst for Electrocatalytic Degradation of Rhodamine B Dye and Removal of Escherichia coli from Simulated Wastewater" Catalysts 12, no. 3: 269. https://doi.org/10.3390/catal12030269
APA StyleChouki, T., Machreki, M., Topić, J., Butinar, L., Stefanov, P., Jez, E., Summers, J. S., Valant, M., Fait, A., & Emin, S. (2022). Iron Phosphide Precatalyst for Electrocatalytic Degradation of Rhodamine B Dye and Removal of Escherichia coli from Simulated Wastewater. Catalysts, 12(3), 269. https://doi.org/10.3390/catal12030269