1. Introduction
Green Chemistry is a new approach to the development of sustainable chemical processes aiming to the minimization or elimination of the utilization and generation of hazardous substances. In the 21st century, incorporation of the principles of green chemistry in organic synthesis has gained increasing interest, since the reduction of toxic, flammable volatile organic solvents (VOCs), and strong catalysts is of high importance. However, in order to achieve more environmentally friendly protocols for the synthesis of organic compounds, the redesign of reactions and modifications of existing chemical processes is often required.
Deep eutectic solvents (DES) were firstly introduced in 2003 by Abbott et al. [
1,
2] and, in the past few years, have emerged as potential substituents to conventional VOCs and have found many applications in various scientific fields such as extraction [
3,
4] and catalysis [
5,
6,
7]. DES derive from simple mixing of at least two components, a hydrogen bond donor (HBD), and a hydrogen bond acceptor (HBA), in appropriate molar ratios, which are able to form intermolecular interactions (hydrogen bonds and van der Waals interactions). Due to these forces, the ability of the components to crystallize is lost, resulting to significant depression of the melting point of the mixture, compared to that of each initial constituent. In case natural derived starting materials, such as amino acids, organic acids, and sugars, are used as HBD and HBA, the eutectic mixture formed is defined as natural deep eutectic solvent (NaDES). NaDES appeared in the literature for the first time in 2011, by Choi et. al. [
8], and, since then, they have been extensively studied due to their numerous advantages, such as low volatility, non-flammability, chemical and thermal stability, low cost, recyclability, and biodegradability, while they can be tailor-made by the proper choice of starting materials giving a plethora of possible forming mixtures and the plasticity of their properties.
Organocatalysis is one of the most important tools in the hands of organic synthetic chemists, and organocatalysts are gaining grounds as a green alternative to metal-containing catalysts. The use of organocatalysts in a synthetic route, instead of metal containing catalysts, provides several advantages, such as stereoselectivity, milder reaction conditions, lower toxicity, and ability to perform the reaction, without using anhydrous conditions, as well as reducing the metal waste and avoiding the contamination of the final product with metal traces [
9].
L-proline, the only secondary proteinogenic amino acid, has been one of the first small molecules to be applied as an organocatalyst. The ability of L-proline to form enamines or imines, when reacting with carbonyl groups, as well as its ability to induce chirality promoted by the cyclic structure of the molecule, have been exploited in a wide range of organic synthetic methodologies [
10]. Moreover, the low cost and high availability of L-proline render this amino acid advantageous for use as an organocatalyst, even for reactions of industrial interest with the prospect of scale up.
A large number of reports have been published showing the potential of proline to act as an organocatalyst for C-C formation reactions, such as aldol and Knoevenagel condensations, Michael addition, and others, in either conventional solvents or green solvents, such as ionic liquids or deep eutectic solvents. Concerning the use of proline in green solvents, the group of Wang et al. have reported the proline-catalyzed Knoevenagel reaction in imidazole ionic liquids [
11], as well as in choline chloride-based deep eutectic solvents [
12], whereas Tasqeeruddin and Asiri have succeeded the synthesis of quinoline derivatives via Knoevenagel condensation reaction catalyzed by proline in the ionic liquid 1,3-dimethylimidazolium methyl sulfate [
13].
Moreover, proline-based ionic liquids can be successfully applied as solvents and basic catalysts for a Michael addition reaction between chalcone and dimethyl malonate, as shown in the work of Bacha et al. [
14]. Proline nitrate, another proline-based ionic liquid, was recently reported to effectively catalyze the synthesis of pyrimidine derivatives in various solvents, as well as in H
2O [
15].
Aurones or 2-benzylidendbenzofuran-3(2
H)-ones are naturally occurring compounds that belong to the flavonoid family (minor flavonoids) and are structural isomers of the flavones. The aurone skeleton constitutes of a benzofuran core and a phenyl group, joined by a carbon-carbon exocyclic double bond (
Scheme 1).
The word “aurone” origins from the latin word “aurum” meaning gold and was used for the first time by Bate-Smith and Geissman in 1951 in order to describe the gold-yellow color of the plants in which they occur [
16,
17]. Because of this characteristic, aurones are classified as anthochlor (from Greek “anthos”, meaning flower, and “khloros”, meaning bright green to yellow), a class of water-soluble pigments [
18]. Apart from the color, aurones play a role in the protection of the plant from several infections as defense agents and are, thus, called phytoalexins [
19,
20]. Although aurones have not been as extensively studied as other flavonoids, it has been proved that they possess a variety of pharmacological activities, such as antimalarial [
21,
22], anticancer [
23], antioxidant [
24,
25], antifungal [
26,
27], tyrosinase inhibitory [
28], antibacterial [
29], antileishmanial [
30], histone deacetylase inhibitory [
31], and more [
32]. Representative naturally occurring aurones, with medicinal properties, are aureusidin, sulfuretin, maritimetin, hispidol, and leptosidin (
Scheme 2) that have been extracted from several flowers of the Asteraceae, Plantaginaceae, Fabaceae families, etc. [
18].
Several synthetic approaches for the synthesis of aurones have been developed and recently reviewed by Sui et al. [
27] and Popova et al. [
33]. The most commonly applied synthetic protocols include the oxidative cyclization of 2′-hydroxychalcones in the presence of various oxidative agents, such as mercury (II) acetate in pyridine or acetic acid (
Scheme 3a),the Knoevenagel condensation of benzofuran, with substituted aromatic aldehydes in the presence of either basic or acidic reagents, such as sodium hydroxide, potassium hydroxide, hydrochloride, and thionyl chloride [
33] (
Scheme 3b) and the intramolecular cyclization of substituted acetylenes in the presence of bases and using appropriate metal catalysts, in order to achieve selectivity towards 5-
exo-dig cyclization [
34,
35] (
Scheme 3c).
The Knoevenagel condensation reaction constitutes an effective C–C formation reaction between carbonyl or heterocarbonyl compounds and any compounds possessing an active methylene group, resulting to the synthesis of important and bioactive organic compounds [
36]. Generally, the reaction is performed in the presence of bases; however, several efforts have been made for the establishment of a green profile [
37,
38,
39].
Recently, there have been some attempts to utilize the DES choline chloride/urea 1:2 using conventional heating [
40] or microwave irradiation [
41] to synthesize aurones via the Knoevenagel condensation reaction between benzofuranone and various aldehydes (
Scheme 4), reducing the reaction time to 30 min and achieving mediocre to satisfactory yields. To our knowledge, these are the only works reporting the greener synthesis of aurones via the Knoevenagel reaction, using DES as alternative reaction media.
Due to the significant properties that aurones demonstrate, numerous studies have been conducted over the years about the synthesis of these bioactive compounds; however, the attempts to develop a more sustainable way to obtain aurones via Knoevenagel condensation are limited. Therefore, the purpose of this project is to design a novel green approach for the synthesis of aurones, using tailor-designed solvents from the natural pool, in combination with high energy techniques. Overall, nine aurones were synthesized and characterized using the optimized methodology. It is of utmost interest that via the proposed methodology the desirable compounds were synthesized in comparable or higher yields, as well as in significantly reduced reaction time. Specifically, regarding the aurones
3c,
3g,
3h, and
3i, the yields range between 59–84% in 14–18 min, while, in the literature, the same compounds were obtained in 58–66% yields in 12–48 h [
40].
2. Results and Discussion
2.1. Synthesis and Structural Characterization of NaDES
Proline-based NaDES were task-specifically designed since the potential of proline to act as an organocatalyst has been studied extensively and it has been applied to numerous reactions, since 1971 [
42]. In this context, we attempted to explore three proline-based NaDES, namely proline/glycerol 1:2 (Pro/Gly), proline/oxalic acid 1:1 (Pro/Ox), and proline/lactic acid/water (Pro/LA/W), both as solvents and catalysts for the Knoevenagel condensation reaction between benzofuranone and benzaldehydes. Herein, it is presented the synthesis of four proline- and choline chloride-based NaDES via the heating and stirring method.
Table 1 shows the detailed preparation parameters for all NaDES shown in this work.
All the NaDES were characterized by 1H and 13C NMR spectroscopy (300 and 600 MHz), confirming the structure of the NaDES and the ratio of the initial components, and by Fourier transform infrared spectroscopy (FTIR) operated in attenuated total reflectance (ATR) mode. The FTIR spectra worked synergistically for the structural elucidation of the NaDES, while the main difference between the proline-based and the choline-based NaDES’ spectra is the characteristic peaks at 1620 cm−1, attributed to the N-H bending and the characteristic peaks at 1220 cm−1, attributed to the C-N stretching, that appear only in the proline-based NaDES.
2.2. Physicochemical Properties of NaDES
Three of the main physicochemical properties of the NaDES were measured, the polarity pH and viscosity, and the results are presented in
Table 2. Moreover, measurements of ethanol and water are also reported for the sake of comparison.
Polarity, pH, and viscosity of the NaDES, were selected as very important parameters to be studied since they have essential impact on chemical reactions and applications such as catalysis.
The polarity of a solvent is a key parameter for chemical reactions that could affect the reaction rate, product yield, and selectivity, as well as reaction mechanism during a chemical reaction process [
43].
Herein, the NaDES polarity is depicted by the E
NR parameter, obtained from solvatochromic shift of the Nile red dye used as a solvatochromic probe. The Nile red polarity scale has been selected due to its extensive use in the study of the effect of DES structural modifications on overall solvent polarity [
44]. The λ
max was determined with an ultraviolet–visible (UV–VIS) spectrophotometer. Following Equation (1), the molar transition energies (E
NR) were calculated. Solvents with higher polarity shift the λ
max of the dye to higher wavelength values, yielding lower E
NR values [
45].
The NaDES polarity is related to the hydrogen bonds that occur between hydrogen bond donors and acceptors (intermolecular or intramolecular) and, therefore, the formation of more hydrogen bonds is expected to increase the polarity of the solvent [
44,
46]. Thus, it can be concluded that the structure of the NaDES components and the interactions between them strongly affect the polarity of the solvents. The obtained results of the present study. indicate that the NaDES possessing carboxylic acids as HBD (
Table 2, entries 1 and 2) are more polar, compared to those where glycerol is used (
Table 2, entries 3 and 4). All the tested NaDES are more polar than ethanol (λ
max > 548, E
NR < 52.17) and less polar (or of similar polarity) than water (λ
max > 593, E
NR < 48.20).
Regarding the pH, it is known that the hydrogen bonds between HBD and HBA have a strong effect on the acidity of the NaDES [
47]. From the measured pH values of the studied NaDES, it can be observed that the strong acidic nature of the Pro/Ox and Pro/LA/W is likely due to the carboxylic functional groups present in the hydrogen bond donors.
The viscosity measurements revealed that all proline-based NaDES present higher viscosity values than the choline chloride-based NaDES, suggesting that the HBA used for the synthesis of NaDES plays a major role in the resulting viscosity. The nature of the HBD seems to also affect the viscosity of the produced NaDES, since the variation of HBD (oxalic acid, glycerol, lactic acid) leads to significantly different viscosity values. Another determining factor for the viscosity of the NaDES is the water content, as it is observed from the viscosity value of the Pro/LA/W NaDES, which is substantially lower than that of the Pro/Ox and Pro/Gly.
2.3. Optimization of the Synthetic Protocol
The optimization of the methodology for the synthesis of aurones via the Knoevenagel reaction was performed on a model reaction between equimolar quantities of benzofuranone (
1) and vanillin (
2) (
Scheme 5).
The synthesized proline-based NaDES were applied to the model reaction, providing the product in moderate to satisfactory yields (
Table 3), with the NaDES Pro/Gly 1:2 giving the best results (yields up to 89%). More specifically, as far as the conventional heating conditions are concerned, Pro/Ox afforded the aurone in moderate yield (57%), after 20 h of reaction, while Pro/LA/W and Pro/Gly gave the aurones in higher yields (70% and 76%, respectively) and significantly less time (5 and 6 h, respectively). These results can be attributed to the very low pH of the NaDES Pro/Ox (pH = 1.89), compared to the other NaDESs, concluding that strong acidic conditions do not favor the reaction. In order to examine the significance of the presence of L-proline in the reaction, two experiments were performed: one using glycerol as the solvent and one using the NaDES choline chloride/glycerol (ChCl/Gly) 1:2. In the presence of glycerol, the reaction did not proceed at all, whereas, in the presence of ChCl/Gly, a mere 10% yield was obtained. These findings indicate that not only glycerol by itself is not adequate to promote the reaction, but also the use of ChCl/Gly NaDES, containing the same HBD as Pro/Gly, had not the same effect in the formation of the product, confirming the importance of proline in the reaction mechanism. Aiming to further explore the catalytic role of proline, an attempt to perform the model reaction using proline in a catalytic amount (0.1 eq) and glycerol, as solvent, was made. The results showed that the yield of the obtained product was very low, suggesting that the quantity of L-proline and the hydrogen bonds, formed in the proline-based NaDES, are key factors in the promotion of the reaction.
In order to further enhance the green character of the novel synthetic protocol, an attempt to exploit high energy techniques was undertaken. Thus, both microwave (MW) and ultrasound irradiation, combined with the proline-based NaDES, were examined as alternatives to conventional heating (
Table 3).
The results revealed that both MW and ultrasound irradiation reduced the reaction time significantly (from hours to minutes) in all three cases of solvents; however, in terms of the product yield, neither MW or ultrasound had a noteworthy positive effect when Pro/Ox and Pro/LA/W were used as solvents and catalysts.
On the other hand, when studying Pro/Gly as the solvent, although MW irradiation did not seem to play any significant role, ultrasound irradiation increased the product yield from 76% to 89%, reducing at the same time, the reaction time from 6 h to 8 min. Therefore, the optimum reaction conditions were found to be Pro/Gly 1:2 as solvent, using ultrasound irradiation (120 W) for 8 min.
2.4. Recyclability and Reusability of the NaDES Pro/Gly
Towards a more sustainable approach, the recyclability and reusability of the NaDES Pro/Gly was examined. The solvent was applied to benzofuranone and vanillin, as model substrates, in a ratio 2 g NaDES/mmol of reactants, under the optimized conditions; upon completion of the reaction, water was added to the mixture, and the precipitate formed was filtered off. The NaDES was recovered after vacuum evaporation of the filtrate and was then used without any further purification for the next reaction. Following this process, the NaDES was recycled and reused up to six times without any significant loss in yield (
Table 4). It should be noted that after the evaporation of water, the remaining amount of NaDES was practically unchanged (that is, there was no significant mass loss after the evaporation of water). However, the NaDES had then to be transferred from the evaporation flask to the vessel appropriate for the ultrasound irradiation and, during this transfer, there was a significant mass loss, due to the high viscosity of the NaDES (5064 cP). Therefore, the observed mass loss is a matter of handling. Due to this fact, the amount of used reagents were different for each run, in order to sustain the same solvent-to-reactants ratio.
This observation is of utmost interest, since the process cost turns out to be significantly lower than when conventional solvents and catalysts are used. This is due to the fact that Pro/Gly acts both as solvent and catalyst and for at least seven runs can be used effectively to obtain the desirable final product in high purity. Furthermore, in terms of sustainability, the proposed reaction method follows many of the green chemistry demands.
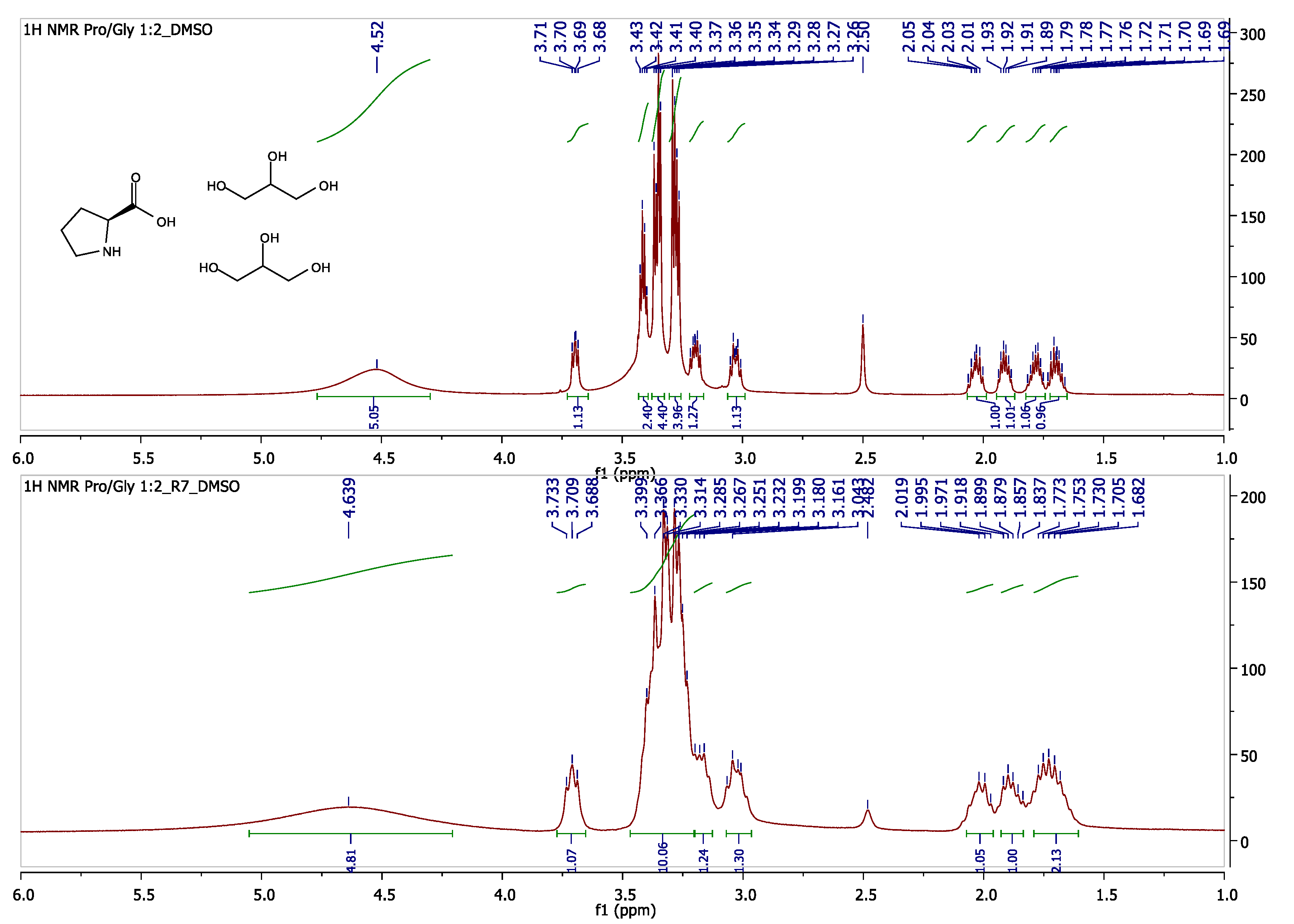
The recycled NaDES, after each run, was controlled, regarding its composition, via
1H NMR, revealing that the composition of NaDES remained the same, even after seven runs (
Figure 1).
2.5. Broadening the Scope of the Reaction
In an effort to examine the scope of the novel synthetic protocol, different substituted aldehydes were used in the Knoevenagel reaction under the optimized conditions (
Scheme 6). Benzaldehydes bearing methoxy, hydroxy, or halogen groups were applied to the reaction, while an attempt to use heterocyclic aldehydes was made, as well, providing the majority of the aurone analogs in satisfactory to good yields and high purity (
Table 5). It is worth mentioning that the developed methodology for the Knoevenagel reaction eliminates the need for protection and deprotection of the hydroxyl group, since for the synthesis of aurones
3a,
3d, and
3e, bearing hydroxy groups in the B ring of the scaffold, the corresponding aldehydes were used in the reaction without further chemical modifications.
The structures of the products were determined by
1H NMR spectroscopy, proving that this novel synthetic protocol yields the Z-geometrical isomer exclusively. Aurones appear in the form of two isomers, E- and Z-isomers, with the later one being the more thermodynamically favored [
20]. In the
1H NMR spectra of the synthesized aurones, the most characteristic peak is one singlet owed to the exocyclic vinylic proton that appears at δ 6.72–6.91 ppm, confirming the formation of a single geometrical isomer the Z-isomer, as can be concluded by comparing the
1H NMR shifts with the literature-reported Z-aurones. In the case of the spectrum of aurone
3h, (
Figure S17, Supplementary Materials), a downfield shift of the vinyl proton, at the region of 7.37–7.22 ppm, was observed. The NMR spectra of compounds
3a–
3i are available in the
Supplementary Materials (Figures S10–S18, Supplementary Materials).
3. Materials and Methods
L-proline (Fluorochem, Glossop, UK, 99%), D,L-lactic acid (LabKem, Barcelona, Spain, 80% aq. Sol.), glycerol anhydrous (Penta, Katovice, Czech Republic, 99.9%), Choline Chloride (Glentham Life Sciences, Corsham, UK, 99%), 2,3-Dihydrobenzo[b]furan-3-one (Fluorochem, 95%), vanillin (Fluka, Dresden, Germany 98%), 3-methoxybenzaldehyde (Aldrich, Saint Louis, MO, USA, 97%), 3,4-dimethoxybenzaldehyde (Aldrich, 99%), 4-hydroxybenzaldehyde (Acros Organics, Geel, Belgium, 99%), 3,4-dihydroxybenzaldehyde (Fluka, 97%), 4-chlorobenzaldehyde (Fluka, 98%), 4-bromobenzaldehyde (Fluorochem, 98%), pyrrole-2-carboxaldehyde (Fluka, 97%), thiophene-2-carboxaldehyde (Fluka, 98%), and Nile red (Glentham Life Sciences) were purchased and used without further purification.
The microwave assisted reactions were performed on the Milestone Start SYNTH-Microwave Synthesis Labstation. The ultrasound assisted reactions were performed using the Sonics VC 400 High Intensity Processor (Sonics and Materials Inc., Newtown, CT, USA), equipped with a piezoelectric converter and 13 mm diameter titanium alloy (Ti-6Al-4V) probe.
1H NMR spectra (300 and 600 MHz) were recorded on a Varian 300 MHz and Varian 600 MHz NMR spectrometer (Palo Alto, CA, USA) at the Institute of Chemical Biology, National Hellenic Research Foundation. Melting points were determined on a Gallenkamp MFB-595 melting point apparatus (Cambridge, UK). and are uncorrected. FTIR spectroscopy was performed on a JASCO FT/IR-4200 spectrometer (Easton, MD, USA). in attenuated total reflectance (ATR) mode, in the range from 4000 to 400 cm−1.
3.1. General Procedure for the NaDES Preparation
The NaDES were prepared using the heating and stirring method, as described in our previous work [
3,
37], with slight modifications. In a round-bottomed flask, the hydrogen bond donor and hydrogen bond acceptor were mixed in the appropriate molar ratio and stirred in an oil bath with a magnetic stirrer at 60 °C, regarding the proline-based NaDES, and 80 °C, for the choline chloride-based NaDES, until a clear liquid is formed. The NaDES are then used without further purification.
3.1.1. NaDES L-Prοline/Oxalic Acid (Pro/Ox) 1:1
L-proline and oxalic acid were mixed in a molar ratio 1:1, and the mixture was stirred at 60 °C overnight, until a colorless transparent liquid was formed. IR
νmax 3544 (N-H stretching), 2991 (C-H stretching), 1737 (C=O stretching), 1625 (N-H bending), 1373 (C-H bending), 1228 (C-O stretching, C-N stretching) cm
−1;
1H NMR (600 MHz, DMSO-
d6): δ (ppm) 6.69 (brs, 3H, 2× -OH, NH), 4.08 (t,
J = 7,8 Hz, 1H, H-2), 3.23–3.19 (m, 1H, H-5a), 3.16–3.10 (m, 1H, H-5b), 2.20–2.14 (m, 1H, H-3a), 1.96–1.89 (m, 1H, H-3b), 1.88–1.77 (m, 2H, H-4);
13C NMR (600 MHz, DMSO-
d6): δ (ppm) 171.03, 164.3, 59.56, 45.25, 28.56, 23.55
| L-proline/Oxalic acid 1:1 | ![Catalysts 12 00249 i016]() |
3.1.2. NaDES L-Prοline/Glycerol (Pro/Gly) 1:2
L-proline and glycerol were mixed in a molar ratio 1:2, and the mixture was stirred at 60 °C for 4 h, until an orange-yellow transparent liquid was formed. IR
νmax 3338 (O-H stretching, N-H stretching), 3012 (C-H stretching), 2969 (C-H stretching), 1737 (C=O stretching), 1621 (N-H bending), 1365 (C-H bending), 1216 (C-O stretching, C-N stretching), 1043 (C-O stretching) cm
−1;
1H NMR (300 MHz, DMSO-
d6): δ (ppm) 4.52 (brs, 5H, 4× -OH, NH), 3.71 (t,
J = 8.4 Hz, 1H, H-2), 3.43–3.40 (m, 2H), 3.37–3.34 (m, 4H), 3.29–3.26 (m, 4H), 3.21–3.19 (m, 1H), 3.5–3.01 (m, 1H), 2.06–2.00 (m, 1H), 1.93–1.89 (m, 1H), 1.81–1.75 (m, 1H), 1.73–1.66 (m, 1H);
13C NMR (600 MHz, DMSO-
d6): δ (ppm) 170.23, 72.58, 63.11, 60.67, 45.22, 29.01, 23.91.
| L-proline/Glycerol 1:2 | ![Catalysts 12 00249 i017]() |
3.1.3. NaDES L-proline/D,L-Lactic Acid (Pro/LA/W) 1:2:2.5
L-proline and D,L-lactic acid (80% aqueous solution) were mixed in a molar ratio 1:2 (the 2.5 eq of water (W) refers to the amount of water that is present in the commercially available D,L-lactic acid), and the mixture was stirred at 60 °C for 4 h, until a yellow transparent liquid was formed. IR
νmax 3459 (O-H stretching, N-H stretching), 2980 (C-H stretching), 1737 (C=O stretching), 1619 (N-H bending), 1367 (C-H bending), 1222 (C-O stretching, C-N stretching), 1130 (C-O stretching) cm
−1;
1H NMR (600 MHz, DMSO-
d6): δ (ppm) 4.03 (q,
J = 6.6 Hz, 2H, 2× CH
3-C
H-), 3.96 (t,
J = 7.2 Hz, 1H, H-2), 3.24–3.20 (m, 1H, H-5a), 3.14–3.10 (m, 1H, H-5b), 2.15–2.09 (m, 1H, H-3a), 1.91–1.85 (m, 1H, H-3b), 1.83–1.73 (m, 2H, H-4), 1.18 (d,
J = 7.8 Hz, 6H, 2× -CH
3);
13C NMR (600 MHz, DMSO-
d6): δ (ppm) 176.68, 171.14, 66.01, 60.41, 45.28, 28.96, 23.85, 20.65.
| L-proline/D,L-Lactic acid 1:2 | ![Catalysts 12 00249 i018]() |
3.1.4. NaDES Choline Chloride/Glycerol (ChCl/Gly) 1:2
Choline chloride was dried under vacuum prior to use. Choline chloride and glycerol were mixed in a molar ratio 1:2, and the mixture was stirred at 80 °C for 2 h, until a colorless transparent liquid was formed. IR
νmax 3342 (O-H stretching), 2944 (C-H stretching), 1481 (C-H bending), 1047 (C-O stretching)
1H NMR (600 MHz, DMSO-
d6): δ (ppm) 5.51 (t,
J = 5.4 Hz, 1H, -NCH
2CH
2O
H), 4.54 (d,
J = 4.8 Hz, 2H, 2× -OH), 4.47 (t,
J = 6.0 Hz, 4H, -NCH
2CH
2-), 3.82 (quint,
J = 5.4 Hz, 2H, 2× -C
H(OH)-), 3.43–3.49 (m, 4H), 3.37–3.33 (m, 4H), 3.29–3.26 (m, 4H), 3.13 (s, 9H, 3× -CH
3).
| Choline Chloride /Glycerol 1:2 | ![Catalysts 12 00249 i019]() |
3.2. Physicochemical Properties of NaDES
3.2.1. Polarity Measurements
The polarity of NaDES was estimated using Nile red (initial stock solution of Nile red in ethanol: 0.1 mM) as a solvatochromic probe as reported in the literature [
45,
48]. The absorption maxima of the solutions (λ
max) were determined with an ultraviolet–visible (UV–VIS) spectrophotometer in the 400–700 nm range and the molar transition energies (E
NR) were calculated using Equation (1).
Nile red exhibits a bathochromic shift in polar solvents and a hypsochromic shift in non-polar solvents. High E
NR values indicate lower polarity of the tested compounds while low E
NR values indicate higher polarity in the Nile red polarity scale [
44].
3.2.2. pH Measurements
After the NaDES preparation, the pH was determined using a 744 pH Meter Metrohm. The electrode was calibrated with the pH 4.0 and pH 7.0 buffer solutions and then the pH of the prepared solvents was determined at 23 ± 2 °C.
3.2.3. Viscosity Measurements
The viscosity of the prepared NaDES was determined using a Brookfield DV1 Viscometer (New Castle, DE, USA). 6.7 mL of each NaDES were used and upon selection of the appropriate spindle speed, the viscosity was measured at 25 °C.
3.3. Synthesis of Aurone Derivative 3a under Conventional Heating
In a round bottom flask benzofuranone (1) (60 mg, 0.45 mmol) and vanillin (2a) (68.1 mg, 0.45 mmol) were added to approximately 0.85 g of the appropriate NaDES. The reaction mixture was refluxed at 60 °C, in an oil bath under inert atmosphere for 5–24 h. The completion of the reaction was monitored by thin layer chromatography (TLC). The reaction mixture was progressively turned into a viscous orange solid mass. At the end of the reaction, appropriate amount of water was added and the precipitate formed was filtered off by vacuum filtration. The product was obtained as an orange-yellow powder.
3.4. Synthesis of Aurone Derivative 3a under Microwave Irradiation in NaDES
Bezofuranone (1) (100 mg, 0.75 mmol) and vanillin (2a) (113.4 mg, 0.75 mmol) were added to approximately 1.5 g of the appropriate NaDES in a microwave quartz tube and the reaction was performed under microwave irradiation. The MW conditions were determined as follows:
T = 60 °C, E = 100 W;
Heating time: 5 min;
Reaction time: 15 min;
Cooling time: 5 min.
The completion of the reaction was monitored by TLC every 5 min. The reaction mixture was progressively turned into a viscous orange solid mass. At the end of the reaction, appropriate amount of water was added, and the precipitate formed was filtered off by vacuum filtration.
3.5. General Procedure for the Synthesis of Aurone Derivatives 3a–3h under Ultrasound Irradiation in NaDES
A mixture of benzofuranone (1 eq) and the appropriate aldehyde (1 eq) were added to approximately 1.5 g of NaDES proline/glycerol 1:2 and the mixture was placed under sonication via an ultrasound probe at 30% amplitude (120 W) for required time with a 9 s ON, 2 s OFF cycle. The completion of the reaction was monitored by TLC. The reaction mixture was progressively turned into a viscous orange solid mass. At the end of the reaction, appropriate amount of water was added, and the precipitate formed was filtered off by vacuum filtration.
| General Structure and numbering scheme for derivatives 3a–3g | ![Catalysts 12 00249 i020]() |
3.5.1. 4′-Hydroxy-3′-methoxyaurone (3a)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and vanillin (
2a) (113.4 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 8 min. After the work-up procedure, the product was obtained as an orange-yellow powder. Yield: 177.1 mg (89%). m.p. 199–200 °C (ref. [
49] m.p. 200–201 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 7.82 (d,
J = 7.8 Hz, 1H, H-4), 7.65 (t,
J = 8.1 Hz, 1H, H-6), 7.50–7.48 (m, 2H, H-2′, H-6′), 7.32 (d,
J = 8.4 Hz, 1H, H-7), 7.22 (t,
J = 7.5 Hz, 1H, H-5), 7.00 (d,
J = 8.7 Hz, 1H, H-5′), 6.87 (s, 1H, H-10), 5.98 (s, 1H, -OH), 4.00 (s, 3H, -OCH
3) (ref. [
49]).
3.5.2. 3′-Methoxyaurone (3b)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 3-methoxybenzaldehyde (
2b) (113.4 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 12 min. After the work-up procedure, the product was obtained upon recrystallization from methanol/dichloromethane as a yellow powder. Yield: 105.9 mg (56%). m.p. 114.0–117 °C (ref. [
25] 120–121 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 7.81 (d,
J = 7.8 Hz, 1H, H-4), 7.67 (t,
J = 7.5 Hz, 1H, H-6), 7.51–7.49 (m, 2H, H-2′, H-4′), 7.41–7.32 (m, 2H, H-6′, H-5), 7.23 (t,
J = 7.5 Hz, 1H, H-5′), 6.97 (dd,
J = 8.1, 1.2 Hz, 1H, H-7), 6.87 (s, 1H, H-10), 3.89 (s, 3H, -OCH
3) (ref. [
50]).
3.5.3. 3′,4′-Dimethoxyaurone (3c)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 3,4-dimethoxybenzaldehyde (
2c) (123.9 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 14 min. After the work-up procedure, the product was obtained as an orange powder. Yield: 177.8 mg (84%). m.p. 155–157 °C (ref. [
25] m.p. 160–162 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 7.82 (d,
J = 6.9 Hz, 1H, H-4), 7.65 (t,
J = 7.5 Hz, 1H, H-6), 7.54 (s, 1H, H-2′), 7.52 (d,
J = 8.4 Hz, 1H, H-6′), 7.32 (d,
J = 8.4 Hz, 1H, H-7), 7.22 (t,
J = 7.2 Hz, 1H, H-5), 6.96 (d,
J = 8.4 Hz, 1H, H-5′), 6.89 (s, 1H, H-10), 3.98 (s, 3H, -OCH
3), 3.95 (s, 3H, -OCH
3) (ref. [
49]).
3.5.4. 4′-Hydroxyaurone (3d)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 4-hydroxybenzaldehyde (
2d) (91.6 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 14 min. After the work-up procedure, the product was obtained as an orange-red powder. Yield: 145.2 mg (81%). m.p. 237–241 °C (ref. [
31] m.p. 230–235 °C);
1H NMR (300 MHz, DMSO-
d6): δ (ppm) 10.26 (brs, 1H, -OH), 7.89 (d,
J = 8.7 Hz, 2H, H-2′, H-6′), 7.81–7.77 (m, 2H, H-4, H-6), 7.55 (d,
J = 8.4 Hz, 1H, H-7), 7.31 (t,
J = 7.2 Hz, 1H, H-5), 6.93–6.90 (m, 3H, H-3′, H-5′, H-10) (ref. [
49]).
3.5.5. 3′,4′-Dihydroxyaurone (3e)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 3,4-dihydroxybenzaldehyde (
2e) (103.4 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 12 min. After the work-up procedure, the product was obtained as an orange-red powder. Yield: 86.6 mg (76%). m.p. 217–221 °C (ref. [
31] 228–231 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 9.60 (brs, 2H, 2× -OH), 7.81–7.77 (m, 2H, H-4, H-6), 7.54–7.51 (m, 2H, H-2′, H-6′), 7.33–7.28 (m, 2H, H-5, H-7), 6.86 (d,
J = 8.4 Hz, 1H, H-5′), 6.82 (s, 1H, H-10) (ref. [
49]).
3.5.6. 4′-Chloroaurone (3f)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 4-chlorobenzaldehyde (
2f) (104.7 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 16 min. After the work-up procedure, the product was obtained as an orange-red powder. Yield: 135.4 mg (70%). m.p. 150–153 °C (ref. [
25] 152–160 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 7.85 (d,
J = 8.7 Hz, 2H, H-2′, H-6′), 7.81 (d,
J = 8.4 Hz, 1H, H-4), 7.67 (t,
J = 7.5, 1H, H-6), 7.42 (d,
J = 8.4 Hz, 2H, H-3′, H-5′), 7.33 (d,
J = 8.1 Hz, 1H, H-7), 7.23–7.21 (m, 1H, H-5), 6.83 (s, 1H, H-10) (ref. [
51]).
3.5.7. 4′-Bromoaurone (3g)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 4-bromobenzaldehyde (
2g) (137.8 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 18 min. After the work-up procedure, the product was obtained as an orange powder. Yield: 151.3 mg (67%). m.p. 162–165 °C (ref. [
40] m.p. 152–158 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 7.82–7.77 (m, 2H, H-2′, H-6′), 7.67 (t,
J = 7.5 Hz, 1H, H-6), 7.58 (d,
J = 8.4 Hz, 2H, H-3′, H-5′), 7.34 (d,
J = 7.8 Hz, 2H, H-4, H-7), 7.24–7.21 (m, 1H, H-5), 6.81 (s, 1H, H-10) (ref. [
40]).
3.5.8. (Z)-2-(thiophen-2-ylmethylene)benzofuran-3(2H)-one (3h)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and 2-Thiophenecarboxaldehyde (
2h) (137.8 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 18 min. After the work-up procedure, the product was obtained as an orange-brown powder. Yield: 101 mg (59%). m.p. 114–117 °C (ref. [
51] m.p. 126–128 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 7.87 (d,
J = 7.5 Hz, 1H, H-4), 7.75–7.62 (m, 3H, H-5, H-6, H-7), 7.42 (d,
J = 8.1 Hz, 1H, H-3′), 7.33–7.22 (m, 3H, H-10, H-4′, H-5′) (ref. [
52]).
(Z)-6,7-dihydroxy-2-(thiophen-2-ylmethylene)benzofuran-3(2H)-one
3h | ![Catalysts 12 00249 i021]() |
3.5.9. (Z)-2-((1H-pyrrol-2-yl)methylene)benzofuran-3(2H)-one (3i)
Prepared according to the general procedure, benzofuranone (
1) (100 mg, 0.75 mmol) and pyrrole-2-carboxaldehyde (
2i) (137.8 mg, 0.75 mmol) was added to the NaDES. The mixture was sonicated for 18 min. After the work-up procedure, the product was obtained upon purification using flash column chromatography (petroleum ether/ethyl acetate 95:5) as an orange powder. Yield: 98.2 mg (62%). m.p. 137–139 °C (ref. [
40] m.p. 138–142 °C);
1H NMR (300 MHz, CDCl
3-
d1): δ (ppm) 9.66 (brs, 1H, -NH-), 7.79 (d,
J = 7.8 Hz, 1H, H-4), 7.61 (d,
J = 8.1 Hz, 1H, H-6), 7.30 (d,
J = 8.1 Hz, 1H, H-7), 7.21 (t,
J = 6.9 Hz, 1H, H-5), 7.14 (s, 1H, H-3′), 6.97 (s, 1H, H-10), 6.72 (s, 1H, H-5′), 6.36 (s, 1H, H-4′) (ref. [
52]).
(Z)-2-((1H-pyrrol-2-yl)methylene)benzofuran-3(2H)-one
3i | ![Catalysts 12 00249 i022]() |
4. Conclusions
In the present work, a simple, efficient, and green methodology for the synthesis of aurones were developed using green solvents and high energy techniques. Four different NaDES were task-specifically prepared: L-proline/glycerol 1:2, L-proline/oxalic acid 1:1, L-proline/D,L-lactic acid/water 1:2:2.5, and choline chloride/glycerol 1:2. The NaDES were structurally characterized and three physicochemical parameters were measured, namely polarity, pH, and viscosity, in order to examine their potential effect on the catalytic activity of the solvents. Then, the efficiency of the NaDES as solvents and catalysts were investigated using a model Knoevenagel reaction. Only the proline-based NaDES afforded the desired aurone in high purity and satisfactory yields, highlighting the significance of proline in the reaction mechanism.
In order to further promote the green character of the synthetic protocol the synergistic effect of microwave and ultrasound irradiation was studied. The results indicated that ultrasound irradiation outweighs microwave irradiation when combined with Pro/Gly as the solvent, giving the highest yield (89%) and reducing the reaction time from 6 h (when performed with conventional heating) to 8 min. Therefore, the optimum conditions for the reaction were chosen as: Pro/Gly 1:2 as solvent and sonication under ultrasound probe for 8 min.
The scope of the reaction was examined using various substituted benzaldehydes bearing substituents such as halogens, methoxy and hydroxyl-groups. An additional advantage of the developed methodology is that the free hydroxyl groups are well tolerated in the conditions of the reaction, thus eliminating the need to protect and deprotect the sensitive OH group. This is in accordance with the 8th Green Chemistry Principle, which states that the use of derivatives and/or protective groups should be minimized if not eliminated in order to develop a greener route to the chemical entities of interest. The presence of free OH groups in the aurone scaffold, apart from being a common structural characteristic in the majority of naturally occurring aurones, is also indispensable for many bioactivities such as antioxidant or enzyme inhibitory activity (for example HDAC or tyrosinase inhibition); thus, it is important to have a methodology that provides direct access to these compounds.
Finally, the recyclability of the NaDES was examined, revealing that Pro/Gly can be used, at least, up to seven times, without significant loss regarding the yield of the reaction, reinforcing the method’s environmentally friendly asset.












 Glycerol
Glycerol Oxalic acid
Oxalic acid D,L-lactic acid *
D,L-lactic acid *
 Glycerol
Glycerol