Microfluidics Biocatalysis System Applied for the Synthesis of N-Substituted Benzimidazole Derivatives by Aza-Michael Addition
Abstract
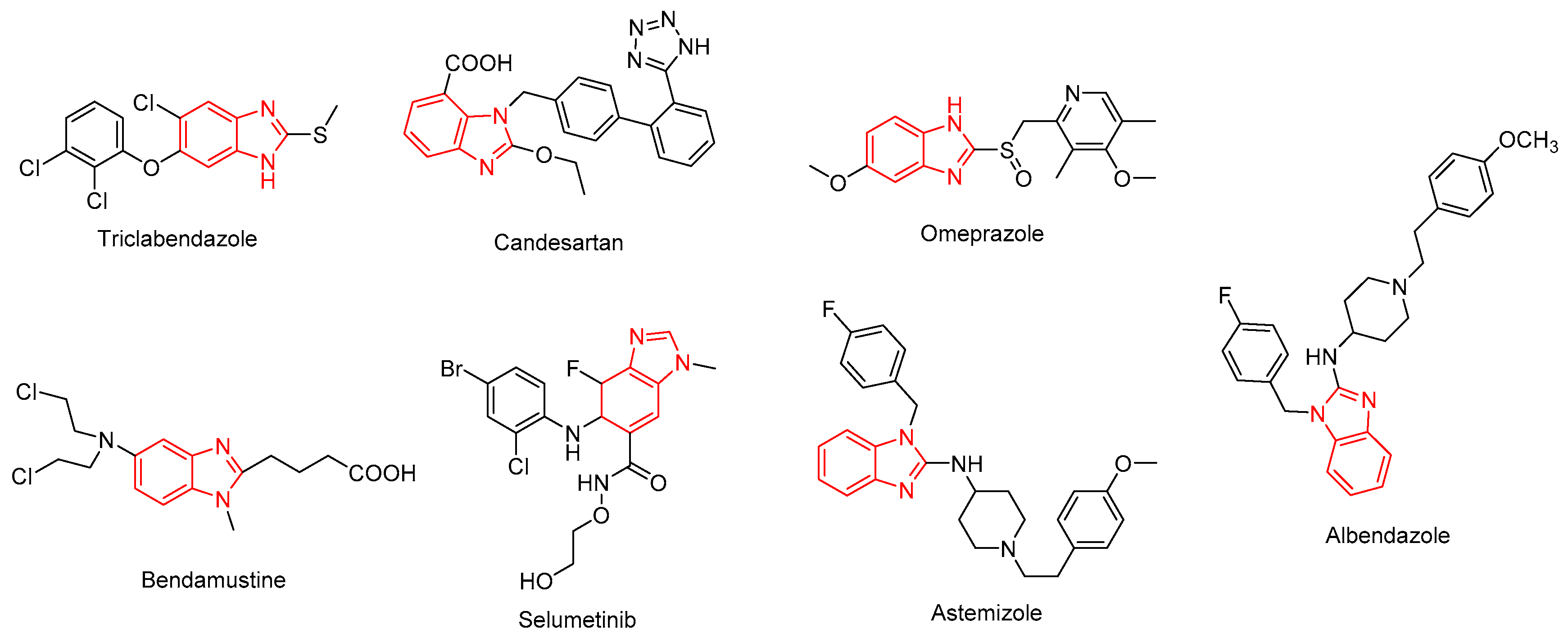
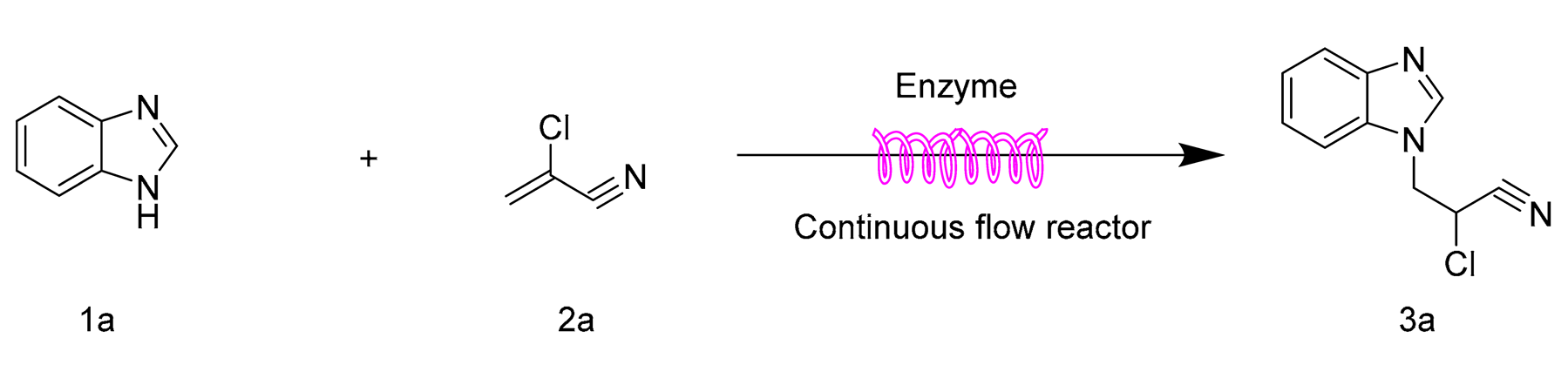
:1. Introduction
2. Results
2.1. Effect of Reaction Solvent
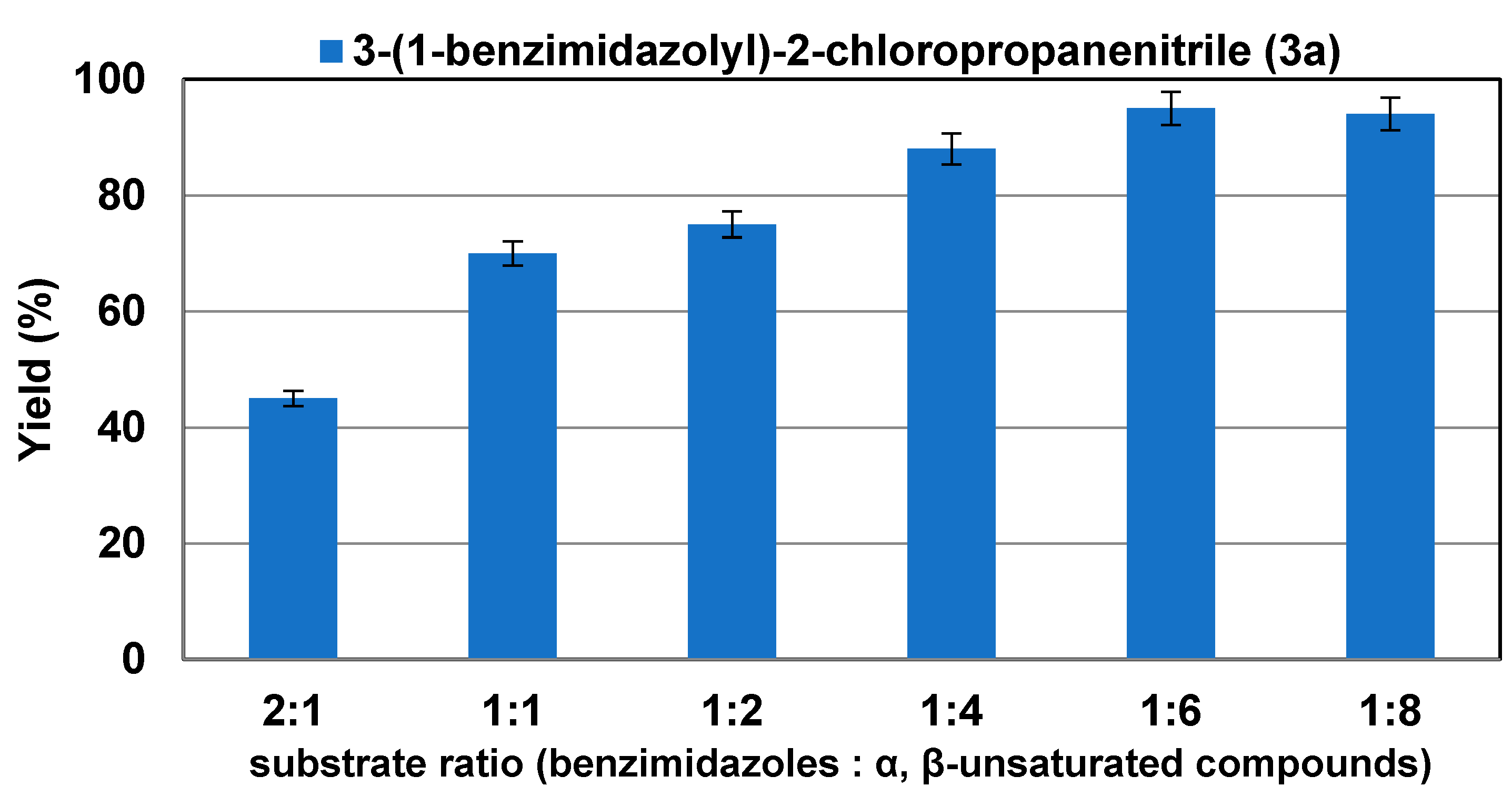
2.2. Effect of Substrate Ratio
2.3. Effect of Reaction Temperature
2.4. Effect of Residence Time
2.5. The Effect of Enzyme Reusability
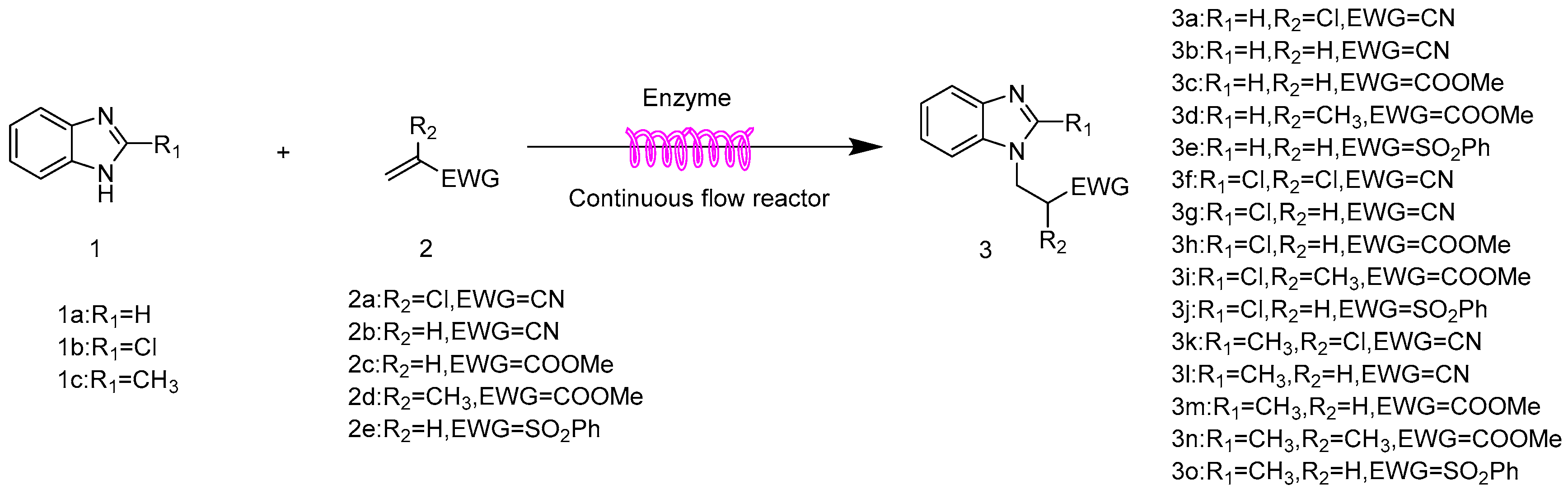
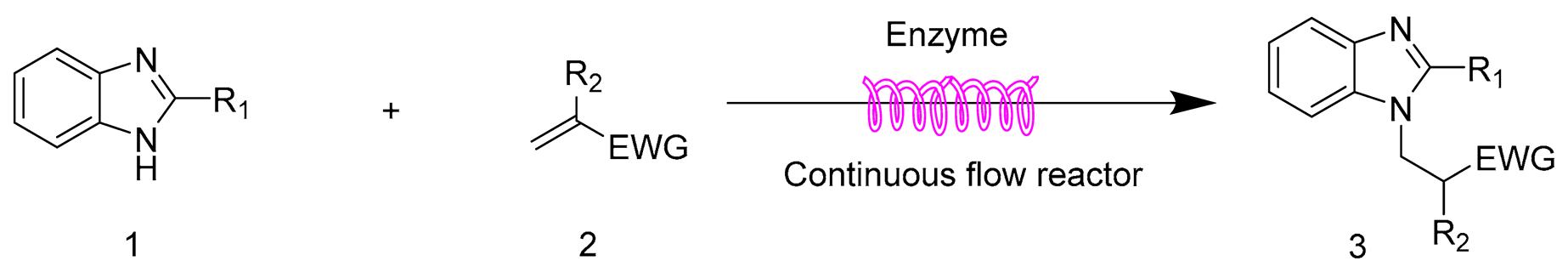
2.6. The Scope and Limitation of the Synthesis of N-Substituted Benzimidazole Derivatives Catalyzed by Lipozyme TL IM in Continuous-Flow Microreactors
3. Materials and Methods
3.1. Materials
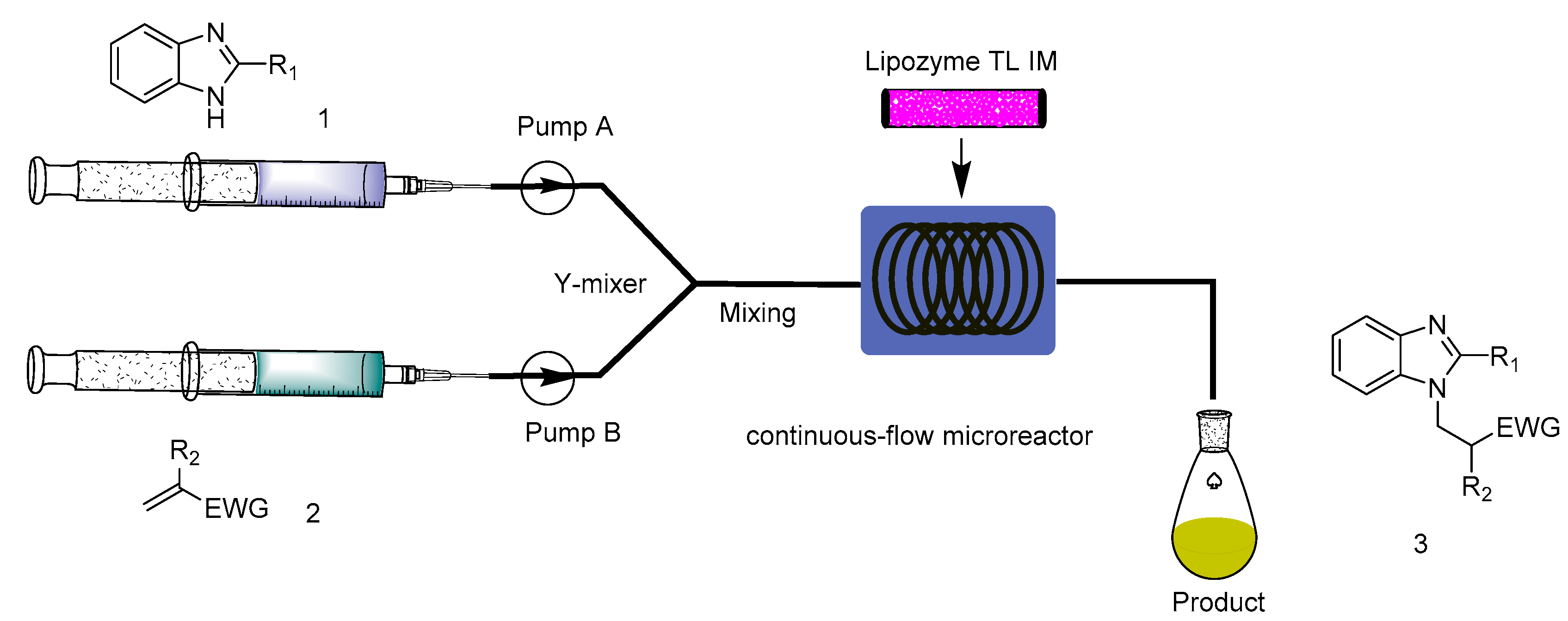
3.2. Experimental Setup and Experiment Conditions
3.3. Analytical Methods
3.3.1. Thin-Layer Chromatography (TLC)
3.3.2. Nuclear Magnetic Resonance (NMR) and High-Resolution Mass Spectrometry (HRMS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, S.; Mohan, C. Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 494–505. [Google Scholar] [CrossRef]
- Francesconi, V.; Cichero, E.; Schenone, S.; Naesens, L.; Tonelli, M. Synthesis and Biological Evaluation of Novel (thio)semicarbazone-Based Benzimidazoles as Antiviral Agents against Human Respiratory Viruses. Molecules 2020, 25, 1487. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.M.; Badaweya, T.K. Synthesis of some substituted cycloalkyl pyrido[1,2-a]benzimidazoles with anticipated antineoplastic activity. Eur. J. Med. Chem. 1999, 34, 633–667. [Google Scholar]
- Wu, Z.; Xia, M.B.; Bertsetseg, D.; Wang, Y.H.; Bao, X.L.; Zhu, W.B.; Tao, X.; Chen, P.R.; Tang, H.S.; Yan, Y.J.; et al. Design, synthesis and biological evaluation of novel fluoro-substituted benzimidazole derivatives with anti-hypertension activities. Bioorg. Chem. 2020, 101, 104042. [Google Scholar] [CrossRef]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S.; Hugar, M.H. Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010, 45, 1753–1759. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, S.K.; Naaz, F.; Sen Gupta, P.S.; Yadav, M.; Singh, V.K.; Singh, A.; Rana, M.K.; Gupta, S.K.; Schols, D.; et al. Alkylated benzimidazoles: Design, synthesis, docking, DFT analysis, ADMET property, molecular dynamics and activity against HIV and YFV. Comput. Biol. Chem. 2020, 89, 107400. [Google Scholar] [CrossRef]
- Monforte, A.M.; Ferro, S.; De Luca, L.; Lo Surdo, G.; Morreale, F.; Pannecouque, C.; Balzarini, J.; Chimirri, A. Design and synthesis of N(1)-aryl-benzimidazoles 2-substituted as novel HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2014, 22, 1459–1467. [Google Scholar] [CrossRef]
- Mavrova, A.; Yancheva, D.; Anastassova, N.; Anichina, K.; Zvezdanovic, J.; Djordjevic, A.; Markovic, D.; Smelcerovic, A. Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazoles. Bioorg. Med. Chem. 2015, 23, 6317–6326. [Google Scholar] [CrossRef]
- Chandrika, N.T.; Shrestha, S.K.; Ngo, H.X.; Garneau-Tsodikova, S. Synthesis and investigation of novel benzimidazole derivatives as antifungal agents. Bioorg. Med. Chem. 2016, 24, 3680–3686. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Tang, X.; Liu, T.; Peng, F.; Zhou, Q.; Luo, H.; He, M.; Xue, W. Antimicrobial evaluation of myricetin derivatives containing benzimidazole skeleton against plant pathogens. Fitoterapia 2021, 149, 104804. [Google Scholar] [CrossRef]
- Kamat, V.; Yallur, B.C.; Poojary, B.; Patil, V.B.; Nayak, S.P.; Krishna, P.M.; Joshi, S.D. Synthesis, molecular docking, antibacterial, and anti-inflammatory activities of benzimidazole-containing tricyclic systems. J. Chin. Chem. Soc. 2021, 68, 1055–1066. [Google Scholar] [CrossRef]
- Desai, N.C.; Dodiya, A.M.; Shihory, N.R. A search of novel antimicrobial based on benzimidazole and 2-pyridone heterocycles. Med. Chem. Res. 2011, 21, 2579–2586. [Google Scholar] [CrossRef]
- Stolic, I.; Cipcic Paljetak, H.; Peric, M.; Matijasic, M.; Stepanic, V.; Verbanac, D.; Bajic, M. Synthesis and structure-activity relationship of amidine derivatives of 3,4-ethylenedioxythiophene as novel antibacterial agents. Eur. J. Med. Chem. 2015, 90, 68–81. [Google Scholar] [CrossRef]
- Shareef, P.A.; Brennan, G.P.; McVeigh, P.; Khan, M.A.; Morphew, R.M.; Mousley, A.; Marks, N.J.; Saifullah, M.K.; Brophy, P.M.; Maule, A.G.; et al. Time-dependent tegumental surface changes in juvenile Fasciola gigantica in response to triclabendazole treatment in goat. Acta Trop. 2014, 136, 108–117. [Google Scholar] [CrossRef]
- Ntountaniotis, D.; Kellici, T.; Tzakos, A.; Kolokotroni, P.; Tselios, T.; Becker-Baldus, J.; Glaubitz, C.; Lin, S.; Makriyannis, A.; Mavromoustakos, T. The application of solid-state NMR spectroscopy to study candesartan cilexetil (TCV-116) membrane interactions. Comparative study with the AT1R antagonist drug olmesartan. Biochim. Biophys. Acta 2014, 1838, 2439–2450. [Google Scholar] [CrossRef] [Green Version]
- Koukoula, M.; Dotsikas, Y.; Molou, E.; Schulpis, K.H.; Thodi, G.; Chatzidaki, M.; Triantafylli, O.; Loukas, Y.L. Study of the effect of CYP2C19 polymorphisms on omeprazole pharmacokinetics by utilizing validated LC-MS/MS and Real Time-PCR methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1047, 173–179. [Google Scholar] [CrossRef]
- Huber, S.; Antoni, F.; Schickaneder, C.; Schickaneder, H.; Bernhardt, G.; Buschauer, A. Stabilities of neutral and basic esters of bendamustine in plasma compared to the parent compound: Kinetic investigations by HPLC. J. Pharm. Biomed. Anal. 2015, 104, 137–143. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Khan, A.A.; Ali, Z.; Dewangan, R.P.; Rafi, M.; Hassan, M.Q.; Akhtar, M.S.; Siddiqui, A.A.; Partap, S.; Pasha, S.; et al. Synthesis of stable benzimidazole derivatives bearing pyrazole as anticancer and EGFR receptor inhibitors. Bioorg. Chem. 2018, 78, 158–169. [Google Scholar] [CrossRef]
- Jakhar, R.; Paul, S.; Bhardwaj, M.; Kang, S.C. Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer Lett. 2016, 372, 89–100. [Google Scholar] [CrossRef]
- Velazquez-Olvera, S.; Salgado-Zamora, H.; Jimenez-Cardoso, E.; Campos-Aldrete, M.E.; Perez-Gonzalez, C.; Ben Hadda, T. In vitro anti-Giardia lamblia activity of 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyridines and -pyrimidines, individually and in combination with albendazole. Acta Trop. 2016, 155, 6–10. [Google Scholar] [CrossRef]
- Sun, C.-C.; Xu, K.; Zeng, C.-C. Transition Metal- and Base-Free Electrochemical aza-Michael Addition of Aromatic aza-Heterocycles or Ts-Protected Amines to α,β-Unsaturated Alkenes Mediated by NaI. ACS Sustain. Chem. Eng. 2018, 7, 2255–2261. [Google Scholar] [CrossRef]
- Gao, X.; Shan, C.; Chen, Z.; Liu, Y.; Zhao, X.; Zhang, A.; Yu, P.; Galons, H.; Lan, Y.; Lu, K. One-pot synthesis of beta-lactams by the Ugi and Michael addition cascade reaction. Org. Biomol. Chem. 2018, 16, 6096–6105. [Google Scholar] [CrossRef]
- Hinds, E.M.; Wolfe, J.P. A Cross-Metathesis/Aza-Michael Reaction Strategy for the Synthesis of Cyclic and Bicyclic Ureas. J. Org. Chem. 2018, 83, 10668–10676. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.; Kim, G.; Lee, Y. Copper-Catalyzed Aza-Michael Addition of Aromatic Amines or Aromatic Aza-Heterocycles to α,β-Unsaturated Olefins. J. Org. Chem. 2016, 81, 4048–4057. [Google Scholar] [CrossRef]
- Wen, J.; Luo, Y.L.; Zhang, H.Z.; Zhao, H.H.; Zhou, C.H.; Cai, G.X. A green and convenient approach toward benzimidazole derivatives and their antimicrobial activity. Chin. Chem. Lett. 2016, 27, 391–394. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Xie, M.; Wu, J.; Qu, G.; Ding, K.; Guo, H. Regio- and Enantioselective Allylic Amination of Aliphatic MBH Adducts with N-Heteroaromatics. Chemistry 2018, 24, 1425–1430. [Google Scholar] [CrossRef]
- Medina, F.; Michon, C.; Agbossou-Niedercorn, F. Intermolecular Mono- and Dihydroamination of Activated Alkenes Using a Recoverable Gold Catalyst. Eur. J. Org. Chem. 2012, 2012, 6218–6227. [Google Scholar] [CrossRef]
- Selvaraju, M.; Wang, Y.-L.; Sun, C.-M. Ruthenium(ii)-catalyzed C–H alkenylation/annulation cascade for the rapid synthesis of benzoimidazoisoindoles. Org. Chem. Front. 2017, 4, 1358–1362. [Google Scholar] [CrossRef]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Otari, S.V.; Patel, S.K.S.; Kalia, V.C.; Lee, J.-K. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour. Technol. 2020, 302, 122887. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Almeida, F.L.C.; Travália, B.M.; Gonçalves, I.S.; Forte, M.B.S. Biodiesel production by lipase-catalyzed reactions: Bibliometric analysis and study of trends. Biofuels Bioprod. Biorefining 2021, 15, 1141–1159. [Google Scholar] [CrossRef]
- Du, L.-H.; Cheng, B.-Z.; Yang, W.-J.; Xu, L.-L.; Luo, X.-P. Markovnikov addition of imidazole derivatives with vinyl esters catalyzed by lipase TL IM from Thermomyces lanuginosus/K2CO3 in a continuous-flow microreactor. RSC Adv. 2016, 6, 59100–59103. [Google Scholar] [CrossRef]
- Kitazume, T.; Murata, K.; Kokusho, Y.; Iwasaki, S. Enzymes Active in Organic Media—Synthesis of Optically-Active Trifluoromethylated Compounds Via Asymmetric Addition-Reactions. J. Fluor. Chem. 1988, 39, 75–86. [Google Scholar] [CrossRef]
- Steunenberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J.P.; Scott, E.L.; Franssen, M.C. Lipase-catalyzed aza-Michael reaction on acrylate derivatives. J. Org. Chem. 2013, 78, 3802–3813. [Google Scholar] [CrossRef]
- Du, L.-H.; Ling, H.-M.; Luo, X.-P. Michael addition of pyrimidine derivatives with acrylates catalyzed by lipase TL IM from Thermomyces lanuginosus in a continuous-flow microreactor. RSC Adv. 2014, 4, 7770. [Google Scholar] [CrossRef]
- Du, L.H.; Dong, Z.; Long, R.J.; Chen, P.F.; Xue, M.; Luo, X.P. The convenient Michael addition of imidazoles to acrylates catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in a continuous flow microreactor. Org. Biomol. Chem. 2019, 17, 807–812. [Google Scholar] [CrossRef]
- Du, L.-H.; Long, R.-J.; Xue, M.; Chen, P.-F.; Yang, M.-J.; Luo, X.-P. Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines. Catalysts 2020, 10, 432. [Google Scholar] [CrossRef]
- Li, H.-P.; You, Z.-N.; Liu, Y.-Y.; Zheng, G.-W.; Gong, H.; Mo, Y.; Zhu, N.; Bai, Y.-P.; Xu, J.-H. Continuous-Flow Microreactor-Enhanced Clean NAD+ Regeneration for Biosynthesis of 7-Oxo-lithocholic Acid. ACS Sustain. Chem. Eng. 2021, 10, 456–463. [Google Scholar] [CrossRef]
- Coloma, J.; Guiavarc'h, Y.; Hagedoorn, P.-L.; Hanefeld, U. Probing batch and continuous flow reactions in organic solvents: Granulicella tundricola hydroxynitrile lyase (GtHNL). Catal. Sci. Technol. 2020, 10, 3613–3621. [Google Scholar] [CrossRef]
- Gkantzou, E.; Govatsi, K.; Chatzikonstantinou, A.V.; Yannopoulos, S.N.; Stamatis, H. Development of a ZnO Nanowire Continuous Flow Microreactor with β-Glucosidase Activity: Characterization and Application for the Glycosylation of Natural Products. ACS Sustain. Chem. Eng. 2021, 9, 7658–7667. [Google Scholar] [CrossRef]
- Yang, A.; Yue, J.; Zheng, S.; Yang, X.; Kong, L.; Zhou, D.; Qin, L.; Zhong, H. Experimental investigation of mononitrotoluene preparation in a continuous-flow microreactor. Res. Chem. Intermed. 2022, 48, 4373–4390. [Google Scholar] [CrossRef]
- Carlucci, C. An Overview on the Production of Biodiesel Enabled by Continuous Flow Methodologies. Catalysts 2022, 12, 717. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.-S.; am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key Green Engineering Research Areas for Sustainable Manufacturing: A Perspective from Pharmaceutical and Fine Chemicals Manufacturers. Org. Process Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- Hessel, V.; Kralisch, D.; Krtschil, U. Sustainability through green processing–novel process windows intensify micro and milli process technologies. Energy Environ. Sci. 2008, 1, 467–478. [Google Scholar] [CrossRef]
- Du, L.-H.; Xue, M.; Yang, M.-J.; Pan, Y.; Zheng, L.-Y.; Ou, Z.-M.; Luo, X.-P. Ring-Opening of Epoxides with Amines for Synthesis of β-Amino Alcohols in a Continuous-Flow Biocatalysis System. Catalysts 2020, 10, 1419. [Google Scholar] [CrossRef]
- Du, L.-H.; Yang, M.-J.; Pan, Y.; Zheng, L.-Y.; Zhang, S.-Y.; Sheng, Z.-K.; Chen, P.-F.; Luo, X.-P. Continuous Flow Biocatalysis: Synthesis of Coumarin Carboxamide Derivatives by Lipase TL IM from Thermomyces lanuginosus. Catalysts 2022, 12, 339. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I.; Moldoveanu, C. Ultrasound assisted synthesis of imidazolium salts: An efficient way to ionic liquids. Ultrason. Sonochem. 2015, 23, 376–384. [Google Scholar] [CrossRef]
- Zhou, M.; Eun, Y.J.; Guzei, I.A.; Weibel, D.B. Structure-activity studies of divin: An inhibitor of bacterial cell division. ACS Med Chem Lett 2013, 4, 880–885. [Google Scholar] [CrossRef]



 | ||||
|---|---|---|---|---|
| Entry | Solvent | Catalysts | Log P | Yield b (%) |
| 1 | Methanol | None | −0.76 | n.d. |
| 2 | Methanol | Lipozyme TL IM | −0.76 | 95.4 ± 1.6 |
| 3 | Tert-amyl alcohol | Lipozyme TL IM | 1.04 | 45.8 ± 1.5 |
| 4 | DMSO | Lipozyme TL IM | −1.3 | 68.2 ± 1.0 |
| 5 | Isopropanol | Lipozyme TL IM | 0.39 | 32.3 ± 0.5 |
| 6 | Acetonitrile | Lipozyme TL IM | −0.33 | 42.5 ± 0.8 |
| 7 | n-Hexane | Lipozyme TL IM | 3.94 | 66.4 ± 1.1 |
| 8 | DMF | Lipozyme TL IM | −1.0 | 43.2 ± 0.7 |
 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | EWG | Product | Yield b (%) |
| 1 | H | Cl | CN | 3a | 95.4 ± 1.6 |
| 2 | H | H | CN | 3b | 90.3 ± 0.8 |
| 3 | H | H | COOMe | 3c | 92.2 ± 1.5 |
| 4 | H | CH3 | COOMe | 3d | trace |
| 5 | H | H | SO2Ph | 3e | 97.1 ± 1.2 |
| 6 | Cl | Cl | CN | 3f | 80.5 ± 0.5 |
| 7 | Cl | H | CN | 3g | 76.8 ± 0.9 |
| 8 | Cl | H | COOMe | 3h | 77.4 ± 1.1 |
| 9 | Cl | CH3 | COOMe | 3i | trace |
| 10 | Cl | H | SO2Ph | 3j | 80.3 ± 0.7 |
| 11 | CH3 | Cl | CN | 3k | 95.6 ± 1.4 |
| 12 | CH3 | H | CN | 3l | 90.4 ± 1.1 |
| 13 | CH3 | H | COOMe | 3m | 93.2 ± 0.6 |
| 14 | CH3 | CH3 | COOMe | 3n | trace |
| 15 | CH3 | H | SO2Ph | 3o | 97.2 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.-K.; Pan, Y.; Du, L.-H.; Zheng, L.-Y.; Sheng, Z.-K.; Zhang, S.-Y.; Lin, H.; Zhang, A.-Y.; Xie, H.-J.; Yang, Z.-K.; et al. Microfluidics Biocatalysis System Applied for the Synthesis of N-Substituted Benzimidazole Derivatives by Aza-Michael Addition. Catalysts 2022, 12, 1658. https://doi.org/10.3390/catal12121658
Jiang R-K, Pan Y, Du L-H, Zheng L-Y, Sheng Z-K, Zhang S-Y, Lin H, Zhang A-Y, Xie H-J, Yang Z-K, et al. Microfluidics Biocatalysis System Applied for the Synthesis of N-Substituted Benzimidazole Derivatives by Aza-Michael Addition. Catalysts. 2022; 12(12):1658. https://doi.org/10.3390/catal12121658
Chicago/Turabian StyleJiang, Rong-Kuan, Yue Pan, Li-Hua Du, Ling-Yan Zheng, Zhi-Kai Sheng, Shi-Yi Zhang, Hang Lin, Ao-Ying Zhang, Han-Jia Xie, Zhi-Kai Yang, and et al. 2022. "Microfluidics Biocatalysis System Applied for the Synthesis of N-Substituted Benzimidazole Derivatives by Aza-Michael Addition" Catalysts 12, no. 12: 1658. https://doi.org/10.3390/catal12121658




