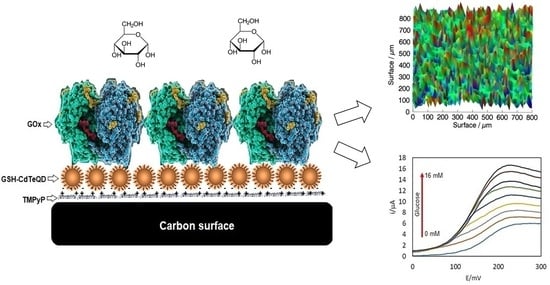
Immobilization of Glucose Oxidase on Glutathione Capped CdTe Quantum Dots for Bioenergy Generation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pysicochemical Characterization of GSH-CdTeQD
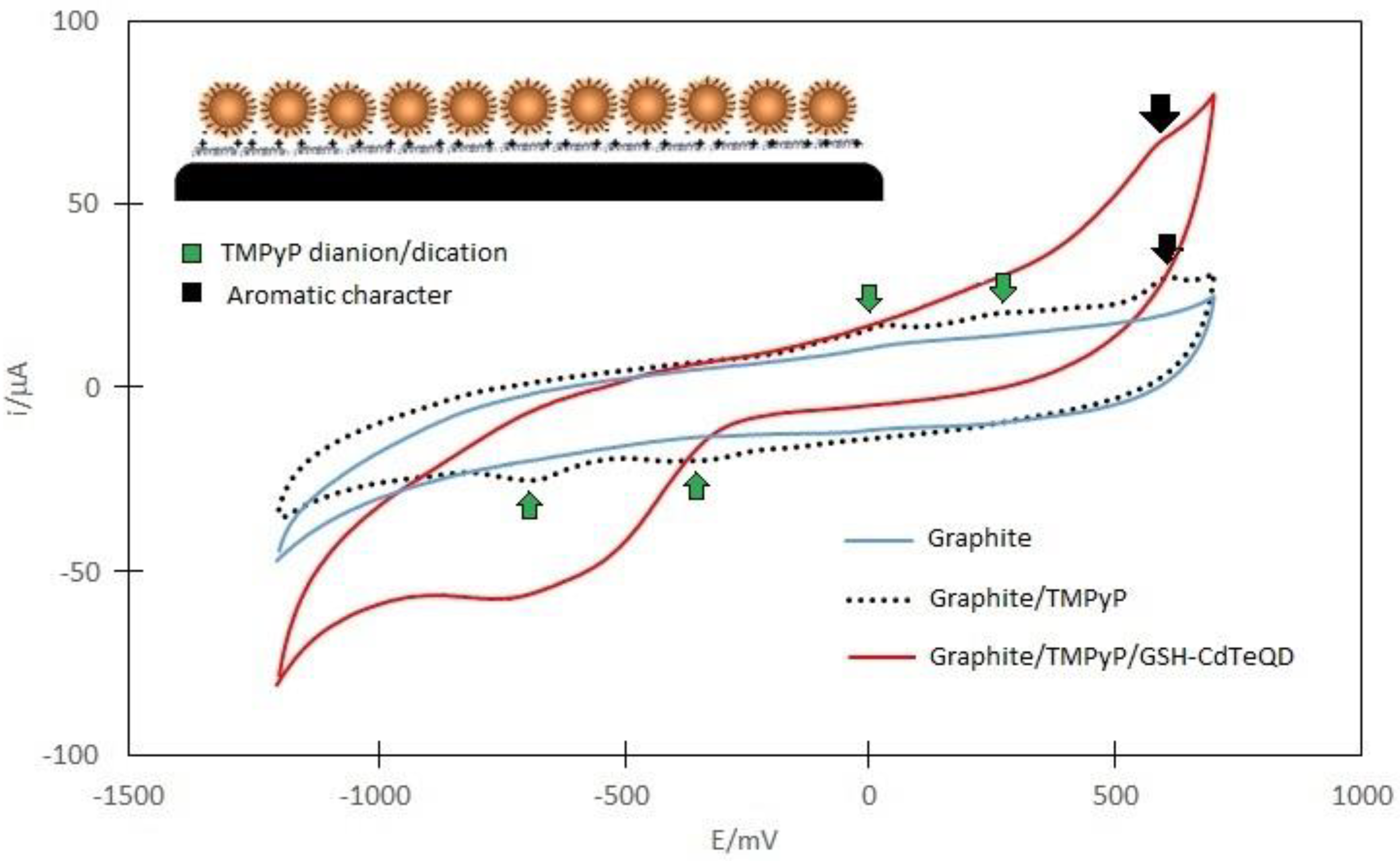
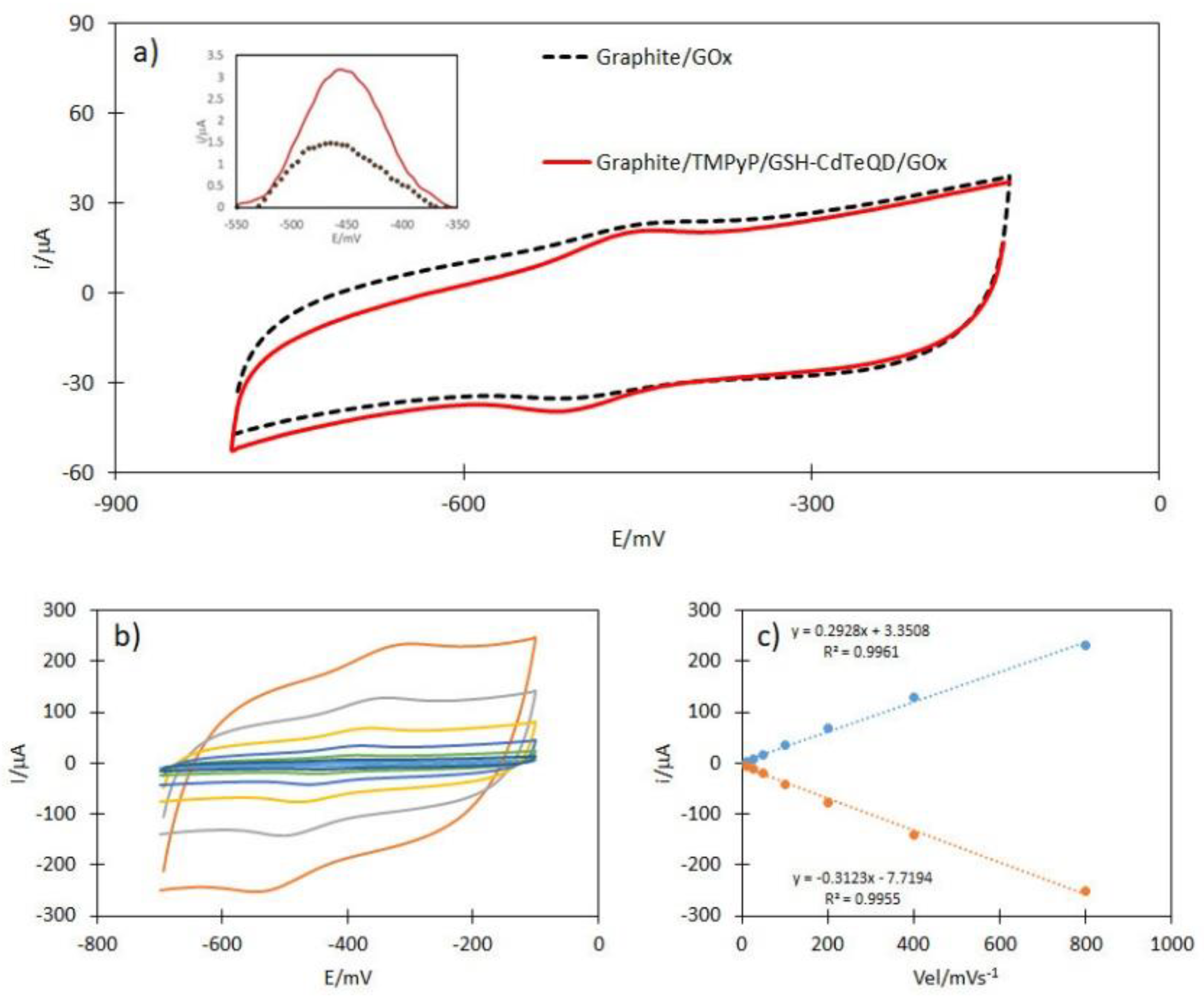
2.2. Electrochemical Analysis of TMPyP, GSH-CdTeQD and TMPyP/GSH-CdTeQD
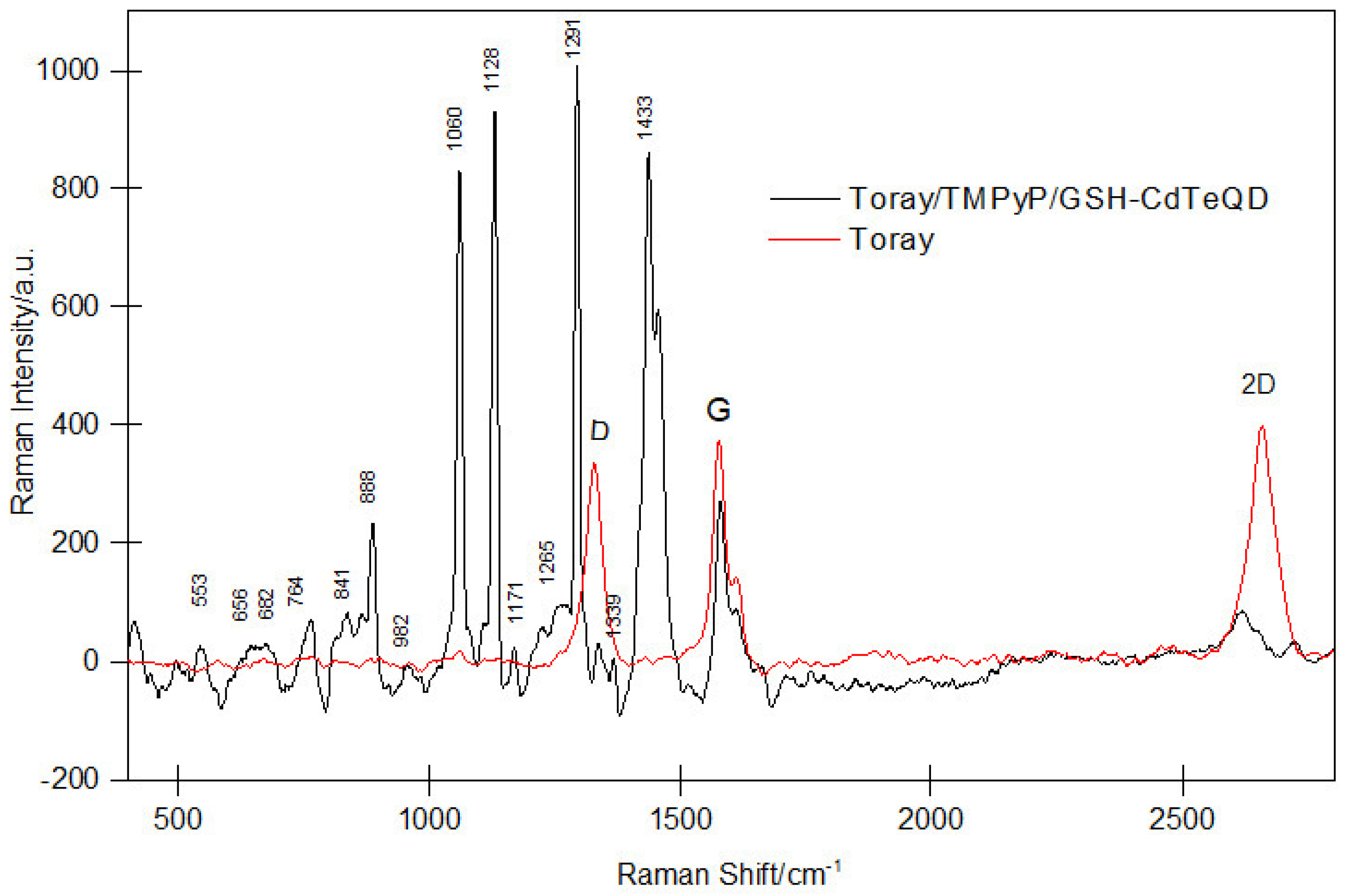
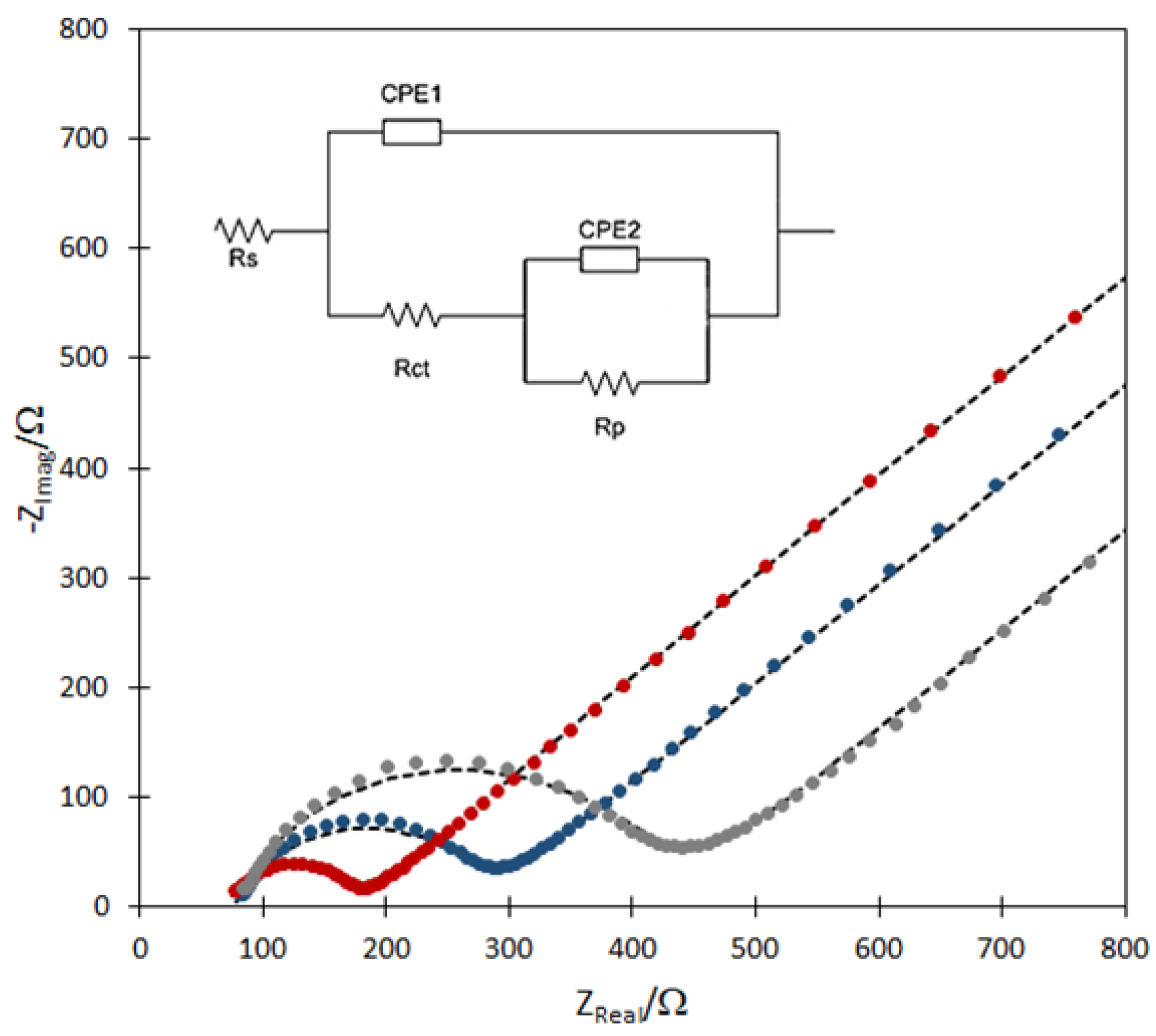
2.3. EIS for TMPyP and TMPyP/GSH-CdTeQD
2.4. Electrochemical Analysis of GOx Immobilization
2.5. Physicochemical Analysis of Anchored GOx
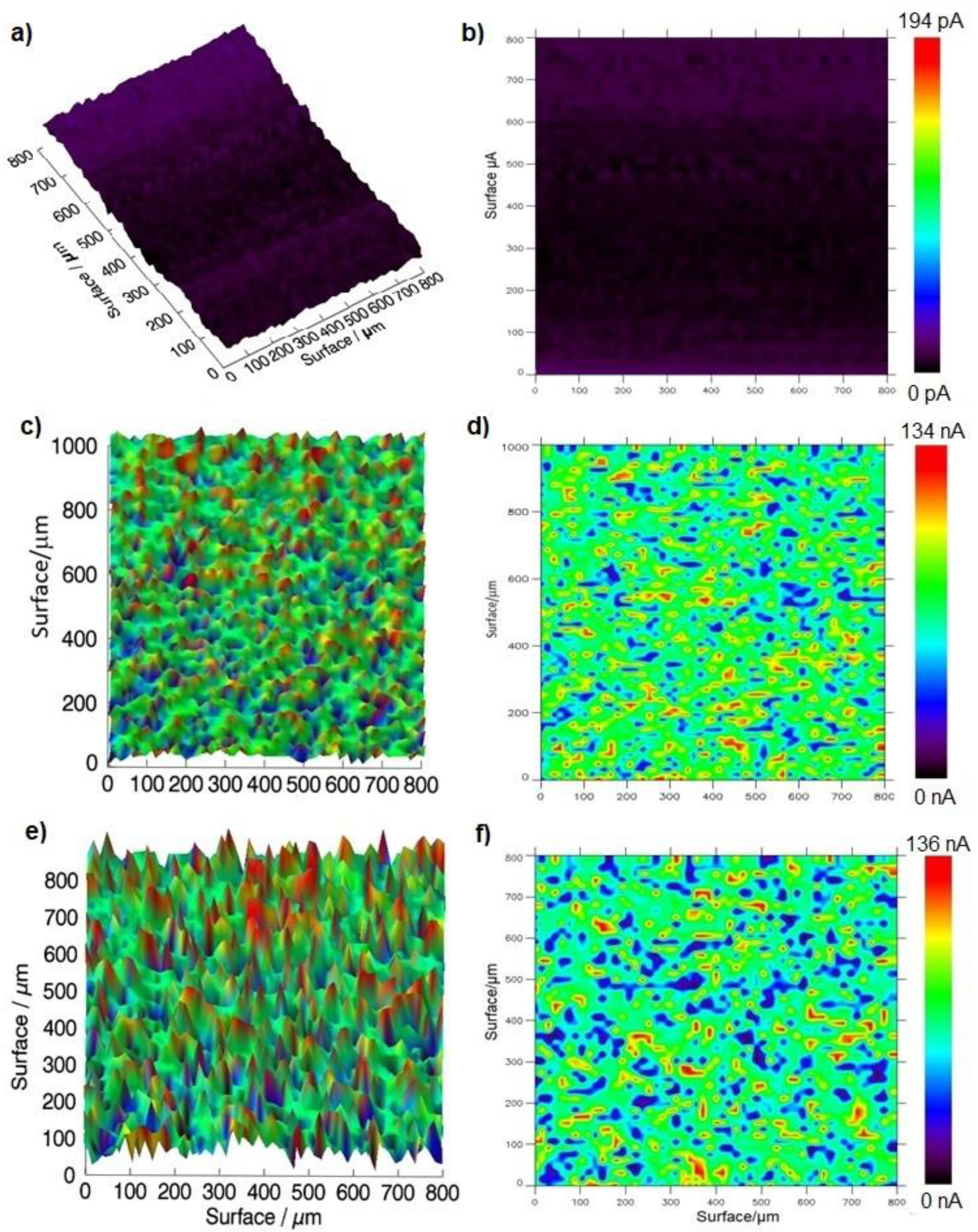
2.6. Evaluation of TMPyP/GSH-CdTQD/GOx Electrodes by Scanning Electrochemical Microscopy
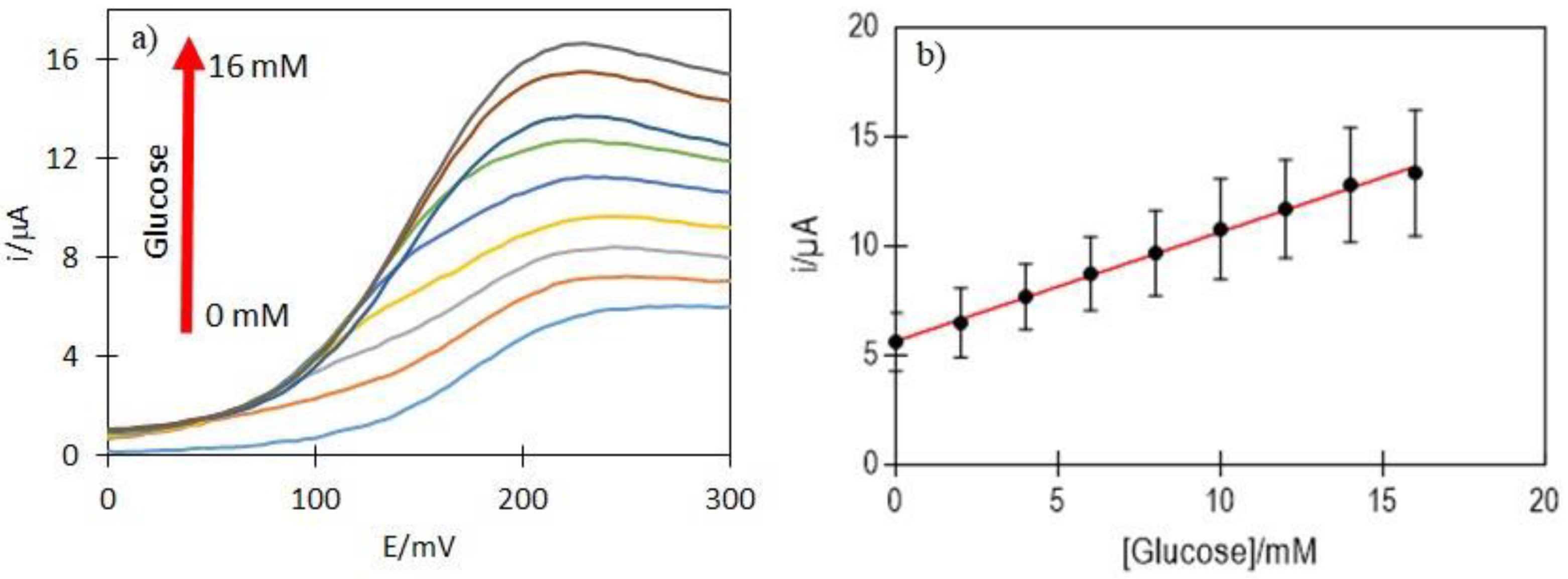
2.7. Amperometric Response of TMPyP/GSH-CdTeQD/GOx Electrodes to Glucose
2.8. Interferents Assay
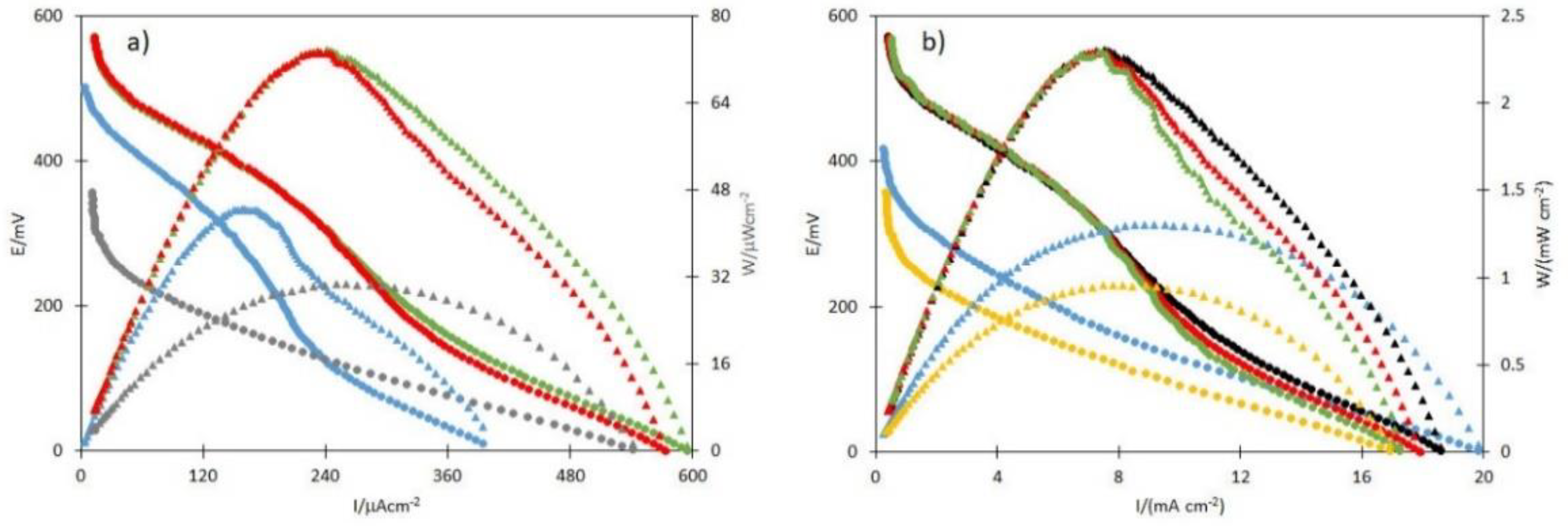
2.9. Performance of Glucose Microfluidic Fuel Cell
3. Materials and Methods
3.1. Synthesis of GSH-CdTeQD
3.2. Preparation of Electrodes
3.3. Electrochemical Characterization
3.4. Design and Operation of the Glucose Microfluidic Cell
3.5. Physicochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, A.; Bhatt, R.; Bajpai, J.; Bajpai, A. Nanomaterials based biofuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 19085–19105. [Google Scholar] [CrossRef]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, S.U.; Duteanu, N.; Ciocan, S.; Nasar, A.; Inamuddin. A review: Evolution of enzymatic biofuel cells. J. Environ. Manag. 2021, 298, 113483. [Google Scholar] [CrossRef] [PubMed]
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices—A review. Int. J. Hydrogen Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Tang, X.; Xu, L.; Min, L.; Xue, Y.; Hu, X.; Yang, Z. Multiwalled carbon nanotubes coated with cobalt(II) sulfide nanoparticles for electrochemical sensing of glucose via direct electron transfer to glucose oxidase. Microchim. Acta 2020, 187, 80. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, A.M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Chomicz, R.; Bystrzejewski, M.; Stolarczyk, K. Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery. Catalysts 2021, 11, 705. [Google Scholar] [CrossRef]
- Estrada-Osorio, D.; Escalona-Villalpando, R.A.; Gutiérrez, A.; Arriaga, L.; Ledesma-García, J. Poly-L-lysine-modified with ferrocene to obtain a redox polymer for mediated glucose biosensor application. Bioelectrochemistry 2022, 146, 108147. [Google Scholar] [CrossRef]
- Haque, S.U.; Nasar, A.; Duteanu, N.; Inamuddin. Carbon based-nanomaterials used in biofuel cells—A review. Fuel 2023, 331, 125634. [Google Scholar] [CrossRef]
- Ahmad, R.; Rizaldo, S.; Shaner, S.E.; Kissel, D.S.; Stone, K.L. Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework. Catalysts 2022, 12, 969. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Panda, A.K. Recent advances in nano-engineered approaches used for enzyme immobilization with enhanced activity. J. Mol. Liq. 2021, 338, 116602. [Google Scholar] [CrossRef]
- Maity, D.; Minitha, C.R.; Rajenda, K.R.T. Glucose oxidase immobilized amine terminated multiwall carbon nanotubes/reduced graphene oxide/polyaniline/gold nanoparticles modified screen-printed carbon electrode for highly sensitive amperometric glucose detection. Mater. Sci. Eng. C 2019, 105, 110075. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Choi, S.H.; Kang, Y.C.; Lee, J.-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3yolk–shell particles: A promising support for enzyme immobilization. Nanoscale 2016, 8, 6728–6738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Zhong, Y.; Wu, X.; Hu, R.; Wang, L.; Zhang, Y.; Fan, G.; Hu, X.; Li, J.; Yang, Z. Synthesis of perovskite-type SrTiO3 nanoparticles for sensitive electrochemical biosensing applications. J. Electroanal. Chem. 2018, 810, 95–99. [Google Scholar] [CrossRef]
- Sağlam, Ö.; Kızılkaya, B.; Uysal, H.; Dilgin, Y. Biosensing of glucose in flow injection analysis system based on glucose oxidase-quantum dot modified pencil graphite electrode. Talanta 2016, 147, 315–321. [Google Scholar] [CrossRef]
- Buk, V.; Pemble, M.E. A highly sensitive glucose biosensor based on a micro disk array electrode design modified with carbon quantum dots and gold nanoparticles. Electrochim. Acta 2019, 298, 97–105. [Google Scholar] [CrossRef]
- Sheng, Z.; Han, H.; Hu, X.; Chi, C. One-step growth of high luminescence CdTe quantum dots with low cytotoxicity in ambient atmospheric conditions. Dalton Trans. 2010, 39, 7017–7020. [Google Scholar] [CrossRef]
- Cao, L.; Ye, J.; Tong, L.; Tang, B. A New Route to the Considerable Enhancement of Glucose Oxidase (GOx) Activity: The Simple Assembly of a Complex from CdTe Quantum Dots and GOx, and Its Glucose Sensing. Chem. A Eur. J. 2008, 14, 9633–9640. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Graphene Quantum Dots Assembled with Metalloporphyrins for “Turn On” Sensing of Hydrogen Peroxide and Glucose. Chem. A Eur. J. 2015, 21, 9343–9348. [Google Scholar] [CrossRef]
- Vaishnavi, E.; Renganathan, R. “Turn-on-off-on” fluorescence switching of quantum dots–cationic porphyrin nanohybrid: A sensor for DNA. Analyst 2014, 139, 225–234. [Google Scholar] [CrossRef]
- Ahmed, G.H.; Aly, S.M.; Usman, A.; Eita, M.S.; Melnikov, V.A.; Mohammed, O.F. Quantum confinement-tunable intersystem crossing and the triplet state lifetime of cationic porphyrin–CdTe quantum dot nano-assemblies. Chem. Commun. 2015, 51, 8010–8013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Tanveer, Z.I.; Jiang, K.; Huang, Q.; Zhang, J.; Wu, Y.; Han, Z. Label-Free Fluorescent Aptasensor for Ochratoxin—A Detection Based on CdTe Quantum Dots and (N-Methyl-4-pyridyl) Porphyrin. Toxins 2019, 11, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Bae, I.; Kim, S.K. Effect of Axial Ligand on the Binding Mode of M-meso-Tetrakis(N-methylpyridinium-4-yl)porphyrin to DNA Probed by Circular and Linear Dichroism Spectroscopies. J. Phys. Chem. B 2012, 116, 12510–12521. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Masoud, M.S. Self-assembly of porphyrin on graphene oxide in aqueous medium: Fabrication, characterization, and photocatalytic studies. Photochem. Photobiol. Sci. 2019, 18, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Zha, S.; Zhu, Y.; Wu, P.; Ehrenberg, B.; Chen, J.-Y. Carbon nanodots featuring efficient FRET for two-photon photodynamic cancer therapy with a low fs laser power density. Biomaterials 2014, 35, 9372–9381. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Fuenzalida, F.; Ruiz, D.; Aguirre, M. Electrochemical, spectroscopic and morphological characterization of electrostatic self-assembled hybrids of tetracationic phosphonium porphyrins and CdTe quantum dots. J. Appl. Electrochem. 2014, 44, 1345–1353. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J.C.X.; Chen, S.; Yu, Y.; Zhang, J. Advanced Noncarbon Materials as Catalyst Supports and Non-noble Electrocatalysts for Fuel Cells and Metal–Air Batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Khan, H.; Tanveer, M.; Nguyen, D.D.; Ullah, A.; Shin, M.C.; Kwak, M.K.; Park, C.W.; Kim, G.M. Efficient stacking of glucose/oxygen microfluidic biofuel cells using a single-streamflow channel. Energy Convers. Manag. 2022, 271, 116270. [Google Scholar] [CrossRef]
- Escalona, R.; Hasan, K.; Milton, R.D.; Moreno-Zuria, A.; Arriaga, L.; Minteer, S.D.; Ledesma-García, J. Performance comparison of different configurations of Glucose/O2 microfluidic biofuel cell stack. J. Power Sources 2019, 414, 150–157. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Z.; Qiao, L.; Liu, B. Advances in microfluidic strategies for single-cell research. TrAC Trends Anal. Chem. 2022, 157, 116822. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, C.; Zhu, X.; Yang, Y.; Ye, D.; Chen, R.; Liao, Q. Elimination of Fuel Crossover in a Single-Flow Microfluidic Fuel Cell with a Selective Catalytic Cathode. Ind. Eng. Chem. Res. 2022, 61, 1955–1964. [Google Scholar] [CrossRef]
- Ibrahim, O.A.; Navarro-Segarra, M.; Sadeghi, P.; Sabaté, N.; Esquivel, J.P.; Kjeang, E. Microfluidics for Electrochemical Energy Conversion. Chem. Rev. 2022, 122, 7236–7266. [Google Scholar] [CrossRef] [PubMed]
- Escalona-Villalpando, R.; Martínez-Maciel, A.; Espinosa-Ángeles, J.; Ortiz-Ortega, E.; Arjona, N.; Arriaga, L.; Ledesma-García, J. Evaluation of hybrid and enzymatic nanofluidic fuel cells using 3D carbon structures. Int. J. Hydrogen Energy 2018, 43, 11847–11852. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Liu, N.; Zhang, R.; Xu, J.; Su, W.; Yu, Y.; Ma, Z.; Li, W.; Chen, K. Enhanced stability and emission intensity of aqueous CdTe/CdS core–shell quantum dots with widely tunable wavelength. Can. J. Phys. 2014, 92, 802–805. [Google Scholar] [CrossRef]
- Libertino, S.; Aiello, V.; Scandurra, A.; Renis, M.; Sinatra, F. Immobilization of the Enzyme Glucose Oxidase on Both Bulk and Porous SiO2 Surfaces. Sensors 2008, 8, 5637–5648. [Google Scholar] [CrossRef]
- Suzuki, N.; Lee, J.; Loew, N.; Takahashi-Inose, Y.; Okuda-Shimazaki, J.; Kojima, K.; Mori, K.; Tsugawa, W.; Sode, K. Engineered Glucose Oxidase Capable of Quasi-Direct Electron Transfer after a Quick-and-Easy Modification with a Mediator. Int. J. Mol. Sci. 2020, 21, 1137. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Segets, D. Analysis of Particle Size Distributions of Quantum Dots: From Theory to Application. KONA Powder Part. J. 2016, 33, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, Z.; Miao, P. One-pot synthesis of GSH-Capped CdTe quantum dots with excellent biocompatibility for direct cell imaging. Heliyon 2018, 4, e00576. [Google Scholar] [CrossRef] [Green Version]
- Lemon, C.M.; Karnas, E.; Han, X.; Bruns, O.T.; Kempa, T.J.; Fukumura, D.; Bawendi, M.G.; Jain, R.K.; Duda, D.G.; Nocera, D.G. Micelle-Encapsulated Quantum Dot-Porphyrin Assemblies as In Vivo Two-Photon Oxygen Sensors. J. Am. Chem. Soc. 2015, 137, 9832–9842. [Google Scholar] [CrossRef]
- He, D.; Yang, X.; He, X.; Wang, K.; Yang, X.; He, X.; Zou, Z. A sensitive turn-on fluorescent probe for intracellular imaging of glutathione using single-layer MnO2 nanosheet-quenched fluorescent carbon quantum dots. Chem. Commun. 2015, 51, 14764–14767. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Yue, Z.; Zhang, Z.; Teng, K.-S.; Li, M.-J.; Yi, C.; Yang, M. Coupling gold nanoparticles to silica nanoparticles through disulfide bonds for glutathione detection. Nanotechnology 2013, 24, 375501. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, G.; Guo, W.; Li, P.; Wang, E.; Wang, J. Glutathione-mediated release of functional plasmid DNA from positively charged quantum dots. Biomaterials 2008, 29, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, Z.; Li, Y.; Ying, J.Y. From Glutathione Capping to a Crosslinked, Phytochelatin-like Coating of Quantum Dots. Adv. Mater. 2008, 20, 3410–3415. [Google Scholar] [CrossRef]
- Gu, Y.-P.; Cui, R.; Zhang, Z.-L.; Xie, Z.-X.; Pang, D.-W. Ultrasmall Near-Infrared Ag2Se Quantum Dots with Tunable Fluorescence for In Vivo Imaging. J. Am. Chem. Soc. 2012, 134, 79–82. [Google Scholar] [CrossRef]
- Deng, Z.; Lie, F.L.; Shen, S.; Ghosh, I.; Mansuripur, M.; Muscat, A.J. Water-Based Route to Ligand-Selective Synthesis of ZnSe and Cd-Doped ZnSe Quantum Dots with Tunable Ultraviolet A to Blue Photoluminescence. Langmuir ACS J. Surf. Colloids 2009, 25, 434–442. [Google Scholar] [CrossRef]
- Li, Y.; Cui, R.; Zhang, P.; Chen, B.-B.; Tian, Z.-Q.; Li, L.; Hu, B.; Pang, D.-W.; Xie, Z.-X. Mechanism-Oriented Controllability of Intracellular Quantum Dots Formation: The Role of Glutathione Metabolic Pathway. ACS Nano 2013, 7, 2240–2248. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef]
- Selvarajan, P.; Chandra, G.; Bhattacharya, S.; Sil, S.; Vinu, A.; Umapathy, S. Potential of Raman spectroscopy towards understanding structures of carbon-based materials and perovskites. Emerg. Mater. 2019, 2, 417–439. [Google Scholar] [CrossRef]
- Wang, R.-X.; Fan, J.-J.; Fan, Y.-J.; Zhong, J.-P.; Wang, L.; Sun, S.-G.; Shen, X.-C. Platinum nanoparticles on porphyrin functionalized graphene nanosheets as a superior catalyst for methanol electrooxidation. Nanoscale 2014, 6, 14999–15007. [Google Scholar] [CrossRef]
- Barszcz, B.; Bogucki, A.; Laskowska, B.; Ion, R.; Graja, A. Spectral Investigations of Fullerene-Porphyrin Complexes. Acta Phys. Pol. A 2007, 112, 143–152. [Google Scholar] [CrossRef]
- Huang, G.G.; Han, X.X.; Hossain, M.K.; Kitahama, Y.; Ozaki, Y. A Study of Glutathione Molecules Adsorbed on Silver Surfaces under Different Chemical Environments by Surface-Enhanced Raman Scattering in Combination with the Heat-Induced Sensing Method. Appl. Spectrosc. 2010, 64, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Dogra, S.D.; Kaur, S.; Tripathi, S.; Prakash, S.; Rai, B.; Saini, G. Structure and vibrations of glutathione studied by vibrational spectroscopy and density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Van Caemelbecke, E.; Derbin, A.; Hambright, P.; Garcia, R.; Doukkali, A.; Saoiabi, A.; Ohkubo, K.; Fukuzumi, S.; Kadish, K.M. Electrochemistry of [(TMpyP)MII]4+(X−)4 (X− = Cl− or BPh4−) and [(TMpyP)MIIICl]4+(Cl−)4 in N,N-Dimethylformamide Where M Is One of 15 Different Metal Ions. Inorg. Chem. 2005, 44, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- Kadish, K.M.; Araullo, C.; Maiya, G.B.; Sazou, D.; Barbe, J.M.; Guilard, R. Electrochemical and spectral characterization of copper, zinc, and vanadyl meso-tetrakis(1-methylpyridinium-4-yl)porphyrin complexes in dimethylformamide. Inorg. Chem. 1989, 28, 2528–2533. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Q.; Swain, G.M. Anthraquinonedisulfonate Electrochemistry: A Comparison of Glassy Carbon, Hydrogenated Glassy Carbon, Highly Oriented Pyrolytic Graphite, and Diamond Electrodes. Anal. Chem. 1998, 70, 3146–3154. [Google Scholar] [CrossRef]
- Lim, Y.; Chu, J.H.; Lee, D.H.; Kwon, S.-Y.; Shin, H. Increase in graphitization and electrical conductivity of glassy carbon nanowires by rapid thermal annealing. J. Alloys Compd. 2017, 702, 465–471. [Google Scholar] [CrossRef]
- Silva, L.A.J.; Stefano, J.S.; Cardoso, R.M.; Prado, N.S.; Soares, P.H.T.; Nossol, E.; Munoz, R.A.A.; Angnes, L.; Richter, E.M. Evaluation of graphite sheets for production of high-quality disposable sensors. J. Electroanal. Chem. 2019, 833, 560–567. [Google Scholar] [CrossRef]
- Available online: https://jrossmacdonald.com/levmlevmw/ (accessed on 15 November 2022).
- Walch, N.J. Graphene Modified Electrodes for Enhanced Electrochemical Detection. Ph.D. Thesis, Cranfield University, Bedford, UK, 2016. [Google Scholar]
- Konwer, S.; Boruah, R.; Dolui, S.K. Studies on Conducting Polypyrrole/Graphene Oxide Composites as Supercapacitor Electrode. J. Electron. Mater. 2011, 40, 2248–2255. [Google Scholar] [CrossRef]
- Pyo, M.; Maeder, G.; Kennedy, R.T.; Reynolds, J.R. Controlled release of biological molecules from conducting polymer modified electrodes: The potential dependent release of adenosine 5′-triphosphate from poly(pyrrole adenosine 5′-triphosphate) films. J. Electroanal. Chem. 1994, 368, 329–332. [Google Scholar] [CrossRef]
- Brown, A.P.; Anson, F.C. Electron transfer kinetics with both reactant and product attached to the electrode surface. J. Electroanal. Chem. Interfac. Electrochem. 1978, 92, 133–145. [Google Scholar] [CrossRef]
- Verhagen, M.F.; Hagen, W.R. Electron transfer mechanisms of flavine adenine dinucleotide at the glassy carbon electrode; a model study for protein electrochemistry. J. Electroanal. Chem. 1992, 334, 339–350. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Razak, K.A.; Lockman, Z. Fabrication and Characterization of Glucose Biosensors by Using Hydrothermally Grown ZnO Nanorods. Sci. Rep. 2018, 8, 13722. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.U.; Nasar, A.; Inamuddin; Asiri, A.M. Preparation and characterization of a bioanode (GC/MnO2/PSS/Gph/Frt/GOx) for biofuel cell application. Int. J. Hydrogen Energy 2019, 44, 7308–7319. [Google Scholar] [CrossRef]
- Kundu, M.; Bhardwaj, H.; Pandey, M.K.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Development of electrochemical biosensor based on CNT–Fe3O4 nanocomposite to determine formaldehyde adulteration in orange juice. J. Food Sci. Technol. 2019, 56, 1829–1840. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose Oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Wu, P.; Shao, Q.; Hu, Y.; Jin, J.; Yin, Y.; Zhang, H.; Cai, C. Direct electrochemistry of glucose oxidase assembled on graphene and application to glucose detection. Electrochim. Acta 2010, 55, 8606–8614. [Google Scholar] [CrossRef]
- Kowalewska, B.; Jakubow, K. The impact of immobilization process on the electrochemical performance, bioactivity and conformation of glucose oxidase enzyme. Sens. Actuators B Chem. 2017, 238, 852–861. [Google Scholar] [CrossRef]
- Wen, D.; Liu, Y.; Yang, G.; Dong, S. Electrochemistry of glucose oxidase immobilized on the carbon nanotube wrapped by polyelectrolyte. Electrochim. Acta 2007, 52, 5312–5317. [Google Scholar] [CrossRef]
- Wu, W.-C.; Huang, J.-L.; Tsai, Y.-C. Direct electron transfer and biosensing of glucose oxidase immobilized at multiwalled carbon nanotube-alumina-coated silica modified electrode. Mater. Sci. Eng. C 2012, 32, 983–987. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electro-chemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef] [PubMed]
- El-Nahass, M.; Zeyada, H.; Aziz, M.; Makhlouf, M. Optical absorption of tetraphenylporphyrin thin films in UV–vis–NIR region. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Jhonsi, M.A.; Renganathan, R. Photoinduced Electron Transfer from Meso-Tetrakis (N-methylpyridyl)porphyrin (TMPyP) to Colloidal CdS. Z. Phys. Chem. 2008, 222, 1601–1610. [Google Scholar] [CrossRef]
- Baghayeri, M.; Veisi, H.; Ghanei-Motlagh, M. Amperometric glucose biosensor based on immobilization of glucose oxidase on a magnetic glassy carbon electrode modified with a novel magnetic nanocomposite. Sens. Actuators B Chem. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, S.H.; Kang, Y.C.; Lee, J.-K. Eco-Friendly Composite of Fe3O4-Reduced Graphene Oxide Particles for Efficient Enzyme Immobilization. ACS Appl. Mater. Interfaces 2017, 9, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Donoso, J.M.; Monrás, J.P.; Bravo, D.; Aguirre, A.; Quest, A.F.; Osorio-Roman, I.O.; Aroca, R.F.; Chasteen, T.G.; Vásquez, C.C. Biomimetic, Mild Chemical Synthesis of CdTe-GSH Quantum Dots with Improved Biocompatibility. PLoS ONE 2012, 7, e30741. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Kalisz, H.; Hendle, J.; Schmid, R.; Schomburg, D. Crystal Structure of Glucose Oxidase from Aspergillus niger Refined at 2.3 A Resolution. J. Mol. Biol. 1993, 229, 153–172. [Google Scholar] [CrossRef]
- Polcari, D.; Dauphin-Ducharme, P.; Mauzeroll, J. Scanning Electrochemical Microscopy: A Comprehensive Review of Experimental Parameters from 1989 to 2015. Chem. Rev. 2016, 116, 13234–13278. [Google Scholar] [CrossRef]
- Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Kisieliute, A.; Bucinskas, V.; Ramanavicius, A. Scanning electrochemical microscopy in the development of enzymatic sensors and immunosensors. Biosens. Bioelectron. 2019, 141, 111411. [Google Scholar] [CrossRef]
- Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Genys, P.; Ramanavicius, A. Evaluation of Enzymatic Kinetics of GOx-based Electrodes by Scanning Electrochemical Microscopy at Redox Competition Mode. Electroanalysis 2017, 29, 1532–1542. [Google Scholar] [CrossRef]
- Carriere, V.M.; Rodrigues, J.P.; Tan, C.; Arumugam, P.; Poh, S. In Vitro Electrochemical Detection of Hydrogen Peroxide in Activated Macrophages via a Platinum Microelectrode Array. Sensors 2021, 21, 5607. [Google Scholar] [CrossRef] [PubMed]
- Conzuelo, F.; Schulte, A.; Schuhmann, W. Biological imaging with scanning electrochemical microscopy. Proc. R. Soc. A Math. Phys. Eng. Sci. Med. 2018, 474, 20180409. [Google Scholar] [CrossRef] [PubMed]
- Raith, T.; Kröninger, A.; Mickert, M.; Gorris, H.H.; Matysik, F.-M. Enhanced resolution of generator-collector studies of enzymatic structures by means of hydrodynamic scanning electrochemical microscopy. Talanta 2020, 214, 120844. [Google Scholar] [CrossRef] [PubMed]
- Preet, A.; Lin, T.-E. A Review: Scanning Electrochemical Microscopy (SECM) for Visualizing the Real-Time Local Catalytic Activity. Catalysts 2021, 11, 594. [Google Scholar] [CrossRef]
- Ganiga, M.; Cyriac, J. An ascorbic acid sensor based on cadmium sulphide quantum dots. Anal. Bioanal. Chem. 2016, 408, 3699–3706. [Google Scholar] [CrossRef]
- Izadyar, A.; Ni Van, M.; Rodriguez, K.A.; Seok, I.; Hood, E.E. A bienzymatic amperometric glucose biosensor based on using a novel recombinant Mn peroxidase from corn and glucose oxidase with a Nafion membrane. J. Electroanal. Chem. 2021, 895, 115387. [Google Scholar] [CrossRef]
- Bahar, T.; Yazici, M.S. Immobilized glucose oxidase biofuel cell anode by MWCNTs, ferrocene, and polyethylenimine: Electrochemical performance. Asia-Pac. J. Chem. Eng. 2018, 13, e2149. [Google Scholar] [CrossRef]
- Khan, H.; Choi, J.H.; Ullah, A.; Kim, Y.H.; Kim, G.M. Continuous Determination of Glucose Using a Membraneless, Microfluidic Enzymatic Biofuel Cell. Micromachines 2020, 11, 1129. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Nguyen, N.-T.; Chan, S.H. A review on membraneless laminar flow-based fuel cells. Int. J. Hydrogen Energy 2011, 36, 5675–5694. [Google Scholar] [CrossRef] [Green Version]
- Arjun, A.M.; Vimal, M.; Sandhyarani, N. A hybrid hydrogel separated biofuel cell with a novel enzymatic anode and glucose tolerant cathode. Int. J. Hydrogen Energy 2019, 44, 27056–27066. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Yu, C.; Dong, J.; Peng, R.; Hu, Z.; Jiao, S. Direct electrochemistry and bioelectrocatalysis of glucose oxidase in CS/CNC film and its application in glucose biosensing and biofuel cells. RSC Adv. 2017, 10, 4572–4579. [Google Scholar] [CrossRef] [Green Version]
- Renaud, L.; Selloum, D.; Tingry, S. Xurography for 2D and multi-level glucose/O2 microfluidic biofuel cell. Microfluid. Nanofluidics 2015, 18, 1407–1416. [Google Scholar] [CrossRef]
- Galindo, R.; Dector, A.; Arriaga, L.; Gutiérrez, S.; Herrasti, P. Maghemite as a catalyst for glucose oxidation in a microfluidic fuel cell. J. Electroanal. Chem. 2012, 671, 38–43. [Google Scholar] [CrossRef]
- Zebda, A.; Renaud, L.; Cretin, M.; Pichot, F.; Innocent, C.; Ferrigno, R.; Tingry, S. A microfluidic glucose biofuel cell to generate micropower from enzymes at ambient temperature. Electrochem. Commun. 2009, 11, 592–595. [Google Scholar] [CrossRef]
- Khan, H.; Kim, C.M.; Kim, S.Y.; Goel, S.; Dwivedi, P.K.; Sharma, A.; Kim, Y.H.; Kim, G.M. Correction to: Fabrication of Enzymatic Biofuel Cell with Electrodes on Both Sides of Microfluidic Channel. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 655. [Google Scholar] [CrossRef] [Green Version]
- Escalona-Villalpando, R.; Dector, A.; Dector, D.; Moreno-Zuria, A.; Durón-Torres, S.; Galván-Valencia, M.; Arriaga, L.; Ledesma-García, J. Glucose microfluidic fuel cell using air as oxidant. Int. J. Hydrogen Energy 2016, 41, 23394–23400. [Google Scholar] [CrossRef]
- Zebda, A.; Gondran, C.; Le Goff, A.; Holzinger, M.; Cinquin, P.; Cosnier, S. Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube-enzyme electrodes. Nat. Commun. 2011, 2, 370. [Google Scholar] [CrossRef] [Green Version]
- Reuillard, B.; Le Goff, A.; Agnès, C.; Holzinger, M.; Zebda, A.; Gondran, C.; Elouarzaki, K.; Cosnier, S. High power enzymatic biofuel cell based on naphthoquinone-mediated oxidation of glucose by glucose oxidase in a carbon nanotube 3D matrix. Phys. Chem. Chem. Phys. 2013, 15, 4892–4896. [Google Scholar] [CrossRef]


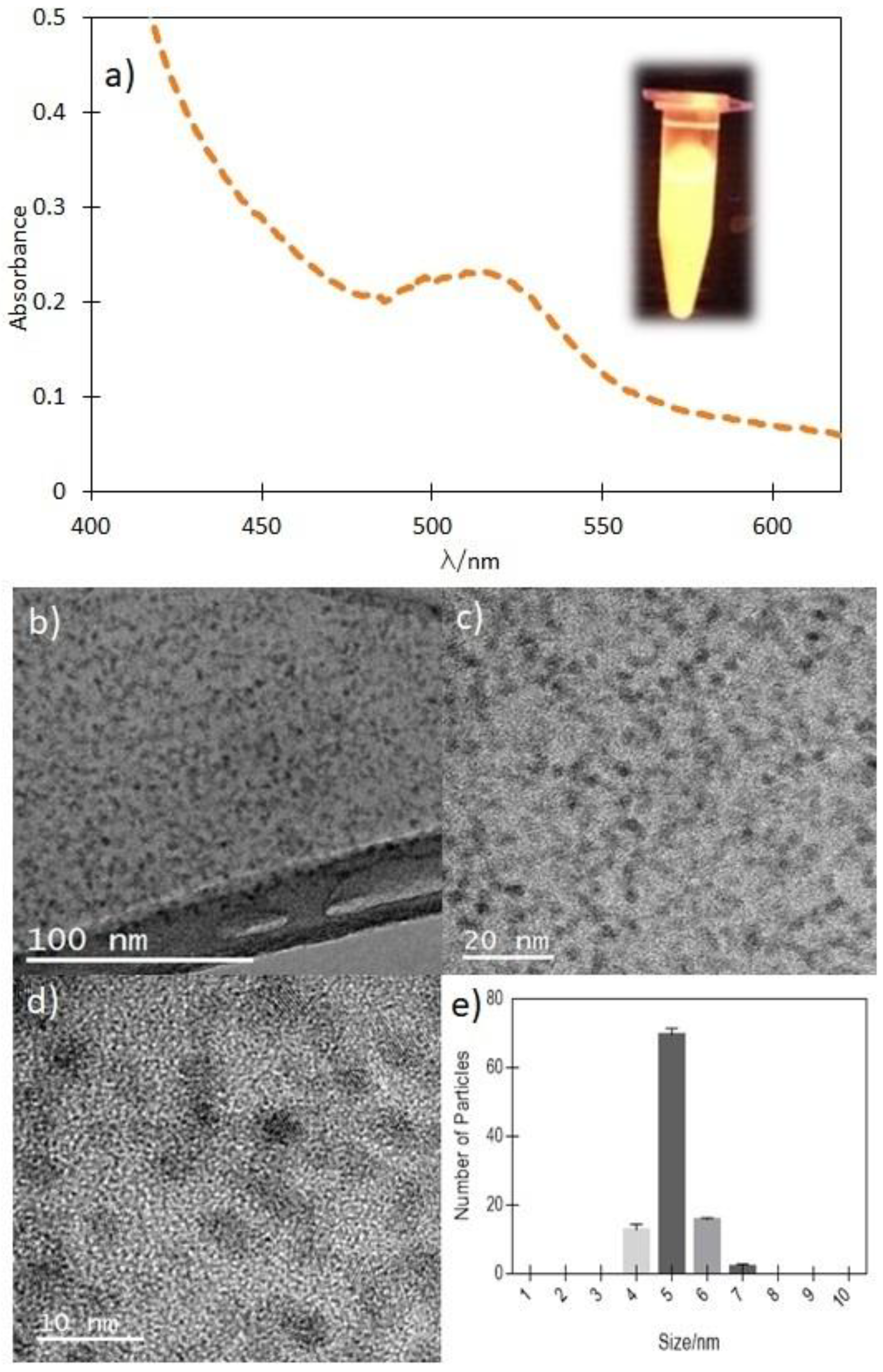



| Sample | Hydrodynamical Ratio/nm | ζ/mV |
|---|---|---|
| TMPyP | - | −4.3 |
| GSH-CdTeQD | 7 | −29.3 |
| TMPyP/GSH-CdTeQD | 328 | −33.9 |
| Element | Weight % | Atomic % |
|---|---|---|
| C | 31 ± 3 | 43 ± 4 |
| O | 36 ± 4 | 37 ± 4 |
| Na | 24 ± 2 | 17 ± 1 |
| S | 2 ± 0.1 | 1 ± 0.1 |
| Cl | 4 ± 0.2 | 2 ± 0.1 |
| Cd | 3 ± 0.3 | 0.4 ± 0.04 |
| Te | 1 ± 0.4 | 0.1 ± 0.05 |
| Anode | OCP/ V | Imax/ mA cm−2 | Wmax/ mW cm−2 | Cell Type | Ref |
|---|---|---|---|---|---|
| Pyr-Carboxilic Acid MWCNTs | 0.140 | 0.006 | 0.06 | Hidrogel | [92] |
| Chitosan-CarbonNanoChips | 0.59 | 0.434 | 0.055 | S. C. 1 | [93] |
| Vinil/Au | 0.333 | 0.045 | 0.013 | Microfluidic | [94] |
| Vulcan/Maghemite/GOx | 0.3 | N. E. | 0.03 | Microfluidic | [95] |
| Au | 0.3 | 0.69 | 0.11 | Microfluidic | [96] |
| ITO/MWCNTs/CrosslinkedGOx | 0.56 | 0.45 | 0.153 | Microfluidic | [97] |
| MWCNTs/GOx/GA | 0.72 | 2.45 | 0.61 | Microfluidic | [98] |
| MWCNTs/GOx | 0.95 | N. E. | 1.25 | S. C. 1 | [99] |
| MWCNTs/Naftoquinone/GOx | 0.76 | 4.47 | 1.54 | S. C. 1 | [100] |
| TMPyP/GSHCdTeQD/GOx | 0.571 | 7.53 | 2.30 | Microfluidic | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-López, D.; Galván-Valencia, M.; Rojas-de Soto, I.; Escalona-Villalpando, R.A.; Ledesma-García, J.; Durón-Torres, S. Immobilization of Glucose Oxidase on Glutathione Capped CdTe Quantum Dots for Bioenergy Generation. Catalysts 2022, 12, 1659. https://doi.org/10.3390/catal12121659
Lozano-López D, Galván-Valencia M, Rojas-de Soto I, Escalona-Villalpando RA, Ledesma-García J, Durón-Torres S. Immobilization of Glucose Oxidase on Glutathione Capped CdTe Quantum Dots for Bioenergy Generation. Catalysts. 2022; 12(12):1659. https://doi.org/10.3390/catal12121659
Chicago/Turabian StyleLozano-López, Daniel, Marisol Galván-Valencia, Ivone Rojas-de Soto, Ricardo A. Escalona-Villalpando, Janet Ledesma-García, and Sergio Durón-Torres. 2022. "Immobilization of Glucose Oxidase on Glutathione Capped CdTe Quantum Dots for Bioenergy Generation" Catalysts 12, no. 12: 1659. https://doi.org/10.3390/catal12121659
APA StyleLozano-López, D., Galván-Valencia, M., Rojas-de Soto, I., Escalona-Villalpando, R. A., Ledesma-García, J., & Durón-Torres, S. (2022). Immobilization of Glucose Oxidase on Glutathione Capped CdTe Quantum Dots for Bioenergy Generation. Catalysts, 12(12), 1659. https://doi.org/10.3390/catal12121659