Enhancing the Thermal Stability of Glutathione Bifunctional Synthase by B-Factor Strategy and Un/Folding Free Energy Calculation
Abstract
1. Introduction
2. Results
2.1. Model Construction and MD of GshFSA
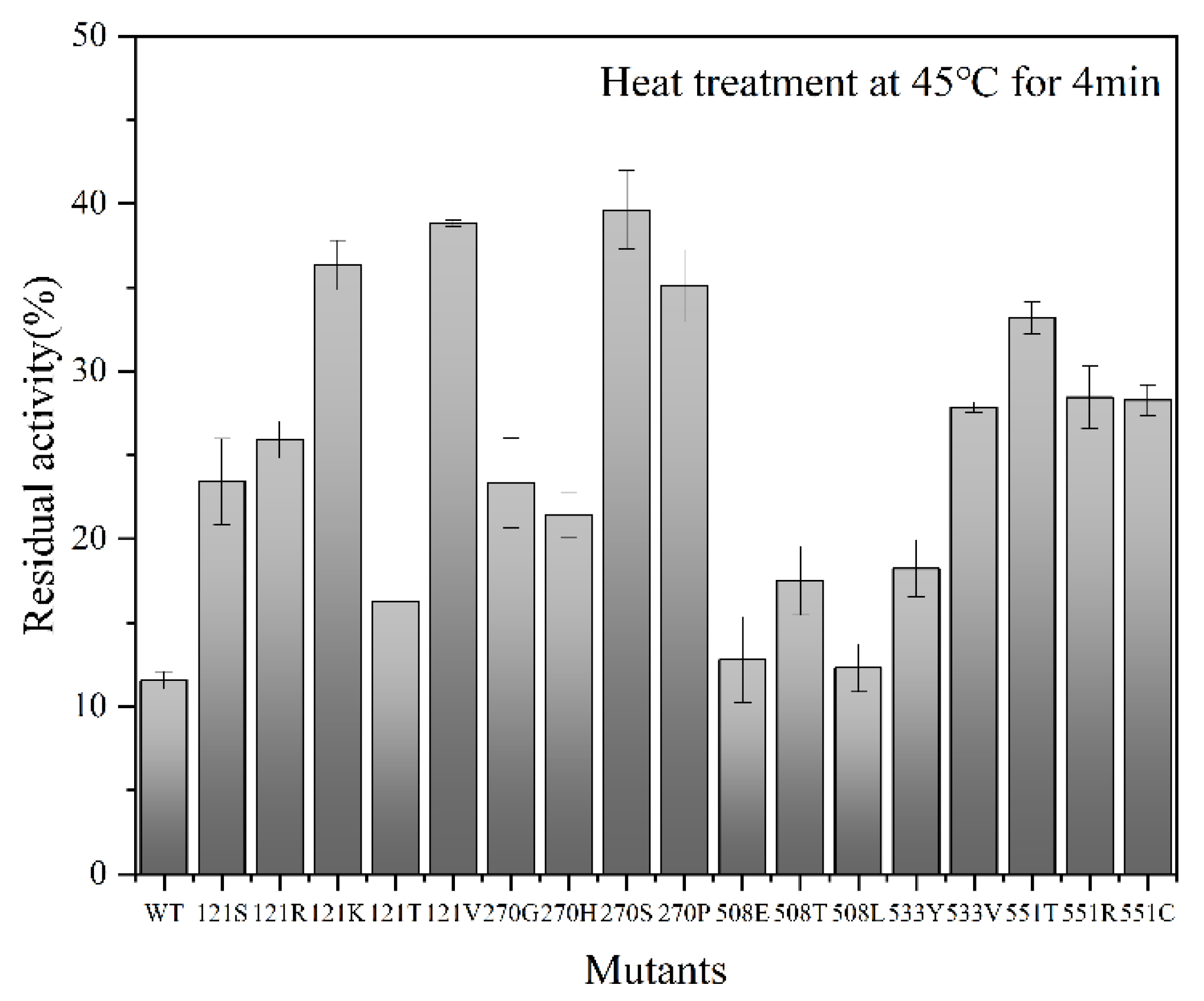
2.2. Screening Results of Point-Saturated Mutant Libraries
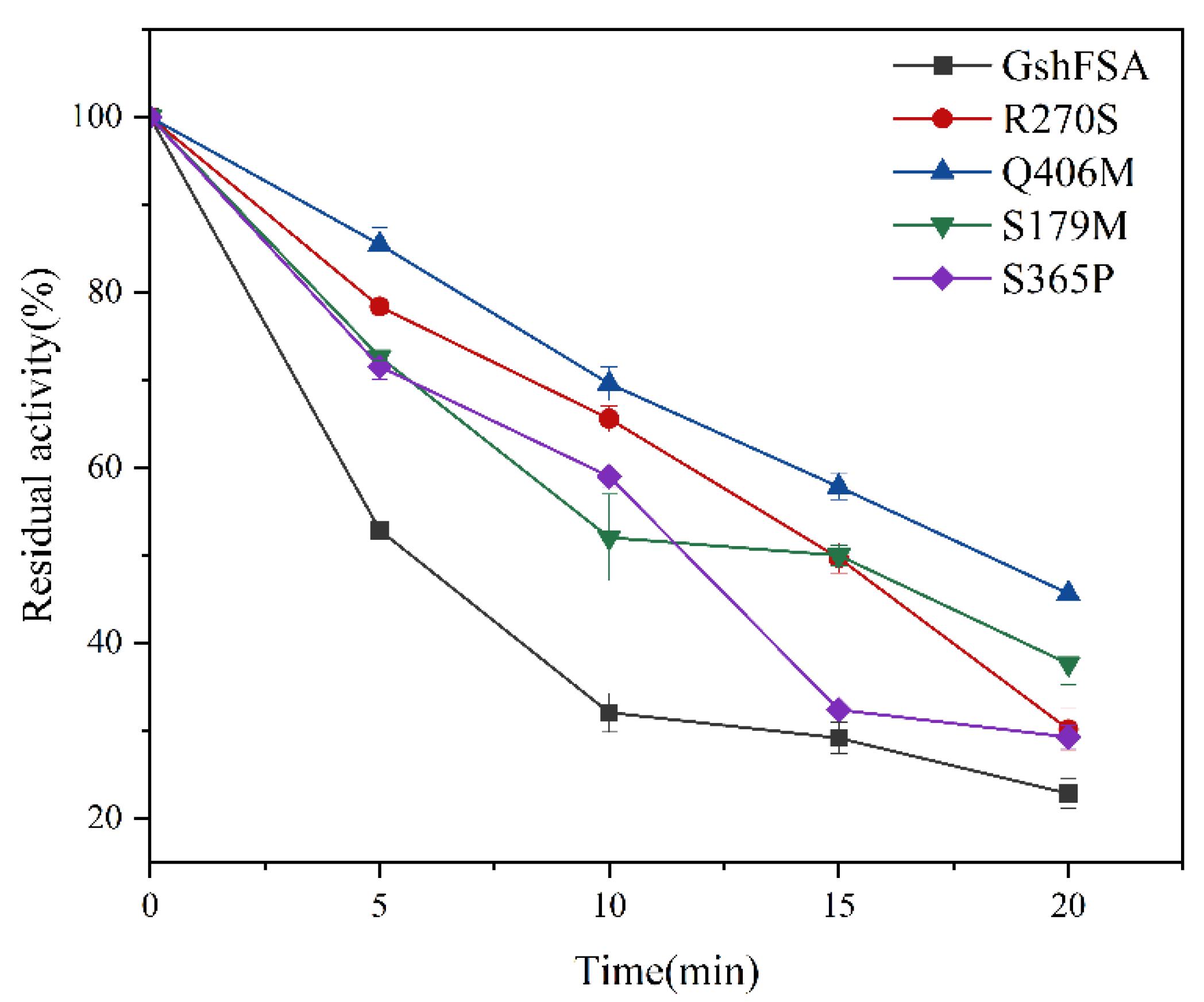
2.3. Calculation of Un/Folding Free Energy and Modification of Single Variants
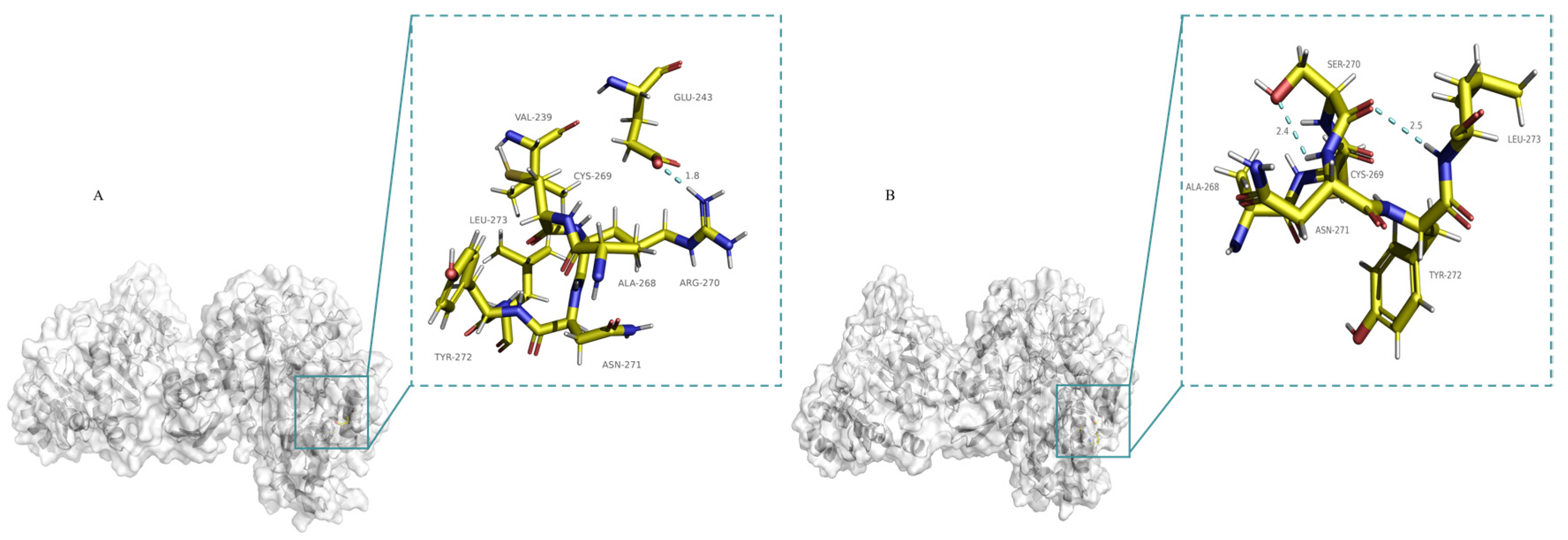
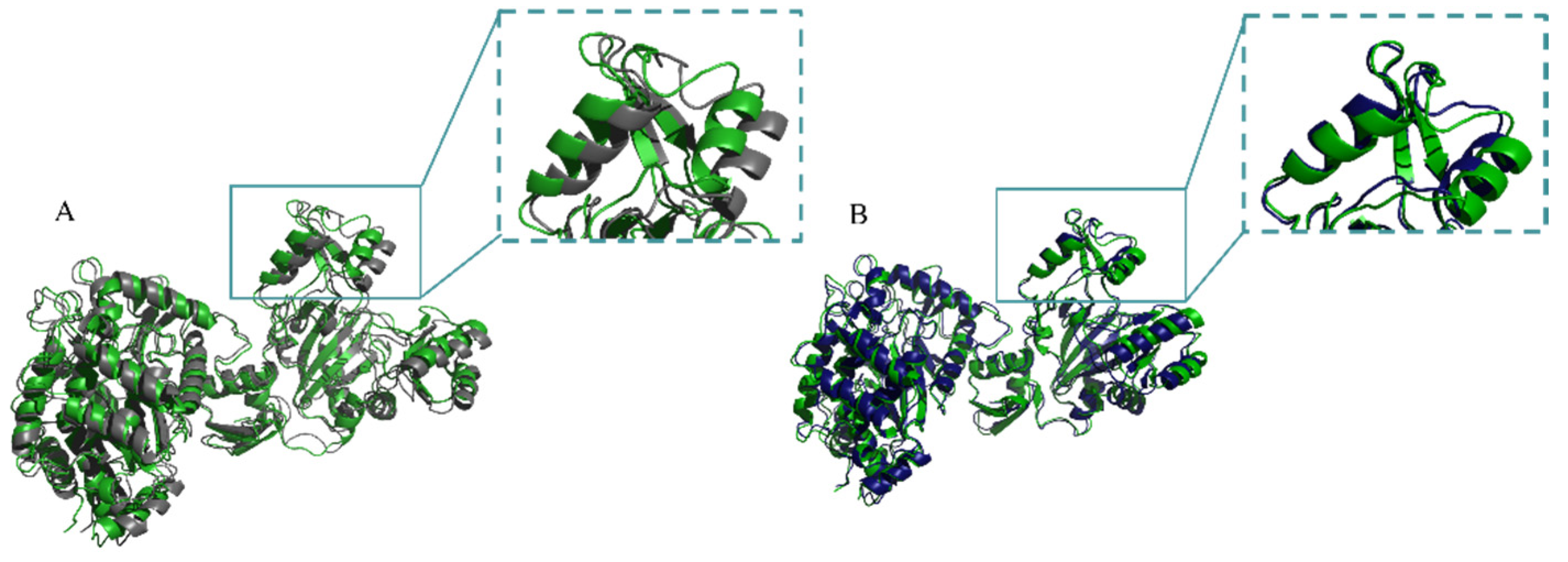
2.4. Mechanistic Analysis
3. Materials and Methods
3.1. Strains and Culture Medium
3.2. Construction of Mutants
3.3. Enzyme Activity and Thermal Stability Assay
3.4. High Throughput Screening
3.5. Model Construction and Molecular Dynamics Simulation
3.6. Un/Folding Energy Calculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, Y.; Ding, F.; Zhu, X.; Yang, L.; Zou, P.; Rao, H.; Zhao, Q.; Wang, X. Colorimetric determination of glutathione in human serum and cell lines by exploiting the peroxidase-like activity of CuS-polydopamine-Au composite. Anal. Bioanal. Chem. 2018, 410, 4805–4813. [Google Scholar] [CrossRef] [PubMed]
- Patzschke, A.; Steiger, M.G.; Holz, C.; Lang, C.; Mattanovich, D.; Sauer, M. Enhanced glutathione production by evolutionary engineering of Saccharomyces cerevisiae strains. Biotechnol. J. 2015, 10, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, G.; Chen, J. Glutathione: A review on biotechnological production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Wu, H.; Li, Z.; Ye, Q. Heterologous gshF gene expression in various vector systems in Escherichia coli for enhanced glutathione production. J. Biotechnol. 2015, 214, 63–68. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Huang, B.; Li, Z.; Ye, Q. One-pot synthesis of glutathione by a two-enzyme cascade using a thermophilic ATP regeneration system. J. Biotechnol. 2017, 241, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Janowiak, B.E.; Griffith, O.W. Glutathione synthesis in Streptococcus agalactiae: One protein accounts for γ-glutamylcysteine synthetase and glutathione synthetase activities. J. Biol. Chem. 2005, 280, 11829–11839. [Google Scholar] [CrossRef] [PubMed]
- Traxlmayr, M.W.; Obinger, C. Directed evolution of proteins for increased stability and expression using yeast display. Arch. Biochem. Biophys. 2012, 526, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tompa, D.R.; Gromiha, M.M.; Saraboji, K. Contribution of main chain and side chain atoms and their locations to the stability of thermophilic proteins. J. Mol. Graph. Model. 2016, 64, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Ulrich Hartl, F. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef]
- Sanchez-Ruiz, J.M. Protein kinetic stability. Biophys. Chem. 2010, 148, 1–15. [Google Scholar] [CrossRef]
- Blum, J.K.; Ricketts, M.D.; Bommarius, A.S. Improved thermostability of AEH by combining B-FIT analysis and structure-guided consensus method. J. Biotechnol. 2012, 160, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Radusky, L.G.; Cianferoni, D.; Serrano, L. FoldX 5.0: Working with RNA, small molecules and a new graphical interface. Bioinformatics 2019, 35, 4168–4169. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zou, G.; Liu, C.; Chai, S.; Yan, X.; Li, X.; Liu, R.; Yang, Y.; Zhou, Z. Improving the thermostability of a GH11 xylanase by directed evolution and rational design guided by B-factor analysis. Enzym. Microb. Technol. 2021, 143, 109720. [Google Scholar] [CrossRef]
- Bell, E.L.; Smithson, R.; Kilbride, S.; Foster, J.; Hardy, F.J.; Ramachandran, S.; Tedstone, A.A.; Haigh, S.J.; Garforth, A.A.; Day, P.J. Directed evolution of an efficient and thermostable PET depolymerase. Nat. Catal. 2022, 5, 673–681. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Pan, L.; Yang, L.; Li, H.; Ji, F.; Zhang, Y.; Tang, H.; Yang, D. Improving the thermostability of alginate lyase FlAlyA with high expression by computer-aided rational design for industrial preparation of alginate oligosaccharides. Front. Bioeng. Biotechnol. 2022, 10, 1011273. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.A.; Krause, A.; Arnold, F.H. Navigating the protein fitness landscape with Gaussian processes. Proc. Natl. Acad. Sci. USA 2013, 110, E193–E201. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Garg, P.; Vanamamalai, V.K.; Jali, I.; Sharma, S. In silico prediction of the animal susceptibility and virtual screening of natural compounds against SARS-CoV-2: Molecular dynamics simulation based analysis. Front. Genet. 2022, 13, 906955. [Google Scholar] [CrossRef]
- Lu, T.; Tan, H.; Lee, D.; Chen, G.; Jia, Z. New insights into the activation of Escherichia coli tyrosine kinase revealed by molecular dynamics simulation and biochemical analysis. Biochemistry 2009, 48, 7986–7995. [Google Scholar] [CrossRef]
- Musil, M.; Stourac, J.; Bendl, J.; Brezovsky, J.; Prokop, Z.; Zendulka, J.; Martinek, T.; Bednar, D.; Damborsky, J. FireProt: Web server for automated design of thermostable proteins. Nucleic Acids Res. 2017, 45, W393–W399. [Google Scholar] [CrossRef]
- Bednar, D.; Beerens, K.; Sebestova, E.; Bendl, J.; Khare, S.; Chaloupkova, R.; Prokop, Z.; Brezovsky, J.; Baker, D.; Damborsky, J. FireProt: Energy-and evolution-based computational design of thermostable multiple-point mutants. PLoS Comput. Biol. 2015, 11, e1004556. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Chen, S.; Zhao, X.; Nie, Y.; Xu, Y. Computation-aided engineering of starch-debranching pullulanase from Bacillus thermoleovorans for enhanced thermostability. Appl. Microbiol. Biotechnol. 2020, 104, 7551–7562. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, H.; Zhao, Y.; Zuo, L. BayeStab: Predicting effects of mutations on protein stability with uncertainty quantification. Protein Sci. 2022, 31, e4467. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Gong, J.-S.; Qin, A.; Li, H.; Li, H.; Qin, J.; Qian, J.-Y.; Xu, Z.-H.; Shi, J.-S. A combination of bioinformatics analysis and rational design strategies to enhance keratinase thermostability for efficient biodegradation of feathers. Sci. Total Environ. 2022, 818, 151824. [Google Scholar] [CrossRef] [PubMed]




| GshFSA | T1/2 at 40 °C (min) | Special Enzyme Activity (U/mg) |
|---|---|---|
| Wild type | 5.69 | 1.47 |
| R270S | 14.88 | 1.53 |
| Mutants | FoldX-ΔΔG (kcal/mol) | Rosetta_ddg-ΔΔG (kcal/mol) | t1/2 at 40 °C (min) |
|---|---|---|---|
| S179M | −3.88 | −2.9 | 15.18 |
| S365P | −2.87 | −4.61 | 11.69 |
| I71W | −2.62 | −10.81 | 7.74 |
| S266M | −2.51 | −6.55 | 6.67 |
| N210G | −2.01 | −9.11 | 6.47 |
| Q206W | −1.94 | −7.33 | 8.17 |
| N444W | −1.91 | −7.85 | 4.66 |
| Q406M | −1.82 | −8.72 | 18.21 |
| L434F | −1.81 | −5.44 | 6.69 |
| A199F | −1.74 | −9.43 | 6.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Sun, H.; Jiang, Q.; Zheng, R.; Wang, Q.; Zhang, Q.; Liu, L.; Cao, H. Enhancing the Thermal Stability of Glutathione Bifunctional Synthase by B-Factor Strategy and Un/Folding Free Energy Calculation. Catalysts 2022, 12, 1649. https://doi.org/10.3390/catal12121649
Zhu W, Sun H, Jiang Q, Zheng R, Wang Q, Zhang Q, Liu L, Cao H. Enhancing the Thermal Stability of Glutathione Bifunctional Synthase by B-Factor Strategy and Un/Folding Free Energy Calculation. Catalysts. 2022; 12(12):1649. https://doi.org/10.3390/catal12121649
Chicago/Turabian StyleZhu, Wenlong, Heming Sun, Qixuan Jiang, Ruonan Zheng, Qingyun Wang, Qinfei Zhang, Luo Liu, and Hui Cao. 2022. "Enhancing the Thermal Stability of Glutathione Bifunctional Synthase by B-Factor Strategy and Un/Folding Free Energy Calculation" Catalysts 12, no. 12: 1649. https://doi.org/10.3390/catal12121649
APA StyleZhu, W., Sun, H., Jiang, Q., Zheng, R., Wang, Q., Zhang, Q., Liu, L., & Cao, H. (2022). Enhancing the Thermal Stability of Glutathione Bifunctional Synthase by B-Factor Strategy and Un/Folding Free Energy Calculation. Catalysts, 12(12), 1649. https://doi.org/10.3390/catal12121649







