CO2 Hydrogenation to Renewable Methane on Ni/Ru Modified ZSM-5 Zeolites: The Role of the Preparation Procedure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Properties
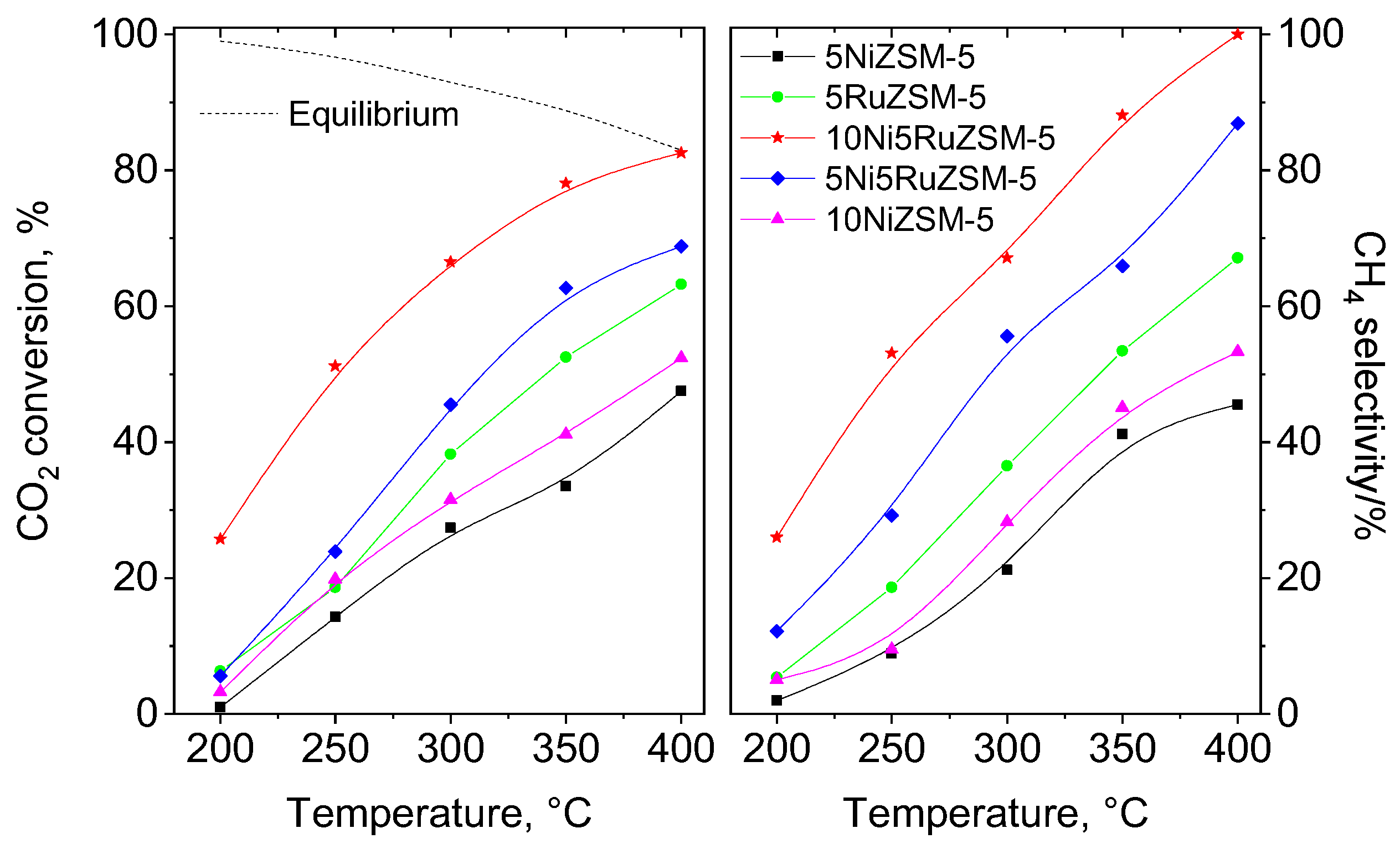
2.2. Catalytic Activity for CO2 Hydrogenation to Methane
3. Experimental
3.1. Materials
3.2. Preparation of Seed Crystals
3.3. Synthesis of ZSM-5 Zeolite
3.4. Modification with Ni or/and Ru Nanoparticles of ZSM-5 Zeolite
3.5. Characterization
3.6. Catalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.-M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; et al. High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature 2008, 453, 379–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.F.; Zantye, M.S.; Kazi, M.-K. Challenges and opportunities in carbon capture, utilization and storage: A process systems engineering perspective. Comput. Chem. Eng. 2022, 166, 107925. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D.; de Coninck, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Centre for Low Carbon Futures: New York, NY, USA, 2011. [Google Scholar]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef] [Green Version]
- Von der Assen, N.; Voll, P.; Peters, M.; Bardow, A. Life cycle assessment of CO2 capture and utilization: A tutorial review. Chem. Soc. Rev. 2014, 43, 7982–7994. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2020, 368, 2–19. [Google Scholar] [CrossRef]
- Wulf, C.; Linssen, J.; Zapp, P. Power-to-Gas—Concepts, Demonstration, and Prospects. In Hydrogen Supply Chains; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–345. [Google Scholar]
- Munnik, P.; Velthoen, M.E.Z.; de Jongh, P.E.; de Jong, K.P.; Gommes, C.J. Nanoparticle Growth in Supported Nickel Catalysts during Methanation Reaction-Larger is Better. Angew. Chem. 2014, 126, 9647–9651. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Tuning Zeolite Properties towards CO2 Methanation: An Overview. ChemCatChem 2019, 11, 2388–2400. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Takht Ravanchi, M. Carbon dioxide utilization for methane production: A thermodynamic analysis. J. Pet. Sci. Eng. 2015, 134, 14–22. [Google Scholar] [CrossRef]
- Held, M.; Schollenberger, D.; Sauerschell, S.; Bajohr, S.; Kolb, T. Power-to-Gas: CO2 Methanation Concepts for SNG Production at the Engler-Bunte-Institut. Chem. Ing. Tech. 2020, 92, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Younas, M.; Loong Kong, L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Paviotti, M.; Faroldi, B.; Cornaglia, L. Ni-based catalyst over rice husk-derived silica for the CO2 methanation reaction: Effect of Ru addition. J. Environ. Chem. Eng. 2021, 9, 105173. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, Á.; Oykova, M.; Lazarova, H.; Koseva, N.; Mihályi, M.R.; Shestakova, P. Selective production of phenol on bifunctional, hierarchical ZSM-5 zeolites. Molecules 2021, 26, 3576. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, Á.; Popova, M.; Mavrodinova, V.; Urbán, M.; Kiricsi, I.; Minchev, C. Synthesis and characterization of Ni-MCM-41 materials with spherical morphology and their catalytic activity in toluene hydrogenation. Microporous Mesoporous Mater. 2007, 99, 149–158. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO2 methanation on Ni-Ru/g-Al2O3 via modulating impregnation sequence and controlling surface active species. RSC Adv. 2014, 4, 16472–16479. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickelmetal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 reduction on supported Ru/Al2O3 catalysts: Cluster size dependence of product selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2016, 293–294, 89–96. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davo-Quinonero, A.; Bailon-García, E.; Lozano-Castello, D.; Gonzalez-Marcos, J.A.; Bueno-Lopez, A.; Gonzalez-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2020, 356, 419–432. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The difference of roles of alkaline-earth metal oxides on silica-supported nickel catalysts for CO2 methanation. RSC Adv. 2014, 4, 58171–58177. [Google Scholar] [CrossRef]
- Mutz, B.; Sprenger, P.; Wang, W.; Wang, D.; Kleist, W.; Grunwaldt, J.-D. Operando Raman spectroscopy on CO2 methanation over alumina-supported Ni, Ni3Fe and NiRh0.1 catalysts: Role of carbon formation as possible deactivation pathway. Appl. Catal. A Gen. 2018, 556, 160–171. [Google Scholar] [CrossRef]
- Alrafei, B.; Polaert, I.; Ledoux, A.; Azzolina-Jury, F. Remarkably stable and efficient Ni and Ni-Co catalysts for CO2 methanation. Catal. Today 2020, 346, 23–33. [Google Scholar] [CrossRef]
- Sache, E.; le Pastor-Perez, L.; Haycock, B.J.; Villora-Pico, J.J.; Sepúlveda-Escribano, A.; Reina, T.R. Switchable catalysts for chemical CO2 recycling: A step forward in the methanation and reverse water–gas shift reactions. ACS Sustain. Chem. Eng. 2020, 8, 4614–4622. [Google Scholar] [CrossRef]
- Ye, T.; Chen, Z.; Chen, Y.; Xie, H.; Zhong, Q.; Qu, H. Green synthesis of ZSM-5 zeolite for selective catalytic reduction of NO via template-free method from tailing residue. J. Environ. Chem. Eng. 2022, 10, 107766. [Google Scholar] [CrossRef]
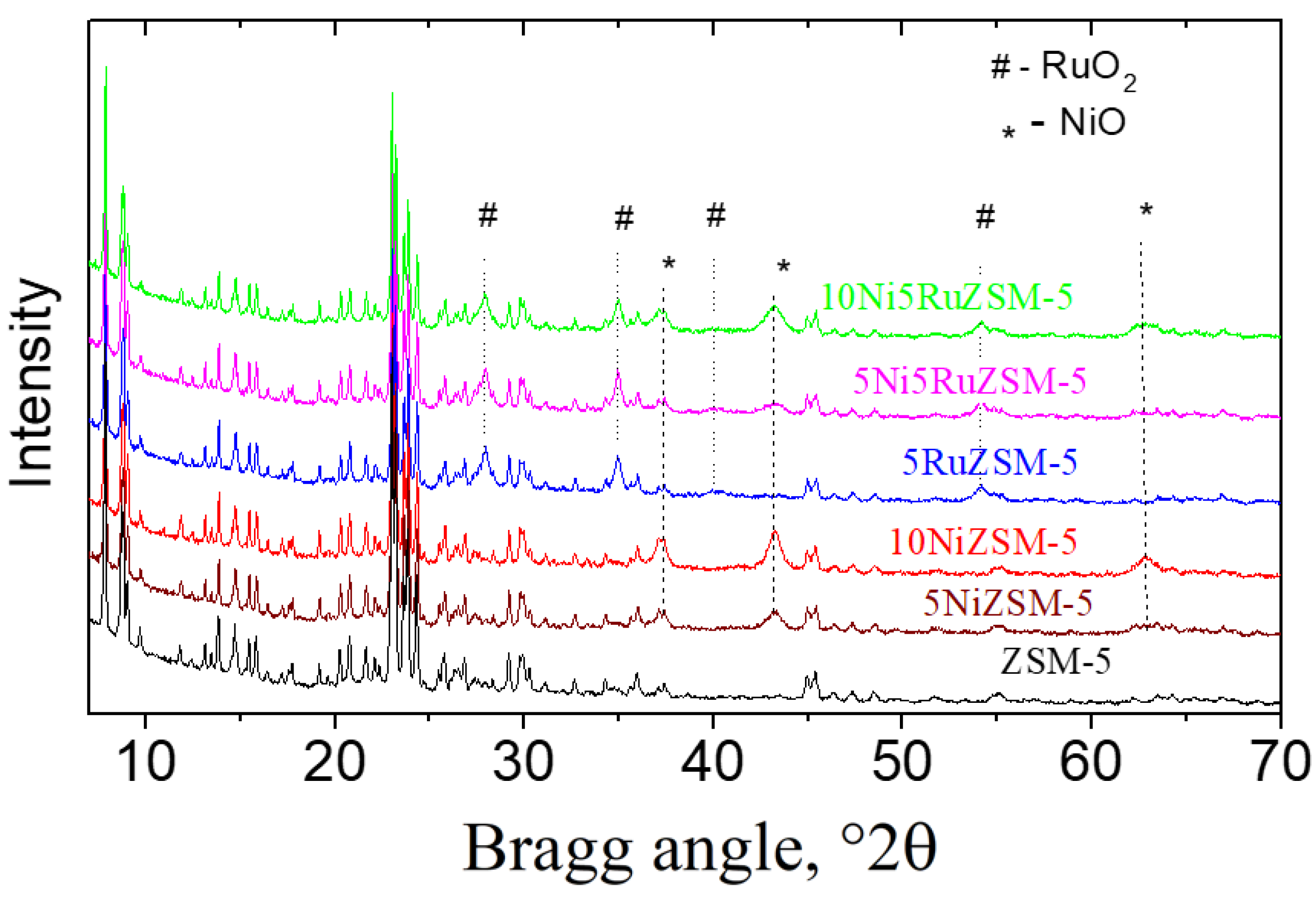

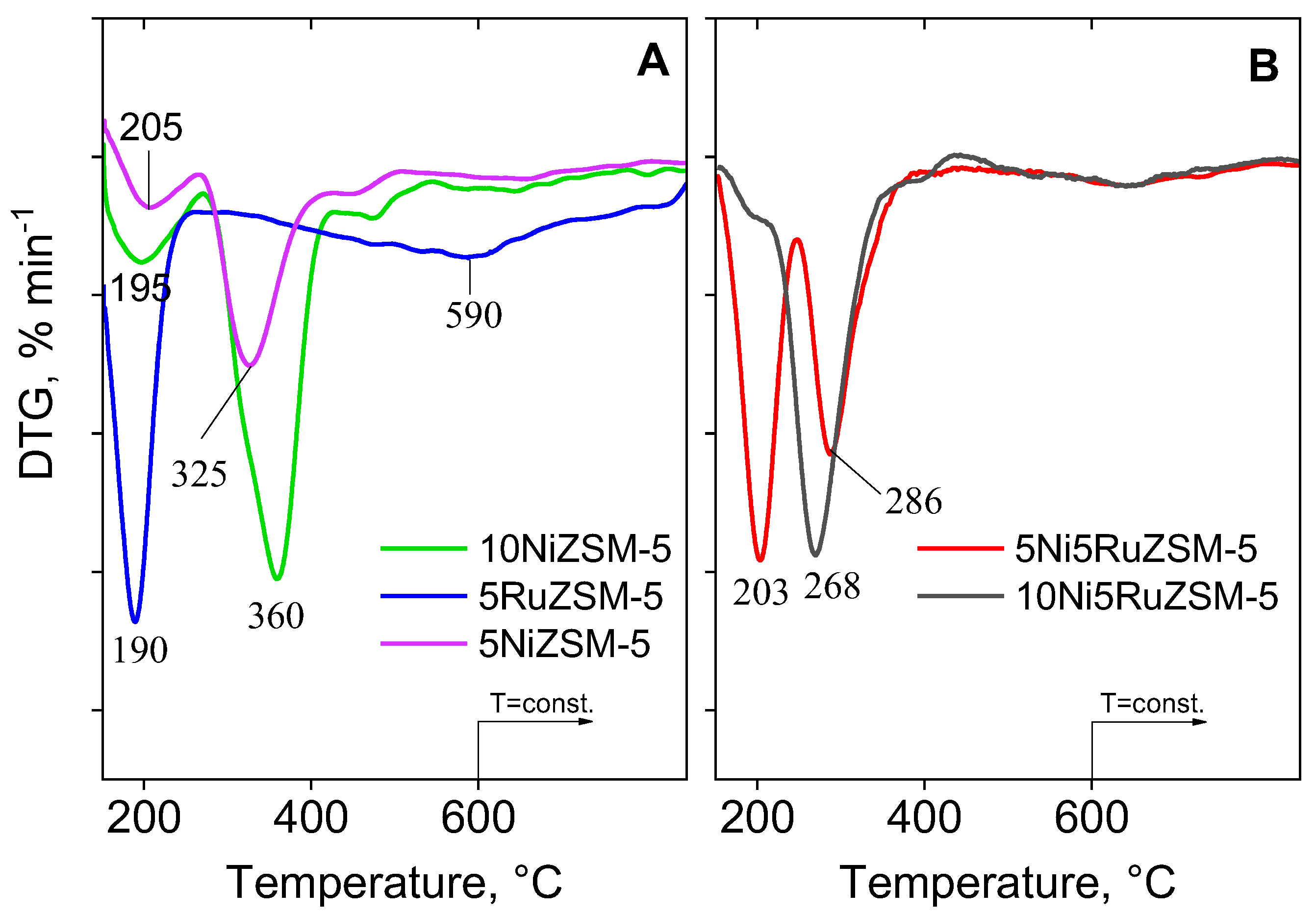
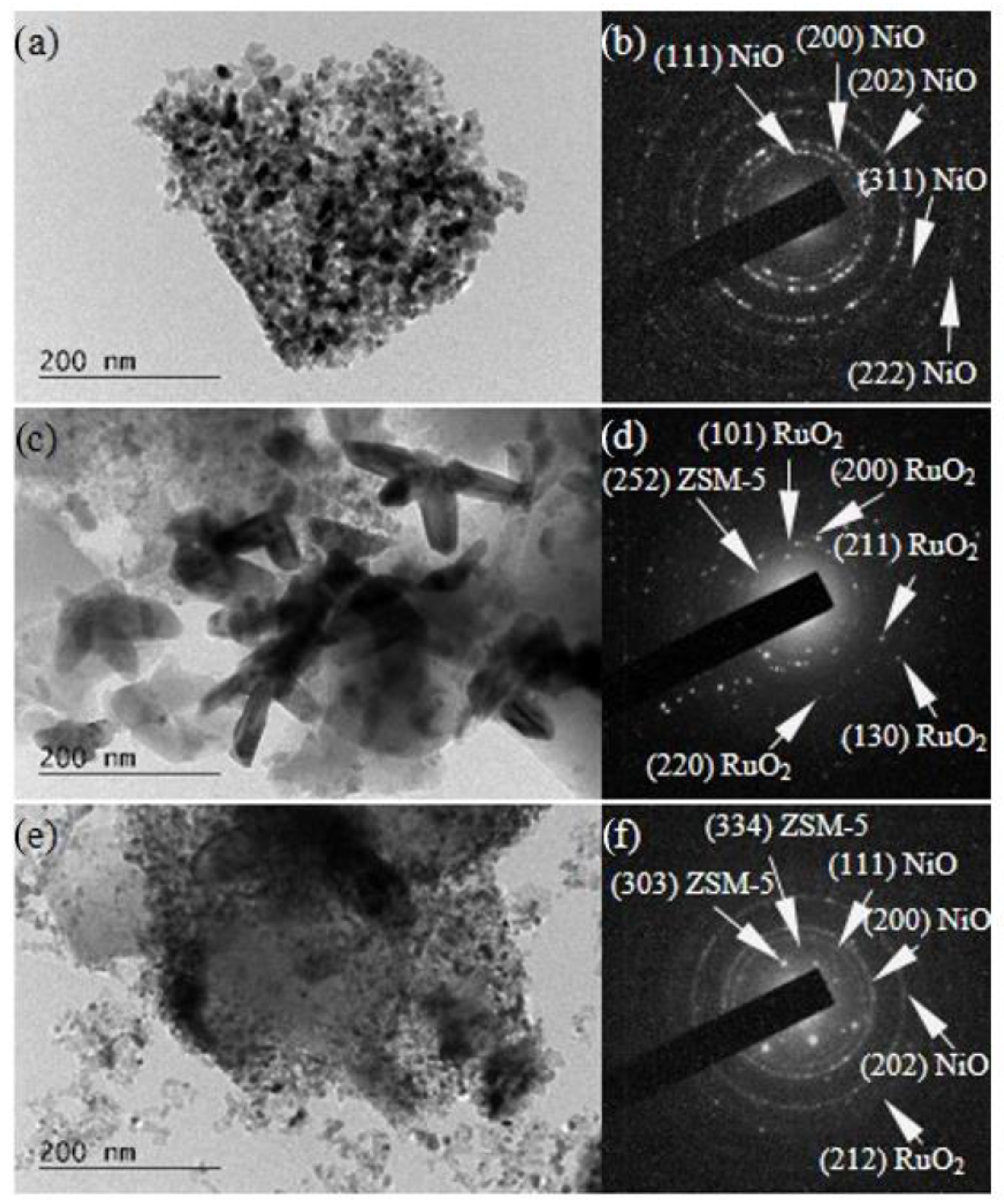
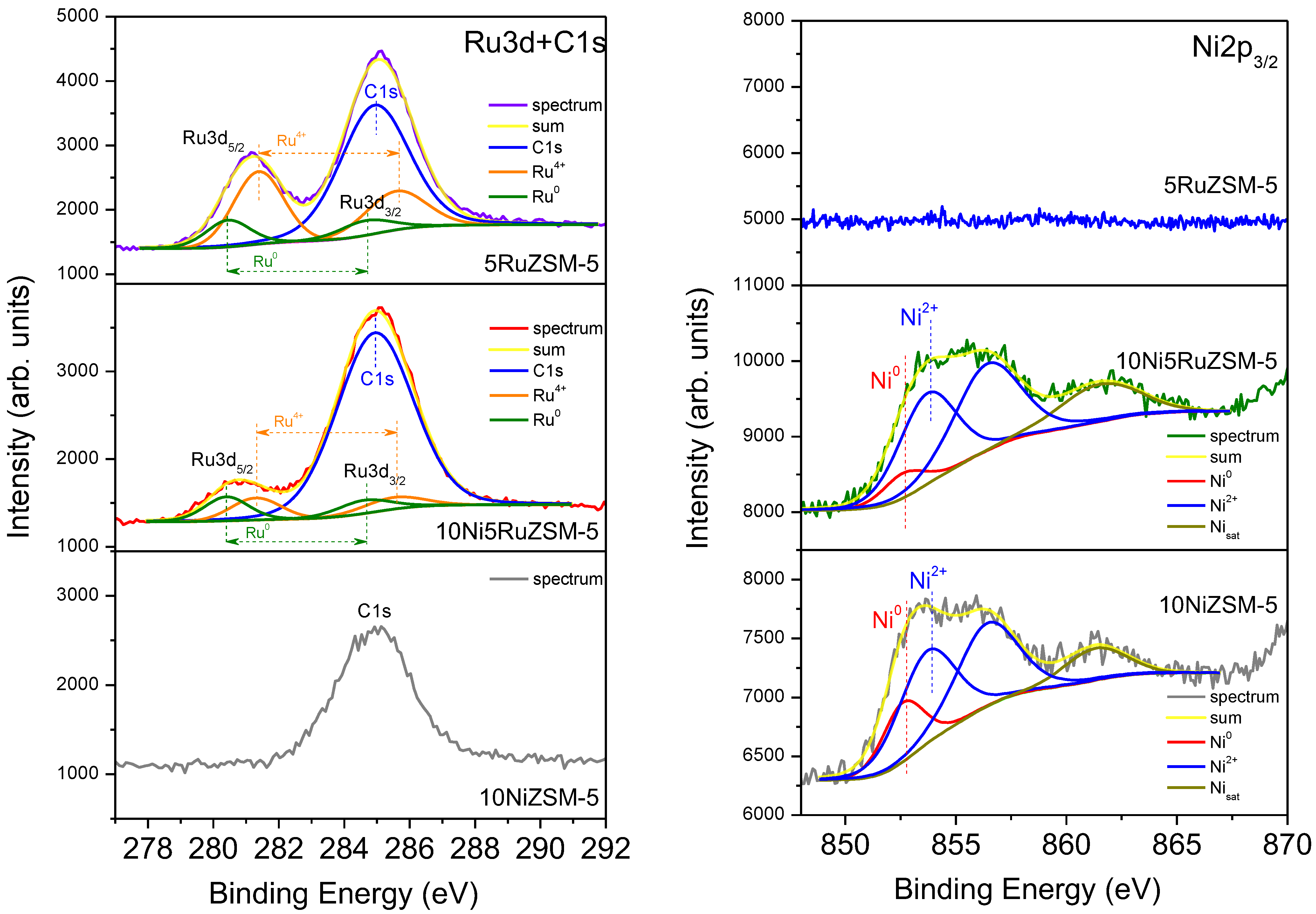
| Sample | Phase Composition | Cell Parameters, Å | Crystallite Size, nm |
|---|---|---|---|
| 10NiZSM-5 | NiO (Fm-3m) | 4.1815 (7) | 16 |
| Spent 10NiZSM-5 | Ni (Fm-3m) | 3.5241 (4) | 33 |
| 5NiZSM-5 | NiO (Fm-3m) | 4.184 (2) | 13 |
| Spent 5NiZSM-5 | Ni (Fm-3m) | 3.534 (1) | 15 |
| 5RuZSM-5 | RuO2 (P42/mnm) | 4.511 (1) 3.114 (1) | 19 |
| Spent 5RuZSM-5 | Ru (P63/mmc) | 2.7098 (8) 4.285 (2) | 19 |
| 5Ni5RuZSM-5 | NiO (Fm-3m) RuO2 (P42/mnm) | 4.186 (3) 4.511 (1) 3.112 (1) | 9 21 |
| Spent 5Ni5RuZSM-5 | Ni (Fm-3m) Ru (P63/mmc) | 3.533 (2) 2.699 (2) 4.271 (6) | 14 9 |
| 10Ni5RuZSM-5 | NiO (Fm-3m) RuO2 (P42/mnm) | 4.186 (1) 4.514 (1) 3.110 (1) | 10 21 |
| Spent 10Ni5RuZSM-5 | Ni (Fm-3m) Ru (P63/mmc) | 3.5318 (9) 2.696 (4) 4.26 (1) | 19 7 |
| Samples | SSA 1 (m2/g) | PV 2 (cm3/g) | Micropore Vol. 3 (cm3/g) | Micropore Surf. Area 3 (m2/g) |
|---|---|---|---|---|
| ZSM-5 | 307 | 0.177 | 0.095 | 231 |
| 5NiZSM-5 | 315 | 0.200 | 0.105 | 256 |
| 10NiZSM-5 | 322 | 0.189 | 0.107 | 261 |
| 5RuZSM-5 | 337 | 0.189 | 0.116 | 282 |
| 5Ni5RuZSM-5 | 306 | 0.180 | 0.103 | 250 |
| 10Ni5RuZSM-5 | 215 | 0.162 | 0.065 | 161 |
| Samples | O (at.%) | Si (at.%) | Ni (at.%) | Al (at.%) | Ru (at.%) |
|---|---|---|---|---|---|
| 10NiZSM-5 | 58.5 | 32.2 | 3.9 | 5.4 | - |
| 5RuZSM-5 | 59.5 | 35.8 | - | 2.7 | 2.0 |
| 10Ni5RuZSM-5 | 54.0 | 29.1 | 6.8 | 8.9 | 1.2 |
| Sample | CH4 Selectivity, % |
|---|---|
| 5NiZSM-5 | 20 |
| 10NiZSM-5 | 19 |
| 5RuZSM-5 | 25 |
| 5Ni5RuZSM-5 | 29 |
| 10Ni5RuZSM-5 | 31 |
| Catalysts | Reaction Conditions | Catalytic Activity, % | Reference |
|---|---|---|---|
| Ni/SiO2 | T = 350 °C; 5 wt.% Ni; GHSV = 15,000 mL gcat−1 h−1 | X = 64.7%; SCH4 = 97.5% | [25] |
| Ni-Rh/Al2O3 | T = 400 °C; 3.1 wt.% Ni; 0.5 wt.% Rh; GHSV = 60,000 mL gcat−1 h−1 | X = 65%; SCH4 = 95% | [26] |
| Ni-Co/Al2O3 | T = 350 °C; 10 wt.% Ni; 10 wt.% Co; GHSV = 133,000 mL gcat−1 h−1 | X = 61.5%; SCH4 = 95% | [27] |
| Ni-Ru/Al2O3 | T = 400 °C; 10 wt.% Ni; 1 wt.% Ru; GHSV = 9000 mL gcat−1 h−1 | X = 82%; SCH4 = 100% | [18] |
| Ru-Ni/CeZr | T = 400 °C; 15 wt.% Ni; 1 wt.% Ru; GHSV = 24,000 mL gcat−1 h−1 | X = 62%; SCH4 = 90% | [28] |
| Ni-Ru/ZSM-5 | T = 400 °C; 10 wt.% Ni; 5 wt.% Ru; GHSV = 30,000 mL gcat−1 h−1 | X = 82%; SCH4 = 100% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, M.; Oykova, M.; Dimitrov, M.; Karashanova, D.; Kovacheva, D.; Atanasova, G.; Szegedi, Á. CO2 Hydrogenation to Renewable Methane on Ni/Ru Modified ZSM-5 Zeolites: The Role of the Preparation Procedure. Catalysts 2022, 12, 1648. https://doi.org/10.3390/catal12121648
Popova M, Oykova M, Dimitrov M, Karashanova D, Kovacheva D, Atanasova G, Szegedi Á. CO2 Hydrogenation to Renewable Methane on Ni/Ru Modified ZSM-5 Zeolites: The Role of the Preparation Procedure. Catalysts. 2022; 12(12):1648. https://doi.org/10.3390/catal12121648
Chicago/Turabian StylePopova, Margarita, Manuela Oykova, Momtchil Dimitrov, Daniela Karashanova, Daniela Kovacheva, Genoveva Atanasova, and Ágnes Szegedi. 2022. "CO2 Hydrogenation to Renewable Methane on Ni/Ru Modified ZSM-5 Zeolites: The Role of the Preparation Procedure" Catalysts 12, no. 12: 1648. https://doi.org/10.3390/catal12121648
APA StylePopova, M., Oykova, M., Dimitrov, M., Karashanova, D., Kovacheva, D., Atanasova, G., & Szegedi, Á. (2022). CO2 Hydrogenation to Renewable Methane on Ni/Ru Modified ZSM-5 Zeolites: The Role of the Preparation Procedure. Catalysts, 12(12), 1648. https://doi.org/10.3390/catal12121648








